ABSTRACT
African swine fever is a devastating disease of swine caused by African swine fever virus (ASFV). The pathogenesis of the disease remains largely unknown, leaving the spread of the disease uncontrolled in many countries and regions. Here, we identified E120R, a structural protein of ASFV, as a key virulence factor and late-phase-expressed protein of the virus. E120R revealed an activity to suppress the host antiviral response through blocking beta interferon (IFN-β) production, and the amino acids (aa) at sites 72 and 73 (amino acids 72-73) in the C-terminal domain were essential for this function. E120R interacted with interferon regulatory factor 3 (IRF3) and interfered with the recruitment of IRF3 to TANK-binding kinase 1 (TBK1), which in turn suppressed IRF3 phosphorylation, decreasing interferon production. A recombinant mutant ASFV was further constructed to confirm the claimed mechanism. The ASFV lacking the complete E120R region could not be rescued, whereas the virus could tolerate the deletion of the 72nd and 73rd residues in E120R (ASFV E120R-Δ72-73aa). ASFV E120R with the two-amino-acid deletion failed to interact with IRF3 during ASFV E120R-Δ72-73aa infection, and the viral infection activated IRF3 phosphorylation highly and induced more robust type I interferon production than its parental ASFV. An unbiased transcriptome-wide analysis of gene expression also confirmed that considerably more IFN-stimulated genes (ISGs) were detected in ASFV E120R-Δ72-73aa-infected porcine alveolar macrophages (PAMs) than in wild-type ASFV-infected PAMs. Together, our findings have identified a novel mechanism evolved by ASFV to inhibit the host antiviral response, and they provide a new target for guiding the development of ASFV live-attenuated vaccine.
IMPORTANCE African swine fever is a highly contagious animal disease affecting the pig industry worldwide, which has brought enormous economic losses. Infection by the causative agent, African swine fever virus (ASFV), causes severe immunosuppression during viral infection, contributing to serious clinical manifestations. Therefore, identification of the viral proteins involved in immunosuppression is critical for ASFV vaccine design and development. Here, for the first time, we demonstrated that E120R protein, a structural protein of ASFV, played an important role in suppression of interferon regulatory factor 3 (IRF3) phosphorylation and type I interferon production by binding to IRF3 and blocking the recruitment of IRF3 to TANK-binding kinase 1 (TBK1). Deletion of the crucial binding sites in E120R critically increased the interferon response during ASFV infection. This study explored a novel antagonistic mechanism of ASFV, which is critical for guiding the development of ASFV live-attenuated vaccines.
KEYWORDS: ASFV, E120R, cGAS, IRF3, immune evasion
INTRODUCTION
African swine fever virus (ASFV) belongs to the genus Asfivirus in the family Asfarviridae and has a double-stranded DNA molecule of 170 to 190 kb encoding more than 150 viral proteins, which play important roles in viral assembly, viral replication, virus-host interaction, and immune evasion (1–6). A complex enveloped DNA virus, ASFV is responsible for African swine fever disease (ASF), which is one of the most serious viral diseases infecting pigs (7). The development of a vaccine has been greatly hindered by knowledge gaps regarding disease pathogenesis and immune evasion (8). Despite extensive research, there are no available vaccines against ASF as of now, and the disease has spread to many countries and regions (9, 10). Depletion of the virulence factors from field viruses to generate live-attenuated viruses (LAVs) is the most promising strategy for development of efficient vaccines so far (11). Therefore, it is critical to identify the virulence and immunosuppressive factors to provide potential targets for vaccine design.
ASFV was originally isolated and identified in Kenya in the 1920s (12), and it subsequently spread to Europe, including Italy, France, Spain, Portugal, Georgia, Ukraine, Estonia, Romania, Moldova, Poland, and Czech Republic (13). To date, it has been identified and reported in more than 37 countries (9, 10). In China, the first occurrence of ASFV infection was reported in Heilongjiang Province in August 2018 and was characterized by obvious enlargement of the spleen and generalized severe hemorrhage in pigs (9, 14, 15). ASFV infections were subsequently reported in more than 23 provinces or regions in China (9). ASFV has threatened more than 50% of the domestic pig population in China, leading to severe economic losses to the pig industry (9).
The innate immune response is the first line of the host’s defense against invading pathogens. Host pattern recognition receptors (PRRs) interact with pathogen-associated molecular patterns (PAMPs) and then initiate the innate immune response. Multiple cytosolic DNA sensors, including cyclic GMP-AMP synthase (cGAS), interferon gamma (IFN-γ)-inducible protein 16, DEAD box helicase 41, DNA-dependent activator of interferon regulatory factors, and RNA polymerase III, have been reported (16). Of these sensors, cGAS is widely studied and plays an important role in recognizing cytosolic or viral DNA (17–19). The cGAS-stimulator of interferon genes (STING) pathway is crucial for the induction of type I IFN production during DNA virus infection. cGAS is activated by viral DNA, which leads to its interaction with STING and further results in the activation of TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3) to induce the production of IFN-β (20).
ASFV, as a double-stranded DNA virus, is recognized by cGAS (21). ASFV infection controls cGAS/STING pathway activation and regulates IFN-β production (21, 22). A recent study has reported that the ASFV protein DP96R inhibits the cGAS/STING/TBK1 signaling pathway by inhibiting TBK1 function (23). E120R, a structural protein of ASFV, is approximately 120 amino acids long (24, 25). E120R is involved in ASFV transport from assembly sites to the plasma membrane, which indicates that E120R plays an important role in virus dissemination but not in virus infectivity (26). The impact of E120R on the host innate immune response remains unknown. In the present study, we found that overexpression of E120R suppressed cGAS/STING-triggered IFN-β promoter activation. E120R inhibited cGAS/STING-induced IFN-β mRNA and protein expression, which was dependent on inhibiting IRF3 phosphorylation. Deletion of E120R was lethal to the virus, suggesting the critical role of E120R for ASFV survival. The amino acids (aa) at sites 72 and 73 (amino acids 72-73, or 72-73aa) in the C-terminal domain of E120R were essential for this inhibitory activity. These two amino acids in E120R critically increased the IFN response in porcine alveolar macrophages (PAMs) and resulted in significantly attenuated replication of the virus. These data clarified a novel antagonistic mechanism of ASFV mediated by the E120R protein and suggested that the E120R gene can be used as a candidate target gene for the ASFV live-attenuated vaccines.
RESULTS
E120R inhibited cGAS/STING-mediated IFN-β activation in HEK-293T cells.
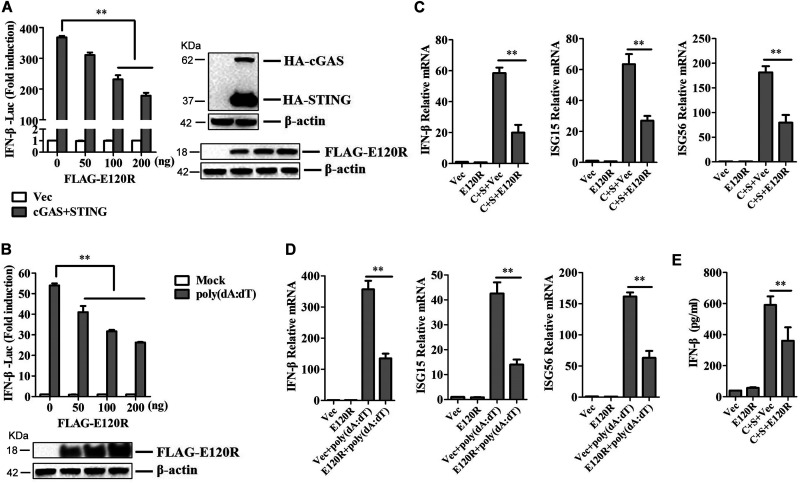
To explore the impact of E120R protein on cGAS/STING-induced signaling responses, HEK-293T cells were transfected with IFN-β-Luc expression plasmid and pRL-TK plasmid along with FLAG vector or FLAG-tagged-E120R (FLAG-E120R), hemagglutinin-tagged-cGAS (HA-cGAS), and HA-STING expression plasmids. At 24 h posttransfection (hpt), the IFN-β promoter activities were determined by using a Dual-Luciferase assay kit. Overexpression of E120R suppressed cGAS/STING-triggered IFN-β promoter activation in a dose-dependent manner in HEK-293T cells (Fig. 1A). To further confirm the impact of E120R on IFN-β promoter activation, HEK-293T cells were cotransfected with FLAG vector or FLAG-E120R and IFN-β-Luc expression plasmids, as well as pRL-TK plasmid. At 24 hpt, the cells were treated with the synthetic double-stranded DNA (dsDNA)-mimetic poly(dA·dT) for 12 h, and the activation of the IFN-β promoter was evaluated. E120R also inhibited poly(dA·dT)-induced IFN-β promoter activation in a dose-dependent manner (Fig. 1B).
FIG 1.
E120R inhibited IFN-β production in HEK-293T cells. (A) HEK-293T cells were transfected with 0.1 μg/well of IFN-β-Luc expression plasmid and 0.01 μg/well of pRL-TK plasmid along with 0.1 μg/well of HA-cGAS and HA-STING expression plasmids, as well as increasing doses of FLAG-E120R expression plasmid (0, 50, 100, and 200 ng). At 24 hpt, the promoter activation of IFN-β was determined by using the Dual-Luciferase assay kit. Three independent experiments were performed with two technical replicates. (B) HEK-293T cells were transfected with 0.1 μg/well of IFN-β-Luc expression plasmid and 0.01 μg/well of pRL-TK plasmid along with increasing doses of FLAG-E120R expression plasmid (0, 50, 100, and 200 ng). At 24 hpt, cells were transfected with poly(dA·dT) (2 μg/ml) for 12 h. The promoter activation of IFN-β was evaluated through the Dual-Luciferase assay. Three independent experiments were performed with two technical replicates. (C) HEK-293T cells cultured in 6-well plates were transfected with 1 μg each of HA-cGAS and HA-STING expression plasmids and 1 μg each of FLAG vector or FLAG-E120R expression plasmid. At 24 hpt, the mRNA expression levels of IFN-β, ISG15, and ISG56 were determined by qPCR assay. Three independent experiments were performed with two technical replicates. C, cGAS; S, STING. (D) HEK-293T cells cultured in 6-well plates were transfected with 1 μg of FLAG vector or FLAG-E120R expression plasmid. At 24 hpt, cells were transfected with poly(dA·dT) (2 μg/ml) for 12 h, and the mRNA expression levels of IFN-β, ISG15, and ISG56 were measured by qPCR assay. Three independent experiments were performed with two technical replicates. (E) Transfection experiments were performed as described in the legend to panel C. The supernatant was collected, and the amounts of secreted IFN-β protein were measured using an ELISA kit. Three technical replicates were performed. Error bars show standard deviations. **, P < 0.01.
