ABSTRACT
Mother-to-child transmission (MTCT) of human immunodeficiency virus type 1 (HIV-1) and human cytomegalovirus (HCMV) may occur during pregnancy, labor, or breastfeeding. These viruses from amniotic fluid, cervicovaginal secretions, and breast milk may simultaneously interact with oropharyngeal and tonsil epithelia; however, the molecular mechanism of HIV-1 and HCMV cotransmission through the oral mucosa and its role in MTCT are poorly understood. To study the molecular mechanism of HIV-1 and HCMV MTCT via oral epithelium, we established polarized infant tonsil epithelial cells and polarized-oriented ex vivo tonsil tissue explants. Using these models, we showed that cell-free HIV-1 and its proteins gp120 and tat induce the disruption of tonsil epithelial tight junctions and increase paracellular permeability, which facilitates HCMV spread within the tonsil mucosa. Inhibition of HIV-1 gp120-induced upregulation of mitogen-activated protein kinase (MAPK) and NF-κB signaling in tonsil epithelial cells, reduces HCMV infection, indicating that HIV-1-activated MAPK and NF-κB signaling may play a critical role in HCMV infection of tonsil epithelium. HCMV infection of tonsil epithelial cells also leads to the disruption of tight junctions and increases paracellular permeability, facilitating HIV-1 paracellular spread into tonsil mucosa. HCMV-promoted paracellular spread of HIV-1 increases its accessibility to tonsil CD4 T lymphocytes, macrophages, and dendritic cells. HIV-1-enhanced HCMV paracellular spread and infection of epithelial cells subsequently leads to the spread of HCMV to tonsil macrophages and dendritic cells. Our findings revealed that HIV-1- and HCMV-induced disruption of infant tonsil epithelial tight junctions promotes MTCT of these viruses through tonsil mucosal epithelium, and therapeutic intervention for both HIV-1 and HCMV infection may substantially reduce their MTCT.
IMPORTANCE Most HIV-1 and HCMV MTCT occurs in infancy, and the cotransmission of these viruses may occur via infant oropharyngeal and tonsil epithelia, which are the first biological barriers for viral pathogens. We have shown that HIV-1 and HCMV disrupt epithelial junctions, reducing the barrier functions of epithelia and thus allowing paracellular penetration of both viruses via mucosal epithelia. Subsequently, HCMV infects epithelial cells, macrophages, and dendritic cells, and HIV-1 infects CD4+ lymphocytes, macrophages, and dendritic cells. Infection of these cells in HCMV- and HIV-1-coinfected tonsil tissues is much higher than that by HCMV or HIV-1 infection alone, promoting their MTCT at its initial stages via infant oropharyngeal and tonsil epithelia.
KEYWORDS: HIV, breast milk, human cytomegalovirus, mother to child transmission, tonsil mucosal epithelium
INTRODUCTION
Mother-to-child transmission (MTCT) is an important pathway for the spread of human immunodeficiency virus-1 (HIV-1) during pregnancy, labor, delivery, and breastfeeding (1–7). Approximately 200,000 infants acquire HIV-1 infection annually despite antiretroviral therapy (ART), suggesting the need for alternative prevention strategies (8, 9). In women not treated with ART, HIV MTCT occurs through the breast milk in 30 to 45% of cases (10). Breast milk of HIV-infected mothers may contain cell-free HIV-1 as well as HIV-infected CD4+ T lymphocytes and macrophages, which may initiate the mucosal transmission of virus (11–13). Infectious HIV-1 may persist in the breast milk despite ART, which significantly reduces MTCT but does not eliminate the virus and its reservoirs (14–16). The lining epithelia of oropharyngeal and gastrointestinal mucosae have well-developed tight junctions, which prevent paracellular penetration of viral pathogens, including HIV-1, and serve as a barrier to mucosal transmission of viruses (17–21). To initiate systemic infection, HIV-1 must first be transmitted across oropharyngeal and/or intestinal mucosal epithelium and then infect susceptible immune cells (21). HIV transmission across fetal/infant oropharyngeal mucosal epithelial cells occurs by transcytosis; i.e., virions transmigrate across cells without infecting them (20). However, cell-free HIV-1 transcytosis via mucosal epithelial cells is not highly efficient; only 0.01 to 0.05% of virions from the initial inoculum may translocate across epithelial cells (20, 22–24). More than 90% of internalized virions are sequestered in the endosomes of epithelial cells, including multivesicular bodies and vacuoles (24, 25). These sequestered virions in the epithelium are infectious and are released by the interaction of activated peripheral blood mononuclear cells (PBMC) and CD4+ T lymphocytes with epithelial cells containing the virus. Furthermore, HIV-1 interaction with mucosal epithelial cells may lead to the disruption of epithelial tight junctions and facilitate paracellular transport of virus, initiating infection of intramucosal and submucosal target cells (21, 26–29).
Human cytomegalovirus (HCMV) is an important human pathogen (30) that is associated with opportunistic infection during HIV/AIDS disease. It manifests as oral mucosal lesions, esophagitis, retinitis, pneumonia, hepatitis, gastrointestinal inflammation, or encephalopathy (31–35). HIV-infected individuals, including pregnant women, are almost universally coinfected with HCMV (36, 37). HCMV MTCT occurs in utero via the placenta and during labor by exposure to cervicovaginal secretions (38, 39). However, most HCMV MTCT occurs postpartum through infected breast milk and oral mucosal secretions (38–40). Almost all HCMV in HCMV-seropositive, HIV-uninfected women is activated by lactation (41); virus is shed into breast milk for only 4 to 6 weeks postpartum (42, 43). In contrast, in HIV-infected women, a high level of HCMV shedding into breast milk may significantly increase for 6 or more months postpartum, facilitating MTCT via infant oropharyngeal and gastrointestinal mucosal epithelia (44). Low CD4+ count and high HIV RNA in the breast milk are correlated with higher levels of HCMV DNA in breast milk (44, 45). In HIV-infected women, the rate of HCMV perinatal and postnatal transmission may increase to 90% of children who acquire a virus during early childhood (32, 39, 46, 47). HCMV infection, or its reactivation in HIV-infected pregnant women, may also play a critical role in HIV-1 MTCT. An increasing level of HCMV in breast milk is associated with an increased risk of postpartum HIV-1 MTCT (44, 47–49).
We and others have shown that HIV-1 cell-free virions and viral tat and gp120 proteins play an important role in the impairment of the mucosal barrier by disrupting epithelial tight junctions (21, 26–29, 50, 51). HIV tat and gp120 are regulatory and envelope proteins, respectively, that activate multiple signaling pathways, including mitogen-activated protein kinase (MAPK) and NF-κB signaling. This may lead to disruption of tight and adherens junctions through induction of epithelial-mesenchymal transition, aberrant internalization of tight junction proteins, and their downregulation and/or proteasome-mediated degradation (52–64). HCMV infection also disrupts tight junctions of intestinal epithelial cells (65). Thus, disruption of mucosal epithelial tight junctions by HIV-1 and HCMV may impair the barrier functions of oropharyngeal and intestinal epithelia, allowing paracellular penetration of these viruses and the initiation of MTCT.
Although accumulated evidence indicates that both HIV and HCMV are simultaneously detected in breast milk and may serve as a synergistic risk factor for MTCT, the mechanism of cotransmission of these viruses through infant oropharyngeal and tonsil mucosal epithelia has been poorly investigated. We hypothesize that HIV-1 and HCMV may synergistically impair the barrier function of oropharyngeal and tonsil mucosal epithelia, facilitating their transmucosal transmission. To test this hypothesis, we investigated the joint role of HIV and HCMV in transmucosal transmission by using monostratified polarized infant tonsil epithelial cells and ex vivo infant tonsil tissue explants. We show here that both HIV-1 and HCMV may promote their transmucosal transmission through disruption of epithelial junctions, which initiates the spread of both viruses within intramucosal and submucosal target cells. That is, the simultaneous interaction of HIV-1 and HCMV with tonsil mucosa can synergistically increase the rate of MTCT of both viruses in HIV-1- and HCMV-coinfected women.
RESULTS
HIV-1 tat- and gp120-mediated disruption of tight junctions leads to paracellular spread of HCMV.
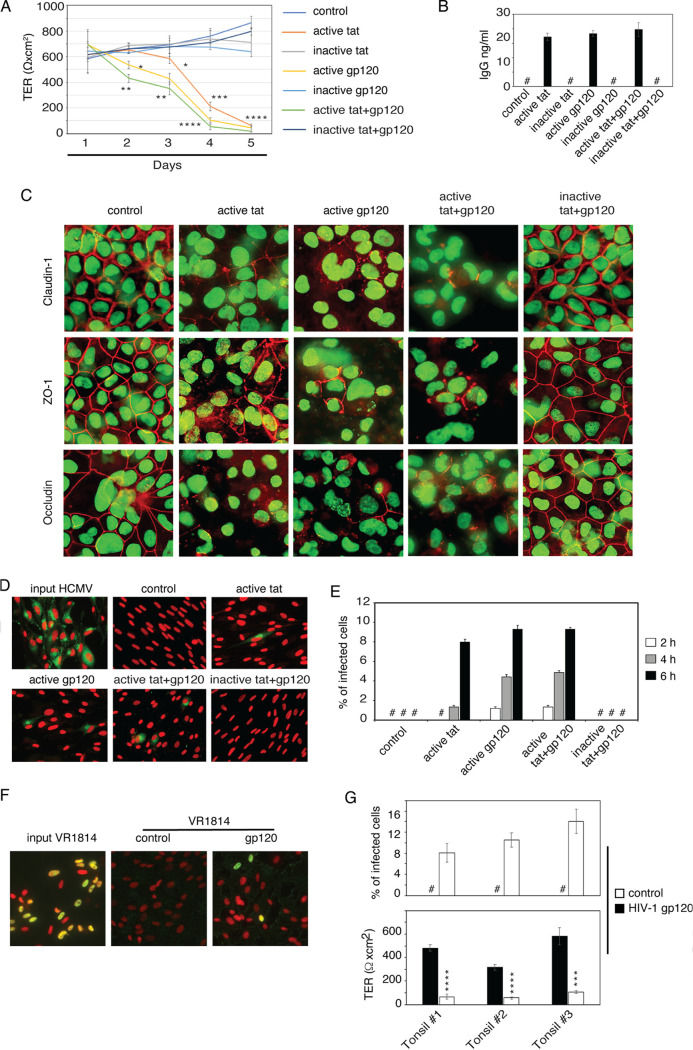
HIV-induced disruption of epithelial junctions may promote paracellular penetration of other viruses, including HCMV. To test this hypothesis, we established cultures of monostratified polarized infant tonsil epithelial cells. Cells were treated separately with recombinant HIV-1 proteins tat and gp120 and with a combination of tat and gp120 to examine their effects on transepithelial resistance (TER), paracellular permeability, and tight junction integrity of polarized cells. Control groups consisted of untreated cells and cells treated with inactive mutant tat, which was generated by substitution of the basic arginine-rich domain at amino acids (aa) 49 to 57 and the integrin-binding RGD motif in the C terminus with alanines (50, 51). Heat-inactivated gp120 was also used as a negative control (50, 51). Cells were treated with active and inactive tat and gp120 proteins, and culture medium was changed every day to add fresh proteins (Fig. 1A). Treatment of tonsil epithelial cells with active tat, gp120, and tat+gp120 for 5 days induced a rapid decrease (90%) in the TER of epithelial monolayers and increased paracellular permeability by 40 to 60% compared with untreated control cells and inactive tat+gp120-treated cells (Fig. 1A and B).
FIG 1.
HIV-1 tat- and gp120-induced disruption of tight junctions facilitates HCMV paracellular spread via polarized tonsil epithetical cells. (A) Polarized tonsil epithelial cells were treated with recombinant HIV-1BAL tat and gp120 proteins (10 ng/ml of each). Untreated cells or inactive tat- and gp120-treated cells served as a control. Culture medium was changed every day to add fresh proteins, and TER was measured. (B) After 5 days of protein treatment, the paracellular permeability of polarized cells was measured by IgG leakage assay. (C) After 5 days of protein treatment, one set of cells was fixed and immunostained for the tight junction proteins claudin-1, occludin, and ZO-1 (all red). Cell nuclei were stained with Sytox green nucleic acid stain (green). Cells were analyzed using fluorescence microscopy (Nikon Eclipse E400). Magnification, ×630. (D and E) After 5 days of protein treatment, HCMV AD169 at an MOI of 1 was added to the apical surface of polarized cells. After 2, 4, or 6 h, basolateral medium was collected and used to infect HFF; 48 h later, cells were immunostained with HCMV gB antibody (D). One set of HFF was directly infected with input virus. HCMV gB-positive cells were counted and presented as a percentage of infection (E). (F and G) After 5 days of HIV-1 gp120 protein treatment or without treatment (control), tonsil epithelial cells were measured for TER (G, lower chamber) and then incubated with clinical strain HCMV VR1814 at an MOI of 1 from apical membranes of polarized cells. After 4 h, basolateral medium was collected and used to infect HFF. HCMV VR1814 infection of HFF was examined by immunostaining of HCMV IE1/2 expression (green) (F). Cell nuclei were stained with propidium iodide solution (red). Merged images are presented, and the yellow signal shows colocalization of IE1/2 with cell nuclei, which indicates HCMV infection. Infected cells were quantitatively evaluated and presented as a percentage of infection (G, top). (A, B, E, and G) Data are means and SD (n = 3) and represent three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (in comparison with the control groups). #, not detected.
Expression of the tight junction proteins claudin-1, occludin, and zonula occludens-1 (ZO-1) in control cells by immunofluorescence assay was detected exclusively in a ring shape, indicating their lateral membrane localization (Fig. 1C). In contrast, expression of claudin-1, occludin, and ZO-1 in epithelial cells treated with active tat and gp120 proteins was in a discontinuous ring shape, cytoplasmic, or negative, showing disruption or a lack of tight junctions (Fig. 1C). The decrease in TER and increased paracellular permeability in the cells treated with active tat and gp120 were correlated with the disruption of tight junction proteins claudin-1, occludin, and ZO-1. These experiments showed that HIV-1 tat and gp120 proteins impair the barrier function of polarized infant tonsil epithelial cells, as shown in our previous work (18, 50, 51).
Next, we examined whether HIV-1-induced disruption of tight junctions of infant tonsil epithelial cells leads to the paracellular spread of HCMV. After treating epithelial monolayers with HIV-1 proteins for 5 days, we added 3 × 105 infectious units (IU) of HCMV AD169 to the apical membranes of epithelial cells for 2, 4, or 6 h. At each time point, the basolateral culture medium from the lower chamber of Transwell inserts was collected for an HCMV infectivity assay using human foreskin fibroblasts (HFF).
Immunofluorescence staining of HFF with monoclonal antibodies against HCMV glycoprotein B (gB) did not detect infectious HCMV virions in the basolateral medium of untreated control cells or inactive tat- and gp120-treated cells, indicating that intact tight junctions of tonsil epithelia prevented paracellular spread of HCMV (Fig. 1D). In contrast, infectious HCMV infection was detected in the basolateral medium of HIV-1 gp120- and tat-treated epithelial cells, showing paracellular spread of HCMV via disrupted tight junctions by HIV-1 proteins. Quantitative analysis of HCMV-infected HFF showed that paracellular spread from the apical to the basolateral compartment was time dependent: initially detected after 2 h at a low level (1.8%) and reaching its highest level (8 to 10%) 6 h after incubation (Fig. 1E). HFF infected directly with 3 × 105 IU of HCMV, which was used for the apical inoculum, showed 96% infection.
To confirm whether these findings were reproducible with the clinical isolate of HCMV, we performed experiments with HCMV VR1814. Polarized tonsil epithelial cells from three independent donors were treated with HIV gp120. Untreated cells served as a control. After 5 days of treatment, gp120 reduced TER in all epithelial cells by 70 to 90%, compared with untreated control cells, indicating substantial disruption of epithelial junctions (Fig. 1G, bottom). HCMV VR1814 (3 × 105 IU) was added to apical membranes of polarized epithelial cells, and after 4 h of incubation, basolateral medium was collected to study HFF infection. HCMV VR-1814 infection was detected in HFF by immunofluorescence assay using a monoclonal antibody against immediate early (IE1/2) proteins of HCMV (Fig. 1F). Quantitative analysis of HCMV VR1814-infected HFF showed that HIV-1 gp120-induced paracellular transport of HCMV was detected in all three tonsil epithelial cell cultures and varied from 8% to 15% (Fig. 1G, top). In contrast, no HCMV VR1814 infection was detected in the medium of untreated control tonsil cells with intact tight junctions. HFF directly infected with 3 × 105 IU of HCMV VR1814 led to 90% infection. These data show that HIV-1 tat- and gp120-induced disruption of infant tonsil epithelial cells facilitates paracellular spread of HCMV.
Cell-free HIV virion-induced disruption of infant tonsil epithelial junctions facilitates paracellular HCMV spread.
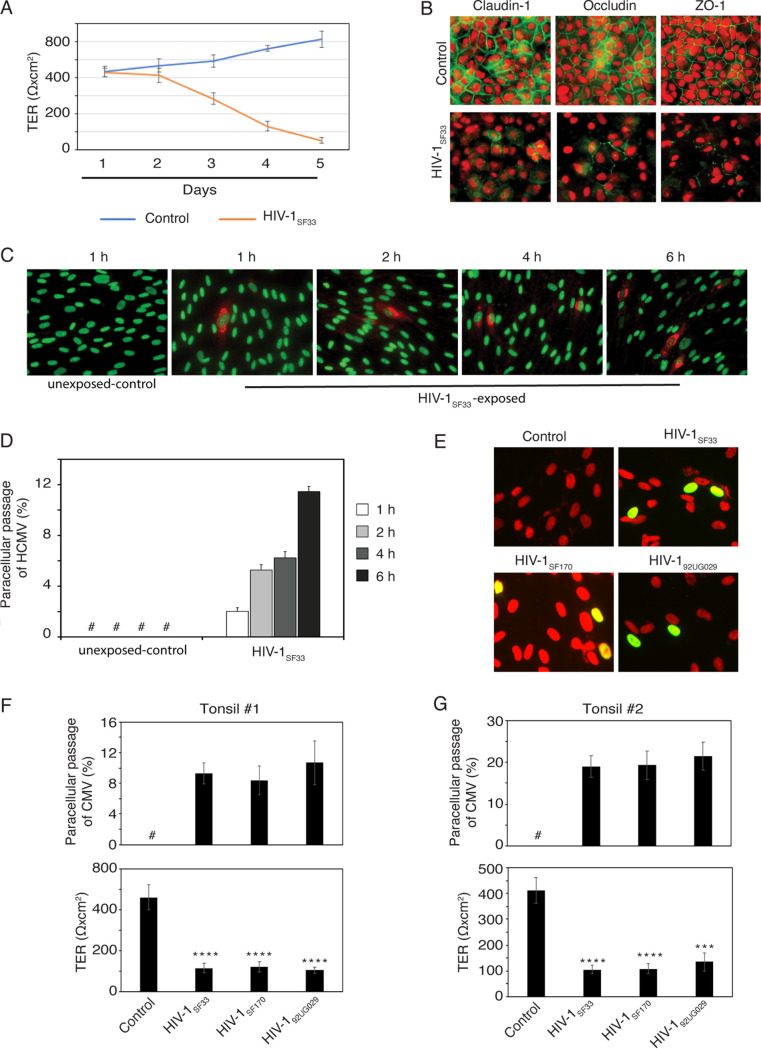
The experiments described above showed that HIV-1 envelope protein gp120 disrupts epithelial tight junctions and facilitates HCMV paracellular spread. To determine if HIV cell-free virions, which contain gp120 on their surface, also promote HCMV paracellular spread, we treated polarized infant tonsil epithelial cells with dually tropic HIV-1SF33 for 5 days. Cell-free HIV virions gradually reduced TER, and after 5 days, the reduction of TER was 95% compared with untreated control cells (Fig. 2A). Immunofluorescence analysis of claudin-1, occludin, and ZO-1 expression in untreated and HIV-1SF33-treated cells at day 5 showed that expression of all three tight junction proteins was lost in 80 to 95% of cells incubated with HIV compared with control cells (Fig. 2B). This indicates that cell-free HIV-1 induces a severe disruption of tonsil epithelial tight junctions.
FIG 2.
Cell-free HIV-1 virions induce tight junction disruption of tonsil epithelial cells and increase HCMV paracellular spread. (A) Polarized tonsil epithelial cells were exposed to dually tropic HIV-1SF33 for 5 days. Each day, the culture medium was changed to add fresh virus. Untreated cells served as a control. TER was measured every day. (B) After 5 days, one set of HIV-1SF33-treated or untreated cells were fixed and immunostained for tight junction proteins claudin-1, occludin, and ZO-1 (all in red). Cell nuclei were stained with propidium iodide solution (red). Cells were analyzed using fluorescence microscopy. Magnification, ×400. (C and D) HCMV AD169 at an MOI of 1 was added to the apical surface (upper chamber) of HIV-1SF33-treated or untreated polarized tonsil cells for 5 days. After 1, 2, 4, or 6 h of HCMV AD169 inoculation, the culture medium from the basolateral chamber was collected and used to infect HFF. HCMV AD169 infection in HFF was examined by immunostaining of HCMV gB (red). Cell nuclei were stained with Sytox green nucleic acid stains (green). Cells were analyzed using fluorescence microscopy (C). Magnification, ×400. HCMV gB-positive HFF were counted in 10 random microscopic fields, and the percentage of cells positive for gB was determined (D). (E, F, and G) Polarized tonsil epithelial cells from two independent donors were exposed to dually tropic HIV-1SF33, R5-tropic HIV-1SF170, or X4-tropic HIV-192UG029 for 5 days, and TER was measured (F and G, bottom). Cells were then incubated for 4 h with HCMV VR1814 at an MOI of 1 from the apical surface. Culture medium from the basolateral chamber was collected and used to infect HFF. HCMV VR1814 infection of HFF was detected by HCMV IE1/2 immunostaining (green) (E). Cell nuclei were stained with propidium iodide solution (red). Cells were analyzed by fluorescence microscopy. Magnification, ×600. Merged panes show the yellow signal that indicates colocalization of IE1/2 with cell nuclei, indicating HCMV infection. Infected cells were quantitatively evaluated and presented as a percentage of infection (F and G, top). (A, D, F, and G) Data are means and SD of triplicate values. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (compared with the control cells). #, not detected.
To determine if HIV-1SF33-induced disruption of tonsil epithelial tight junctions facilitates HCMV paracellular spread, we incubated polarized tonsil epithelial cells exposed to HIV-1SF33 with HCMV AD169 for 1, 2, 4, or 6 h. At each time point, the basolateral medium was collected and examined in the HCMV infectivity assay using HFF. Cells not exposed to HIV-1SF33 served as a negative control. Immunostaining of HFF for HCMV gB showed that HCMV infection was not detected in control cells (Fig. 2C), indicating a lack of paracellular spread of HCMV. In contrast, HCMV infection was detected in HFF incubated with basolateral medium from epithelial cells exposed to HIV-1SF33. Quantitative analysis showed that paracellular transport of HCMV was time dependent starting with 2% at 2 h after apical inoculation of HIV-1SF33 and reaching 11.5% at 6 h after inoculation (Fig. 2D).
We next examined the role of the dually tropic HIV-1SF33 lab strain and the clinical isolates of R5-tropic HIV-1SF170 and X4-tropic HIV-192UG029 viruses in the paracellular transport of HCMV clinical strain VR-1814. Polarized tonsil epithelial cells from two independent donors were exposed to HIV-1 virions for 5 days, and HCMV paracellular transport was evaluated by examining the infectivity of HFF in basolateral medium by detection of the IE protein of HCMV (Fig. 2E). All three HIV-1 strains reduced TER of epithelial cells by 80 to 90% compared with tonsil cells not exposed to HIV-1, indicating that dually tropic, X4-tropic, and R5-tropic viruses disrupt epithelial junctions (Fig. 2F and G, bottom). Paracellular transport of HCMV showed that all three HIV strains facilitated HCMV VR1814 paracellular spread via disrupted tonsil epithelial cells from independent donors (Fig. 2F and G, top). However, the paracellular spread of HCMV VR1814 was higher in cells from donor 2 (20%) than in cells from donor 1 (8 to 9%). These findings indicate that the paracellular spread of the clinical strain of HCMV via tonsil epithelium can be facilitated by dually tropic, X4-tropic, and R5-tropic HIV-1 strains by disruption of epithelial junctions.
HIV-1-induced disruption of tonsil epithelial tight junctions increased HCMV infection of epithelial cells.
We have shown that cell-free HIV-1 and the interaction of gp120 and tat proteins with tonsil epithelial cells activate MAPK and NF-κB signaling pathways, which disrupt tight and adherens junctions and depolarize the epithelial cells (50–52). To test the hypothesis that these events may modulate HCMV infection, we examined HCMV infection in polarized cells exposed to cell-free HIV-1 and its proteins tat and gp120.
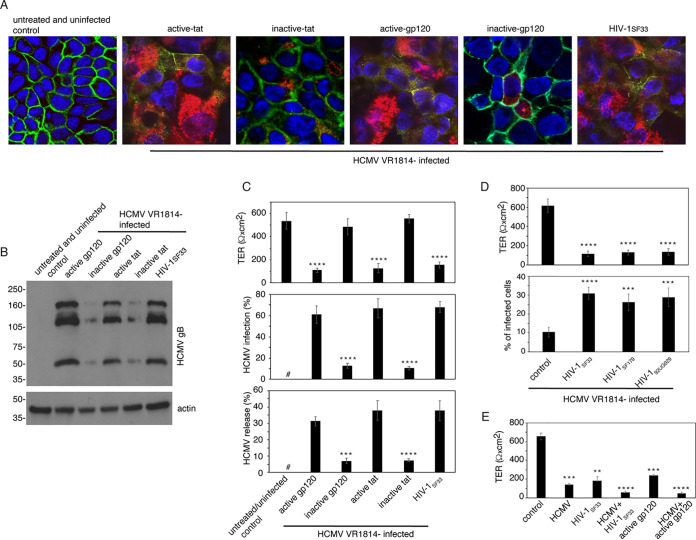
Polarized tonsil epithelial cells were exposed to active and inactive HIV-1 tat and gp120 and cell-free HIV-1SF33 for 5 days, and then cells were infected with HCMV VR1814 from the apical surface. Cells without HIV proteins, HIV virions, and HCMV served as negative controls. Three days after HCMV infection, cells were coimmunostained for occludin and HCMV gB. Confocal microscopy showed that almost 100% of control cells had strong occludin expression. In contrast, cells exposed to HIV-1SF33 virions and its active proteins did not express occludin or substantially reduce its expression, indicating severe disruption of tight junctions (Fig. 3A). In cells treated with inactive tat and gp120, occludin expression was not reduced and was similar to that of untreated control cells. Analysis of HCMV gB protein expression in epithelial cells exposed to HIV proteins and virions showed that high-level gB expression was detected in a majority of cells treated with active tat and gp120 and cell-free HIV-1SF33 virions (Fig. 3A). Expression of gB in tonsil cells treated with inactive HIV-1 tat and gp120 was at a low level. This result was confirmed in a parallel experiment examining gB expression by Western blotting. A substantial increase in gB expression was observed only in tonsil epithelial cells treated with active tat and gp120 and HIV-1 virions, in contrast to cells treated with inactive tat and gp120 (Fig. 3B).
FIG 3.
HIV-1-induced disruption of tonsil epithelial tight junctions increases HCMV infection. (A and B) Polarized tonsil epithelial cells were exposed for 5 days to cell-free HIV-1SF33 and its active and inactive proteins tat and gp120. Untreated cells served as a control. Cells were then infected with HCMV VR1814 at an MOI of 1. After 3 days, one set of cells was coimmunostained for HCMV gB (red) and tight junction protein occludin (green) (A). Cell nuclei were counterstained with DAPI (blue). Cells were analyzed by fluorescence microscopy. Magnification, ×630. The next set of cells was lysed, and HCMV VR1814 infection was examined by Western blotting, which detected HCMV gB (B). Sample loading for the Western blot was controlled by detecting β-actin. Immunoblots were performed at least twice, and representative results are shown. (C) Polarized tonsil epithelial cells were exposed for 5 days to cell-free HIV-1SF33 and its active and inactive proteins tat and gp120, as described for Fig. 3A. TER of polarized cells was then measured (top), and cells were infected with HCMV VR1814 at an MOI of 1. After 3 days, cells were immunostained for HCMV gB, and infected cells were quantitatively analyzed (middle). Culture medium containing HCMV VR1814 released from apical and basolateral membranes was collected, combined, and used to infect HFF (bottom). (D) Polarized tonsil epithelial cells were exposed for 5 days to dually tropic HIV-1SF33, R5-tropic HIV-1SF170, or X4-tropic HIV-192UG029 viruses and at day 5 the TER was measured (top). Cells were then infected with HCMV VR1814 at an MOI of 1 for 3 days. Cells were immunostained for HCMV gB, and gB-expressing cells were quantitatively evaluated (bottom). (E) Polarized tonsil epithelial cells were infected with HCMV VR1814 with or without HIV-1SF33. The next set of cells were treated with gp120 alone or gp120 with HCMV VR1814. After 5 days, the TER was measured. (C, D, and E) Data are means and SD from one of two or three independent experiments, each in triplicate (n = 3). **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (compared with control cells). #, not detected.
Next, we quantified the effect of cell-free HIV-1 and its proteins tat and gp120 on the level of HCMV infection in polarized tonsil epithelial cells. Polarized tonsil epithelial cells were exposed to active and inactive HIV-1 tat and gp120 and cell-free HIV-1SF33. After 5 days, TER showed a drastic reduction in cells exposed to active tat and gp120 and virions, compared with untreated cells and cells treated with inactive HIV proteins (Fig. 3C, top). Then these cells were infected with HCMV VR1814, and one set of cells was fixed and immunostained for HCMV gB protein. Quantitative analysis of gB-expressing cells showed that 60 to 65% of cells treated with active HIV-1 tat and gp120 and HIV virions were infected with HCMV VR1814. HCMV infection in tonsil cells treated with inactive HIV-1 tat and gp120 was 10 to 12%, indicating a substantially lower level of HCMV VR1814 infection (Fig. 3C, middle) compared with tonsil cells exposed to HIV proteins and cell-free virions.
To examine the release of HCMV VR1814 progeny from infected cells, we collected the apical and basolateral medium of polarized cells and used it to infect HFF (Fig. 3C, bottom). Quantitative analysis of HCMV IE expression in HFF showed that a higher number of released infectious HCMV virions were detected in the media of cells treated with active tat and gp120 and cell-free HIV-1SF33 (∼30 to 35%) than in inactive tat- and gp120-treated tonsil cells (∼12%).
To examine the role of X4-tropic and R5-tropic HIV-1 in HCMV infection of tonsil epithelial cells, we exposed polarized tonsil epithelial cells to dually tropic HIV-1SF33, R5-tropic HIV-1SF170, and X4-tropic HIV-192UG029 for 5 days. All three HIV-1 strains reduced TER by ∼60%, indicating disruption of tight junctions (Fig. 3D, top). Cells were then infected with HCMV VR1814; after 3 days, cells were immunostained for HCMV gB. Quantitative analysis showed that pre-exposure of tonsil epithelial cells to the dually tropic, X4-tropic, and R5-tropic HIV-1 strains led to an increase in HCMV VR1814 of 2- to 3-fold, compared with control cells not exposed to HIV-1 (Fig. 3D, bottom).
Altogether, these data clearly demonstrate that HIV-induced disruption of tight junctions of polarized infant tonsil epithelial cells substantially increases HCMV VR1814 infection and release of its progeny virions, indicating that disruption of epithelial tight junctions increases productive HCMV infection.
Because HCMV infection can also disrupt epithelial junctions, we examined HIV- and HCMV-induced disruption of tight junctions. Polarized tonsil epithelial cells were infected with HCMV VR1814 and HIV-1SF33 both separately and together. In parallel experiments, cells were treated with gp120 alone and gp120 with HCMV VR1814. After 5 days, the reduction of TER was significantly higher in cells treated with HCMV VR1814 and HIV-1SF33 or with recombinant gp120 than in cells treated with HCMV VR1814 and HIV-1SF33 viruses and gp120 alone. This indicates that the combination of HCMV and HIV-1 and the combination of HCMV and gp120 may synergistically disrupt tight junctions of tonsil epithelium (Fig. 3E).
HIV-1 envelope protein gp120 may play a key role in the promotion of HCMV infection of infant tonsil epithelial cells.
To better understand the role of HIV gp120 in HCMV infection, we inactivated cell-free HIV-1SF33 by UV irradiation, which prevents expression of viral genes without changing the envelope structure. The purified recombinant gp120 was inactivated by heat treatment. Polarized tonsil epithelial cells were exposed to active and inactive HIV-1SF33 and gp120 for 5 days. Both active and inactive HIV-1SF33 reduced the TER of polarized cells by 90% compared with untreated control cells (Fig. 4A, bottom). This indicates that noninfectious cell-free virus induces TER reduction; i.e., disruption of junctions is mediated by envelope gp120. Inactive gp120 did not reduce TER, unlike active gp120, which substantially reduced TER in polarized epithelial cells, confirming the functional role of gp120. These cells were then infected with HCMV VR1814 and, after 3 days, cells were immunoassayed for HCMV gB expression. Quantitative analysis showed that both UV-irradiated and active HIV-1SF33 increased HCMV VR1814 infection by 3- to 4-fold compared with control cells (Fig. 4A, top). Treatment of cells with active gp120 also increased HCMV VR1814 infection compared with inactive gp120. Thus, both purified recombinant gp120 and authentic gp120 from the virion envelope can disrupt tight junctions and increase HCMV VR1814 infection.
FIG 4.
HIV-1 gp120-induced disruption of tight junctions in tonsil epithelial cells may play a critical role in increasing HCMV infection through activation of MAPK and NF-κB signaling. (A) Polarized tonsil epithelial cells were exposed to cell-free HIV-1SF33 and UV-inactivated HIV-1SF33. In parallel experiments, cells were treated with recombinant gp120 and heat-inactivated gp120. After 5 days, TER was measured (bottom), and cells were then infected with HCMV VR1814 at an MOI of 1 for 3 days. Cells were immunostained for HCMV gB and quantitatively evaluated (top). (B) Polarized tonsil epithelial cells were treated for 5 days with recombinant gp120 proteins from HIV-1BAL, HIV-1IIIB, HIV-1CN54, and HIV-196ZM65. Untreated cells served as a control. After 5 days, TER was measured (bottom). Cells were then infected with HCMV VR1814 at an MOI of 1, and 3 days later, infection was examined by gB immunostaining, and gB-positive cells were quantitatively analyzed and presented as a percentage of infected cells (top). (C) Tonsil polarized epithelial cells from 10 independent donors were treated with HIV-1BAL gp120 for 5 days, and TER was measured. Cells were then infected with HCMV VR1814 at an MOI of 1, and infection was quantified by counting gB-expressing cells (top). (D and E) Polarized tonsil epithelial cells were treated with gp120 and tat proteins in the presence or absence of the chemical compounds U0126 and MG-132, which are inhibitors of MAPK and NF-κB, respectively. After 5 days, TER was measured (bottom), and cells were then infected with HCMV VR1814 at an MOI of 1. After 3 days, one set of cells was quantitatively evaluated for HCMV infection by gB immunostaining (top) (D). The next set of cells was analyzed for expression of HCMV IE1/2, and total and phosphorylated ERK1/2, IκB, and NF-κB were measured by Western blotting assay (E). (F) Polarized tonsil epithelial cells were treated with HIV-1 gp120 for 4 days and then maintained without gp120 for the next 4 days. Control cells were not treated with gp120. TER was measured every day (left). One set of cells treated with gp120 for 4 days and one set of cells untreated for the next 4 days were infected with HCMV VR1814. Untreated control cells were also infected with HCMV VR1814 at days 4 and 8. HCMV VR1814-infected cells were immunostained for gB, and gB-positive cells were counted (right). (A, B, C, D, and F) Data represent one of three independent experiments and are means and SD of triplicate values. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (compared with control cells).
To further confirm the role of gp120 in the promotion of HCMV infection in tonsil epithelial cells, we treated polarized cells for 5 days with recombinant gp120 from the following strains of HIV-1: HIV-1BAL, HIV-1IIIB, HIV-1CN54, and HIV-196ZM65. HIV-1BAL, HIV-1IIIB, and HIV-196ZM65 reduced TER by 75 to 80% compared with gp120 of HIV-1CN54, which did not reduce TER (Fig. 4B, bottom). Infection of these cells with HCMV VR1814 showed that disruption of epithelial tight junctions by gp120 of HIV-1BAL, HIV-1IIIB, and HIV-196ZM65 strains increased HCMV infection by 3- to 5-fold compared with untreated control cells (Fig. 4B, top). HCMV VR1814 infection was not increased in tonsil cells treated with gp120 from HIV-1CN54, which also did not reduce TER and therefore did not disrupt tight junctions. These findings indicate that gp120 glycoprotein from three of four HIV-1 strains (75%) disrupts epithelial tight junctions and increases HCMV infection.
To assess donor-to-donor variation, we used polarized tonsil epithelial cells isolated from 10 independent donors and treated them with gp120. All cells reduced TER compared with untreated control cells (Fig. 4C, bottom). However, analysis of HCMV VR1814 infection in these cells showed that six cultures (60%) increased HCMV infection by 2- to 6-fold compared with untreated control cells (Fig. 4C, top). Two cultures (numbers 5 and 9) did not show a significant increase in HCMV infection. Two other cultures (numbers 7 and 8) were not infected by HCMV regardless of gp120 treatment or reduction of TER. These data indicate that HIV-1 gp120 may play a key role in the disruption of tonsil epithelial tight junctions and the promotion of HCMV infection in most (60%) tonsil epithelial cultures from independent donors.
Inhibition of HIV-1 gp120-induced MAPK and NF-κB signaling reduced HCMV infection and disruption of tonsil epithelial junctions.
Previously, we showed that HIV-induced activation of MAPK and NF-κB signaling plays a critical role in the disruption of tight and adherens junctions (50–52). To examine the role of HIV-1 gp120-activated MAPK and NF-κB signaling in HCMV infection, we treated polarized tonsil epithelial cells with HIV-1 gp120 for 5 days in the presence or absence of a nontoxic concentration of inhibitors of MAPK and NF-κB signaling, U0126 and MG-132, respectively. Both MAPK and NF-κB inhibitors lessened gp120-induced TER reduction by ∼70% (Fig. 4D, bottom). Cells were then infected with HCMV VR1814, and 24 h later, were analyzed for IE1/2 protein expression by immunostaining. Quantitative analysis showed that, in gp120-treated cells, both MAPK and NF-κB inhibitors reduced HCMV VR1814 infection by ∼35% and 45%, respectively, compared with gp120-treated control cells (Fig. 4D, top).
In parallel experiments, one set of these cells was collected and lysed to examine MAPK and NF-κB activation and HCMV infection by Western blotting assay (Fig. 4E). Exposure of polarized tonsil epithelial cells to HIV-1 gp120 led to the induction of MAPK/ERK1/2, IκBα, and NF-κB phosphorylation. It has been shown that MAPK-induced NF-κB activation is initiated by phosphorylation and degradation of IκBα protein. This leads to NF-κB translocation to the nucleus, where it binds DNA and induces transcription of target genes (66, 67). In the presence of the MAPK inhibitor U0126 and the NF-κB inhibitor MG-132, gp120-induced phosphorylation of MAPK/ERK1/2, IκBα, and NF-κB was abolished; this was correlated with the reduction of IE1/2 expression, i.e., HCMV VR1814 infection (Fig. 4E). These data indicate that HIV-1 gp120-induced activation of MAPK/ERK1/2 and NF-κB signaling in tonsil epithelial cells is critical for the activation of HCMV infection.
It is well known that the development of epithelial cell polarity and the assembly of epithelial cell junctions are regulated by multiple factors. Under various stimuli, the disassembly of cell junctions and the depolarization of epithelial cells could be dynamically changed to reassembly of their junctions and repolarization of epithelial cells (68–71). To test whether HIV-1 gp120-initiated disruption of cell junctions and depolarization of epithelial cells is reversible in the absence of gp120, we treated polarized cells with gp120 for 4 days; cells were then maintained without gp120 for the next 4 days (Fig. 4F). gp120 significantly reduced TER for 4 days of treatment compared with untreated control cells (Fig. 4F, left). However, removal of gp120 from treated cells during the next 4 days led to the gradual recovery of TER. This indicates that gp120-induced disruption of their junctions and depolarization of epithelial cells is reversible; i.e., in the absence of gp120 these cells may become polarized with reassembled junctions.
To examine if this reversible process would change HIV-enhanced HCMV infection, we infected one set of tonsil epithelial cells with HCMV VR1814 at day 4 of gp120 treatment. Next, we infected the same set of cells with HCMV VR1814 after 4 days without gp120 treatment (Fig. 4F, right). gp120-untreated control cells were also infected with HCMV. HCMV infection was examined by quantitative analysis of gB expression, which showed that HCMV infection was 2-fold higher in the gp120-disrupted epithelial cells than in the recovered cells maintained without gp120 treatment. These findings suggested that HIV-1-enhanced HCMV infection of tonsil epithelial cells depends on the presence of HIV-1 gp120 for a few days in the tonsil mucosal environment. In the absence of HIV-1 gp120, the epithelial cells may recover and become polarized again, with reassembled junctions, and thus become less prone to infection by HCMV.
HCMV infection of ex vivo tonsil tissue explants.
Although HIV-1 infection of ex vivo tonsil tissue explants has been shown (29, 72), the ex vivo tonsil tissue model for HCMV infection has not yet been established. To generate an ex vivo tonsil tissue explant for HCMV infection, we propagated polarized-oriented tissue explants from palatine tonsil collected from two HIV-uninfected children under 5 years of age, as described in our previous work (18, 21). Tissue explants were maintained for 6 days; to examine the status of tight junctions of tonsil epithelium, one set of explants each day was fixed and immunostained for tight junction protein occludin from day 0 through day 6. After confocal microscopy from day 0 to day 3, occludin was detected at the lateral membrane of the entire multistratified epithelium in a ring shape, indicating preservation of intact tight junctions (Fig. 5A). However, starting at day 4, the pattern of occludin became discontinuous, and at day 5 and 6, occludin localization became diffusely cytoplasmic, showing the loss of tight junctions. The stratified structure of mucosal epithelium was also dissociated in the same areas of tonsil explants; however, the necrosis and destruction of cells were not observed.
FIG 5.
HCMV infection of ex vivo infant tonsil explants. (A) Explants were placed in Transwell inserts with the mucosal surface facing up to be accessible for viral inoculation, and the lateral edges were sealed with 4% agarose. Tissues were maintained for 6 days, and culture medium was changed every 2 or 3 days. At the end of each day, one set of explants was fixed, sectioned, and immunostained for the tight junction protein occludin (red). Cell nuclei were stained for DAPI (blue). Cells were analyzed by fluorescence microscopy. Magnification, ×400. EP, epithelium; LP, lamina propria. (B) Polarized-oriented infant tonsil explants were infected with 3 × 105 IU of HCMV VR1814 from the mucosal (apical) surface of tonsil epithelium. After 1 day, one set of explants was immunostained for HCMV IE1/2, and after 3 and 5 days, one set of explants was immunostained for HCMV gB (both green). Cell nuclei were counterstained with DAPI (blue). Cells were analyzed by fluorescence microscopy. Magnification, ×100 (two lower left panels) and ×400 (all others). (C and D) Tonsil explants from three independent donors were infected with HCMV VR1814. Uninfected explants served as a control. After 3, 6, and 9 days, culture medium was collected from the upper and lower chambers, combined, and used to infect HFF with HCMV. Infection was quantified by IE immunostaining (C). Data are means and SD (n = 3). At day 9, the infected and control tissue explants were homogenized, lysed, and examined for HCMV gB and IE1/2 by Western blotting (D).
To examine HCMV infection of tonsil explants, we added HCMV VR1814 to the mucosal surface; after 1 day, tissues were immunostained for HCMV IE protein, and after 3 and 5 days, tissues were examined for gB protein. An immunofluorescence assay showed that IE protein expression was detected in nuclei of the upper part of the squamous epithelium 1 day after infection (Fig. 5B), and gB expression was detected in the cytoplasm of epithelial cells starting at 3 days after infection. At 5 days, HCMV-infected cells showed virus-specific cytopathogenic changes, with large cytoplasm and round or oval masses in nuclei separated by a halo from the nuclear membrane (Fig. 5B).
In the next experiments, we infected tonsil tissue explants from three independent donors with HCMV VR1814; culture medium was collected at 3, 6, and 9 days after infection and used to infect HFF. Culture medium from uninfected tissues was also collected and used for HFF infection, and these cells served as a control. Immunostaining of HFF showed that IE expression was detected in the medium of all three HCMV-infected tonsil tissues (Fig. 5C). A quantitative assay of IE-positive HFF showed that HCMV infection gradually increased at 6 and 9 days after infection. None of the culture medium from three uninfected tonsil explants showed HCMV infection in HFF, indicating a lack of endogenous HCMV infection of tonsil tissues.
Tonsil tissues at day 9 were collected, homogenized, lysed, and used in a Western blot assay for detection of HCMV gB. HCMV gB protein was detected in all three HCMV-infected tissues and was not detected in three uninfected control tissues (Fig. 5D).
These data indicate that ex vivo tonsil explants can be infected by HCMV and may serve as a model for HCMV and HIV coinfection.
HIV-1 gp120- and tat-induced disruption of tight junctions of tonsil epithelial tissues increases HCMV infection.
To investigate the role of HIV proteins gp120 and tat in HCMV infection of infant tonsil tissues, we established polarized-oriented ex vivo tonsil tissue explants from two independent donors. The tissue explants were treated with inactive HIV-1 gp120+tat or active gp120+tat for 5 days (Fig. 6A). The tissues were then immunostained for occludin. Confocal microscopy showed that active gp120+tat treatment substantially changed occludin expression 3 days after treatment from a membrane pattern to a cytoplasmic pattern, indicating disruption of tight junctions. In contrast, at day 3 posttreatment, inactive gp120+tat treatment did not change occludin localization, i.e., the occludin staining pattern was in a ring shape, which indicates intact tight junctions (Fig. 6A). At day 4 posttreatment, occludin expression in tissues treated with active gp120+tat substantially decreased and become cytoplasmic compared to tissues treated with inactive HIV-1 gp120+tat, which still showed intact tight junctions in approximately 50% of cells. However, at day 5 posttreatment, both active gp120+tat- and inactive gp120+tat-treated tissues showed disruption of tight junctions (Fig. 6A).
FIG 6.
HIV-1 tat and gp120 disrupt tonsil epithelial junctions and facilitate HCMV infection. (A) Tonsil tissue explants were treated with active or inactive HIV-1 tat and gp120 proteins for 5 days; culture medium was changed daily to add fresh proteins. After each day, one set of explants was immunostained for occludin (red). (B) One set of tonsil explants treated with active tat and gp120 for 3 days was infected with 3 × 105 IU of HCMV VR1814; after 2 days, tissues were coimmunostained for HCMV gB (green) and occludin (red). Cell nuclei were stained with DAPI (blue), and cells were analyzed by fluorescence microscopy. Magnification, ×400. EP, epithelium; LP, lamina propria. (C) Tonsil explants from two independent donors were treated with active tat and gp120. After 3 days, explants were infected with 3 × 105 IU of HCMV VR1814. After 2 more days, tissues were immunostained for HCMV gB; gB-expressing cells were counted, and values are presented as a percentage of infection. (D) Tonsil tissues from six donors (#1 to #6) were treated with HIV-1 active or inactive tat and gp120 for 3 days. Untreated tissues served as a control. Tissues were then infected with HCMV VR1814 for 3 days. Tissues were immunostained for HCMV gB, and HCMV gB-expressing cells were quantitatively evaluated. (A and B) Data are means and SD of triplicate values. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (compared with the controls treated with inactive tat+gp120).
In the next experiments, tonsil tissue explants were treated with active gp120 or tat and inactive gp120+tat for 3 days. Then, these tissue explants were infected with HCMV VR1814, and after 2 days, tissues were coimmunostained for HCMV gB and occludin (Fig. 6B). By confocal immunofluorescence microscopy, HCMV gB expression was detected in the cytoplasm of epithelial cells within the upper layers of tonsil epithelium (Fig. 6B). Quantitative analysis of cells expressing HCMV gB showed that the number of cells expressing these proteins was 2- to 4-fold higher in the gp120- and tat-treated tissue explants than in explants treated with inactive tat+gp120, indicating that active tat and gp120 increased HCMV infection (Fig. 6C). Uninfected tissue did not show gB signals, indicating a lack of endogenous HCMV infection of tonsil tissues.
To examine donor-to-donor variation in tonsil tissues from six independent donors, we treated tissues with gp120 and tat and their inactive forms (Fig. 6D). Then, tissues were infected with HCMV VR1814, and at day 3 after infection, tissues were homogenized and supernatant was used to infect HFF. Quantitative analysis of HFF expressing HCMV IE protein showed that a higher level of infection was detected in 4 of 6 tissues treated with active gp120 and tat proteins (64%) compared with explants treated with inactive HIV-1 proteins (Fig. 6D). One of 6 tissues (16.6%) showed a similar level of HCMV infection in active and inactive tat- and gp120-treated tissues. One of 6 tissues (16.6%) was negative for HCMV IE.
These data indicate that HCMV infection increased in 64% of tonsil tissues treated with HIV-1 gp120 and tat, compared with tissues treated with inactive gp120+tat.
HCMV-induced disruption of tonsil epithelial tight junctions facilitates HIV-1 paracellular spread.
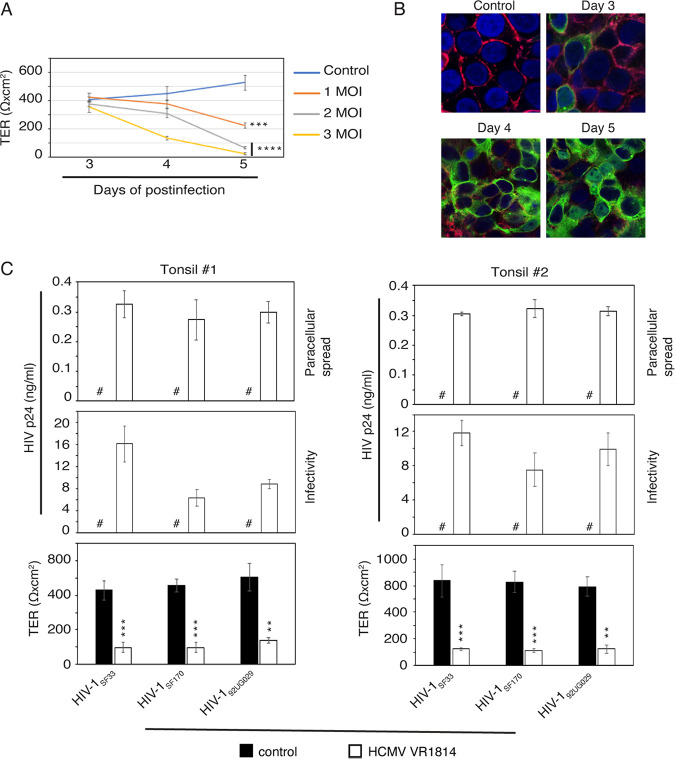
Figure 3E shows that HCMV infection of polarized tonsil epithelial cells reduces TER, indicating disruption of epithelial junctions, which could facilitate HIV paracellular passage and increase HIV-1 transmucosal transmission. To test this possibility, we infected polarized tonsil epithelial cells with HCMV VR1814 at a multiplicity of infection (MOI) of 1, 2, and 3, and TER was measured after 3, 4, and 5 days of postinfection. TER was reduced in accordance with HCMV VR1814 dose and duration of infection (Fig. 7A). A more drastic reduction of TER (90%) was detected with higher MOI and longer time of infection. Coimmunostaining of HCMV gB and occludin in the HCMV-infected cells showed that a higher expression of gB is correlated with a drastic reduction in membrane localization of occludin or the downregulation of its expression (Fig. 7B), indicating disruption of tight junctions by HCMV infection.
FIG 7.
HCMV infection disrupts tonsil epithelial junctions and facilitates paracellular HIV-1 spread. (A and B) Polarized tonsil epithelial cells were infected with 1, 2, or 3 MOI of HCMV VR1814, and TER was measured after 3, 4 and 5 days (A). One set of cells infected with HCMV VR1814 at an MOI of 3 was coimmunostained for occludin (red) and HCMV gB (green) after 3, 4, or 5 days (B). Uninfected polarized cells served as a control. Cell nuclei were stained with DAPI (blue), and cells were analyzed by fluorescence microscopy. Magnification, ×400. (C) Polarized tonsil epithelial cells from two independent donors were infected with HCMV VR1814 for 5 days. TER was measured (bottom), and the apical surface of cells was exposed for 2 h to dually tropic HIV-1SF33, R5-tropic HIV-1SF170, and X4-tropic HIV-192UG029 (3 ng/insert). Culture medium from the basolateral surface was collected and examined for p24 (top) using ELISA. Culture medium was also tested for HIV-1 infectivity in PBMC (middle). (A and C) Data are means and SD. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (compared with the control cells). #, not detected.
To examine the role of HCMV-induced disruption of tight junctions in paracellular HIV-1 spread, we infected polarized tonsil epithelial cells from two independent donors with HCMV VR1814 at 3 MOI. Five days later, TER was reduced by 70 to 80% in infected cells compared with uninfected control cells (Fig. 7C, middle and bottom). Then, cell-free HIV-1SF33 at 3 ng/insert was added to the apical chamber of inserts. After 2 h, medium from basolateral chambers was collected and examined for HIV-1 p24 by enzyme-linked immunosorbent assay (ELISA). Approximately 300 pg/insert p 24 of HIV-1 SF33 was detected in the basolateral chamber of HCMV-infected polarized cells from both donors, which is ∼10% of apically inoculated virions (Fig. 7C, top). In contrast, no HIV-1SF33 was detected in the basolateral chamber of HCMV-uninfected control cells, indicating that polarized cells with intact tight junctions do not allow paracellular HIV-1SF33 viral spread. Thus, the HCMV-induced disruption of epithelial junctions facilitates HIV-1 paracellular spread. To examine the infectivity of transmigrated HIV-1, we added activated PBMC to the basolateral medium. Paracellular transport of HIV-1 virions from HCMV-infected tonsil cells infected the PBMC (Fig. 7C, middle), indicating that HCMV-induced disruption of tonsil epithelial junctions may play a critical role in HIV-1 transmucosal transmission.
HIV and HCMV coinfection of tonsil epithelium may jointly promote their spread.
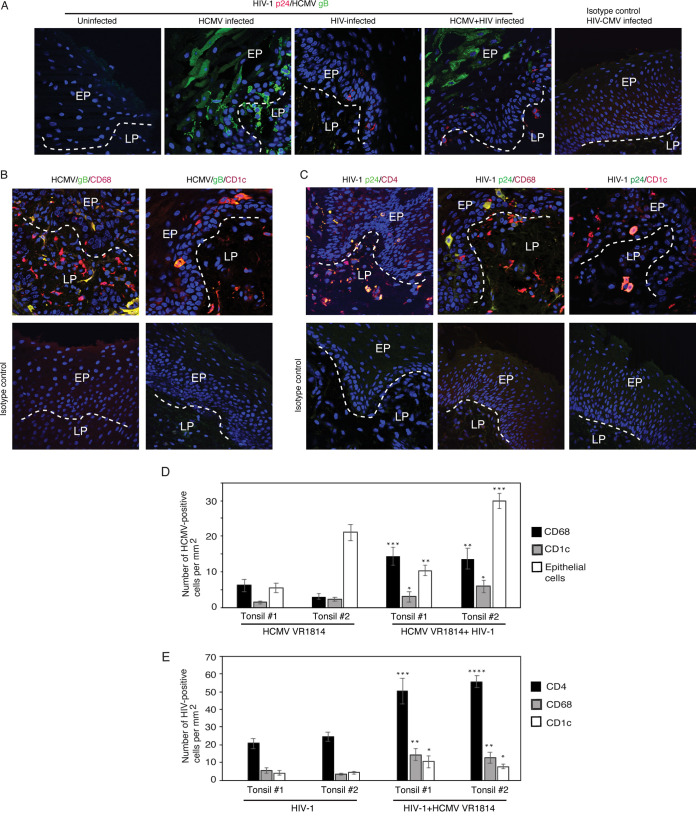
The experiments described above clearly show that HIV-1- and HCMV-induced disruption of tonsil epithelial junctions jointly promote their paracellular spread, suggesting that in HIV-1- and HCMV-coinfected individuals, these two viruses may synergistically promote their transmucosal transmission. To examine the role of HCMV and HIV-1 in their spread and infection, we infected polarized-oriented tonsil tissue explants with HCMV VR1814 and HIV-1SF33 independently or with two viruses from the apical surface of tissue explants. Uninfected tissues served as a negative control. After 3 days, tissue sections were coimmunostained for HCMV gB and HIV-1 p24. Confocal microscopy showed that the epithelial cells of HCMV-infected tissues were positive for gB, and cells expressing gB were detected in the epithelium and lamina propria, suggesting that HCMV may also infect nonepithelial cells in the lamina propria (Fig. 8A). HIV-1-infected tissues were positive for p24 and had a lymphocyte phenotype. In the HCMV- and HIV-1-coinfected tissues, both HCMV gB and HIV-1 p24 were detected in the cells with epithelial and lymphocyte morphology, respectively. HIV-1 and HCMV coinfected cells were not detected.
FIG 8.
HCMV and HIV-1 coinfection of ex vivo tonsil tissues. (A) Polarized-oriented tonsil explants were infected with HCMV VR1814 or HIV-1SF33 or coinfected with both viruses. Uninfected tissues served as a control. After 5 days, tissue sections were coimmunostained for HCMV gB (green) and HIV-1 p24 (red). (B) HCMV VR1814 and HIV-1SF33 -coinfected tissues were costained for HCMV gB (green) and for markers of macrophages CD68 and DC CD1c (both red). (C) HCMV VR1814- and HIV-1SF33-coinfected tissues were costained for HIV-1 p24 (green) and for markers of CD4 T lymphocytes (CD4), macrophages (CD68), and DC (CD1c) (all red). Merged panels are shown, and the yellow signal indicates HCMV or HIV infection of CD4 T lymphocytes, macrophages, and DC. Cell nuclei were stained with DAPI (blue), and cells were analyzed by confocal microscopy. Magnification, ×400. EP, epithelium; LP, lamina propria. (D and E) Tonsil explants from two independent donors were infected with HCMV VR1814 or HIV-1SF33 or coinfected with both. After 5 days, tissue sections were costained for HCMV gB (green) and for markers of macrophages and DC (red). In parallel experiments, tissue sections were immunostained for HIV-1 p24 (green) and for markers of CD4 T lymphocytes, macrophages, and DC (red). HCMV-infected macrophages and DC (D), and HIV-1-infected CD4 T lymphocytes, macrophages, and DC (E) were quantitatively evaluated and are presented as number of cells per mm2. Similarly, HCMV-infected epithelial cells also were quantitatively evaluated. Data are means and SD. *, P < 0.05; **, P < 0.01 and ***, P < 0.001; ****, P < 0.0001 (explants coinfected with both viruses were compared with explants infected with only HIV-1 or HCMV).
HCMV may infect macrophages and dendritic cells (DC) in vivo and in vitro (73–75); thus, it is possible that HCMV may infect tonsil tissues macrophages and DC ex vivo. To identify HCMV-infected macrophages and DC, we coimmunostained tissue sections with HCMV gB and CD68 or CD1c, which are markers for macrophages and DC, respectively. Confocal microscopy revealed that HCMV gB was colocalized with CD68 and CD1c markers, suggesting that these cells were infected with HCMV (Fig. 8B). It is possible that some of the HCMV gB-positive macrophages and DCs may have gB signals due to phagocytosis of fragments from other infected cells.
To examine HIV-1-infected immune cells, we coimmunostained tissue sections with HIV-1 p24 with markers of CD4+ T lymphocytes, CD68 macrophages, or CD1c DC. HIV-1 p24 was colocalized with CD4, CD68, and CD1c markers, indicating that these cells were infected with HIV-1 (Fig. 8C).
Quantitative analysis showed that the number of HCMV-infected CD68+ macrophages and CD1c+ DC was 2- to 3-fold higher in HCMV- and HIV-coinfected tissues than in tissues infected by HCMV alone (Fig. 8D). HCMV gB-positive epithelial cells were also counted, and the number of HCMV-infected epithelial cells was increased two to three times in tissues infected by both viruses compared to tissues infected only with HCMV. This indicates that HIV-1 infection may promote HCMV infection of these tissues (Fig. 8D). Similarly, HCMV- and HIV-1-coinfected tissues had more HIV-1-infected CD4+ T lymphocytes, CD68+ macrophages, and CD1c+ DC than did tissues infected by HIV alone. This shows that HCMV and HIV-1 coinfection may increase HIV-1 infection of these tissues (Fig. 8E).
To further investigate the role of HIV-1 and HCMV synergism in their infectivity, we infected tonsil explants from four independent donors with HIV-1SF33 and/or HCMV VR1814 in the presence or absence of the anti-HIV-1 drug lamivudine (3TC) or the anti-HCMV drug ganciclovir (GCV) for 9 days. Untreated tissues served as a control. Culture medium was collected at 3, 6, and 9 days after infection and examined for HIV-1 by ELISA p24 assay and for HCMV by infection of HFF. Results from tissues of two donors showed that both HIV-1 and HCMV infection were significantly increased in the HIV-HCMV-coinfected tissues compared with tissues infected separately (Fig. 9A and B). Treatment of coinfected tissues with anti-HIV-1 lamivudine significantly reduced HCMV infection, and anti-HCMV GCV significantly reduced HIV-1 infection (Fig. 9A and B).
FIG 9.
HCMV and HIV-1 coinfection of tonsil tissues synergistically promotes infection of both viruses. (A) Tonsil tissue explants from four independent donors were infected with HIV-1SF33, and tissues were cultured with or without GCV or lamivudine for 9 days. The next set of tissue explants from the same donors were coinfected with HIV-1SF33 and HCMV VR1814 in the presence or absence of GCV. After 3, 6, and 9 days, culture medium was collected and examined for HIV-1 p24 using ELISA. p24 values were normalized per 50 mg of tissue. (B) Tonsil explants from three independent donors were infected with HCMV VR1814, and tissues were cultured with or without GCV or lamivudine for 9 days. The next set of tonsil explants was coinfected with HCMV VR1814 and HIV-1SF33 with or without GCV. Tissue culture medium was collected after 3, 6, and 9 days and tested for HCMV infection of HFF. HCMV IE-positive HFF were quantitatively evaluated and are presented as a percentage of HCMV-infected cells (normalized per 50 mg of tissue). (C) Tonsil tissue explants from two independent donors were infected with only HCMV VR1814 or coinfected with HCMV VR1814 and dually tropic HIV-1SF33, R5-tropic HIV-1SF170, or X4-tropic HIV-192UG029 at 3 ng/tissue. At 3, 6, and 9 days of culture, the medium was collected and tested for HIV-1 p24 by ELISA. All data are means and SD of triplicate values. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (compared with coinfected explants and explants infected with only HIV-1 or HCMV). #, not detected.
However, HIV-1SF33 infection in the explant of the third donor was not detected after days 3 and 6; only by day 9 was infection detected. HCMV VR1814 infection was also not highly detectable after days 3 and 6, but some infection was observed at day 9. Nevertheless, the trend of a higher level of HIV-1 and HCMV infection was observed in the coinfected explants, which was reduced by anti-HIV or anti-HCMV drug treatments. In the fourth donor’s tissues explants, the peak of HIV-1SF33 infection was detected after day 6, and at day 9 it was reduced. In the HCMV-HIV-1SF33 -coinfected explants, HIV-1SF33 infection was significantly increased compared with tissues infected only with HIV-1SF33. However, a significant increase of HCMV VR1814 infection in the HCMV- and HIV-1SF33-coinfected explants was not detected.
These data revealed that coinfection of tissue explants with HIV-1 and HCMV may synergistically increase the infectibility of each virus in 75% of tonsil tissues.
To examine the role of HCMV infection in dually tropic, R5-tropic, and X4-tropic HIV-1 strains, tonsil explants from two independent donors were coinfected with HCMV VR1814 and dually tropic HIV-1SF33, R5-tropic HIV-1SF170, or X4-tropic HIV-192UG029. As a control, explants were infected with only HIV-1 strains. At 3, 6, and 9 days after infection, culture medium was collected and examined for HIV-1 p24. ELISA p24 data showed that HIV-1 infection from all three HIV-1 strains was 3- to 6-fold higher in HIV-1- and HCMV-coinfected explants than in explants infected with HIV-1 alone (Fig. 9C). These data indicate that HCMV infection of tonsil tissues promotes the infectious activity of dually tropic, R5-tropic, and X4-tropic HIV-1 strains.
DISCUSSION
We have shown here that HIV-1 and HCMV synergistically promote each other’s infection and spread in polarized tonsil epithelial cells and infant tonsil mucosal epithelial tissues. This finding may partially explain the mechanisms of these viruses' synergistic roles in MTCT at its initial stage.
In HIV- and HCMV-coinfected women, both viruses are present in the breast milk (11, 12, 76), which may interact with the oropharyngeal and gut mucosal epithelium of the child. Our findings show that cell-free HIV-1- and recombinant gp120-induced disruption of tight junctions opens the paracellular space between tonsil epithelial cells, leading to the paraepithelial spread of HCMV. Thus, cell-free HIV-1 may disrupt the tight junctions of tonsil epithelium and facilitate HCMV paracellular transport from breast milk; i.e., the primary HCMV MTCT transmission would be initiated. It is also possible that, during systemic HIV/AIDS disease in infants, the HIV-1 gp120 and tat proteins may be secreted into blood and saliva (18, 77–80), which may disrupt oral mucosal epithelial junctions (18, 21, 27–29, 50, 51), again promoting HCMV MTCT. Paracellular transmigrated HCMV via infant tonsil epithelium is infectious in the human fibroblast, indicating that HIV-promoted spread of HCMV MTCT via tonsil epithelium can initiate systemic HCMV infection in the child.
The HIV tat- and gp120-induced increase of HCMV infection in infant tonsil epithelial cells indicates that the depolarized epithelial cells with disrupted junctions are more susceptible to HCMV infection. We showed that HIV tat and gp120 induce the activation of MAPK and NF-κB signaling in tonsil epithelial cells (50–52). It is well documented that NF-κB activation is required for transactivation of the major IE promoter of HCMV and expression of viral IE proteins (81–83). Furthermore, the activation of MAPK is required for HCMV replication and production of viral progeny (84). Indeed, in our studies, inhibition of MAPK and NF-κB in HIV-1 gp120-treated tonsil epithelial cells substantially reduced HCMV infection, confirming that HIV-induced activation of these signaling pathways is critical for the increase in HCMV infection of tonsil epithelium.
Lack of tight junction disruption and activation of HCMV infection by one of the gp120 proteins from the HIV-1 CN54 strain suggested that mutation and alteration of HIV-1 gp120 may lead to reduction of viral binding to heparan sulfate proteoglycan (HSPG) and galactosylceramide (GalCer) of epithelial cells via the V3 domain or other domains of gp120 (85–89), which is critical for activation of MAPK and NF-κB signaling (90–92). This may reduce the role of gp120 in the activation of HCMV infection.
We have shown that HIV tat- and gp120-induced disruption of tight and adherens junctions initiated the development of the epithelial-mesenchymal transition, which leads to depolarization of epithelial cells and acquisition of a fibroblast-like phenotype (52). This may lead to redistribution of HCMV receptors from polarized epithelial membrane domains into nonpolarized fibroblast-like membranes, which may allow more accessibility of HCMV receptors to the virus. It is known that infectivity of HCMV in fibroblasts is significantly higher than that in epithelial cells (93, 94). Multiple cell surface receptors are involved in HCMV infection, including epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor alpha (PDGFRα), integrins, and neuropilin-2 (95–102); some of these receptors could be expressed predominantly in epithelial cells or in fibroblasts. Because epithelial-mesenchymal transition cells may have a hybrid phenotype from epithelial cells and fibroblasts, these cells may express both epithelial cell- and fibroblast-specific receptors, allowing a higher level of HCMV infection.
The increase of HCMV-infected macrophages and DC in HIV-1- and HCMV-coinfected tonsil tissues suggests that HIV-1 infection can also enhance HCMV infection of intraepithelial and subepithelial macrophages and DC. It was reported that HIV-1 proteins gp120 and tat may activate monocytes/macrophages and DC (103–105). It is possible that in the HIV-1-infected tonsil tissue environment, secreted and shed tat and gp120 proteins may activate monocytes/macrophages and DC, respectively, in a paracrine manner. Activation of these cells is critical for productive HCMV infection (106–109). Thus, HIV-1 infection can promote HCMV infection of tonsil epithelial cells as well as intraepithelial/subepithelial monocytes/macrophages and DC, thus playing a critical role in HCMV spread and dissemination within the tonsil tissues.
The increase of HIV-1 paracellular spread via disrupted tonsil epithelial cells by HCMV indicates that HCMV infection of tonsil mucosal epithelium can initiate HIV-1 transmucosal transmission. It has been shown that HCMV induces disruption of tight junctions in intestinal epithelial cells through activation of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) by productive viral infection (65). It is possible that these molecules could also be involved in the disruption of tight junctions of tonsil epithelial cells by HCMV infection, which facilitates HIV paracellular transmission.
Furthermore, the increase of HIV-1 infection in HIV- and HCMV-coinfected ex vivo tonsil tissues showed that HCMV-initiated paracellular penetration of HIV-1 via tonsil epithelium facilitates viral spread to intraepithelial and subepithelial CD4+ T lymphocytes, monocytes/macrophages, and DC. In addition, HCMV-infected epithelial cells, macrophages, and DC cells in the mucosal environment may express and secrete multiple bioactive molecules, including chemokines/cytokines and chemoattractants such as IL-8, IL-6, and TNF-α, monocyte chemoattractant protein-1 (MCP-1), regulated on activation normal T cell expressed and secreted (RANTES), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and stromal cell-derived factor 1 (SDF1) (110–116), which can activate bystander intraepithelial and subepithelial CD4+ T lymphocytes, monocytes/macrophages, and DC, thus priming them for HIV-1 infection.
HCMV expresses its own chemokine-cytokine and cytokine receptor homologs, including UL33, UL78, UL146, UL147, and US27 (112, 117–119), which could create an inflammation-like response in the infection site. This may lead to the activation of HIV-susceptible cells in the HCMV-infected tonsil mucosal/submucosal environment and an increase in migration of HIV target cells to the epithelial site. This can contribute to an increase in HIV-1 infection of its target cells in HCMV-coinfected tonsil tissues.
We have observed some donor variability of HIV-1 and HCMV infection of tonsil cells and tissues, which could be due to multiple factors, including lack or different level of expression of viral receptors, chemokine/cytokines, and antiviral innate proteins in tonsil epithelial and immune cells.
In our previous work, we have shown that tight and adherens junctions of oral and genital epithelia of HIV-infected individuals were disrupted, and this correlated with the presence of HIV-1-infected CD4+ T lymphocytes, macrophages, and dendritic cells expressing gp120 and tat proteins within the mucosal environment (18, 52). It is also well known that reactivation of HCMV is associated with opportunistic infections during HIV/AIDS disease (30). In HIV-infected individuals, HCMV replicates and spreads in the epithelial cells of the oral mucosa, lung, liver, and gut (31–35). Thus, it is possible that HIV and HCMV may interact in vivo within the various tissue environments, promoting each other’s infection and spread.
In summary, we have shown that HIV-1 and HCMV coinfection of infant tonsil mucosal epithelium synergistically promotes their transmission via tonsil mucosa (Fig. 10), which is a critical step for initiation of systemic HIV-1 and HCMV infection. The interaction of cell-free HIV-1 and its proteins gp120 and tat with the tonsil mucosal epithelium disrupts the tight junctions of epithelial cells, facilitating HCMV paracellular transport. HIV-induced depolarization of epithelial cells and activation of MAPK and NF-κB signaling increase HCMV infection of epithelial cells. HCMV infection of tonsil epithelial cells initiates the spread of virus to the intramucosal and submucosal macrophages and DC.
FIG 10.

Model showing how HIV-1 and HCMV coinfection of tonsil tissues may synergistically enhance both viral infections. (A) Well-developed tight and adherens junctions of oropharyngeal and tonsil epithelia play a critical role in the establishment of barrier function, which prevents the paracellular spread of viral pathogens, including HIV-1 and HCMV. (B) Most MTCT of HIV-1 and HCMV may occur during labor and breastfeeding, when these viruses may simultaneously interact with mucosal epithelial cells, leading to disruption of their integrity. Cell-free HIV-1 from cervicovaginal secretions and breast milk may induce the disruption of oropharyngeal and tonsil mucosal epithelium junctions by gp120 on the viral surface. Furthermore, in HIV-1-infected infants, the secreted tat may also disrupt mucosal tight junctions. Thus, HIV-1-induced disruption of oropharyngeal mucosal epithelia may enhance HCMV paracellular spread, initiating HCMV MTCT. HCMV infection of oropharyngeal mucosal epithelium also disrupts oropharyngeal and tonsil mucosal epithelia and enhances HIV-1 paracellular spread, which may initiate HIV-1 MTCT. (C) HIV-1- and HCMV-induced disruption of mucosal epithelium integrity may promote the paracellular spread of HIV-1, increasing its accessibility to intramucosal and submucosal CD4 T lymphocytes, macrophages, and DC. HIV-1- and HCMV-induced disruption of mucosal epithelium also enhances HCMV paracellular spread and infection of epithelial cells, which subsequently spread HCMV to intraepithelial and submucosal macrophages and DC. Thus, HIV-1 and HCMV viruses may synergistically promote MTCT through infant tonsil mucosal epithelium.
HCMV infection of tonsil mucosal epithelium facilitates HIV-1 paracellular transmission by disruption of tonsil epithelial tight junctions (Fig. 10). HCMV infection also promotes HIV-1 infection of intraepithelial and submucosal CD4+ T lymphocytes, monocytes/macrophages, and DC. HCMV-promoted HIV-1 infection could be initiated by activation of HIV-1-susceptible cells via a direct or indirect effect of HCMV infection, resulting in the expression and secretion of chemokines and cytokines in the tonsil tissue environment.
The development of therapeutic approaches to inhibit MAPK and NF-κB signaling of tonsil and/or intestinal epithelia of an HIV-1- and HCMV-coinfected mother may prevent HIV-1- and HCMV-induced disruption of tonsil epithelial junctions. This can preserve or restore the barrier function of the epithelial mucosal lining of the child and reduce paracellular spread of HIV-1 and HCMV from cervicovaginal secretions and breast milk. This could lead to a reduction of subsequent infection and spread of both viruses within the epithelial and subepithelial environment and may reduce HIV-1 and HCMV MTCT via oropharyngeal and tonsil mucosal epithelium.
MATERIALS AND METHODS
Ethics statement.
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Committee on Human Research of the University of California–San Francisco (IRB approval number H8597-30664-03). All subjects provided written informed consent for the collection of samples and subsequent analysis.
Viruses, viral proteins, and cells.
Laboratory-adapted dually (X4-R5) tropic HIV-1SF33 and the primary isolates R5-tropic HIV-1SF170 and X4-tropic HIV-192UG029 were grown in PBMC activated with 2.5 μg/ml phytohemagglutinin (Sigma) and 1 μg/ml IL-2 (BD Biosciences) for 3 days. Viral stocks were titrated by p24 concentration using HIV-1 p24 ELISA (PerkinElmer) according to the manufacturer’s instructions.
Recombinant HIV-1 (Bal strain) wild-type tat and inactive mutant tat proteins were purchased from ImmunoDX (Woburn, MA). Mutant tat was generated by substitution of the basic arginine-rich domain at aa 49 to 57 and the integrin-binding RGD motif in the C terminus with alanines. Recombinant gp120 proteins from HIV-1BAL, HIV-1IIIB, HIV-1CN-54, and HIV-196ZM651 strains were provided by the NIH AIDS Reagent Program.
Primary tonsil epithelial keratinocytes were established from palatine tonsil tissue from HIV-negative children <5 years of age after routine tonsillectomy at the clinic of the Department of Otolaryngology, University of California—San Francisco. The keratinocytes were grown in keratinocyte growth medium (KGM Gold) (Lonza). Keratinocytes were used at early passages and frozen in liquid nitrogen. Keratinocyte culture from each donor was verified for the absence of HIV-1 and HCMV infection.
HFF (Lonza) were maintained in fibroblast growth medium (Lonza). Human umbilical vein endothelial cells (HUVEC) (Lonza) were maintained in endothelial cell growth medium (Lonza).
HCMV strain AD169 and clinical strain VR1814 were kindly provided by Lenore Pereira, University of California—San Francisco. HCMV AD169 viral stock was propagated and titrated in HFF. HCMV VR1814 was propagated in primary HUVEC (Lonza) and titrated in HFF.
Establishment of polarized tonsil epithelial cells.
To establish polarized cells from primary tonsil epithelial cells, we propagated primary keratinocytes from tonsil tissue samples, as described in our previous work (19, 120). Tonsil polarized epithelial cells were established in 3-μm Transwell two-chamber filter inserts (12 well), as described previously (17, 19, 20, 24, 25). The polarity of epithelial cells was confirmed by immunodetection of tight junction proteins occludin, claudin-1, and ZO-1 and measurement of TER and paracellular permeability (19). TER was measured with a Millicell ERS epithelial volt-ohm meter (Millipore Corp., Billerica, MA). Paracellular permeability was evaluated by adding horseradish peroxidase-conjugated goat anti-donkey IgG F(ab′)2 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) to the upper chamber and photometrically assaying the medium from the lower chamber for horseradish peroxidase with o-phenylenediamine dihydrochloride as the substrate (19, 121). Detection of IgG in the lower chamber indicated leakage of IgG from the upper chamber.
Establishment of polarized-oriented tonsil tissue explants.
Tonsil tissues without visible inflammation were collected from HIV-negative children under 5 years of age who had obstructive sleep apnea. In this condition, breathing is difficult owing partly or completely to blockage of the upper airway during sleep. This makes diaphragm and chest muscles work harder to open the obstructed airway and pull air into the lungs. About 50 to 70% of these patients lack inflammation in tonsil tissues (122–125).
Palatine tonsil tissues were collected from the clinic of the Department of Otolaryngology, University of California—San Francisco 1 to 2 h after routine tonsillectomy surgery. Explants, ∼5 by 5 mm, dissected from tonsil epithelium containing lamina propria without the lymphoid part of tonsil tissue were placed with the mucosal side facing up in the upper chamber of Millicell filter inserts (12-mm diameter and 3-μm pore size) (Millipore). The lateral edges of the explants were sealed with 3% agarose, as described in our previous work (18, 21). Tonsil tissues from each donor were verified for absence of HIV-1 infection by p24 ELISA. Similarly, tonsil tissues were verified for absence of HCMV infection by immunofluorescence of tissue sections for HCMV gB and IE1/2 proteins and by detection of HCMV infection in HFF. Also, each tonsil tissue after each experiment was examined for the integrity of tight junctions by immunostaining for occludin. Tissues with disrupted junctions were excluded from further study.
Treatment of polarized cells with cell-free HIV virions and HIV proteins tat and gp120.
Cell-free HIV-1 virions were purified by using Amicon Ultra-15 columns as described elsewhere (26, 52). Infectious or UV-inactivated HIV-1 was added to the apical surface of polarized epithelial cells or tissues at 10 ng/ml of p24 antigen. HIV-1 was inactivated by UV irradiation at 100 mJ/cm2 (26). gp120 was inactivated by incubation at 85°C for 30 min (50). Inactive tat was generated by substitution of the basic arginine-rich domain at aa 49 to 57 and the integrin-binding RGD motif in the C terminus with alanines (ImmunoDX). Active and inactive recombinant tat and/or gp120 (10 ng/ml of each) were added to polarized tonsil cells from apical surfaces. Culture medium was changed daily to add fresh virus or proteins. Disruption of tight junctions was examined by immunostaining their marker proteins, ZO-1, occludin, and claudin-1. Expression and localization of tight junction proteins as a ring shape was considered an intact junction. Diffuse, dot-like patterns in the cytoplasm were considered disrupted junctions (18). Disruption of epithelial junctions was also monitored by measuring TER and paracellular permeability (17, 20).
Assay for HIV-1 and HCMV spread via the paracellular route of polarized tonsil epithelial cells.
Cell-free HIV-1 or HCMV virion were added to the apical surface of polarized tonsil epithelial cell monolayers in 12-well plates with a 3-μm pore size. At each time point, the basolateral medium was collected and examined for viruses, which migrated via the paracellular route. HIV-1 detection in basolateral medium was determined by ELISA p24 assay. The infectivity of HIV-1 was examined via infection of activated PBMC in basolateral medium. To examine HIV-1 paracellular spread via HCMV-infected cells, the PBMC were treated with 20 μM GCV, which inhibits the possible infection of PBMC with HCMV.
Detection of HCMV in basolateral medium was examined by virus infectivity assay in HFF. Forty-eight or 24 h after infection, HFF were immunostained for HCMV gB or IE1 proteins, respectively. For HCMV gB and IE1/2 immunostaining, we used mouse monoclonal antibodies CH177 and CH160 (126, 127), respectively (both provided by Lenore Pereira, University of California—San Francisco).
Infection of polarized tonsil epithelial cells and ex vivo tonsil tissue explants with HCMV and HIV-1.
All stock viruses of HIV-1 and HCMV were purified by using Amicon Ultra-15 columns as described elsewhere (26, 52). Polarized tonsil epithelial cells were infected with HCMV AD169 or VR1814 strains at an MOI of 1 from the apical surface of polarized cells as described in our previous work (128). Polarized-oriented tonsil tissue explants were infected with HCMV VR1814 at 3 × 105 IU per explant from the apical/mucosal surface of tonsil epithelium. Tonsil tissue explants were infected with HIV-1 at 1 ng/p24/explant from the mucosal surface of tonsil epithelium. After 2 h, cells or tissues were washed 3 times and maintained with culture medium. To inhibit HCMV infection, we treated tissue explants for 9 days with 20 μM GCV (InvivoGen) (65). To inhibit HIV-1 infection, we treated tissue explants with 10 μM lamivudine (Sigma-Aldrich) for 1 day before infection and for 9 days after infection (72). For inhibition of MAPK and NF-κB signaling, cells were treated with a 10 μM concentration of the MAPK inhibitor U0126 or with a 10 μM concentration of the NF-κB inhibitor MG-132 (both from Sigma-Aldrich). The absence of a toxic effect by GCV, lamivudine, U0126, or SB431542 was confirmed in tonsil keratinocytes by the MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] cell viability assay (Biotium). For each experimental condition, used duplicated tissue explants were used.
Immunofluorescence assay.
Polarized epithelial cells were fixed with 4% paraformaldehyde in phosphate-buffered saline for 15 min, permeabilized with 0.01% Triton X-100 in 4% paraformaldehyde for 5 min, and infiltrated with 2% sucrose. Tonsil explants were fixed in 3% paraformaldehyde, infiltrated with 5 to 15% sucrose, embedded in optimal cutting temperature compound, and frozen in liquid nitrogen, as described in our previous work. The following antibodies were used for detection of HIV-1 and cellular proteins: (i) mouse anti-HIV-1 p24 (5 μg/ml) (NIH AIDS Reagent Program), goat anti-HIV-1 p24 (5 μg/ml) (ViroStat), and goat anti-HIV-1 (5 μg/ml) (US Biological); (ii) mouse antibodies to CD4, CD68, and CD3 (1 μg/ml of each) (all from BD Biosciences); (iii) rabbit anti-CD68 (Abcam) and CD1c (LS BioPath); and (iv) mouse anti-ZO-1 (1 μg/ml), anti-occludin (1 μg/ml), and anti-claudin-1 (5 μg/ml) (all from Invitrogen). For detection of HCMV IE1/2 and gB proteins, we used mouse monoclonal antibodies CH160 and CH177, respectively (126, 127) (provided by Lenore Pereira, University of California—San Francisco).
Secondary antibodies labeled with DyLight 488, DyLight 594, and Alexa Fluor were purchased from Jackson ImmunoResearch. Cell nuclei were counterstained with TO-PRO-3 iodide or DAPI (4′,6-diamidino-2-phenylindole; blue) (Molecular Probes). The specificity of each antibody was confirmed by negative staining with the corresponding primary isotype control antibody. Cells were analyzed by using a Leica SP5 confocal laser microscope (Leica Microsystems) or Nikon Eclipse E400 fluorescence microscope (Nikon).
For quantitative evaluation of HIV p24- and HCMV gB-expressing immune cells, sections were coimmunostained for viral proteins with immune cell markers (CD68 for macrophages, CD1c for DC, and CD4 for lymphocytes), and immune cells expressing HIV-1 proteins were counted in the epithelium and lamina propria. Cells were counted in 10 randomly selected microscopic fields (×200) per section in at least three sections for each explant. Results are presented as the average number of positive cells per square millimeter. All cell counts were performed in a blind fashion by two investigators (I.S. and R.H.).
Western blot assay.
Cells were extracted with 1% Triton X-100 buffer (150 mM NaCl, 10 mM Tris/HCl, pH 8.0) and a cocktail of protease inhibitors (Roche). Tissue explants were homogenized by using motor-driven grinders connected with disposable pellet pestles (Kimble Kontes pellet pestle cordless motor) and then extracted with 1% Triton X-100 buffer. Proteins were separated on an SDS-polyacrylamide gel with a 4-to-20% gradient. HCMV gB and IE1/2 were detected by using mouse monoclonal antibodies (provided by Lenore Pereira, University of California—San Francisco). For detection of gB, we used a pool of CH28, CH45, CH86, CH386, and CH44. HCMV IE1/2 was detected by CH160. An equal protein load was confirmed by the use of anti-β-actin mouse monoclonal antibody (MAb) (Ambion). MAPK activity was measured by detection of phosphorylated and total ERK1/2 protein using anti-phospho-p42/44 MAPK (Erk1/2) (Cell Signaling Technology) and total p42/44 MAPK (Erk1/2) antibodies (Cell Signaling Technology). To determine NF-κB activity, we used anti-phospho-IκBα (Ser32) (14D4) (Cell Signaling Technology), total anti-IκBα (44D4) rabbit MAb (Cell Signaling Technology), anti-phospho-NF-κB p65 (Se536) (93H1) rabbit MAb (Cell Signaling Technology), and anti-NF-κB p65 (C22B4) rabbit MAb (Cell Signaling Technology).
Statistical analysis.
Statistical comparisons were made by a two-tailed Student's t test. A P value of <0.05 was considered significant. Results are expressed as means and standard deviations (SD).
ACKNOWLEDGMENTS
We thank Lenore Pereira (University of California—San Francisco) for providing HCMV strains and antibodies and for useful discussions of the paper.
This project was supported by NIDCR R01DE028129 and NCI R01CA232887 grants (to S.M.T.) and by the Directorate General of Higher Education, Ministry of National Education Indonesia (grant 431/UN6.3.1/PL/2016) (to I.S.).
Contributor Information
Sharof M. Tugizov, Email: sharof.tugizov@ucsf.edu.
Joanna L. Shisler, University of Illinois at Urbana-Champaign
REFERENCES
- 1.Mundy DC, Schinazi RF, Gerber AR, Nahmias AJ, RandallHW, Jr.. 1987. Human immunodeficiency virus isolated from amniotic fluid. Lancet 2:459–460. 10.1016/S0140-6736(87)91001-4. [DOI] [PubMed] [Google Scholar]
- 2.Jaspan HB, Robinson JE, Amedee AM, Van Dyke RB, Garry RF. 2004. Amniotic fluid has higher relative levels of lentivirus-specific antibodies than plasma and can contain neutralizing antibodies. J Clin Virol 31:190–197. 10.1016/j.jcv.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Maiques V, Garcia-Tejedor A, Perales A, Cordoba J, Esteban RJ. 2003. HIV detection in amniotic fluid samples. Amniocentesis can be performed in HIV pregnant women? Eur J Obstet Gynecol Reprod Biol 108:137–141. 10.1016/s0301-2115(02)00405-0. [DOI] [PubMed] [Google Scholar]
- 4.Willumsen JF, Filteau SM, Coutsoudis A, Uebel KE, Newell ML, Tomkins AM. 2000. Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv Exp Med Biol 478:211–223. 10.1007/0-306-46830-1_19. [DOI] [PubMed] [Google Scholar]
- 5.Nussenblatt V, Lema V, Kumwenda N, Broadhead R, Neville MC, Taha TE, Semba RD. 2005. Epidemiology and microbiology of subclinical mastitis among HIV-infected women in Malawi. Int J STD AIDS 16:227–232. 10.1258/0956462053420248. [DOI] [PubMed] [Google Scholar]
- 6.Semba RD. 2000. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci 918:156–162. 10.1111/j.1749-6632.2000.tb05484.x. [DOI] [PubMed] [Google Scholar]
- 7.Semba RD, Neville MC. 1999. Breast-feeding, mastitis, and HIV transmission: nutritional implications. Nutr Rev 57:146–153. 10.1111/j.1753-4887.1999.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 8.Harris K, Yudin MH. 2020. HIV infection in pregnant women: a 2020 update. Prenat Diagn 40:1715–1721. 10.1002/pd.5769. [DOI] [PubMed] [Google Scholar]
- 9.Phillips TK, Teasdale CA, Geller A, Ng'eno B, Mogoba P, Modi S, Abrams EJ. 2021. Approaches to transitioning women into and out of prevention of mother-to-child transmission of HIV services for continued ART: a systematic review. J Int AIDS Soc 24:e25633. 10.1002/jia2.25633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White AB, Mirjahangir JF, Horvath H, Anglemyer A, Read JS. 2014. Antiretroviral interventions for preventing breast milk transmission of HIV. Cochrane Database Syst Rev 2014:CD011323. 10.1002/14651858.CD011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, Biggar RJ, Broadhead R, Miotti PG, Sokoll LJ, van der Hoeven L, Chiphangwi JD. 1999. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis 180:93–98. 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn L, Kim HY, Walter J, Thea DM, Sinkala M, Mwiya M, Kankasa C, Decker D, Aldrovandi GM. 2013. HIV-1 concentrations in human breast milk before and after weaning. Sci Transl Med 5:181ra51. 10.1126/scitranslmed.3005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satomi M, Shimizu M, Shinya E, Watari E, Owaki A, Hidaka C, Ichikawa M, Takeshita T, Takahashi H. 2005. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J Infect Dis 191:174–181. 10.1086/426829. [DOI] [PubMed] [Google Scholar]
- 14.Redmond AM, McNamara JF. 2015. The road to eliminate mother-to-child HIV transmission. J Pediatr (Rio J) 91:509–511. 10.1016/j.jped.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Lehman DA, Chung MH, John-Stewart GC, Richardson BA, Kiarie J, Kinuthia J, Overbaugh J. 2008. HIV-1 persists in breast milk cells despite antiretroviral treatment to prevent mother-to-child transmission. AIDS 22:1475–1485. 10.1097/QAD.0b013e328302cc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Perre P, Rubbo PA, Viljoen J, Nagot N, Tylleskar T, Lepage P, Vendrell JP, Tuaillon E. 2012. HIV-1 reservoirs in breast milk and challenges to elimination of breast-feeding transmission of HIV-1. Sci Transl Med 4:143sr3. 10.1126/scitranslmed.3003327. [DOI] [PubMed] [Google Scholar]
- 17.Tugizov SM, Berline JW, Palefsky JM. 2003. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med 9:307–314. 10.1038/nm830. [DOI] [PubMed] [Google Scholar]
- 18.Tugizov SM, Herrera R, Chin-Hong P, Veluppillai P, Greenspan D, Michael Berry J, Pilcher CD, Shiboski CH, Jay N, Rubin M, Chein A, Palefsky JM. 2013. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology 446:378–388. 10.1016/j.virol.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tugizov SM, Herrera R, Palefsky JM. 2013. Epstein-Barr virus transcytosis through polarized oral epithelial cells. J Virol 87:8179–8194. 10.1128/JVI.00443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. 2011. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology 409:211–222. 10.1016/j.virol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. 2012. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. J Virol 86:2556–2570. 10.1128/JVI.06578-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bomsel M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med 3:42–47. 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 23.Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. 2007. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol 81:395–405. 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasen A, Herrera R, Rosbe K, Lien K, Tugizov SM. 2018. HIV internalization into oral and genital epithelial cells by endocytosis and macropinocytosis leads to viral sequestration in the vesicles. Virology 515:92–107. 10.1016/j.virol.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasen A, Herrera R, Rosbe K, Lien K, Tugizov SM. 2017. Release of HIV-1 sequestered in the vesicles of oral and genital mucosal epithelial cells by epithelial-lymphocyte interaction. PLoS Pathog 13:e1006247. 10.1371/journal.ppat.1006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. 2010. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 6:e1000852. 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, Hope TJ. 2013. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol 87:11388–11400. 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinh MH, Anderson MR, McRaven MD, Cianci GC, McCoombe SG, Kelley ZL, Gioia CJ, Fought AJ, Rademaker AW, Veazey RS, Hope TJ. 2015. Visualization of HIV-1 interactions with penile and foreskin epithelia: clues for female-to-male HIV transmission. PLoS Pathog 11:e1004729. 10.1371/journal.ppat.1004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maher D, Wu X, Schacker T, Larson M, Southern P. 2004. A model system of oral HIV exposure, using human palatine tonsil, reveals extensive binding of HIV infectivity, with limited progression to primary infection. J Infect Dis 190:1989–1997. 10.1086/425423. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths P, Baraniak I, Reeves M. 2015. The pathogenesis of human cytomegalovirus. J Pathol 235:288–297. 10.1002/path.4437. [DOI] [PubMed] [Google Scholar]
- 31.Drew WL. 1992. Cytomegalovirus infection in patients with AIDS. Clin Infect Dis 14:608–615. 10.1093/clinids/14.2.608-a. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs A, Schluchter M, Easley K, Demmler G, Shearer W, La Russa P, Pitt J, Cooper E, Goldfarb J, Hodes D, Kattan M, McIntosh K. 1999. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med 341:77–84. 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nigro G, Krzysztofiak A, Gattinara GC, Mango T, Mazzocco M, Porcaro MA, Provvedi S, Booth JC. 1996. Rapid progression of HIV disease in children with cytomegalovirus DNAemia. AIDS 10:1127–1133. [PubMed] [Google Scholar]
- 34.Chandwani S, Kaul A, Bebenroth D, Kim M, John DD, Fidelia A, Hassel A, Borkowsky W, Krasinski K. 1996. Cytomegalovirus infection in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J 15:310–314. 10.1097/00006454-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Gie RP, Goussard P. 2017. CMV pneumonia in HIV-infected and HIV-uninfected infants: a neglected disease? Int J Tuber Lung Dis 21:1209–1210. 10.5588/ijtld.17.0714. [DOI] [PubMed] [Google Scholar]
- 36.Gianella S, Letendre S. 2016. Cytomegalovirus and HIV: a dangerous pas de deux. J Infect Dis 214(Suppl 2):S67–S74. 10.1093/infdis/jiw217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gianella S, Massanella M, Wertheim JO, Smith DM. 2015. The sordid affair between human herpesvirus and HIV. J Infect Dis 212:845–852. 10.1093/infdis/jiv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pass RF, Anderson B. 2014. Mother-to-child transmission of cytomegalovirus and prevention of congenital infection. J Pediatric Infect Dis Soc 3(Suppl 1):S2–S6. 10.1093/jpids/piu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slyker JA. 2016. Cytomegalovirus and paediatric HIV infection. J Virus Erad 2:208–214. 10.1016/S2055-6640(20)30873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmink S, Kruszon-Moran D, Dollard SC, Lanzieri TM. 2017. Effect of breastfeeding and additional household children on cytomegalovirus seroprevalence among U.S. children 1 to 5 years of age. Clin Vaccine Immunol 24:e00243-17. 10.1128/CVI.00243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier J, Lienicke U, Tschirch E, Kruger DH, Wauer RR, Prosch S. 2005. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol 43:1318–1324. 10.1128/JCM.43.3.1318-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamprecht K, Witzel S, Maschmann J, Dietz K, Baumeister A, Mikeler E, Goelz R, Speer CP, Jahn G. 2003. Rapid detection and quantification of cell free cytomegalovirus by a high-speed centrifugation-based microculture assay: comparison to longitudinally analyzed viral DNA load and pp67 late transcript during lactation. J Clin Virol 28:303–316. 10.1016/s1386-6532(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda A, Kimura H, Hayakawa M, Ohshiro M, Kato Y, Matsuura O, Suzuki C, Morishima T. 2003. Evaluation of cytomegalovirus infections transmitted via breast milk in preterm infants with a real-time polymerase chain reaction assay. Pediatrics 111:1333–1336. 10.1542/peds.111.6.1333. [DOI] [PubMed] [Google Scholar]
- 44.Viljoen J, Tuaillon E, Nagot N, Danaviah S, Peries M, Padayachee P, Foulongne V, Bland R, Rollins N, Newell ML, van de Perre P. 2015. Cytomegalovirus, and possibly Epstein-Barr virus, shedding in breast milk is associated with HIV-1 transmission by breastfeeding. AIDS 29:145–153. 10.1097/QAD.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 45.Gantt S, Leister E, Jacobsen DL, Boucoiran I, Huang ML, Jerome KR, Jourdain G, Ngo-Giang-Huong N, Burchett S, Frenkel L. 2016. Risk of congenital cytomegalovirus infection among HIV-exposed uninfected infants is not decreased by maternal nelfinavir use during pregnancy. J Med Virol 88:1051–1058. 10.1002/jmv.24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slyker JA, Lohman-Payne BL, John-Stewart GC, Maleche-Obimbo E, Emery S, Richardson B, Dong T, Iversen AK, Mbori-Ngacha D, Overbaugh J, Emery VC, Rowland-Jones SL. 2009. Acute cytomegalovirus infection in Kenyan HIV-infected infants. AIDS 23:2173–2181. 10.1097/QAD.0b013e32833016e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang TS, Wiener J, Dollard SC, Amin MM, Ellington S, Chasela C, Kayira D, Tegha G, Kamwendo D, Jamieson DJ, van der Horst C, Kourtis AP, Team BANS, BAN Study Team . 2015. Effect of cytomegalovirus infection on breastfeeding transmission of HIV and on the health of infants born to HIV-infected mothers. AIDS 29:831–836. 10.1097/QAD.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin LY, Sheth PM, Persad D, Kovacs C, Kain T, Diong C, Su D, Ostrowski M, Raboud JM, Kaul R. 2014. Impact of CMV therapy with valganciclovir on immune activation and the HIV viral load in semen and blood: an observational clinical study. J Acquir Immune Defic Syndr 65:251–258. 10.1097/01.qai.0000435256.34306.c1. [DOI] [PubMed] [Google Scholar]
- 49.Adland E, Klenerman P, Goulder P, Matthews PC. 2015. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front Microbiol 6:1016. 10.3389/fmicb.2015.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sufiawati I, Tugizov SM. 2014. HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread. PLoS One 9:e88803. 10.1371/journal.pone.0088803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sufiawati I, Tugizov SM. 2018. HIV-induced matrix metalloproteinase-9 activation through mitogen-activated protein kinase signalling promotes HSV-1 cell-to-cell spread in oral epithelial cells. J Gen Virol 99:937–947. 10.1099/jgv.0.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lien K, Mayer W, Herrera R, Rosbe K, Tugizov SM. 2019. HIV-1 proteins gp120 and tat induce the epithelial–mesenchymal transition in oral and genital mucosal epithelial cells. PLoS One 14:e0226343. 10.1371/journal.pone.0226343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toschi E, Bacigalupo I, