Abstract
Cancer cachexia (CC) is a devastating syndrome characterized by weight loss, reduced fat mass and muscle mass that affects approximately 80% of cancer patients and is responsible for 22%–30% of cancer-associated deaths. Understanding underlying mechanisms for the development of CC are crucial to advance therapies to treat CC and improve cancer outcomes. CC is a multi-organ syndrome that results in extensive skeletal muscle and adipose tissue wasting; however, CC can impair other organs such as the liver, heart, brain, and bone as well. A considerable amount of CC research focuses on changes that occur within the muscle, but cancer-related impairments in other organ systems are understudied. Furthermore, metabolic changes in organ systems other than muscle may contribute to CC. Therefore, the purpose of this review is to address degenerative mechanisms which occur during CC from a whole-body perspective. Outlining the information known about metabolic changes that occur in response to cancer is necessary to develop and enhance therapies to treat CC. As much of the current evidences in CC are from pre-clinical models we should note the majority of the data reviewed here are from pre-clinical models.
Keywords: Muscle atrophy, Tumor-bearing mouse, Lewis lung carcinoma, Protein turnover, Mitochondrial dysfunction
Introduction
Cancer is one of the leading causes of death worldwide with the World Health Organization reporting 9.6 million deaths and 18.1 million new cases in 2018.1,2 Cancer-cachexia (CC) is a wasting syndrome that occurs in up to 80% of cancer patients.3,4 CC is the primary cause of death for 22%–30% of cancer patients,3,4 with incidence predicted to grow in years to come.5 Despite CC's widespread implications, it is often poorly diagnosed and often missed completely. Typically, there is an examination of weight loss, body mass index (BMI), and skeletal muscle, and clinically requires greater than 5% loss of body weight over a 6-month period, BMI less than 20 kg/m2, and low muscle mass (sarcopenia) to be diagnosed.6,7 However, CC is often irreversible once weight loss begins to occur; therefore, using weight loss as diagnostic criteria is therapeutically inadequate and outlines the need to identify a pre-cachectic signature both for diagnostic and treatment purposes. Therefore, the purpose of this review is to examine the development of CC from bench to bedside in multiple organ systems. To begin we will describe demographics in cancer patients and degenerative mechanisms in the skeletal muscle during CC. Additionally, we will address molecular alterations in other organs in CC. Notably, as much of the existing evidence in CC is from pre-clinical models the bulk of our discussion on mechanisms of CC comes from pre-clinical models and needs further validation in clinical studies.
Cancer cachexia patient demographics and clinical progression
As previously stated, CC can affect as much as 80% of cancer patients depending on the type of cancer.3,4 However, CC outcomes following cancer diagnosis and treatment are influenced by factors such as age, body composition, biological sex, type of cancer and activity level.8, 9, 10 Herein, we will examine CC occurrence as a function of age, biological sex and type of cancer.
CC is of particular importance in the elderly demographic, since sarcopenia, age-related loss of muscle mass and function, commonly occurs alongside CC.11 In fact, among patients in a specialized geriatric oncology clinic, up to 65% presented with CC.12 Despite the prevalence of CC in aged populations, older subjects continue to be poorly represented in pre-clinical models of CC and randomized phase II and III CC clinical trials.13, 14, 15 Understanding the development of CC in the aged population is crucial for developing effective therapies as especially when considering other common comorbidities in this population CC onset and progression may differ in young patients when compared to aged patients.
In addition, biological sex has been recently reported as an important factor in terms of cancer incidence and mortality.16 Although both male and female subjects exhibit CC, the development of CC may differ between sexes.17 A recent study shows cancer incidence is 20% higher in males between sexes and the male-to-female incidence ratio was distinctly different in many primary malignant tumor developments such as liver, bladder, oral cavity, esophageal, and thyroid cancer in the US.18 Moreover, the mortality rate in malignant cancers is nearly 30% higher in males compared to females in various age groups.19,20 Moreover, cancer-induced cachexia generally occurs in 40%–50% of female patients and 40%–60% of male patients over 60 years of age21,22 and overall prevalence of severe muscle loss criteria among cancer patients was much higher in males (61%) than females (31%).23 These distinct underlying differences between sexes in cancer incidence and mortality are complicated to delineate, but this phenomenon can be attributed by both external (lifestyle, alcohol consumption, smoking, and delayed diagnosis) and internal factors (hormone levels, and biological sex-based molecular changes).16,17 We will discuss more detailed biological sex-based molecular changes in the skeletal muscle later in this review.
All cancers can present with cachexia and common cancers such as those of the pancreas, lung, and liver are responsible for approximately half of all cancer-related deaths worldwide.24 Cancers that are more commonly associated with cachexia are often diagnosed at an advanced stage and have direct effects on digestion.25 Furthermore, tumor characteristics such as tumor proliferation rates and inflammatory profiles contribute to CC.3,26 Baseline assessments of pre-cachexia (early stage of CC without marked weight loss; i.e., ≤5%)6 or mass loss history are critical for predicting CC trajectory at later stages of cancer progression.27 To predict if CC will occur in patients, there is a need for a defined pre-cachectic signature, which would allow for the development and implementation of preventative treatments to decrease the severity of CC or even prevent CC.
Recently, many clinical studies have taken place to identify characteristics of CC across several different types of cancers in humans. Surprisingly, results of clinical studies often show different outcomes than pre-clinical animal models. For example, in pre-clinical animal models there is consistent data showing that muscle proteolysis is occurring with CC; however, the results from clinical studies are much more variable.28 Our group recently reviewed common pre-clinical models of CC,29 notably current efforts are ongoing to develop pre-clinical models of CC to more accurately recapitulate human CC. One excellent example of these efforts is the recent development of the KPP mouse model of pancreatic ductal adenocarcinoma (PDA).30 Notably, the KPP mouse exhibits similar gene ontology to human patients and a more protracted time course of development than common allografted tumor models.30 One source of variation could be that in pre-clinical animal models’ experimental conditions such as tumor size are tightly controlled for, while in clinical studies the progression of cancer is often not controlled for. More data from clinical models is needed in order to fully understand the development of CC in humans. For example, mitochondrial dysfunction is a common finding in pre-clinical animal models that is understudied in clinical models. Dolly et al. recently published a more in-depth review of recent clinical studies.28 Below are some common findings detailing the progression of CC in pre-clinical animal models.
Considering the varying impacts of CC across age groups, biological sex, types of cancer and others, there is a strong need to identify novel biomarkers associated with the progression of CC that could be useful for diagnostic purposes prior to marked weight loss.31 In fact, identification of biomarkers is among a series of identified priorities in cancer cachexia and critical initiatives in this effort have been recently established.32 Recent studies have identified different types of potential bloodborne biomarkers in various cancer models.32 For instance, Talbert and coworkers33 found elevated circulating levels of monocyte chemoattractant protein-1 (MCP-1) in cachectic pancreatic cancer patients which was absent in their weight stable counterparts, providing a potential biomarker for CC. A similar finding has been reported that higher plasma C18-ceramide to C24-ceramide (C18:C24) levels were found in cachectic pancreatic cancer patients compared to non-cachectic patients.34 In addition, bloodborne biomarkers to identify pre-cachexia could include inflammatory cytokines,35 microRNA's,36 and lipolysis markers.31 Specifically, interleukin (IL)-6 is elevated in blood in pre-clinical models of CC.37 Downstream of IL-6, signaling transducer and activator of transcription (STAT) 3 signaling is linked to phenotypes of cancer-cachexia including skeletal muscle wasting, cardiac dysfunction and hypothalamic inflammation.38, 39, 40, 41 Mitogen-activated protein kinase (MAPK/ERK) is another downstream target of IL-6 that contributes to increased mitochondrial fission factors in tumor-bearing mice.42,43 Intriguingly, the induction of cancer-cachexia is IL-6 independent in female mice.44 Furthermore, our recent data show plasma-derived proteins such as Serum Amyloid A1 (SAA1) content was exclusively higher in mice with LLC-induced cachexia even when compared to models of disuse-induced muscle atrophy.45 However, it should be noted that biomarkers may differ depending on the type of cancer.46 More recently, clinical trials have been conducted to identify novel biomarkers for CC in humans. Some common biomarkers observed in clinical trials include c-reactive protein, albumin, insulin-like growth factor-1 (IGF-1), free fatty acids, IL-6, and IL-1028. One issue with looking at biomarkers in humans is that the biomarkers are screened for in all stages of cancer, which makes it difficult to identify a potential biomarker for early detection of CC. Hence, studies using pre-clinical models that are looking for these bloodborne biomarkers during different stages and types of cancer are needed to identify novel biomarkers that can be utilized to effectively diagnose and prevent it.
Role of chemotherapy and radiation therapy in cancer cachexia development
Common classes of chemotherapeutics such as taxanes (e.g., paclitaxel or taxol) or vinka alkaloids (e.g., vinblastine) are used to treat multiple cancers including breast cancer, lung cancer, pancreatic cancer, and gastrointestinal cancer.47,48 Chemo- or radiation therapy reduces the physical fitness of cancer patients,49 which has negative impacts on CC outcomes. Early muscle loss during chemotherapy is associated with poor treatment response and reduced survival.50 Pre-clinical models and clinical studies show that chemotherapies such as taxanes or vinka alkaloids induce severe muscle weakness, impaired neuronal activities, and mitochondrial dysfunctions.51, 52, 53, 54 While some data suggest common mechanisms may exist between cancer-induced and chemotherapy-induced cachexia,55 unfortunately to date few pre-clinical studies have attempted to investigate the combinatory effects of cancer and cancer treatment on cachexia outcomes. This is largely impacted by the confounding effects of cancer treatment on tumor volume. Many preclinical models, especially allograft models, rely on tumor mass as a cue for CC, with cancer treatments tumor volume is often restricted or reversed creating difficulty in studying CC in an appropriately matched study. Further investigation of these combinatory effects are required to advance our understanding of CC in more clinically apt conditions.
Clinical trials
The National Institute of Health's definition of a clinical trial is a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. Despite the clear need to treat CC, there have not been many clinical trials with the goal of treating CC. Clinical trials that have taken place have been largely unsuccessful. Recently, both anamorelin and enobosarm were tested in clinical trials, but failed to reach primary endpoint measurements.56 Exercise has been successfully used as a preventative intervention for CC.57 This said, an accompanying article by Halle et al. more specifically addresses the potential therapeutic impacts of exercise on CC.58 One explanation for why these trials were unsuccessful is that mechanisms that lead to the initial development of CC are understudied.
Changes in skeletal muscle during the development of CC
A summary of alterations to the skeletal muscle during pre- and cachectic states can be found in Table 1.
Table 1.
A summary of alterations to the skeletal muscle during pre- and cachectic states.
| Marker | Pre-cachexia | Cachexia | Reference |
|---|---|---|---|
| Protein synthesis | ↔ | ↓ | 52, 53, 54, 65, 110 |
| Protein degradation | ↔ | ↑ | 52, 53, 54 |
| Atrogenes (Atrogin-1 & MuRF-1) | ↔ | ↑ | 52, 53, 93, 110 |
| Deptor | ? | ↑ | 53, |
| Regeneration | ? | ↓ | 74 |
| Cell cycling & Myogensis | ↓ | ↔ | 53 |
| Autophagy | ↔ | ↑ | 40, 52, 53, 82 |
| ATP production | ? | ↓ | 40, 78 |
| MAPK | ↔ | ↑ | 53, 86, 93, 100 |
| Muscle contractility | ↓ | ↓ | 84, 86, 87 |
| Calcium homeostasis | ? | ↓ | 76, 89 |
| Mitochondrial content | ↔ | ↓ | 75, 79 |
| ROS emission | ↑ | ↑ | 40, 75, 83 |
| Oxidative stress | ↑ | ↑ | 40, 75 |
| Mitochondrial fusion | ↓ | ↓ | 40, 36, 75 |
| Mitochondrial fission | ↔ | ↑ | 40, 36, 75 |
| Mitophagy | ↔ | ↑ | 75 |
| Mitochondrial respiration | ↔ | ↓ | 40, 54, 75 |
| Antioxidant activity | ↔ | ↔ | 75 |
| Apoptosis | ? | ↑ | 36, 65, 82 |
| Inflammation | ↑ | ↑ | 32, 36, 86, 93, 100 |
Protein turnover during the development and progression of CC
The major feature of CC is the net loss of skeletal muscle protein, which occurs when protein degradation exceeds protein synthesis.59 Prior evidence shows pre-clinical models of CC have elevated protein degradation while protein synthesis is suppressed.60, 61, 62, 63 Two major protein degradation processes, the ubiquitin proteasome system (UPS) and the autophagy-lysosomal pathway (ALP) are often activated during muscle wasting conditions including CC.64 In the UPS, ubiquitin is conjugated to target proteins that are tagged and recognized through E3-E2 ubiquitin ligases, which are then degraded via the ATP-dependent 26S proteasome.65 Two major atrophy-related genes are commonly accepted, atrogin-1 (also known as MAFbx)66,67 and MuRF1 (muscle RING finger protein 1)66 and both atrogenes encode E3 ligases, which initiate ubiquitination of target protein substrates,68, 69, 70 thereby upregulating muscle atrophy. It should be noted that other related atrogenes have been recently described.71 These markers are elevated in various models of atrophy including CC, disuse, and denervation-induced muscle wasting.62,72,73 Recent evidence from our group also showed that elevated mRNA content of atrogin-1 and MuRF1 and protein content of ubiquitin were found in the skeletal muscle of Lewis Lung Carcinoma (LLC)-induced tumor-bearing mice 4 weeks following tumor implantation (cachectic state).62 Furthermore, a nearly 40% lowered fractional synthesis rate (FSR) of mixed proteins was observed in muscle tissues in LLC tumor-bearing mice at this point.62 Similar findings using the ApcMin/+ mouse (colon cancer model) have been previously reported by the Carson group.74
Moreover, muscle protein synthesis is one of the two major components when it comes to net protein balance while its role in CC has not been clearly understood. While our data62,63 and other works60,61 clearly demonstrate reduced protein anabolism in pre-clinical animal models of CC, understanding of the suppression of anabolic signaling remains incomplete.60,75 The primary cellular signaling pathway of muscle growth known as Akt/mTOR signaling is an essential part of muscle protein synthesis.76,77 Downstream signaling of the mammalian target of rapamycin (mTOR) promotes mRNA translation initiation, thereby leading to generation of newly synthesized protein and increasing muscle mass,78 and mTOR activation can be directly suppressed via its own inhibitors, DEP domain-containing mTOR-interacting protein (DEPTOR)79 and regulated in development and DNA damage response 1 (REDD1).80 Our recent work also found elevated Deptor protein content concomitant with reduced FSR of muscle protein in LLC-induced cachectic mice, indicating mTOR inhibition via Deptor as an essential hub for regulation of muscle protein synthesis in CC.62
Skeletal muscle regeneration during the development and progression of CC
An alternative factor that may contribute to muscle wasting in CC is altered skeletal muscle regeneration,81 a more detailed review on muscle regeneration during cancer cachexia has been published.82 Skeletal muscle has an efficient homeostatic capacity to regenerate itself in response to injury and it can be hypothesized that skeletal muscle undergoes the regeneration process in response to muscle wasting during CC. Accordingly, it is plausible that cancer-induced muscle wasting is in part due to impaired muscle regeneration capacity dependently or independently of cellular signaling of protein synthesis. Interestingly, our group has observed reduced myogenic and cell cycle-associated markers including Cyclin D1, Pax7, MyoD, and Myogenin at the early stages of cancer in tumor-bearing mice before marked weight loss,62 indicating muscle atrophy might be initiated via impairment of the muscle regeneration processes during the early stages of cancer. Other work has shown that skeletal muscle regeneration is impaired in tumor-bearing mice.83 However, only few pre-clinical and clinical studies have observed the role of the muscle regeneration process in cancer-associated muscle wasting, making it difficult to advance the development of therapeutics to prevent CC. Collectively, prior studies suggest both protein degradation and synthesis contribute to protein turnover during CC although its individual contribution has not been well understood. Therefore, further investigations on regulation of muscle protein turnover including regeneration mechanisms during pre-cachexia are needed to prevent muscle wasting in CC.
Mitochondrial alterations during the development and progression of CC
Mitochondrial health and function have long been implicated with various human diseases including CC.84, 85, 86 Degeneration in muscle from cachectic mice has been shown in multiple accounts; however, mitochondrial changes that occur during the development of CC are underexplored. Previous studies have reported that both in vivo and in vitro models of CC can lead to impaired mitochondrial function including reduced respiratory capacity, ATP production, and elevated reactive oxygen species (ROS) emission.43,63,87,88 However, our recent findings reveal mitochondrial ROS generation and mitochondrial oxidative stress precedes the development of CC in LLC tumor-bearing mice.84 Elevated ROS emission is associated with impaired mitochondrial quality control (MQC) mechanisms including mitochondrial dynamics (fusion and fission) and autophagy (mitophagy).89,90 Our recent study shows elevated ROS occurs concomitantly with reduced content of mitochondrial fusion regulatory proteins during the pre-cachectic stage in tumor-bearing mice. In addition, markers for mitophagy and mitochondrial fission were upregulated, and mitochondrial respiration were impaired during the cachectic phase.84 Similar results were reported by others of reduced mitochondrial fusion, biogenesis, elevated mitochondrial fission, ROS emission, and protein markers associated with autophagosome formation in both cachectic mice43 and cachectic cancer patients.91 Chronic elevation of mitochondrial ROS generation can increase rates of protein degradation via activation of the ubiquitin proteasome-mediated protein breakdown process, leading to muscle wasting.92 To our knowledge, this was the first data that shows alterations in mitochondrial health and function during pre-cachexia, and currently it is unknown if early targeting of H2O2 and mitochondrial fusion may prevent mitochondrial degeneration observed during CC. Hence, additional supportive investigations on targeting on H2O2 and mitochondrial fusion are needed to elucidate mitochondrial regulations during the development and progression of CC.
Skeletal muscle contractile function during the development and progression of CC
In addition to a loss of muscle mass, reduced muscle strength is another indicator of the survival rate among patients and rodents with advanced cancer.93, 94, 95 CC is associated with impaired muscle contractility and accelerated muscle fatigue in both tumor-bearing mice,95,96 and advanced cancer patients.93 Previous evidence revealed that cachectic mice showed impaired contractility in both force production and fatigability and, notably, increased muscle fatigability was present in weight stable ApcMin/+ mice,95 suggesting increased muscle fatigability in pre-cachexia may serve as an early functional marker for CC. Muscle fatigue is the decline in ability of muscles to generate force. Force production is controlled by contractile properties such as fiber type, motor unit, ATPase activity, and neuromuscular connection.97,98 In addition, altered calcium homeostasis is a hallmark of cancer cells and prior studies have shown that altered calcium homeostasis including mitochondrial calcium retention capacity, voltage-gated calcium channels, and SR stress levels are observed in the skeletal muscle of cancer patients.85,99 Since calcium is an essential molecule that enables the cross-bridge muscle contraction cycle100 and is involved in signaling for mitochondrial ATP production,101 it is suggested that altered calcium homeostasis in CC may play an important role in inducing impaired muscle contractile function.
Muscle force is strongly associated with the integrity of the extracellular matrix (ECM) in that muscle force generated by the actomyosin cross-bridge mechanism transmits force to external tendons via ECM.102,103 ECM structure in ApcMin/+ mice is disrupted by the accumulation of non-contractile tissue.104 The matrix metalloproteinase (MMP) are a family of zinc-dependent endopeptidases, which plays an important role in regulating the integrity of ECM via degrading essential ECM components such as collagens.105 MMP contents are elevated during the development of CC, which may promote ECM remodeling.106 In addition, disrupted ECM integrity can be partially explained via the accumulation of fibrotic tissues in CC.107 Recent evidence showed with novel histological data that disrupted skeletal muscle integrity is accounted for with the replacement of muscle with collagen, fat, and fibrosis tissues and this result was supported by altered transcriptional profiling of TGF-β (regulator of tissue fibrosis) in pancreatic cancer patients.107 However, the regulation of ECM in CC is not completely understood and additional investigations are required to delineate its elusive roles of ECM during the development and progression of CC.
Sex differences in skeletal muscle during the development and progression of CC
CC occurs in both male and female populations; however, mechanisms that drive muscle loss differ between male and female subjects.17,108 In fact, female mice are less sensitive to inflammation-mediated activation of the ubiquitin-proteasome system and subsequent muscle protein breakdown, common in CC, as compared to males.109, 110, 111 Differences in the inflammation and protein breakdown pathways observed in prior works show distinct variations in cancer-induced muscle degradation mechanisms that occur between sexes in mice.17,108 More studies are needed to delineate the ambiguous roles of biological sex during the development and progression of CC. Considering differences in CC susceptibility by biological sex, explorations of mechanistic differences in CC between biological sexes may provide valuable insight to therapeutic approaches to prevent or attenuate this condition. The 4-core genotype model has recently been developed in order to delineate whether sex specific changes are primarily caused from hormonal changes, chromosomal differences or a combination of both.112 However, tools such as the 4-core genotype model have never been used to delineate mechanisms behind sex specific differences in CC.
Alterations to non-skeletal muscle tissues during development and progression of CC
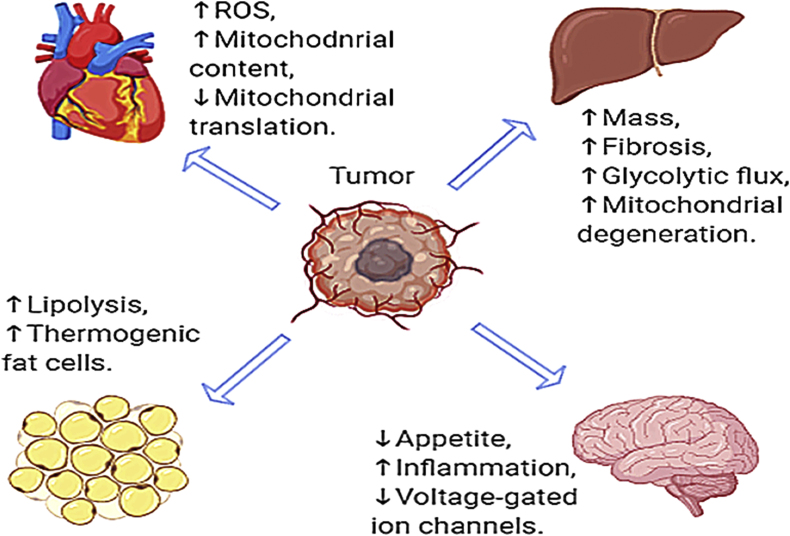
Though the skeletal muscle is by definition a primary focus and definitive aspect of CC, this condition affects multiple organ systems. In many cases these alterations to tissues such as the liver, adipose tissue, cardiac muscle, brain and others are relatively underexplored. A summary of alterations to the non-skeletal muscle tissues during pre- and cachectic states can be found in Fig. 1. In addition to those discussed, further evidences strongly indicates a potential role of altered immune function in CC, these alterations have been recently described in a review by VanderVeen et al.113 and thus will not be described here. However, they may be critical to understanding the full spectra of this condition and appropriate therapeutic strategies.
Fig. 1.
Summary of alterations previously demonstrated in non-skeletal muscle tissues during cancer cachexia.
