ABSTRACT
Glucosinolates (GSLs) are a group of cancer chemopreventive sulfur-containing compounds found primarily in Brassica vegetables. The goals of this study were to summarize the current knowledge and discuss the challenges of developing a dietary GSL database for US foods. A systematic literature search was conducted for the period 1980–2020. Thirty articles were found to meet all inclusion and exclusion criteria; 27 GSLs were reported in 16 different vegetables. GSLs identified and quantified ranged from 3 for winter cress to 16 for cabbage. In general, the experimental designs of these 30 studies did not fully consider the factors related to the data quality. Enormous variations of GSLs are observed between different vegetables and in the same vegetables. In conclusion, the studies on GSLs in commonly consumed vegetables are still limited, and some data may be outdated. Currently available data are not sufficient to develop a valid GSL database in the United States.
Keywords: Brassica, cruciferous, database, food composition, glucosinolate, isothiocyanate
Introduction
Glucosinolates (GSLs) are sulfur-rich, anionic plant secondary metabolites (Figure 1) found principally in the plant order Brassicales. The genus Brassica in Brassicaceae (older name, Cruciferae) contains most of the commonly consumed vegetables (1), such as broccoli, kale, cabbages, cauliflower, and Brussels sprouts, which are often referred to as Brassica or cruciferous vegetables. In addition, a number of non-Brassica edible plants, such as radish, papaya, and winter cress, also contain GSLs. Evidence of human usage of GSL-containing plants as foods or condiments dates back thousands of years (2). At present, some Brassica vegetables, including broccoli, cabbage, and cauliflower, are among the mostly commonly consumed vegetables in the world (3).
FIGURE 1.
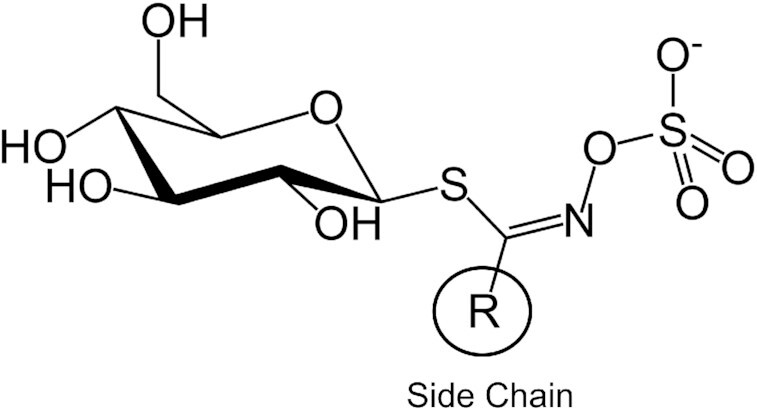
Core chemical structure of glucosinolates.
In plants, GSLs coexist with an endogenous β-thioglucosidase called myrosinase; but they are stored separately in different cells or in different intracellular compartments. Upon plant tissue disruption, myrosinase comes into contact with GSLs resulting in rapid hydrolysis to form a variety of hydrolytic products (4). The hydrolytic products exhibit toxicity or feeding deterrence to a wide range of potential insect herbivores. In humans, epidemiological evidence from prospective cohort studies and retrospective case-control studies has linked consumption of cruciferous vegetables to reduced risk of various types of cancer, including lung (5), gastric (6), colorectal (7), breast (6), bladder (8), and prostate cancer (9). Large numbers of animal studies and human clinical trials have also been performed to reveal the possible molecular mechanisms, such as detoxification of carcinogens (10, 11), antioxidant activities (12), modulation of inflammation (13), and induction of apoptosis (14). The evidence accumulated in the past decades suggested that the cancer chemopreventive effects of cruciferous vegetables could largely be attributed to the hydrolytic products of GSLs such as isothiocyanates and indole-3-carbinol (15).
Types of GSL and their concentrations vary enormously among different plants, both qualitatively and quantitatively, according to the species and cultivar, tissue type, growing stage, environmental factors, insect attack, and microorganism intrusion (16, 17). The total GSL intake was estimated as 14.2 ± 1.1 mg/d for men and 14.8 ± 1.3 mg/d for women in a German population (18); and 6.5 mg/d, among which 35% were of indole type, in a Spanish adult population (19). The national mean daily intake in the United Kingdom was calculated to be 46.1 mg in fresh materials, and 29.4 mg from cooked foods (20). However, these crude dietary intake estimates were made based on very limited data or dietary exposure to GSL-containing vegetables. There is currently no dietary intake estimation of GSLs in the United States, due to lack of adequate dietary GSL composition data.
Food composition data are considered as the foundation of dietetic practice and nutritional research (21). In the United States, our group has developed several databases for the dietary bioactive compounds of public health interest such as flavonoids, which have been widely used as a valuable tool to assess their dietary intakes (22, 23). Recent interests of the scientific community in dietary GSLs center on their chemoprotective effects against cancer (24–26). The relations between consumption of Brassica vegetables and/or GSLs and the risk of cancers have been investigated in many nutritional epidemiological studies (27–30). To better understand the association between GSL intake and the risk of cancer and other chronic diseases, it is critical to precisely estimate the dietary intake of GSLs. A valid composition database of dietary GSLs would be expected to provide fundamental information in calculating the GSL intake.
An extensive literature search found only one published study that attempted to develop a dietary GSL database (31). This article was published in 2003 and has since been broadly cited in epidemiological studies and used to estimate the dietary intake of dietary GSLs. However, data from only 18 references were included in developing the database, and among them 10 studies used the glucose-release methods, 5 used HPLC, and 3 used GC-based methods. Because of the limitation of analytical methods, no data on individual GSLs were provided. With significantly more data generated in the last two decades, and most importantly, the advancement of new analytical methods, it is possible to develop a new dietary GSL database. Development of a food composition database is a multistep process, generally including data acquisition, data evaluation, data compilation, data aggregation, and data dissemination (32, 33). Data collection and the quality evaluation are the core tasks of developing a valid food composition database (23, 34). The primary goals of this study were to summarize the currently available GSL data, evaluate the data quality, and discuss the challenges on the development and application of a dietary GSL database for the foods grown and/or sold in the United States.
Methods
A systemic literature search was conducted for the period 1980–2020 based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (35). PubMed, SciFinder, and Google Scholar were selected as primary search databases. The following keywords were used in different combinations: glucosinolate, Brassica, cruciferous, food composition, analysis, isothiocyanate, vegetable. Reference lists of the retrieved research and review articles were reviewed to identify references not found using electronic search engines. Only data in original research articles were included; secondary data in review articles or books were excluded. The detailed inclusion and exclusion criteria are described as follows:
Because the database is intended to be developed for the US foods, plant materials obtained from retailers or fields in the United States were included. According to USDA's Economic Research Service, Mexico and Canada are the major exporters of GSL-containing vegetables to the United States (https://www.ers.usda.gov/data-products/vegetables-and-pulses-data/by-commodity). Therefore, data from the studies conducted in these two countries, if any, were also included.
Information on the source and identification, ideally full scientific names and cultivars, of samples were provided. Only the data on fresh raw samples were included.
Only the data of commonly consumed plants and the edible parts of the samples were included. Wild plants or those used as folk medicine, animal feeds, nonedible parts, or by-products of the plants, such as seeds of certain vegetables (36), were excluded.
The analytical methods must be carefully described. Because studies have shown that different GSLs and isothiocyanates had different biological effects, only the methods that quantify individual GSLs were included. Thus, the publications that only reported total GSLs were excluded.
For the publications that aimed to study the factors or the effects of certain treatments or manipulations on the concentrations of GSLs, only the data from untreated or control groups were included.
Data extracted from the studies were converted to the unit of milligrams per 100 g fresh weight (FW). Values reported based on dry weight were converted into an FW basis using the water content provided in the specific study, or the moisture level reported in USDA National Nutrient Database for Standard Reference, Legacy Release (37) if such information is not available in the literature.
Results
Overview of the publications
After several sequential stages of screening (35), 30 articles were found to meet all inclusion and exclusion criteria (Figure 2), including 28 articles published by US researchers and 2 by Canadian researchers. The summary of these 30 studies, including the year of publication, foods being analyzed, location of samples, and the analytical method, is presented in Table 1. The number of publications almost equally distributed in each decade from 1980 to 2020. But the number of publications for different foods is unequal. Broccoli is the most studied one, which appeared in 16 studies. Cabbage and Brussels sprouts were each reported in 7 studies, cauliflower was reported in 4 studies, and kale in 3 studies. The rest of the foods were only presented in 1–2 studies (Figure 3).
FIGURE 2.
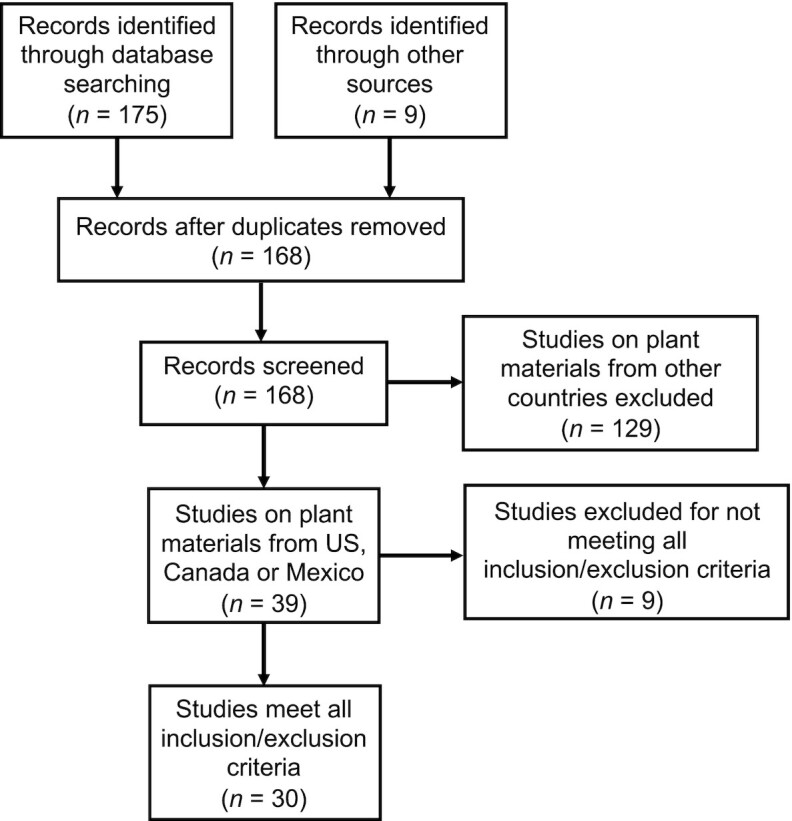
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of literature search and screening.
TABLE 1.
