Abstract
Injections of the GABAA antagonist bicuculline into the medial ventral pallidum (VPm) induce marked increases in food intake, but nothing is known about the way in which these injections alter the distribution of intake in a macronutrient selection situation. We investigated this topic by adapting rats to a diet containing independent sources of protein, carbohydrate and fat, and then examining the effects of intra-VPm bicuculline on diet selection. Under these conditions, bicuculline produced a massive, preferential increase in fat intake with subjects consuming a mean of 97% of their calories from fat. Furthermore, all treated subjects ate fat before any other macronutrient, suggesting that the animals’ behavior was directed selectively towards this dietary component even before consumption had begun. Similar effects were not observed following food deprivation, which exerted its largest effect on carbohydrate intake. To compare the intra-VPm bicuculline response to that seen after activation of GABA receptors in the nucleus accumbens shell (AcbSh), a major source of projections to the VPm, we conducted similar experiments with intra-AcbSh injections of muscimol and baclofen. These injections also enhanced food intake, but did not reproduce the selective preference for fat seen after intra-VPm bicuculline. These experiments provide the first demonstration of preferential enhancement of fat intake following manipulations of a nonpeptide neurotransmitter. Since mean intakes of fat under baseline conditions and after deprivation tended to be lower than those of carbohydrates, it seems unlikely that the effects of intra-VPm bicuculline are related to the intrinsic “rewarding” properties of fat, but might rather reflect the induction of a state of “fat craving.”
Keywords: macronutrient, carbohydrate, feeding, food deprivation, DAMGO, bicuculline
1.0 Introduction
When a variety of foods are available, animals must decide not only how much to eat, but also how to distribute their intake across the accessible items. One could imagine that the available foods could simply be ranked in an order of preference determined in part by innate factors and in part by past experiences, and that animals would always tend to consume the largest quantities of the most preferred items. Evidence against this type of “fixed hierarchy model” is, however, provided by observations that the relative preferences of subjects for different foods may vary depending on the internal state of the animal. For example, sodium depletion is associated with increased intake of salty foods [1], and extreme food deprivation may lead to increased intake of foods rich in fat [2].
Although much less effort has been devoted to food selection than to the control of total intake, substantial evidence suggests that a number of different brain systems may participate in mediating the relative preferences expressed by animals for different foods. For example, in animals given a choice between foods rich in fat and those rich in carbohydrates, systemic injections of opiates tend to preferentially increase the intake of fatty foods, whereas intraventricular injections of NPY tend to preferentially increase the intake of carbohydrates [2]. Some of the most convincing evidence in this regard has been obtained following manipulations of the paraventricular nucleus of the hypothalamus (PVN), a brain region from which local injections of the peptides enkephalin and galanin induce a marked preference for fat [3–5], whereas activation of NPY or norepinephrine receptors preferentially increases carbohydrate intake [6, 7]. Injections of serotonin at this site also specifically decrease carbohydrate intake [8]. Such results indicate that ingestive behavior must involve more than simply gating ingestive systems and that brain mechanisms are able to influence not only how much, but also what types of food will be eaten.
Brain mechanisms controlling feeding are not restricted to the hypothalamus, and in recent years it has been shown that the nucleus accumbens (Acb), a structure located in the basal telencephalon, also exerts a powerful influence on ingestive behavior. Pronounced increases in food intake can be induced by injections of a variety of drugs into the Acb, especially its shell region (AcbSh), and these injections alter activity at a number of sites in the hypothalamus, including the PVN [9, 10]. In the current context, it is important that some of these injections alter relative food preferences. For example, in animals given a choice of high-fat and high-carbohydrate diets, injections of the μ-opioid agonist D-Ala2,N,Me-Phe4,Gly-ol5-enkephalin (DAMGO) into the core of the Acb induce a strong preference for fat, irrespective of the baseline preferences of the animals [11]. In contrast, rats made hyperphagic by intra-AcbSh injections of the GABAA agonist muscimol into AcbSh show similar increases in both fat and carbohydrate intake [12].
Although the circuit through which the Acb may mediate macronutrient intake has yet to be identified, it is known that projections from GABAergic medium spiny neurons in the Acb synapse on cells in the medial ventral pallidum (VPm) [13–16], and that this structure in turn can influence the lateral hypothalamus (LH) and PVN [17, 18]. While the LH is well known as a brain region involved in the control of ingestion [19], the medial ventral pallidum also has a demonstrated role in the control of food intake. Injections of either GABAA antagonists, glutamate agonists, or μ-opioid agonists into the VPm induce intense feeding in satiated rats [18, 20–22]. Furthermore, VPm lesions reduce the feeding induced by muscimol injections in the AcbSh through specific disruption of the AcbSh-VPm circuit [23]. It has been proposed that the Acb, VPm, and LH work together to form a functional circuit controlling some aspects of feeding behavior [10, 23, 24]. While the ability of chemical stimulation of the VPm to induce intense hyperphagia is well documented, nothing is known about whether these procedures also alter the relative preference of rats for different dietary components in a fashion similar to that seen after intra-Acb injections of DAMGO. In the current set of studies, we therefore investigated the effects of intra-VPm injections of the GABAA antagonist bicuculline on food selection in ad-libitum fed rats consuming a diet containing independent sources of fat, carbohydrate and protein. We also examined deprivation-induced feeding in these same subjects in order to determine whether this manipulation produced a pattern of ingestion similar to that seen after bicuculline injections. Since certain aspects of our procedure differed from those employed by earlier investigators, we additionally examined macronutrient selection in subjects with injections of several orexigenic compounds into the AcbSh.
2.0 Materials and methods
2.1 Subjects
The subjects were 15 male Sprague–Dawley rats (Charles River), weighing between 290 and 350 g at the time of surgery. The rats were housed individually in plastic cages (L 43 X W 22 X H 21 cm) with wire floors on a 12-h light:12-h dark cycle at a constant room temperature (~21°C). Water and food were available ad libitum, except as noted below. For the period before surgery and for the first week after surgery, food was provided in the form of standard lab chow (Harlan Teklad), after which time animals were switched for the remainder of the experiments to a nutritionally-complete diet consisting of three separate macronutrients components, described below. Throughout the experiments, rats were handled and weighed on a daily basis. All experiments conformed to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UIC Institutional Animal Care and Use Committee.
2.2 Surgery
Surgery was performed using standard, aseptic, flat-skull stereotaxic techniques under sodium pentobarbital (60 mg/kg) anesthesia. In 8 experimental subjects, bilateral 22-gauge stainless steel guide cannulae (Plastics One, Roanoke, VA), aimed so as to terminate 2.0 mm dorsal to the VPm, were implanted at coordinates of AP: −0.2, LM: ±1.8, DV: −6.7. In the remaining subjects, bilateral 22-gauge cannulae, aimed so as to terminate 2.0 mm dorsal to the AcbSh, were implanted at coordinates of AP: 1.6, LM: ±0.8, DV: −6.1. The guide cannulae were held in place using stainless steel screws and denture lining material and a stainless steel obturator was inserted into the lumen of each cannula to help maintain patency. After surgery, the rats received an injection of the analgesic carprofen (5 mg/kg, sc) and the antibiotic cefazolin (60 mg/kg). Each rat was allowed to recover for at least 7 days before the start of behavioral testing.
