Dilated erythrocyte membrane specifically transfer iPSC to spleen for effective tumor prevention.
Abstract
The major obstacles for tumor vaccine to be surmounted are the lack of versatile property and immunity-inducing effectiveness. Induced pluripotent stem cells (iPSCs) expressed various antigens the same as multiple types of tumors, providing a promising source of wide-spectrum cancer vaccines. The damaged erythrocyte membrane entrapped by spleen could be developed as antigen deliverer for enhancing acquired immunity. Here, the modified lipid materials were used to dilate erythrocyte membrane to fabricate coalescent nanovector, which not only preserved the biological characteristics of erythrocyte membrane but also remedied the defect of insufficient drug loading capacity. After wrapping iPSC protein, the nanovaccine iPSC@RBC-Mlipo exhibited obvious splenic accumulation, systemic specific antitumor immunity evocation, and effective tumor expansion and metastasis inhibition in mice. Hence, our research may provide a prospective strategy of efficient tumor vaccine for clinical practice.
INTRODUCTION
Cancer immunotherapy, which can stimulate the body’s immune system to rapidly evoke a nonspecific or specific immune function (1, 2) and activate relevant immune cells and factors to attack tumor tissue (3, 4), is an ongoing research hotspot in the field of cancer treatment (5, 6). Among them, tumor vaccine can prevent and treat cancer by presenting tumor-associated antigen to provoke specific antitumor immunity (7–9). Although the research of tumor vaccine has made some progress, there are still many obstacles such as wide-spectrum effect, specific targeting ability, and efficient immune mobilization, which would be the formidable challenges in the field of tumor vaccine (10, 11).
In recent years, the preparation technology of induced pluripotent stem cells (iPSCs) is widely used in organ transplantation and other fields (12–14). Kooreman et al. (15) reported that iPSCs and cancer cells have common epitopes and iPSCs share a larger cancer-related epitope library, which indicates that vaccines based on it could provide a large number of tumor antigens for the immune system (16, 17), so as to establish wide-spectrum antitumor immunity for durable tumor regression in a broad variety of cancers (18), but the intact cells as vaccine have serious defects such as the following: (i) The iPSCs that have the ability of differentiation must be treated with mitomycin C or γ-radiation before use to avoid tumorigenicity, so intact iPSCs as vaccine have potential safety hazards (15). (ii) The size of intact iPSC is 10 to 15 μm, and the relatively large size makes it easy to be intercepted by the lung and other tissues after being injected into the body (19). (iii) Interception also results in inadequate contact between intact iPSCs and immune cells. Thus, here, the iPSC total protein was extracted to achieve a wide-spectrum and promising tumor vaccine with low tumorigenicity and high immunogenicity.
Spleen is an important lymphoid organ that contains several immune factors such as antigen-presenting cells (APCs) (20, 21). Therefore, spleen targeting could rouse an efficient and systemic immune response to attack tumor tissues (22). Besides, working as the important “filter” in human body, spleen is also the “blood bank” of the human body (23). When the blood is transported to the spleen, the red blood cells will be “shunted”; that is, the newborn red blood cells can continue to stay in the plasma and through the spleen because of the strong deformation ability of the cell membrane, and the aged or damaged red blood cells will be intercepted and then cleared by phagocytes (24, 25). Therefore, it is suggested that the use of damaged erythrocyte membrane may have the potential of “targeting” retention of spleen.
However, the poor drug loading efficiency of erythrocyte membrane astricted its further application (26, 27). On the basis of this, liposome that contains phospholipid bilayer was applied to fuse with erythrocyte membrane (28, 29). The fusion drug delivery not only retained the excellent biocompatibility and targeting spleen ability of erythrocyte membrane but also overcame the drug loading defect (30). At the same time, liposome as the drug carrier appears frequently in clinical treatment in recent years, which means that it has advantageous clinical transformed potentiality (31, 32). The pair of mannose and mannose receptors can recognize and capture antigen molecules, promote antigen presentation, and regulate the maturation and differentiation of immune cells (33–35). Thus, we prepared mannose-modified liposomes to encourage the combination with APCs and stimulate maturation so as to activate the specific immunity of the body. Furthermore, the nanovaccine can protect the antigen from rapid degradation and elimination by the body to effectively prolong the action time of antigen (36, 37) and targeted the delivery of antigen to lymphoid tissue (38, 39). It can also achieve the simultaneous interpretation of tumor antigen and immunomodulator, emerging synergistic immune stimulation (40, 41).
In this study, we report that a robust nanovaccine based on erythrocyte membrane fused with mannose-liposome and wrapping iPSC protein, iPSC@RBC-Mlipo, can adequately accumulate at the spleen, positively integrate with APCs through the mannose receptor, and elicit antitumor immunity. Subsequently, the mature APCs activated lymphocytes through antigen cross-presentation and then attacked cancer cells. Results showed that the vaccine could effectively activate the immune system and inhibit tumor growth, metastasis, and recurrence. Overall, iPSC@RBC-Mlipo could be an efficient versatile tumor vaccine, holding the prospect of clinical application in cancer therapy.
RESULTS
Synthesis and characterization of iPSC@RBC-Mlipo
To construct the nanovaccine iPSC@RBC-Mlipo, erythrocyte membrane was used to decorate mannose-modified liposomes and then loaded with iPSC protein (Fig. 1A). Transmission electron microscopy (TEM) images revealed that the iPSC@RBC-Mlipo nanocomposites that contained iPSC protein formed stable nanoparticulate vehicles and displayed more complex morphology (Fig. 1B). The hydrodynamic diameter of liposome increased from 125 to 140 nm after fusion and further increased to 184 nm with the loading of iPSC protein, and the nanovaccine presented lower potential (4.7 mV) than M-liposome (13.9 mV) for binding negative-potential erythrocyte membrane and wrapping negatively charged iPSC protein (Fig. 1C). To further prove that M-liposome was fused with erythrocyte membrane, colocalization of green fluorescence representing erythrocyte membrane with intrinsic red fluorescence representing M-liposome was observed after fusion operations compared with free mixture group (Fig. 1D), and the nanovaccine iPSC@RBC-Mlipo appeared with the same colocalization (fig. S1). Flow cytometry also revealed the same results by observing the community gathered (Fig. 1E). SDS–polyacrylamide gel electrophoresis (SDS-PAGE) verified that the nanovaccine iPSC@RBC-Mlipo had similar protein expression with iPSC protein and erythrocyte membrane (Fig. 1F). Compared with erythrocyte membrane, the fusion vector RBC-Mlipo appeared with higher protein loading capacity (PLC) and protein loading efficiency (PLE) (fig. S2). Last, the empty carrier RBC-Mlipo and the nanovaccine iPSC@RBC-Mlipo owned a small size fluctuation in fetal bovine serum (FBS) or phosphate-buffered saline (PBS), respectively, proving the stability of nanocomposite in the internal environments (Fig. 1G and fig. S3).