Summary of the studies that contained the original data of glucosinolate contents of foods in the United States and Canada (1980–2020)1
| Year | Sample location | Foods | Analytical method | Reference |
|---|---|---|---|---|
| 2003 | Urbana, IL, USA | Broccoli | HPLC after desulfation | (41) |
| 1994 | Washington DC, USA | Broccoli (green sprouting broccoli) | HPLC after desulfation | (47) |
| 2002 | IL, USA | Broccoli | HPLC after desulfation | (72) |
| 1981 | Madison, WI, USA | Rutabaga, turnip | GC | (73) |
| 1987 | Madison, WI, USA | Broccoli, Brussels sprouts, cauliflower, collards, kale, mustard greens, kohlrabi | GC | (74) |
| 1985 | Madison, WI, USA | Radish | GC | (75) |
| 1987 | Madison, WI, USA | Turnip | GC | (76) |
| 2000 | Knoxville, TN, USA | Broccoli | HPLC after desulfation | (77) |
| 2005 | Knoxville, TN, USA | Broccoli, Brussels sprouts, red cabbage, cauliflower, kale | HPLC after desulfation | (43) |
| 1980 | OR, NY, WI, USA | Cabbage | GC-MS | (78) |
| 2014 | USA | Brussels sprouts, cabbage | HPLC after desulfation | (79) |
| 1988 | Syracuse, NY, USA | Broccoli, Brussels sprouts, green cabbage | HPLC after desulfation | (53) |
| 1989 | USA | Brussels sprouts, broccoli | HPLC after desulfation | (52) |
| 1995 | Salinas, CA, USA | Broccoli | HPLC after desulfation | (80) |
| 2011 | USA | Broccoli | HPLC after desulfation | (81) |
| 2014 | IL, USA | Broccoli | HPLC after desulfation | (82) |
| 2015 | IL, USA | Horseradish | HPLC after desulfation | (83) |
| 1999 | Champaign, IL, USA | Cabbage, broccoli, kale, Brussels sprouts, cauliflower | HPLC after desulfation | (48) |
| 2004 | Champaign, IL, USA | Horseradish | HPLC after desulfation | (84) |
| 2001 | Champaign, IL, USA | Broccoli | GC-MS | (44) |
| 2005 | Wooster, OH, USA | Green cabbage | HPLC after desulfation | (85) |
| 2014 | ME, OR, USA | Broccoli | HPLC after desulfation | (86) |
| 2005 | CA, USA | Broccoli | GC-MS | (87) |
| 2005 | Becker, MN, USA | Red cabbage, green cabbage | HPLC after desulfation | (42) |
| 1991 | Ontario, Canada | Rutabaga | HPLC after desulfation | (88) |
| 1993 | Ontario, Canada | Broccoli | HPLC after desulfation | (54) |
| 2017 | College Station, TX, USA | Green kohlrabi, green cabbage | HPLC after desulfation | (89) |
| 2015 | MD, USA | Broccoli microgreens | UPLC after desulfation | (90) |
| 2005 | Columbus, OH, USA | Broccoli, broccoli sprouts, Brussels sprouts, cauliflower | HPLC-MS | (91) |
| 1997 | Canada; Harmony, NJ, USA | Winter cress | HPLC-MS | (92) |
1UPLC, ultra performance liquid chromatography.
FIGURE 3.
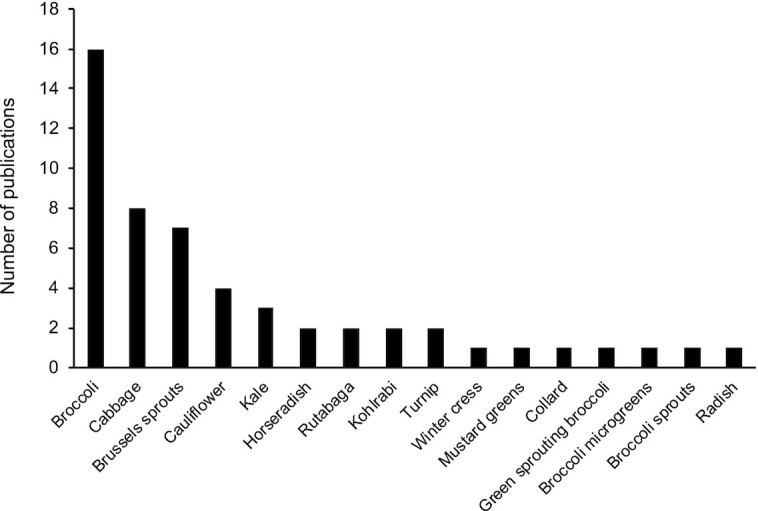
Numbers of publications on glucosinolate-containing vegetables in the United States and Canada from 1980 to 2020.
Overview of the data
GSLs in 16 different vegetables were reported, including 13 Brassica vegetables and 3 non-Brassica vegetables. Of the 13 Brassica vegetables, 10 of them are different varieties of Brassica oleracea L., which represent the most commonly consumed cruciferous vegetable in the United States. The common names of these vegetables and their scientific names are listed in Table 2. In terms of the analytical method, HPLC analysis of desulfated GSLs (38) was found to be the primary quantification method. GC or GC-MS were used in several early studies, and HPLC-MS was adopted in 2 studies. Although >100 GSLs have been identified or tentatively identified in plants thus far (39), only 27 GSLs were reported in all 16 vegetables, included 19 aliphatic, 4 aromatic, and 4 indole GSLs (Table 3). The concentrations of individual GSLs in each food are presented in Tables 4–8. For studies with >1 sample, the range of the values is presented. The number of GSLs identified and quantified varied considerably between different vegetables, ranging from 3 for winter cress to 16 for cabbage (Table 9).
TABLE 2.
Scientific and common names of 16 glucosinolate-containing plant foods
| Scientific name | Common name |
|---|---|
| Armoracia rusticana | Horseradish |
| Barbarea vulgaris | Winter cress |
| Brassica campestris ssp. rapifera | Turnip |
| Brassica juncea var. rugosa | Mustard greens |
| Brassica napus ssp. rapifera | Rutabaga |
| Brassica oleracea var. acephala | Kale |
| Brassica oleracea L. acephala group (var. sabellica) | Collard |
| Brassica oleracea var. botrytis | Cauliflower |
| Brassica oleracea var. botrytis subvar. cymosa Lam. | Broccoli, calabrese, green sprouting broccoli |
| Brassica oleracea var. capitata | Cabbage, red cabbage, green cabbage |
| Brassica oleracea var. gemmifera | Brussels sprouts |
| Brassica oleracea var. gongylodes | Kohlrabi |
| Brassica oleracea var. italica | Broccoli |
| Brassica oleracea var. italica | Broccoli microgreens |
| Brassica oleracea var. italica | Broccoli sprouts |
| Raphanus sativus ssp. radicola | Radish (red, white, and black) |
TABLE 3.
Glucosinolates reported in 16 glucosinolate-containing plant foods and their trivial and semisystematic names1
| Trivial name | Semisystematic name | Molecular weight |
|---|---|---|
| Aliphatic GSLs | ||
| Glucoalyssin | 5-Methylsulfinylpentyl GSL | 451 |
| Glucoberteroin | 5-Methylthiopentyl GSL | 435 |
| Glucobrassicanapin | 4-Pentenyl GSL | 387 |
| Glucocochlearin | 1-Methylpropyl GSL | 375 |
| Glucoerucin | 4-Methylthiobutyl GSL | 421 |
| Glucoerysolin | 4-Methylsulfonylbutyl GSL | 453 |
| Glucoiberin | 3-(Methylsulfinyl)propyl GSL | 423 |
| Glucoiberverin | 3-(Methylthio)propyl-GSL | 407 |
| Glucokohlrabiin | Pentyl GSL | 389 |
| Gluconapin | 3-Butenyl GSL | 373 |
| Glucoraphanin | 4-Methylsulfinylbutyl GSL | 437 |
| Glucoraphasatin | 4-Methylthiobut-3-enyl GSL | 419 |
| Glucoraphasativusain | Hexyl GSL | 403 |
| Glucoraphenin | 4-Methylsulfinyl-3-butenyl GSL | 435 |
| Napoleiferin | 2-Hydroxy-4-pentenyl GSL | 403 |
| Progoitrin | 2(R)-Hydroxy-3-butenyl GSL | 389 |
| Epiprogoitrin | 2(S)-Hydroxy-3-butenyl GSL | 389 |
| Sinigrin | 2-Propenyl GSL | 359 |
| N/A | 4-Hydroxybutyl GSL | 391 |
| Aromatic GSLs | ||
| (2R)-Glucobarbarin | (2R)-2-Hydroxy-2-phenylethyl GSL | 439 |
| (2S)-Glucobarbarin | (2S)-2-Hydroxy-2-phenylethyl GSL | 439 |
| Gluconasturtiin | 2-Phenethyl GSL | 423 |
| Glucotropaeolin | Benzyl GSL | 409 |
| Indolyl GSLs | ||
| Glucobrassicin | 3-Indolylmethyl GSL | 448 |
| 4-hydroxyglucobrassicin | 4-Hydroxy-3-indolylmethyl GSL | 464 |
| 4-methoxyglucobrassicin | 4-Methoxy-3-indolylmethyl GSL | 478 |
| Neoglucobrassicin | 1-Methoxy-3-indolylmethyl GSL | 478 |
1GSL, glucosinolate; N/A, not available.
TABLE 4.
