Abstract
SOX proteins bind similar DNA motifs through their high-mobility-group (HMG) domains, but their action is highly specific with respect to target genes and cell type. We investigated the mechanism of target selection by comparing SOX1/2/3, which activate δ-crystallin minimal enhancer DC5, with SOX9, which activates Col2a1 minimal enhancer COL2C2. These enhancers depend on both the SOX binding site and the binding site of a putative partner factor. The DC5 site was equally bound and bent by the HMG domains of SOX1/2 and SOX9. The activation domains of these SOX proteins mapped at the distal portions of the C-terminal domains were not cell specific and were independent of the partner factor. Chimeric proteins produced between SOX1 and SOX9 showed that to activate the DC5 enhancer, the C-terminal domain must be that of SOX1, although the HMG domains were replaceable. The SOX2-VP16 fusion protein, in which the activation domain of SOX2 was replaced by that of VP16, activated the DC5 enhancer still in a partner factor-dependent manner. The results argue that the proximal portion of the C-terminal domain of SOX1/2 specifically interacts with the partner factor, and this interaction determines the specificity of the SOX1/2 action. Essentially the same results were obtained in the converse experiments in which COL2C2 activation by SOX9 was analyzed, except that specificity of SOX9-partner factor interaction also involved the SOX9 HMG domain. The highly selective SOX-partner factor interactions presumably stabilize the DNA binding of the SOX proteins and provide the mechanism for regulatory target selection.
An important question of transcriptional regulation is how transcription factors select their correct target genes within the genome. DNA-binding specificity of individual protein factors must be one of the major determinants of target gene selection. A family of closely related transcription factors often have highly similar DNA-binding properties, most often recognizing only ∼6-bp DNA sequences, with allowance of considerable degeneracy. Nevertheless, these transcription factors activate or repress each specific set of target genes via these binding motifs and exert quite different biological effects even within a protein family. The Sox gene family investigated in this work is a good example of such a family. The problem is the mechanism which enables each SOX protein to select the correct subset of binding sites to achieve its proper in vivo function.
Sox genes have been identified on the basis of their homology to the high-mobility-group (HMG) box of the mammalian testis-determining gene Sry (17; for a review, see reference 36). They comprise a large gene family of more than 20 members in mammals and are found in all investigated species of the animal kingdom (10, 49). SOX proteins are grouped into subfamilies based on the amino acid sequence of the HMG domain (49). Within each subfamily, the amino acid sequence of the HMG domain is ≥90% identical and sequence similarity extends outside this domain. Although the homology exhibited within the HMG domain is ∼60% between distantly related subfamilies, DNA-binding characteristics of the various SRY and SOX family proteins tested so far appear very similar to each other. They recognize essentially the same sets of sequences with very similar affinities (11, 19, 24, 26) and induce sharp bends at similar angles (7, 12, 31).
Sox genes are found to be expressed in a wide variety of tissues during development. Neural tissues express high levels of many Sox genes, e.g., Sox1, Sox2, Sox3, Sox6, Sox9, and Sox11 (6, 8, 18, 34, 37, 39, 42, 45). Sox2 is also expressed in the lens and gut epithelium in chicken embryos (24, 25, 45), and in teratocarcinoma and embryonic stem cells (50). Sox9 is expressed in prechondrogenic and chondrogenic tissues (34, 47, 48, 51) and in the genital ridge (9, 27), sites which are consistent with the skeletal malformations and sex reversal found in campomelic dysplasia patients with mutations in the gene. Sox11 is widely expressed throughout embryos at early stages, but its expression becomes restricted in tissues, e.g., central and peripheral nervous systems, at later stages (18, 45).
In these Sox-expressing tissues, the products of the Sox genes are each expected to have a unique biological function and to regulate different sets of genes. A limited number of authentic target genes of SOX proteins have been identified, but SOX proteins activate the genes in all known cases. An important observation is that activation of a particular gene by a SOX protein occurs only in a subset of cells or tissues among those expressing the SOX protein.
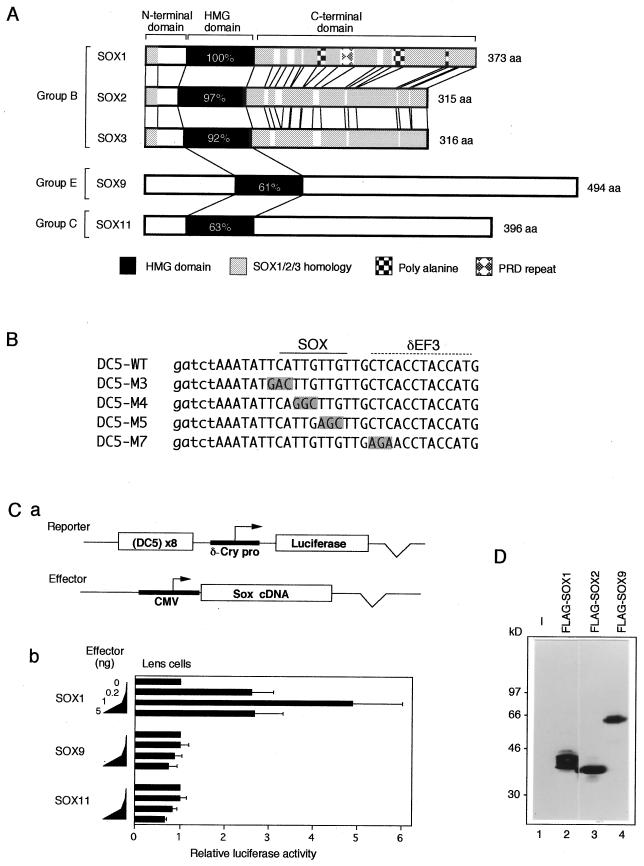
Best-characterized examples of SOX target genes are the lens-specific chicken δ1-crystallin and mouse γ-crystallin genes, regulated by three highly related SOX proteins, SOX1, -2, and -3 (collectively called SOX1/2/3) (24, 25, 35). Lens-specific activity of the intronic enhancer of the chicken δ1-crystallin gene (20) is dependent on the 30-bp-long DC5 fragment, which by itself has a lens-specific enhancer activity (22). It has been demonstrated that SOX1/2/3 (Fig. 1A) bind to the 5′ half of the DC5 sequence (Fig. 1B), and binding of one of these SOX proteins is essential for the DC5 enhancer (24, 25). It is important that in activation of the DC5 enhancer, SOX1/2/3 require the putative partner factor δEF3, which interacts with the 3′-half of the DC5 sequence and is present in lens cells but absent in fibroblasts (22, 24, 25). Mutations of the DC5 sequence that inhibit binding of either SOX1/2/3 (e.g., M4) or δEF3 (e.g., M7) abolish the enhancer activity (22, 24) (Fig. 1B). It has been demonstrated that overexpression of SOX2 increases the enhancer activity of the wild-type DC5 (DC5-WT) in lens cells, but this does not occur when the δEF3 site is mutated to M7 sequence (24). It has also been demonstrated that lens-specific γ-crystallin promoters have an essential SOX1/2/3 binding site (24, 35). Importance of SOX1/2/3 in crystallin gene expression is confirmed by the fact that Sox1-deficient mice lack expression of all members of γ-crystallin genes in the stage when only SOX1 is normally expressed in the lens (35).
FIG. 1.
Comparison of SOX1/2/3, SOX9, and SOX11 with respect to protein structure and activation of the DC5 enhancer. (A) Schematic presentation of chicken SOX proteins used in this study. Percentages of amino acid identity with SOX1 in the HMG domain are given. (B) Sequences of the DC5 fragments used in this study. In the DC5 mutant sequences (22), altered bases are shaded. The SOX binding site and the putative δEF3 site are indicated at the top. Lowercase letters indicate bases introduced to generate BglII and BamHI restriction sites. (C) (a) Scheme of the cotransfection assay. The luciferase reporter plasmid contains octamerized DC5 fragment positioned upstream of δ1-crystallin minimal promoter (δ-Cry pro; −51 to +57). The cDNAs of Sox genes were expressed by the CMV enhancer-promoter. (b) Comparison of effects of SOX1, SOX9, and SOX11 on the DC5 enhancer. Lens cells were transfected with the luciferase reporter plasmid and with various amounts of effector vectors (0, 0.2, 1, and 5 ng) encoding one of the SOX proteins. Luciferase activity generated by the reporter in the absence of exogenous SOX was taken as 1. (D) Expression of FLAG-tagged SOX proteins in lens cells. Lens cells were transfected with pCMV/SV-Flag1 plasmids encoding no protein (−) or the indicated SOX proteins. Whole-cell lysates were subjected to Western blot analysis using anti-FLAG antibody. Calculated molecular masses of FLAG-SOX proteins are as follows: FLAG-SOX1, 39.2 kDa; FLAG-SOX2, 35.8 kDa; and FLAG-SOX9, 56.4 kDa. The sizes of molecular mass markers are indicated on the left.
Other established targets of regulation by SOX are the human (COL2A1) and mouse (Col2a1) genes encoding type II collagen, the major extracellular matrix component of cartilage (2, 31). Expression of Sox9 parallels that of Col2a1 during chondrogenesis, and abnormal regulation of COL2A1 expression is presumed to be a cause of the skeletal abnormalities associated with campomelic dysplasia (34, 51). Chondrocyte-specific expression of Col2a1/COL2A1 is regulated by conserved sequences in the first intron (2, 30, 32). SOX9 is shown to bind to at least two sites, COL2C1 and COL2C2, of the 309-bp human COL2A1 enhancer (2). Mutations of these SOX9 binding sequences abolish chondrocyte-specific activity of the human COL2A1 enhancer in transgenic mice (2). In the case of mouse Col2a1, the conserved 18-bp enhancer has been demonstrated to be the minimal enhancer in chondrosarcoma cells (32); the 18-bp sequence is identical to human COL2C2. Analogous to the DC5 enhancer, the COL2C2 activity is dependent on both the SOX binding site and a site for another enhancer binding factor (31, 32). SOX9 has been shown to activate the COL2C2-containing enhancer fragments in transfected cells of various origins (31). Ectopic expression of SOX9 also transactivates reporter constructs containing COL2C1 and COL2C2 in transgenic mice (2).
In this report, we investigated the mechanism by which SOX proteins selectively activate particular enhancers, taking advantage of two established natural target sequences, δ1-crystallin DC5 for SOX1/2/3 and Col2a1/COL2A1 COL2C2 for SOX9. The data indicate that enhancer activation by SOX proteins requires DNA-binding partner factors which specifically interact with each subclass of SOX proteins. This cooperation likely occurs not at the step of transactivation but presumably at the step of establishing high-affinity DNA binding. Through the specific interaction with the partners, a specific SOX protein is selectively employed by a particular SOX site.
MATERIALS AND METHODS
Chicken Sox cDNAs.
cDNAs of Sox9 and Sox11 used in this study were obtained from cDNA libraries of chicken embryonic (14-day-old) brain. DNA database accession numbers are AB012236 for Sox9 and AB012237 for Sox11. Chicken Sox2 (24) and chicken Sox1 and Sox3 (25) cDNAs have been described elsewhere.
Plasmid construction.
To express Sox cDNAs in cultured cells, we inserted cDNA fragments into cytomegalovirus (CMV) enhancer-promoter-driven expression vectors pCMV/SV1, pCMV/SV2, and/or pCMV/SV-Flag1. pCMV/SV1 was constructed by inserting the simian virus 40 replication origin into pCMVX (22). pCMV/SV2 is the same as pCMV/SV1 except that it has the cloning sites of pcDNAI (Invitrogen). pCMV/SV-Flag1 was constructed by replacing the cloning site of pCMV/SV2 with the Met-FLAG sequence and the cloning sites of pCITE-3 (Novagen).
Plasmid constructs of SOX1 C-terminal deletions were made by digestion of SmaI (ΔC323, ΔC216, and ΔC192) or ScaI (ΔC132) and by inserting the fragments into pCMV/SV2(EcoStop), in which the EcoRI-NotI fragment was replaced by a sequence containing three-frame stop codons. The internal deletion Δ132-247 construct was made by removing ScaI-PstI fragment. In the SOX1 ΔN45 mutant, the AvaII site was preceded by Kozak sequence and a methionine codon. The deletions are indicated by the amino acids that have been deleted.
Constructs coding for SOX1-SOX9 chimeric proteins were made by exchanging cDNA fragments corresponding to the N-terminal, HMG, and/or C-terminal domains of the proteins, using XmnI and BbsI sites which are located at the N- and C-terminal ends, respectively, of the HMG domain. As the natural Sox9 sequence does not contain the BbsI site, we generated the junctional BbsI site by PCR using BbsI-tagged primers. BbsI site-bearing Sox9 cDNA coded for SOX9(+BbsI) with alteration of the amino acid sequence (underlined in the sequence below) at the junction of the HMG and C-terminal domains, but this alteration did not affect the activation potential as SOX9 in cotransfection experiments (data not shown). Amino acid sequences at the junctions of the N-terminal, HMG, and C-terminal domains are as follows: SOX1, KAGQ/DRVK-----PRRKTK/TLLKK; SOX9, SKNK/PHVK-----PRRRKS/VKNGQ; SOX9(+BbsI), SKNK/PHVK-----PRRKTK/T/VKNGQ; SOX9-1-9, SKNK/PHVK-----PRRKTK/T/VKNGQ; and SOX1-9-1, KAGQ/DRVK-----PRRKTK/TLLKK.
The expression vector for GAL4 fusions was made by inserting the HindIII-EcoRI fragment encoding the DNA-binding domain (GAL4DBD; amino acids [aa] 1 to 147) from pSGVP (40) into pCMV/SV2, resulting in pCMV/SV2-GAL4DBD. The fragments encoding the C-terminal domains of SOX proteins were inserted downstream of the GAL4 sequence.
Luciferase reporter plasmids containing the octamerized DC5 and COL2C2 derivatives were constructed as described by Kamachi and Kondoh (22). To make the luciferase reporter for the GAL4 fusion assay, a fragment containing a tetramerized GAL4 site was inserted upstream of the δ1-crystallin minimal promoter of pδ51LucII.
For in vitro protein synthesis, portions of Sox cDNAs indicated in Fig. 2 were inserted into a pCITE-3 vector (Novagen) so that SOX proteins were made as S-Tag (Novagen) fusions.
FIG. 2.
Comparison of DNA binding and DNA bending by SOX proteins. (A) Schematic presentation of the S-Tag fusion proteins synthesized in vitro by coupled transcription/translation (TNT system). S-Tag sequence at the N terminus is hatched. (B) pBend2-DC5 contained the DC5 fragment (black bar) in the middle of the duplicated multi-restriction site sequences. pBend2-DC5 DNA was cleaved at the restriction sites indicated on the map and used as probes in the circular permutation analysis. (C) Circular permutation analysis of DNA bending induced by SOX1, -2, and -9. Gel mobility shift experiments were done with S-Tag–SOX proteins (2 ng) and the permuted probes A to G. (D) The relative mobilities of the protein-DNA complexes were plotted against the flexure displacement of the probes (12).
Cell culture and transfection analysis.
Cultures of lens cells and fibroblasts were prepared from 14-day-old chicken embryos as described by Hayashi et al. (20). We have recently found that a patched form of lens epithelia gives a higher activity of the DC5 enhancer than dissociated lens cells, and it was used in the experiment represented in Fig. 5. The cultures were prepared as follows. Lenses were disrupted with forceps and incubated in Hanks’ saline containing 0.1% collagenase for 30 min at 37°C. The epithelial cell clumps derived from one lens equivalent were plated in a 35-mm-diameter dish coated with type I collagen (Iwaki Glass) and cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum (FCS). Two days later, the medium was replaced with Ham’s F12 medium with 10% FCS, and cells were cultured for another 24 h before transfection. 10T1/2 cells were cultured in Dulbecco’s modified Eagle medium containing 10% FCS and seeded at 5 × 104 cells per 35-mm-diameter dish 1 day before transfection.
FIG. 5.
Effects of altering orientation and spacing between the SOX and δEF3 sites in the DC5 enhancer. (A) The SOX binding site was mutated in DC5-M4 and reversed in orientation in DC5-SR. A 4-bp spacer was added between the SOX and δEF3 sites in DC5-I4a. Altered bases are shaded. The binding sites for SOX and δEF3 are shown at the top. (B) Octamerized DC5 fragments were placed upstream of the δ1-crystallin minimal promoter (−51 to +57) in both normal (N) and reverse (R) orientations relative to the direction of transcription. Activity of the mutated DC5 enhancers in panel A was assessed by transfecting luciferase reporter plasmids into patched cultures of lens cells and fibroblasts. Luciferase expression by reporter plasmids was normalized to that of the enhancerless plasmid pδ51LucII (−).
Cells cultured in a 35-mm-diameter dish were transfected with 1.5 μg of plasmid DNA by a calcium phosphate precipitation method (5). Cotransfection was performed with plasmid DNA containing 1.3 μg of luciferase reporter, 0.1 μg (total) of Sox expression vector-empty vector mixture, and 0.1 μg of β-galactosidase reference reporter (pSV-β-Galactosidase [Promega] or pMiwZ [44]). In the transfections shown in Fig. 5, DNA containing 1.4 μg of luciferase reporter and 0.1 μg of pSV-β-Galactosidase was transfected. Luciferase activity was measured 48 h after transfection as described by Kamachi and Kondoh (22) and normalized to β-galactosidase activity, determined by using 4-methylumbelliferyl-β-d-galactopyranoside (13). Transfections were carried out at least three times, and the averages are shown with the standard deviations.
Western blotting.
Lens cells cultured in a 90-mm-diameter dish were transfected with 10 μg of plasmid DNA encoding FLAG-tagged SOX proteins, and whole-cell lysates were prepared in 100 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer; 15 μl of the lysates was loaded onto a sodium dodecyl sulfate–10% polyacrylamide gel and transferred to Immobilon-P polyvinylidene difluoride membrane (Millipore) with 10 mM 3-(cyclohexylamino)-1-propanesulfonic acid (pH 11.0) and 10% methanol. FLAG-SOX proteins were detected with anti-FLAG M5 antibody (IBI Kodak).
Protein preparation and gel mobility shift assay.
For in vitro protein synthesis, a transcription/translation coupled reticulocyte lysate system (TNT; Promega) was used with pCITE-3 containing Sox cDNAs. Synthesized S-Tag fusion proteins were estimated by S-Tag rapid assay (Novagen), and 0.7 to 0.9 μl of the lysate containing 2 ng of fusion proteins was used in binding assays. Gel mobility shift assays were carried out under the conditions described by Kamachi and Kondoh (22).
Circular permutation assay.
The DC5 fragment was cloned between the BamHI and BglII sites of the circular permutation vector pBend2 (28). Circularly permuted DNA fragments were excised with one of the restriction enzymes indicated in Fig. 2B and labeled by using either Klenow enzyme and [α-32P]dCTP or T4 polynucleotide kinase and [γ-32P]ATP. Gel mobility shift assays were performed as described by Kamachi and Kondoh (22). Bending angles were estimated according to the method of Ferrari et al. (12).
RESULTS
δ1-crystallin DC5 enhancer is activated by SOX1/2/3 but not by SOX9 or SOX11.
We have previously shown that overexpression of SOX1/2/3 activates the DC5 enhancer in lens cells but not in fibroblasts (24, 25). Since SOX9 and SOX11 resemble SOX1/2/3 only in the HMG domain (Fig. 1A), we inquired whether such distantly related SOX proteins could equally activate the DC5 enhancer. Lens cells were transfected with a luciferase reporter gene carrying the DC5 enhancer and with various amounts of effector vectors expressing either SOX9 or SOX11 (Fig. 1Ca). In these experiments, a 25-fold range of effector vectors (0.2 to 5 ng) was used to compensate for possible difference of expression levels of SOX proteins (Fig. 1Cb). In contrast to SOX1 or SOX2/3 (reference 25; see also Fig. 3A), neither SOX9 nor SOX11 was capable of activating the DC5 enhancer activity in lens cells (Fig. 1Cb) or in fibroblasts (data not shown). The same SOX9 construct activated the COL2C2 enhancer (see Fig. 7) in lens cells (data not shown), indicating expression of functional SOX9 in transfected lens cells. When analogous expression vectors coding for FLAG-tagged SOX proteins were transfected to lens cells, synthesis of these proteins was demonstrated by Western blotting (Fig. 1D for SOX1, -2, and -9; data for SOX11 in a separate experiment not shown) and by nuclear accumulation of the proteins by immunofluorescence (data not shown). These results confirmed that all exogenous SOX proteins were expressed in the transfected lens cells, but only SOX1/2/3 were capable of activating the DC5 enhancer.
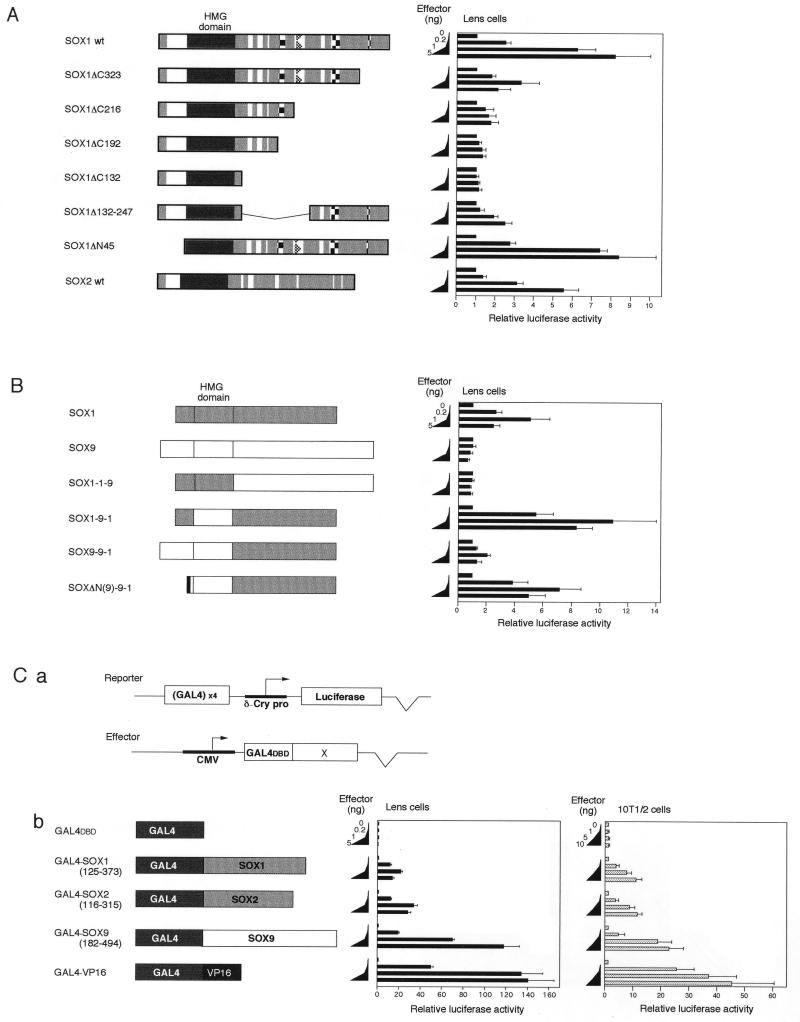
FIG. 3.
Requirement of the SOX1 C-terminal domain for activation of the DC5 enhancer. (A) Transcriptional activity of SOX1, its deletion mutants, and SOX2. Schematic structures of the SOX proteins are shown on the left. Symbols are as in Fig. 1A. Cotransfection assays were done as described for Fig. 1C. (B) Activity of the SOX1-SOX9 chimeric proteins in activation of the DC5 enhancer. Schematic structures of chimeric proteins made by combinations of the domains derived from SOX1 and SOX9 are shown on the left. The chimeric proteins are designated by the origin of N-terminal, HMG, and C-terminal domains. For instance, SOX1-1-9 had the N-terminal and HMG domains of SOX1 and the C-terminal domain of SOX9. SOX1 portions are indicated by shaded boxes, and SOX9 portions are indicated by open boxes. SOXΔN(9)-9-1 protein lacked the N-terminal 96 aa of SOX9 and instead had Met-FLAG tag sequence indicated by the black box. Cotransfection assays were done as for Fig. 1C. (C) C-terminal domains of the SOX proteins have transactivation potential. (a) The luciferase reporter plasmid contained tetramerized GAL4 binding sites in the upstream of δ1-crystallin minimal promoter (δ-Cry pro). C-terminal domains of SOX proteins (×) were fused in frame to GAL4DBD. (b) Various amounts of effector plasmids expressing GAL4-SOX fusion proteins were transfected into lens cells and 10T1/2 cells together with the luciferase reporter plasmid and compared with GAL4DBD and GAL4-VP16. Luciferase activity generated by the reporter in the absence of GAL4 fusion proteins was taken as 1.
FIG. 7.
Specific activation of the COL2C2 enhancer by SOX9. (A) The luciferase reporter plasmid contained an octamerized COL2C2 fragment placed upstream of the δ1-crystallin minimal promoter. (B) Regulation of the COL2C2 enhancer by various SOX protein derivatives. 10T1/2 cells were transfected with the luciferase reporter plasmid and various amounts of SOX expression plasmid (0, 1, 5, and 10 ng). Luciferase activity generated by the reporter in the absence of exogenous SOX was taken as 1. (C) The luciferase reporter plasmid containing either COL2C2 WT or COL2C2 M(SC) was cotransfected as for panel B. (D) Various amounts of effector vectors (0, 1, 5, and 10 ng) bearing genes encoding either SOX9 (a) or SOX9(1-264)-VP16 (Fig. 4A) (b) were cotransfected into 10T1/2 cells with luciferase reporter plasmids carrying COL2C2 WT (■), C2M(1-4) (•), or C2M(9-14) (▴). Luciferase activity generated by the reporter containing COL2C2 WT in the absence of exogenous SOX proteins was taken as 1. The VP16 activation domain could not overcome the requirement of the partner factor for SOX9 to activate the COL2C2 enhancer.
DNA-binding and DNA-bending properties are indistinguishable among the HMG domains of different SOX proteins.
To examine the molecular basis of selective activation of the DC5 enhancer by SOX1/2/3, we analyzed which parts of the molecules are involved in the selectivity by comparing SOX1/2/3 with SOX9 and SOX11. SOX1 and SOX2 were analyzed among the former mainly because of their high activation potentials (25), while SOX9 was chosen as the counterpart of SOX1/2/3 for comparison because its natural target gene is known.