2.3 Macronutrient diet components and adaptation
The rats were allowed to select their food from three separate sources of pure macronutrients (protein, carbohydrate, and fat) presented simultaneously in glass jars with dimensions of approximately 62 X 60 mm (diameter X height) which were placed in the corners of the animals’ home cages. The detailed composition of each of the dietary components is presented in Table 1; all the ingredients were obtained from Harlan Laboratories (Indianapolis, IN) except for sucrose (Domino Foods, Chicago, IL), lard (Armour, Peoria, IL) and dibasic calcium phosphate (Sigma-Aldrich, St. Louis, MO). The dietary components were similar to those used in previous studies by other authors [2, 6] except that we used custom modified versions of the AIN-93 vitamin and mineral mixes in which cellulose was substituted for sucrose. Thus our protein and fat components contained no sugar, whereas the sucrose concentrations in the mixtures used by earlier workers have been as high as 2%. Placement of the food jars containing various components was changed daily to prevent the development of position preferences. Subjects were given two weeks to adapt to the multicomponent diet before the start of drug injections. On the last three days of the adaptation period, 24-h intakes of each of the three dietary components were determined, correcting for spillage.
TABLE 1.
Composition of Dietary Components (g/kg)
| Ingredient | Protein | Fat | Carbohydrate |
|---|---|---|---|
| Casein, VFT | 920.45 | 0 | 0 |
| L-Cystine | 13.8 | 0 | 0 |
| * AIN-93-Vitamin Mix | 15 | 15 | 15 |
| * AIN-93 Mineral Mix (W/O Ca, P) | 20 | 20 | 20 |
| Calcium Carbonate, anhydrous | 27 | 5.4 | 5.4 |
| Calcium Phosphate, Dibasic, anhydrous | 0 | 30.2 | 30.2 |
| Choline Bitartrate | 3.75 | 3.75 | 3.75 |
| Lard | 0 | 925.65 | 0 |
| Sucrose | 0 | 0 | 365.65 |
| Dextrin | 0 | 0 | 280 |
| Corn Starch | 0 | 0 | 280 |
| kcal/g | 3.4 | 8.5 | 3.6 |
Cellulose was substituted for the sucrose normally found in these mixes.
2.4 Drugs
Bicuculline (1(S),9(R)-(—)-bicuculline methbromide, molecular weight (MW) = 462.3), muscimol (5-Aminomethyl-3-hydroxy-isoxazole, MW = 114.1) and DAMGO ([D-Ala2, N-Me-Phe4, Gly5-ol]-Enkephalin, MW = 513.6) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and baclofen ((RS)-4-amino-3-(4-chlorophenyl)butanoic acid, MW = 213.7) from Tocris Bioscience (Bristol, UK). All compounds were prepared in sterile 0.15 M saline.
2.5 Intracerebral injection technique and feeding tests
During the intracerebral injections, the rats were restrained gently, the obturators removed, and 28-gauge injection cannulae, extending 2.0 mm beyond the ventral tip of the guide, were inserted into each guide cannula. In order to acclimate the animals to the injection procedure, all subjects were given a “sham injection,” in which the injectors were inserted but no injection made, two days prior to the first actual injection. Injections were made in a volume of 0.5 μl at a rate of 0.33 μl/min using motor-driven microsyringe pumps connected to the injection cannulae with fluid-filled polyethylene tubing. After the infusion, the injection cannulae were left in place for an additional 60 s in order to minimize leakage up the cannula track. The obturators were then replaced and the rats were returned to their home cages which contained freshly weighed sources of macronutrients. Intake was assessed after 60 or 120 min, as detailed below, and subsequently corrected for spillage. During this period video cameras were located above the animals’ cages to record their behavior during the test sessions. The recordings were later examined to determine which of the three macronutrient components was first sampled by the subjects. Additionally, locomotor activity following bicuculline or the matched saline injections was assessed by dividing the animals’ cages into four quadrants and manually counting the numbers of times that animals crossed from one quadrant to another.
2.6 Perfusion and histology
When behavioral testing was completed, each of the rats was deeply anesthetized using sodium pentobarbital (150 mg/kg) and perfused transcardially with 50 mL of a 0.15 M saline solution followed immediately by 500 ml of a 10% buffered formalin solution at pH 7.3. The brains were removed and stored in the formalin solution for at least 7 days. They were then frozen and 50 μm-thick coronal sections were taken throughout the extent of the VPm or the AcbSh and stained with cresyl violet. The locations of cannula tips were plotted on atlas sections [25] matched as closely as possible to the level of the placements.
2.7 Experimental protocol in rats with VPm cannulae
Two weeks following placement on the macronutrient diets the responses to injections of saline and bicuculline were examined in a counterbalanced fashion. Half of the subjects were given bilateral injections of bicuculline (100 ng/side) into the VPm and the remainder sterile saline. After the injections, the rats were returned immediately to their home cages where intake of the three macronutrient diets was assessed for 60 min. Three days later, subjects received the converse injection and intake was again assessed. After another 3 day break in testing, half of the subjects were deprived of all food for 24 hr after which the three macronutrient components were returned, and intake measured over a 60 min period. In the remaining nondeprived animals, the foods were briefly removed from animals’ cages and then returned, after which intakes were again measured 60 min later. Finally, three days later, animals were tested under the opposite condition.
2.8 Experimental protocol in rats with AcbSh cannulae
Rats with AcbSh cannulae were tested using the same basic approach as described above. Following the two week adaptation period, 60 min intakes were measured following counterbalanced bilateral injections of muscimol (100 ng/side) or the sterile saline vehicle. Subsequently, the rats received counterbalanced bilateral injections of DAMGO (125 ng/side) or vehicle, after which intakes were measured for periods of 120 min. Four days later, 60 min intakes in all subjects were measured following bilateral injections of baclofen (200 ng/side).
2.9 Statistical Analysis
Data for food intake expressed as either grams or calories were analyzed by two way repeated measures analyses of variance (ANOVAs). When significant interaction effects were observed, the source of these was investigated using the Fisher LSD test. Correlations and locomotor activity were evaluated using t tests. A criterion of p<.05 was adopted for all tests.