Glucosinolates identified in broccoli grown in the Unites States1
| Scientific name | Plant material | Glucosinolates | Concentration range, mg/100 g FW) | Refs |
|---|---|---|---|---|
| Brassica oleracea var. italica | 21 cultivars | Aliphatic GSL | (41) | |
| Epiprogoitrin | 0–2.90 | |||
| Glucoalyssin | 0–7.22 | |||
| Gluconapin | 0–6.58 | |||
| Glucoraphanin | 1.09–58.94 | |||
| Napoleiferin | 0–6.26 | |||
| Progoitrin | 0.72–19.41 | |||
| Indole GSLs | ||||
| Glucobrassicin | 1.09–15.66 | |||
| 4-Hydroxyglucobrassicin | 0–4.82 | |||
| 4-Methoxyglucobrassicin | 0–2.59 | |||
| Neoglucobrassicin | 0–22.61 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0.43–5.20 | |||
| Brassica oleracea L. (Botrytis Group) (Brassica oleracea var. italica) | 10 genotypes | Aliphatic GSLs | (72) | |
| Glucoraphanin | 3.74–107.88 | |||
| Progoitrin | 0–32.88 | |||
| Sinigrin | 0–1.54 | |||
| Brassica oleracea var. italica | 6 cultivars | Aliphatic GSLs | (74) | |
| Glucoerucin | 0.46–1.56 | |||
| Glucoiberin | 0.59–4.74 | |||
| Gluconapin | 0–0.37 | |||
| Glucoraphanin | 13.02–38.59 | |||
| Progoitrin | 0–1.44 | |||
| Sinigrin | 0–0.57 | |||
| Indole GSL | ||||
| Glucobrassicin | 18.91–32.12 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0–0.80 | |||
| N/A | 8 cultivars | Aliphatic GSLs | (77) | |
| Glucoiberin | 0–4.53 | |||
| Glucoraphanin | 1.92–71.78 | |||
| Progoitrin | 0–8.91 | |||
| Indole GSLs | ||||
| 4-Hydroxyglucobrassicin | 0–1.19 | |||
| Glucobrassicin | 0.48–26.32 | |||
| Neoglucobrassicin | 0.05–34.57 | |||
| Brassica oleracea var. italica | 2 cultivars growing during 2 fall and 2 spring seasons | Aliphatic GSLs | (43) | |
| Gluconapin | 0–0.40 | |||
| Glucoraphanin | 6.55–69.67 | |||
| Indole GSLs | ||||
| 4-Hydroxyglucobrassicin | 0.99–2.48 | |||
| 4-Methoxyglucobrassicin | 2.05–4.09 | |||
| Glucobrassicin | 17.26–35.95 | |||
| Neoglucobrassicin | 5.63–46.03 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0–3.17 | |||
| Brassica oleracea | 1 cultivar | Aliphatic GSLs | (53) | |
| Glucobrassicanapin | 1.21 | |||
| Glucoiberin | 13.97 | |||
| Glucoraphanin | 48.95 | |||
| Progoitrin | 0.66 | |||
| Sinigrin | 0.55 | |||
| Indole GSLs | ||||
| Glucobrassicin | 54.56 | |||
| Neoglucobrassicin | 7.70 | |||
| Brassica oleracea | 1 cultivar | Aliphatic GSLs | (52) | |
| Glucobrassicanapin | 1.18 | |||
| Glucoiberin | 13.59 | |||
| Glucoraphanin | 47.62 | |||
| Progoitrin | 0.64 | |||
| Sinigrin | 0.54 | |||
| Indole GSLs | ||||
| Glucobrassicin | 53.07 | |||
| Neoglucobrassicin | 7.49 | |||
| Brassica oleracea var. italica | 1 cultivar | Aliphatic GSLs | (80) | |
| Glucoiberin | 14.48 | |||
| Glucoraphanin | 217.90 | |||
| Indole GSLs | ||||
| 4-Methoxyglucobrassicin | 8.69 | |||
| Glucobrassicin | 50.81 | |||
| Neoglucobrassicin | 9.72 | |||
| Brassica oleracea ssp. italica | 5 cultivars | Aliphatic GSLs | (81) | |
| Gluconapin | 11.57–39.11 | |||
| Glucoraphanin | 9.82–28.06 | |||
| Indole GSLs | ||||
| Glucobrassicin | 11.98–47.94 | |||
| Neoglucobrassicin | 5.63–46.03 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 5.43–13.58 | |||
| Brassica oleracea ssp. italica | 1 cultivar | Aliphatic GSL | (82) | |
| Glucoraphanin | 9.59 | |||
| Indole GSL | ||||
| Neoglucobrassicin | 23.17 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 5.79 | |||
| Brassica oleracea var. italica | 50 genotypes | Aliphatic GSLs | (mean of 50 genotypes) | (48) |
| Glucoalyssin | 0.97 | |||
| Glucobrassicanapin | 1.24 | |||
| Glucoiberin | 0.45 | |||
| Gluconapin | 3.99 | |||
| Glucoraphanin | 33.20 | |||
| Napoleiferin | 3.02 | |||
| Progoitrin | 4.16 | |||
| Sinigrin | 0.38 | |||
| Indole GSLs | ||||
| 4-Hydroxyglucobrassicin | 0.99 | |||
| 4-Methoxyglucobrassicin | 2.05 | |||
| Glucobrassicin | 5.27 | |||
| Neoglucobrassicin | 1.02 | |||
| Aromatic GSLs | ||||
| Gluconasturtiin | 1.81 | |||
| Brassica oleracea var. italica | 4 cultivars | Aliphatic GSLs | (44) | |
| Glucoraphanin | 23.38–33.20 | |||
| N/A | 23 cultivars | Aliphatic GSL | (86) | |
| Glucoraphanin | 5.38–34.65 | |||
| Indole GSLs | ||||
| Glucobrassicin | 6.47–17.30 | |||
| Neoglucobrassicin | 2.76–24.50 | |||
| Brassica oleracea | 1 cultivar | Aliphatic GSL | (87) | |
| Glucoraphanin | 22.91 | |||
| Brassica oleracea var. italica | 2 cultivars at 3 locations | Aliphatic GSLs | (54) | |
| Gluconapin | 0–0.01 | |||
| Glucoraphanin | 8.70–24.22 | |||
| Progoitrin | 0–5.83 | |||
| Indole GSLs | ||||
| 4-Hydroxyglucobrassicin | 0.05–1.24 | |||
| 4-Methoxyglucobrassicin | 1.69–5.83 | |||
| Glucobrassicin | 18.65–49.57 | |||
| Neoglucobrassicin | 2.25–21.53 | |||
| N/A | 1 cultivar | Aliphatic GSLs | (91) | |
| Glucoalyssin | 0.47 | |||
| Glucoerucin | 0.43 | |||
| Glucoiberin | 5.46 | |||
| Gluconapin | 0.02 | |||
| Glucoraphanin | 45.89 | |||
| Progoitrin | 0.03 | |||
| Sinigrin | 0.05 | |||
| Indole GSLs | ||||
| 4-Methoxyglucobrassicin | 18.40 | |||
| Glucobrassicin | 16.89 | |||
| Neoglucobrassicin | 5.45 |
Data reported as dry weight in the literature were converted into fresh weight using the water content in USDA National Nutrient Database for Standard Reference, Legacy Release (37), unless they were provided in the literature. FW, fresh weight; GSL, glucosinolate; N/A, not available; Refs, references.
TABLE 8.
Glucosinolates identified in other foods in the United States1
| Scientific name | Plant material | Glucosinolates | Concentration range (mg/100 g FW) | Refs |
|---|---|---|---|---|
| Armoracia rusticana | Horseradish root, 6 accessions | Aliphatic GSL | (83) | |
| Sinigrin | 137.25–386.96 | |||
| Indole GSL | ||||
| Glucobrassicin | 0–10.33 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 15.97–72.38 | |||
| Armoracia rusticana | Horseradish root, 27 accessions | Aliphatic GSL | (84) | |
| Sinigrin | 8.47–1092.94 | |||
| Indole GSL | ||||
| Glucobrassicin | 0–15.86 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0–242.58 | |||
| Brassica napus ssp. rapifera | Rutabaga, 6 groups containing 12 cultivars | Aliphatic GSLs | (73) | |
| Glucoalyssin | 0–7.67 | |||
| Glucoberteroin | 1.74–25.67 | |||
| Glucocochlearin | 0.19–21.00 | |||
| Glucoerucin | 6.32–21.89 | |||
| Gluconapin | 1.49–24.25 | |||
| Napoleiferin | 0–4.43 | |||
| Progoitrin | 20.62–41.62 | |||
| Indole GSL | ||||
| Glucobrassicin | 10.30–41.22 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 10.58–22.00 | |||
| Brassica napus ssp. rapifera | Rutabaga, 1 cultivar | Aliphatic GSLs | (88) | |
| Glucoerucin | 0.93 | |||
| Gluconapin | 1.70 | |||
| Glucoraphanin | 2.96 | |||
| Progoitrin | 33.02 | |||
| Indole GSLs | ||||
| 4-Hydroxyglucobrassicin | 0.88 | |||
| Neoglucobrassicin | 0.25 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 5.28 | |||
| Brassica oleracea var. gongylodes | Kohlrabi, 1 cultivar | Aliphatic GSLs | (74) | |
| Glucoerucin | 14.15 | |||
| Glucoiberin | 0.97 | |||
| Glucoiberverin | 3.95 | |||
| Glucoraphanin | 1.88 | |||
| Progoitrin | 0.12 | |||
| Indole GSL | ||||
| Glucobrassicin | 12.41 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0.76 | |||
| Brassica oleracea var. gongylodes | Green kohlrabi, 1 cultivar | Aliphatic GSLs | (89) | |
| Glucoerucin | 4.36† | |||
| Glucoiberin | 5.33† | |||
| Glucoibervirin | 2.20† | |||
| Gluconapin | 0.70† | |||
| Glucoraphanin | 5.58† | |||
| Progoitrin | 8.86 | |||
| Sinigrin | 6.07 | |||
| Indole GSLs | ||||
| Glucobrassicin | 5.24† | |||
| 4-Methoxyglucobrassicin | 1.29† | |||
| Neoglucobrassicin | 14.28 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0.61 | |||
| Brassica campestris ssp. rapifera | Turnip, 8 groups containing 29 cultivars | Aliphatic GSLs | (73) | |
| Glucobrassicanapin | 2.32–18.58 | |||
| Glucoberteroin | 3.48–20.01 | |||
| Glucocochlearin | 0.38–14.25 | |||
| Glucoerucin | 1.26–12.63 | |||
| Gluconapin | 0.75–25.74 | |||
| Napoleiferin | 0–11.28 | |||
| Progoitrin | 5.84–38.90 | |||
| Indole GSL | ||||
| Glucobrassicin | 5.38–34.50 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 10.58–39.76 | |||
| Brassica campestris ssp. rapifera | Turnip greens, 19 cultivars harvested from 2 consecutive years | Aliphatic GSLs | (mean) | (76) |
| Glucobrassicanapin | 22.64 | |||
| Glucoberteroin | 0.17 | |||
| Glucocochlearin | 2.81 | |||
| Glucoerucin | 0 | |||
| Gluconapin | 27.04 | |||
| Napoleiferin | 1.21 | |||
| Progoitrin | 2.33 | |||
| Indole GSL | ||||
| Glucobrassicin | 3.81 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 4.02 | |||
| Turnip roots, 19 cultivars harvested from 2 consecutive years | Aliphatic GSLs | (mean) | ||
| Glucobrassicanapin | 13.55 | |||
| Glucoberteroin | 10.01 | |||
| Glucocochlearin | 1.31 | |||
| Glucoerucin | 5.26 | |||
| Gluconapin | 15.48 | |||
| Napoleiferin | 2.62 | |||
| Progoitrin | 8.36 | |||
| Indole GSL | ||||
| Glucobrassicin | 9.41 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 44.63 | |||
| Brassica oleracea var. botrytis subvar. cymosa Lam. | Green sprouting broccoli, 1 cultivar | Aliphatic GSLs | (47) | |
| Epiprogoitrin | 0.32 | |||
| Glucoiberin | 1.54 | |||
| Progoitrin | 0.25 | |||
| Sinigrin | 0.16 | |||
| Indole GSL | ||||
| Glucobrassicin | 4.26 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0.81 | |||
| Barbarea vulgaris | Winter cress, 1 cultivar | Aromatic GSLs | (92) | |
| (2R)-Glucobarbarin | 3.00 | |||
| (2S)-Glucobarbarin | 2.00 | |||
| Gluconasturtiin | 230.00 | |||
| Brassica juncea var. rugosa | Mustard greens, 2 cultivar | Aliphatic GSLs | (74) | |
| Gluconapin | 1.01–3.17 | |||
| Glucoraphanin | 0–0.92 | |||
| Sinigrin | 249.11–279.80 | |||
| Indole GSL | ||||
| Glucobrassicin | 1.88–5.47 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 2.20–3.21 | |||
| Brassica oleracea L. acephala group (var. sabellica) | Collards, 6 cultivars | Aliphatic GSLs | (74) | |
| Glucoiberin | 0–21.01 | |||
| Gluconapin | 2.16–14.21 | |||
| Glucoraphanin | 0–5.77 | |||
| Progoitrin | 6.54–50.69 | |||
| Sinigrin | 22.44–70.83 | |||
| Indole GSL | ||||
| Glucobrassicin | 30.11–74.05 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0.30–2.24 | |||
| N/A | Broccoli sprouts, 1 cultivar | Aliphatic GSLs | (91) | |
| Glucoalyssin | 0.05 | |||
| Glucoerucin | 42.94 | |||
| Glucoiberin | 25.34 | |||
| Gluconapin | 0.91 | |||
| Glucoraphanin | 58.12 | |||
| Progoitrin | 4.12 | |||
| Sinigrin | 1.34 | |||
| Indole GSLs | ||||
| 4-Methoxyglucobrassicin | 26.48 | |||
| Glucobrassicin | 1.77 | |||
| Neoglucobrassicin | 14.91 | |||
| Brassica oleracea var. italica | Broccoli microgreens, 1 cultivar | Aliphatic GSLs | (90) | |
| Glucoerucin | 71.77 | |||
| Glucoiberin | 8.93 | |||
| Glucoiberverin | 20.38 | |||
| Glucokohlrabiin | 2.71 | |||
| Glucoraphanin | 2.34 | |||
| Glucoraphasativusain | 10.57 | |||
| Indole GSLs | ||||
| 4-Hydroxyglucobrassicin | 4.08 | |||
| Neoglucobrassicin | 40.96 | |||
| Raphanus sativus ssp. radicola | Red radish, 36 cultivars | Aliphatic GSLs | (75) | |
| Glucoraphanin | 0–0.87 | |||
| Glucoraphasatin | 26.82–78.35 | |||
| Glucoraphenin | 0.44–6.53 | |||
| White radish, 7 cultivars | Aliphatic GSLs | |||
| Glucoraphanin | 0–0.87 | |||
| Glucoraphasatin | 34.36–77.10 | |||
| Glucoraphenin | 0.44–3.92 | |||
| Indole GSL | ||||
| Glucobrassicin | 0.90–8.51 | |||
| Black radish, 1 cultivar | Aliphatic GSLs | |||
| Glucoraphanin | 0.87 | |||
| Glucoraphasatin | 98.05 | |||
| Glucoraphenin | 7.83 |
Data reported as dry weight in the literature were converted into fresh weight using the water contents in USDA National Nutrient Database for Standard Reference, Legacy Release (37), unless they were provided in the literature. FW, fresh weight; GSL, glucosinolate; N/A, not available; Refs, references.