Fig. 1. Synthesis and characterization of iPSC@RBC-Mlipo.
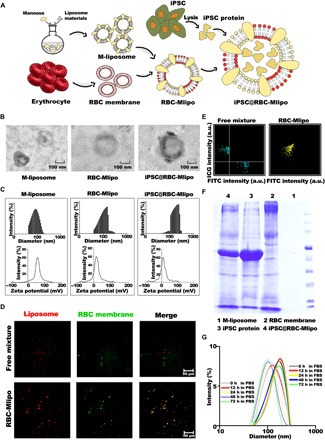
(A) Schematic of preparation of iPSC@RBC-Mlipo by fusing erythrocyte membrane with M-liposome and then packaging iPSC protein. (B) TEM images of M-liposome, RBC-Mlipo, and iPSC@RBC-Mlipo. Scale bars, 100 nm. (C) Hydrated particle size and zeta potential of M-liposome, RBC-Mlipo, and iPSC@RBC-Mlipo. a.u., arbitrary unit. (D) Confocal laser scanning microscopy images of group 1 untreated mixture of erythrocyte membrane and M-liposome and group 2 of RBC-Mlipo. Red for rhodamine-labeled M-liposome; green for fluorescein isothiocyanate (FITC)–anti-MPP1/EMP55 (erythrocyte membrane protein 55–specific antibody)–labeled erythrocyte membrane. Scale bars, 50 μm. (E) Flow cytometric analysis of distribution of nanoparticles to prove the effective fusion of liposome and erythrocyte membrane. (F) SDS-PAGE pattern of proteins from (1) M-liposome, (2) red blood cell (RBC) membrane, (3) iPSC protein, and (4) iPSC@RBC-Mlipo lysates. (G) Size changes of iPSC@RBC-Mlipo nanoparticles in FBS and PBS to prove stability.
APCs activation by iPSC@RBC-Mlipo
The ability of nanocomposites to combine and stimulate APCs in spleen tissue is crucial to light the “switch” of adaptive immunity (Fig. 2A). Colocalization of fluorescent signals was observed after coincubation with macrophages; compared with liposomes, mannose-modified liposomes were easier to be absorbed by macrophages and so was the fusion vector RBC-Mlipo. However, the ingestion of macrophages to nanocomposites weakened when macrophages were first incubated with mannose, which blocked the mannose receptors on the macrophage surface (Fig. 2B and fig. S4). Flow cytometry analysis also indicated the same results that mannose modification promoted ingestion and mannose block weakened this specific binding, which may explain the mechanism that mannose receptor mediates internalization of nanovaccine to further promote the maturation and differentiation of APCs (Fig. 2C). Colocalization of nanovaccine and macrophage lysosome further confirmed that APCs absorbed antigen via mannose-lysosome pathway and presented it to downstream cells after lysosome treatment (fig. S5). According to Fig. 2E, the high expression of SIINFEKL/H-2Kb on the APCs stimulated by nanovaccine elucidated the antigen cross-presentation by dendritic cells (DCs) to T lymphocytes via the peptide major histocompatibility complex I (pMHCI) complex/T cell receptor signal. MicroRNA-155 (miRNA-155), an indicator of APC maturation and differentiation after antigen capture, was 5.05 times higher or 3.67 times higher in the iPSC@RBC-Mlipo group than that in blank macrophages or DCs, respectively (Fig. 2D). Meanwhile, the morphological changes of macrophages were recorded to confirm APC maturation and differentiation (Fig. 2F). Macrophages change from spherical to spindle shaped, elongated, and dendritic after treatment with groups. Together, iPSC@RBC-Mlipo had the superior ability of promoting APC recruitment, maturation, and antigen cross-presentation.
Fig. 2. iPSC@RBC-Mlipo target and stimulate APC maturation and differentiation.
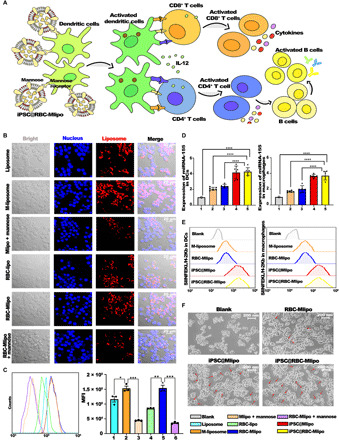
(A) Schematic of immune-stimulating effect of iPSC@RBC-Mlipo by targeting DCs and stimulating their differentiation. IL-12, interleukin-12. (B) Confocal laser scanning microscopy images of macrophages treated respectively. Blue for Hoechst labeled macrophage nucleus; Red for rhodamine-labeled liposome. Scale bars, 20 μm. (C) Flow cytometric analysis of cellular uptake of nanoparticles and the quantified intracellular fluorescence uptake level calculation. 1, liposome; 2, M-liposome; 3, Mlipo + mannose; 4, RBC-lipo; 5, RBC-Mlipo; and 6, RBC-Mlipo + mannose. MFI, mean fluorescence intensity. (D) Using SYBR Green quantitative polymerase chain reaction to analyze the expression quantity of miRNA-155 in DCs and macrophages to show differentiation degree. 1, blank; 2, M-liposome; 3, RBC-Mlipo; 4, iPSC@Mlipo; and 5, iPSC@RBC-Mlipo. (E) Antigen cross-presentation in DC2.4 cells and macrophages coincubated with groups. 1, blank; 2, M-liposome; 3, RBC-Mlipo; 4, iPSC@Mlipo; and 5, iPSC@RBC-Mlipo. (F) Morphological changes of macrophages before and after nanoparticles were treated. The red arrow points to the differentiated macrophages. Scale bars, 200 nm. Data were expressed as means ± SEM. Statistical significance was calculated by one-way analysis of variance (ANOVA) with Tukey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001.
