Abstract
Cellular senescence is a cell fate that occurs in response to numerous types of stress and can promote tissue repair or drive inflammation and disruption of tissue homeostasis depending on the context. Aging and obesity lead to an increase in the senescent cell burden in multiple organs. Senescent cells release a myriad of senescence-associated secretory phenotype factors that directly mediate pancreatic β-cell dysfunction, adipose tissue dysfunction, and insulin resistance in peripheral tissues, which promote the onset of type II diabetes mellitus. In addition, hyperglycemia and metabolic changes seen in diabetes promote cellular senescence. Diabetes-induced cellular senescence contributes to various diabetic complications. Thus, type II diabetes is both a cause and consequence of cellular senescence. This review summarizes recent studies on the link between aging, obesity, and diabetes, focusing on the role of cellular senescence in disease processes.
Keywords: Diabetes, cellular senescence, aging, obesity, senotherapeutics, inflammation
Introduction
Type II diabetes (T2D) is a growing public health problem. Older adults make up the largest segment of people with T2D, and as the number of individuals over 65 is growing rapidly, so is the incidence of T2D (1). One hundred and eleven million cases of T2D were reported in the year 2019 among people over 65 years of age. In this age group, it is estimated that 1 out of 5 people have T2D (2). Aging and obesity are the predominant T2D risk factors and are also associated with an increased burden of senescent cells (SnCs). While cellular senescence is reported to contribute to the pathogenesis of diabetes, the diabetic microenvironment also seems to increase the burden of SnCs (3).
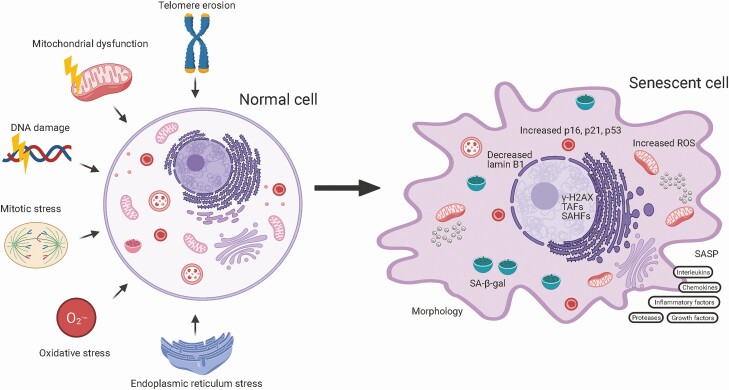
Cellular senescence is a state of indefinite cessation of the cell cycle. Senescence can be triggered by mitotic stress, DNA damage, telomere erosion, mitochondrial dysfunction, oxidative stress, inflammation, mechanical stress, and oncogenic activation (Fig. 1). Some, but not all, SnCs develop a senescence-associated secretory phenotype (SASP), comprising a variety of factors that include proinflammatory interleukins, chemokines, growth factors, proteases, receptors, enzymes that modify the extracellular matrix (ECM), stem cell/progenitor toxins, reactive metabolites, bioactive lipids, microRNAs, and extracellular vesicles. Evidence suggests that the role of SASP is to induce the immune system to clear the damaged SnCs, since they themselves are often resistant to apoptosis. However, with age, immune system function wanes contributing to an increased burden of SnCs in older organisms. Senescence is a conserved tumor suppressor mechanism that prevents replication of a damaged genome and mutagenesis (4). No known physiologic stimuli can cause SnCs to re-enter the cell cycle (5, 6). Thus, the cell cycle arrest in SnCs is considered irreversible. Expression of p16INK4a, a cell cycle inhibitor and tumor suppressor, increases exponentially with chronologic age, suggesting that SnCs accumulate more rapidly in old age (7), likely due to both an increased induction and decreased immune clearance (8). Chronic senescence is established to play a causative role in aging and many age-related diseases (9). However, an acute increase in SnCs plays a positive role in tissue repair, likely through the secreting the factors that remodel tissue locally and activate the immune system to limit that remodeling (10). In young healthy animals, senescence is induced transiently upon tissue injury and is essential for the restoration of tissue homeostasis (10-12).
Figure 1.
Characteristics of senescent cells. SnCs have upregulation of the cell cycle inhibitors p16INK4a and p21CIP1. In addition, SnCs show telomere shortening or damage (TAF), and epigenetic changes (SAHF). SnCs have decreased levels of nuclear Lamin B1 and increased lysosomal SA-β-gal activity. Many SnCs have a SASP comprised of chemokines, inflammatory factors and interleukins, growth factors and regulators, extracellular matrix components, soluble receptors, proteases and regulators, reactive metabolites, bioactive lipids, microRNAs, and extracellular vesicles. Early in senescence, SnCs secrete HMBG1, a key DAMP (an endogenous molecule that activates the innate immune system), which amplifies the SASP. There is no senescent-specific marker and not all SnCs express the same markers, especially in terms of the SASP. ROS, reactive oxygen species; SA-β-gal, senescence-associated β-galactosidase; SAHF, senescence-associated heterochromatin foci; SASP, senescence-associated secretory phenotype; TAF, telomere-associated foci. Figure created with BioRender.com.
Hallmarks of Cellular Senescence
Cell cycle arrest, mediated by upregulation of the cell cycle inhibitors p16INK4a (p16), p15INK4b (p15), p19ARF, and p21CIP (p21), is a universal characteristic of SnCs (Fig. 1). However, it is not a specific marker of senescence, because multiple cellular mechanisms can drive cell cycle arrest. Upregulation of cell cycle inhibitors is also not a pan-senescence marker since various cell cycle regulators are engaged in different cell types and also in response to difference inducers of senescence. SnCs do not respond to mitogenic signaling, which distinguishes them from quiescent cells (13).
Most, if not all, SnCs show signs of DNA damage regardless of the mechanism inducing senescence, including increased γ-H2AX foci, 53BP1 foci, telomere-associated foci (TAF), and de-condensation of pericentromeric satellite heterochromatin (senescence-associated heterochromatin foci) (14) (Fig. 1). DNA damage is a direct inducer of senescence through DNA damage response (DDR) signaling mechanism (15). For example, double-strand breaks are potent activators of the DDR initiate by identification of a broken DNA end by the MRE11/RAD50/NBS1 complex and recruitment of ataxia-telangiectasia mutated (ATM) kinase to that site. ATM functions as a signal transducer, activating hundreds of effector proteins through its kinase activity to promote DNA repair. This includes phosphorylation of the histone H2AX, leading to γ-H2AX foci at sites of DNA repair (16). ATM also phosphorylates and activates p53, which leads to the transcriptional activation of many genes that can mediate senescence if the DDR signaling persists (17). γ-H2AX nuclear foci or phosphorylated p53 are commonly used as markers of senescence, although, again, they are not specific to SnCs.