TABLE 9.
Glucosinolates identified and quantified in 16 different foods1
| Glucosinolate | BR | CB | BS | CF | KL | RB | GSB | TN | KR | MG | CL | BRS | BRM | HR | WC | RD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliphatic GSLs | ||||||||||||||||
| 4-Hydroxybutyl GSL | X | |||||||||||||||
| Epiprogoitrin | X | X | ||||||||||||||
| Glucoalyssin | X | X | X | X | X | |||||||||||
| Glucoberteroin | X | X | ||||||||||||||
| Glucobrassicanapin | X | X | X | X | X | X | ||||||||||
| Glucocochlearin | X | X | ||||||||||||||
| Glucoerucin | X | X | X | X | X | X | X | X | X | |||||||
| Glucoerysolin | X | |||||||||||||||
| Glucoiberin | X | X | X | X | X | X | X | X | X | X | ||||||
| Glucoiberverin | X | X | X | X | X | |||||||||||
| Glucokohlrabiin | X | |||||||||||||||
| Gluconapin | X | X | X | X | X | X | X | X | X | X | ||||||
| Glucoraphanin | X | X | X | X | X | X | X | X | X | X | X | |||||
| Glucoraphasatin | X | |||||||||||||||
| Glucoraphasativusain | X | |||||||||||||||
| Glucoraphenin | X | |||||||||||||||
| Napoleiferin | X | X | X | X | X | |||||||||||
| Progoitrin | X | X | X | X | X | X | X | X | X | X | X | |||||
| Sinigrin | X | X | X | X | X | X | X | X | X | X | ||||||
| Indole GSLs | ||||||||||||||||
| Glucobrassicin | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| 4-Hydroxyglucobrassicin | X | X | X | X | X | X | X | |||||||||
| 4-Methoxyglucobrassicin | X | X | X | X | X | X | ||||||||||
| Neoglucobrassicin | X | X | X | X | X | X | X | |||||||||
| Aromatic GSLs | ||||||||||||||||
| (2R)-Glucobarbarin | X | |||||||||||||||
| (2S)-Glucobarbarin | X | |||||||||||||||
| Gluconasturtiin | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Glucotropaeolin | X |
This table was prepared based on the data presented in Tables 4–8. BR, broccoli; BRM, broccoli microgreens; BRS, broccoli sprouts; BS, Brussels sprouts; CB, cabbage; CF, cauliflower; CL, collard; GSB, green sprout broccoli; GSL, glucosinolate; HR, horse radish; KL, kale; KR, kohlrabi; MG, mustard greens; RB, rutabaga; RD, radish; TN, turnip; WC, winter cress.
TABLE 5.
Glucosinolates identified in cabbages in the United States1
| Scientific name | Plant material | Glucosinolates | Concentration range, mg/100 g FW | Refs |
|---|---|---|---|---|
| Brassica oleracea var. capitata | Cabbage, 2 cultivars, 3 y, 2 seasons | Aliphatic GSLs | (43) | |
| Glucoiberin | 2.26–75.13 | |||
| Gluconapin | 0.80–5.99 | |||
| Glucoraphanin | 0.47–28.99 | |||
| Progoitrin | 0.42–6.66 | |||
| Sinigrin | 1.15–40.72 | |||
| Indole GSLs | ||||
| Glucobrassicin | 10.07–59.44 | |||
| 4-Hydroxyglucobrassicin | 0.50–2.98 | |||
| 4-Methoxyglucobrassicin | 1.53–8.69 | |||
| Neoglucobrassicin | 0.51–13.30 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0–1.36 | |||
| N/A | Cabbage, 3 varieties in 3 locations | Aliphatic GSLs | (78) | |
| Glucoerucin | 0.03–1.24 | |||
| Glucoerysolin | 0–0.44 | |||
| Glucoiberin | 10.75–12.56 | |||
| Glucoiberverin | 0.80–4.00 | |||
| Gluconapin | 0.63–0.96 | |||
| Glucoraphanin | 0.21–1.67 | |||
| Progoitrin | 0.29–2.38 | |||
| Sinigrin | 12.64–18.54 | |||
| Indole GSL | ||||
| Glucobrassicin | 4.41–12.34 | |||
| Aromatic GSLs | ||||
| Gluconasturtiin | 0.24–0.98 | |||
| Glucotropaeolin | 0.03–0.13 | |||
| N/A | Cabbage, 1 cultivar | Indole GSL | (79) | |
| Glucobrassicin | 21.24 | |||
| Brassica oleracea var. capitata | Cabbage, 1 cultivar | Aliphatic GSLs | (53) | |
| Glucoiberin | 11.28 | |||
| Glucoraphanin | 1.62 | |||
| Progoitrin | 2.70 | |||
| Sinigrin | 15.72 | |||
| Indole GSLs | ||||
| Glucobrassicin | 8.64 | |||
| 4-Hydroxyglucobrassicin | 1.20 | |||
| 4-Methoxyglucobrassicin | 0.84 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0.12 | |||
| Brassica oleracea var. capitata | Green cabbage, 1 cultivar | Aliphatic GSLs | (89) | |
| Glucoiberin | 1.492 | |||
| Glucoibervirin | 2.232 | |||
| Glucoraphanin | 0.682 | |||
| Sinigrin | 15.78 | |||
| Indole GSLs | ||||
| Glucobrassicin | 21.27 | |||
| 4-Hydroxyglucobrassicin | 0.042 | |||
| 4-Methoxyglucobrassicin | 6.352 | |||
| Neoglucobrassicin | 0.042 | |||
| Brassica oleracea var. capitata | Cabbage, 6 genotypes | Aliphatic GSLs | (mean of 6 genotypes) | (48) |
| Glucobrassicanapin | 0.61 | |||
| Gluconapin | 2.04 | |||
| Glucoraphanin | 0.34 | |||
| Progoitrin | 0.61 | |||
| Sinigrin | 21.90 | |||
| Indole GSLs | ||||
| Glucobrassicin | 3.15 | |||
| 4-Hydroxyglucobrassicin | 1.09 | |||
| 4-Methoxyglucobrassicin | 1.12 | |||
| Neoglucobrassicin | 0.75 | |||
| Aromatic GSLs | ||||
| Gluconasturtiin | 0.99 | |||
| Brassica oleracea var. capitata | Cabbage, 1 cultivar, 2 y | Aliphatic GSLs | (85) | |
| Glucoiberin | 33.08–41.68 | |||
| Progoitrin | 6.39–11.26 | |||
| Sinigrin | 23.58–24.70 | |||
| Indole GSLs | ||||
| Glucobrassicin | 9.46–30.48 | |||
| Brassica oleracea var. capitata | Green cabbage, 1 cultivar | Aliphatic GSLs | (42) | |
| 4-Hydroxybutyl GSL | 1.60 | |||
| Gluconapin | 0.15 | |||
| Glucoraphanin | 0.39 | |||
| Progoitrin | 0.58 | |||
| Sinigrin | 8.98 | |||
| Indole GSLs | ||||
| Glucobrassicin | 37.05 | |||
| 4-Hydroxyglucobrassicin | 1.39 | |||
| 4-Methoxyglucobrassicin | 13.53 | |||
| Neoglucobrassicin | 5.78 | |||
| Red cabbage, 1 cultivar | Aliphatic GSLs | |||
| 4-Hydroxybutyl GSL | 4.65 | |||
| Gluconapin | 1.31 | |||
| Glucoraphanin | 3.80 | |||
| Progoitrin | 1.36 | |||
| Sinigrin | 7.86 | |||
| Indole GSLs | ||||
| Glucobrassicin | 105.55 | |||
| 4-Hydroxyglucobrassicin | 8.54 | |||
| 4-Methoxyglucobrassicin | 15.01 | |||
| Neoglucobrassicin | 8.37 |
Data reported as dry weight in the literature were converted into fresh weight using the water content in USDA National Nutrient Database for Standard Reference, Legacy Release (37), unless they were provided in the literature. FW, fresh weight; GSL, glucosinolate; N/A, not available; Refs, references.
Estimated from graphic data.
TABLE 6.