We first compared the HMG domains of SOX1/2/3 and SOX9 with respect to DNA binding and bending. The identity of amino acid sequence of the HMG domain is ca. 60% between SOX1/2/3 and SOX9 (Fig. 1A). Approximately 200-aa-long polypeptides containing the HMG domain of SOX1, -2, or -9 were produced as fusion proteins with S-Tag by an in vitro coupled transcription-translation system (Fig. 2A). All of these HMG domain fusion proteins formed a complex with the DC5 sequence placed in pBend2 (Fig. 2B and C). The complexes were sensitive to competition by the WT DC5 sequence and γF-crystallin promoter sequence but not to competition by the mutated DC5 sequences, M3, M4, and M5, in which the SOX binding sequence was altered (data not shown) equally to the full-length SOX2 as reported previously (24). These results indicated that HMG domains of SOX1, SOX2, and SOX9 are indistinguishable from each other in DNA binding to the regulatory sites of δ1- and γF-crystallin genes.
The same pBend2-DC5 DNA was used to compare the abilities of the HMG domains to induce DNA bending at the binding site by a circular permutation assay. The circularly permuted DNA fragments bound by the S-Tag–SOX fusion proteins were subjected to a gel mobility shift assay (Fig. 2C). The angle of the protein-induced bend in a DNA fragment by all three proteins was estimated at 66° (Fig. 2D).
Thus, SOX1/2/3 and SOX9 are indistinguishable in binding to and bending at the DC5 sequence (data not shown for SOX3). Therefore, the interaction of the HMG domain with the DC5 DNA is not involved in the mechanism to discriminate SOX1/2/3 from SOX9 and other SOX proteins in activation of the DC5 enhancer.
The C-terminal domain of SOX1/2 is involved not only in transactivation but in selective action to DC5.
It is important to know which domain of the SOX proteins is involved in selective activation of the DC5 enhancer in lens cells. We conceptually divided the SOX proteins into three domains, the N-terminal, HMG, and C-terminal domains (Fig. 1A). To determine domains required for DC5 activation, we analyzed SOX1 mutants with deletions of the C-terminal and N-terminal domains. We introduced a set of successive C-terminal truncations in SOX1 and tested their effects on the ability to activate the DC5 enhancer in a cotransfection assay. As shown in Fig. 3A, removal of 51 aa from the C terminus of SOX1 (ΔC323) significantly reduced the ability to stimulate the DC5 enhancer activity. Transactivation activity was totally lost upon extended truncation to position 192. Thus, the HMG domain of SOX1 alone is not sufficient but the association of the C-terminal domain is essential for activation of the DC5 enhancer. An internal deletion of aa 132 to 247 also resulted in a loss of activation, indicating that at least two subdomains, aa 132 to 247 and aa 323 to 373, of the C-terminal domain are required for activation of the DC5 enhancer by SOX1. In contrast, the N-terminal domain of SOX1 was dispensable for activation of DC5 (Fig. 3A). Essentially the same result was obtained with SOX2, consistent with the amino acid sequence conservation between SOX1 and SOX2 (23, 25).
To determine which domains establish the functional specificity of SOX proteins at the DC5 enhancer, we produced cDNA encoding chimeric proteins in which the N-terminal, HMG, and C-terminal domains of SOX1 and SOX9 were interchanged in various combinations (Fig. 3B). Synthesis and DNA-binding activities of these chimeric proteins were confirmed in COS-7 cells transfected with the expression vectors (data not shown). The chimeric proteins were tested for the ability to stimulate the DC5 enhancer by cotransfection assays (Fig. 3B). Since the chimeric protein SOX1-1-9 (SOX1 N-terminal domain, SOX1 HMG domain plus SOX9 C-terminal domain) failed to stimulate enhancer activity, the C-terminal domain of SOX1 is essential for the specific transactivation. On the other hand, the chimeric protein SOX1-9-1 showed even higher transactivation than SOX1, confirming the requirement of SOX1 C-terminal domain in activation of DC5 in lens cells. This finding also indicates that the HMG domain is interchangeable between SOX1 and SOX9 and is not significantly involved in discrimination of SOX1 over SOX9 in transactivation of the DC5 enhancer. In this regard, it was rather surprising that the chimeric protein SOX9-9-1 showed fairly modest transactivation in spite of having the SOX1 C-terminal domain. However, removal of the N-terminal portion of the protein [SOXΔN(9)-9-1] resulted in transactivation comparable to that for SOX1, suggesting that the N-terminal domain of SOX9 has an inhibitory effect on activation of the DC5 enhancer by the SOX1 C-terminal domain in lens cells. In a gel mobility shift assay, SOX9-9-1 and SOXΔN(9)-9-1 bound to the DC5 probe indistinguishably. On the other hand, removal of the N-terminal domain from SOX9 did not cause activation of DC5 by SOX9 (data not shown). From these results, we concluded that the C-terminal domain of SOX is determinative in specific activation of the DC5 enhancer.
We inquired whether the C-terminal domains of SOX1/2/3 have transactivation potential when isolated from the HMG domains and fused to GAL4DBD (Fig. 3C). Lens cells were transfected with the GAL4DBD fusion constructs and a luciferase reporter plasmid carrying GAL4-binding sites (Fig. 3Ca). While GAL4DBD alone caused no increase of luciferase activity, GAL4-SOX1 and GAL4-SOX2 stimulated luciferase expression 20- to 40-fold (Fig. 3Cb). It was also observed that these GAL4DBD-SOX fusion proteins similarly activated the luciferase reporter in chicken fibroblasts (data not shown) and 10T1/2 cells (Fig. 3Cb). These results indicate that the C-terminal domains of SOX1 and SOX2 when isolated all display transactivation potential without dependence on the cell type. The same conclusion has been reached in studies using the mouse SOX1 C-terminal domain (L. Pevny and R. Lovell-Badge, unpublished result cited in reference 25). Südbeck et al. (43) and Ng et al. (34) recently reported that human and mouse SOX9 have a potent transactivation domain in their C termini. The failure of SOX9 to stimulate the DC5 enhancer cannot be accounted for by models assuming cell-type-dependent activity of the activation domain of SOX9, since exogenous SOX9 activated the COL2C2 enhancer in lens cells as described above. Furthermore, GAL4-SOX9 fusion protein carrying the C-terminal domain of chicken SOX9 and GAL4DBD activated expression of the GAL4-dependent reporter gene in lens cells (Fig. 3C) and fibroblasts (data not shown), in sharp contrast to the failure of intact SOX9 to activate DC5 in either cells. The level of stimulation by GAL4-SOX9 was even higher than those by GAL4-SOX1/2 and was comparable to that by GAL4-VP16, in which GAL4DBD was fused to an acidic activation domain of the herpes simplex virus protein VP16 (40). Thus, the activation domain of SOX9 does not function at the DC5 enhancer when linked to the intrinsic SOX9 HMG domain, in spite of binding of the SOX9 HMG domain to the DC5 sequence in in vitro experiments (Fig. 2).
The above results indicate that the C-terminal domain of SOX1/2 was essential and unable to be replaced with that of SOX9 for selective activation of the DC5 enhancer, while C-terminal domains of SOX1/2 and SOX9 exhibited nonspecific transactivation potential when tested as GAL4DBD fusions. This finding implies that a portion of the C-terminal domain, one not involved in transactivation by itself, may play a role in selective action of SOX proteins.
The VP16 activation domain joined to the SOX HMG domain does not overcome the requirement of the partner factor δEF3 in activation of the DC5 enhancer.
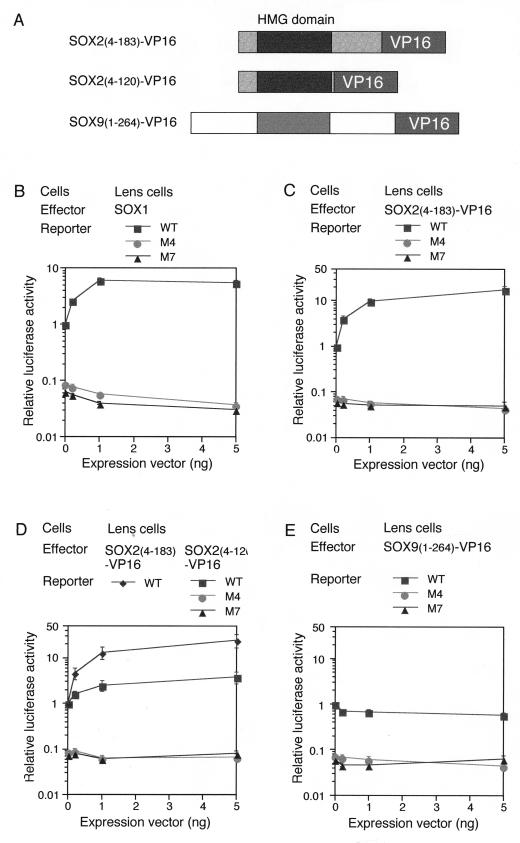
Previously, we reported that activation of the DC5 enhancer by SOX2 requires the integrity of a sequence neighboring the SOX binding site which is assigned to the δEF3 binding site (24). For instance, mutation M7 of the putative δEF3 site (Fig. 1B) totally inactivated the DC5 enhancer activity, although it did not affect SOX2 binding to DC5 in vitro (24). The requirement for δEF3 was also investigated for SOX1, SOX3, and the chimeric SOX1-9-1. It was demonstrated that activation of the DC5 enhancer by these SOX proteins was lost by mutation M7 of the δEF3 site similarly to mutation M4 in the SOX binding site (Fig. 4B; data not shown for SOX3 and SOX1-9-1). This result confirmed that all SOX proteins capable of activation of DC5 depend on binding of the partner factor δEF3 at the nearby site.
FIG. 4.
Fusion proteins of SOX HMG domains bearing the VP16 activation domain still require the partner factor for transactivation. (A) Schematic structures of SOX-VP16 fusion proteins. C-terminal domains of SOX2 and SOX9 carrying the transactivation potential were replaced with the VP16 activation domain. (B to E) Luciferase reporter plasmids were the same as in Fig. 1 and carried the WT, M4 (loss of SOX binding), or M7 (loss of δEF3 binding) form of the DC5 enhancer. Luciferase reporter plasmids were cotransfected with various amounts of effector vectors (0, 0.2, 1, and 5 ng) expressing either SOX1 (B), SOX2(4-183)-VP16 (C and D), SOX2(4-120)-VP16 (D), or SOX9(1-264)-VP16 (E) into lens cells. Luciferase activity generated by the reporter containing DC5-WT in the absence of exogenous SOX proteins was taken as 1 in each experiment. Luciferase expression of the reporters carrying the M4 or M7 mutation was ∼15-fold lower than that of the DC5-WT-carrying reporter. This finding indicates that in lens cells, the transfected reporter is already activated by endogenous SOX1/2/3. Therefore, the overall activation level of DC5 enhancer by SOX1/2/3 in lens cells is estimated to be 30- to 100-fold, and that by SOX2(4-183)-VP16 is ∼300-fold.
The above results indicated that in the absence of a proper partner factor bound at a nearby site of the enhancer, the transactivation domains of SOX do not stimulate transcription when they are linked to the SOX HMG domain, which binds to the DC5 sequence in in vitro experiments. These results were in contrast to the case of the GAL4DBD-SOX1/2 C-terminal domain fusion protein which was independent of the partner factor (Fig. 3C). To test whether properties of the activation domains of SOX have unique characteristics to account for this behavior, we examined the performance of the typical acidic activation domain of VP16 when it is linked to SOX proteins (Fig. 4A). We first made a fusion protein in which the VP16 activation domain was fused to a truncated SOX2 protein [SOX2(4-183)] that had lost its own activation potential (25) (Fig. 3A shows comparable data for SOX1). This SOX2(4-183)-VP16 fusion protein strongly stimulated the DC5 enhancer activity when expressed in lens cells (Fig. 4C); this activation was dependent on the SOX binding site as expected (Fig. 4C; compare WT with M4). It was remarkable that when the DC5-M7 mutant lacking the partner δEF3 site was used, SOX2(4-183)-VP16 was totally inactive in inducing an enhancer activity in lens cells (Fig. 4C). In fibroblasts which lacks δEF3 activity, overexpressed SOX2(4-183)-VP16 did not stimulate transcription even through DC5-WT (data not shown). These results show that SOX2(4-183)-VP16 cannot activate transcription by itself and is still dependent on cooperation with δEF3 to stimulate the DC5 enhancer. The SOX9(1-264)-VP16 fusion, in which the VP16 activation domain was linked to the truncated SOX9, did not stimulate the activity of the DC5 enhancer even in lens cells, just as with the intact SOX9 protein (Fig. 4E). These results indicate that the requirement for δEF3 for SOX1/2/3 activation of the DC5 enhancer is not overcome by replacement of the SOX activation domain with the strong VP16 activation domain. Thus, the mechanism rendering transactivation by SOX1/2/3 dependent on cooperation by the partner factor δEF3 is not associated with the activation domain per se but is attributed to some other characteristic of the SOX proteins, possibly the direct protein-protein interactions with δEF3 at the closely positioned DNA-binding sites.
Where in the SOX2 protein is the interaction interface with δEF3 located? Since SOX2(4-183)-VP16 efficiently activated the DC5 enhancer, the interaction surface must be between aa 4 and 183. We made another SOX2-VP16 fusion, in which SOX2(4-120) was fused to the VP16 activation domain, and compared it with SOX2(4-183)-VP16. In contrast to SOX2(4-183)-VP16, SOX2(4-120)-VP16 activated the DC5 enhancer only three- to fourfold (Fig. 4D), indicating that the domain from aa 121 to 183 is important for SOX1/2/3-δEF3 interaction. It is to be noted, however, that SOX2(4-120)-VP16 still activated DC5, albeit ineffectively, and this activation was lost with DC5-M7, indicating dependence on δEF3. Thus, the region from aa 4 to 120 including the HMG domain seems to provide a minor interface for δEF3 interaction.
The DC5 enhancer activity depends on orientation and spacing of the SOX and δEF3 binding sites.
As described above, SOX1/2/3 require cooperation with δEF3 to activate the DC5 enhancer. If a direct protein interaction between the two factors is involved, orientation and spacing between their binding sites should have a critical effect on the cooperation. We thus examined the effect of altering their relative positions by using mutated forms of the DC5 enhancer. In the SR mutation, the SOX binding site ATTGTT has been altered to AACAAT, producing CATTGTTG sequence in reverse orientation; the I4a mutation has a 4-bp spacer inserted between the SOX and δEF3 binding sites (Fig. 5A). In gel mobility shift assays, it was confirmed that DC5-SR and DC5-I4a sequences were bound by SOX2 protein to the same extent as DC5-WT (data not shown). As shown in Fig. 5B, both mutations eliminated the DC5 enhancer activity in lens cells. Exogenous expression of SOX2 did not induce any enhancer activity of DC5-SR or DC5-I4a (data not shown). The data strongly support the model that direct protein interaction between SOX1/2/3 and δEF3 is required for SOX1/2/3 to activate DC5.
Activation of the Col2a1 enhancer by SOX9 also requires specific cooperation with another enhancer-binding factor.
Bell et al. (2) and Lefebvre et al. (31) identified COL2A1/Col2a1 as a target gene of SOX9 during chondrogenesis. The human 309-bp COL2A1 enhancer has two SOX9 binding sequences, COL2C1 and COL2C2 (Fig. 6A), both of which are essential for its enhancer function in transgenic mice (2). The COL2C2 sequence, the 18-bp minimal enhancer of Col2a1, is perfectly conserved among human, mouse, and rat cells. In addition, Lefebvre et al. (31) showed that the mouse 48-bp Col2a1 enhancer containing the COL2C2 sequence is activated by SOX9 but not by SOX4 or SOX5. In this study, activation of the COL2C2 enhancer by SOX9 was dependent not only on the binding site of SOX itself but also on a nearby sequence which is likely to be the binding site of another protein factor. This provided us with an opportunity to compare the mechanism of selective action of SOX9 on the Col2a1 enhancer with that of SOX1/2/3 on the DC5 enhancer. We first tested the specificity of SOX9 in activation of the minimal enhancer COL2C2. As shown in Fig. 7A and B, eight copies of the COL2C2 sequence placed upstream of the δ-crystallin promoter were strongly activated by SOX9 in 10T1/2 cells but only marginally by SOX1 and SOX2 and not at all by SOX11. This result indicated that the determinant of SOX specificity resides in the 18-bp sequence of COL2C2.
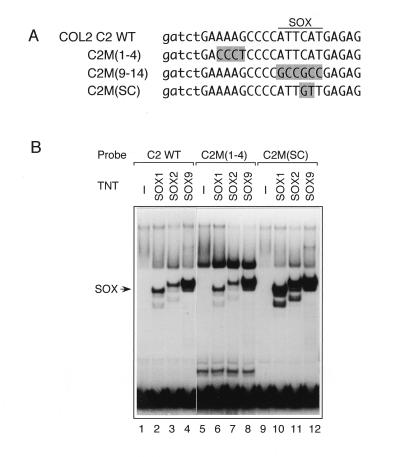
FIG. 6.
Binding of SOX proteins to the Col2a1 enhancer. (A) WT and mutant sequences of the COL2C2 enhancer. To designate COL2C2 mutations, we followed the nucleotide numbering of Lefebvre et al. (32). The C2M(9-14) mutation is the same as C2M in reference 2, which abolishes SOX binding. In C2M(SC), the SOX site was converted to the SOX consensus sequence. In the mutant sequences, altered bases are shaded. Lowercase letters indicate the base sequences added to generate BglII and BamHI restriction sites. (B) Comparison of binding of SOX1, SOX2, and SOX9 to COL2C2 sequences. Gel mobility shift assays were performed with either unprogrammed TNT lysate (−) or S-Tag–SOX proteins (Fig. 2), using the indicated probes. SOX9 bound to the COL2C2 WT and M(1-4) sequences more efficiently than did SOX1 or SOX2. Alteration to C2M(SC) resulted in binding of SOX1 and SOX2 comparable to that of SOX9.
To determine which domain of SOX9 is required for activation of the COL2C2 enhancer, we tested various SOX derivatives, including SOX9 deletion mutants and SOX1-SOX9 chimeric proteins, for the ability to activate COL2C2 in cotransfection assays (Fig. 7B). SOX9ΔC265 failed to activate the COL2C2 enhancer, confirming the previous notion that SOX9 requires the C-terminal activation domain for transactivation (34, 43). The chimeric proteins SOX9-9-1 and SOX1-9-1 were defective in activation of COL2C2, which indicates that activation of COL2C2 requires the SOX9 C-terminal domain. This is similar to the case of activation of DC5, where the SOX1 C-terminal domain was essential. However, in addition to the C-terminal domain, the HMG domain of SOX9 was also required for activation of COL2C2, since SOX9-1-9 and SOX1-1-9 were inefficient, indicating that the HMG domain could not be substituted by that of SOX1. Among SOX1-SOX9 chimeric proteins, only SOX1-9-9 was as active as SOX9, confirming that both the HMG and C-terminal domains of SOX9 are essential to fully activate COL2C2.
To dissect the molecular events involved in selective utilization of SOX9 in activation of the COL2C2 enhancer, we compared the binding affinities of SOX proteins to the COL2C2 sequences. Interestingly, SOX9 bound to the wild-type COL2C2 probe more efficiently than SOX1 and SOX2 (Fig. 6B, lane 2 to 4), while the three proteins bound to the DC5 sequence with similar affinities (Fig. 2C and data not shown).
Differential binding to and requirement of the SOX9 HMG domain in activation of COL2C2 prompted us to examine to what extent the higher affinity of SOX9 to COL2C2 sequence contributes to specific activation by SOX9. To this end, we changed the SOX9 binding sequence ATTCAT in COL2C2 to the SOX consensus ATTGTT (SC mutation), creating COL2C2M(SC) (Fig. 6A). It was confirmed in a gel mobility shift assay that SOX1, -2, and -9 bound to the COL2C2 M(SC) probe with almost the same affinity (Fig. 6B, lane 10 to 12). In transactivation assays using transfected 10T1/2 cells, SOX9 activated the COL2C2 M(SC) enhancer to levels comparable to those of the COL2C2 WT enhancer (Fig. 7C). The SC mutation augmented activation by SOX1 and by SOX9-1-9 only slightly (Fig. 7C), indicating that differential binding of COL2C2 plays only a minor role in the selective activity of SOX9.
Because of the presence of the neighboring binding motif influencing COL2C2 enhancer activity (31), we focused on the interactions with this site. Earlier findings and our results described above suggest that interaction between SOX9 and the partner factor is crucial for activation of the COL2C2 enhancer as observed in regulation of the DC5 enhancer by SOX1/2/3. To analyze the nature of the interaction of SOX9 with the partner factor, we tested the ability of the SOX9(1-264)-VP16 fusion protein (Fig. 4A) to activate transcription through the WT or two mutant forms of COL2C2: COL2C2 M(9-14), in which the SOX9 binding site is mutated in a way to abolish SOX9 binding (2), and COL2C2 M(1-4), in which the putative binding site of the partner factor is ablated without affecting SOX binding (Fig. 6B, lane 6 to 8). Similar mutations have been shown to abolish enhancer activity in chondrocytes (32). We confirmed that exogenous SOX9 could not transactivate expression via mutant sequence COL2C2 M(9-14) or COL2C2 M(1-4) in 10T1/2 cells (Fig. 7Da). Similarly, neither of the mutant forms of the COL2C2 enhancer was activated by SOX9(1-264)-VP16 (Fig. 7Db), which indicates that activation by SOX9(1-264)-VP16 is also dependent on interaction with the partner factor.
DISCUSSION
Although the binding sequences of all known SOX proteins are barely distinguishable from each other, each SOX protein regulates a distinct set of target genes. How are specific SOX proteins used in regulation of particular genes? Further, regulation of a target gene by SOX protein does not always occur but is cell dependent. For instance, SOX1, SOX2, and SOX3 activate expression of crystallin genes only in lens cells, although expression of Sox1, -2, and -3 genes is not confined to the lens but is also found in other tissues, e.g., the central nervous system. What is the mechanism of this cell dependence? To address these related questions, we investigated regulation of the two established SOX target enhancers, the δ1-crystallin DC5 enhancer activated by SOX1/2/3 and the Col2a1 COL2C2 enhancer activated by SOX9. An important clue to the answers was that these enhancers carry not only the binding site of SOX proteins but also an essential element next to it which is considered a putative binding site for a partner factor. The questions concern the nature of the interaction of the SOX and the partner factor in activation of an enhancer.
We analyzed the contribution of individual domains of SOX proteins in the selective activation of the DC5 and COL2C2 enhancers. It was first shown that the process of binding of the individual HMG domains to the DC5 enhancer does not discriminate between the SOX proteins and thus plays no role in this selective action. The chimeric proteins were made in various combinations of the domains derived from SOX1 and SOX9. It was demonstrated that activation of the DC5 enhancer required the SOX1 C-terminal domain, but the HMG domain could be replaced by that of SOX9. By contrast, activation of the COL2C2 enhancer required the SOX9 HMG domain in addition to the SOX9 C-terminal domain. The differential binding affinities of the SOX HMG domains to the COL2C2 element do not account for this specific requirement, and a unique property of the SOX9 HMG domain other than that of DNA binding is essential for activation of the COL2C2 enhancer. These and other data argue that the SOX proteins interact with the partner factor by using an interface involving the proximal half of the C-terminal domain and the HMG domain. The interaction with the partner factor stabilizes binding of SOX proteins to the target site and renders the transactivation potential associated with the C-terminal domain effective. The portions of the interface which are employed in specific interaction with the partner factor are dependent on the particular SOX-partner combination. The rationales for this model are discussed below.
DNA binding by HMG domains of the SOX proteins.
When tested for binding to the DC5 sequence, SOX9 was indistinguishable from SOX1/2/3 in binding affinity and DNA bending. The observed bending angle of 66° is slightly different from those previously reported for SRY (76°) (12, 38), SOX5 (74°) (7), and SOX9 (57°) (31), but these differences are likely ascribed to the difference in the binding sequence used, which is known to affect the degree of bending (38). Consistent with the similar properties of DNA binding and bending, a domain swap experiment indicated that the HMG domain of SOX1 can be replaced by that of SOX9 in activation of the DC5 enhancer. These findings argue against the model that selective DNA binding by individual HMG domains is the major mechanism of the target selection by SOX proteins.
In contrast to the case of the DC5 enhancer, COL2C2 has a SOX binding sequence fairly different from the SOX consensus, and it was bound by SOX9 more strongly than SOX1 and SOX2. This is the first clear observation that each SOX protein may have a sequence preference in DNA recognition. Even in this case, however, the sequence preference cannot be the major determinant of the specificity of SOX action. The COL2C2 M(SC) enhancer was bound equally well by SOX9 and SOX1 HMG domains, yet it was not effectively activated by SOX1 or SOX9-1-9. Therefore, it is a property of the SOX9 HMG domain other than DNA binding that is required. As will be discussed, this requirement concerns the interaction with the partner factor.
Transcriptional activation by the activity of the C-terminal domains requires DNA-binding partner factors.
C-terminal domains of SOX proteins bear the transactivation potential, which is demonstrated by the fusion of these domains with GAL4DBD. However, this activation potential is cryptic in the native protein without cooperation of the specific partner factor which is available only in the proper context of the enhancer sequence and in the proper cell types.
Mutational analysis of SOX1 (Fig. 3A) and SOX2 (25) revealed that the distal part of the C-terminal domain is required for transactivation of the DC5 enhancer. The transactivation potential of C-terminal domains of SOX1/2/3 and SOX9 displayed by fusion with GAL4DBD was not cell dependent (Fig. 3C) and does not account for the cell-specific activities of SOX proteins.
An important observation was made in assays using the artificial SOX proteins in which the C-truncated SOX2 and SOX9 were fused to the strong activation domain VP16. These SOX-VP16 fusion proteins showed the same specificity of transactivation as native SOX2 and SOX9, still being dependent on interaction with the partner factor to activate the DC5 and COL2C2 enhancers, respectively. Thus, activation domains of VP16, SOX1/2, and SOX9 all functioned non-cell specifically when bound to DNA through GAL4DBD, but the same activation domains were cell and enhancer specific when bound to DNA through the SOX HMG domain. These different outcomes between HMG domain-mediated and GAL4-mediated DNA bindings probably lie in their affinity to DNA. GAL4DBD binds DNA as a dimer at a high affinity (Kd = 2 × 10−11 M) (3), whereas SOX or SRY binds as a monomer at moderate affinities, e.g., Kds of 2 × 10−9 M for SOX5 (7) and 10−8 to 3 × 10−9 M for SRY (16, 38), (but 3 × 10−11 M for SOX4 [46]). The Kd of SOX1 was estimated at ∼3 × 10−9 M in our preliminary experiment (23). Presumably SOX, when it binds DNA by its HMG domain, is not stable enough to initiate transactivation and requires a binding partner to achieve stable binding to the regulatory target site. Kjærulff et al. (29) reported a similar observation for the yeast HMG protein Ste11; they suggested that Ste11 does not bind to the Ste11 sites of M-specific genes by itself in P cells but instead binds to the sites through interaction with Mat1-Mc and acquires the capacity to activate transcription in M cells.
It is highly possible that SOX1/2/3 directly interact with δEF3 upon binding to the DC5 enhancer and make a complex. This view is supported by the finding that displacement of the δEF3 binding site by 4 bp away from the original position or flipping the SOX binding site while maintaining the same distance from the δEF3 site resulted in total loss of enhancer activity (Fig. 5).
Under ordinary conditions of gel mobility shift assay using lens nuclear extract, a δEF3-DC5 complex has not been detected, but a lens-specific δEF3 footprint on the genomic DC5 sequence has been demonstrated by in vivo footprinting using isolated nuclei (41). Molecular cloning of δEF3 which is under way will clarify the nature of interaction between SOX1/2/3 and δEF3.
The interface of interaction between the SOX proteins and their partner factors.
The interaction between SOX1/2/3 and δEF3 must involve the HMG-proximal portion of the C-terminal domain (Fig. 8A). This is most clearly demonstrated by the result that SOX2(4-183), which by itself was defective in activating the DC5 enhancer, strongly activated the same enhancer by fusion of the VP16 activation domain (Fig. 4C); this activation was totally dependent on the δEF3 binding site and occurred only in lens cells (i.e., dependent on δEF3). The same analysis further indicated that the HMG domain of SOX2 is also involved in the interaction with δEF3. Although the SOX(4-120)-VP16 fusion protein activated DC5 enhancer only marginally, this activating effect was still dependent on δEF3 (Fig. 4D). Corresponding domains of SOX9 showed no such interaction with δEF3 (Fig. 4E). Thus, the HMG domain and the proximal portion of the C-terminal domain of SOX1/2/3 form the interface between δEF3 and the SOX proteins. However, since SOX1-9-1 strongly activated the DC5 enhancer in a δEF3-dependent manner (data not shown), it appears that the C-terminal domain of SOX1/2/3 provides the major and determinative portion of the interface.
FIG. 8.
Model of SOX-partner factor interactions essential for activation of a specific target gene by a SOX protein. (A) Functional domains of SOX proteins. Major transactivation potential is mapped to the distal portion of the C-terminal domain for both SOX2 (25) and SOX9 (34, 43). The interface region possibly interacting with the partner factors is indicated by broken lines. (B) Model for selection of a target site by SOX1/2/3 and SOX9. In the absence of the partner factor, binding of the SOX proteins to the SOX site occurs with low affinity (Kd of 3 × 10−9 M for SOX1 binding to DC5) and is not stable enough to exert a significant effect in an in vivo situation. Only when the partner factor lies next to the SOX site do SOX proteins more stably bind to their sites (e.g., Kd of 10−11 M, assuming affinity analogous to that for the GAL4DBD-GAL4 binding site) and elicit transactivation.
Participation of the HMG domain in interfacing between a SOX protein and the partner factor is more clearly demonstrated in the case of SOX9. SOX9(1-264)-VP16 efficiently activated COL2C2 (Fig. 7D), indicating that the region from aa 1 to 264 spanning the HMG domain and the proximal portion of the C-terminal domain was sufficient for interaction with the partner factor. However, SOX1-SOX9 chimeric proteins must have the SOX9 HMG domain and the SOX9 C-terminal domain to activate the COL2C2 enhancer (Fig. 7B). The HMG domain of SOX9 could not be replaced by that of SOX1 even when the SOX site of COL2C2 was changed to COL2C2M(SC) sequence, which is bound efficiently by the SOX1 HMG domain (Fig. 7C).
Thus, although the proximal portion of the C-terminal domain and the HMG domain of a SOX protein may generally form the interface to the partner factors (Fig. 8A), which portion of the interface provides the major interaction sites seems to depend greatly on the nature of the partner factor of the particular enhancer to be activated by the SOX protein, as indicated by the difference between SOX1/2 and SOX9. This notion has been further corroborated by a recent report on the analysis of the fibroblast growth factor 4 enhancer (1). Activity of this enhancer in embryonal carcinoma cells is dependent on synergistic interaction of SOX2 and Oct-3, which bind nearby sites (50), and this synergism relies on direct interactions between the SOX2 HMG domain and Oct-3 POU domain (1).
Consideration of analogous cases.
LEF-1 is one of the best-characterized members of the TCF/SOX family. It has been shown that LEF-1 cannot activate transcription on its own but that it must act in concert with factors that bind to other nearby sites in the T-cell receptor alpha-chain enhancer. In contrast to the C-terminal activation domains of SOX, the context-dependent transactivation domain of LEF-1, when linked to GAL4DBD, is still dependent on the enhancer context and does not activate promoters bearing only GAL4 DNA-binding sites (4, 15). In this respect, SOX and LEF-1 proteins are very different, although they have many properties in common. Considering, however, that the DNA-binding affinity of LEF-1 (Kd = 10−9 M [14]) is comparable to that of SOX proteins, protein interaction between LEF-1 and other enhancer binding factors may be crucial to form a stable complex on the enhancer to activate transcription.
Other transcription factor families might select their target genes in a manner similar to that of SOX proteins. For example, the homeodomain is similar to the HMG domain in that it binds to DNA as a monomer with dissociation constants of 10−8 to 10−9 M and in that homeodomains have only a 6-bp consensus binding site (21). Moreover, it is shown that some of the HOX proteins cooperatively bind DNA with the partner PBX/EXD proteins, which is suggested to be important for HOX proteins to carry out their in vivo functions (33). Thus, the model proposed here for SOX proteins provides a paradigm of a general mechanism of target gene selection.
The mechanism of specific action of the SOX proteins.
We have investigated the mechanism by which a given SOX protein activates a limited set of genes in a cell-specific manner. What is the mechanism behind the regulation of δ1-crystallin and Col2a1 genes through their SOX-dependent enhancers? SOX2 expression is activated in the lens area of lateral head ectoderm by induction by the retina primordium and continues to be expressed together with SOX1 and SOX3 in the lens cells (24, 25), but this induction alone does not explain the lens-specific expression of δ-crystallin, since SOX1, SOX2, and SOX3 are expressed in the central nervous system and several other tissues as well (6, 25, 37, 39, 42, 45). It is likely that the tissue distribution of the partner factor δEF3 is highly restricted. In fact, we could not detect the activity of δEF3, which activates the DC5 enhancer in cooperation with SOX2, in cells other than the lens (24). It is likely that the unique combination of SOX1/2/3 and δEF3 is present only in the lens cells, and this is the basis of lens-specific activation of the δ-crystallin enhancer.
By contrast, it has been shown that high Sox9 expression closely parallels Col2a1 expression (34, 51). Lefebvre et al. (31) and we have indeed observed activation of COL2C2 enhancer by exogenous SOX9 in a variety of cell types (data not shown), indicating that the SOX9 partner factor is widely distributed. Because of this, activation of the Col2a1 gene appears primarily dependent on the expression of SOX9, and the specific interaction of SOX9 and its partner factor may be primarily employed in precluding the action of an inappropriate class of SOX protein from Col2a1 activation. In fact, ectopic expression of SOX9 in transgenic mice activated expression of the endogenous Col2a1 gene and COL2A1 enhancer-bearing reporter gene in the majority of the ectopic SOX9 sites (2). However, there is a difference in expression between Sox9 and Col2a1. For example, the genital ridge expresses Sox9 at fairly high levels but no Col2a1 transcript is detectable (9, 27, 34, 51), implying that the SOX9 partner factor employed in activation of COL2C2 is lacking. Consistent with this notion is the observation that in the above-mentioned transgenic mice with ectopic SOX9 expression, transactivation of the COL2A1 enhancer reporter gene did not occur in some sites of ectopic SOX9 expression (2).
The model of target site selection by the SOX HMG domain proteins derived from the present study is summarized in Fig. 8. (i) In an in vivo situation, the HMG domain of SOX is not sufficient to form a stable protein-DNA complex at a SOX site by itself. (ii) SOX proteins require binding partner factors unique to each SOX or SOX subfamily in order to achieve stable complex formation with the target DNA, which leads to transcriptional activation. (iii) The HMG domain and proximal portion of the C-terminal domain of the SOX proteins provide the interface with partner factors. (iv) The portions of the interface used in selection of the authentic partner factor vary depending on the combination of the SOX and its partner.
ACKNOWLEDGMENTS
We are grateful to S. Adhya for his gift of the pBend2 vector.
This work was supported by grants from the Ministry of Education, Science and Culture of Japan and Science and Technology Agency of Japan to Y.K. and H.K.