To investigate whether E120R protein affected the expression of IFN-β and IFN-stimulated genes (ISGs), we measured the mRNA expression of antiviral genes in cells that were cotransfected with HA-cGAS and HA-STING expression plasmids or treated with poly(dA·dT). The results showed that E120R inhibited both cGAS/STING-induced (Fig. 1C) and poly(dA·dT)-induced (Fig. 1D) IFN-β, ISG15, and ISG56 mRNA expression. In addition, E120R significantly inhibited cGAS/STING-induced IFN-β protein expression (Fig. 1E). Taken together, these results indicated that ASFV E120R inhibited cGAS/STING- and poly(dA·dT)-induced IFN-β production in the model cell line.
E120R inhibited cGAS/STING-mediated IFN-β activation in PK-15 cells.
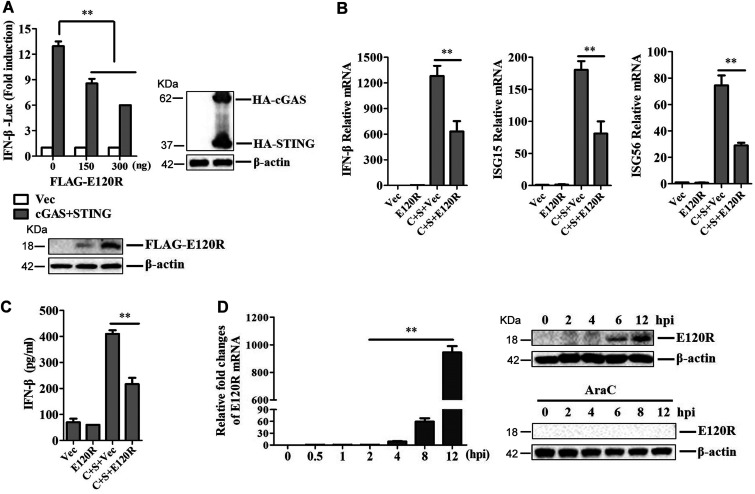
To further confirm the inhibitory effect of E120R on IFN-β activation, porcine kidney 15 (PK-15) cells were transfected with porcine IFN-β-Luc expression plasmid and pRL-TK plasmid, along with FLAG vector or FLAG-E120R, HA-cGAS, and HA-STING expression plasmids. At 24 hpt, the activation of the IFN-β promoter was determined. E120R inhibited cGAS/STING-induced porcine IFN-β promoter activation in a dose-dependent manner (Fig. 2A). The expression of IFN-β, ISG15, and ISG56 in PK-15 cells that were cotransfected with HA-cGAS and HA-STING expression plasmids was also evaluated. As expected, E120R significantly inhibited cGAS/STING-induced IFN-β, ISG15, and ISG56 mRNA expression (Fig. 2B). E120R also inhibited cGAS/STING-induced IFN-β protein secretion (Fig. 2C). Taken together, these data indicated that E120R suppressed the activation of the cGAS/STING pathway and blocked type I IFN production in porcine cells.
FIG 2.
E120R inhibited IFN-β activation in PK-15 cells. (A) PK-15 cells were transfected with IFN-β-Luc expression plasmid and pRL-TK plasmid along with HA-cGAS and HA-STING expression plasmids, as well as increasing doses of FLAG-E120R expression plasmid (0, 150, and 300 ng). At 24 hpt, the promoter activation of IFN-β was determined by using the Dual-Luciferase assay kit. The expression of HA-cGAS, HA-STING, and FLAG-E120R was confirmed by Western blotting. (B and C) PK-15 cells cultured in 6-well plates were transfected with 1 μg each of HA-cGAS and HA-STING expression plasmids and 1 μg each of FLAG vector or FLAG-E120R expression plasmid. (B) At 24 hpt, the mRNA expression levels of IFN-β, ISG15, and ISG56 were determined by qPCR assay. (C) The supernatants were collected, and the amounts of secreted IFN-β protein were measured using an ELISA kit. C, cGAS; S, STING. (D) PAMs were infected with ASFV for 0, 0.5, 1, 2, 4, 6, 8, or 12 h with or without AraC (40 μg/ml). Expression of E120R mRNA was determined by qPCR assay. Expression of E120R protein at 0, 2, 4, 6, 8, or 12 hpi was detected by Western blotting. Three independent experiments were performed for all of the above-described experiments. Error bars show standard deviations. **, P < 0.01.
The expression of E120R in PAMs during ASFV infection was subsequently investigated to confirm its presence in ASFV-infected cells. The mRNA expression of E120R increased from 4 h postinfection (hpi) and reached an extremely high level at 12 hpi, and a small amount of E120R protein could be detected at 6 hpi during ASFV infection in PAMs (Fig. 2D). E120R protein has different low-molecular-mass forms, ranging from 12 to 25 kDa (27). Here, a band of about 18 kDa was detected in ASFV-infected PAMs using an anti-E120R polyclonal antibody prepared in our laboratory (Fig. 2D). To further confirm the presence of E120R during ASFV infection, cytosine arabinoside (AraC), an inhibitor of late-phase ASFV gene replication, was used in our study. The results showed that incubation with AraC completely inhibited the expression of E120R (Fig. 2D). This suggested that E120R was synthesized in the late stage after ASFV infection, and thus, the E120R gene was a late-phase-expressed gene of ASFV.
Amino acids 72-73 in E120R were responsible for its inhibitory effect on type I IFN production.
E120R is a structural protein of ASFV that is composed of 123 amino acids. To identify the crucial domains or sites in E120R that were associated with its immunosuppressive function, two plasmids expressing mutants FLAG-E120R 1-61aa and FLAG-E120R 62-123aa were first generated. HEK-293T cells were cotransfected with FLAG vector or FLAG-E120R or FLAG-E120R mutant expression plasmid and HA-cGAS and HA-STING expression plasmids. The cGAS/STING-induced activation of the IFN-β promoter was evaluated at 24 hpt. E120R and E120R 62-123aa but not E120R 1-61aa suppressed IFN-β promoter activation (Fig. 3A), suggesting that the C-terminal domain of E120R was responsible for blocking cGAS/STING signaling pathway activation. The expression of E120R 1-61aa and E120R 62-123aa was verified by Western blotting (Fig. 3A).
FIG 3.
Amino acids 72 and 73 of E120R were essential for the E120R-mediated inhibitory effect on IFN-β production. (A to D) HEK-293T cells were transfected with 0.1 μg/well of IFN-β-Luc expression plasmid and 0.01 μg/well of pRL-TK plasmid along with 0.1 μg/well each of HA-cGAS and HA-STING expression plasmids and FLAG vector or FLAG-E120R or FLAG-E120R mutant expression plasmid. At 24 hpt, the promoter activation of IFN-β was determined by using the Dual-Luciferase assay kit. The expression of various E120R mutants was determined by Western blotting. C, cGAS; S, STING. Three independent experiments were performed with two technical replicates. Error bars show standard deviations. *, P < 0.05; **, P < 0.01.
We subsequently screened the functional domains in E120R 62-123aa that were essential for its inhibitory activity. A series of truncated E120R mutants (E120R Δ62-65, Δ66-70, Δ71-75, Δ76-80, Δ81-85, Δ86-90, Δ91-95, Δ96-100, Δ101-105, Δ106-110, Δ111-115, and Δ116-123) were constructed and used for the IFN-β-Luc reporter assay. The IFN-β promoter activity in the cells transfected with FLAG vector or FLAG-E120R or FLAG-E120R mutant expression plasmid and HA-cGAS and HA-STING expression plasmids was evaluated. Deletion of the amino acid 71 to 75 region in E120R completely abrogated the inhibitory effect of E120R on IFN-β promoter activation, while E120R and other E120R truncates still inhibited the IFN-β promoter activation (Fig. 3B), suggesting that the amino acid 71 to 75 region of E120R was essential for its inhibitory activity against type I IFN production. The critical sites in the amino acid 71 to 75 region of E120R that were essential for the inhibitory activity were further identified. Five single-site mutants of E120R (E120R E71A [E120R bearing a mutation of E to A at residue 71], E72A, E73A, D74A, and S75A) were constructed and analyzed. The results showed that mutation of amino acid 72 or 73 in E120R abrogated the inhibitory activity of E120R on IFN-β promoter activation (Fig. 3C). Therefore, we subsequently constructed a truncated mutant protein with deletion of both the 72nd and 73rd residues in E120R, which was designated FLAG-E120R dm (FLAG-E120R-Δ72-73aa). Obviously, the deletion of residues 72 and 73 in E120R (E120R-Δ72-73aa) failed to inhibit cGAS/STING-mediated IFN-β promoter activation (Fig. 3D). These results indicated that the C-terminal region of E120R suppressed the activation of cGAS/STING signaling pathway, and the amino acids at sites 72 and 73 in E120R were critical for its inhibitory function against type I IFN production.
ASFV E120R-Δ72-73aa recombinant virus infection induced significantly higher IFN-β expression than wild-type (WT) ASFV in PAMs.