Glucosinolates identified in Brussels sprouts in the United States1
| Scientific name | Plant material | Glucosinolates | Concentration range (mg/100 g FW) | Refs |
|---|---|---|---|---|
| Brassica oleracea var. gemmifera | 6 cultivars | Aliphatic GSLs | (74) | |
| Glucoerucin | 0.17–0.51 | |||
| Glucoiberin | 1.02–7.99 | |||
| Glucoiberverin | 0–0.08 | |||
| Gluconapin | 0.19–4.55 | |||
| Glucoraphanin | 0.17–9.88 | |||
| Progoitrin | 0.39–9.88 | |||
| Sinigrin | 1.40–8.15 | |||
| Indole GSL | ||||
| Glucobrassicin | 146.85–217.33 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0.13–0.59 | |||
| Brassica oleracea var. gemmifera | 2 cultivars, 3 y, 2 seasons | Aliphatic GSLs | (43) | |
| Glucoiberin | 8.29–39.09 | |||
| Gluconapin | 1.04–6.79 | |||
| Glucoraphanin | 1.84–18.35 | |||
| Progoitrin | 5.99–22.33 | |||
| Sinigrin | 13.07–24.12 | |||
| Indole GSLs | ||||
| Glucobrassicin | 27.60–119.80 | |||
| 4-Hydroxyglucobrassicin | 1.95–6.50 | |||
| 4-Methoxyglucobrassicin | 4.02–7.36 | |||
| Neoglucobrassicin | 0–12.71 | |||
| N/A | 1 cultivar | Indole GSL | (79) | |
| Glucobrassicin | 74.05 | |||
| Brassica oleracea var. gemmifera | 1 cultivar | Aliphatic GSLs | (53) | |
| Glucoiberin | 22.80 | |||
| Glucoraphanin | 35.55 | |||
| Progoitrin | 44.10 | |||
| Sinigrin | 23.70 | |||
| Indole GSLs | ||||
| Glucobrassicin | 267.15 | |||
| 4-Hydroxyglucobrassicin | 9.30 | |||
| 4-Methoxyglucobrassicin | 42.90 | |||
| Brassica oleracea | 1 cultivar | Aliphatic GSLs | (52) | |
| Glucoiberin | 21.28 | |||
| Glucoraphanin | 33.18 | |||
| Progoitrin | 41.16 | |||
| Sinigrin | 22.12 | |||
| Indole GSLs | ||||
| Glucobrassicin | 249.34 | |||
| 4-Hydroxyglucobrassicin | 8.68 | |||
| 4-Methoxyglucobrassicin | 40.04 | |||
| Brassica oleracea var. gemmifera | 4 genotypes | Aliphatic GSLs | (mean of 4 genotypes) | (48) |
| Glucoalyssin | 0.63 | |||
| Glucobrassicanapin | 2.71 | |||
| Gluconapin | 36.03 | |||
| Glucoraphanin | 6.12 | |||
| Napoleiferin | 2.26 | |||
| Progoitrin | 13.07 | |||
| Sinigrin | 44.73 | |||
| Indole GSLs | ||||
| 4-Hydroxyglucobrassicin | 3.90 | |||
| 4-Methoxyglucobrassicin | 2.68 | |||
| Glucobrassicin | 20.07 | |||
| Neoglucobrassicin | 1.34 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 2.96 | |||
| N/A | 1 cultivar | Aliphatic GSLs | (91) | |
| Glucoalyssin | 4.96 | |||
| Glucoiberin | 24.83 | |||
| Gluconapin | 40.28 | |||
| Glucoraphanin | 6.60 | |||
| Progoitrin | 51.74 | |||
| Sinigrin | 55.65 | |||
| Indole GSLs | ||||
| 4-Methoxyglucobrassicin | 40.34 | |||
| Glucobrassicin | 167.55 |
Data reported as dry weight in the literature were converted into fresh weight using the water content in USDA National Nutrient Database for Standard Reference, Legacy Release (37), unless they were provided in the literature. FW, fresh weight; GSL, glucosinolate; N/A, not available; Refs, references.
TABLE 7.
Glucosinolates identified in cauliflower and kale grown in the United States1
| Scientific name | Plant material | Glucosinolates | Concentration range (mg/100 g FW) | Refs |
|---|---|---|---|---|
| Brassica oleracea L. botrytis | Cauliflower, 5 cultivars | Aliphatic GSLs | (74) | |
| Glucoerucin | 0.08–0.55 | |||
| Glucoiberin | 0–9.64 | |||
| Glucoiberverin | 0.65–3.01 | |||
| Gluconapin | 0–0.04 | |||
| Glucoraphanin | 0–0.74 | |||
| Sinigrin | 1.04–5.92 | |||
| Indole GSL | ||||
| Glucobrassicin | 8.42–46.91 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0–0.17 | |||
| Brassica oleracea L. botrytis | Cauliflower, 2 cultivars, 3 y, 2 seasons | Aliphatic GSLs | (43) | |
| Glucoiberin | 1.01–10.40 | |||
| Gluconapin | 0–0.30 | |||
| Glucoraphanin | 0–1.04 | |||
| Progoitrin | 0–0.93 | |||
| Sinigrin | 1.14–13.10 | |||
| Indole GSLs | ||||
| Glucobrassicin | 9.95–28.78 | |||
| 4-Hydroxyglucobrassicin | 0.37–5.15 | |||
| 4-Methoxyglucobrassicin | 0.76–2.27 | |||
| Neoglucobrassicin | 1.90–25.40 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0–5.03 | |||
| Brassica oleracea L. botrytis | Cauliflower, 3 genotypes | Aliphatic GSLs | (mean of 3 genotypes) | (48) |
| Glucobrassicanapin | 0.31 | |||
| Gluconapin | 0.89 | |||
| Glucoraphanin | 1.73 | |||
| Napoleiferin | 0.64 | |||
| Progoitrin | 0.93 | |||
| Sinigrin | 26.48 | |||
| Indole GSLs | ||||
| Glucobrassicin | 4.62 | |||
| 4-Hydroxyglucobrassicin | 5.89 | |||
| 4-Methoxyglucobrassicin | 3.79 | |||
| Neoglucobrassicin | 0.76 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 1.34 | |||
| N/A | Cauliflower, 1 cultivar | Aliphatic GSLs | (91) | |
| Glucoalyssin | 2.11 | |||
| Glucoerucin | 1.09 | |||
| Glucoiberin | 17.60 | |||
| Gluconapin | 0.50 | |||
| Glucoraphanin | 1.98 | |||
| Progoitrin | 3.33 | |||
| Sinigrin | 15.08 | |||
| Indole GSLs | ||||
| 4-Methoxyglucobrassicin | 19.31 | |||
| Glucobrassicin | 68.54 | |||
| Neoglucobrassicin | 11.19 | |||
| Brassica oleracea var. acephala | Curly kale, 2 cultivars; smooth-leafed kale, 1 cultivar | Aliphatic GSLs | (74) | |
| Glucoiberin | 2.70–29.18 | |||
| Gluconapin | 0–1.53 | |||
| Glucoraphanin | 0.31–3.36 | |||
| Progoitrin | 0–8.75 | |||
| Sinigrin | 7.40–10.30 | |||
| Indole GSL | ||||
| Glucobrassicin | 19.80–31.14 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 0.34–0.93 | |||
| Brassica oleracea var. acephala | Kale, 2 cultivars, 3 y, 2 seasons | Aliphatic GSLs | (43) | |
| Glucoiberin | 2.19–28.51 | |||
| Glucoraphanin | 0–1.36 | |||
| Progoitrin | 0–0.81 | |||
| Sinigrin | 1.49–7.07 | |||
| Indole GSLs | ||||
| Glucobrassicin | 8.36–32.02 | |||
| 4-Hydroxyglucobrassicin | 0.48–1.92 | |||
| 4-Methoxyglucobrassicin | 0.50–1.49 | |||
| Neoglucobrassicin | 0.99–4.96 | |||
| Brassica oleracea var. acephala | Kale, 2 genotypes | Aliphatic GSLs | (mean of 2 genotypes) | (48) |
| Glucobrassicanapin | 0.40 | |||
| Gluconapin | 3.87 | |||
| Glucoraphanin | 4.53 | |||
| Progoitrin | 2.42 | |||
| Sinigrin | 38.72 | |||
| Indole GSLs | ||||
| Glucobrassicin | 5.57 | |||
| 4-Hydroxyglucobrassicin | 0.48 | |||
| 4-Methoxyglucobrassicin | 0.99 | |||
| Neoglucobrassicin | 0.50 | |||
| Aromatic GSL | ||||
| Gluconasturtiin | 1.75 |
Data reported as dry weight in the literature were converted into fresh weight using the water contents in USDA National Nutrient Database for Standard Reference, Legacy Release (37), unless they were provided in the literature. FW, fresh weight; GSL, glucosinolate; N/A, not available; Refs, references.
Discussion
Data source: domestic compared with international data
Before developing a database of certain dietary component(s) based on the literature data, the first question to ask is, “What data should be included?” This question should be answered based on the purpose of developing such a database. GSLs are not essential nutrients for human beings. The main purpose of developing a dietary GSL database is to estimate the dietary intake, which can then be used in the nutritional epidemiological studies to evaluate the diet-disease relations (31). Notably, almost all nutritional epidemiological studies were conducted in specific countries or regions, as was indicated in a recent review article (24). Hence, calculating the GSL composition in 1 country or region, rather than combining all international data, would be more relevant in assessing the dietary intake of these compounds. Furthermore, types and concentrations of GSLs are highly variable based on the genetic background and various environmental factors (17). Inclusion of international data will add unnecessary variations to the dataset. Therefore, only the data of the vegetables that people in the United States have access to, including those obtained from the markets in the United States or grown in the United States, Canada, and Mexico, were included in this study.
Evaluation of data quality
Many factors need to be considered when discussing the quality of food composition data. To ensure data quality, the USDA Methods and Application of Food Composition Laboratory (formerly the Nutrient Data Laboratory) has developed a comprehensive data quality evaluation system (34, 40). Five categories, namely, sampling plan, number of samples, sample handling, analytical method, and analytical quality control, must be carefully assessed to ensure data quality. It is noteworthy that none of the 30 articles included in this study was designed to produce data for the purpose of developing a composition database. These studies were carried out for various other purposes, such as the comparison between different cultivars (41), effects of fertilization (42), or the seasonal variation (43). Hence, no standard procedure was followed, and the experimental design generally did not fully consider the factors discussed above. For a certain study (44), only GSLs of interest, not the full profile, were analyzed and reported.
As for the 5 categories, sampling plans of these studies were generally not comprehensive. Samples in some studies were collected from the field with information about cultivars, location, and growing conditions, whereas others were obtained from the local supermarket without further information. Another commonly neglected issue is the insufficient or incorrect description of the plant materials. For example, in 5 studies, only common names of the samples were provided. Because many GSL-containing vegetables have been consumed by human beings for hundreds or even thousands of years, it is very common that one species/variety has multiple common names, and one common name can refer to different varieties or even species (15, 45, 46). As an example, Brassica oleracea var. botrytis subvar. cymosa Lam. was referred to as broccoli in one study (47), whereas the scientific name of broccoli should be Brassica oleracea var. italica. And the lack of the major broccoli GSL glucoraphanin in Brassica oleracea var. botrytis subvar. cymosa Lam. confirmed its difference from Brassica oleracea var. italica (Table 4). To avoid misuse of the plant materials, samples need to be described with correct and complete scientific names in addition to the common names.
The number of samples varied considerably based on specific experimental designs. Fifty genotypes of broccoli were included in one study to investigate the variation of GSLs in Brassica vegetables (48), whereas in many other studies, only 1 sample was analyzed (Tables 4–8). Sample handling was also different between different studies. The plant materials were analyzed either as raw or dry forms, with or without cold storage. For the analytical methods, HPLC analysis of desulfated GSL is the predominant analytical method, which was used in 21 of 30 studies. GC or GC-MS were mainly used in the early published articles, and HPLC-MS was used in only 2 recent studies. With the same method, the sample preparation and detailed analytical procedure were also different between different studies. Lastly, the analytical quality control, which ensures that the results of analysis are consistent, accurate, and comparable, was unfortunately not mentioned in any of the 30 studies.
Data variation
In plants, GSLs belong to an extraordinarily diverse group of plant secondary metabolites. They play important roles to help plants cope with continuous environmental challenges (49). GSLs are one of the best-known groups of plant secondary metabolites for plant antiherbivore defenses (50). In contrast to the primary metabolites, the diversity and variability of plant secondary metabolites are much greater (51). It has been shown that the type and concentration(s) of GSLs in GSL-containing plants can be greatly affected by the genetic background, agricultural practices, and various environmental factors (17). As shown in Tables 4–8, there are substantial variations in the values between different vegetables and different cultivars/genotypes and growing conditions in the same vegetables.
Because more studies were conducted for broccoli than for any other foods summarized in this study, it was selected as an example to discuss the data variation and the major influential factors. First of all, except for 2 studies, which appear to have been conducted by the same group on the same material (52, 53), the number of GSLs reported in broccoli is different for all other studies (Table 10), ranging from 1 (44) to 13 (48). It should be mentioned that the study that reported only 1 GSL (glucoraphanin) focused on isothiocyanate sulforaphane, and glucoraphanin was quantified as the precursor of sulforaphane. The major GSLs detected in broccoli are glucoraphanin and progoitrin of aliphatic GSLs, and glucobrassicin and neoglucobrassicin of indole GSLs. Yet glucoraphanin is the only GSL that was identified and quantified in all 16 studies. Based on the published data, several key factors are discussed as follows:
TABLE 10.
Distribution of glucosinolates in broccoli from different studies1
| Glucosinolate | Ref (41) | Ref (72) | Ref (74) | Ref (77) | Ref (43) | Ref (53) | Ref (52) | Ref (80) | Ref (81) | Ref (82) | Ref (48) | Ref (44) | Ref (86) | Ref (87) | Ref (54) | Ref (91) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliphatic GSLs | ||||||||||||||||
| Epiprogoitrin | X | |||||||||||||||
| Glucoalyssin | X | X | X | |||||||||||||
| Glucobrassicanapin | X | X | X | |||||||||||||
| Glucoerucin | X | X | ||||||||||||||
| Glucoiberin | X | X | X | X | X | X | X | |||||||||
| Gluconapin | X | X | X | X | X | X | X | |||||||||
| Glucoraphanin | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Napoleiferin | X | X | ||||||||||||||
| Progoitrin | X | X | X | X | X | X | X | X | X | |||||||
| Sinigrin | X | X | X | X | X | X | ||||||||||
| Indole GSLs | ||||||||||||||||
| Glucobrassicin | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| 4-Hydroxyglucobrassicin | X | X | X | X | X | |||||||||||
| 4-Methoxyglucobrassicin | X | X | X | X | X | X | ||||||||||
| Neoglucobrassicin | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Aromatic GSL | ||||||||||||||||
| Gluconasturtiin | X | X | X | X | X | X |
1GSL, glucosinolate; Ref, reference.
The plant's genetic background
In one study (41), 21 cultivars were grown on the same field in the same year. The concentration ranges of the 4 major GSLs were extremely wide: glucoraphanin (1.09–58.94 mg/100 g FW), progoitrin (0.72–19.41 mg/100 g FW), glucobrassicin (1.09–15.66 mg/100 g FW), and neoglucobrassicin (0–22.61 mg/100 g FW), respectively. The variabilities of minor GSLs were even greater.
Growing condition
As shown in 1 study (54), the same cultivars grown at 3 locations in Ontario, Canada, resulted in a ≤10-fold difference for certain GSLs (e.g., neoglucobrassicin). In another study (43), contents of GSLs were found to be significantly different in broccoli grown in the spring and fall. The variation was further complicated by different cultivars.
Analytical methods
Even with the same method, analytical variation is unavoidable between studies due to different sample handling protocols, extraction methods, operation, and instrumentation. Changes in analytical methods, such as from GC to HPLC after desulfation, might lead to very different GSL values. In addition, because of their coexistence with myrosinase in plant tissues, enzyme deactivation is proved to be a critical step to avoid hydrolytic loss and/or de novo synthesis during sample preparation. Different ways of myrosinase deactivation or not deactivating it will inevitably alter the GSL profile and their concentrations (55).
Challenges and considerations of developing a valid dietary GSL database
Insufficient data
Overall, the number of studies on the quantification of GSLs in commonly consumed vegetables is quite limited, with only 30 studies conducted in the United States and Canada across 4 decades. Eleven vegetables were studied in only 1 or 2 articles, which by no means would reflect the variabilities of their GSL concentrations. Not only do we have insufficient data, but some of the data could be outdated. Nearly half of the studies were published >20 y ago. One of the major considerations in developing food composition databases has been providing and maintaining data that reflect the foods that are being currently consumed (56). The food composition, including the plant secondary metabolites such as GSLs, changes over time due to the developments of new cultivars, changes in growing conditions and agricultural practices, as well as the advances of new analytical methodology.
Data expression and utilization
One of the major applications of the food composition databases is to calculate dietary intakes of the food components for clinical and health-related research. However, this process is often criticized as too unreliable because food components vary greatly. As discussed in the previous sections, GSLs exhibit extremely high variation. Taking broccoli as an example, variability of GSLs identified in broccoli between different studies (Table 10) is almost as great as that in different foods (Table 4). The values of individual GSLs varied considerably between different studies. As an example, the concentration of glucoraphanin, the major GSL in broccoli, ranged from 1.92 to 217.90 mg/100 g FW (Table 4). In several available databases on dietary bioactive compounds, such as the flavonoid databases developed by our group, the data are usually expressed as mean, median, and range (23, 33). Users of food composition data often do not pay much attention to the information beyond simple mean values, nor are they sufficiently aware of the natural variation in the composition of a food affected by various genetic, environmental, and management factors (57). The professionals who calculate dietary intakes based on the dietary database are often unable to decide whether the database entries represent the actual foods eaten by the specific population they are interested in. Better descriptions of the source of data and the genetic background, growing conditions, storage, and processing will minimize the uncertainty but still be unable to completely address the huge variation of the data.
Application of a dietary GSL database in nutritional epidemiological research
Nutritional research has shifted from addressing nutrient deficiency to the role of diet in preventing chronic and degenerative diseases, as well as in overall well-being and longevity. Nutritional epidemiological research plays a central role in the field of nutritional sciences, through which the diet-disease relations first observed or hypothesized in the laboratory can be examined at the level of free-living populations and clinically defined subgroups (58). Well-conducted, large, prospective epidemiological studies have been crucial in updating the dietary guidelines (59). However, nutritional epidemiology has recently been criticized for several major shortcomings (60), one of which is the inability to accurately measure the diet intake (61). In 1 recent study, the intake of GSLs and the risk of type 2 diabetes was assessed in 3 prospective cohorts of US men and women (62). The major flaw of this article, argued in a “Letter to the Editor” (63), is that the authors adopted the GSL intake data based on a publication (18) that only provided mean values but did not consider the effects of processing or the preparation of vegetables (63).
The observation that only hydrolytic products of GSLs are actual in vivo bioactive compounds posted additional concerns about using the GSL database to assess the intake for nutritional epidemiological studies. For example, the yield of isothiocyanates from myrosinase-catalyzed hydrolysis of GSLs is influenced by a number of factors. In a study that compared total isothiocyanate yield of 9 commonly consumed raw cruciferous vegetables in the United States (64), a wide range (as much as 41-fold difference) of isothiocyanate yield was observed across the vegetables. Striking variation in isothiocyanate yield was also observed within each vegetable. For example, 9 samples of mustard green showed a 345-fold difference in isothiocyanate yield. Cooking is another important factor that can significantly alter the isothiocyanate yields. It has been shown in another study (65) that boiling, stewing, and chip-baking reduced isothiocyanate yields, whereas stir-frying, steaming, and microwaving increased isothiocyanate yields from cruciferous vegetables. The extent of change was significant. For instance, a maximum 11-fold increase by stir-frying in broccoli and a 99% reduction by stewing in cabbage. In addition to hydrolysis by myrosinase, GSLs can be hydrolyzed by other “myrosinase-like” enzymes; and nonisothiocyanate breakdown compounds of GSLs are also produced through thermal processing or chemical degradation (17). Pathway(s) of GSL hydrolysis/degradation and the conditions determine the type and amount of breakdown compounds being produced. Therefore, the dietary intake of GSLs might not correlate with the concentration of isothiocyanates in the human body. Studies have shown that generally intake of cruciferous vegetables was only weakly correlated with the urinary isothiocyanate concentrations (66).
Because intake of GSLs might not correlate with their actual bioactive forms in vivo, nutritional epidemiological studies that attempted to establish the association between the intake of GSLs and cancer risk can lead to inconsistent or even false results. Alternative methods to quantify the hydrolytic or breakdown compounds of GSLs in the human body could provide more relevant information. One such method is the measurement of urinary isothiocyanates. Isothiocyanates are excreted in urine, and thus provide a sensitive and specific dietary biomarker of GSL intake. Indeed, studies have been carried out to investigate the association between urinary isothiocyanates and cancer risk (67–70). Interestingly, in 1 of these studies (68), a urinary isothiocyanate biomarker was found to be associated with significantly reduced breast cancer risk in Chinese women, whereas total Brassica intake did not show such an association. Nevertheless, measuring urinary isothiocyanate is obviously very much resource dependent. It might not be feasible when the nutritional epidemiological studies are performed with a large human population for multiple years. GSL intake estimated by an FFQ and GSL database/dataset could still be useful. But it is important for the researchers to understand the limitations of using such information when interpreting the data or explaining the discrepancies.
Conclusion
The studies on GSLs in commonly consumed GSL-containing vegetables in the United States are still quite limited, and some of the data could be outdated. The data quality of these studies varies but is generally unsatisfactory because none of them were designed to provide data for the development of a composition database. Enormous variations of GSL data are observed between different foods and between different studies and cultivars in the same foods. Currently available data are not sufficient to develop a valid GSL database in the United States, and more comprehensive studies are needed, especially for the understudied foods. For these reasons, the total GSL concentration in a typical American diet was not calculated in this study to avoid misleading. Another consideration is that GSLs can also be found in various dietary supplements, and they could contribute significantly to the daily intake. Unfortunately, the information on GSL contents in dietary supplements is largely unavailable.
Because intake of GSLs might not correlate with their actual bioactive forms in vivo, making an association between GSL intake and the disease risk factors can lead to inconsistent or discrepant results in nutritional epidemiological studies. Alternative methods such as the measurement of urinary isothiocyanates as biomarkers of GSL intake could provide more relevant information (71). Researchers should be encouraged to include alternative measurements if possible; or use them to verify the data calculated from a dietary composition database/dataset, to avoid bias. Finally, even though the discussion in this study was made specifically for the dietary GSLs, the similar challenges and concerns might also be related to other dietary bioactive compounds regarding the development and applications of the composition databases.
ACKNOWLEDGEMENTS
We thank Seema A Bhagwat for assistance with the literature search and data analysis. The authors’ responsibilities were as follows—XW, PRP: designed the study; XW: performed the systematic literature review and data analysis; XW, PRP: wrote the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the US Department of Agriculture, Agricultural Research Service.
Author disclosures: The authors report no conflicts of interest.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Contributor Information
Xianli Wu, Email: xianli.wu@usda.gov, Methods and Application of Food Composition Laboratory, USDA ARS Beltsville Human Nutrition Research Center, Beltsville, MD, USA.
Pamela R Pehrsson, Methods and Application of Food Composition Laboratory, USDA ARS Beltsville Human Nutrition Research Center, Beltsville, MD, USA.
References
- 1.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. [DOI] [PubMed] [Google Scholar]
- 2.Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 1983;18:123–201. [DOI] [PubMed] [Google Scholar]
- 3.Francisco M, Tortosa M, Martínez-Ballesta MdC, Velasco P, García-Viguera C, Moreno DA. Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Ann Appl Biol. 2017;170:273–85. [Google Scholar]
- 4.Redovniković IR, Glivetić T, Delonga K, Vorkapić-Furač J. Glucosinolates and their potential role in plant. Period Biol. 2008;110:297–309. [Google Scholar]
- 5.Wu QJ, Yang G, Zheng W, Li HL, Gao J, Wang J, Gao YT, Shu XO, Xiang YB. Pre-diagnostic cruciferous vegetables intake and lung cancer survival among Chinese women. Sci Rep. 2015;5:10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosetti C, Filomeno M, Riso P, Polesel J, Levi F, Talamini R, Montella M, Negri E, Franceschi S, La Vecchia C. Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol. 2012;23:2198–203. [DOI] [PubMed] [Google Scholar]
- 7.Azeem S, Gillani SW, Siddiqui A, Jandrajupalli SB, Poh V, Syed Sulaiman SA. Diet and colorectal cancer risk in Asia—a systematic review. Asian Pac J Cancer Prev. 2015;16:5389–96. [DOI] [PubMed] [Google Scholar]
- 8.Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers MP. Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. Eur J Epidemiol. 2016;31:811–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan R, Lok K, Woo J. Prostate cancer and vegetable consumption. Mol Nutr Food Res. 2009;53:201–16. [DOI] [PubMed] [Google Scholar]
- 10.Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Muñoz A, Johnson JL, Groopman JDet al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res. 2014;7:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan JM, Murphy SE, Stepanov I, Wang R, Carmella SG, Nelson HH, Hatsukami D, Hecht SS. 2-Phenethyl isothiocyanate, glutathione S-transferase M1 and T1 polymorphisms, and detoxification of volatile organic carcinogens and toxicants in tobacco smoke. Cancer Prev Res. 2016;9:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riso P, Del Bo C, Vendrame S, Brusamolino A, Martini D, Bonacina G, Porrini M. Modulation of plasma antioxidant levels, glutathione S-transferase activity and DNA damage in smokers following a single portion of broccoli: a pilot study. J Sci Food Agric. 2014;94:522–8. [DOI] [PubMed] [Google Scholar]
- 13.López-Chillón MT, Carazo-Díaz C, Prieto-Merino D, Zafrilla P, Moreno DA, Villaño D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin Nutr. 2019;38:745–52. [DOI] [PubMed] [Google Scholar]
- 14.Abbaoui B, Riedl KM, Ralston RA, Thomas-Ahner JM, Schwartz SJ, Clinton SK, Mortazavi A. Inhibition of bladder cancer by broccoli isothiocyanates sulforaphane and erucin: characterization, metabolism, and interconversion. Mol Nutr Food Res. 2012;56:1675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke DB. Glucosinolates, structures and analysis in food. Anal Methods. 2010;2:310–25. [Google Scholar]
- 16.Holst B, Williamson G. A critical review of the bioavailability of glucosinolates and related compounds. Nat Prod Rep. 2004;21:425–47. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Huang H, Childs H, Wu Y, Yu L, Pehrsson PR. Glucosinolates in Brassica vegetables: characterization and factors that influence distribution, content, and intake. Annu Rev Food Sci Technol. 2021;12:485–511. [DOI] [PubMed] [Google Scholar]
- 18.Steinbrecher A, Linseisen J. Dietary intake of individual glucosinolates in participants of the EPIC-Heidelberg cohort study. Ann Nutr Metab. 2009;54:87–96. [DOI] [PubMed] [Google Scholar]
- 19.Agudo A, Ibanez R, Amiano P, Ardanaz E, Barricarte A, Berenguer A, Dolores Chirlaque M, Dorronsoro M, Jakszyn P, Larranaga Net al. Consumption of cruciferous vegetables and glucosinolates in a Spanish adult population. Eur J Clin Nutr. 2008;62:324–31. [DOI] [PubMed] [Google Scholar]
- 20.Sones K, Heaney RK, Fenwick GR. An estimate of the mean daily intake of glucosinolates from cruciferous vegetables in the UK. J Sci Food Agric. 1984;35:712–20. [Google Scholar]
- 21.Pennington JA, Stumbo PJ, Murphy SP, McNutt SW, Eldridge AL, McCabe-Sellers BJ, Chenard CA. Food composition data: the foundation of dietetic practice and research. J Am Diet Assoc. 2007;107:2105–13. [DOI] [PubMed] [Google Scholar]
- 22.Haytowitz DB, Wu X, Bhagwat SA. USDA database for the flavonoid content of selected foods. Release 3.3 [Internet]. USDA Agricultural Research Service; 2018; [cited November 16, 2020]. Available from:http://www.ars.usda.gov/nutrientdata/flav. [Google Scholar]
- 23.Bhagwat SA, Haytowitz DB, Wasswa-Kintu SI, Pehrsson PR. Process of formulating USDA's Expanded Flavonoid Database for the Assessment of Dietary intakes: a new tool for epidemiological research. Br J Nutr. 2015;114:472–80. [DOI] [PubMed] [Google Scholar]
- 24.Ngo SNT, Williams DB. Protective effect of isothiocyanates from cruciferous vegetables on breast cancer: epidemiological and preclinical perspectives. Anticancer Agents Med Chem. 2020;21:1413–30. [DOI] [PubMed] [Google Scholar]
- 25.Melrose J. The glucosinolates: a sulphur glucoside family of mustard anti-tumour and antimicrobial phytochemicals of potential therapeutic application. Biomedicines. 2019;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soundararajan P, Kim JS. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules. 2018;23:2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. [DOI] [PubMed] [Google Scholar]
- 29.Steinbrecher A, Nimptsch K, Husing A, Rohrmann S, Linseisen J. Dietary glucosinolate intake and risk of prostate cancer in the EPIC-Heidelberg cohort study. Int J Cancer. 2009;125:2179–86. [DOI] [PubMed] [Google Scholar]
- 30.van Poppel G, Verhoeven DT, Verhagen H, Goldbohm RA. Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv Exp Med Biol. 1999;472:159–68. [DOI] [PubMed] [Google Scholar]
- 31.McNaughton SA, Marks GC. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr. 2003;90:687–97. [DOI] [PubMed] [Google Scholar]
- 32.Holden JM, Bhagwat SA, Haytowitz DB, Gebhardt SE, Dwyer JT, Peterson J, Beecher GR, Eldridge AL, Balentine D. Development of a database of critically evaluated flavonoids data: application of USDA's data quality evaluation system. J Food Compos Anal. 2005;18:829–44. [Google Scholar]
- 33.Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart Det al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database. 2010;2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holden JM, Bhagwat SA, Patterson KY. Development of a multi-nutrient data quality evaluation system. J Food Compos Anal. 2002;15:339–48. [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West LG, Meyer KA, Balch BA, Rossi FJ, Schultz MR, Haas GW. Glucoraphanin and 4-hydroxyglucobrassicin contents in seeds of 59 cultivars of broccoli, raab, kohlrabi, radish, cauliflower, brussels sprouts, kale, and cabbage. J Agric Food Chem. 2004;52:916–26. [DOI] [PubMed] [Google Scholar]
- 37.Haytowitz DB, Ahuja JKC, Wu X, Somanchi M, Nickle M, Nguyen QA, Roseland JM, Williams JR, Patterson KY, Li Yet al. USDA national nutrient database for standard referencelegacy release. USDA;2019; [cited November 16, 2020] [Internet]. Available from:https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release. [Google Scholar]
- 38.International Standards Organization. EEC . Rapeseed – determination of glucosinolates content – part 1: method using high-performance liquid chromatography. ISO 9167-1. Geneva: International Organization for Standardization; 1992. p. 1–9. [Google Scholar]
- 39.Blazevic I, Montaut S, Burcul F, Olsen CE, Burow M, Rollin P, Agerbirk N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry. 2020;169:112100. [DOI] [PubMed] [Google Scholar]
- 40.Bhagwat SA, Patterson KY, Holden JM. Validation study of the USDA's Data Quality Evaluation System. J Food Compos Anal. 2009;22:366–72. [Google Scholar]
- 41.Baik HY, Juvik JA, Jeffery EH, Wallig MA, Kushad M, Klein BP. Relating glucosinolate content and flavor of broccoli cultivars. J Food Sci. 2003;68:1043–50. [Google Scholar]
- 42.Rosen CJ, Fritz VA, Gardner GM, Hecht SS, Carmella SG, Kenney PM. Cabbage yield and glucosinolate concentrations as affected by nitrogen and sulfur fertility. HortScience. 2005;40:1493–8. [Google Scholar]
- 43.Charron CS, Saxton AM, Sams CE. Relationship of climate and genotype to seasonal variation in the glucosinolate-myrosinase system. I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. J Sci Food Agric. 2005;85:671–81. [Google Scholar]