3.0 Results
3.1 Animals with VPm cannulae
3.1.1 Histology
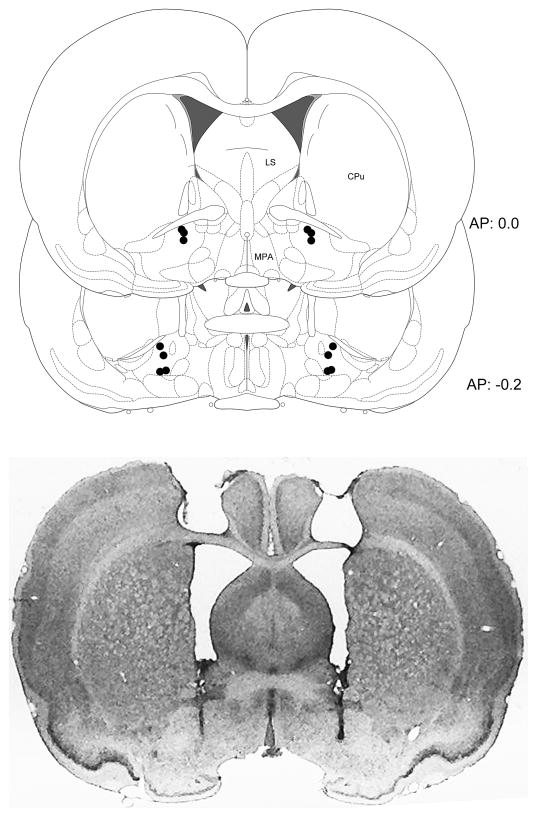
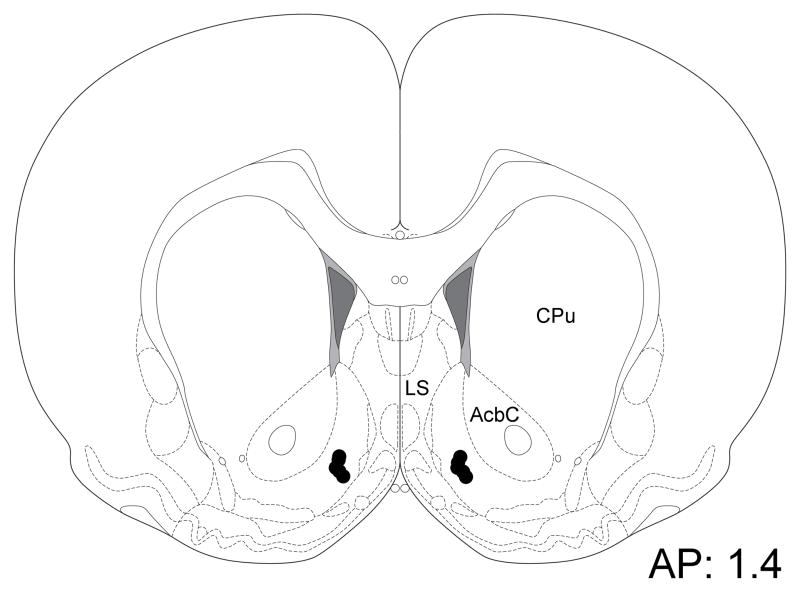
In one animal, the injector tips terminated just below the ventral border of the VPm; data from this subject was not included in the analysis, although its behavioral results were similar to those seen in the remaining animals. In all of the other subjects, injector tips terminated within the boundaries of the VPm as illustrated schematically in Fig. 1. No correlation could be observed between behavioral responses and location of the cannula tips within the VPm.
Figure 1.
Schematic illustration showing individual VPm injection placements (upper panel) and a cresyl violet stained coronal section showing a typical cannula placement in the VPm (lower panel). CPu: caudate-putamen; LS: lateral septum; MPA: medial preoptic area
3.1.2 Bicuculline injections
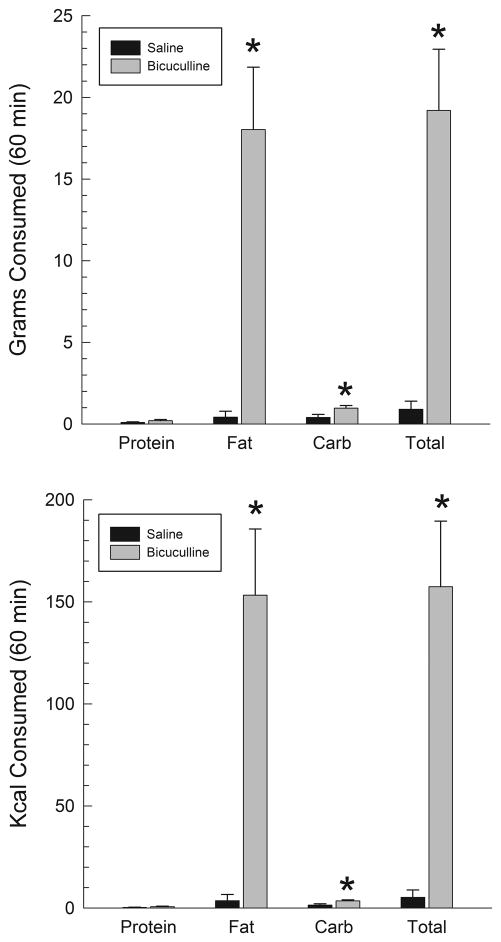
As can be seen on the right-hand columns of the upper and lower panels of Fig. 2, injections of bicuculline into the VPm produced a large increase in total 60 min intakes in the macronutrient selection paradigm. The left sides of these figures show intakes of the three individual macronutrient mixtures. Whether measured in terms of grams or calories consumed (upper and lower panels of Fig. 2, respectively), fat intake was potentiated to a much greater extent than intakes of the other macronutrients. Examination of grams eaten by means of a 2 X 3 (bicuculline X macronutrient) repeated-measures ANOVA indicated a significant effect of bicuculline (F(1,6)=24.8, p<.002), of nutrient (F(2,12)=20.1, p<.001) and of the bicuculline X nutrient interaction (F(2,12)=20.5, p<.001). Post hoc contrasts indicated that bicuculline significantly increased intakes of fat (p<.005) and carbohydrate (p<.05), but not protein, although a small trend was seen in that case as well. The increase in fat intake was significantly larger than the increase in carbohydrate intake (p<.005). Similar results were observed for calories consumed where there was a significant effect of bicuculline (F(1,6) = 23.1, p<.003), macronutrient (F(2,12)=21.38, p<.001) and of the bicuculline X macronutrient interaction (F(2,12)=21.3, p<.001). Post hoc tests again indicated that the increases in fat and carbohydrate were both significant (p<.005 and p<.05 respectively) and that the increase in fat calories was larger than that in carbohydrate calories (p<.005). Examination of the video recordings indicated that fat was the first macronutrient consumed by all 7 subjects after bicuculline injections; in contrast only 2 of the seven subjects consumed fat first after saline injections. These distributions differ significantly using the Fisher exact probability test (p<.01). Locomotor activity, measured as quadrants entered over the test period, tended to be higher after bicuculline than saline (55.2±6.5 vs. 44.4±12.2), but this difference was not significant (p>.20).
Figure 2.
Grams (upper panel) and calories (lower panel) consumed following injections of bicuculline (100 ng/side) or saline into the VPm. *p<.05 vs. control.