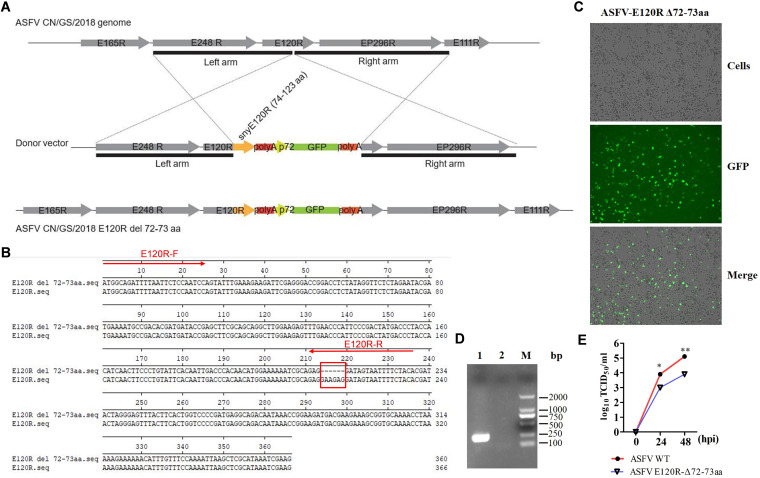
Construction of recombinant virus with a mutation in a viral protein is a good strategy to explore the function of the viral protein (24). The recombinant E120R mutant ASFV was further constructed using homologous recombination (Fig. 4A) to demonstrate both the functional role of E120R and the mechanism involved in the control of the cGAS/STING pathway. Complete deletion of the E120R region led to the inability to rescue ASFV (data not shown), which suggested that the E120R protein was essential for ASFV replication. In contrast, the virus with the deletion of the 72nd and 73rd residues in E120R (ASFV E120R-Δ72-73aa) could be successfully rescued. The deletion of E120R amino acids 72 and 73 was confirmed by the NGS sequencing analysis, which showed that the targeted amino acids were successfully deleted (Fig. 4B) and there were no additional off-target mutations (data not shown). ASFV E120R-Δ72-73aa replicated well in BMDM cells (Fig. 4C). PCR amplification of one fragment of the E120R coding sequence (CDS) using a specific pair of primers confirmed that the recombinant ASFV was obtained without contamination by its parental ASFV (Fig. 4D). The locations of the specific primers are indicated in Fig. 4B. The replication characteristics of ASFV E120R-Δ72-73aa were evaluated in PAMs as well. The results showed that the yields of ASFV E120R-Δ72-73aa were approximately 10-fold lower than the yields of its parental ASFV (Fig. 4E).
FIG 4.
The strategy for construction of ASFV E120R-Δ72-73aa. (A) The recombinant E120R mutant ASFV was constructed via the homologous recombination method as described in Materials and Methods. (B) Alignment of the E120R and E120R-Δ72-73aa sequences. Red arrows represent the locations of E120R-F/-R primers. (C) The growth status of ASFV E120R-Δ72-73aa (Tenth generation virus, F10) in BMDM cells is indicated. GFP, green fluorescent protein. (D) The partial E120R fragment was amplified from the recombinant ASFV and its parental ASFV using the E120R-F/-R primers. The PCR products were detected by electrophoresis. M, DNA marker. (E) The replication characteristics of ASFV E120R-Δ72-73aa and its parental virus were analyzed. PAM cells were infected with ASFV E120R-Δ72-73aa or its parental virus (multiplicity of infection [MOI] of 0.01), and the viral titers were measured at the indicated time points. TCID50, 50% tissue culture infective dose. *, P < 0.05; **, P < 0.01.
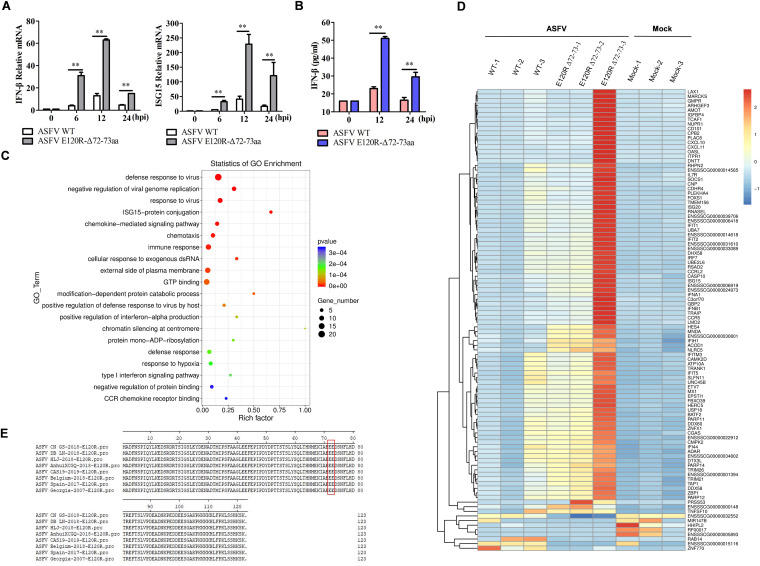
ASFV E120R-Δ72-73aa and its parental virus (ASFV WT) were then used to infect PAMs, and the expression levels of IFN and ISGs were measured and compared. ASFV WT induced weak expression of IFN-β and ISG15 early in the infection. However, ASFV E120R-Δ72-73 infection triggered IFN-β and ISG15 expression significantly (Fig. 5A). The secretion of IFN-β protein in the supernatant was evaluated using an enzyme-linked immunosorbent assay (ELISA) kit as well. The secretion of IFN-β protein in ASFV E120R-Δ72-73-infected PAMs was increased remarkably compared with its secretion in PAMs infected by ASFV WT (Fig. 5B).
FIG 5.
ASFV E120R-Δ72-73aa infection induced higher expression levels of IFN-β and ISGs in PAMs than infection by ASFV WT. (A and B) PAMs were infected with ASFV WT or ASFV E120R-Δ72-73aa (MOI of 1) for 0, 6, 12, or 24 h. (A) The cells were collected for detection of IFN-β and ISG15 mRNA expression. (B) The supernatants were collected at 0, 12, or 24 hpi, and the secreted IFN-β protein was measured using an ELISA kit. Two independent experiments were performed with similar results. Error bars show standard deviations. **, P < 0.01. (C) PAMs were mock infected or infected by equal amounts of ASFV WT or ASFV E120R-Δ72-73aa for 12 h. Transcriptome analysis was then carried out. Gene Ontology (GO) enrichment analysis of DEGs in PAMs infected by ASFV WT or ASFV E120R-Δ72-73aa was performed, and the top 20 most significant enrichment terms were extracted (P < 0.05). Three technical replicates were performed. (D) The heat map shows the top 100 significant DEGs in PAMs in response to ASFV WT or ASFV E120R-Δ72-73aa infection. Three technical replicates were performed. (E) Alignment of the E120R protein sequences of different ASFV strains reported in recent years.
An unbiased transcriptomic analysis of the expression landscape and cellular responses in PAMs in response to ASFV WT or ASFV E120R-Δ72-73 infection was subsequently performed. The Gene Ontology (GO) enrichment analysis showed that many differentially expressed genes (DEGs) were enriched in the “immune response,” “ISG15-protein conjugation,” “response to virus,” and “type I interferon signaling pathway” GO terms (ASFV E120R-Δ72-73aa versus ASFV WT) (Fig. 5C). The heat map in Fig. 5D shows the top 100 significant DEGs. In these DEGs, IFN-β, IFN-α, and numerous ISGs, including OASL, ISG20, RNASEL, IFIT1, IFIT2, DHX58 (LGP2), IRF7, ISG15, GBP2, IFIH1 (MDA5), IFITM3, IFIT5, MX1, IFI44, and DDX58 (RIG-I), were significantly increased in the ASFV E120R-Δ72-73aa-infected PAMs compared to their expression levels in the ASFV WT-infected PAMs, suggesting that ASFV E120R-Δ72-73aa revealed increased activity in the induction of type I IFN production and ISG expression compared to ASFV WT. The differences in the results for the ASFV E120R-Δ72-73aa-infected PAMs may be due to the fact that that the samples were prepared in parallel three times. However, a clearly elevated response was observed all three times. The data for transcriptomic analysis are included in Tables S1 to S4 in the supplemental material.
Furthermore, the E120R amino acid sequences from different ASFV strains isolated in recent years were aligned and analyzed, showing 100% amino acid identity (Fig. 5E). This indicated that the E120R protein, which plays an important role in virus replication in host cells, is highly conserved among different ASFV strains. The 72nd and 73rd residues in E120R were critically involved in inhibition of type I IFN production and ISG expression.
IRF3 might be the potential target of ASFV E120R protein.
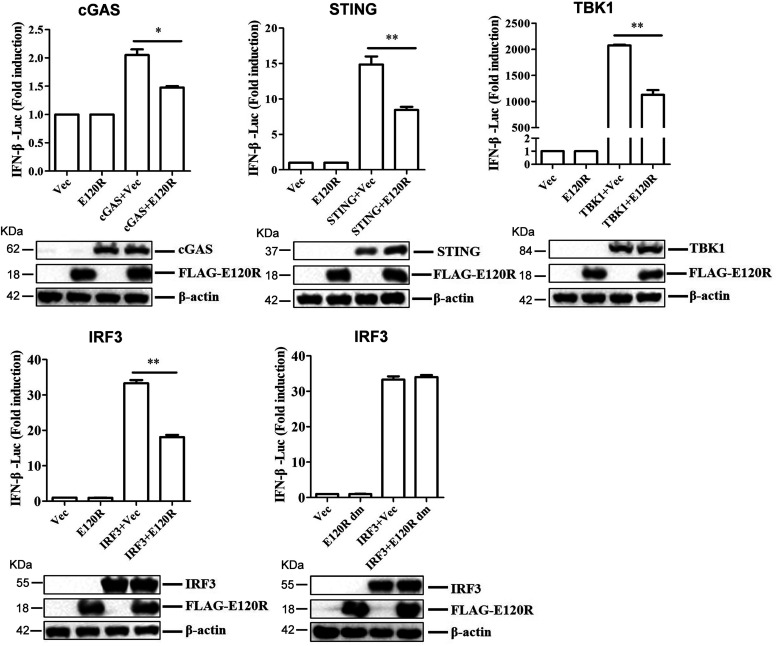
cGAS is responsible for recognition of viral DNA and induction of type I IFN during DNA virus infection. The observed inhibition of type I IFN production by the ASFV E120R protein raises the possibility that E120R targets one or several components of the cGAS/STING signaling pathway. To identify the potential target regulated by E120R, HEK-293T cells were cotransfected with FLAG-E120R expression plasmid and plasmids expressing each component of the cGAS/STING signaling pathway (including cGAS, STING, TBK1, and IRF3), together with IFN-β-Luc and pRL-TK plasmids. The activation of the IFN-β promoter was determined at 24 hpt. Overexpression of these component molecules activated IFN-β promoter activity, while overexpression of E120R protein considerably inhibited the activation of the IFN-β promoter induced by all of these molecules (Fig. 6). Overexpression of FLAG-E120R dm did not inhibit IRF3-mediated IFN-β promoter activation (Fig. 6). Meanwhile, the results of Western blotting indicated that ASFV E120R protein did not affect the protein expression of these components. Therefore, we speculated that ASFV E120R protein targeted IRF3 to block type I IFN production.
FIG 6.
IRF3 was the potential target of ASFV E120R protein for suppression of IFN-β production. HEK-293T cells were transfected with 0.1 μg/well of IFN-β-Luc expression plasmid and 0.01 μg/well of pRL-TK plasmid along with 0.1 μg/well of FLAG vector or FLAG-E120R or FLAG-E120R dm expression plasmid and HA-tagged-cGAS, -STING, -TBK1, or -IRF3 expression plasmid. At 24 hpt, the activation of the IFN-β promoter was evaluated with the Dual-Luciferase assay. Three independent experiments were performed with similar results. Error bars show standard deviations. *, P < 0.05; **, P < 0.01.
E120R protein inhibited IRF3 phosphorylation.
Given that ASFV E120R protein might target IRF3 to inhibit type I IFN production and E120R did not affect the expression of IRF3 protein, we investigated the effect of E120R on the posttranslational modification of IRF3. The phosphorylation of IRF3 plays important roles in the induction of IFN-β production (28). To explore how E120R induced the reduction of IFN-β, we measured IRF3 phosphorylation in HEK-293T cells transfected with FLAG vector or FLAG-E120R and HA-cGAS and HA-STING expression plasmids. At 24 hpt, the cells were collected and analyzed by Western blotting. E120R did not affect the protein expression of endogenous IRF3, but it inhibited cGAS/STING-induced IRF3 phosphorylation (Fig. 7A). The phosphorylation levels of IRF3 in the absence or presence of E120R in HEK-293T and PK-15 cells treated with poly(dA·dT) were further evaluated. E120R inhibited poly(dA·dT)-mediated IRF3 phosphorylation (Fig. 7B).
FIG 7.
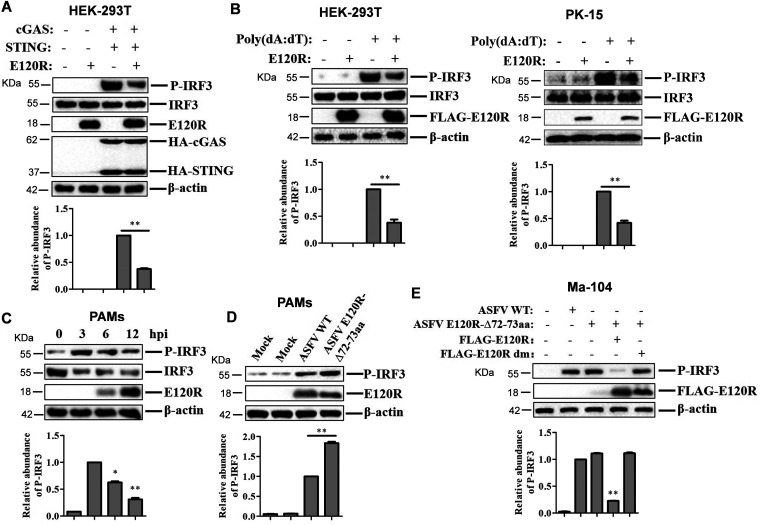
E120R protein inhibited IRF3 phosphorylation. (A) HEK-293T cells were transfected with 2 μg of empty FLAG vector, 1 μg of HA vector, or 1 μg each of HA-cGAS and HA-STING expression plasmids and 2 μg of FLAG-E120R expression plasmid. At 24 hpt, the expression of IRF3, P-IRF3, E120R, cGAS, and STING proteins was detected by Western blotting. Three independent experiments were performed. (B) HEK-293T cells were transfected with 2 μg each of FLAG vector and/or FLAG-E120R expression plasmids. PK-15 cells were transfected with 1 μg each of FLAG vector and/or FLAG-E120R expression plasmids. At 24 hpt, the transfected cells were transfected with poly(dA·dT) (2 μg/ml) for 12 h. Expression of IRF3, P-IRF3, and E120R proteins was detected by Western blotting. Three independent experiments were performed. (C) PAMs were infected with ASFV (MOI of 2) for 0, 3, 6, or 12 h. Expression of IRF3, P-IRF3, and E120R proteins was detected by Western blotting. Three independent experiments were performed. (D) PAMs were mock infected or infected with ASFV WT or ASFV E120R-Δ72-73aa (MOI of 2) for 8 h. Expression of the P-IRF3 and E120R proteins was detected by Western blotting. Three independent experiments were performed. (E) Ma-104 cells cultured in 6-well plates were transfected with 4 μg of FLAG vector or FLAG-E120R or FLAG-E120R dm expression plasmid. At 24 hpt, the cells were infected with ASFV or ASFV E120R-Δ72-73aa (MOI of 2) for 4 h, and the levels of IRF3 phosphorylation were detected by Western blotting. Three independent experiments were performed. The change in abundance of P-IRF3 was determined by densitometric analysis using ImageJ Software and normalized to β-actin. Error bars show standard deviations. *, P < 0.05; **, P < 0.01.
The impact of ASFV infection on IRF3 phosphorylation was also evaluated. PAMs were infected with ASFV for 0, 3, 6, or 12 h, and the levels of IRF3 expression and phosphorylated IRF3 were then analyzed by Western blotting. ASFV infection promoted IRF3 phosphorylation at 3 hpi, while the IRF3 phosphorylation subsequently decreased over time (Fig. 7C), which was consistent with previous results (21). The phosphorylation of IRF3 in PAMs that were infected by ASFV WT or ASFV E120R-Δ72-73aa was further measured and compared. ASFV E120R-Δ72-73aa infection induced higher levels of phosphorylation of IRF3 in PAMs than did ASFV WT (Fig. 7D). To further confirm the impacts of ASFV WT and ASFV E120R-Δ72-73aa on IRF3 phosphorylation, Ma-104 monkey kidney epithelial cells were transfected with FLAG vector or FLAG-E120R and FLAG-E120R dm expression plasmids. At 24 hpt, the cells were infected with ASFV for 4 h, and then the expression of phosphorylated IRF3 was analyzed by Western blotting. ASFV WT and ASFV E120R-Δ72-73aa induced the same amounts of phosphorylated IRF3 at 4 hpi, while E120R but not E120R dm inhibited ASFV-induced IRF3 phosphorylation (Fig. 7E). Taken together, these results indicated that ASFV infection inhibited IRF3 phosphorylation and that the E120R protein was critically involved in this process. The amino acid 72-73 site was the crucial region in E120R that was responsible for this inhibitory function.
E120R interacted with IRF3 during viral infection.
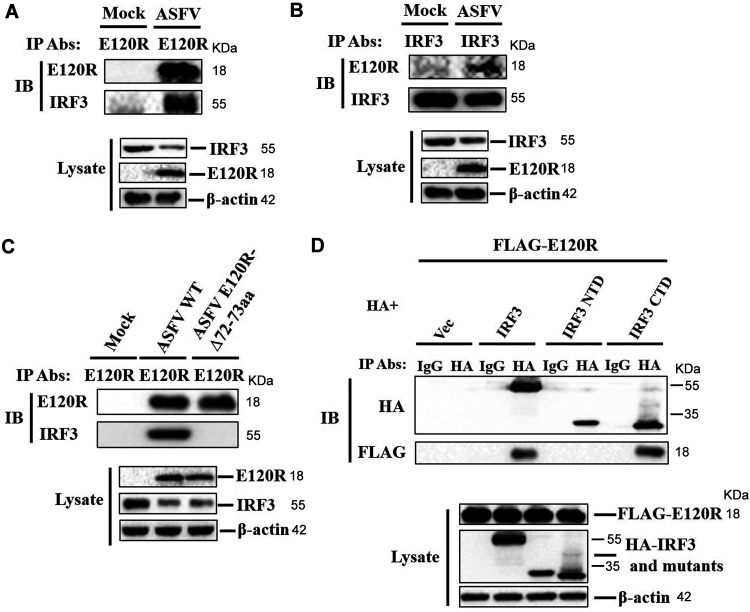
To explore a possible interaction between E120R and IRF3 during viral infection, PAMs were mock infected or infected with ASFV, and the cell lysates were immunoprecipitated with anti-E120R antibody and subjected to Western blotting. E120R pulled down IRF3 (Fig. 8A). A reverse immunoprecipitation experiment was also performed using anti-IRF3 antibody, which showed that IRF3 also immunoprecipitated E120R (Fig. 8B). We also identified the crucial region in E120R that was essential for E120R-IRF3 interaction. PAMs were infected with ASFV WT or ASFV E120R-Δ72-73aa. The cell lysates were immunoprecipitated with anti-E120R antibody and analyzed by Western blotting. IRF3 was pulled down by E120R, whereas E120R-Δ72-73aa did not pull down IRF3 (Fig. 8C), which indicated that amino acids 72 and 73 of E120R were essential for the E120R-IRF3 interaction. The critical region in IRF3 responsible for E120R-IRF3 interaction was further identified. The N-terminal domain (NTD, amino acids 1 to 197) and C-terminal domain (CTD, amino acids 198 to 427) of IRF3 were used to investigate the binding domain in IRF3 (29, 30). The results showed that the IRF3 CTD, but not the IRF3 NTD, interacted with E120R (Fig. 8D). Taken together, these results indicated that E120R interacted with IRF3 in the context of viral infection and that amino acids 72 and 73 of E120R and the IRF3 CTD were essential for the E120R-IRF3 interaction.
FIG 8.
E120R interacted with IRF3 during viral infection. (A) PAMs were mock infected or infected with ASFV (MOI of 1) for 12 h. The cell lysates were immunoprecipitated with anti-E120R antibody and subjected to Western blotting using the indicated antibodies. Three independent experiments were performed. IP, immunoprecipitation; IB, immunoblotting. (B) A reverse immunoprecipitation assay was performed using anti-IRF3 antibody as described in the legend to panel A. The antibody-antigen complexes were detected using anti-E120R or anti-IRF3 antibody. Three independent experiments were performed. (C) PAMs were mock infected or infected with ASFV WT or ASFV E120R-Δ72-73aa (MOI of 1) for 12 h. The cell lysates were immunoprecipitated with anti-E120R antibody and subjected to Western blotting using the indicated antibodies. Three independent experiments were performed. (D) HEK-293T cells were transfected with 8 μg of FLAG-E120R expression plasmid along with 8 μg of HA vector or HA-IRF3, HA-IRF3 NTD, or HA-IRF3 CTD expression plasmid for 24 h. Cells were lysed, and the lysates were immunoprecipitated with anti-HA antibody and subjected to Western blotting. NTD, N-terminal domain; CTD, C-terminal domain. Three independent experiments were performed.
E120R impaired the interaction of IRF3 with TBK1.
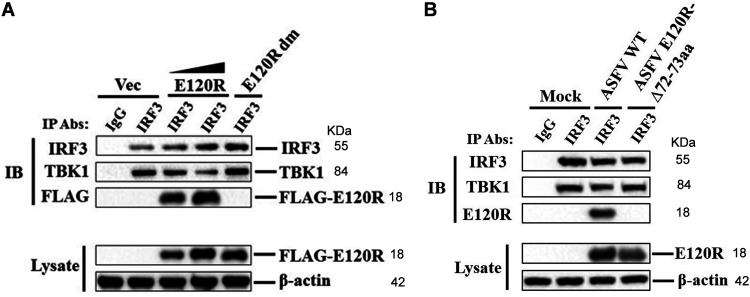
The interaction between TBK1 and IRF3 is critical for phosphorylation of IRF3 and type I IFN production (31). To explore how E120R induced the reduction of IRF3 phosphorylation, we detected the interaction between TBK1 and IRF3. PK-15 cells were transfected with FLAG vector or FLAG-E120R or FLAG-E120R-Δ72-73aa expression plasmid (FLAG-E120R dm). At 24 hpt, the cells were treated with poly(dA·dT) and the cell lysates were immunoprecipitated with anti-IRF3 antibody and analyzed by Western blotting. IRF3 pulled down TBK1, and FLAG-E120R inhibited the interaction between IRF3 and TBK1 in a dose-dependent manner. However, FLAG-E120R dm did not impair the interaction between IRF3 and TBK1 (Fig. 9A). To confirm the impact of ASFV infection on the interaction between IRF3 and TBK1, PAMs were mock infected or infected with ASFV WT or ASFV E120R-Δ72-73aa. The cell lysates were immunoprecipitated with anti-IRF3 antibody and analyzed by Western blotting. TBK1 was efficiently pulled down by IRF3, while ASFV infection inhibited the interaction between IRF3 and TBK1 and ASFV E120R-Δ72-73aa infection did not affect the interaction (Fig. 9B). Taken together, these results indicated that E120R impaired the interaction between IRF3 and TBK1 and that amino acids 72 and 73 of E120R were essential for the suppression of this interaction.
FIG 9.
E120R impaired the interaction between IRF3 and TBK1. (A) PK-15 cells were transfected with 10 μg of FLAG vector or 5 μg or 10 μg of FLAG-E120R or 10 μg of FLAG-E120R dm expression plasmid. At 24 hpt, cells were transfected with poly(dA·dT) (2 μg/ml) for 12 h. Cells were lysed, and the lysates were immunoprecipitated with anti-IRF3 antibody and subjected to Western blotting. The antibody-antigen complexes were detected using anti-TBK1, anti-IRF3, or anti-FLAG antibody. Three independent experiments were performed with similar results. (B) PAMs were mock infected or infected with ASFV WT or ASFV E120R-Δ72-73aa (MOI of 1) for 8 h. Cell lysates were immunoprecipitated with anti-IRF3 antibody and subjected to Western blotting. The antibody-antigen complexes were detected using anti-TBK1, anti-IRF3, or anti-E120R antibody. Three independent experiments were performed with similar results. IP, immunoprecipitation; IB, immunoblotting.
DISCUSSION
RIG-like receptor (RLR)-, NOD-like receptor (NLR)-, Toll-like receptor (TLR)-, and cGAS-mediated innate immunity form the first line of defense that protects hosts from invasion by RNA or DNA viruses. After DNA virus infection, viral DNA can be recognized by the cytosolic DNA sensor cGAS, and the IFN-β signal pathway is then activated to induce IFN-β and ISG expression to limit the initial viral replication and initiate the appropriate adaptive immune response. IRF3, a key transcription factor, plays important roles in the induction of type I IFN and is essential for giving rise to the expression of many genes involved in the innate immune response (32). IRF3 is shared by various PRRs to trigger IFN production, which further reveals its critical role in host innate immunity (29). However, many viruses, such as Seneca valley virus (SVV), foot-and-mouth disease virus (FMDV), peste des petits ruminants virus (PPRV), herpes simplex virus (HSV), Theiler’s virus, varicella-zoster virus, and poxviruses, have evolved multiple strategies to inhibit the activation of IRF3 to evade the host’s antiviral innate immune response (29, 30, 33–35). ASFV, containing more than 150 genes, is a highly contagious pathogen that causes a devastating and economically significant disease of pigs. However, little is known about how ASFV blocks the innate immune response (36, 37). In the present study, we determined that ASFV E120R protein targeted IRF3 to evade the host innate immune response, revealing a novel mechanism evolved by ASFV to inhibit the host antiviral response through disruption of the TBK1-IRF3 axis.
Different antagonistic mechanisms targeting IRF3 have been identified in various viruses. For instance, SVV and FMDV inhibit the expression of IRF3 protein to attenuate type I IFN production (29, 38). PPRV infection impairs the interaction of IRF3 with TBK1 and inhibits IRF3 nuclear translocation, resulting in the suppression of IFN synthesis (39). The nonessential accessory protein ML of Thogotovirus suppresses host type I IFN production by blocking the interaction of IRF3 with the transcriptional coactivator CREB-binding protein (CBP) (40). Ebola virus suppresses host type I IFN production by blocking the dimerization and phosphorylation of IRF3 (41). HSV ICP27 protein associates directly with IRF3 to inhibit its phosphorylation and nuclear translocation, resulting in the inhibition of IFN-β induction (42). Like ASFV, poxviruses are large DNA viruses that encode large amounts of proteins. Virulent poxviruses inhibit DNA sensing by preventing STING activation (43). In addition, the poxvirus N2 protein inhibits IRF3 activity in the nucleus (35), and the viral C6 protein inhibits IRF3 activation and translocation into the nucleus, resulting in the inhibition of IFN-β production (44). The NTD of IRF3 contains the DNA binding domain (45), and the CTD of IRF3 (containing the Ser385, Ser386, and Ser396 phosphorylation sites) includes the crucial region for its phosphorylation (46). Here, our data indicated that ASFV E120R protein interacted with the IRF3 CTD and disrupted the interaction between TBK1 and IRF3, resulting in the inhibition of IRF3 phosphorylation.
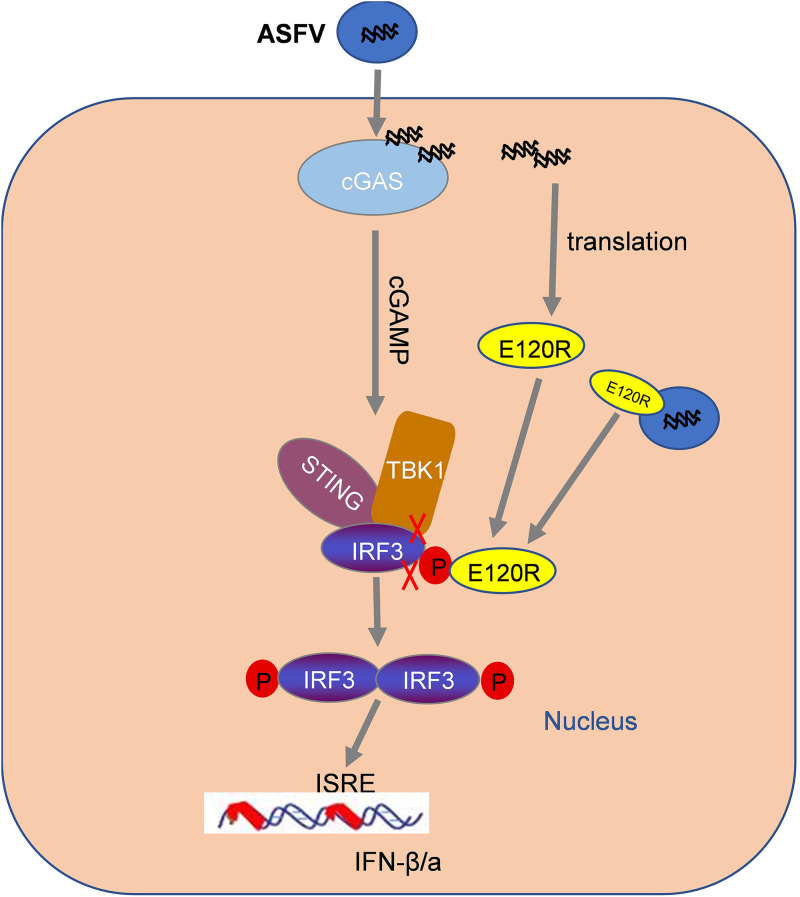
Further detailed analysis revealed that the deletion of amino acids 72 and 73 in the C-terminal region of E120R abrogated the interaction between IRF3 and E120R, which recovered the interaction between IRF3 and TBK1 and enhanced IRF3 phosphorylation during ASFV reapplication. Altogether, we determined that the interaction between E120R and IRF3 CTD impaired the interaction of TBK1 with IRF3 and then decreased the phosphorylation of IRF3 induced by TBK1, resulting in the inhibition of IFN-β and ISG expression, which finally benefited ASFV replication. Together, our data identified an antagonistic mechanism by which the ASFV E120R protein blocked type I IFN production (Fig. 10). The reduced ASFV E120R-Δ72-73aa replication may also lead to an increase in the IFN response, since the expression of other known ASFV IFN inhibitory proteins would be reduced, leading to an increase in the type I IFN response. E120R, a structural protein, is localized in the cytoplasm. Additionally, E120R can bind to the major capsid protein p72. Immunoelectron microscopy revealed that E120R protein localizes at the surface of the intracellular virions. The incorporation of E120R into the virus particle is dependent on p72 expression (27). E120R might be involved in the transport of ASFV particles from the viral factories to the plasma membrane (27). Therefore, we speculated that E120R protein inhibits IFN-β production in both the virus particle and the cytoplasm.