- 44.Matusheski NV, Wallig MA, Juvik JA, Klein BP, Kushad MM, Jeffery EH. Preparative HPLC method for the purification of sulforaphane and sulforaphane nitrile from Brassica oleracea. J Agric Food Chem. 2001;49:1867–72. [DOI] [PubMed] [Google Scholar]
- 45.Possenti M, Baima S, Raffo A, Durazzo A, Giusti AM, Natella F. Glucosinolates in food. In: Mérillon J-M, Ramawat KGeditors. Glucosinolates. Switzerland: Springer International Publishing; 2016. p. 1–46. [Google Scholar]
- 46.Johnson TL, Dinkova-Kostova AT, Fahey JW. Glucosinolates from the Brassica vegetables and their health effects. In: Caballero B, Finglas P, Toldra Feditors. Encyclopedia of food and health. Oxford: Academic Press; 2016. p. 248–55. [Google Scholar]
- 47.Betz JM, Fox WD. High-performance liquid chromatographic determination of glucosinolates in Brassica vegetables. In: Food phytochemicals for cancer prevention I: fruits and vegetables. Huang M-T, Osawa T, Ho C-T, Rosen RTeditors. ACS Publications; 1994. p. 181–96. [Google Scholar]
- 48.Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47:1541–8. [DOI] [PubMed] [Google Scholar]
- 49.Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–7. [DOI] [PubMed] [Google Scholar]
- 50.Hopkins RJ, van Dam NM, van Loon JJ. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol. 2009;54:57–83. [DOI] [PubMed] [Google Scholar]
- 51.Hartmann T. Diversity and variability of plant secondary metabolism: a mechanistic view. Entomol Exp Appl. 1996;80:177–88. [Google Scholar]
- 52.Goodrich RM, Anderson JL, Stoewsand GS. Glucosinolate changes in blanched broccoli and Brussels sprouts. J Food Process Preserv. 1989;13:275–80. [Google Scholar]
- 53.Goodrich RM, Parker RS, Lisk DJ, Stoewsand GS. Glucosinolate, carotene and cadmium content of Brassica oleracea grown on municipal sewage sludge. Food Chem. 1988;27:141–50. [Google Scholar]
- 54.Shelp BJ, Liu L, McLellan D. Glucosinolate composition of broccoli (Brassica oleracea var. italica) grown under various boron treatments at three Ontario sites. Can J Plant Sci. 1993;73:885–8. [Google Scholar]
- 55.Wu X, Sun J, Haytowitz DB, Harnly JM, Chen P, Pehrsson PR. Challenges of developing a valid dietary glucosinolate database. J Food Compos Anal. 2017;64:78–84. [Google Scholar]
- 56.Kapsokefalou M, Roe M, Turrini A, Costa HS, Martinez-Victoria E, Marletta L, Berry R, Finglas P. Food composition at present: new challenges. Nutrients. 2019;11:1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sevenhuysen GP. Food composition databases: current problems and solutions. Food, Nutrition and Agriculture - 12 - Food Composition Data. FAO; 1994. [Google Scholar]
- 58.Tarasuk VS, Brooker AS. Interpreting epidemiologic studies of diet-disease relationships. J Nutr. 1997;127:1847–52. [DOI] [PubMed] [Google Scholar]
- 59.Hu FB, Willett WC. Current and future landscape of nutritional epidemiologic research. JAMA. 2018;320:2073–4. [DOI] [PubMed] [Google Scholar]
- 60.Ioannidis JPA. The challenge of reforming nutritional epidemiologic research. JAMA. 2018;320:969–70. [DOI] [PubMed] [Google Scholar]
- 61.Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr. 2015;6:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma L, Liu G, Sampson L, Willett WC, Hu FB, Sun Q. Dietary glucosinolates and risk of type 2 diabetes in 3 prospective cohort studies. Am J Clin Nutr. 2018;107:617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oliviero T, Verkerk R, Dekker M. Reply to “Dietary glucosinolates and risk of type 2 diabetes in 3 prospective cohort studies”. Am J Clin Nutr. 2018;108:425. [DOI] [PubMed] [Google Scholar]
- 64.Tang L, Paonessa JD, Zhang Y, Ambrosone CB, McCann SE. Total isothiocyanate yield from raw cruciferous vegetables commonly consumed in the United States. J Funct Foods. 2013;5:1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Kwan ML, Pratt R, Roh JM, Kushi LH, Danforth KN, Zhang Y, Ambrosone CB, Tang L. Effects of cooking methods on total isothiocyanate yield from cruciferous vegetables. Food Sci Nutr. 2020;8:5673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogtmann E, Yang G, Li HL, Wang J, Han LH, Wu QJ, Xie L, Cai Q, Li GL, Waterbor JWet al. Correlates of self-reported dietary cruciferous vegetable intake and urinary isothiocyanate from two cohorts in China. Public Health Nutr. 2015;18:1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Epplein M, Wilkens LR, Tiirikainen M, Dyba M, Chung FL, Goodman MT, Murphy SP, Henderson BE, Kolonel LN, Le Marchand L. Urinary isothiocyanates; glutathione S-transferase M1, T1, and P1 polymorphisms; and risk of colorectal cancer: the Multiethnic Cohort Study. Cancer Epidemiol Biomarkers Prev. 2009;18:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, Cheng JR, Shu XO, Gao YT, Zheng W. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–6. [PubMed] [Google Scholar]
- 69.Fowke JH, Gao YT, Chow WH, Cai Q, Shu XO, Li HL, Ji BT, Rothman N, Yang G, Chung FLet al. Urinary isothiocyanate levels and lung cancer risk among non-smoking women: a prospective investigation. Lung Cancer. 2011;73:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fowke JH, Shu XO, Dai Q, Shintani A, Conaway CC, Chung FL, Cai Q, Gao YT, Zheng W. Urinary isothiocyanate excretion, brassica consumption, and gene polymorphisms among women living in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003;12:1536–9. [PubMed] [Google Scholar]
- 71.Sun J, Charron CS, Novotny JA, Peng B, Yu L, Chen P. Profiling glucosinolate metabolites in human urine and plasma after broccoli consumption using non-targeted and targeted metabolomic analyses. Food Chem. 2020;309:125660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown AF, Yousef GG, Jeffery EH, Klein BP, Wallig MA, Kushad MM, Juvik JA. Glucosinolate profiles in broccoli: variation in levels and implications in breeding for cancer chemoprotection. J Amer Soc Hort Sci. 2002;127:807–13. [Google Scholar]
- 73.Carlson DG, Daxenbichler ME, VanEtten CH, Tookey HL, Williams PH. Glucosinolates in crucifer vegetables: turnips and rutabagas. J Agric Food Chem. 1981;29:1235–9. [DOI] [PubMed] [Google Scholar]
- 74.Carlson DG, Daxenbichler ME, VanEtten CH, Kwolek WF, Williams PH. Glucosinolates in crucifer vegetables: broccoli, brussels sprouts, cauliflower, collards, kale, mustard greens, and kohlrabi. J Am Soc Hortic Sci. 1987;112:173–8. [Google Scholar]
- 75.Carlson DG, Daxenbichler ME, VanEtten CH, Hill CB, Williams PH. Glucosinolates in radish cultivars. J Amer Soc Hort Sci. 1985;110:634–8. [Google Scholar]
- 76.Carlson DG, Daxenbichler ME, Tookey HL, Kwolek WF, Hill CB, Williams PH. Glucosinolates in turnip tops and roots: cultivars grown for greens and/or roots. J Am Soc Hort Sci. 1987;112:179–83. [Google Scholar]
- 77.Charron CS, Sams CE, Canaday CH. Impact of glucosinolate content in broccoli (Brassica oleracea (Italica group)) on growth of Pseudomonas marginalis, a causal agent of bacterial soft rot. Plant Dis. 2002;86:629–32. [DOI] [PubMed] [Google Scholar]
- 78.Daxenbichler ME, VanEtten CH, Williams PH. Glucosinolate products in commercial sauerkraut. J Agric Food Chem. 1980;28:809–11. [DOI] [PubMed] [Google Scholar]
- 79.Fujioka N, Ainslie-Waldman CE, Upadhyaya P, Carmella SG, Fritz VA, Rohwer C, Fan Y, Rauch D, Le C, Hatsukami DKet al. Urinary 3,3'-diindolylmethane: a biomarker of glucobrassicin exposure and indole-3-carbinol uptake in humans. Cancer Epidemiol Biomarkers Prev. 2014;23:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hansen M, Møller P, Sørensen H, de Trejo MC. Glucosinolates in broccoli stored under controlled atmosphere. J Amer Soc Hortic Sci. 1995;120:1069–74. [Google Scholar]
- 81.Kim HS, Juvik JA. Effect of selenium fertilization and methyl jasmonate treatment on glucosinolate accumulation in broccoli florets. J Amer Soc Hort Sci. 2011;136:239–46. [Google Scholar]
- 82.Ku KM, Jeffery EH, Juvik JA. Optimization of methyl jasmonate application to broccoli florets to enhance health-promoting phytochemical content. J Sci Food Agric. 2014;94:2090–6. [DOI] [PubMed] [Google Scholar]
- 83.Ku K-M, Jeffery EH, Juvik JA, Kushad MM. Correlation of quinone reductase activity and allyl isothiocyanate formation among different genotypes and grades of horseradish roots. J Agric Food Chem. 2015;63:2947–55. [DOI] [PubMed] [Google Scholar]
- 84.Li X, Kushad MM. Correlation of glucosinolate content to myrosinase activity in horseradish (Armoracia rusticana). J Agric Food Chem. 2004;52:6950–5. [DOI] [PubMed] [Google Scholar]
- 85.Radovich TJK, Kleinhenz MD, Streeter JG. Irrigation timing relative to head development influences yield components, sugar levels, and glucosinolate concentrations in cabbage. J Amer Soc Hort Sci. 2005;130:943–9. [Google Scholar]
- 86.Renaud EN, Van Bueren ETL, Myers JR, Paulo MJ, Van Eeuwijk FA, Zhu N, Juvik JA. Variation in broccoli cultivar phytochemical content under organic and conventional management systems: implications in breeding for nutrition. PLoS One. 2014;9:e95683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robbins RJ, Keck A-S, Banuelos G, Finley JW. Cultivation conditions and selenium fertilization alter the phenolic profile, glucosinolate, and sulforaphane content of broccoli. J Med Food. 2005;8:204–14. [DOI] [PubMed] [Google Scholar]
- 88.Shattuck VI, Kakuda Y, Shelp BJ. Effect of low temperature on the sugar and glucosinolate content of rutabaga. Sci Hortic. 1991;48:9–19. [Google Scholar]
- 89.Singh J, Jayaprakasha GK, Patil BS. Rapid and efficient desulfonation method for the analysis of glucosinolates by high-resolution liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Agric Food Chem. 2017;65:11100–8. [DOI] [PubMed] [Google Scholar]
- 90.Sun J, Kou L, Geng P, Huang H, Yang T, Luo Y, Chen P. Metabolomic assessment reveals an elevated level of glucosinolate content in CaCl2 treated broccoli microgreens. J Agric Food Chem. 2015;63:1863–8. [DOI] [PubMed] [Google Scholar]
- 91.Tian Q, Rosselot RA, Schwartz SJ. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Biochem. 2005;343:93–9. [DOI] [PubMed] [Google Scholar]
- 92.Zrybko CL, Fukuda EK, Rosen RT. Determination of glucosinolates in domestic and wild mustard by high-performance liquid chromatography with confirmation by electrospray mass spectrometry and photodiode-array detection. J Chromatogr A. 1997;767:43–52. [Google Scholar]