Changes in other tissues during the development and progression of CC: adipose tissue
Adipose tissue along with the skeletal muscle are two major tissues that undergo significant weight loss during CC, however, changes in adipose tissue are less understood than muscle during cancer development. A recent investigation shed light on the importance of fat wasting during CC.114 In this study, three cachexia phenotypes were identified (64% had muscle and fat wasting, 17% had fat-only wasting, and 19% had no wasting) and surprisingly, survival rate for cancer patients with fat-only wasting was as poor as for those with muscle and fat wasting,114 indicating adipose tissue may play an essential role in cancer-induced wasting. Loss of fat mass could result from either elevated lipolysis or reduced lipogenesis. In patients suffering from CC, elevated free fatty acid concentrations have been reported, which may suggest activated lipolysis,115 increasing free-fatty acids. Increased cytosolic free-fatty acids are then transferred to the mitochondrial matrix, where they are used for β-oxidation,116 thereby losing fat storage.
In addition, the activation of thermogenic fat cells (brown and beige fat) has been described in both pre-clinical models and cachectic patients.117, 118, 119 In fact, recent studies observed elevated thermogenic activity and energy expenditure of adipose tissue occur in pre-clinical animal models of CC.120, 121, 122 Thermogenic adipocytes such as brown and beige cells burn calories to help maintain body temperature and can be activated in response to cold exposure or sympathetic stimulation in normal physiological conditions. However, abnormally upregulated thermogenesis via beiging of white fat depots contributes to energy wasting, thereby inducing weight loss.123 A recent investigation using a loss of PRDM16, a transcription factor required for the beiging process, prevented adipose wasting in tumor-bearing mice.120 These prior works indicate tumors activate thermogenesis when it is not necessary during CC, exacerbating cachectic phenotype although the underlying mechanism is not completely understood. Collectively, these prior studies suggest an important role for adipose tissue in progressive wasting in CC. Further studies are required to determine more precise mechanisms on adipose tissue during CC and if targeting components of adipose tissue develops in the treatment of CC.
Changes in other tissues during the development and progression of CC: liver
CC is closely implicated with liver metabolic dysfunctions as cancer-induced pro-inflammatory cytokines play a crucial role in regulating metabolic homeostasis such as energy production and glycogen storage in the liver.124, 125, 126, 127 Accordingly, changes in hepatic function and regulation may contribute to cancer-induced weight loss.128 In fact, prior investigations have suggested that cachectic mice exhibit depleted liver glycogen content and increased mRNA content of glycolytic and gluconeogenic enzymes, ER-stress, and inflammatory signalings in cachectic mice.126,127
Furthermore, it has been described that an enlarged liver mass is present in tumor-bearing cachectic rodents with pronounced inflammation markers.129, 130, 131 This increased liver mass during CC is accompanied by increased collagen deposition and elevated MMPs,130 suggesting that the enlarged liver mass during CC may in part be due to elevated fibrosis and/or collagen deposition via the upregulation of MMPs. Moreover, the regulation of hepatic metabolism can be largely controlled by mitochondrial function as mitochondria are the major whole-body ATP producer, especially, in liver and muscle. Dumas et al.132 further observed reduced efficiency of oxidative phosphorylation, elevated ROS emission, and fatty acid accumulation in hepatic mitochondria of rats with CC. Our recent investigation revealed impaired content of mitochondrial quality regulators in the liver of pre-cachectic mice,130 which may contribute to hepatic mitochondrial dysfunction observed in tumor-bearing rodents. Alterations in hepatic metabolic regulation may contribute to cancer-induced weight loss.128 Due to high levels of protein catabolism and glycolytic flux in cancer patients, elevated plasma contents of lactate and amino acids are often observed in those patients.133, 134, 135 Elevated plasma amino acids and lactate are then used as a substrate to exploit hepatic gluconeogenesis, leading to increased glucose supply for tumor growth.136 Overall, prior evidence has indicated various molecular alterations in liver in CC, however, to our knowledge, only one study,130 has directly assessed timecourse aberration in hepatic physiology during development and progression of CC in rodents. Considering the critical function of liver in whole body metabolism better clarifying mechanisms of hepatic degeneration in CC may be critical to prevention and reversal of this condition.
Changes in other tissues during the development and progression of CC: heart
Cancer and heart disease are the two leading causes of death accounting for approximately 50% of total deaths in the U.S.137 while decreased cardiac function limits overall survival for cancer patients.138,139 Cardiac impairments such as reduced cardiac mass, pathological signaling, and impaired left ventricular systolic function are often present in subjects suffering from CC.140,141 Furthermore, cardiac dysfunction and atrophy during CC are correlated with skeletal muscle atrophy.142 Our recent evidence indicates that cardiac tissue from LLC-induced tumor-bearing mice and in vitro models of cardiac cachexia exhibit elevated mitochondria content (likely driven by reduced mitophagy suggesting accumulation of damaged mitochondria), decreased mitochondrial mRNA translation rate, and increased mitochondrial ROS emission with reduced scavenging capacity.143 Unfortunately, to date cardiac cachexia remains significantly understudied. Therefore, there remains a critical need to identify key underlying mechanisms in development of cardiac cachexia.
Changes in other tissues during the development and progression of CC: brain
Progressive wasting is indeed a hallmark and defining feature of CC and it can be partially driven by loss of appetite (anorexia) and abnormal energy metabolism6 that are controlled via neurons converging on nuclei in the hypothalamus.144,145 Although loss of appetite is not the sole culprit for marked weight loss in CC, most advanced cancer patients undergo some degree of anorexia.146 In addition, primary anorexia is associated with neurohormonal signaling, inflammatory cytokines, and peripheral appetite signaling147,148 while the pathophysiology behind the primary anorexia remains understudied. The inflammatory environment associated with cancer may influence the appetite. Specifically, pro-inflammatory cytokines such as IL-6 are elevated during CC.35 Pro-inflammatory cytokines can be sensed by the hypothalamus, which then activates the central melanocortin system, which controls energy homeostasis.149 When activated, the central melanocortin system signals to reduce food intake, thereby promoting muscle and adipose wasting.149
Furthermore, prior evidence has revealed that cancer reduces the capacity of voltage-gated ion channels in the brain of rats,150 which is associated with mitochondrial dysfunction, calcium imbalance, and mtDNA damage.52,151 Despite of this information, little attention has still been received in brain physiology during CC. Therefore, more attention should be paid to brain activities including atrophic signaling, mitochondrial function, and appetite regulations during the development and progression of CC.
Conclusions
Pronounced muscle loss in cancer patients is a hallmark for deteriorating conditions, which affects numerous cancer patients. Understanding the molecular mechanisms involved in muscle loss during the development and progression of CC is an urgent issue to improve cancer outcomes. The problem with waiting until symptoms of CC occur is that cancer-related changes in metabolism often occur prior to the onset of phenotypic CC62,84,130,152; therefore, preventative approaches prior to CC onset may be necessary. Furthermore, a large portion of CC research focuses on skeletal muscle. Tumor burden affects multiple organ systems, and many of these organ systems contribute to the weight loss associated with cancer. Another important aspect is that pre-clinical animal models of CC has mainly investigated using male animals although distinct sex differences in cachectic phenotype have been reported in CC.17,21,153 Taken together, future studies should more focus on biological sex and both muscle and non-muscle organs from a chronological perspective during the development and progression of CC to advance the study of CC.
Submission statement
This manuscript is an original work that has not been previously published, nor will it be under consideration for publication by any other journal before a decision has been made by Sports Medicine and Health Science. If accepted, this manuscript will not be published elsewhere.
Authors’ contribution
Seongkyun Lim and Jacob L. Brown wrote/edited the manuscript. Tyrone A. Washington and Nicholas P. Greene edited/revised/approved of manuscript.
Conflict of interest
The authors declare no financial or other conflicts of interest that could influence the interpretations of this work.
Acknowledgements
Authors would like to thank the dedicated faculty, staff and students at both the Exercise Science Research Center at the University of Arkansas and the Aging and Metabolism Research Program at the Oklahoma Medical Research Foundation for their consistent support of this research. Work presented here was funded by National Institutes of Health under Award Number R15AR069913 and R01AR075794 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of General Medical Sciences. Author Jacob L. Brown is supported by National Institute of Aging at the National Institutes of Health 5T32AG052363-02.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Canc. Mar 1 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Fearon Kenneth C.H., Glass David J., Guttridge Denis C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metabol. 8/8/2012;16(2):153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Onesti J.K., Guttridge D.C. Inflammation based regulation of cancer cachexia. BioMed Res Int. 2014;2014:168407. doi: 10.1155/2014/16840710.1155/2014/168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith B.D., Smith G.L., Hurria A., Hortobagyi G.N., Buchholz T.A. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol : Off. J. Am. Soc. Clin. Oncol. Jun 10 2009;27(17):2758–2765. doi: 10.1200/jco.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 6.Fearon K., Strasser F., Anker S.D., et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. May 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 7.Mattox T.W. vol. 32. official publication of the American Society for Parenteral and Enteral Nutrition; Oct 2017. Cancer Cachexia: Cause, Diagnosis, and Treatment. Nutrition in Clinical Practice; pp. 599–606. 5. [DOI] [PubMed] [Google Scholar]
- 8.Tisdale M.J. Cachexia in cancer patients. Nat Rev Canc. 2002;2(11):862–871. doi: 10.1007/s00520-017-3902-6. doi: 10.1007/s00520-017-3902-6. Nov. [DOI] [PubMed] [Google Scholar]
- 9.Deluche E., Leobon S., Desport J.C., Venat-Bouvet L., Usseglio J., Tubiana-Mathieu N. Impact of body composition on outcome in patients with early breast cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. Mar. 2018;26(3):861–868. doi: 10.1007/s00520-017-3902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cormie P., Zopf E.M., Zhang X., Schmitz K.H. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev. 1 2017;39(1):71–92. doi: 10.1093/epirev/mxx007. Jan. [DOI] [PubMed] [Google Scholar]
- 11.Williams G.R., Rier H.N., McDonald A., Shachar S.S. Sarcopenia & aging in cancer. J Geriatr Oncol. May 2019;10(3):374–377. doi: 10.1016/j.jgo.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne R.F., Roussel B., Culakova E., et al. Characterizing cancer cachexia in the geriatric oncology population. J Geriatr Oncol. May 2019;10(3):415–419. doi: 10.1016/j.jgo.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temel J.S., Abernethy A.P., Currow D.C., et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17(4):519–531. doi: 10.1016/S1470-2045(15)00558-6. Apr. [DOI] [PubMed] [Google Scholar]
- 14.Solheim T.S., Laird B.J.A., Balstad T.R., et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia, Sarcopenia and Muscle. Oct 2017;8(5):778–788. doi: 10.1002/jcsm.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maccio A., Madeddu C., Gramignano G., et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecologic oncology. Mar 2012;124(3):417–425. doi: 10.1016/j.ygyno.2011.12.435. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y., Shao X., Wang X., Liu L., Liang H. Sex disparities in cancer. Canc Lett. 2019;466:35–38. doi: 10.1016/j.canlet.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Rosa-Caldwell M.E., Greene N.P. Muscle metabolism and atrophy: let's talk about sex. Biol Sex Differ. 2019;10(1):1–14. doi: 10.1186/s13293-019-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebecca S., Siegel M.P.H., Kimberly D., Miller M.P.H., Ahmedin Jemal D.V.M. Cancer statistics. CA A Canc. J Clin. 2017;67(27):7–30. doi: 10.3322/caac.21387. [DOI] [Google Scholar]
- 19.Cook M.B., Dawsey S.M., Freedman N.D., et al. Sex disparities in cancer incidence by period and age. Canc Epidemiol Prevent Biomark. 2009;18(4):1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook M.B., McGlynn K.A., Devesa S.S., Freedman N.D., Anderson W.F. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomark Prev. 2011;20(8):1629–1637. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson L.J., Liu H., Garcia J.M. Springer; 2017. Sex Differences in Muscle Wasting. Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity; pp. 153–197. [DOI] [Google Scholar]
- 22.Vagnildhaug O.M., Blum D., Wilcock A., et al. The applicability of a weight loss grading system in cancer cachexia: a longitudinal analysis. J Cachexia, Sarcopenia and Muscle. 2017;8(5):789–797. doi: 10.1002/jcsm.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baracos V.E., Reiman T., Mourtzakis M., Gioulbasanis I., Antoun S. Body composition in patients with non− small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91(4):1133S–1137S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 24.Amano K., Maeda I., Morita T., et al. C-reactive protein, symptoms and activity of daily living in patients with advanced cancer receiving palliative care. J Cachexia, Sarcopenia Muscle. 2017;8(3):457–465. doi: 10.1002/jcsm.12184. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stojcev Z., Matysiak K., Duszewski M., Banasiewicz T. The role of dietary nutrition in stomach cancer. Contemp Oncol. 2013;17(4):343–345. doi: 10.5114/wo.2013.37213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laine A., Iyengar P., Pandita T.K. The role of inflammatory pathways in cancer-associated cachexia and radiation resistance. Mol Canc Res. 2013;11(9):967–972. doi: 10.1158/1541-7786.MCR-13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stene G.B., Balstad T.R., Leer A.S.M., et al. Deterioration in muscle mass and physical function differs according to weight loss history in cancer cachexia. Cancers. Dec 3 2019;11(12) doi: 10.3390/cancers11121925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolly A., Dumas J.F., Servais S. Cancer cachexia and skeletal muscle atrophy in clinical studies: what do we really know? J Cachexia, Sarcopenia and Muscle. 2020 doi: 10.1002/jcsm.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosa-Caldwell M.E., Fix D.K., Washington T.A., Greene N.P. Muscle alterations in the development and progression of cancer-induced muscle atrophy: a review. J Appl Physiol. 2020;128(1):25–41. doi: 10.1152/japplphysiol.00622.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talbert E.E., Cuitiño M.C., Ladner K.J., et al. Modeling human cancer-induced cachexia. Cell Rep. 2019;28(6):1612–1622. doi: 10.1016/j.celrep.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loumaye A., Thissen J.P. Biomarkers of cancer cachexia. Clin Biochem. Dec 2017;50(18):1281–1288. doi: 10.1016/j.clinbiochem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Dunne R.F., Mustian K.M., Garcia J.M., et al. Research priorities in cancer cachexia: the university of rochester cancer center NCI community oncology research Program (NCORP) research base symposium on cancer cachexia and sarcopenia. Curr Opin Support Palliat Care. 2017;11(4):278. doi: 10.1097/SPC.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talbert E.E., Lewis H.L., Farren M.R., et al. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naïve pancreatic cancer patients. J Cachexia, Sarcopenia and Muscle. 2018;9(2):358–368. doi: 10.1097/SPC.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakedis J.M., Dillhoff M.E., Schmidt C.R., et al. Plasma ceramides as a sexually dimorphic biomarker of pancreatic cancer-induced cachexia. medRxiv. 2020 doi: 10.1101/2020.06.01.20111492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carson J.A., Baltgalvis K.A. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. Oct 2010;38(4):168–176. doi: 10.1097/JES.0b013e3181f44f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D.E., Brown J.L., Rosa-Caldwell M.E., et al. Cancer cachexia-induced muscle atrophy: evidence for alterations in microRNAs important for muscle size. Physiol Genom. 2017;49(5):253–260. doi: 10.1152/physiolgenomics.00006.2017. [DOI] [PubMed] [Google Scholar]