FIG 10.
Schematic overview of the working model of how ASFV E120R protein negatively regulates type I IFN production. ASFV E120R protein negatively regulates type I IFN production by interacting with IRF3 and impairing the interaction between TBK1 and IRF3 to block IRF3 phosphorylation. E120R protein may inhibit IFN-β production both in the virus particle and in the cytoplasm. ISRE, IFN-stimulated response element.
BA71V is a genotype I ASFV strain (47). Previous reports showed that the expression of E120R can be detected at >10 h after viral infection in BA71V-infected Vero cells (a late-phase-expressed ASFV protein) (27). Besides, BA71V lacking the complete E120R region could survive and replicate in Vero cells (27, 48). Our results showed that ASFV lacking the complete E120R region could not be rescued in porcine BMDM cells, suggesting an essential role of E120R for ASFV replication in porcine cells. The ASFV strain used in the present study belonged to genotype II. There are many genomic sequence differences between genotype I and genotype II ASFV. E120R might play different roles during the replication of different genotypes of ASFV in different cells. In addition, our findings indicated that E120R is also a late gene during genotype II ASFV infection in PAMs, which is in accordance with previous results (48). However, in the present study, Western blotting showed that a small amount of E120R protein could be detected at 6 hpi in ASFV-infected PAMs, which is earlier than in BA71V-infected Vero cells. The different genotypes of ASFV or different cells used in these experiments might have contributed to the different manifestations.
Comprehensive investigation of the functions of ASFV proteins and clarification of the immune evasion mechanisms used by ASFV are critical to understanding the pathogenesis of ASFV. The deletion of specific virulence factors in ASFV using recombination technologies (49–51) or the CRISPR-Cas9 genome editing system (52) has made the generation of safer LAVs much easier (53, 54), and the available LAVs are coming closer to the market. This work identifies a novel mechanism evolved by ASFV to inhibit the host antiviral response and provides a new target to guide the design and development of ASFV LAVs. Deletion of the codons encoding amino acids 72 and 73 from the E120R gene of ASFV might further improve the safety and effectiveness of previously reported LAVs.
MATERIALS AND METHODS
Viruses and cells.
Human embryonic kidney 293T (HEK-293T) cells, porcine kidney 15 (PK-15) cells, and Ma-104 monkey kidney epithelial cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco) containing 10% inactivated fetal bovine serum (FBS; Gibco) and maintained at 37°C (5% CO2). Porcine alveolar macrophages (PAMs) and bone marrow-derived macrophages (BMDM) were cultured in RPMI 1640 medium (Gibco) containing 15% inactivated FBS and maintained at 37°C (5% CO2). The ASFV strain, a genotype II ASFV named ASFV CN/GS/2018, was isolated and stored in our laboratory (55).
Plasmids and antibodies.
The full-length sequence of ASFV E120R was cloned into the p3×FLAG-CMV-7.1 vector (Sigma-Aldrich, St. Louis, MO, USA) to yield the FLAG-tagged expression construct (FLAG-E120R). HA-cGAS, HA-STING, HA-TBK1, and HA-IRF3 expression plasmids were stored by our laboratory previously. FLAG-E120R mutant expression plasmids were constructed by mutagenesis PCR. All of the plasmids constructed were analyzed and verified by DNA sequencing. The plasmids were transfected into HEK-293T and PK-15 cells using Lipofectamine 3000 (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The plasmids were transfected into Ma-104 cells using Polyplus-transfection reagent (jetPEI) according to the manufacturer’s protocol.
The commercial antibodies used in this study included anti-FLAG polyclonal antibody (F7425, 1:1,000; Sigma-Aldrich), anti-FLAG monoclonal antibody (sc-166355, 1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA), anti-IRF3 rabbit monoclonal antibody (4302S, 1:1,000; Cell Signaling Technology), anti-IRF3 mouse monoclonal antibody (sc-33641, 1:1,000; Santa Cruz Biotechnology), anti-phosphorylated-IRF3 (P-IRF3) monoclonal antibody (37829S, 1:1,000; Cell Signaling Technology), anti-TBK1 monoclonal antibody (ab40676, 1:1,000; Abcam, Cambridge, MA, USA), anti-HA monoclonal antibody (26183, 1:2,000; Thermo Scientific), and anti-β-actin monoclonal antibody (sc-8432, 1:1,000; Santa Cruz Biotechnology). Anti-E120R polyclonal antibody was produced in rabbit by immunization with ASFV E120R protein.
Virus titration.
ASFV WT and ASFV E120R-Δ72-73aa strains were quantified using the hemadsorption (HAD) assay as described previously (56). Briefly, PAMs were cultured in 96-well plates, and the viruses were added to the 96-well plates and titrated in triplicate using 10-fold serial dilutions. HAD was determined at 7 hpi, and 50% HAD doses (HAD50) were calculated using the Reed and Muench method (57).
Coimmunoprecipitation and Western blotting.
HEK-293T cells or PAMs were cultured in 10-cm dishes, and the monolayer cells were transfected with the indicated plasmids or infected with ASFV. Cells were collected and lysed, and the lysates were immunoprecipitated using anti-E120R, anti-IRF3, or anti-HA antibody as described previously (58). For Western blotting, the target proteins were analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto Immobilon-P membrane (EMD Millipore, Billerica, MA, USA), which was blocked with 5% skim milk in Tris-buffered saline with Tween 20 (TBST) at room temperature for 2 h and then incubated with the appropriate primary antibody at 4°C overnight. To avoid interference between heavy chain and IRF3 bands, the heavy-chain-free secondary antibody (1:5,000; Abbkine Scientific Co., Ltd.) was incubated at room temperature for 2 h. Antibody-antigen complexes were visualized with chemiluminescence detection reagents (Thermo Scientific).
RNA extraction and qPCR.
Total RNAs in the cells sample were extracted using TRIzol reagent (Invitrogen). Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA) and random hexamer primers (TaKaRa, Shiga, Japan) were used to synthesize cDNAs. SYBR premix Ex Taq reagents (TaKaRa) and the Mx3005P qPCR system (Agilent Technologies) were used to quantify the transcription levels of mRNAs. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal control. The relative fold changes of mRNA were calculated with the comparative cycle threshold (2−ΔΔCT) method (59). The qPCR primers used in this study are listed in Table 1.
TABLE 1.
qPCR primers used in this study
| Primer | Sequence (5′–3′) | Target gene |
|---|---|---|
| pIFN-β-F | GCTAACAAGTGCATCCTCCAAA | Porcine IFN-β gene |
| pIFN-β-R | AGCACATCATAGCTCATGGAAAGA | |
| pISG15-F | GATCGGTGTGCCTGCCTTC | Porcine ISG15 gene |
| pISG15-R | CGTTGCTGCGACCCTTGT | |
| pISG56-F | TTAGAAAACAGGGTCTTGGAGGAG | Porcine ISG56 gene |
| pISG56-R | CGTAAGGTAATACAGCCAGGCATA | |
| pGAPDH-F | ACATGGCCTCCAAGGAGTAAGA | Porcine GAPDH gene |
| pGAPDH-R | GATCGAGTTGGGGCTGTGACT | |
| E120R-F | GACCCTACCACATCAACTTCCCT | ASFV E120R gene |
| E120R-R | ACCGCTTTCTTCGTCATCTTCCG | |
| hISG56-F | CTTGAGCATCCTCGGGTTCATC | Human ISG56 gene |
| hISG56-R | AAGTCAGCAGCCAGGTTTAGGG | |
| hISG15-F | TGGACAAATGCGACGAACC | Human ISG15 gene |
| hISG15-R | CCCGCTCACTTGCTGCTT | |
| hIFN-β-F | GACATCCCTGAGGAGATTAAG | Human IFN-β gene |
| hIFN-β-R | ATGTTCTGGAGCATCTCATAG | |
| hGAPDH-F | CGGGAAGCTTGTGATCAATGG | Human GAPDH gene |
| hGAPDH-R | GGCAGTGATGGCATGGACTG |
ELISA.
Cell supernatants were collected and assayed for porcine IFN-β using a porcine IFN-β ELISA kit (Solarbio). The measured value was compared with the standard according to the manufacturer’s instructions.
Luciferase reporter gene assay.