As described above, SnCs secrete cytokines, chemokines, and proteases, which are collectively referred to as the SASPs (Fig. 1). SASP factors also are not specific to SnCs and can play both positive and negative roles in several biological processes such as wound healing and cancer progression (10, 18). The upregulation of SASP factors is mediated by the proinflammatory transcription factor nuclear factor kappa B (NF-κB) (19). A major inducer of NF-κB activation is the DDR. GATA4 and C/EBPβ are other transcription factors involved in the regulation of SASP genes (20, 21). However, these transcription factors play myriad roles in cells and are not unique to SnCs.
Other characteristics of SnCs include increased protein content, partly regulated through increased mTOR activity, increased expression of senescent cell antiapoptotic proteins, and increased activity of lysosomal senescence-associated beta-galactosidase (SA-β-gal) (22). SnCs are metabolically active with increased AMP:ATP and ADP:ATP ratios (23). Multiple conditions, such as oxidative stress, mutations, infections, and lack of chaperones, can cause endoplasmic reticulum (ER) stress, leading to the accumulation and aggregation of proteins. ER stress initiates the unfolded protein response (UPR), resulting in reduced protein synthesis, enlarged ER, and export of misfolded proteins. SnCs have an increased UPR, possibly in response to the increased protein demand of SASP (24, 25).
A key morphologic feature of SnCs, at least in vitro, is an enlarged and irregularly shaped cell body (26) (Fig. 1). This is driven by cytoskeleton rearrangement, primarily vimentin filaments (24). Another hallmark of SnCs is the loss of lamin B1, a structural protein of the nuclear lamina. The destabilization of the nuclear integrity caused by reduced lamin B1 results in other nuclear changes, such as the loss of condensation of constitutive heterochromatin and the appearance of cytoplasmic chromatin fragments enriched in epigenetic marks associated with DNA damage (27). The most consistent change in the plasma membrane composition in SnCs is the upregulation of caveolin-1, an essential component of cholesterol-enriched microdomains called caveolae. Caveolin-1 contributes to the morphology of SnCs (28).
SnCs have upregulation of many lysosomal proteins and increased content, detected as increased SA-β-gal activity (29) (Fig. 1). Increased lysosomal content could result from the metabolic demands of SnCs and enhanced lysosomal biogenesis. However, residual bodies, namely lipofuscins in SnCs, support the lack of lysosomal removal (30). SnCs also have increased mitochondria content. The membrane potential of SnC mitochondria is often decreased, leading to the release of mitochondrial enzymes and increased reactive oxygen species (ROS) production (31) (Fig. 1). The major source of the extra-mitochondrial content is the accumulation of dysfunctional mitochondria due to reduced mitophagy (32). This is, at least in part, a consequence of reduced mitochondrial fission and increased fusion (33).
Senotherapeutics
Molecules that can specifically kill SnCs (senolytics) or dampen their SASP and other senescence markers (senomorphics) have been identified (34). Senolytics often target pro-survival pathways upregulated in SnCs, which include BCL-2-BCL-XL, p53-p21, and PI3K-AKT, resulting apoptosis of the SnC. The first senolytic drugs identified were the natural product fisetin (35), the combination of dasatinib and quercetin (D + Q) (34), HSP90 inhibitors (36), and Navitoclax (37). Additional natural products, novel compounds, and re-purposed FDA approved drugs with senolytic activity were identified more recently (38, 39). Drugs with senomorphic activity include the mTOR inhibitor rapamycin, used clinically as an immunosuppressant, and inhibitors of IKK-NF-kB and JAK-STAT (40). In addition, metformin, a drug used to treat T2D, prevents NF-κB activation (41), thereby reducing SASP and acting as a senomorphic.
Senescence Drives Aging
Various markers of senescence, including SA-β-gal, p16, TAFs, and activation of the DDR, are increased in tissues of aged mammals, including rodents (42-44), baboons (45, 46), and humans (47-49), suggesting a link between SnCs and age-associated pathologies. Studies performed using BubR1H/H mice, which age rapidly due to an insufficiency of a pivotal mitotic checkpoint protein, revealed a causal link between SnCs and aging (50). Prematurely aged tissues in this model, including skeletal muscle, eye, and adipose tissue (AT), accumulate high-level of p16Ink4a-positive SnCs, which contribute to the sarcopenia, cataracts, and lipodystrophy. The onset of these disease is delayed in BubR1H/H mice when p16Ink4a-expressing cells are ablated via drug-induced activation of transgenic caspase-8, expression of which is driven by the p16 promotor (referred to as the INK-ATTAC construct) (51). Elimination of SnCs in aged wild-type INK-ATTAC mice results in an extension in the median but not maximal lifespan, suggesting that SnCs contribute to mortality in mice. The Ercc1−/Δ mouse model of a human progeroid syndrome (52) has reduced expression of the ERCC1-XPF endonuclease, which is essential for multiple DNA repair mechanisms. Ercc1−/Δ mice spontaneously develop senescence in multiple organs, including AT, liver, and pancreas, and interestingly a similar distribution of SnCs is seen in aged wild-type mice (53-55). Removal of SnCs using senolytic drugs blunts multiple aging features in Ercc1−/Δ mice and aged wild-type mice, including renal dysfunction, liver damage, pancreatic damage, and neurodegeneration, while improving strength and endurance, and activity (9, 35, 56). Collectively, these studies demonstrate that SnCs drive age-related morbidity and mortality (57).
Cellular Senescence in Pathogenesis of Diabetes
The role of SnCs in diabetes is complex. SnCs play a part in T2D pathophysiology through a direct effect on pancreatic β-cell function, participation in AT dysfunction, and SASP-mediated tissue damage (58, 59). Metabolic changes observed in diabetes, such as high circulating glucose and altered lipid metabolism, can also stimulate SnC formation (60). Thus, SnCs could be part of a pathogenic loop in diabetes, as both a cause and effect of metabolic changes and tissue damage (58).
Pancreatic β-Cells
Defective insulin secretion in T2D is caused by β-cell dysfunction and reduced β-cell mass. β-cell dysfunction is caused by chronic hyperglycemia and/or hyperlipidemia, referred to as glucotoxicity and lipotoxicity, respectively (61). Chronically high levels of free fatty acids (FFAs) and glucose are toxic to β-cells, which can manifest in the form of ER stress, the production of ROS, and/or mitochondrial dysfunction (62, 63). To further complicate matters, β-cells respond to FFA by producing and secreting inflammatory cytokines (interleukin [IL]-1β, IL-6, IL-8) and chemokines (CCL2, CXCL1) (64), of which IL-1β is a major player and its secretion affects the activation of resident immune cells (65). β-cells mediate their response to FFA through the toll-like receptor 4 (TLR4)-MyD88 pathway, which leads to the activation of NF-κB (64, 66). Experiments with isolated human islet cells or with mouse cell lines (MIN6, INS-1) revealed that TLR4 is required for the secretion of IL-1β following exposure to high FFA concentrations (66). Knock-down of TLR4 or Myd88 expression, or blocking the signaling pathway, attenuates production of IL-1β, and insulin production is restored.
Numerous studies have documented the accumulation of macrophages (MΦ) within islets of T2D patients and mouse models of obesity and diabetes (66-69). Infiltration and polarization of MΦ from an anti-inflammatory (M2) profile towards a proinflammatory (M1) profile (CD11b+Ly-6C- MΦ) are characteristic of islets in T2D (64). Several mouse models of obesity, as well as mice placed on a high-fat diet (HFD), display a similar increase in islet MΦ. The secretion of chemokines like CCL2 and CXCL1 help to recruit circulating monocytes that convert into CD11b+Ly-6C+ MΦ. T cells are not increased in islets of mouse models of T2D, however, increased B cells are detected (70). In fact, a small percentage of T2D patients test positive for autoantibodies to islet proteins (71, 72) (insulin, insulinoma-associated protein-2, glutamic acid decarboxylase), which occurs most frequently in older patients who have had diabetes for a longer duration. Additionally, T2D patients can also have auto-reactive T cells, which in some elderly patients can occur in the absence of autoantibodies.
Glucose tolerance worsens with age in T2D, reflecting a progressive reduction in the responsiveness of β-cells to glucose stimulation and reduced sensitivity of peripheral tissues to insulin. Prior to the onset of hyperglycemia, β-cells can compensate for increased metabolic demands with increased insulin secretion. However, this compensation becomes limited by the age-related decline in β-cell proliferation seen in both rodents (73) and humans (74). This lack of proliferation in response to increased insulin demand may arise partially from the accumulation of senescent β-cells (75).
Evidence for senescent β-cells includes diminished β-cell proliferation and increased expression of senescence markers are found in diet-induced T2D mice (76) and the telomere length in β-cells from patients with T2D is significantly shorter than in the control subjects (77). High-glucose drives oxidative stress in β-cells, which may contribute to telomerase dysfunction those cells (78). Haploinsufficiency of Tert in mice shortens telomeres, including in β-cells. This leads to fasting hyperglycemia, increased expression of p16Ink4a in pancreatic islets, and impaired mitochondrial membrane hyperpolarization and Ca2+ mobilization β-cells, which are crucial for insulin exocytosis (79).
The number of SA-β-gal+ cells is increased in islets isolated from the pancreas of healthy older people compared to young, and this is increased further in islets from T2D patients compared to nondiabetic, suggesting that β-cell senescence might contribute to the pathogenesis of T2D (80). In mice, SA-β-gal+ β-cells have decreased expression of β-cell identity genes (Ins1, Pdx1, Mafa, Neurod1) with a concomitant increase in expression of markers of senescence (p16 and p21), and SASP (Ccl2, Il1α, Il6, Tnfα). Senescent β-cells have increased basal insulin secretion (81) and are transcriptionally rewired, displaying decreased expression of genes required for cellular depolarization, incretin signaling, and production of insulin granules (82), all necessary for insulin secretion.
Senescent β-cells have also occurred in the pancreas of patients with type 1 autoimmune diabetes (T1D) compared with nondiabetic people and individuals who are autoantibody positive prior to onset of T1D (83). This suggests a correlation between disease progression and the level of senescent β-cells. In the nonobese diabetic mouse, an animal model of T1D, SnCs and SASP increase with time in pancreatic islets (83). Senolytics reduce senescence markers and the incidence of diabetes in these mice (83). In mice placed on an HFD, the onset of insulin resistance (IR) is associated with senescent β-islet cells and senolytics can restore glucose metabolism (84). p16INK4a expression increases in human and mouse β-cells with age, and this contributes to their reduction in regenerative capacity (79, 85, 86). Clearance of p16Ink4a+ cells in mice, using the INK-ATTAC construct, improves glucose metabolism and insulin secretion, while decreasing the expression of proinflammatory SASP factors in islets (80). The senolytic ABT263 (Navitoclax), reverses hyperglycemia and restores the normal β-cell gene expression profile mice with induced insulin resistance caused by the insulin receptor antagonist S961 (80). The BCL-2 pathway, 1 of several anti-apoptotic pathways, is upregulated in SA-β‐gal+ β‐cells, consistent with ABT263 killing SA-β‐gal+ islet cells in vitro (80).
Adipose Tissue Senescence and Inflammation
AT is the largest and most active endocrine organ for regulating energy balance, glucose and lipid homeostasis, and immunity in response to the fluctuation in nutritional status, environment, lifestyle, and aging (87). White adipose tissue (WAT) stores surplus energy in the form of triglycerides that are hydrolyzed into FFA and glycerol for energy supply during nutrient deprivation. Brown AT (BAT) or beige adipocytes convert chemical energy, mainly FFA, into heat via the mitochondrial uncoupling reaction to maintain core body temperature and energy balance. The amount and function of AT are closely associated with age, calorie intake, physical activity, and health status. An increase in visceral WAT (vWAT), but not subcutaneous WAT (sWAT) with obesity is causatively associated with T2D and cardiovascular diseases. vWAT is more prone to senescence compared to sWAT (88). Senescent AT impairs lipid handling, while promoting IR, defective adaptive thermogenesis, aberrant adipocytokine production, and interferes with normal physiological functions of other metabolic organs via SASP.
BubR1 H/H mice display dramatic loss of sWAT and AT senescence (89). Clearance of these p16Ink4a+ cells in BubR1H/H; INK-ATTAC mice reverses AT senescence, thereby, preventing aging-related lipodystrophy (9). AT from obese individuals exhibits increased oxidative stress and telomere shortening, which induce senescence. Intracellular ROS activates p38 mitogen-activated protein kinases, which in turn induces p53-p21–dependent senescence (87). IR is one of the consequences of AT senescence when p53 is activated. Transgenic mice with overexpression of p53 have increased senescence and IR, whereas p53 inactivation results in the opposite effects in diabetic mice (59, 90).
Metabolic stress induced by high calorie intake induces an inflammatory microenvironment, in particular in AT. This affects not only resident immune cells, but also infiltrating immune cells, which heighten inflammation and contribute to IR through secretion of inflammatory cytokines (82, 91-93). Excessive nutrient intake activates adipocytes to internalize and accumulate FFA through a CD36-driven mechanism regulated by PPARγ (94-96). Adipocytes respond to the accumulation of lipids by enlarging and proliferating and initiating an inflammatory response through an HIF1α-NF-kB–driven mechanism (97-99). Similar to adipocytes, adipose resident MΦ also accumulate FFA through CD36. These MΦ further contribute to the inflammatory response by secreting TNF-α, IL-6, Il-1β, MCP-1, CX3CL1, and IL-8 (94, 95, 100). vWAT of wild-type and db/db mice on a HFD exhibit elevated markers of senescence (SA-β-gal, p16Ink4a) (101), a diminished capacity to clear glucose upon challenge, and IR. Senolytics significantly improve glucose tolerance and insulin sensitivity (101). Exercise has a similar effect as senolytics in these diabetic mice (102).
There are numerous interactions between the immune system and AT during obesity that contribute to IR (Table 1). This inflammatory response, and the subsequent hyperglycemia, enhances the formation of SnCs, which can exacerbate IR (83, 84). The number of senescent adipocytes increases the number of MΦ positive for the cyclic ADP ribose hydrolase CD38 (103). CD38 expression increases along with inflammatory and senescence markers in the AT of old mice, in addition to CD68, a marker of active MΦ (103). Increased CD38 drives depletion of the critical metabolite NAD+ (104). The increase in IR is paralleled by an increase in senescent T cells both in humans and mice (105). Older T2D patients have elevated levels of CD8+CD57+CD28– T cells, which exhibit markers of senescence. Levels of senescent CD8+ T cells are increased in pre-diabetics compared to nondiabetic controls and may be predictive of the development of hyperglycemia (105, 106). Senescent T cells in mice, defined as CD8+CD44+PD-1+, are elevated in old wild-type mice placed on a HFD for 2 months, which leads to IR, inflamed AT, and increased SnCs in AT and liver (105). Notably, driving senescence in the immune system alone, is sufficient to drive increased expression of senescence markers in pancreatic tissue and organ damage, as determined by serum amylase levels (107).
Table 1.
A short list of the interactions occurring between immune cells and inflamed adipose tissue
| Immune cell subsets | Impact on adipose tissue |
|---|---|
| T cells | Peripheral CD8+ T cells infiltrate inflamed AT (92, 93, 100, 136) |
| Infiltrating T cells adopt a Th1 (interferon secreting) or a Th17 (IL-17-secreting) profile (93, 137-139) | |
| Decreased antigen specificities recognized by infiltrating CD8+ T cells (100) | |
| Resident CD4+ regulatory T cells maintain AT homeostasis through PPARγ. However, during obesity, the frequency of resident CD4+ regulatory T cells decreases (136, 140-143) | |
| Macrophage | Infiltration into AT mediated by MCP-1 chemokine secreted by resident T cells (97, 136) |
| Excessive nutrients induce activation of MΦ driven through HIF1α, NF-κB, and IL-1β (94, 95, 136, 144) | |
| DC | Infiltration into AT mediated by CX3CL1 chemokine secreted by resident T cells (97, 145) |
| In lean mice, DCs exhibit a CD11c+CD103+ phenotype that induces CD4+Foxp3+ Tregs to maintain AT homeostasis. In obese or db/db knockout mice, DC exhibit a CD11c+F4/80lo phenotype and induce Th17 cells (145, 146) | |
| Other innate cells | In lean conditions, innate lymphoid cells type-2 induce anti-inflammatory responses and attenuate insulin resistance, but this is lost during obesity (147-149) |
Abbreviations: AT, adipose tissue; DC, dendritic cell; IL, interleukin; NF-κB, nuclear factor kappa B.
Peripheral Tissues
Fat is redistributed outside fat depots with aging, accumulating in muscle, liver, bone marrow, and other ectopic sites. Insulin-resistant hypertrophic adipocytes have augmented lipolytic activity and diminished ability to take up FFA, resulting in a redirection of lipids toward nonadipose tissues (ectopic fat deposition) (108). If fatty acid β-oxidation in the mitochondria cannot keep up with the increased supply of FFA to nonadipose tissues accumulation of lipid intermediates like diacylglycerol and ceramides occurs. These lipid intermediates lead to activation of serine/threonine kinases that phosphorylate insulin receptor substrates on serine residues. Phosphorylated insulin receptor substrates do not function properly, leading to impaired insulin signaling and disruption of normal metabolic processes. Accumulation of lipid intermediates promotes ER stress, mitochondrial dysfunction, and apoptosis (109). Dysregulation of liver and skeletal muscle metabolism can strongly impact whole-body glucose homeostasis and insulin sensitivity (110).
Cellular Senescence in Diabetic Complications
People with diabetes are more likely to develop age-related morbidities, such as frailty, Alzheimer’s disease, mild cognitive impairment, cardiovascular disease, osteoporosis, visual impairment, and bladder and renal dysfunction, indicating that T2D itself might represent a proaging state (3). Advanced glycation end products (AGEs) are proteins or lipids that become glycated when combined with sugars. AGEs contribute to multiple microvascular and macrovascular complications by forming of cross-links between molecules in the basement membrane of the ECM and by engaging the receptor for advanced glycation end products (111). In diabetes, the increased AGE formation induces endothelial cell senescence (112). Ex vivo, endothelial cells from healthy aged and T2D individuals show chronic NF-κB activation. Blockade of NF-κB-mediated inflammatory responses in endothelial cells prolongs the lifespan of mice by preventing IR (113). Endothelial cell senescence impairs systemic metabolic health (114) (Fig. 2). The presence of elevated oxidized low-density lipoproteins, commonly observed in diabetics, reduces the number of circulating endothelial progenitor cells and drives their senescence (115).
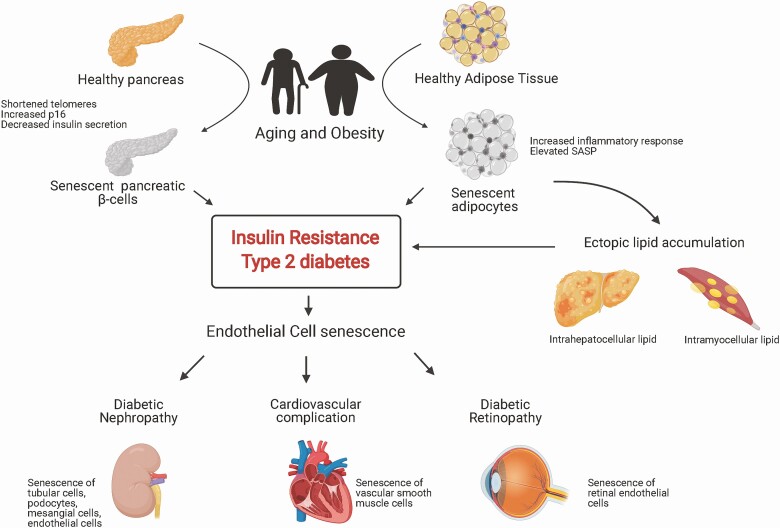
Figure 2.
Diabetes and cellular senescence. Aging and obesity induce inflammation and cellular senescence in pancreatic β-cells and adipocytes. Senescence of β-cells results in β-cell dysfunction and decreased insulin exocytosis. Senescence of adipocytes leads to ectopic lipid accumulation and insulin resistance. Together these contribute to progression of T2D. Hyperglycemia-induced endothelial senescence plays a major role in development of various diabetic complications such as diabetic retinopathy, diabetic nephropathy, and cardiovascular complications. Figure created with BioRender.com.
Diabetic Retinopathy
In diabetic retinopathy (DR), the microvessels in the retina become damaged, degenerate, and regrow in an aberrant manner. Diseased vessels show upregulation of senescence marker expression (116). In retinas of patients with DR and in a mouse model of proliferative DR, a high level of senescent p16INK4a-expressing cells accumulates. Senolysis by means of genetic approaches that clear p16Ink4a expressing cells or small molecule inhibitors of the antiapoptotic protein BCL-xL, suppress pathological angiogenesis (116). In DR, endothelial cells are the primary targets of glucose-induced damage. Diabetes-induced retinal endothelial cell senescence involves sequential events initiated by NADPH oxidase-2 (NOX2) activation, increased expression of its target arginase-1 (Arg1), and ROS production (117). Increased Arg1 expression promotes senescence through a mechanism involving increases in the expression of p16Ink4a, p21Cip1, and p53 (118). Arg1 gene deletion or pharmacology inhibition protects against endothelial cell senescence in diabetic mice (118).
Glucose-induced downregulation of SIRT1 in endothelial cells promotes senescence and increases the production of several ECM proteins and vasoactive factors in diabetes (119). MicroRNAs (miR-34a, miR-1, miR-19b, miR-320a) are translational suppressors of SIRT1 and also implicated in endothelial cell senescence (120, 121). Oxidative stress suppression of SIRT6 activity also drives endothelial cell senescence and likely contributes to the pathogenesis of DR (122). Endothelial cell senescence is reduced by Donepezil, which increases SIRT1 activity (123). Senescent endothelial cells have a secretome that attracts neutrophils and triggers the production of neutrophil extracellular traps (NETs). NETs ultimately clear senescent endothelial cells and remodel unhealthy blood vessels. Genetic or pharmacological inhibition of NETosis prevents the clearance of senescent endothelial cells and promotes DR (124).
Cardiovascular Complications
Vascular aging contributes to the high morbidity and mortality of cardiovascular diseases observed in patients with diabetes. Vascular smooth muscle cells (VSMCs) are vital components of the vascular walls and senescence of these cells plays a key role in vascular aging. For instance, VSMC senescence can promote vascular calcification and atherosclerosis (125). Vascular calcification is a key component of vasculopathy commonly seen in patients with T2D. SnCs in proximity to blood vessel walls (eg, in perivascular AT) may contribute to the cellular dysfunction that underlies the development and progression of vascular diseases such as aneurysm and atherosclerosis (126). In VSMCs, adiponectin can reduce high-glucose-induced senescence and may be a potential therapeutic agent for cardiovascular complications in diabetic patients (127).
Diabetic Nephropathy
Diabetic nephropathy is the leading cause of chronic kidney disease and renal failure in developed countries. In the past two decades, the morbidity and mortality of diabetic nephropathy have risen rapidly globally (128). The mechanisms driving diabetic nephropathy involve a multifactorial interaction of metabolic and hemodynamic factors, including high blood glucose, AGE, the renin–angiotensin system, and protein kinase-C–induced generation of ROS, which mediates the activation of the transcription factor NF-κB (129). Diabetic nephropathy is strongly associated with accelerated senescence of renal tubular cells, podocytes, mesangial cells, and endothelial cells. Hyperglycemia can directly induce senescence in mesangial (130) and tubular cells (131). Interestingly, high glucose can also induce MΦ to secrete inflammatory factors, creating a state of low-grade inflammation (129).
In both T1D and T2D, telomere attrition is associated with renal cell senescence, proteinuria, and progression of diabetic nephropathy (132, 133). In streptozotocin-induced T1D mice, renal expression of p21 is increased dramatically along with an increase in SA-β-gal staining in tubular epithelial cells. However, other cyclin-dependent kinase inhibitors such as p16Ink4a and p27Kip1 were not altered. In the T1D kidney, tubular senescence was induced by hyperglycemia via a sodium-glucose transport protein 2 (SGLT2) (134). Glomerular mesangial cells, which provide structural support for the glomerular capillary loops and regulate the ultrafiltration surface for glomerular filtration, are directly affected by hyperglycemia. A high extracellular glucose concentration promotes ECM accumulation, cell cycle arrest, cellular hypertrophy, and induces senescence in cultured glomerular mesangial cells (135).
Conclusion
The accumulation of SnCs during aging and obesity is involved in the pathogenesis of T1D and T2D. High glucose induces senescence of a variety of cell types, which contributes to the development of diabetic complications, including nephropathy, retinopathy, vasculopathy, and cardiovascular disease. Thus, SnCs play a major role in initiating and exacerbating diabetes. Given that oral administration of senolytic combination dasatinib and quercetin (D+Q) improves insulin sensitivity and reduces the AT inflammation in obese mice (101), 2 phase 2, randomized clinical trials of D+Q and the senolytic fisetin were initiated for subjects with diabetic kidney disease at Mayo Clinic (NCT03325322 and NCT02848131). For the D+Q study, senolytic treatment has already been demonstrated to reduce the senescent cell burden in AT biopsied from obese diabetic subjects (56). Importantly, no serious drug side effects have been observed to date (56). Therefore, it is possible that senolytics, once optimized, will be effective in delaying the onset and reducing the severity of diabetic complications, particularly in obese individuals.
Acknowledgements
Financial Support: This work was supported by the NIH/NIA grants P01 R01 AG063543, R01 AG043376, U19 AG056278, and P01 AG062413.
Conflicts of Interest: Pending patents on senolytic drugs and their uses are held the University of Minnesota (L.J.N., P.D.R.). L.J.N. and P.D.R. are co-founders of NRTK Biosciences, a startup focused on the development of novel senolytics. L.J.N. has consulted for Merck and Ono Pharma on senescent cells as a therapeutic target. L.J.N. and P.D.R. are on the Scientific Advisory Board for Innate Biologics, developing bacterial effector proteins, and P.D.R. is co-founder and member of the Scientific Advisory Board for Genascence Corporation, a gene therapy company focused on osteoarthritis.
Glossary
Abbreviations
- AGE
advanced glycation end product
- AT
adipose tissue
- BAT
brown adipose tissue
- DDR
DNA damage response
- DR
diabetic retinopathy
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- IL
interleukin
- IR
insulin resistance
- FFA
free fatty acid
- NET
neutrophil extracellular trap
- NF-κB
nuclear factor kappa B
- ROS
reactive oxygen species
- SA-β-gal
senescence-associated beta-galactosidase
- SAHF
senescence-associated heterochromatin foci
- SASP
senescence-associated secretory phenotype
- SnC
senescent cell
- T2D
type II diabetes
- UPR
unfolded protein response
- VSMC
Vascular smooth muscle cell
- WAT
white adipose tissue
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Kalyani RR, Golden SH, Cefalu WT. Diabetes and aging: unique considerations and goals of care. Diabetes Care. 2017;40(4):440-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, Zhao Y.. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Medicina (Kaunas) 2019;55(9):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmer AK, Gustafson B, Kirkland JL, Smith U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. 2019;62(10):1835-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Regulski MJ. Cellular Senescence: what, why, and how. Wounds. 2017;29(6):168-174. [PubMed] [Google Scholar]
- 6. Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28(6):436-453. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8(4):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He S, Sharpless NE. Senescence in Health and Disease. Cell. 2017;169(6):1000-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Storer M, Mas A, Robert-Moreno A, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155(5):1119-1130. [DOI] [PubMed] [Google Scholar]
- 12. Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10(1):51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dimri GP, Campisi J. Molecular and cell biology of replicative senescence. Cold Spring Harb Symp Quant Biol. 1994;59:67-73. [DOI] [PubMed] [Google Scholar]
- 14. Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY). 2016;8(1):3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White RR, Vijg J. Do DNA Double-Strand Breaks Drive Aging? Mol Cell. 2016;63(5):729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Celeste A, Petersen S, Romanienko PJ, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296(5569):922-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turenne GA, Paul P, Laflair L, Price BD. Activation of p53 transcriptional activity requires ATM’s kinase domain and multiple N-terminal serine residues of p53. Oncogene. 2001;20(37):5100-5110. [DOI] [PubMed] [Google Scholar]
- 18. Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohanna M, Giuliano S, Bonet C, et al. Senescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS). Genes Dev. 2011;25(12):1245-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019-1031. [DOI] [PubMed] [Google Scholar]
- 21. Kang C, Xu Q, Martin TD, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015;349(6255):aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15(11):1139-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James EL, Michalek RD, Pitiyage GN, et al. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J Proteome Res. 2015;14(4):1854-1871. [DOI] [PubMed] [Google Scholar]
- 24. Druelle C, Drullion C, Deslé J, et al. ATF6α regulates morphological changes associated with senescence in human fibroblasts. Oncotarget. 2016;7(42):67699-67715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pluquet O, Pourtier A, Abbadie C. The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol. 2015;308(6):C415-C425. [DOI] [PubMed] [Google Scholar]
- 26. Bent EH, Gilbert LA, Hemann MT. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev. 2016;30(16):1811-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sadaie M, Salama R, Carroll T, et al. Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev. 2013;27(16):1800-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zou H, Stoppani E, Volonte D, Galbiati F. Caveolin-1, cellular senescence and age-related diseases. Mech Ageing Dev. 2011;132(11-12):533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee BY, Han JA, Im JS, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187-195. [DOI] [PubMed] [Google Scholar]
- 30. Georgakopoulou EA, Tsimaratou K, Evangelou K, et al. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging (Albany NY). 2013;5(1):37-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Passos JF, Saretzki G, Ahmed S, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5(5):e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tai H, Wang Z, Gong H, et al. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy. 2017;13(1):99-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalle Pezze P, Nelson G, Otten EG, et al. Dynamic modelling of pathways to cellular senescence reveals strategies for targeted interventions. PLoS Comput Biol. 2014;10(8):e1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. Ebiomedicine. 2018;36:18-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schafer MJ, White TA, Iijima K, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robbins PD, Jurk D, Khosla S, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol. 2021;61:779-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borghesan M, Hoogaars WMH, Varela-Eirin M, Talma N, Demaria M. A senescence-centric view of aging: implications for longevity and disease. Trends Cell Biol. 2020;30(10):777-791. [DOI] [PubMed] [Google Scholar]
- 40. Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanigur Sultuybek G, Soydas T, Yenmis G. NF-κB as the mediator of metformin’s effect on ageing and ageing-related diseases. Clin Exp Pharmacol Physiol. 2019;46(5):413-422. [DOI] [PubMed] [Google Scholar]
- 42. Burd CE, Sorrentino JA, Clark KS, et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell. 2013;152(1-2):340-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8(3):311-323. [DOI] [PubMed] [Google Scholar]
- 45. Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311(5765):1257. [DOI] [PubMed] [Google Scholar]
- 46. Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128(1):36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int. 2004;65(2):510-520. [DOI] [PubMed] [Google Scholar]
- 48. Ressler S, Bartkova J, Niederegger H, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5(5):379-389. [DOI] [PubMed] [Google Scholar]
- 49. Chkhotua AB, Gabusi E, Altimari A, et al. Increased expression of p16(INK4a) and p27(Kip1) cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am J Kidney Dis. 2003;41(6):1303-1313. [DOI] [PubMed] [Google Scholar]
- 50. Hanks S, Coleman K, Reid S, et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet. 2004;36(11):1159-1161. [DOI] [PubMed] [Google Scholar]
- 51. Baker DJ, Perez-Terzic C, Jin F, et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol. 2008;10(7):825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Niedernhofer LJ, Garinis GA, Raams A, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444(7122):1038-1043. [DOI] [PubMed] [Google Scholar]
- 53. Gregg SQ, Robinson AR, Niedernhofer LJ. Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair (Amst). 2011;10(7):781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robinson AR, Yousefzadeh MJ, Rozgaja TA, et al. Spontaneous DNA damage to the nuclear genome promotes senescence, redox imbalance and aging. Redox Biol. 2018;17:259-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yousefzadeh MJ, Zhao J, Bukata C, et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020;19(3):e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16(10):718-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Regulski M. Understanding diabetic induction of cellular senescence: a concise review. Wounds. 2018;30(4):96-101. [PubMed] [Google Scholar]
- 61. Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119-S124. [DOI] [PubMed] [Google Scholar]
- 62. Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab. 2011;22(7):266-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lytrivi M, Castell AL, Poitout V, Cnop M. Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes. J Mol Biol. 2020;432(5):1514-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eguchi K, Nagai R. Islet inflammation in type 2 diabetes and physiology. J Clin Invest. 2017;127(1):14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110(6):851-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15(4):518-533. [DOI] [PubMed] [Google Scholar]
- 67. Chan JY, Lee K, Maxwell EL, Liang C, Laybutt DR. Macrophage alterations in islets of obese mice linked to beta cell disruption in diabetes. Diabetologia. 2019;62(6):993-999. [DOI] [PubMed] [Google Scholar]
- 68. Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56(9):2356-2370. [DOI] [PubMed] [Google Scholar]
- 69. Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52(8):1686-1688. [DOI] [PubMed] [Google Scholar]
- 70. Butcher MJ, Hallinger D, Garcia E, et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57(3):491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li R, Huang J, Yu Y, Yang Y. Islet autoantibody patterns in patients with type 2 diabetes aged 60 and higher: a cross-sectional study in a Chinese hospital. Front Endocrinol (Lausanne). 2018;9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brooks-Worrell B, Narla R, Palmer JP. Islet autoimmunity in phenotypic type 2 diabetes patients. Diabetes Obes Metab. 2013;15(Suppl 3):137-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54(9):2557-2567. [DOI] [PubMed] [Google Scholar]
- 74. Gregg BE, Moore PC, Demozay D, et al. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97(9):3197-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li N, Liu F, Yang P, et al. Aging and stress induced β cell senescence and its implication in diabetes development. Aging (Albany NY). 2019;11(21):9947-9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia. 2005;48(1):58-67. [DOI] [PubMed] [Google Scholar]
- 77. Tamura Y, Izumiyama-Shimomura N, Kimbara Y, et al. β-cell telomere attrition in diabetes: inverse correlation between HbA1c and telomere length. J Clin Endocrinol Metab. 2014;99(8):2771-2777. [DOI] [PubMed] [Google Scholar]
- 78. Matsui-Hirai H, Hayashi T, Yamamoto S, et al. Dose-dependent modulatory effects of insulin on glucose-induced endothelial senescence in vitro and in vivo: a relationship between telomeres and nitric oxide. J Pharmacol Exp Ther. 2011;337(3):591-599. [DOI] [PubMed] [Google Scholar]
- 79. Guo N, Parry EM, Li LS, et al. Short telomeres compromise β-cell signaling and survival. PLoS One. 2011;6(3):e17858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aguayo-Mazzucato C, Andle J, Lee TB Jr, et al. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019;30(1):129-142.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Aguayo-Mazzucato C, van Haaren M, Mruk M, et al. β Cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab. 2017;25(4):898-910.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Aguayo-Mazzucato C. Functional changes in beta cells during ageing and senescence. Diabetologia. 2020;63(10):2022-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Thompson PJ, Shah A, Ntranos V, Van Gool F, Atkinson M, Bhushan A. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab. 2019;29(5):1045-1060.e10. [DOI] [PubMed] [Google Scholar]
- 84. Aguayo-Mazzucato C, Andle J, Lee TB Jr, et al. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019;30(1):129-142.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443(7110):453-457. [DOI] [PubMed] [Google Scholar]
- 86. Chen H, Gu X, Su IH, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23(8):975-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu Z, Wu KKL, Jiang X, Xu A, Cheng KKY. The role of adipose tissue senescence in obesity- and ageing-related metabolic disorders. Clin Sci (Lond). 2020;134(2):315-330. [DOI] [PubMed] [Google Scholar]
- 88. Yamada T, Kamiya M, Higuchi M, Nakanishi N. Fat depot-specific differences of macrophage infiltration and cellular senescence in obese bovine adipose tissues. J Vet Med Sci. 2018;80(10):1495-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Baker DJ, Jeganathan KB, Cameron JD, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36(7):744-749. [DOI] [PubMed] [Google Scholar]
- 90. Minamino T, Orimo M, Shimizu I, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15(9):1082-1087. [DOI] [PubMed] [Google Scholar]
- 91. Ehrhardt N, Cui J, Dagdeviren S, et al. Adiposity-independent effects of aging on insulin sensitivity and clearance in mice and humans. Obesity (Silver Spring). 2019;27(3):434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol. 2020;11:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842(3):446-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7(1):48-52. [DOI] [PubMed] [Google Scholar]
- 95. Kennedy DJ, Kuchibhotla S, Westfall KM, Silverstein RL, Morton RE, Febbraio M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc Res. 2011;89(3):604-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2(72):re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Daryabor G, Kabelitz D, Kalantar K. An update on immune dysregulation in obesity-related insulin resistance. Scand J Immunol. 2019;89(4):e12747. [DOI] [PubMed] [Google Scholar]
- 98. Liu W, Shen SM, Zhao XY, Chen GQ. Targeted genes and interacting proteins of hypoxia inducible factor-1. Int J Biochem Mol Biol. 2012;3(2):165-178. [PMC free article] [PubMed] [Google Scholar]
- 99. Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013;33(5):904-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McDonnell WJ, Koethe JR, Mallal SA, et al. High CD8 T-cell receptor clonality and altered CDR3 properties are associated with elevated isolevuglandins in adipose tissue during diet-induced obesity. Diabetes. 2018;67(11):2361-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Palmer AK, Xu M, Zhu Y, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18(3):e12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schafer MJ, White TA, Evans G, et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016;65(6):1606-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Covarrubias AJ, Kale A, Perrone R, et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat Metab. 2020;2(11):1265-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chini CCS, Peclat TR, Warner GM, et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat Metab. 2020;2(11):1284-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yi HS, Kim SY, Kim JT, et al. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis. 2019;10(3):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lau EYM, Carroll EC, Callender LA, et al. Type 2 diabetes is associated with the accumulation of senescent T cells. Clin Exp Immunol. 2019;197(2):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yousefzadeh MJ, Flores RR, Zhu Y, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594(7861):100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Blüher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117(6):241-250. [DOI] [PubMed] [Google Scholar]
- 109. Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90(5A):11G-18G. [DOI] [PubMed] [Google Scholar]
- 111. Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597-605. [DOI] [PubMed] [Google Scholar]
- 112. Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hasegawa Y, Saito T, Ogihara T, et al. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012;125(9):1122-1133. [DOI] [PubMed] [Google Scholar]
- 114. Barinda AJ, Ikeda K, Nugroho DB, et al. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat Commun. 2020;11(1):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Imanishi T, Hano T, Sawamura T, Nishio I. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol. 2004;31(7):407-413. [DOI] [PubMed] [Google Scholar]
- 116. Crespo-Garcia S, Tsuruda PR, Dejda A, et al. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab. 2021;33(4):818-832.e7. [DOI] [PubMed] [Google Scholar]
- 117. Rojas M, Lemtalsi T, Toque HA, et al. NOX2-induced activation of arginase and diabetes-induced retinal endothelial cell senescence. Antioxidants (Basel). 2017;6(2):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shosha E, Xu Z, Narayanan SP, et al. Mechanisms of diabetes-induced endothelial cell senescence: role of arginase 1. Int J Mol Sci. 2018;19(4):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8(1):e54514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Thounaojam MC, Jadeja RN, Warren M, et al. MicroRNA-34a (miR-34a) mediates retinal endothelial cell premature senescence through mitochondrial dysfunction and loss of antioxidant activities. Antioxidants (Basel). 2019;8(9):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Liu J, Chen S, Biswas S, et al. Glucose-induced oxidative stress and accelerated aging in endothelial cells are mediated by the depletion of mitochondrial SIRTs. Physiol Rep. 2020;8(3):e14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Liu R, Liu H, Ha Y, Tilton RG, Zhang W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed Res Int. 2014;2014:902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang T, Tian F, Wang J, et al. Donepezil attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells through SIRT1 activation. Cell Stress Chaperones. 2015;20(5):787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Binet F, Cagnone G, Crespo-Garcia S, et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science. 2020;369(6506):eaay5356. [DOI] [PubMed] [Google Scholar]
- 125. Liu Y, Drozdov I, Shroff R, Beltran LE, Shanahan CM. Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ Res. 2013;112(10):e99-109. [DOI] [PubMed] [Google Scholar]
- 126. Parvizi M, Ryan ZC, Ebtehaj S, Arendt BK, Lanza IR. The secretome of senescent preadipocytes influences the phenotype and function of cells of the vascular wall. Biochim Biophys Acta Mol Basis Dis. 2021;1867(1):165983. [DOI] [PubMed] [Google Scholar]
- 127. Cui XJ, Lin X, Zhong JY, et al. Adiponectin attenuates the premature senescence of vascular smooth muscle cells induced by high glucose through mTOR signaling pathway. Aging Med (Milton). 2020;3(3):178-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Xiong Y, Zhou L. The signaling of cellular senescence in diabetic nephropathy. Oxid Med Cell Longev. 2019;2019:7495629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhang X, Chen X, Wu D, et al. Downregulation of connexin 43 expression by high glucose induces senescence in glomerular mesangial cells. J Am Soc Nephrol. 2006;17(6):1532-1542. [DOI] [PubMed] [Google Scholar]
- 131. Chen K, Dai H, Yuan J, et al. Optineurin-mediated mitophagy protects renal tubular epithelial cells against accelerated senescence in diabetic nephropathy. Cell Death Dis. 2018;9(2):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fyhrquist F, Tiitu A, Saijonmaa O, Forsblom C, Groop PH; FinnDiane Study Group . Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. J Intern Med. 2010;267(3):278-286. [DOI] [PubMed] [Google Scholar]
- 133. Sampson MJ, Hughes DA. Chromosomal telomere attrition as a mechanism for the increased risk of epithelial cancers and senescent phenotypes in type 2 diabetes. Diabetologia. 2006;49(8):1726-1731. [DOI] [PubMed] [Google Scholar]
- 134. Kitada K, Nakano D, Ohsaki H, et al. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complications. 2014;28(5):604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Guo J, Zheng HJ, Zhang W, et al. Accelerated Kidney Aging in Diabetes Mellitus. Oxid Med Cell Longev. 2020;2020:1234059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914-920. [DOI] [PubMed] [Google Scholar]
- 137. Chuang HC, Sheu WH, Lin YT, et al. HGK/MAP4K4 deficiency induces TRAF2 stabilization and Th17 differentiation leading to insulin resistance. Nat Commun. 2014;5:4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Surendar J, Frohberger SJ, Karunakaran I, et al. Adiponectin limits IFN-γ and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Front Immunol. 2019;10:2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhao RX, He Q, Sha S, et al. Increased AHR transcripts correlate with pro-inflammatory T-helper lymphocytes polarization in both metabolically healthy obesity and type 2 diabetic patients. Front Immunol. 2020;11:1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bapat SP, Myoung Suh J, Fang S, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528(7580):137-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cipolletta D, Feuerer M, Li A, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486(7404):549-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cucak H, Vistisen D, Witte D, Philipsen A, Rosendahl A. Reduction of specific circulating lymphocyte populations with metabolic risk factors in patients at risk to develop type 2 diabetes. PLoS One. 2014;9(9):e107140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sharma M, Boytard L, Hadi T, et al. Enhanced glycolysis and HIF-1α activation in adipose tissue macrophages sustains local and systemic interleukin-1β production in obesity. Sci Rep. 2020;10(1):5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bertola A, Ciucci T, Rousseau D, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61(9):2238-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Galle-Treger L, Sankaranarayanan I, Hurrell BP, et al. Costimulation of type-2 innate lymphoid cells by GITR promotes effector function and ameliorates type 2 diabetes. Nat Commun. 2019;10(1):713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Shafiei-Jahani P, Hurrell BP, Galle-Treger L, et al. DR3 stimulation of adipose resident ILC2s ameliorates type 2 diabetes mellitus. Nat Commun. 2020;11(1):4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhao XY, Zhou L, Chen Z, et al. The obesity-induced adipokine sST2 exacerbates adipose Treg and ILC2 depletion and promotes insulin resistance. Sci Adv. 2020;6(20):eaay6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.