- 37.Puppa M.J., White J.P., Sato S., Cairns M., Baynes J.W., Carson J.A. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim Biophys Acta. Dec 2011;1812(12):1601–1606. doi: 10.1016/j.bbadis.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmers T.A., Fishel M.L., Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol. Jun 2016;54:28–41. doi: 10.1016/j.semcdb.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White J.P., Puppa M.J., Sato S., et al. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skeletal Muscle. 2012;2:14. doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serrano A.L., Baeza-Raja B., Perdiguero E., Jardi M., Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metabol. Jan 2008;7(1):33–44. doi: 10.1152/ajpendo.00410.2012. [DOI] [PubMed] [Google Scholar]
- 41.White J.P., Puppa M.J., Gao S., Sato S., Welle S.L., Carson J.A. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metabol. 2013;304(10):E1042–E1052. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fix D.K., VanderVeen B.N., Counts B.R., Carson J.A. Regulation of skeletal muscle DRP-1 and FIS-1 protein expression by IL-6 signaling. Oxid Med Cell Longev. 2019;2019:8908457. doi: 10.1155/2019/8908457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VanderVeen B.N., Fix D.K., Carson J.A. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: a role for inflammation. Oxid Med Cell Longev. 2017:3292087. doi: 10.1155/2017/3292087. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hetzler K.L., Hardee J.P., Puppa M.J., et al. Sex differences in the relationship of IL-6 signaling to cancer cachexia progression. Biochim Biophys Acta. May 2015;1852(5):816–825. doi: 10.1016/j.bbadis.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim S., Dunlap K.R., Rosa-Caldwell M.E., et al. Comparative plasma proteomics in muscle atrophy during cancer-cachexia and disuse: the search for atrokines. Physiol Rep. 2020;8(19) doi: 10.14814/phy2.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsey M.L., Talbert E., Ahn D., et al. Circulating interleukin-6 is associated with disease progression, but not cachexia in pancreatic cancer. Pancreatology : official journal of the International Association of Pancreatology (IAP) 2019;19(1):80–87. doi: 10.1016/j.pan.2018.11.002. [et al.]. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Constantinou M., Tsai J.Y., Safran H. Paclitaxel and concurrent radiation in upper gastrointestinal cancers. Canc Invest. 2003;21(6):887–896. doi: 10.1081/CNV-120025092. [DOI] [PubMed] [Google Scholar]
- 48.Beard E.L., Jr. The american society of health system pharmacists. JONA's Healthc Law, Ethics, Regul. 2001;3(3):78–79. doi: 10.1097/00128488-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Canada J.M., Trankle C.R., Carbone S., et al. Determinants of cardiorespiratory fitness following thoracic radiotherapy in lung or breast cancer survivors. Am J Cardiol. Dec 26 2019 doi: 10.1016/j.amjcard.2019.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki S., Oki E., Saeki H., et al. Skeletal muscle loss during systemic chemotherapy for colorectal cancer indicates treatment response: a pooled analysis of a multicenter clinical trial (KSCC 1605-A) Int J Clin Oncol. Oct 2019;24(10):1204–1213. doi: 10.1007/s10147-019-01460-8. [DOI] [PubMed] [Google Scholar]
- 51.Ghoreishi Z., Esfahani A., Djazayeri A., et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Canc. 2012;12(1):355. doi: 10.1186/1471-2407-12-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han Y., Smith M.T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN) Front Pharmacol. Dec 18 2013;4:156. doi: 10.3389/fphar.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howarth F.C., Calaghan S.C., Boyett M.R., White E. Effect of the microtubule polymerizing agent taxol on contraction, Ca2+ transient and L-type Ca2+ current in rat ventricular myocytes. J Physiol. 1999;516(Pt 2):409. doi: 10.1111/j.1469-7793.1999.0409v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konishi S., Kishida S. Studies on the morphological changes of skeletal muscle induced by vincristine, vinblastine and colchicine. Bull Osaka Med Sch. Jul 1984;30(1):19–40. https://europepmc.org/article/med/6544604 [PubMed] [Google Scholar]
- 55.Barreto R., Mandili G., Witzmann F.A., Novelli F., Zimmers T.A., Bonetto A. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol. 2016;7:472. doi: 10.3389/fphys.2016.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le-Rademacher J.G., Crawford J., Evans W.J., Jatoi A. Overcoming obstacles in the design of cancer anorexia/weight loss trials. Crit Rev Oncol-Hematol. 2017;117:30–37. doi: 10.1016/j.critrevonc.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lira F.S., Neto J.C.R., Seelaender M. Exercise training as treatment in cancer cachexia. Appl Physiol Nutr Metabol. 2014;39(6):679–686. doi: 10.1139/apnm-2013-0554. [DOI] [PubMed] [Google Scholar]
- 58.Halle M., Schoenberg M.H. Physical activity in the prevention and treatment of colorectal carcinoma. Deutsches Ärzteblatt Int. 2009;106(44):722. doi: 10.3238/arztebl.2009.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fanzani A., Conraads V.M., Penna F., Martinet W. Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia, Sarcopenia and Muscle. 2012;3(3):163–179. doi: 10.1007/s13539-012-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith K.L., Tisdale M.J. Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br J Canc. 1993;67(4):680–685. doi: 10.1038/bjc.1993.126. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White J.P., Baynes J.W., Welle S.L., et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc Min/+ mouse. PloS One. 2011;6(9) doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown J.L., Lee D.E., Rosa-Caldwell M.E., et al. Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice. J Cachexia, Sarcopenia and Muscle. Oct 2018;9(5):987–1002. doi: 10.1002/jcsm.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown J.L., Lawrence M.M., Ahn B., et al. Cancer cachexia in a mouse model of oxidative stress. J Cachexia, Sarcopenia and Muscle. 2020/09/12 2020 doi: 10.1002/jcsm.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45(10):2121–2129. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie Y., Varshavsky A. Physical association of ubiquitin ligases and the 26S proteasome. Proc Natl Acad Sci Unit States Am. 2000;97(6):2497–2502. doi: 10.1073/pnas.060025497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bodine S.C., Latres E., Baumhueter S., et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 67.Gomes M.D., Lecker S.H., Jagoe R.T., Navon A., Goldberg A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci Unit States Am. 2001;98(25):14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petroski M.D., Deshaies R.J. Mechanism of lysine 48-linked ubiquitin-chain synthesis by the cullin-RING ubiquitin-ligase complex SCF-Cdc34. Cell. 2005;123(6):1107–1120. doi: 10.1016/j.cell.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 69.Yamao F. Ubiquitin system: selectivity and timing of protein destruction. J Biochem. 1999;125(2):223–229. doi: 10.1093/oxfordjournals.jbchem.a022277. [DOI] [PubMed] [Google Scholar]
- 70.Winston J.T., Koepp D.M., Zhu C., Elledge S.J., Harper J.W. A family of mammalian F-box proteins. Curr Biol. 1999;9(20) doi: 10.1016/S0960-9822(00)80021-4. 1180-S3. [DOI] [PubMed] [Google Scholar]
- 71.Seaborne R.A., Hughes D.C., Turner D.C., et al. UBR5 is a novel E3 ubiquitin ligase involved in skeletal muscle hypertrophy and recovery from atrophy. J Physiol. Jul 2019;597(14):3727–3749. doi: 10.1113/JP278073. [DOI] [PubMed] [Google Scholar]
- 72.Cohen S., Brault J.J., Gygi S.P., et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. JCB (J Cell Biol) 2009;185(6):1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao J., Zhang Y., Zhao W., et al. Effects of nandrolone on denervation atrophy depend upon time after nerve transection. Muscle Nerve: Off J Am Assoc Electrodiag Med. 2008;37(1):42–49. doi: 10.1002/mus.20888. [DOI] [PubMed] [Google Scholar]
- 74.White J.P., Puppa M.J., Narsale A., Carson J.A. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open. Dec 15 2013;2(12):1346–1353. doi: 10.1242/bio.20136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacDonald A.J., Johns N., Stephens N., et al. Habitual myofibrillar protein synthesis is normal in patients with upper GI cancer cachexia. Clin Canc Res. Apr 1 2015;21(7):1734–1740. doi: 10.1158/1078-0432.CCR-14-2004. [DOI] [PubMed] [Google Scholar]