HEK-293T or PK-15 cells cultured in 24-well plates were transfected with 0.1 μg/well IFN-β-Luc expression plasmid along with 0.01 μg/well pRL-TK and other expression plasmids. At 24 hpt, the cells were treated or mock treated with poly(dA·dT) (2 μg/ml) for 12 h. The cells were lysed, and the firefly and Renilla luciferase activities in the lysates were analyzed with the Dual-Luciferase assay kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
Construction of the recombinant ASFV using the homologous recombination method.
The recombinant ASFV was generated by homologous recombination using the parental ASFV genome and a recombination transfer vector, as described previously (60). The detailed construction strategy for the gene deletion mutant ASFV is shown in Fig. 4A. Briefly, BMDM cells were transfected with the constructed transfer vectors by using jetPEI-macrophage DNA transfection reagent (Polyplus Transfection, Illkirch, France), and the cells were then infected with ASFV. The purification of the recombinant ASFV was performed by successive limiting dilution and purification. The purity of the recombinant ASFV was detected using specific primers targeting E120R 72-73aa (Fig. 4B). The primer sequences were as follows: E120R-F, ATGGCAGATTTTAATTCTCCAATCC, and E120R-R, GTAGAAAATTACTATCCTCTTCCTC. The sequencing of ASFV WT and ASFV E120R-Δ72-73aa recombinant virus genomes was performed using next-generation sequencing (NGS) as previously described (56, 61). Briefly, purified DNA was used to construct an Illumina standard shotgun library, followed by using the NEBNext ultra DNA library preparation kit (catalog no. E7370; NEB), and then the DNA was sequenced using the Illumina NovaSeq 6000 platform with the dual-end sequencing model. We sequenced 7,637,663 total reads, from which 37,424 reads were identified as ASFV-specific reads. The sequencing had an average depth of 16,131×.
RNA sequencing.
For transcriptomic analysis, RNA from mock-infected, ASFV WT-infected, or ASFV E120R-Δ72-73aa-infected PAMs was extracted using TRIzol reagent. All experiments were carried out in triplicate. The quality and integrity of extracted RNA was determined using 1% (wt/vol) agarose gel and the RNA Nano 6000 assay kit for the Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, USA), respectively. All of the extracted RNA samples were submitted to Lc-Bio Technologies Co. Ltd. (Hangzhou, China) for transcriptome sequencing (RNA-seq). Gene Ontology (GO) enrichment analysis and bioinformatics analysis were performed to analyze the data generated by RNA-seq.
Statistical analysis.
Statistical analysis was performed using SPSS Statistics for Windows, version 17.0 (SPSS, Inc., Chicago, IL, USA). Student’s t test was used for comparison of the data from three independent experiments. A P value of <0.05 was considered statistically significant (*), and a P value of <0.01 was considered highly statistically significant (**).
Data availability.
The newly determined sequences are available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.34tmpg4km.
ACKNOWLEDGMENTS
This research was supported by grants from the National Natural Sciences Foundation of China (grant number 31941002), the National Science and Technology Infrastructure Program (grant number 2018YFC0840402), technology major projects of Gansu province (grant number 20ZD7A006 and NCC0006), the Chinese Academy of Agricultural Science and Technology Innovation Project (grants number CAAS-ZDRW202006 and CAAS-ASTIP-2021-LVRI), the Key-Area Research and Development Program of Guangdong Province (grant number 2019B020211003), Excellent Youth Science and Technology Project of Lanzhou Veterinary Research Institute (grant number 1610312021009), and research funding from Pulike Biological Engineering, Inc.
We declare no competing financial interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Haixue Zheng, Email: haixuezheng@163.com.
Joanna L. Shisler, University of Illinois at Urbana Champaign
REFERENCES
- 1.Dixon LK, Chapman DA, Netherton CL, Upton C. 2013. African swine fever virus replication and genomics. Virus Res 173:3–14. 10.1016/j.virusres.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Revilla Y, Perez-Nunez D, Richt JA. 2018. African swine fever virus biology and vaccine approaches. Adv Virus Res 100:41–74. 10.1016/bs.aivir.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Galindo I, Alonso C. 2017. African swine fever virus: a review. Viruses 9:103. 10.3390/v9050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G, Liu X, Yang M, Zhang G, Wang Z, Guo K, Gao Y, Jiao P, Sun J, Chen C, Wang H, Deng W, Xiao H, Li S, Wu H, Wang Y, Cao L, Jia Z, Shang L, Yang C, Guo Y, Rao Z. 2020. Crystal structure of African swine fever virus pS273R protease and implications for inhibitor design. J Virol 94:e02125-19. 10.1128/JVI.02125-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, Wang C, Yang M, Cao L, Fu D, Liu X, Sun D, Chen C, Wang Y, Jia Z, Yang C, Guo Y, Rao Z. 2020. Structural insight into African swine fever virus dUTPase reveals a novel folding pattern in the dUTPase family. J Virol 94:e01698-19. 10.1128/JVI.01698-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon LK, Sun H, Roberts H. 2019. African swine fever. Antiviral Res 165:34–41. 10.1016/j.antiviral.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 7.De la Torre A, Bosch J, Iglesias I, Munoz MJ, Mur L, Martinez-Lopez B, Martinez M, Sanchez-Vizcaino JM. 2015. Assessing the risk of African swine fever introduction into the European Union by wild boar. Transbound Emerg Dis 62:272–279. 10.1111/tbed.12129. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Chai Y, Song H, Weng C, Qi J, Sun Y, Gao GF. 2019. Crystal structure of African swine fever virus dUTPase reveals a potential drug target. mBio 10:e02483-19. 10.1128/mBio.02483-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao D, Liu R, Zhang X, Li F, Wang J, Zhang J, Liu X, Wang L, Zhang J, Wu X, Guan Y, Chen W, Wang X, He X, Bu Z. 2019. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg Microbes Infect 8:438–447. 10.1080/22221751.2019.1590128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisimwa PN, Ongus JR, Tiambo CK, Machuka EM, Bisimwa EB, Steinaa L, Pelle R. 2020. First detection of African swine fever (ASF) virus genotype X and serogroup 7 in symptomatic pigs in the Democratic Republic of Congo. Virol J 17:135. 10.1186/s12985-020-01398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch-Camós L, López E, Rodriguez F. 2020. African swine fever vaccines: a promising work still in progress. Porc Health Manag 6:17. 10.1186/s40813-020-00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arzt J, White WR, Thomsen BV, Brown CC. 2010. Agricultural diseases on the move early in the third millennium. Vet Pathol 47:15–27. 10.1177/0300985809354350. [DOI] [PubMed] [Google Scholar]
- 13.Iglesias I, Rodriguez A, Feliziani F, Rolesu S, de la Torre A. 2017. Spatio-temporal analysis of African swine fever in Sardinia (2012-2014): trends in domestic pigs and wild boar. Transbound Emerg Dis 64:656–662. 10.1111/tbed.12408. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Sun Y, Qiu HJ. 2018. African swine fever: an unprecedented disaster and challenge to China. Infect Dis Poverty 7:111. 10.1186/s40249-018-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Lee MJ, Lee SK, Kim DY, Seo SJ, Kang HE, Nam HM. 2019. African swine fever virus in pork brought into South Korea by travelers from China, August 2018. Emerg Infect Dis 25:1231–1233. 10.3201/eid2506.181684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe T, Marutani Y, Shoji I. 2019. Cytosolic DNA-sensing immune response and viral infection. Microbiol Immunol 63:51–64. 10.1111/1348-0421.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang B, Zhang L, Lu M, Li J, Lv Y. 2018. PCV2 infection activates the cGAS/STING signaling pathway to promote IFN-beta production and viral replication in PK-15 cells. Vet Microbiol 227:34–40. 10.1016/j.vetmic.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Zhao J, Xu S, Li J, He S, Zeng Y, Xie L, Xie N, Liu T, Lee K, Seo GJ, Chen L, Stabell AC, Xia Z, Sawyer SL, Jung J, Huang C, Feng P. 2018. Species-specific deamidation of cGAS by herpes simplex virus UL37 protein facilitates viral replication. Cell Host Microbe 24:234–248.e5. 10.1016/j.chom.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gekara NO, Jiang H. 2019. The innate immune DNA sensor cGAS: a membrane, cytosolic, or nuclear protein? Sci Signal 12:eaax3521. 10.1126/scisignal.aax3521. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Sun L, Chen ZJ. 2016. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 17:1142–1149. 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Belmonte R, Perez-Nunez D, Pittau M, Richt JA, Revilla Y. 2019. African swine fever virus Armenia/07 virulent strain controls interferon beta production through the cGAS-STING pathway. J Virol 93:e02298-18. 10.1128/JVI.02298-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razzuoli E, Franzoni G, Carta T, Zinellu S, Amadori M, Modesto P, Oggiano A. 2020. Modulation of type I interferon system by African swine fever virus. Pathogens 9:361. 10.3390/pathogens9050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Wu J, Wu Y, Chen H, Zhang S, Li J, Xin T, Jia H, Hou S, Jiang Y, Zhu H, Guo X. 2018. Inhibition of cGAS-STING-TBK1 signaling pathway by DP96R of ASFV China 2018/1. Biochem Biophys Res Commun 506:437–443. 10.1016/j.bbrc.2018.10.103. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Pomares L, Simon-Mateo C, Lopez-Otin C, Vinuela E. 1997. Characterization of the African swine fever virus structural protein p14.5: a DNA binding protein. Virology 229:201–211. 10.1006/viro.1996.8434. [DOI] [PubMed] [Google Scholar]
- 25.Jia N, Ou Y, Pejsak Z, Zhang Y, Zhang J. 2017. Roles of African swine fever virus structural proteins in viral infection. J Vet Res 61:135–143. 10.1515/jvetres-2017-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfonso P, Quetglas JI, Escribano JM, Alonso C. 2007. Protein pE120R of African swine fever virus is post-translationally acetylated as revealed by post-source decay MALDI mass spectrometry. Virus Genes 35:81–85. 10.1007/s11262-006-0015-6. [DOI] [PubMed] [Google Scholar]
- 27.Andrés G, García-Escudero R, Viñuela E, Salas ML, Rodríguez JM, 2001. African swine fever virus structural protein pE120R is essential for virus transport from assembly sites to plasma membrane but not for infectivity. J Virol 75:6758–6768. 10.1128/JVI.75.15.6758-6768.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiscott J. 2007. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem 282:15325–15329. 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 29.Xue Q, Liu H, Zhu Z, Yang F, Ma L, Cai X, Xue Q, Zheng H. 2018. Seneca Valley virus 3C(pro) abrogates the IRF3- and IRF7-mediated innate immune response by degrading IRF3 and IRF7. Virology 518:1–7. 10.1016/j.virol.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Manivanh R, Mehrbach J, Knipe DM, Leib DA. 2017. Role of herpes simplex virus 1 gamma34.5 in the regulation of IRF3 signaling. J Virol 91:e01156-17. 10.1128/JVI.01156-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao W. 2013. Negative regulation of TBK1-mediated antiviral immunity. FEBS Lett 587:542–548. 10.1016/j.febslet.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canivet C, Rheaume C, Lebel M, Piret J, Gosselin J, Boivin G. 2018. Both IRF3 and especially IRF7 play a key role to orchestrate an effective cerebral inflammatory response in a mouse model of herpes simplex virus encephalitis. J Neurovirol 24:761–768. 10.1007/s13365-018-0666-9. [DOI] [PubMed] [Google Scholar]
- 33.Delhaye S, van Pesch V, Michiels T. 2004. The leader protein of Theiler’s virus interferes with nucleocytoplasmic trafficking of cellular proteins. J Virol 78:4357–4362. 10.1128/JVI.78.8.4357-4362.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu H, Zheng C, Xing J, Wang S, Li S, Lin R, Mossman KL. 2011. Varicella-zoster virus immediate-early protein ORF61 abrogates the IRF3-mediated innate immune response through degradation of activated IRF3. J Virol 85:11079–11089. 10.1128/JVI.05098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferguson BJ, Benfield CTO, Ren H, Lee VH, Frazer GL, Strnadova P, Sumner RP, Smith GL. 2013. Vaccinia virus protein N2 is a nuclear IRF3 inhibitor that promotes virulence. J Gen Virol 94:2070–2081. 10.1099/vir.0.054114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correia S, Ventura S, Parkhouse RM. 2013. Identification and utility of innate immune system evasion mechanisms of ASFV. Virus Res 173:87–100. 10.1016/j.virusres.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Zhuo Y, Guo Z, Ba T, Zhang C, He L, Zeng C, Dai H. 2021. African swine fever virus MGF360-12L inhibits type I interferon production by blocking the interaction of importin α and NF-κB signaling pathway. Virol Sin 36:176–186. 10.1007/s12250-020-00304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, Fang L, Luo R, Ye R, Fang Y, Xie L, Chen H, Xiao S. 2010. Foot-and-mouth disease virus leader proteinase inhibits dsRNA-induced type I interferon transcription by decreasing interferon regulatory factor 3/7 in protein levels. Biochem Biophys Res Commun 399:72–78. 10.1016/j.bbrc.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Z, Li P, Yang F, Cao W, Zhang X, Dang W, Ma X, Tian H, Zhang K, Zhang M, Xue Q, Liu X, Zheng H. 2019. Peste des petits ruminants virus nucleocapsid protein inhibits beta interferon production by interacting with IRF3 to block its activation. J Virol 93:e00362-19. 10.1128/JVI.00362-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jennings S, Martínez-Sobrido L, García-Sastre A, Weber F, Kochs G. 2005. Thogoto virus ML protein suppresses IRF3 function. Virology 331:63–72. 10.1016/j.virol.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Basler CF, Mikulasova A, Martinez-Sobrido L, Paragas J, Mühlberger E, Bray M, Klenk HD, Palese P, García-Sastre A. 2003. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J Virol 77:7945–7956. 10.1128/jvi.77.14.7945-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan X, Zhang M, Fu M, Luo S, Hu Q. 2019. Herpes simplex virus type 2 immediate early protein ICP27 inhibits IFN-β production in mucosal epithelial cells by antagonizing IRF3 activation. Front Immunol 10:290. 10.3389/fimmu.2019.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgana I, Sumner RP, Towers GJ, Carlos M. 2018. Virulent poxviruses inhibit DNA sensing by preventing STING activation. J Virol 92:e02145-17. 10.1128/JVI.02145-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unterholzner L, Sumner RP, Baran M, Ren H, Mansur DS, Bourke NM, Randow F, Smith GL, Bowie AG. 2011. Vaccinia virus protein C6 is a virulence factor that binds TBK-1 adaptor proteins and inhibits activation of IRF3 and IRF7. PLoS Pathog 7:e1002247. 10.1371/journal.ppat.1002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honda K, Taniguchi T. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol 6:644–658. 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 46.Jing T, Zhao B, Xu P, Gao X, Chi L, Han H, Sankaran B, Li P. 2020. The structural basis of IRF-3 activation upon phosphorylation. J Immunol 205:1886–1896. 10.4049/jimmunol.2000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malogolovkin A, Burmakina G, Titov I, Sereda A, Gogin A, Baryshnikova E, Kolbasov D. 2015. Comparative analysis of African swine fever virus genotypes and serogroups. Emerg Infect Dis 21:312–315. 10.3201/eid2102.140649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cackett G, Matelska D, Sýkora M, Portugal R, Malecki M, Bähler J, Dixon L, Werner F. 2020. The African swine fever virus transcriptome. J Virol 94:e00119-20. 10.1128/JVI.00119-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.García R, Almazán F, Rodríguez JM, Alonso M, Viñuela E, Rodríguez JF. 1995. Vectors for the genetic manipulation of African swine fever virus. J Biotechnol 40:121–131. 10.1016/0168-1656(95)00037-q. [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Zhao D, He X, Liu R, Wang Z, Zhang X, Li F, Shan D, Chen H, Zhang J, Wang L, Wen Z, Wang X, Guan Y, Liu J, Bu Z. 2020. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci China Life Sci 63:623–634. 10.1007/s11427-020-1657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borca MV, Ramirez-Medina E, Silva E, Vuono E, Rai A, Pruitt S, Holinka LG, Velazquez-Salinas L, Zhu J, Gladue DP. 2020. Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J Virol 94:e02017-19. 10.1128/JVI.02017-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borca MV, Holinka LG, Berggren KA, Gladue DP. 2018. CRISPR-Cas9, a tool to efficiently increase the development of recombinant African swine fever viruses. Sci Rep 8:3154. 10.1038/s41598-018-21575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gladue DP, O’Donnell V, Ramirez-Medina E, Rai A, Pruitt S, Vuono EA, Silva E, Velazquez-Salinas L, Borca MV. 2020. Deletion of CD2-like (CD2v) and C-type lectin-like (EP153R) genes from African swine fever virus Georgia-Δ9GL abrogates its effectiveness as an experimental vaccine. Viruses 12:1185. 10.3390/v12101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Donnell V, Risatti GR, Holinka LG, Krug PW, Carlson J, Velazquez-Salinas L, Azzinaro PA, Gladue DP, Borca MV. 2017. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. J Virol 91:e01760-16. 10.1128/JVI.01760-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li D, Liu Y, Qi X, Wen Y, Li P, Ma Z, Liu Y, Zheng H, Liu Z. 2021. African swine fever virus MGF-110-9L-deficient mutant has attenuated virulence in pigs. Virol Sin 36:187–195. 10.1007/s12250-021-00350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li D, Yang W, Li L, Li P, Ma Z, Zhang J, Qi X, Ren J, Ru Y, Niu Q, Liu Z, Liu X, Zheng H. 2021. African swine fever virus MGF-505-7R negatively regulates cGAS-STING-mediated signaling pathway. J Immunol 206:1844–1857. 10.4049/jimmunol.2001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramakrishnan MA. 2016. Determination of 50% endpoint titer using a simple formula. World J Virol 5:85–86. 10.5501/wjv.v5.i2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li D, Yang W, Yang F, Liu H, Zhu Z, Lian K, Lei C, Li S, Liu X, Zheng H, Shu H. 2016. The VP3 structural protein of foot-and-mouth disease virus inhibits the IFN-beta signaling pathway. FASEB J 30:1757–1766. 10.1096/fj.15-281410. [DOI] [PubMed] [Google Scholar]
- 59.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 60.Ramirez-Medina E, Vuono E, Pruitt S, Rai A, Silva E, Zhu J, Velazquez-Salinas L, Gladue DP, Borca MV. 2020. X69R is a non-essential gene that, when deleted from African swine fever, does not affect virulence in swine. Viruses 12:918. 10.3390/v12090918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krug PW, Holinka LG, O’Donnell V, Reese B, Sanford B, Fernandez-Sainz I, Gladue DP, Arzt J, Rodriguez L, Risatti GR, Borca MV. 2015. The progressive adaptation of a Georgian isolate of African swine fever virus to Vero cells leads to a gradual attenuation of virulence in swine corresponding to major modifications of the viral genome. J Virol 89:2324–2332. 10.1128/JVI.03250-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 (GO enrichment analysis).<br>. Download JVI.00824-21-s0001.xlsx, XLSX file, 0.02 MB (25.3KB, xlsx)
Table S2 (KEGG pathway analysis).<br>. Download JVI.00824-21-s0002.xlsx, XLSX file, 0.02 MB (23.6KB, xlsx)
Table S3 (Differential expression analysis).<br>. Download JVI.00824-21-s0003.xlsx, XLSX file, 0.02 MB (17.2KB, xlsx)
Table S4 (Differential expression analysis of genes in the mock-, ASFV-, and ASFV E120R Δ72-73aa-infected cells).<br>. Download JVI.00824-21-s0004.xlsx, XLSX file, 0.05 MB (48.9KB, xlsx)
Data Availability Statement
The newly determined sequences are available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.34tmpg4km.