Biodistribution and durable immune response elicitation of iPSC@RBC-Mlipo in vivo
The biodistribution of iPSC@Mlipo, RBC-Mlipo, and iPSC@RBC-Mlipo were analyzed in Institute of Cancer Research (ICR) mice, respectively. Compared with the iPSC@Mlipo group, with the fusion of erythrocyte membrane, the fusion vector RBC-Mlipo and the nanovaccine iPSC@RBC-Mlipo emerged with obvious accumulation in the spleens 2 hours after intravenous injection, and the fluorescence signal can be continuously monitored within 10 hours (Fig. 3A and fig. S6). The dissected spleens in group RBC-Mlipo and iPSC@RBC-Mlipo appeared with higher fluorescence intensity 6 hours after tail vein injection (Fig. 3, B and D), and comparative analysis presented that iPSC@RBC-Mlipo displayed threefold higher spleen distribution than that of the free iPSC@Mlipo (Fig. 3C). At the same time, a little fluorescent signal was also detected in the liver and kidney as the main metabolism route of the nanoparticles (Fig. 3, B and D). However, unlike the limited diffusion and quick clearance of other groups, the nanovaccine facilitated deep spleen penetration, diffused throughout the whole areas of the spleen, and showed a long-time retention (fig. S7).
Fig. 3. Biodistribution and immune-stimulating ability of iPSC@RBC-Mlipo in vivo.
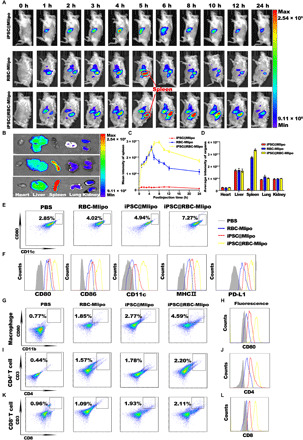
(A) Fluorescence imaging of ICR mice for hours after intravenous injection of group 1 iPSC@Mlipo, group 2 RBC-Mlipo, and group 3 iPSC@RBC-Mlipo. The fluorescent signal is expressed in the form of radiant efficiency. (B) Fluorescence imaging of dissected organs 6 hours after intravenous injection. (C) Mean fluorescence intensity of the spleen at different time points in vivo after treatment with three groups. (D) Average fluorescence intensity of dissected organs 6 hours after intravenous injection. (E) Flow cytometric analysis of activated splenic DCs 12 hours after treatment with groups. (F) Fluorescence of various maturation markers expressed on the surface of splenic DCs 12 hours after treatment with groups. Flow cytometric analysis of activated macrophages (G), CD4+ T cells (I), and CD8+ T cells (K). (H, J, and L) Fluorescence of various activation markers expressed on splenic immune cells 12 hours after intravenous injection of groups. Data were expressed as means ± SEM. Statistical significance was calculated by one-way ANOVA with Tukey’s post hoc test.
DCs play crucial roles in initiating and regulating the antitumor immune response by presenting tumor antigens to T lymphocytes (42). Flow cytometry analysis measured the frequency of matured DCs and demonstrated that the nanovaccine iPSC@RBC-Mlipo markedly accelerated DC maturation in vivo (Fig. 3, E and F, and fig. S8). It also expressed high antigen cross-presentation ability (fig. S9). We further evaluated the systemic immune response induced by DC maturation by analyzing the changes of immune cells from spleen and peripheral blood after treatment. Macrophages, CD4+ T cells, and CD8+ T cells in the spleen were expanded and activated by the nanovaccine iPSC@RBC-Mlipo compared with other groups (Fig. 3, G to L, and fig. S10). The obvious increase of immune cells in peripheral blood indicated that splenic immune cells could extend to peripheral blood after being vaccinated by iPSC@RBC-Mlipo (fig. S11), along with the elevated secretion of the relating cytokines and immunoglobulin G (IgG) in serum (fig. S12). We also proved the safety of the empty carrier RBC-Mlipo and the nanovaccine iPSC@RBC-Mlipo for no organ toxicity (fig. S13) and the normal physiological and biochemical indexes (tables S1 and S2). To sum up, all results in this part confirmed that the nanovaccine iPSC@RBC-Mlipo had the potential of the specific spleen targeting, accurate distribution, and specific immunity elicitation of the nanovaccine iPSC@RBC-Mlipo.
Antitumor effect of the nanovaccine iPSC@RBC-Mlipo
First, the antitumor effect was tested by setting B16F10 tumor–bearing mice model after being treated with the nanovaccine iPSC@RBC-Mlipo twice (Fig. 4A). Compared with PBS group, the nanovaccine iPSC@RBC-Mlipo prolonged the survival of 40% mice by day 100 with no obvious weight change (Fig. 4C and fig. S15) and lead to puissant tumor suppression (Fig. 4, D and E). Tumors were dissected after sacrifice (Fig. 4H and fig. S14), and tumor weights in different groups appear with significant differences (Fig. 4B). Immunofluorescence confirmed that the nanovaccine iPSC@RBC-Mlipo–mediated immunotherapy significantly raised the frequency of infiltrating CD4+ T cells and CD8+ T cells in tumor tissues (Fig. 4F). TUNEL [terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate (DUTP) nick end labeling] assay performed on tumor sections presented significant apoptosis and necrosis of the tumor cells in the group treated with the nanovaccine iPSC@RBC-Mlipo (Fig. 4G), indicating that sufficient immune infiltration led to the disruption of the established tumors. Meanwhile, histological stained analysis with hematoxylin and eosin (H&E) (Fig. 4I) and Ki67 (cell proliferation–related proteins) (Fig. 4J) of tumor slides further confirmed that the nanovaccine iPSC@RBC-Mlipo decreased the malign grade of tumor tissue.
Fig. 4. Preventive antitumor effect of the nanovaccine iPSC@RBC-Mlipo.