- 76.Bodine S.C., Stitt T.N., Gonzalez M., et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 77.Bodine S.C. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38(11):1950–1957. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- 78.Preiss T., Hentze M W. Starting the protein synthesis machine: eukaryotic translation initiation. Bioessays. 2003;25(12):1201–1211. doi: 10.1002/bies.10362. [DOI] [PubMed] [Google Scholar]
- 79.Peterson T.R., Laplante M., Thoreen C.C., et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gordon B.S., Steiner J.L., Lang C.H., Jefferson L.S., Kimball S.R. Reduced REDD1 expression contributes to activation of mTORC1 following electrically induced muscle contraction. Am J Physiol Endocrinol Metabol. 2014;307(8):E703–E711. doi: 10.1152/ajpendo.00250.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bossola M., Marzetti E., Rosa F., Pacelli F. Skeletal muscle regeneration in cancer cachexia. Clin Exp Pharmacol Physiol. 2016;43(5):522–527. doi: 10.1111/1440-1681.12559. [DOI] [PubMed] [Google Scholar]
- 82.Talbert E.E., Guttridge D.C. Impaired regeneration: a role for the muscle microenvironment in cancer cachexia. Semin Cell Dev Biol. Jun 2016;54:82–91. doi: 10.1016/j.semcdb.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He W.A., Berardi E., Cardillo V.M., et al. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest. 2013;123(11):4821–4835. doi: 10.1172/JCI68523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown J.L., Rosa-Caldwell M.E., Lee D.E., et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia, Sarcopenia and Muscle. 2017;8(6):926–938. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wallace D.C. Mitochondrial genetic medicine. Nat Genet. Dec 2018;50(12):1642–1649. doi: 10.1038/s41588-018-0264-z. [DOI] [PubMed] [Google Scholar]
- 86.Argilés J.M., López-Soriano F.J., Busquets S. Muscle wasting in cancer: the role of mitochondria. Curr Opin Clin Nutr Metab Care. 2015;18(3):221–225. doi: 10.1097/MCO.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 87.Tzika A.A., Fontes-Oliveira C.C., Shestov A.A., et al. Skeletal muscle mitochondrial uncoupling in a murine cancer cachexia model. Int J Oncol. 2013;43(3):886–894. doi: 10.3892/ijo.2013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guigni B.A., Callahan D.M., Tourville T.W., et al. Skeletal muscle atrophy and dysfunction in breast cancer patients: role for chemotherapy-derived oxidant stress. Am J Physiol Cell Physiol. 2018;315(5):C744–C756. doi: 10.1152/ajpcell.00002.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakellariou G.K., Pearson T., Lightfoot A.P., et al. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep. 2016;6:33944. doi: 10.1038/srep33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ježek J., Cooper K.F., Strich R. Reactive oxygen species and mitochondrial dynamics: the yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants. 2018;7(1):13. doi: 10.3390/antiox7010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Castro G.S., Simoes E., Lima J.D.C.C., et al. Human cachexia induces changes in mitochondria, autophagy and apoptosis in the skeletal muscle. Cancers. 2019;11(9):1264. doi: 10.3390/cancers11091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Russell S.T., Eley H., Tisdale M.J. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal. 2007;19(8):1797–1806. doi: 10.1016/j.cellsig.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Kilgour R.D., Vigano A., Trutschnigg B., Lucar E., Borod M., Morais J.A. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Canc. 2013;21(12):3261–3270. doi: 10.1007/s00520-013-1894-4. [DOI] [PubMed] [Google Scholar]
- 94.Al-Majid S., McCarthy D.O. Resistance exercise training attenuates wasting of the extensor digitorum longus muscle in mice bearing the colon-26 adenocarcinoma. Biol Res Nurs. 2001;2(3):155–166. doi: 10.1177/109980040100200301. [DOI] [PubMed] [Google Scholar]
- 95.VanderVeen B.N., Hardee J.P., Fix D.K., Carson J.A. Skeletal muscle function during the progression of cancer cachexia in the male ApcMin/+ mouse. J Appl Physiol. 2018;124(3):684–695. doi: 10.1152/japplphysiol.00897.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murphy K.T., Chee A., Trieu J., Naim T., Lynch G.S. Importance of functional and metabolic impairments in the characterization of the C-26 murine model of cancer cachexia. Dis Models & Mechan. 2012;5(4):533–545. doi: 10.1242/dmm.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Close R.I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- 98.Van Eijden T., Turkawski S.J.J. Morphology and physiology of masticatory muscle motor units. Crit Rev Oral Biol Med. 2001;12(1):76–91. doi: 10.1177/10454411010120010601. [DOI] [PubMed] [Google Scholar]
- 99.Isaac S.T., Tan T.C., Polly P. Endoplasmic reticulum stress, calcium dysregulation and altered protein translation: intersection of processes that contribute to cancer cachexia induced skeletal muscle wasting. Curr Drug Targets. 2016;17(10):1140–1146. doi: 10.2174/1389450116666150416115721. [DOI] [PubMed] [Google Scholar]
- 100.Szent-Györgyi A.G. Calcium regulation of muscle contraction. Biophys J. 1975;15(7):707–723. doi: 10.1016/S0006-3495(75)85849-8. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Griffiths E.J., Rutter G.A. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta Bioenerg. 2009;1787(11):1324–1333. doi: 10.1016/j.bbabio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 102.Patel T.J., Lieber R.L. Force transmission in skeletal muscle: from actomyosin to external tendons. Exerc Sport Sci Rev. 1997;25:321–363. https://europepmc.org/article/med/9213097 [PubMed] [Google Scholar]
- 103.Bloch R.J., Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31(2):73–78. doi: 10.1097/00003677-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 104.Hardee J.P., Mangum J.E., Gao S., et al. Eccentric contraction-induced myofiber growth in tumor-bearing mice. J Appl Physiol. 2016;120(1):29–37. doi: 10.1152/japplphysiol.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Canc. 2002;2(3):161–174. doi: 10.1038/nrc745. Mar. [DOI] [PubMed] [Google Scholar]
- 106.Devine R.D., Bicer S., Reiser P.J., Velten M., Wold L.E. Metalloproteinase expression is altered in cardiac and skeletal muscle in cancer cachexia. Am J Physiol Heart Circ Physiol. Aug 15 2015;309(4):H685–H691. doi: 10.1152/ajpheart.00106.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Judge S.M., Nosacka R.L., Delitto D., et al. Skeletal muscle fibrosis in pancreatic cancer patients with respect to survival. JNCI Cancer Spectr. 2018;2(3):pky043. doi: 10.1093/jncics/pky043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Montalvo R.N., Counts B.R., Carson J.A. Understanding sex differences in the regulation of cancer-induced muscle wasting. Curr Opin Support Palliat Care. Dec 2018;12(4):394–403. doi: 10.1097/SPC.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oliván S., Calvo A.C., Manzano R., Zaragoza P., Osta R. Sex differences in constitutive autophagy. BioMed Res Int. 2014;2014 doi: 10.1155/2014/652817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ogawa M., Kitano T., Kawata N., et al. Daidzein down-regulates ubiquitin-specific protease 19 expression through estrogen receptor β and increases skeletal muscle mass in young female mice. J Nutr Biochem. 2017;49:63–70. doi: 10.1016/j.jnutbio.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 111.Picard M., Ritchie D., Thomas M.M., Wright K.J., Hepple R.T. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Aging Cell. 2011;10(6):1047–1055. doi: 10.1111/j.1474-9726.2011.00745.x. [DOI] [PubMed] [Google Scholar]
- 112.Itoh Y., Mackie R., Kampf K., et al. Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes. 2015;8 doi: 10.1186/s13104-015-0986-2. 69-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.VanderVeen B.N., Murphy E.A., Carson J.A. The impact of immune cells on the skeletal muscle microenvironment during cancer cachexia. Review. Front Physiol. 2020-August-31 2020;11(1037) doi: 10.3389/fphys.2020.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kays J.K., Shahda S., Stanley M., et al. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J Cachexia, Sarcopenia and Muscle. 2018;9(4):673–684. doi: 10.1002/jcsm.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drott C., Persson H., Lundholm K. Cardiovascular and metabolic response to adrenaline infusion in weight-losing patients with and without cancer. Clin Physiol. Oct 1989;9(5):427–439. doi: 10.1111/j.1475-097X.1989.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 116.Santos C.R., Schulze A. Lipid metabolism in cancer. FEBS J. Aug 2012;279(15):2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 117.Shellock F.G., Riedinger M.S., Fishbein M.C. Brown adipose tissue in cancer patients: possible cause of cancer-induced cachexia. J Canc Res Clin Oncol. 1986;111(1):82–85. doi: 10.1007/BF00402783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brooks S.L., Neville A.M., Rothwell N.J., Stock M.J., Wilson S. Sympathetic activation of brown-adipose-tissue thermogenesis in cachexia. Biosci Rep. 1981;1(6):509–517. doi: 10.1007/BF01121584. [DOI] [PubMed] [Google Scholar]
- 119.Bing C., Brown M., King P., Collins P., Tisdale M.J., Williams G. Increased gene expression of brown fat uncoupling protein (UCP) 1 and skeletal muscle UCP2 and UCP3 in MAC16-induced cancer cachexia. Canc Res. 2000;60(9):2405–2410. https://pubmed.ncbi.nlm.nih.gov/10811117 [PubMed] [Google Scholar]
- 120.Kir S., White J.P., Kleiner S., et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–104. doi: 10.1038/nature13528. Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petruzzelli M., Schweiger M., Schreiber R., et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metabol. Sep 2 2014;20(3):433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 122.Tsoli M., Moore M., Burg D., et al. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Canc Res. Sep 1 2012;72(17):4372–4382. doi: 10.1158/0008-5472.CAN-11-3536. [DOI] [PubMed] [Google Scholar]
- 123.Cohen P., Levy J.D., Zhang Y., et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156(1-2):304–316. doi: 10.1016/j.cell.2013.12.021. Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ryan A.M., Power D.G., Daly L., Cushen S.J., Bhuachalla Ē Ní, Prado C.M. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. May 2016;75(2):199–211. doi: 10.1017/S002966511500419X. [DOI] [PubMed] [Google Scholar]
- 125.Petruzzelli M., Wagner E.F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016;30(5):489–501. doi: 10.1101/gad.276733.115. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Narsale A.A., Enos R.T., Puppa M.J., et al. Liver inflammation and metabolic signaling in ApcMin/+ mice: the role of cachexia progression. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0119888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Narsale A.A., Puppa M.J., Hardee J.P., et al. Short-term pyrrolidine dithiocarbamate administration attenuates cachexia-induced alterations to muscle and liver in ApcMin/+ mice. Oncotarget. 2016;7(37):59482. doi: 10.18632/oncotarget.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Argilés J.M., Stemmler B., López-Soriano F.J., Busquets S. Nonmuscle tissues contribution to cancer cachexia. Mediat Inflamm. 2015;2015:182872. doi: 10.1155/2015/182872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirai K., Ishiko O., Tisdale M. Mechanism of depletion of liver glycogen in cancer cachexia. Biochem Biophys Res Commun. Dec 8 1997;241(1):49–52. doi: 10.1006/bbrc.1997.7732. [DOI] [PubMed] [Google Scholar]
- 130.Rosa-Caldwell M.E., Brown J.L., Lee D.E., et al. Hepatic alterations during the development and progression of cancer cachexia. Appl Physiol Nutr Metabol. 2019 doi: 10.1139/apnm-2019-0407. (ja) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Narsale A.A., Carson J.A. Role of IL-6 in cachexia–therapeutic implications. Curr Opin Support Palliat Care. 2014;8(4):321. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dumas J.F., Goupille C., Julienne C.M., et al. Efficiency of oxidative phosphorylation in liver mitochondria is decreased in a rat model of peritoneal carcinosis. J Hepatol. 2011;54(2):320–327. doi: 10.1016/j.jhep.2010.08.012. Feb. [DOI] [PubMed] [Google Scholar]
- 133.Grasmann G., Smolle E., Olschewski H., Leithner K. Gluconeogenesis in cancer cells - repurposing of a starvation-induced metabolic pathway? Biochim Biophys Acta Rev Canc. Aug 2019;1872(1):24–36. doi: 10.1016/j.bbcan.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bongaerts G.P., van Halteren H.K., Verhagen C.A., Wagener D.J. Cancer cachexia demonstrates the energetic impact of gluconeogenesis in human metabolism. Med Hypotheses. 2006;67(5):1213–1222. doi: 10.1016/j.mehy.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 135.Lai H.S., Lee J.C., Lee P.H., Wang S.T., Chen W.J. Plasma free amino acid profile in cancer patients. Semin Canc Biol. Aug 2005;15(4):267–276. doi: 10.1016/j.semcancer.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 136.Porporato P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. Feb 22 2016;5(2) doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Centers for Disease Control and P Leading causes of death 2012. Im Internet: 2013. https://wwwcdcgov/nchs/fastats/leading-causes-of-death htm
- 138.Hamo C.E., Bloom M.W. Cancer and heart failure: understanding the intersection. Card Fail Rev. 2017;3(1):66. doi: 10.15420/cfr. 2016:24:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Miguel Sánchez C., Elustondo S.G., Estirado A., et al. Palliative performance status, heart rate and respiratory rate as predictive factors of survival time in terminally ill cancer patients. J Pain Symptom Manag. 2006;31(6):485–492. doi: 10.1016/j.jpainsymman.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 140.Manne N.D., Lima M., Enos R.T., Wehner P., Carson J.A., Blough E. Altered cardiac muscle mTOR regulation during the progression of cancer cachexia in the ApcMin/+ mouse. Int J Oncol. Jun 2013;42(6):2134–2140. doi: 10.3892/ijo.2013.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tian M., Nishijima Y., Asp M.L., Stout M.B., Reiser P.J., Belury M.A. Cardiac alterations in cancer-induced cachexia in mice. Int J Oncol. 2010;37(2):347–353. doi: 10.3892/ijo_00000683. [DOI] [PubMed] [Google Scholar]
- 142.Cosper P.F., Leinwand L.A. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Canc Res. Mar 1 2011;71(5):1710–1720. doi: 10.1158/0008-5472.CAN-10-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee DE, Brown JL, Rosa-Caldwell ME, et al. Cancer-induced cardiac atrophy adversely affects myocardial redox state and mitochondrial oxidative characteristics. JCSM Rapid Commun. 10.1002/rco2.18. [DOI] [PMC free article] [PubMed]
- 144.Leibowitz S.F., Alexander J.T. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol Psychiatr. 1998;44(9):851–864. doi: 10.1016/S0006-3223(98)00186-3. [DOI] [PubMed] [Google Scholar]
- 145.Meguid M.M., Fetissov S.O., Varma M., et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16(10):843–857. doi: 10.1016/S0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 146.Tisdale M.J. Cancer anorexia and cachexia. Nutrition. 2001;17(5):438–442. doi: 10.1016/S0899-9007(01)00506-8. [DOI] [PubMed] [Google Scholar]
- 147.Fearon K., Arends J., Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 148.Granda-Cameron C., DeMille D., Lynch M.P., et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1) doi: 10.1188/10.CJON.72-80. [DOI] [PubMed] [Google Scholar]
- 149.Molfino A., Rossi-Fanelli F., Laviano A. The interaction between pro-inflammatory cytokines and the nervous system. Nat Rev Canc. 2009;3(224) doi: 10.1038/nrc2507-c1. [DOI] [PubMed] [Google Scholar]
- 150.Coma M., Vicente R., Busquets S., et al. Impaired voltage-gated K+ channel expression in brain during experimental cancer cachexia. FEBS (Fed Eur Biochem Soc) Lett. Feb 11 2003;536(1-3):45–50. doi: 10.1016/S0014-5793(03)00009-7. [DOI] [PubMed] [Google Scholar]
- 151.Bennett G.J., Doyle T., Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol. Jun 2014;10(6):326–336. doi: 10.1038/nrneurol.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blackwell T.A., Cervenka I., Khatri B., et al. Transcriptomic analysis of the development of skeletal muscle atrophy in cancer-cachexia in tumor-bearing mice. Physiol Genom. Dec 1 2018;50(12):1071–1082. doi: 10.1152/physiolgenomics.00061.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Montalvo R.N., Counts B.R., Carson J.A. Understanding sex differences in the regulation of cancer-induced muscle wasting. Curr Opin Support Palliat Care. 2018;12(4):394–403. doi: 10.1097/SPC.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]