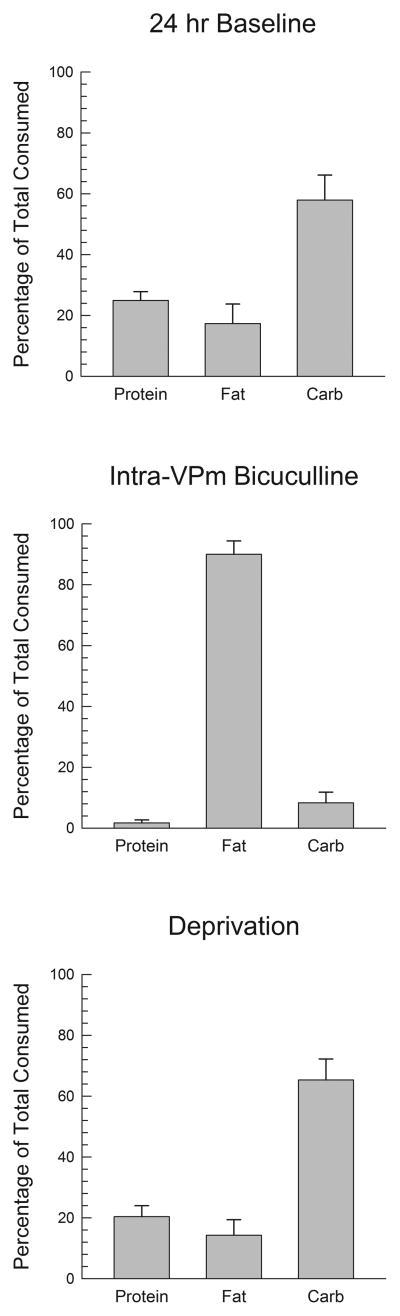
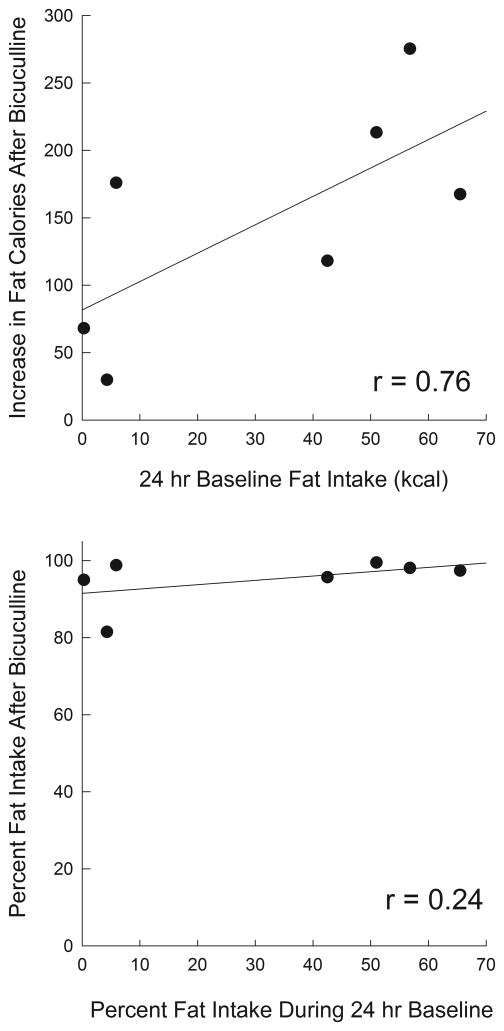
Given the strong preference for fat seen following bicuculline injections, we examined whether nutrient preferences after this drug were related to baseline preferences. Fig 3 shows mean proportional intakes of each of the three macronutrients averaged over the three days before the start of testing (upper panel). In contrast to the dramatic fat preference shown following bicuculline injections (middle panel), animals during 24-h ad libitum feeding periods did not significantly differ in their fat and carbohydrate intake, and actually tended to consume more carbohydrates than fat. The difference in the proportionate consumption of fat under bicuculline and deprivation conditions was significant (F(1,6)=70.1, p<.0001). The upper panel of Fig. 4 shows that baseline 24-h fat intake was significantly correlated with the increase in fat intake produced by bicuculline (p<.05). It should be noted, however, that even rats that ate little or no fat under free feeding conditions still showed large increases in fat intake after bicuculline and that even subjects who, under baseline condition consumed less than 10% of their calories from fat, consumed 80–98% of their calories from fat after bicuculline (Fig. 4, lower panel).
Figure 3.
Percentages of the total number of grams consumed which were taken from the protein, fat, and carbohydrate dietary components under several conditions in subjects with VPm cannulae. The upper panel shows baseline percentages averaged across the three days before the start of intracranial injections. The middle panel shows percentages based on intakes in the 60 min following bicuculline injections, and the lower panel percentages based on intakes in the 60 min period following the return of food after overnight food deprivation.
Figure 4.
Upper panel: Scatter plot and least squares regression line showing the relation between mean baseline caloric fat intake (abscissa) and the increase in absolute caloric intake of fat after bicuculline injections into the VPm, versus saline injections (ordinate). Lower panel: Scatter plot and least squares regression line showing the relation between the percent of caloric intake which consisted of fat during the 3 day baseline period (abscissa) and in the 60 min following bicuculline injections (ordinate). Pearson correlation coefficients are indicated on the figures.
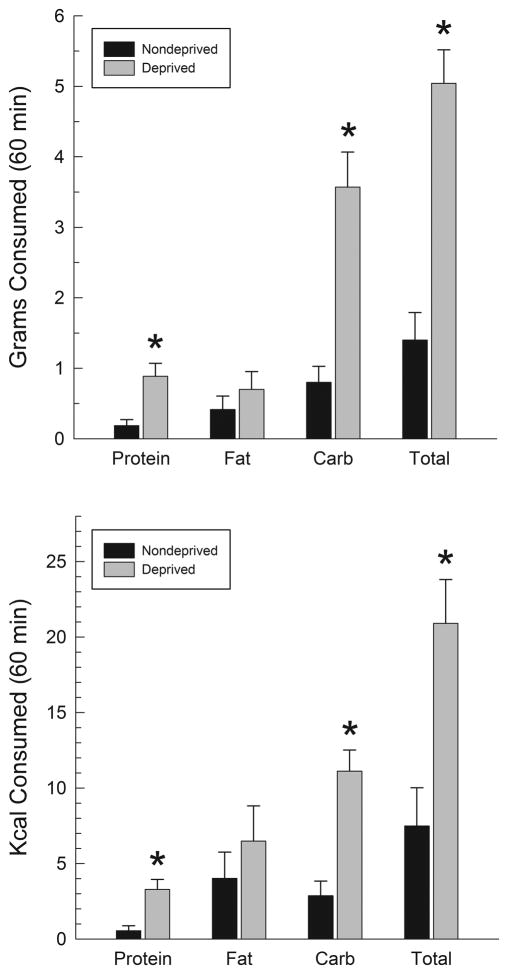
3.1.3 Food deprivation
One rat became sick before completing the deprivation tests, so the data presented below represent the remaining six subjects. The upper panel of Fig 5 displays 60 min macronutrient intake following food deprivation or under nondeprived conditions. Analysis of the data for grams consumed indicated a significant effect of deprivation (F(1,5)=73.3, p<.001), p<.001), macronutrient (F(2,10)=7.2, p<.001) and of the deprivation X macronutrient interaction (F(2,10)=3.2, p<.002). Post hoc comparisons indicated that intakes of protein and carbohydrate were increased significantly (p<.05), while a nonsignificant trend in the same direction was seen with respect to fat intake (p<.10). Furthermore the increase in carbohydrate intake was significantly larger than the increase in fat intake (p<.05). Analogous results in each case were obtained with respect to caloric intake (lower panel of Fig. 5). The proportional intakes of the three macronutrients are shown in the lower panel of Fig. 3, which demonstrates that deprivation did not produce a pattern of fat preference similar to that seen after bicuculline. Subjects consumed a significantly smaller proportion of their total food from fat after deprivation than they did after bicuculline infusions (F(1,5)=108.2, p<.001). Video recordings of the deprivation sessions were available for five animals of which only two sampled fat first.
Figure 5.
Grams (upper panel) and calories (lower panel) consumed in 60 min periods following 24-h food deprivation or a nondeprived control condition. *p<.05 vs control.
3.2 Animals with AcbSh cannulae
3.2.1 Histology
In two animals, the injector tracts passed out the ventral surface of the brain; in both of these subjects, drug injections had little or no effect on ingestive behavior. In the remaining subjects, injector tips terminated in the ventral portion of the medial AcbSh as illustrated schematically in Fig. 6.
Figure 6.
Schematic illustration showing individual AcbSh injection placements. AcbC: nucleus accumbens, core; CPu: caudate-putamen; LS: lateral septum
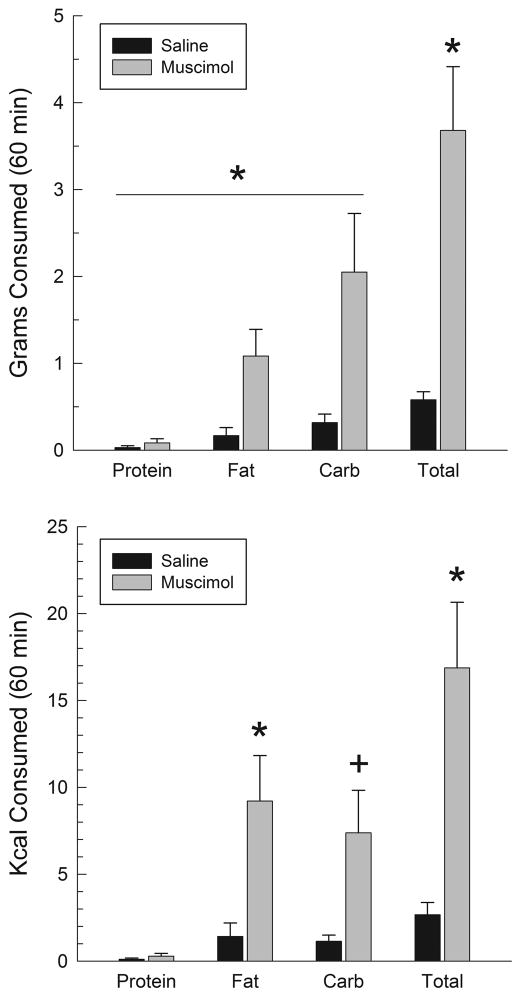
3.2.2 Muscimol injections
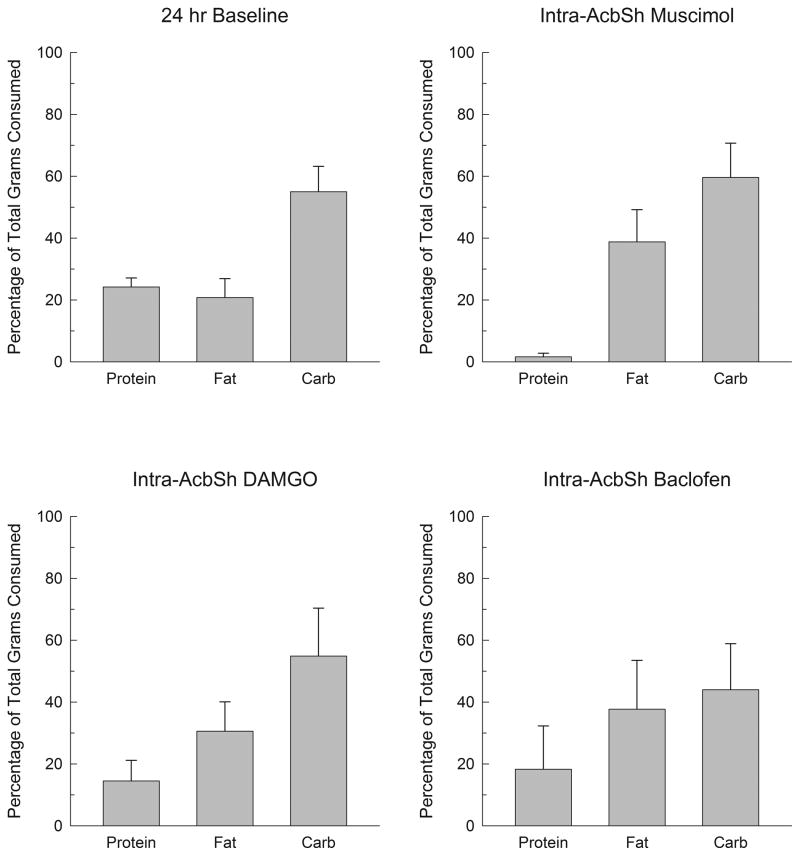
Fig. 7 displays macronutrient intakes following injections of saline or muscimol into the AcbSh. Muscimol significantly increased overall intakes; when intakes were measured as grams eaten, the effect on carbohydrates tended to be larger than that on fat (upper panel), whereas the opposite tendency was seen when the data were expressed as calories consumed (lower panel). Analysis of grams eaten indicated a significant overall effect of muscimol (F(1,4)=17.2, p<.02) and macronutrient (F(2,8)=8.5, p<.01) but the muscimol X macronutrient interaction was not significant (p>.05). Analysis of caloric intakes indicated a significant effect of muscimol (F(1,4)=29.4, p<.01), macronutrient (F(2,8)=6.7, p<.02) and of the muscimol X macronutrient interaction (F(2,8)=5.2, p<.05). Post hoc contrasts in this case indicated that muscimol significantly increased caloric intake of fat (p<.05) and tended to increase carbohydrate intake (p<.06), but had no effect on protein intake (F<1). The muscimol-induced increases in caloric intake of fat and carbohydrate did not differ statistically (F<1). The upper right panel of Fig. 9 illustrates intakes following muscimol, expressed as a percent of total number of grams consumed, and shows that the carbohydrates tended to make up a larger proportion of total intake than did fats, although these differences were not significant (F<1). As was the case in the animals with VPm cannulae, subjects in the current experiment tended overall to consume more carbohydrate than fat under baseline conditions (Fig. 9, upper left panel).
Figure 7.
Grams (upper panel) and calories (lower panel) consumed following injections of saline or muscimol (100 ng/side) into the AcbSh. *p<.05, +p<.06.
Figure 9.
Percentages of the total number of grams consumed that were taken from the protein, fat, and carbohydrate dietary components under several conditions in subjects with AcbSh cannulae. The upper left panel shows the distribution of baseline percentages averaged across the three days prior to the start of injections; the upper right panel shows percentages after muscimol (100 ng/side) injections in the AcbSh; the lower left panel after injections of DAMGO (125 ng/side) in the AcbSh; and the lower right panel after injections of baclofen (200 ng/side) into the AcbSh.
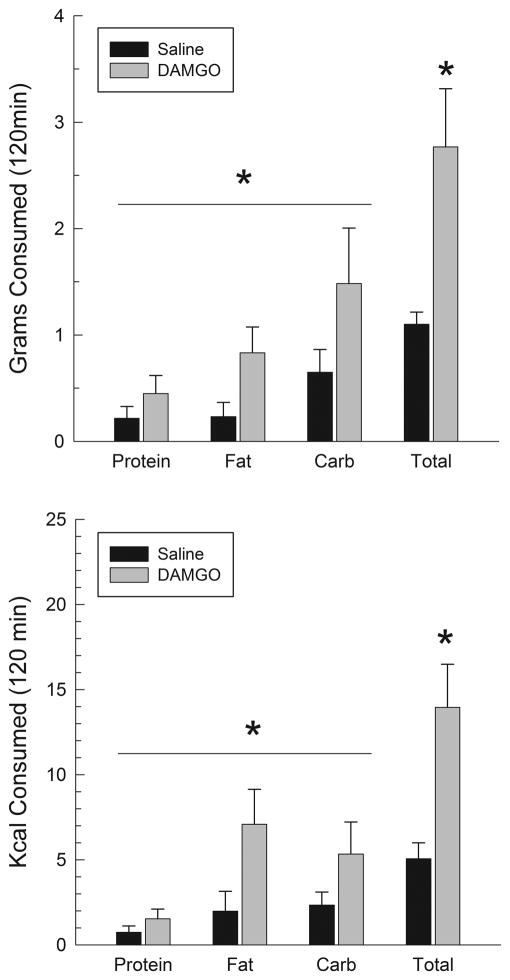
3.2.3 DAMGO injections
Behavioral data following DAMGO injections are shown in Fig. 8 where it can be seen that the opioid agonist produced a small but significant increase in food intake. Similar to muscimol, DAMGO tended to have a larger effect on carbohydrate than fat in terms of grams eaten, whereas this small tendency was reversed when data were analyzed in terms of calories consumed. An ANOVA on grams consumed indicated a significant effect of DAMGO (F(1,4)=12.1, p<.02), but neither the macronutrient effect nor the DAMGO X macronutrient interaction were significant (p>.05), indicating that the effects of DAMGO on the various macronutrients did not differ significantly from one another. Equivalent results were obtained by analyzing data for caloric intakes which indicated a significant effect of DAMGO (F(1,4)=12.2, p<.02), but not of macronutrient or the interaction. The lower left panel of Fig. 9 shows that rats tended to consume a larger proportion of their intake from carbohydrates than fat after DAMGO injections, and the proportionate intake of fat after DAMGO did not differ from their 24-h baseline (p>.10).
Figure 8.
Grams (upper panel) and calories (lower panel) consumed following injections of DAMGO (125 ng/side) into the AcbSh. *p<.05.
3.2.4 Baclofen injections
Rats injected with baclofen into the AcbSh consumed a total of 6.9 ±1.4 g of food, which corresponded to a caloric intake of 29.3 ± 7.4 kcal. Although a separate saline control group was not run for these animals, these intakes were considerably larger than those we observed after either muscimol or DAMGO injections. In terms of grams eaten, the baclofen treated animals consumed primarily the carbohydrate mix (mean intake 4.1 ± 1.3g), while consuming less of the protein mix (0.6 ±0.2g) or the fat mix (2.2 ± 0.9 g). The lower right panel of Fig. 9 shows that baclofen treated animals tended to consume a larger proportion of their intake from carbohydrates than fat, and the proportion of their intake from fat did not differ from their 24-h baselines.
4.0 Discussion
Our results confirm previous reports that blocking GABAA receptors in the VPm increases food intake [18, 20–22, 26]. As the Acb sends a strong GABAergic projection to the VPm [27, 28], it is likely that this effect results from a disruption of inhibitory influences of the Acb on the VPm, but further studies will be required to unequivocally establish this conclusion. Additionally, our findings show that in animals offered a choice between three sources of individual macronutrients, this hyperphagia manifests itself as a selective and dramatic increase in the amount of fat consumed. To the best of our knowledge, this is the first time that pronounced alterations in fat preference in a choice situation have been observed following central manipulations of a nonpeptide neurotransmitter system. It is striking that results similar to those obtained here, although not quite as pronounced, have also been observed after injections of the mu-opioid agonist DAMGO into the nucleus accumbens core (AcbC) [11]. Given that there are dense, reciprocal interconnections between the Acb and the VPm [13–16], it is likely that there is some relation between the influences of these two regions on fat intake. Further studies will, however, be necessary to determine the nature of this relationship as a number of circuits consistent with these results are theoretically possible.
All of the subjects in the present study showed a strong preference for fat following bicuculline injections into the VPm. Although fat intake after bicuculline was correlated with baseline intake, even animals who spontaneously consumed only tiny amounts of lard obtained 80–98% of their calories from this substance following bicuculline injections. These findings demonstrate that blocking GABA transmission in the VPm does not simply increase the inclination of animals to select those foods preferred under baseline conditions. Similar results have been reported following injections of DAMGO into the Acb [11]. It seems likely, therefore, that the selectivity of these manipulations on fat intake has little to do with the intrinsic palatability of the lard. Indeed, there is little in the data obtained from this group of subjects to suggest that the lard component is an especially palatable or generally preferred substance in untreated animals. The notion that effects on fat intake can be accounted for by a special role of Acb opioids in the intake of palatable or “rewarding” foods, appears to have arisen less from empirical data than from a prior belief that the Acb is a component of a “reward system.” In contrast, the current and previous results [11] suggest that, in macronutrient selection situations, the VPm and Acb are able to reorder the ingestive preferences of subjects in a way that has little to do with their preferences in the absence of experimental treatments. It is possible that Acb-VPm circuitry selectively influences preferences for substances with the sensory characteristics (textures, taste or odors) of lipids or for foods with higher caloric densities, an attribute which subjects could have learned about during dietary adaptation period.
Analysis of video recordings showed that all of the bicuculline treated animals, regardless of their baseline preferences or the specific location of the food jars, initially oriented themselves toward the jar containing lard and ingested the fat before either of the other macronutrients, a pattern not seen following food deprivation. Observations of this type have not, to our knowledge, been made in prior macronutrient selection studies. These observations suggest that the injections may not only alter fat intake, per se, but may also alter the incentive properties of the fat component in such a way as to direct the animal’s behavior towards it, even before any oral contact has been made. The effects of the injections on fat intake therefore cannot be entirely the result of factors which arise during ingestion of the diet and one could speculate that the injections may induce a form of “fat craving,” which might be analogous to other forms of food cravings which have been described in the literature [29, 30]. It should be stressed, however, that the orexigenic effects of intra-VPm bicuculline are not restricted to high-fat foods. In the current study, a small but significant increase in carbohydrate intake was also seen, and in previous studies, we have observed substantial increases in the intakes of relatively low-fat diets such as 6% fat lab chow [21] or 3.8% fat Bioserve pellets [22] when these were the only foodstuffs offered.
The feeding seen after bicuculline injections into the VPm was fundamentally different from that we observed after 24 hours of food deprivation in terms of both magnitude and macronutrient profile. Both conditions increased total caloric intake during the 60 min test, but after intra-VPm bicuculline, the rats consumed approximately 7.5 times the number of calories that they did following overnight deprivation. In terms of macronutrient profile, intake after 24-h food deprivation was characterized by significant increases in carbohydrate and protein intake with a smaller effect on fat consumption, a pattern very different from the highly selective fat intake seen following bicuculline. These results indicate that, under comparable conditions, these injections do not generate a state resembling that induced by 24-h food deprivation. It is, of course, possible that larger effects on fat intake might be produced by longer periods of deprivation. It should also be stressed that our lard component contained no sugar, whereas the high fat components studied by many earlier workers contained sucrose in concentrations as high as 2% e.g., [2, 12, 31], a difference which might tend to promote fat intake under a variety of conditions.
Information about the central mechanisms underlying food preferences is so limited that that it is not currently possible to identify the pathways through which the effects of intra-VPm bicuculline are exerted. These effects may be mediated through GABAergic neurons in the VPm, but cells utilizing glutamate and enkephalin as transmitters are also found in this nucleus [32–34] and might also be involved. Evidence indicates that the lateral hypothalamus plays a role in mediating some of the ingestive effects of VPm activation [18, 22], although only limited data exist suggesting that this region is able to influence food choice [35, 36]. It is interesting, however, that acute injections of NMDA into the VPm are able to induce strong Fos expression in the paraventricular hypothalamic nucleus (PVN) [18], the brain region most closely associated with elicited alterations in macronutrient selection, demonstrating a functional link between the two structures. The possibility that the large preferential increase in fat intake we observed is due to VPm influences on PVN cells is consistent with a report that intra-PVN injections of a NPY antagonist suppress intake of high-fat chow induced by DAMGO injections in the Acb, a region sharing dense reciprocal connections with the VPm [37]. Since only a single food source was examined in that study, however, it is impossible to determine whether this effect is specifically related to the fat content of the diet, or simply reflects a general suppression of feeding. Intra-ventricular injections of NPY antagonists, for example, are also able to suppress the intake of low-fat chow induced by injections of muscimol in the AcbSh [38]. Although most work suggests that acute manipulations of the NPY system in the PVN selectively influence carbohydrate intake [6, 7], chronic intra-PVN administration of NPY significantly increases both carbohydrate and fat intake [39] and manipulations of other neuropeptide systems there do selectively alter fat intake [3–5]. Further studies will be necessary to determine the extent and nature of the involvement of the LH and PVN in the effects on feeding and macronutrient selection observed after manipulations of the VPm.
It has been reported previously that the vigorous feeding induced by injections of the GABAA agonist muscimol in the accumbens shell is associated with roughly equivalent increases in the caloric consumption fat and carbohydrate [12]. This result is surprising, as some evidence suggests that the ingestive effects of muscimol at this site may be mediated in part through projections to the VPm [23], a structure which, as the current results show, is capable of preferentially increasing fat intake. The methodology used in the current study, however, differed from that of previous studies in several potentially important details including the greater length of time we allowed for adaptation to the diet and the absence of utilizable carbohydrates in our fat mixture. It seemed possible, therefore, that the observed differences in fat selectivity between our current VPm results and earlier experiments with muscimol in the AcbSh might result from these discrepancies. In order to investigate this possibility, we examined the effects of intra-AcbSh muscimol under conditions identical to those used in the first experiment on the VPm. Our data suggest that methodological differences are not responsible for these varying outcomes as intra-AcbSh muscimol produced roughly equivalent increases in fat and carbohydrate intake under conditions identical to those in which bicuculline in the VPm selectively affected fat consumption. We also observed a similar pattern after injections of the GABAB agonist baclofen, a compound which has been shown to induce feeding when injected in the AcbSh [40, 41], but has not previously been investigated in a macronutrient selection paradigm. Finally, we again failed to obtain a selective effect on fat ingestion with injections of DAMGO into the AcbSh. This result differs from those reported previously with DAMGO injections into the AcbC [11], but several studies have suggested that the effects of DAMGO on fat intake may be related specifically to the core region of the Acb [31, 42, 43]. (Additionally, the injection sites studied here were ventral to those shell sites where some of the ingestive effects of DAMGO are most pronounced [44]). These results suggest that there are two different mechanisms in the Acb which can influence feeding: a system affected by stimulation of GABAA and GABAB receptors in the AcbSh that nonselectively increases fat and carbohydrate intake and a system affected by DAMGO in the AcbC which preferentially affects fat consumption. Other data is also consistent with this notion: for example, injections of DAMGO into the AcbC and muscimol into the AcbSh have markedly different effects on the intake of saccharine, salt, and ethanol solutions [12, 45–47]. The current study suggests that in a macronutrient selection test, the nature of the feeding induced by disinhibition of the VPm more closely resembles that produced by DAMGO in the AcbC than that seen after GABA agonists in the AcbSh. Further work will be needed to determine if this pattern holds in other test situations, and to identify the circuitry underlying these resemblances.
In summary, our results indicate that blockade of GABAA receptors in the VPm induces a dramatic increase in fat intake and preference and also preferentially directs animals’ approach behavior towards fat. This effect has a different profile from those observed after food deprivation or injections of GABA or μ-opioid agonists into the ventral AcbSh. The magnitude and preferential nature of VPm-induced fat intake suggests that this structure is important in the regulation of both food intake and macronutrient selection and that circuitry involving the Acb and the VPm may play a major role in controlling fat intake.
RESEARCH HIGHLIGHTS.