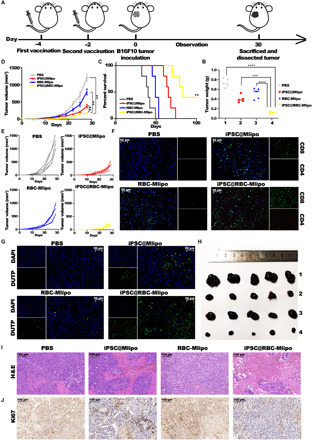
(A) Schematic illustration of setting a B16F10 tumor model after vaccinating different groups twice. (B) Dissected tumors weights in groups. (C) Survival curves, (D) average tumor volume curves, and (E) individual tumor growth kinetics in different groups (n ≥ 5). (F) Immunofluorescence images of tumor slides to present CD4+ T cells and CD8+ T cells infiltrating into the tumor tissues. Red for CD4 antibody, green for CD8 antibody, and blue for 4′,6-diamidino-2-phenylindole (DAPI)–labeled nucleus. Scale bars, 50 μm. (G) Fluorescence TUNEL analysis of apoptosis in the tumor sections after vaccinating different groups. Scale bars, 50 μm. (H) Dissected tumors in groups 1, PBS; 2, iPSC@Mlipo; 3, RBC-Mlipo; and 4, iPSC@RBC-Mlipo. (I) Images of H&E-stained and (J) Ki67-stained tumor slides after vaccinating different groups. Scale bars, 50 μm. Data were expressed as means ± SEM. Statistical significance was calculated by one-way ANOVA with Tukey’s post hoc test. **P < 0.01, ***P < 0.005, and ****P < 0.001.
At the same time, we investigated the therapeutic effect of nanovaccine combined with immunosuppressive checkpoint inhibitor aPD-1 (anti–programmed cell death protein 1) on preventing postoperative tumor recurrence. The nanovaccine iPSC@RBC-Mlipo and aPD-1 were injected intravenously after H22 tumor model was removed by surgery (fig. S16A). As shown in fig. S16 (B to E), compared with other groups, the nanovaccine iPSC@RBC-Mlipo combined with aPD-1 treatment controlled the recurrence of the postoperative tumor, extended mice survival dates, and exhibited obvious tumor structural failure (fig. S16F). Together, these results proved the efficient antitumor ability of the nanovaccine iPSC@RBC-Mlipo, providing valuable insights for treating broad types of solid tumors.
Inhibition of tumor metastasis by the nanovaccine iPSC@RBC-Mlipo
To analyze the antimetastatic potential of the nanovaccine iPSC@RBC-Mlipo, 4T1-luc cells were intravenously injected into mice to mimic the metastasis of cancer cells after iPSC@RBC-Mlipo was vaccinated twice (Fig. 5A). Demonstrated by the bioluminescence imaging in vivo, mice treated with PBS presented obvious cancer metastasis 7 days after 4T1-luc cell injection and so were the iPSC@Mlipo group and RBC-Mlipo group. In contrast, mice vaccinated with iPSC@RBC-Mlipo showed the weaker fluorescence signal and elucidated that it could further delay the tumor growth and metastasis (Fig. 5B). Meanwhile, fluorescence quantitative analysis also verified the significant difference between PBS group and iPSC@RBC-Mlipo group. The survival rate was 60% for mice treated with the nanovaccine iPSC@RBC-Mlipo by 4 weeks (Fig. 5D), and there were no notable changes in body weight (Fig. 5E). In contrast, all the mice in the other groups wasted away and almost died in 21 days. Furthermore, the nanovaccine also increased the frequency of cytokines secreted by several immune cells (Fig. 5F). The antimetastatic effect can also be demonstrated from fewer pulmonary tumor nodules and more normal lung volumes after sacrifice. H&E staining revealed the same results by comparison of pulmonary nodule structure differences in groups (Fig. 5G). To sum up, the nanovaccine iPSC@RBC-Mlipo had excellently preventive antitumor metastatic effect due to the systemic antitumor immunity.
Fig. 5. Preventive antimetastatic effect of the nanovaccine iPSC@RBC-Mlipo.
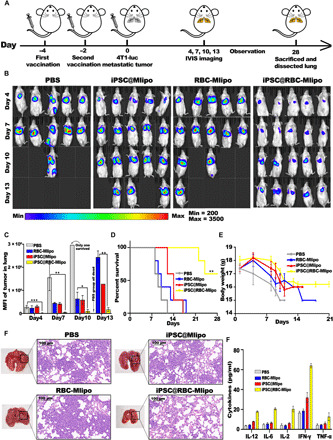
(A) Schematic illustration of setting a 4T1-luc pulmonary metastasis model after vaccinating different groups twice. (B) In vivo bioluminescence imaging of 4T1-luc lung metastasis in different groups. The fluorescent signal is expressed in the form of counts. (C) Mean fluorescence intensity of tumor in lungs. (D) Body weights and (E) survival curves in different groups (n ≥ 5). (F) Cytokines levels in serum after treatment with groups. (G) Dissected lungs in groups and images of H&E-stained lung slides after vaccinating different groups. Scale bars, 50 μm. All data were expressed as means ± SEM. Statistical significance was calculated by one-way ANOVA with Tukey’s post hoc test. *P < 0.05, **P < 0.01, and ***P < 0.005. IFN-γ, interferon-γ; TNF-α, tumor necrosis factor–α.
DISCUSSION
As an important lymphoid organ that produced immunological effect to protect the body, the spleen contains a large number of immune cells, cytokines, and other immune substances, which can be secreted into peripheral blood to directly attack tumor cells and tissues. Therefore, targeting and stimulating severe immune response in the spleen is a feasible way to develop efficient antitumor means. Previous studies have well established that an aged or damaged erythrocyte membrane was easily blocked by the molecular sieve structure of spleen tissue because of its loss of deformability, which can achieve the goal of spleen targeting. At the same time, as an excellent carrier with inherent biocompatibility, the erythrocyte membrane is an inherent component of the body, which is not blocked by the endothelial network system so as to achieve the purpose of long circulation. Moreover, the erythrocyte membrane is easy to obtain, so it has a broad clinical application prospect. Therefore, we use erythrocyte membrane as an antigen carrier, which can effectively transport tumor antigen to the spleen and effectively induce antitumor immunity.