REFERENCES
- 1.Ambrosetti D-C, Basilico C, Dailey L. Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct-3 depends on protein-protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol Cell Biol. 1997;17:6321–6329. doi: 10.1128/mcb.17.11.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell D M, Leung K K H, Wheatley S C, Ng L J, Zhou S, Ling K W, Sham M H, Koopman P, Tam P P L, Cheah K S E. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 3.Carey M, Kakidani H, Leatherwood J, Mostashari F, Ptashne M. An amino-terminal fragment of GAL4 binds DNA as a dimer. J Mol Biol. 1989;209:423–432. doi: 10.1016/0022-2836(89)90007-7. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson P, Waterman M L, Jones K A. The hLEF-1/TCF-1α HMG protein contains a context-dependent transcriptional activation domain that induces the TCRα enhancer in T cells. Genes Dev. 1993;7:2418–2430. doi: 10.1101/gad.7.12a.2418. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow P N, Lovell-Badge R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development. 1996;122:509–520. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- 7.Connor F, Cary P D, Read C M, Preston N S, Driscoll P C, Denny P, Crane-Robinson C, Ashworth A. DNA binding and bending properties of the post-meiotically expressed Sry-related protein Sox-5. Nucleic Acids Res. 1994;22:3339–3346. doi: 10.1093/nar/22.16.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor F, Wright E, Denny P, Koopman P, Ashworth A. The Sry-related HMG box-containing Sox6 is expressed in the adult testis and developing nervous system of the mouse. Nucleic Acids Res. 1995;23:3365–3372. doi: 10.1093/nar/23.17.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva S M, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 10.Denny P, Swift S, Brand N, Dabhade N, Barton P, Ashworth A. A conserved family of genes related to the testis determining gene, SRY. Nucleic Acids Res. 1992;20:2887. doi: 10.1093/nar/20.11.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denny P, Swift S, Connor F, Ashworth A. An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein. EMBO J. 1992;11:3705–3712. doi: 10.1002/j.1460-2075.1992.tb05455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari S, Harley V R, Pontiggia A, Goodfellow P N, Lovell-Badge R, Bianchi M E. SRY, like HMG1, recognizes sharp angles in DNA. EMBO J. 1992;11:4497–4506. doi: 10.1002/j.1460-2075.1992.tb05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiering S N, Roederer M, Nolan G P, Micklem D R, Parks D R, Herzenberg L A. Improved FACS-Gal: flow cytometric analysis and sorting of viable eukaryotic cells expressing reporter gene constructs. Cytometry. 1991;12:291–301. doi: 10.1002/cyto.990120402. [DOI] [PubMed] [Google Scholar]
- 14.Giese K, Amsterdam A, Grosschedl R. DNA-binding properties of the HMG domain of the lymphoid-specific transcriptional regulator LEF-1. Genes Dev. 1991;5:2567–2578. doi: 10.1101/gad.5.12b.2567. [DOI] [PubMed] [Google Scholar]
- 15.Giese K, Grosschedl R. LEF-1 contains an activation domain that stimulates transcription only in a specific context of factor-binding sites. EMBO J. 1993;12:4667–4676. doi: 10.1002/j.1460-2075.1993.tb06155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giese K, Pagel J, Grosschedl R. Distinct DNA-binding properties of the high mobility group domain of murine and human SRY sex-determining factors. Proc Natl Acad Sci USA. 1994;91:3368–3372. doi: 10.1073/pnas.91.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 18.Hargrave M, Wright E, Kun J, Emery J, Cooper L, Koopman P. Expression of the Sox11 gene in mouse embryos suggests roles in neuronal maturation and epithelio-mesenchymal induction. Dev Dyn. 1997;210:79–86. doi: 10.1002/(SICI)1097-0177(199710)210:2<79::AID-AJA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Harley V R, Lovell-Badge R, Goodfellow P N. Definition of a consensus DNA binding site for SRY. Nucleic Acids Res. 1994;22:1500–1501. doi: 10.1093/nar/22.8.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi S, Goto K, Okada T S, Kondoh H. Lens-specific enhancer in the third intron regulates expression of the chicken δ1-crystallin gene. Genes Dev. 1987;1:818–828. doi: 10.1101/gad.1.8.818. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi S, Scott M P. What determines the specificity of action of Drosophila homeodomain proteins? Cell. 1990;63:883–894. doi: 10.1016/0092-8674(90)90492-w. [DOI] [PubMed] [Google Scholar]
- 22.Kamachi Y, Kondoh H. Overlapping positive and negative regulatory elements determine lens-specific activity of the δ1-crystallin enhancer. Mol Cell Biol. 1993;13:5206–5215. doi: 10.1128/mcb.13.9.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamachi, Y., and H. Kondoh. Unpublished data.
- 24.Kamachi Y, Sockanathan S, Liu Q, Breitman M, Lovell-Badge R, Kondoh H. Involvement of SOX proteins in lens-specific activation of crystallin genes. EMBO J. 1995;14:3510–3519. doi: 10.1002/j.1460-2075.1995.tb07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development. 1998;125:2521–2532. doi: 10.1242/dev.125.13.2521. [DOI] [PubMed] [Google Scholar]
- 26.Kanai Y, Kanai-Azuma M, Noce T, Saido T C, Shiroishi T, Hayashi Y, Yazaki K. Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis. J Cell Biol. 1996;133:1–15. doi: 10.1083/jcb.133.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kent J, Wheatley S C, Andrews J E, Sinclair A H, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Zwieb C, Wu C, Adhya S. Bending of DNA by gene regulatory proteins: construction and use of a DNA bending vector. Gene. 1989;85:15–23. doi: 10.1016/0378-1119(89)90459-9. [DOI] [PubMed] [Google Scholar]
- 29.Kjærulff S, Dooijes D, Clevers H, Nielsen O. Cell differentiation by interaction of two HMG-box proteins: Mat1-Mc activates M cell-specific genes in S. pombe by recruiting the ubiquitous transcription factor Ste11 to weak binding sites. EMBO J. 1997;16:4021–4033. doi: 10.1093/emboj/16.13.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebsbach P H, Nakata K, Bernier S M, Hatano O, Miyashita T, Rhodes C S, Yamada Y. Identification of a minimum enhancer sequence for the type II collagen gene reveals several core sequence motifs in common with the link protein gene. J Biol Chem. 1996;271:4298–4303. doi: 10.1074/jbc.271.8.4298. [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre V, Huang W, Harley V R, Goodfellow P N, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the proα1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefebvre V, Zhou G, Mukhopadhyay K, Smith C N, Zhang Z, Eberspaecher H, Zhou X, Sinha S, Maity S N, de Crombrugghe B. An 18-base-pair sequence in the mouse proα1(II) collagen gene is sufficient for expression in cartilage and binds nuclear proteins that are selectively expressed in chondrocytes. Mol Cell Biol. 1996;16:4512–4523. doi: 10.1128/mcb.16.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mann R S, Chan S-K. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 34.Ng L J, Wheatley S, Muscat G E O, Conway-Campbell J, Bowles J, Wright E, Bell D M, Tam P P L, Cheah K S E, Koopman P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 35.Nishiguchi S, Wood H, Sockanathan S, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the γ-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–781. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pevny L H, Lovell-Badge R. Sox genes find their feet. Curr Opin Genet Dev. 1997;7:338–344. doi: 10.1016/s0959-437x(97)80147-5. [DOI] [PubMed] [Google Scholar]
- 37.Pevny L H, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- 38.Pontiggia A, Rimini R, Harley V R, Goodfellow P N, Lovell-Badge R, Bianchi M E. Sex-reversing mutations affect the architecture of SRY-DNA complexes. EMBO J. 1994;13:6115–6124. doi: 10.1002/j.1460-2075.1994.tb06958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rex M, Orme A, Uwanogho D, Tointon K, Wigmore P M, Sharpe P T, Scotting P J. Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn. 1997;209:323–332. doi: 10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 40.Sadowski I, Ma J, Triezenberg S, Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 41.Sekido, R., Y. Kamachi, and H. Kondoh. Unpublished data.
- 42.Streit A, Sockanathan S, Pérez L, Rex M, Scotting P J, Sharpe P T, Lovell-Badge R, Stern C D. Preventing the loss of competence for neural induction: HGF/SF, L5 and Sox-2. Development. 1997;124:1191–1202. doi: 10.1242/dev.124.6.1191. [DOI] [PubMed] [Google Scholar]
- 43.Südbeck P, Schmitz M L, Baeuerle P A, Scherer G. Sex reversal by loss of the C-terminal transactivation domain of human SOX9. Nat Genet. 1996;13:230–232. doi: 10.1038/ng0696-230. [DOI] [PubMed] [Google Scholar]
- 44.Suemori H, Kadokawa Y, Goto K, Araki I, Kondoh H, Nakatsuji N. A mouse embryonic stem cell line showing pluripotency of differentiation in early embryos and ubiquitous β-galactosidase expression. Cell Differ Dev. 1990;29:181–186. doi: 10.1016/0922-3371(90)90120-l. [DOI] [PubMed] [Google Scholar]
- 45.Uwanogho D, Rex M, Cartwright E J, Pearl G, Healy C, Scotting P J, Sharpe P T. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- 46.van de Wetering M, Oosterwegel M, van Norren K, Clevers H. Sox-4, an Sry-like HMG box protein, is a transcriptional activator in lymphocytes. EMBO J. 1993;12:3847–3854. doi: 10.1002/j.1460-2075.1993.tb06063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli F D, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 48.Wright E, Hargrave M R, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryo. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- 49.Wright E M, Snopek B, Koopman P. Seven new members of the Sox gene family expressed during development. Nucleic Acids Res. 1993;21:744. doi: 10.1093/nar/21.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan H, Corbi N, Basilico C, Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995;9:2635–2645. doi: 10.1101/gad.9.21.2635. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Q, Eberspaecher H, Lefebvre V, de Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]