INTRODUCTION:
Achalasia is a primary esophageal motility disorder with heterogeneous manometric subtypes and prognosis, characterized by degeneration of the esophageal myenteric plexus, and reduction in interstitial cells of Cajal (ICCs). This study aimed to explore the histopathologic characteristics of lower esophageal sphincter (LES) muscle from patients with achalasia with different subtypes and different prognosis.
METHODS:
We examined specimens of LES muscle from 122 patients with achalasia who underwent peroral endoscopic myotomy and from 10 control patients who underwent esophagectomy for esophageal cancer. Hematoxylin–eosin staining was performed to assess inflammation infiltration, fibrosis, and atrophy. Specific immunohistochemical staining was performed to identify ICCs and neuronal nitric oxide synthase (nNOS).
RESULTS:
The number of ICCs in patients with type I achalasia was significantly lower than that in patients with type II achalasia, followed by that in control patients (type I vs type II vs control group= 0.4 vs 1.2 vs 9.5; P < 0.001). The number of nNOS-positive cells was significantly lower in patients with achalasia than that in control patients (type I vs type II vs control group = 0.0 vs 0.0 vs 8.0; P < 0.001). Nonrecurrent group had significantly more ICCs than recurrent group (type I: nonrecurrent vs recurrent = 1.0 vs 0.1; P = 0.010; type II: nonrecurrent vs recurrent = 2.0 vs 0.4; P = 0.004).
DISCUSSION:
ICCs and nNOS-positive cells reduced significantly in LES muscle of patients with achalasia. The number of ICCs differed among different achalasia subtypes and was related to patients' clinical prognosis.
INTRODUCTION
Achalasia is a major esophageal motility disorder, characterized by impaired lower esophageal sphincter (LES) relaxation and abnormal peristalsis during swallowing (1). The prevalence of achalasia is approximately 30 in 100,000 individuals (2). Patients with achalasia usually present with dysphagia, regurgitation of undigested food, unintentional weight loss, and chest pain (3). The disease not only brings huge economic burden to the society but also influences the life expectancy of the patients (4).
The etiology of achalasia is not fully understood. The muscle layer histopathology in achalasia was previously studied and is characterized by degeneration of the esophageal myenteric plexus and reduction in interstitial cells of Cajal (ICCs) (5,6). Animal studies also found that the reduction of ICCs and neuronal nitric oxide synthase (nNOS) can cause dysfunction of the LES and esophageal peristalsis (7). ICCs are the cells of mesenchymal origin that occur within and around the muscle layers. Apart from serving as pacemakers, they also involve in the transfer of neurotransmitters. The nNOS is a kind of inhibitory neurotransmitter, which is related to muscle relaxation. The reason for the reduction of ICCs and nNOS-positive cells remains unknown. However, inflammation infiltration might play an important role (8).
According to Chicago classification v3.0, achalasia can be further divided into 3 subtypes (1). Type I is characterized by 100% failed contractions in combination with elevated integrated relaxation pressure (IRP). Type II and type III are defined by panesophageal pressurization and spasm contractions for at least 20% of the swallows in combination with elevated IRP, respectively. The peristaltic pattern and prognosis differ among different subtypes (9). Treatment outcome of type II achalasia is the best, whereas that of type III is the worst (10). Studies focusing on the reason of the heterogeneity in achalasia are lacking. Therefore, it is necessary to compare the histological features among patients with different subtypes. Clarifying the causes of heterogeneity in achalasia might help to improve the treatment outcome and develop individual treatment project.
Considering the different motility patterns and prognosis among achalasia, we hypothesized that different subtypes and different prognosis of achalasia might have different etiology. Therefore, the purpose of this study was to explore the histopathology characteristics in the LES muscle layer in patients with achalasia with different subtypes and different prognosis.
METHODS
Study subjects
We included patients who were diagnosed with achalasia by high-resolution manometry (HRM) and received peroral endoscopic myotomy (POEM) in our hospital from January 2015 to September 2020. Patients who had any of the following conditions were excluded: (i) previous upper gastrointestinal tract invasive treatment; (ii) a history of gastroesophageal tumor; (iii) rheumatic or immune system disease; and (iv) current infection or receiving antibiotics. We also included 10 patients with esophagus cancer as control patients and collected normal LES muscle specimens from them after the esophagectomy. This study was approved by the Ethical Review Board of Sun Yat‐sen University (no. 2014290) and was conducted following the tenets of the Declaration of Helsinki.
HRM
As a routine procedure in our hospital, patients were asked to record their demographic information before the HRM test. HRM procedure was performed as previously reported (11). Patients were required to stop swallowing for 30 seconds to record the basal pressure. Ten 5-mL water swallows were then performed in the supine position. HRM parameters analysis was conducted using Manoview analysis software (Medtronic Inc. Minneapolis, MN) by 2 certificated investigators. Achalasia was defined as described in Chicago Classification 3.0 (1).
POEM and outcome measurements
POEM was performed based on the protocol described by Inoue et al. (12). A submucosal tunnel was created first. The sphincter muscle was dissected across the gastroesophageal junction. After completion of myotomy, the mucosal entry site was closed with hemostatic clips.
The length of dissected muscle during POEM was recorded. Patients were scheduled to follow-up at the center at 3 months, 6 months, and 1-year postoperation and then yearly afterward for symptom assessment and physical examination. Disease recurrence was defined by Eckardt scores >3 or receiving invasive treatment (esophagus dilatation, POEM, or laparoscopic surgery) at least 3 months after POEM.
Biopsy process and histopathological assessment
For patients with achalasia, 4 LES muscle specimens (each with a diameter of about 5 mm) were collected form muscularis propria using biopsy forceps after myotomy during POEM procedure. For patients with esophagus cancer, 4 LES specimens were collected from muscularis propria after esophagectomy. Specimens of patients with esophagus cancer were at least 5 cm away from the tumor and were pathologically confirmed as normal specimen. Specimens were fixed in 10% formalin and then cleared using phosphate-buffered saline. The specimens were cut into 4-μm sections after being dehydrated by alcohol, dealcoholized with xylene, and wrapped in a paraffin film.
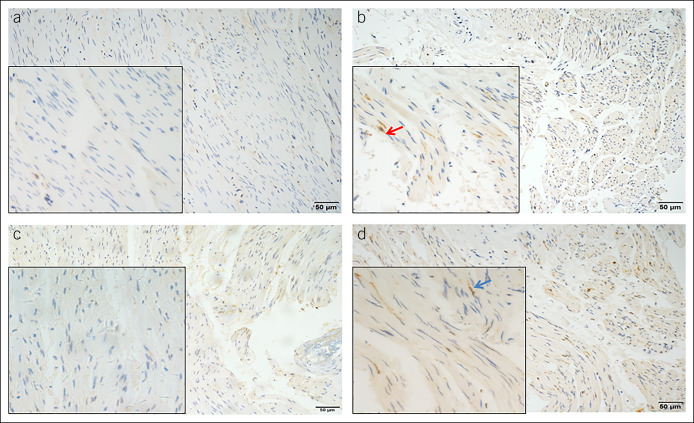
Immunohistochemical (IHC) staining using c-kit (PB0240; Boster Biological Technology, Wuhan, China) was performed to assess the number of ICCs. Only elongated nuclear–stained cells were considered to be ICCs (Figure 1a,b) (13). IHC staining for nNOS was used to identify nitrergic neurons (Figure 1c,d) (24,287; Immunostar, Hudson, WI). Both ICCs and nNOS-positive cells were calculated at 400× magnification in 5 randomly selected microscopy fields. The mean of the 5 randomly selected fields was calculated to obtain the number of ICCs and nNOS-positive cells.
Figure 1.
IHC staining of LES muscle using c-kit: (a) patients with achalasia (200× magnification); (b) control patients (200× magnification). (c) IHC staining for nNOS in LES muscle from patients with achalasia (200× magnification). (d) IHC staining for nNOS in LES muscle from control patients (200× magnification). Insets show pictures at 400× magnification. Red arrow indicates ICCs. Blue arrow indicates nitrergic neurons. ICCs, interstitial cells of Cajal; IHC, immunohistochemical; LES, lower esophageal sphincter; nNOS, neuronal nitric oxide synthase.
Hematoxylin–eosin staining was also performed. Two certificated pathologists, who were blinded to patients' clinical data, assessed the degree of atrophy, fibrosis, and inflammation of the LES muscle independently. Atrophy was graded as follows: (i) no to mild atrophic change with vacuolative degeneration; (ii) moderate atrophic change, with shrinkage of the volume of muscle bundles; and (iii) severe atrophic change, with replacement of smooth muscle bundles by fibrosis (14). Fibrosis was graded as follows: (i) no to mild fibrosis; (ii) moderate fibrosis, fibrotic tissue extension to intersmooth muscle bundles; and (iii) severe fibrosis, bridging fibrosis between neighboring muscle bundles (14). Inflammation cells were counted at 400× magnification.
Statistical analysis
For categorical variables, the percentage form and χ2 tests were used for comparisons. The mean (SD) of normally distributed continuous variables were presented, which were compared by one-way abstract analysis of variance. Median (25th, 75th) and Kruskal-Wallis test were adopted for continuous data that were not normally distributed. Pairwise comparison was conducted after statistical significant difference was observed. Linear regression model was used to identify whether there is a linear relationship between the parameters. The statistical significance was set at P < 0.05. Analysis was performed by 2 investigators separately using SPSS software, version 22 (IBM, Armonk, NY).
RESULTS
In total, we included 29 patients with type I achalasia, 90 patients with type II achalasia, 3 patients with type III achalasia, and 10 control patients. The median follow-up time was 31.0 months. Patients in the control group were significantly older and had a higher body mass index than patients with type I and type II achalasia (Table 1). No significant difference was found in sex, the duration of disease course, and Eckardt scores before the POEM procedure among groups. As for HRM parameters, LES basal pressure and IRP were significantly higher in patients with type II achalasia than those in patients with type I achalasia (Table 1). No significant differences were found in the length of dissected sphincter muscle and disease recurrent rate among patients with achalasia (Table 1).
Table 1.
Baseline data comparison among achalasia patients and control patients
| Type I Group N = 29 |
Type II Group N = 90 |
Type III Group N = 3 |
Control Group N = 10 |
P Value | |
| Men (%) | 48.2 | 40.0 | 100.0 | 60.0 | 0.095 |
| Age (yr, mean [SD]) | 40.8 (12.2) | 39.1 (12.8) | 33 (14.0) | 57.1 (9.6) | <0.001 |
| BMI (kg/m2, median [25th, 75th]) | 18.3 (16.7–20.9) | 19.1 (17.3–21.5) | 16.4 (6.4) | 20.7 (19.0–21.9) | 0.034 |
| Course of disease (mo, mean [SD]) | 95.8 (127.1) | 42.3 (42.5) | 80 (49.9) | — | 0.076 |
| Eckardt scores (median [25th, 75th]) | 7.0 (4.0–8.3) | 5.0 (3.0–7.5) | 3.5 (1.2) | — | 0.081 |
| LES basal pressure (mm Hg, median [25th, 75th]) | 27.7 (19.9–41.2) | 38.0 (29.6–50.1) | 35.6 (12.4) | — | 0.003 |
| Integrated relaxation pressure (mm Hg, median [25th, 75th]) | 21.5 (16.4–29.9) | 30.0 (23.5–39.2) | 17.0 (6.7) | — | 0.002 |
| The length of dissected muscle during POEM (cm, median [25th, 75th]) | 7.0 (6.0–9.0) | 8.0 (7.0–9.0) | 14 (6.1) | — | 0.084 |
| Disease recurrent rate (%) | 34.4 | 22.4 | — | 0.344 |
LES = lower esophageal sphincter; POEM = peroral endoscopic myotomy.
Histopathology comparison among patients with achalasia and control patients
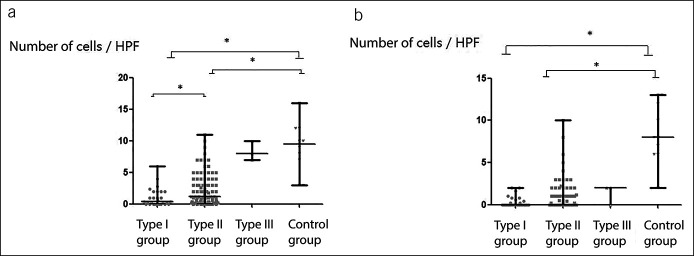
Most patients with achalasia had only no to mild atrophy (92.6%) and fibrosis (86.8%). However, inflammation infiltration was considered to be the course of degeneration of the esophageal myenteric plexus, and the results of studies focusing on the inflammation cells in achalasia varied greatly. In this study, inflammation cells were found in the LES muscle of only 7.2% of patients with achalasia, suggesting that inflammation infiltration might be intermittent. No significant differences were found in atrophy, fibrosis, and inflammation infiltration among groups (Table 2). The number of ICCs of patients with type I achalasia was significantly lower than that of patients with type II achalasia, followed by that of control patients (type I vs type II vs type III vs control group = 0.4 [0.0–2.0] vs 1.2 [0.4–4.0] vs 8.3 [1.5] vs 9.5 [6.0–12.0]; P < 0.001; Figure 2a). More than half of patients with achalasia (58.1%) had complete loss of nNOS-positive cells. The number of nNOS-positive cells was significantly lower in patients with type I and type II achalasia than that in control patients (type I vs type II vs type III vs control group = 0.0 [0.0–0.6] vs 0.0 [0.0–1.0] vs 1.3 [1.2] vs 8.0 [6.0–12.3]; P < 0.001; Figure 2b). When comparing among different subtypes of achalasia, no significant difference was found in nNOS-positive cells. Linear regression model was also conducted to figure out whether there is any linear correlation among ICCs, nNOS-positive cells, the course of disease, LES basal pressure, IRP, and other parameters. However, no significant correlation was found.
Table 2.
Histopathology comparison among achalasia patients and control patients
| Type I Group N = 29 |
Type II Group N = 90 |
Type III Group N = 3 |
Control Group N = 10 |
P Value | |
| Fibrosis grade (%) | 0.181 | ||||
| 1 | 79.3 | 88.8 | 100.0 | 100.0 | |
| 2 | 20.6 | 11.2 | 0.0 | 0.0 | |
| 3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Atrophy grade (%) | 0.629 | ||||
| 1 | 93.1 | 93.3 | 66.6 | 100.0 | |
| 2 | 6.8 | 6.7 | 33.4 | 0.0 | |
| 3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Inflammation infiltration (%) | |||||
| Lymphocyte | 3.4 | 1.1 | 0.0 | 0.434 | |
| Neutrophil | 0.0 | 5.5 | 0.0 | 0.331 | |
| Eosinophils | 3.4 | 1.1 | 0.0 | 0.433 |
Figure 2.
Immunohistochemical comparison of patients with achalasia and control patients (*P < 0.05). (a) ICCs; (b) nNospositive cells. HPF, high-power field; ICCs, interstitial cells of Cajal; nNOS, neuronal nitric oxide synthase.
Comparison between different prognoses of patients
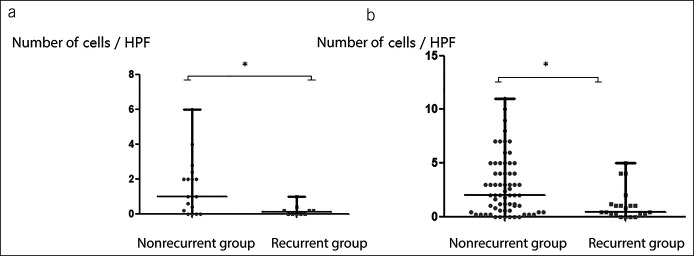
Disease recurrence was found in 34.4% of patients with type I achalasia and 22.4% of patients with type II achalasia. No recurrence was found in patients with type III achalasia. The median time of recurrence was 16.0 and 18.0 months for patients with type I and type II achalasia, respectively. For patients with type I achalasia, 20% of patients with disease recurrence received POEM again and 10% received esophagus dilatation. For patients with type II achalasia, 27.2% of patients with disease recurrence received POEM again and 18.2% received esophagus dilatation. No significant difference was found in demographic data, HRM parameters, and surgery data between nonrecurrent and recurrent groups. No significant difference was found in fibrosis, atrophy, inflammation, and the number of nNOS-positive cells. As for ICCs, nonrecurrent group had significantly more Cajal cells than recurrent group regardless of type I or type II achalasia (type I: nonrecurrent vs recurrent = 1.0 [0.2–2.1] vs 0.1 [0.0–0.3]; P = 0.010; Figure 3a) (type II: nonrecurrent vs recurrent = 2.0 [0.6–4.3] vs 0.4 [0.2–1.1]; P = 0.004; Figure 3b).
Figure 3.
The number of ICCs comparison between nonrecurrent and recurrent groups with the same achalasia subtypes (*P < 0.05). (a) ICCs in type I achalasia; (b) ICCs in type II achalasia. HPF, high-power field; ICCs, interstitial cells of Cajal.
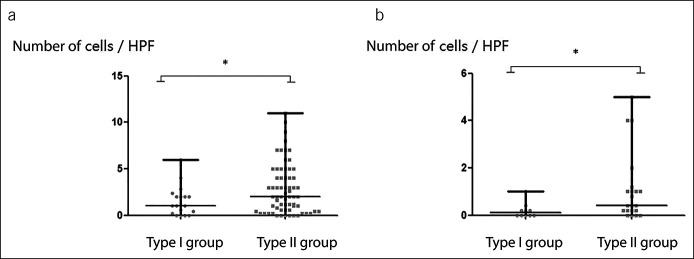
We also compared the histological differences between type I and type II achalasia with the same prognosis. No significant difference was found in demographic data, HRM parameters, surgery data, fibrosis, atrophy, inflammation, or the number of nNOS-positive cells between patients with type I and type II. As for ICCs, irrelevant of recurrent or nonrecurrent group, patients with type II achalasia still had significantly more ICCs than those with type I achalasia (nonrecurrent: type I vs type II = 1.0 [0.2–2.1] vs 2.0 [0.6–4.3]; P = 0.049; Figure 4a; recurrent: type I vs type II = 0.1 [0.0–0.3] vs 0.4 [0.2–1.1]; P = 0.021; Figure 4b).
Figure 4.
The number of ICCs comparison between patients with type I and type II achalasia with the same prognosis (*P < 0.05). (a) ICCs in nonrecurrent patients; (b) ICCs in recurrent patients. HPF, high-power field; ICCs, interstitial cells of Cajal.
DISCUSSION
The etiology of achalasia remains unknown. Considering the heterogeneities of motility pattern (1) and prognosis (10) in achalasia, it was speculated that different subtypes of achalasia may have different pathological mechanisms. In this study, the number of ICCs and nNOS-positive cells in LES muscle were found to be significantly lower in patients with achalasia than those in control patients. Patients with type III achalasia seemed to have the highest number of ICCs and nNOS-positive cells in LES muscle. However, only 3 patients with type III achalasia were included in this study, with no significant difference was found. The number of ICCs differed between patients with type I and type II achalasia. The number of ICCs was also found to be relevant to patients' prognosis after POEM procedure.
Several studies had been conducted to compare the muscle layer between patients with achalasia and control patients. A 1996 study by Goldblum et al. (8) compared the histological features between 11 patients with achalasia and 8 patients who underwent esophagectomy for intramucosal adenocarcinoma. The reduction of ganglion cells, myenteric inflammation, and neural fibrosis were more severe in patients with achalasia than that in control patients. Zarate et al. (15) also found that LES muscles form patients with achalasia were characterized by reduction of ICCs and nNOS-positive cells. Another study that included 53 patients with achalasia found that reduction in nNOS-positive cells and ICCs was correlated with patients' severity of dysphagia (13). The function of ICCs and nNOS-positive cells was further explored in animal studies where intraluminal esophageal manometry was performed in NO-deficient (nNOS−/−), ICCs-deficient (W/Wv)−, and wild-type mice. It was found that nNOS−/− in comparison with wild-type mice showed a significantly higher LES mean resting pressure with an impaired swallow-induced relaxation, whereas W/Wv mice had a hypotensive LES with decreased relaxation (7). Considering the heterogeneities in achalasia, it was necessary to compare the histological features among patients with different subtypes. A study retrospectively examined surgical muscularis propria biopsies obtained from 46 patients with achalasia during laparoscopic esophagomyotomy and found the degree of ganglion cells loss was significantly higher in type I achalasia than type II (16). Besides ganglion cells, ICCs was also found to be different among patients with different subtype (14). It should be noted that limited research has tapped into the histological features among patients with different subtypes of disease. Furthermore, the sample size of these studies was small. The treatment outcomes of patients with achalasia after POEM procedure varies greatly. None of these aforementioned studies explored the relationship between ICCs, nNOS-positive cells, and patients' symptom outcome. Whether the prognosis was related to ICCs and nNOS-positive cells in LES muscle remained uncertain. Compared with former studies, this study had the largest sample size. To our knowledge, this was also the first study that compared the histological features between patients with different achalasia and prognosis.
Consistent with previous studies, most patients with achalasia in this study presented complete loss of nNOS-positive cells (16). This finding further supports that achalasia is a motility disease related to loss of inhibitory neurons in LES. Although inflammation infiltration was considered to play an important role in the reduction of ganglion cells, the results of different studies focusing on the inflammation cells in muscle layer varied widely. Tøttrup et al. (17) studied esophagomyotomy specimens from 9 patients with achalasia using specific IHC methods, with all patients presenting with infiltration of eosinophil. A study using tryptase staining to identify mast cells found that in patients with achalasia, mast cells infiltration in the LES muscle is increased in association with loss of ICCs and neuronal degeneration (18). However, inflammation cell infiltration was found to be absent in most patients with achalasia in some other studies (8,16). Different disease course and different staining methods in these studies might explain the difference. The infiltration of inflammatory cells into muscle tissue may also be intermittent. ICCs are the cells of mesenchymal origin that occur within and around the muscle layers in the gastrointestinal tract. The function of ICCs was not fully understood. Apart from serving as pacemakers, they also involve in the transfer of neurotransmitters including substance P, vesicular acetylcholine transporter, and nNOS (19–21). Although no significant difference in nNOS-positive cells was found, we did find that the reduction degree of ICCs was different among patients with different subtypes of disease and between patients with different prognosis.
Although most patients presented complete loss of nNOS-positive cells, the presence of ICCs enabled the transfer of other neurotransmitters in muscularis propria of patients with type II and type III . This might help explain the clinical heterogeneities in achalasia. Even in the same type of achalasia, ICCs were found to be correlated with better prognosis after POEM. This finding further emphasized the importance of ICCs in achalasia. Although there were a variety of treatment options (such as pneumatic dilatation, POEM, and Heller myotomy) for achalasia, the long-term treatment failure rates of achalasia were between 18% and 35% (22). A recent meta-analysis that included 75 studies (8 randomized controlled trials, 27 prospective cohort studies, and 40 retrospective studies) found that patients' age and manometric subtype were associated with achalasia treatment outcomes (22). However, these factors were not manageable. Because higher number of ICCs was correlated with better prognosis after POEM, further studies exploring the mechanism of the loss of ICCs are needed. Preventing the progressive loss of ICCs and promoting the number of ICCs might become the potential therapeutic target for achalasia.
This study had some limitations. First, only 3 patients with type III achalasia were included. Patients with type III achalasia seemed to have the highest number of ICCs and nNOS-positive cells in LES muscle. However, because of the small sample size in this group, no significant difference could be observed. Further studies focusing on the histological features in type III achalasia are needed. Second, we did not use specific IHC method to identify inflammation cells in muscle layer. This might partly explain why inflammation cells were found in LES muscle of only 7.2% of patients with achalasia. Third, only patients undergoing POEM were included. Patients who underwent other surgery methods and who did not receive surgery were excluded from this study. The samples included in this study might not be representative of the entire population with achalasia. Finally, further mechanism studies are needed to prove and explain the correlation between ICCs and prognosis.
In conclusion, this study demonstrated that ICCs and nNOS-positive cells reduced significantly in LES muscle of patients with achalasia. The number of ICCs differed among patients with different subtypes of disease and was related to patients' symptom outcome. Further studies are still needed to prove and explain this correlation.
CONFLICTS OF INTEREST
Guarantor of the article: Yinglian Xiao, MD, PhD.
Specific author contributions: Songfeng Chen, MD, and Mengyu Zhan, MD, PhD contributed equally to this study. S.C. and M.Z.: acquisition of data, analysis and interpretation of data, and drafting of the manuscript; M.L. and N.T.: interpretation of data and drafting of the manuscript. Y.C., J.W., X.X., Z.Y., and Q.Z.: interpretation of data. Y.X.: study concept and design, analysis of data, and finalizing and approving the manuscript.
Financial support: The study was supported by grants from the National Natural Science Foundation of China (81770544 and 81970479) and the Medical Scientific Research Foundation of Guangdong Province of China (A2019510).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Achalasia is characterized by reduction of interstitial cells of Cajal (ICCs) and neuronal nitric oxide synthase (nNOS)-positive cells.
WHAT IS NEW HERE
✓ The number of ICCs differs among different achalasia subtypes.
✓ The number of ICCs is related to patients' symptom outcome.
Contributor Information
Songfeng Chen, Email: chensf5@mail2.sysu.edu.cn.
Mengyu Zhang, Email: zhangmny@mail2.sysu.edu.cn.
Mengya Liang, Email: infisdsums@163.com.
Niandi Tan, Email: tanniandi@126.com.
Yi Cui, Email: gzcuiyi@163.com.
Jinhui Wang, Email: jinhuiwang100@msn.com.
Xiangbin Xin, Email: xxbken@163.com.
Ziyin Ye, Email: yeziyin@mail.sysu.edu.cn.
Qianjun Zhuang, Email: zhuangxj6@mail2.sysu.edu.cn.
REFERENCES
- 1.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samo S, Carlson DA, Gregory DL, et al. Incidence and prevalence of achalasia in Central Chicago, 2004-2014, since the widespread use of high-resolution manometry. Clin Gastroenterol Hepatol 2017;15:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel DA, Kim HP, Zifodya JS, et al. Idiopathic (primary) achalasia: A review. Orphanet J Rare Dis 2015;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadowski DC, Ackah F, Jiang B, et al. Achalasia: Incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil 2010;22:e256–61. [DOI] [PubMed] [Google Scholar]
- 5.Misiewicz JJ, Waller SL, Anthony PP, et al. Pharmacology and histopathology of the cardiac sphincter in achalasia. Gut 1968;9:726–7. [PubMed] [Google Scholar]
- 6.Csendes A, Smok G, Braghetto I, et al. Histological studies of Auerbach's plexuses of the oesophagus, stomach, jejunum, and colon in patients with achalasia of the oesophagus: Correlation with gastric acid secretion, presence of parietal cells and gastric emptying of solids. Gut 1992;33:150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller M, Colcuc S, Drescher DG, et al. Murine genetic deficiency of neuronal nitric oxide synthase (nNOS(-/-) ) and interstitial cells of Cajal (W/W(v) ): Implications for achalasia? J Gastroenterol Hepatol 2014;29:1800–7. [DOI] [PubMed] [Google Scholar]
- 8.Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology 1996;111:648–54. [DOI] [PubMed] [Google Scholar]
- 9.Oude Nijhuis RAB, Prins LI, Mostafavi N, et al. Factors associated with achalasia treatment outcomes: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18:1442–53. [DOI] [PubMed] [Google Scholar]
- 10.Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 2013;144:718–4. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Liang M, Zhang M, et al. A study of proximal esophageal baseline impedance in identifying and predicting laryngopharyngeal reflux. J Gastroenterol Hepatol 2020;35:1509–14. [DOI] [PubMed] [Google Scholar]
- 12.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265–71. [DOI] [PubMed] [Google Scholar]
- 13.Gockel I, Bohl JR, Eckardt VF, et al. Reduction of interstitial cells of Cajal (ICC) associated with neuronal nitric oxide synthase (n-NOS) in patients with achalasia. Am J Gastroenterol 2008;103:856–64. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima N, Sato H, Takahashi K, et al. Muscle layer histopathology and manometry pattern of primary esophageal motility disorders including achalasia. Neurogastroenterol Motil 2017 Mar;29(3). (doi: 10.1111/nmo.12968) [E-pub ahead of print October 3, 2016.] [DOI] [PubMed] [Google Scholar]
- 15.Zarate N, Wang XY, Tougas G, et al. Intramuscular interstitial cells of Cajal associated with mast cells survive nitrergic nerves in achalasia. Neurogastroenterol Motil 2006;18:556–68. [DOI] [PubMed] [Google Scholar]
- 16.Sodikoff JB, Lo AA, Shetuni BB, et al. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil 2016;28:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tøttrup A, Fredens K, Funch-Jensen P, et al. Eosinophil infiltration in primary esophageal achalasia. A possible pathogenic role. Dig Dis Sci 1989;34:1894–9. [DOI] [PubMed] [Google Scholar]
- 18.Liu ZQ, Chen WF, Wang Y, et al. Mast cell infiltration associated with loss of interstitial cells of Cajal and neuronal degeneration in achalasia. Neurogastroenterol Motil 2019;31:e13565. [DOI] [PubMed] [Google Scholar]
- 19.Ward SM. Interstitial cells of Cajal in enteric neurotransmission. Gut 2000;47(suppl 4):iv40-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckett EA, Takeda Y, Yanase H, et al. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol 2005;493:193–206. [DOI] [PubMed] [Google Scholar]
- 21.Iino S, Horiguchi K. Interstitial cells of Cajal are involved in neurotransmission in the gastrointestinal tract. Acta Histochem Cytochem 2006;39:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oude Nijhuis RAB, Prins LI, Mostafavi N, et al. Factors associated with achalasia treatment outcomes: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18:1442–53. [DOI] [PubMed] [Google Scholar]