However, as a drug carrier, the application of erythrocyte membrane is limited by the low drug loading ability. To address the challenges, liposome was herein used to fuse with erythrocyte membrane as the similar phospholipid bilayer structure; the fused membrane significantly improved drug loading ability. Mannose-modified liposomes can target the mannose receptor family on the surface of APCs and effectively induce stimulation of the maturation of APCs such as DCs and macrophages, thus turning on the switch of immune function. At the same time, mannose modification could reverse the decrease of internalization caused by erythrocyte membrane, which will lead to immune evasion as an inherent component of the body. In Fig. 2 (D and E), the effect of group M-liposome was similar to that of RBC-Mlipo, indicating that the immunostimulatory effect was mainly produced by mannose-modified liposomes rather than RBC. The empty vector RBC-Mlipo holds immune evoking effect and antitumor ability due to the stimulating effect of mannose on APCs. In cell experiment, the immune effect of erythrocyte membrane is not obvious, but it could mediate the aggregation of nanovaccine in the spleen and produces efficient acquired immunity in vivo. From tumor inhibition tests in vivo (Figs. 4 and 5), although iPSC@Mlipo has the tumor inhibitory effect in some degree, it is far from that of the iPSC@RBC-Mlipo group, which could target aggregation in the spleen due to the erythrocyte membrane. As an excellent drug carrier, the fusion nanosystem not only keeps the biological function of targeting spleen but also presents well biocompatibility, high drug loading ability, and low toxicity, so it has a wide range of clinical application prospects.
iPSCs have been widely used in organ transplantation and other fields in recent years. Recently, researchers confirmed the common epitopes between iPSCs and cancer cells through the “two-way immunization” experiment, and iPSCs shared a larger cancer-related epitope library, indicating that autologous iPSCs can provide more accurate and representative tumor-related antigens. Therefore, the nanovaccine based on iPSCs as tumor antigen can provide a large number of tumor antigens for the immune system so as to establish a wide-spectrum antitumor immunity for a variety of cancer types. At the same time, it overcomes the defect of difficult-to-obtain tumor antigen for patients in current research, so it has the potential of clinical application as tumor vaccine. However, the vaccine prepared with intact iPSCs present several defects such as tumorigenicity, easily intercepted because of large size. Therefore, we chose iPSC protein to overcome the potential safety hazard and interception.
We used the fusion of erythrocyte membrane and mannose-modified liposome and encapsulated iPSC protein to prepare the antitumor nanovaccine (Fig. 6). However, the empty carrier RBC-Mlipo and the nanovaccine iPSC@RBC-Mlipo showed different retention time in the spleen; we concluded two reasons such as the following: (i) The hydration particle size of the empty RBC-Mlipo group was about 140 nm, and after protein inclusion, the hydration particle size of nanoparticle iPSC@RBC-Mlipo increased to 184 nm (Figure 1C). The larger-sized nanoparticles are trapped by the dense structure of splenic tissue, leading to slower metabolism. (ii) Compared with the empty RBC-Mlipo group, the vaccine iPSC@RBC-Mlipo group that contained the iPSC protein as tumor-associated antigen recruited more immunocytes to produce a series of immune reactions. Sufficient immune cells increased the uptake of the antigen, so fluorescent signals are continuously monitored. Therefore, slow metabolism and more uptake led to longer duration of fluorescence signal together.
Fig. 6. Schematic illustration of the nanovaccine design and the immune process in vivo.
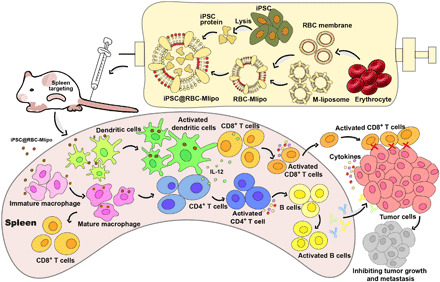
Our work confirmed that the nanovaccine iPSC@RBC-Mlipo could sufficiently prevent B16F10 tumor and 4T1 lung metastasis tumor. In combination with immunosuppressive checkpoint inhibitors, nanovaccine can effectively inhibit tumor recurrence after surgery, making it both preventive and therapeutic, thus enriching the clinical application forms. In view of the wide-spectrum antitumor properties of the nanovaccine, and the whole-body scale antitumor effect, we believe that it could be further explored on the prevention and treatment of malignant leukemia and a broad diversity of solid tumors in future research.
In summary, we used the mannose-modified liposome to dilate erythrocyte membrane and encapsulated iPSC protein to prepare the antitumor nanovaccine, which can target and stimulate spleen APCs to mature and produce high-efficiency antitumor immune response. It can inhibit the generation of primary tumors, attack metastatic tumors, and effectively prevent tumor recurrence after surgery, achieving preventive and therapeutic functions. Therefore, our study proposed a nanovaccine that can produce systemic extensive antitumor immunity for a variety of cancer types. Considering the availability of materials and versatility of nanovaccine, our strategy is emerging as a promising approach for cancer prevention and immunotherapy.
MATERIALS AND METHODS
Materials
The phospholipid 1,2-dihexadecanoyl-snglycero-3-phosphocholine (DPPC) was purchased from A.V.T (Shanghai). Cholesterol, distearoyl phosphatidylethanolamine polyethylene glycol 2000 (DSPE-PEG2000), dimethyldodecyldimethylammonium bromide (DDAB), mannosamine, palmitic acid, and dicyclohexylcarbodiimide (N,N′-dicyclohexylcarbodiimide) were purchased from Shanghai Yuanye Bio-Technology Co. Ltd., and α-tocopherol was purchased from Beijing Solebao Technology Co. Ltd. Fluorescein isothiocyanate (FITC)–anti-membrane protein palmitoylated 1 (MPP1)/EMP55 (erythrocyte membrane protein 55–specific antibody) was purchased from Shanghai Kelei Biotechnology Co. Ltd. Fluorescent labeled antibiotics against cell surface markers for flow cytometry (fluorescent activated cell sorting) asset were purchased from eBioscience.
Cell
iPSCs were purchased from Anhui Zhongsheng Traceability Biotechnology Co. Ltd. with prepared iPSC medium, working solution, and resuscitation medium for auxiliary culture. Cells were derived from peripheral blood, induced by the company’s non-integrated reprogramming technology, without exogenous gene insertion, with normal differentiation ability and normal chromosome karyotype. All cell lines used in the experiment were purchased from the American Type Culture Collection (Shanghai). The cells used in this experiment were cultured in the Biomedical Engineering Laboratory of China Pharmaceutical University at 37°C and 5% CO2.
Animals
ICR, BALB/c mice (6 to 8 weeks) were purchased from the Nanjing Qinglongshan animal farm. Mice were treated under protocols approved by the Institutional Animal Care and Use Committee.
Synthesis of the nanovaccine iPSC@RBC-Mlipo
Mannose-modified lipid was prepared by ultrasonic film dispersion method. Weigh palmitic acid:mannosamine:DCC = 1:1:1 into a round bottom flask. Add appropriate amount of dimethyl sulfoxide to dissolve the solid and shake for 5 hours in a water bath at 37°C and 200 rpm. After centrifuging at 3000 rpm for 10 min, 5 ml of distilled water was added to the supernatant, and the precipitate was collected by vacuum filtration. After drying, the precipitate was dissolved with an appropriate amount of chloroform. The solvent in the filtrate was removed by rotary evaporation and dried sufficiently. The molar ratio is DPPC:cholesterol:DSPE-PEG2000:DDAB:mannose lipid:α-tocopherol = 50:40:5:3:2:0.2, and it was placed in a round bottom flask, fully dissolved with chloroform, rotated at 55°C, 40 rpm for 30 min, and then evaporated for 30 min to form a uniform liposome membrane. The liposome dispersion system was prepared by adding PBS after being dried with nitrogen for 15 min, rotated at 55°C at 40 rpm for 30 min, and extruded 20 times at 400 nm and 20 times at 200 nm by liposome extruder.
The membrane vesicles of red blood cells were extracted by hypotonic burst method. After the whole blood of mice was centrifuged and washed to obtain pure red blood cells, suspended red blood cells were precipitated in 3× volume of red blood cells precooled with hypotonic normal saline and then stood for 25 min at 4°C for cell burst. Repeat centrifugation at 4°C at 12,000 rpm for 10 min was done five times until the supernatant was clear. The lower precipitation was suspended with precooled isotonic saline, and the vesicles were extruded 20 times at 400 nm and 20 times at 200 nm, respectively, to obtain erythrocyte membrane vesicles.
Ultrasonic extrusion was used for membrane fusion. The erythrocyte membrane was mixed with liposome in the ratio of 1:1, and then, it was completely suspended in ice water bath at 100 W. Ultrasonic treatment was conducted for 30 min until the system became slightly transparent. The system was placed in a water bath at 37°C and shaken for 1 hour. The RBC-Mlipo fusion membrane was obtained by extrusion 20 times at 400 nm and 20 times at 200 nm.
Extraction of total cell protein
The cells were resuspended with PBS and centrifuged after digestion, mixed with lysate solution [lysate buffer + phosphatase inhibitor + protease inhibitor + phenylmethanesulfonylfluoride (PMSF)], and then lysed on ice for 30 min. The cells lysates were centrifuged at 4°C at 12,000 rpm for 15 min. Bicinchoninic acid (BCA) protein quantitative method was used for protein quantification. A BCA protein quantitative kit was purchased from Thermo Fisher Scientific.
The tumor vaccine was prepared by mechanical coextrusion. The fusion membrane was centrifuged at 10,000 rpm for 5 min, and the precipitate in the lower layer was resuspended with PBS. The PBS solution of iPSC protein was added. After 100-W ultrasound for 20 min, the liposome extruder was used 20 times at 400 nm and 20 times at 200 nm to obtain the tumor vaccine iPSC@RBC-Mlipo. The concentration of iPSC protein in iPSC@RBC-Mlipo nanovaccine was about 3.5 μg/μl. Each mouse was vaccinated twice with 200 μl each time in every model.
iPSC@RBC-Mlipo characterization
The morphology of the composite was characterized by TEM, and the material was pretreated by phosphotungstic acid negative staining. The hydration radius and potential of nanoparticles were measured by dynamic light scattering method in the following environment: refractive index, 1.440 and medium viscosity, 0.80 centipoise; the measurement system including control PBS solution, measurement material, measurement environment, and so on need to be balanced at 25°C for 5 min.
PLC and PLE were used to evaluate the protein loading capacity of M-liposome, erythrocyte membrane, and RBC-Mlipo
Dialysis method was used to measure the protein release curve. Phosphate buffer solution with different pH was used as the release medium. Peripheral buffer solution (1 ml) was taken at the set time point, and the same amount of blank buffer was added in time. Protein content was determined by BCA protein quantitative method.
Confocal imaging
For membrane fusion and cell uptake experiments, all erythrocyte membrane was labeled with EMP55 (FITC–anti-MPP1/EMP55), APC nucleus was labeled with Hoechst, and liposome was labeled with rhodamine. After coincubation, each dish was washed three times. The results were observed by inverted fluorescence microscopy at 430, 480, and 530 nm. The standard curve of fluorescence concentration of rhodamine-labeled liposomes was used to keep the same dosage for each group.
For immunofluorescence images of CD4+ and CD8+ T cell infiltration and fluorescence TUNEL analysis of apoptosis in the tumor slides, phycoerythrin (PE)–labeled CD4 antibody and FITC-labeled CD8 antibody were used to treat the tumor slides, 4′,6-diamidino-2-phenylindole was used to label the nucleus, and DUTP was used to label nucleic acids of apoptotic cells.
Quantitative polymerase chain reaction
For analysis of miRNA-155 expression in DCs and macrophages, the primers and small interfering RNA used were purchased from Sangon Biotechnology Co. Ltd., Shanghai. The primer sequences were as follows: sense (5′-3′), TACactCCAGCTGGGTTAATGCTAATCGTG and antisense (5′-3′), CTCAACTGGTGTCGTGGAGT. The polymerase chain reaction amplification incubator and quantitative polymerase chain reaction fluorescence quantitative analyzer used were from Thermo Fisher Scientific.
Flow cytometry
For characterization of the nanovaccine iPSC@RBC-Mlipo and the uptake of nanovaccine by cells in vitro, the double dye–labeled RBC-Mlipo was prepared by FITC–anti-MPP1/EMP55–labeled erythrocyte membrane and rhodamine-labeled liposome. APC-SIINKEKL/H-2Kb was used to analyze the expression of SIINFEKL/H-2Kb on the APCs. The coincubation time of cells and nanoparticles was 4 hours.
For evaluating the immune stimulation effect of the vaccine in vivo, the mice were randomly divided into three groups: M-liposome, RBC-Mlipo, and vaccine iPSC@RBC-Mlipo. Three hours later, Peripheral blood mononuclear cell (PBMC) was extracted from peripheral blood of mice, and the spleen was dissected to prepare the single-cell suspension. The DCs of PBMC and spleen cells were analyzed by PE-CD80, PE-CD86, FITC-CD11c, PE-MHCII, and PE–PD-L1 (programmed cell death ligand 1). Antigen cross-presentation was analyzed by APC-SIINKEKL/H-2Kb. Immunocytes were analyzed by PE-CD80, FITC-CD11c, FITC-CD4, and PE-CD8.
Distribution in vivo and analysis of spleen fluorescence signal
The liposomes were labeled with near-infrared dye indocyanine green (ICG) to prepare ICG-Mlipo, ICG–RBC-Mlipo, and ICG–iPSC@RBC-Mlipo. IVIS (In Vivo Imaging System) spectral imaging system was used at 0, 1, 2, 3, 4, 5, 6, 8, 10, 12, and 24 hours after injection. The exposure time of bioluminescence imaging was 5 min.
Cytokine detection
Serum samples of each group were collected and diluted for analysis after the different treatments. IgG antibody, interleukin-12P70 (IL-12P70), IL-6, IL-2, interferon-γ (IFN-γ), and tumor necrosis factor–α (TNF-α) cytokine levels were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s protocols. All the test kits were purchased from Thermo Fisher Scientific.
Construction of tumor model
In the B16F10 solid tumor model, mice were weighed and randomly divided into different groups (n ≥ 5). The mice in each group were injected with the same dose of different vaccine twice before subcutaneously injecting B16F10 (1 × 106). To analyze antitumor ability via tumor model, the tumors were measured with a digital caliper. Three weeks later, we dissected mice to obtain the tumor and measure the immune status of the tumor site and detected the tumor tissue by H&E and Ki67 staining.
In the metastatic model, 4T1-luc cells (1 × 106) were intravenously injected into mice to establish lung metastasis model after vaccination and randomly divided into different groups (n ≥ 5). An IVIS bioluminescence imaging system was used to monitor cancer cells. Three weeks later, we dissected mice to obtain the tumor in lungs and detected the tumor tissue by H&E staining. Serum samples of each group were collected and diluted for analysis after the different treatments. IL-12P70, IL-6, IL-2, IFN-γ, and TNF-α levels were measured by ELISA kits according to the manufacturer’s protocols.
In the recurrence model, H22 cells (1 × 106) were subcutaneously injected into the left flank of BALB/c mice and grew for 2 weeks. Then, we surgically removed the cancer, vaccinated twice, and injected anti-PD-1 antibody (5 mg/kg) on days 9, 12, 15, and 18 via the tail vein. The recurrence tumors were measured by digital caliper and detected by H&E staining. The ellipsoid formula V = π/6 × L × W2 was used to evaluate all tumor volumes.
Statistical analysis
All results were expressed as means ± SEM or SD. Single-factor analysis of variance (ANOVA) was used to analyze multiple groups. The significance was determined by Student’s t test and Tukey’s posttest in normal test. All statistical analyses were performed using GraphPad Prism software, and the statistical significance was set as *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001.
Acknowledgments
Funding: We are grateful to National Natural Science Foundation of China (NSFC 81971743, 81801835, 81827803, 8172900016, 81727804, and 91859204) and project supported by the Natural Science Foundation of Jiangsu Province, China (BK20200083) for their financial support. Author contributions: Y.Z. and X.H. contributed equally to this work. Y.Z. and S.L. conceived the project. Y.Z., X.H., Y.L., R.H., and Y.M. performed the experiments and analyzed the results. P.G., Z.Q. and Y.G. provided useful suggestions to this work. Y.Z. and S.L. wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/35/eabi6326/DC1
REFERENCES AND NOTES
- 1.Yang Y., Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Invest. 125, 3335–3337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riley R. S., June C. H., Langer R., Mitchell M. J., Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia A. L., Xu Y., Lu X. J., Cancer immunotherapy: Challenges and clinical applications. J. Med. Genet. 56, 1–3 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Chung C. K., Da Silva C. G., Kralisch D., Chan A., Ossendorp F., Cruz L. J., Combinatory therapy adopting nanoparticle-based cancer vaccination with immune checkpoint blockade for treatment of post-surgical tumor recurrences. J. Control. Release 285, 56–66 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Li J., Luo Y., Li B., Xia Y., Wang H., Fu C., Implantable and injectable biomaterial scaffolds for cancer immunotherapy. Front. Bioeng. Biotechnol. 8, 612950 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Z., Zheng L., Chen W., Weng W., Song J., Ji J., Delivery strategies of cancer immunotherapy: Recent advances and future perspectives. J. Hematol. Oncol. 12, 126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng M., Mo Y., Wang Y., Wu P., Zhang Y., Xiong F., Guo C., Wu X., Li Y., Li X., Li G., Xiong W., Zeng Z., Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 18, 128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahin U., Türeci Ö., Personalized vaccines for cancer immunotherapy. Science 359, 1355–1360 (2018). [DOI] [PubMed] [Google Scholar]
- 9.S. Thomas, G. C. Prendergast, in Methods in Molecular Biology (Humana Press Inc., 2016), vol. 1403, pp. 755–761. [Google Scholar]
- 10.Patel A., Kaufman H. L., Disis M. L., Next generation approaches for tumor vaccination. Chin. Clin. Oncol. 6, 19 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Bowen W. S., Svrivastava A. K., Batra L., Barsoumian H., Shirwan H., Current challenges for cancer vaccine adjuvant development. Expert Rev. Vaccines 17, 207–215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondo Y., Toyoda T., Inagaki N., Osafune K., iPSC technology-based regenerative therapy for diabetes. J. Diabetes Investig. 9, 234–243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.K. Christensen, F. Roudnicky, C. Patsch, M. Burcin, in Advances in Biochemical Engineering/Biotechnology (Springer Science and Business Media Deutschland GmbH, 2018), vol. 163, pp. 207–220. [DOI] [PubMed] [Google Scholar]
- 14.Malik N., Rao M. S., A review of the methods for human iPSC derivation. Methods Mol. Biol. 997, 23–33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kooreman N. G., Kim Y., de Almeida P. E., Termglinchan V., Diecke S., Shao N. Y., Wei T. T., Yi H., Dey D., Nelakanti R., Brouwer T. P., Paik D. T., Sagiv-Barfi I., Han A., Quax P. H. A., Hamming J. F., Levy R., Davis M. M., Wu J. C., Autologous iPSC-based vaccines elicit anti-tumor responses in vivo. Cell Stem Cell 22, 501–513.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaddanapudi K., Li C., Eaton J. W., Vaccination with induced pluripotent stem cells confers protection against cancer. Stem Cell Investig. 5, 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hailemichael Y., Singh M., Overwijk W., Vaccinating with stem cells to stop cancer. Trends Mol. Med. 24, 524–526 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Bernardes de Jesus B., Neves B. M., Ferreira M., Nóbrega-Pereira S., Strategies for cancer immunotherapy using induced pluripotency stem cells-based vaccines. Cancers 12, 3581 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell A., Wanczyk H., Jensen T., Finck C., Human induced pluripotent stem cells ameliorate hyperoxia-induced lung injury in a mouse model. Am. J. Transl. Res. 12, 292–307 (2020). [PMC free article] [PubMed] [Google Scholar]
- 20.Uchi S.-H., Yanai R., Kobayashi M., Hatano M., Kobayashi Y., Yamashiro C., Nagai T., Tokuda K., Connor K. M., Sonoda K. H., Kimura K., Dendritic cells mediate the anti-inflammatory action of omega-3 long-chain polyunsaturated fatty acids in experimental autoimmune uveitis. PLOS ONE 14, e0219405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbins J. D., Ancelet L. R., Osmond T. L., Petersen T. R., Hermans I. F., Splenic dendritic cells involved in cross-tolerance of tumor antigens can play a stimulatory role in adoptive T-cell therapy. J. Immunother. 38, 321–329 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Dogra P., Rancan C., Ma W., Toth M., Senda T., Carpenter D. J., Kubota M., Matsumoto R., Thapa P., Szabo P. A., Li Poon M. M., Li J., Arakawa-Hoyt J., Shen Y., Fong L., Lanier L. L., Farber D. L., Tissue determinants of human NK cell development, function, and residence. Cell 180, 749–763.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd D. M., Strickland A. D., Surgery of the spleen. Surgery 28, 229–233 (2010). [Google Scholar]
- 24.Li H., Lu L., Li X., Buffet P. A., Dao M., Karniadakis G. E., Suresh S., Mechanics of diseased red blood cells in human spleen and consequences for hereditary blood disorders. Proc. Natl. Acad. Sci. U.S.A. 115, 9574–9579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X., Shen S., Fan Q., Chen G., Archibong E., Dotti G., Liu Z., Gu Z., Wang C., Red blood cell-derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci. Adv. 5, eaaw6870 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng J., Yang Q., Li W., Tan L., Xiao Y., Chen L., Hao Y., Qian Z., Erythrocyte-membrane-coated prussian blue/manganese dioxide nanoparticles as H2O2-responsive oxygen generators to enhance cancer chemotherapy/photothermal therapy. ACS Appl. Mater. Interfaces 9, 44410–44422 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Liu B., Wang W., Fan J., Long Y., Xiao F., Daniyal M., Tong C., Xie Q., Jian Y., Li B., Ma X., Wang W., RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials 217, 119301 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Alipour E., Halverson D., McWhirter S., Walker G. C., Phospholipid bilayers: Stability and encapsulation of nanoparticles. Annu. Rev. Phys. Chem. 68, 261–283 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Dovydenko I. S., Laricheva Y. A., Korchagina K. V., Grigoryeva A. E., Ryabchikova E. I., Kompankov N. B., Pischur D. P., Gushchin A. L., Apartsin E. K., Sokolov M. N., Interaction of hydrophobic tungsten cluster complexes with a phospholipid bilayer. J. Phys. Chem. B 123, 8829–8837 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Chu Y., Zhang J., Pan H., Shi J., Wang J., Chen L., Preparation and evaluation of long circulating erythrocyte membrane-cloaked anti-cancer drug delivery system. Drug Deliv. Transl. Res. 10, 1278–1287 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Nguyen T. X., Huang L., Gauthier M., Yang G., Wang Q., Recent advances in liposome surface modification for oral drug delivery. Nanomedicine 11, 1169–1185 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Li M., Du C., Guo N., Teng Y., Meng X., Sun H., Li S., Yu P., Galons H., Composition design and medical application of liposomes. Eur. J. Med. Chem. 164, 640–653 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Yuba E., Fukaya Y., Yanagihara S., Kasho N., Harada A., Development of mannose-modified carboxylated curdlan-coated liposomes for antigen presenting cell targeted antigen delivery. Pharmaceutics 12, 754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W. J., Tang X. F., Shuai X. X., Jiang C. J., Liu X., Wang L. F., Yao Y. F., Nie S. P., Xie M. Y., Mannose receptor mediates the immune response to ganoderma atrum polysaccharides in macrophages. J. Agric. Food Chem. 65, 348–357 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y., Do D. C., Ishmael F. T., Squadrito M. L., Tang H. M., Tang H. L., Hsu M. H., Qiu L., Li C., Zhang Y., Becker K. G., Wan M., Huang S. K., Gao P., Mannose receptor modulates macrophage polarization and allergic inflammation through miR-511-3p. J. Allergy Clin. Immunol. 141, 350–364.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irvine D. J., Swartz M. A., Szeto G. L., Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 12, 978–990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irvine D. J., Hanson M. C., Rakhra K., Tokatlian T., Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 115, 11109–11146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Z., Wang Y., Zhang L., Huang L., Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano 8, 3636–3645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz L. J., Rosalia R. A., Kleinovink J. W., Rueda F., Löwik C. W. G. M., Ossendorp F., Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8+ T cell response: A comparative study. J. Control. Release 192, 209–218 (2014). [DOI] [PubMed] [Google Scholar]
- 40.De Titta A., Ballester M., Julier Z., Nembrini C., Jeanbart L., Van Der Vlies A. J., Swartz M. A., Hubbell J. A., Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. U.S.A. 110, 19902–19907 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox C. B., Sivananthan S. J., Duthie M. S., Vergara J., Guderian J. A., Moon E., Coblentz D., Reed S. G., Carter D., A nanoliposome delivery system to synergistically trigger TLR4 AND TLR7. J. Nanobiotechnol. 12, 17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D., Wang T., Yu H., Feng B., Zhou L., Zhou F., Hou B., Zhang H., Luo M., Li Y., Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 4, eaau6584 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/35/eabi6326/DC1