Blocking GABA receptors in the VPm greatly increases preference for fat
The treatment yields a 4300% increase in caloric intake with 97% derived from fat
This macronutrient selection profile differs from that seen after food deprivation
Intra-AcbSh muscimol or baclofen increases intake of carbohydrate and fat equally
GABA receptors in the VPm, but not AcbSh, may specifically control fat intake
Acknowledgments
Supported in part by NIH grants R03DA020802 and R01DK071738 and by funds from the UIC Campus Research Board. No funding agency had any role in the design of the study, the collection, analysis, or interpretation of data, in writing the report, or in the decision to publish the manuscript. We would like to thank Dr. Tina M. Herfel for her help with the design of the diets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schulkin J. Sodium Hunger: The Search for a Salty Taste. New York: Cambridge University Press; 1991. [Google Scholar]
- 2.Welch CC, Grace MK, Billington CJ, Levine AS. Preference and diet type affect macronutrient selection after morphine, NPY, norepinephrine, and deprivation. Am J Physiol. 1994;266:R426–33. doi: 10.1152/ajpregu.1994.266.2.R426. [DOI] [PubMed] [Google Scholar]
- 3.Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol. 2007;293:R99–105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- 4.Kyrkouli SE, Stanley BG, Hutchinson R, Seirafi RD, Leibowitz SF. Peptide-amine interactions in the hypothalamic paraventricular nucleus: analysis of galanin and neuropeptide Y in relation to feeding. Brain Res. 1990;521:185–91. doi: 10.1016/0006-8993(90)91541-n. [DOI] [PubMed] [Google Scholar]
- 5.Tempel DL, Leibowitz KJ, Leibowitz SF. Effects of PVN galanin on macronutrient selection. Peptides. 1988;9:309–14. doi: 10.1016/0196-9781(88)90265-3. [DOI] [PubMed] [Google Scholar]
- 6.Leibowitz SF, Weiss GF, Yee F, Tretter JB. Noradrenergic innervation of the paraventricular nucleus: specific role in control of carbohydrate ingestion. Brain Res Bull. 1985;14:561–7. doi: 10.1016/0361-9230(85)90105-4. [DOI] [PubMed] [Google Scholar]
- 7.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985;6:1205–11. doi: 10.1016/0196-9781(85)90452-8. [DOI] [PubMed] [Google Scholar]
- 8.Shor-Posner G, Grinker JA, Marinescu C, Brown O, Liebowitz SF. Hypothalamic serotonin in the control of meal patterns and macronutrient selection. Brain Res Bull. 1986;17:663–71. doi: 10.1016/0361-9230(86)90198-x. [DOI] [PubMed] [Google Scholar]
- 9.Stratford TR. Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Res. 2005;1048:241–50. doi: 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–8. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–14. [PubMed] [Google Scholar]
- 12.Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–36. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- 13.Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- 14.Churchill L, Dilts RP, Kalivas PW. Changes in gamma-aminobutyric acid, mu-opioid and neurotensin receptors in the accumbens-pallidal projection after discrete quinolinic acid lesions in the nucleus accumbens. Brain Res. 1990;511:41–54. doi: 10.1016/0006-8993(90)90223-x. [DOI] [PubMed] [Google Scholar]
- 15.Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–46. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- 16.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 17.Urstadt K, Stanley BG. Anatomical evidence that the ventral pallidum interconnects the accumbens shell and the lateral hypothalamus. Society for Neuroscience Annual Meeting; San Diego, CA. 2013. [Google Scholar]
- 18.Rivero-Covelo I, Wirtshafter D, Stratford TR. Unilateral activation of the medial ventral pallidum using NMDA induces feeding and Fos expression in the lateral hypothalamus. Society for Neuroscience Annual Meeting; San Diego, CA. 2013. [Google Scholar]
- 19.Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev. 1996;20:189–287. doi: 10.1016/0149-7634(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 20.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–49. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stratford TR, Kelley AE, Simansky KJ. Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res. 1999;825:199–203. doi: 10.1016/s0006-8993(99)01239-1. [DOI] [PubMed] [Google Scholar]
- 22.Stratford TR, Wirtshafter D. Lateral hypothalamic involvement in feeding elicited from the ventral pallidum. Eur J Neurosci. 2013;37:648–53. doi: 10.1111/ejn.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratford TR, Wirtshafter D. Evidence that the nucleus accumbens shell, ventral pallidum, and lateral hypothalamus are components of a lateralized feeding circuit. Behav Brain Res. 2012;226:548–54. doi: 10.1016/j.bbr.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stratford TR. The nucleus accumbens shell as a model of integrative subcortical forebrain systems regulating food intake. In: Kirkham TC, Cooper SJ, editors. Appetite and Body Weight: Integrative Systems and the Development of Anti-Obesity Drugs. London: Elsevier; 2007. pp. 27–65. [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. San Diego: Academic Press; 2007. [Google Scholar]
- 26.Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23:1596–604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- 27.Walaas I, Fonnum F. The distribution and origin of glutamate decarboxylase and choline acetyltransferase in ventral pallidum and other basal forebrain regions. Brain Res. 1979;177:325–36. doi: 10.1016/0006-8993(79)90783-2. [DOI] [PubMed] [Google Scholar]
- 28.Zahm DS, Zaborszky L, Alones VE, Heimer L. Evidence for the coexistence of glutamate decarboxylase and Met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res. 1985;325:317–21. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]
- 29.Weingarten HP, Elston D. The phenomenology of food cravings. Appetite. 1990;15:231–46. doi: 10.1016/0195-6663(90)90023-2. [DOI] [PubMed] [Google Scholar]
- 30.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–77. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 32.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–43. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study. J Comp Neurol. 2005;483:351–73. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- 34.Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–60. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- 35.Leibowitz SF, Shor-Posner G, Maclow C, Grinker JA. Amphetamine: effects on meal patterns and macronutrient selection. Brain Res Bull. 1986;17:681–9. doi: 10.1016/0361-9230(86)90200-5. [DOI] [PubMed] [Google Scholar]
- 36.Morganstern I, Chang GQ, Karatayev O, Leibowitz SF. Increased orexin and melanin-concentrating hormone expression in the perifornical lateral hypothalamus of rats prone to overconsuming a fat-rich diet. Pharmacol Biochem Behav. 2010;96:413–22. doi: 10.1016/j.pbb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Townsend RL, Shin AC, Patterson LM, Phifer CB, Berthoud HR. High-fat intake induced by mu-opioid activation of the nucleus accumbens is inhibited by Y1R-blockade and MC3/4R- stimulation. Brain Res. 2010;1350:131–8. doi: 10.1016/j.brainres.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stratford TR, Wirtshafter D. NPY mediates the feeding elicited by muscimol injections into the nucleus accumbens shell. Neuroreport. 2004;15:2673–6. doi: 10.1097/00001756-200412030-00024. [DOI] [PubMed] [Google Scholar]
- 39.Stanley BG, Anderson KC, Grayson MH, Leibowitz SF. Repeated hypothalamic stimulation with neuropeptide Y increases daily carbohydrate and fat intake and body weight gain in female rats. Physiol Behav. 1989;46:173–7. doi: 10.1016/0031-9384(89)90251-5. [DOI] [PubMed] [Google Scholar]
- 40.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–40. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward BO, Somerville EM, Clifton PG. Intraaccumbens baclofen selectively enhances feeding behavior in the rat. Physiol Behav. 2000;68:463–8. doi: 10.1016/s0031-9384(99)00197-3. [DOI] [PubMed] [Google Scholar]
- 42.Katsuura Y, Heckmann JA, Taha SA. mu-Opioid receptor stimulation in the nucleus accumbens elevates fatty tastant intake by increasing palatability and suppressing satiety signals. Am J Physiol. 2011;301:R244–54. doi: 10.1152/ajpregu.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenard NR, Zheng H, Berthoud HR. Chronic suppression of mu-opioid receptor signaling in the nucleus accumbens attenuates development of diet-induced obesity in rats. Int J Obes (Lond) 2010;34:1001–10. doi: 10.1038/ijo.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do μ-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirtshafter D, Covelo IR, Salija I, Stratford TR. Effects of muscimol in the nucleus accumbens shell on salt appetite and sucrose intake: a microstructural study with a comment on the sensitization of salt intake. Behav Neurosci. 2013;126:699–709. doi: 10.1037/a0029641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–23. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- 47.Stratford TR, Wirtshafter D. Opposite effects on the ingestion of ethanol and sucrose solutions after injections of muscimol into the nucleus accumbens shell. Behav Brain Res. 2011;216:514–8. doi: 10.1016/j.bbr.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]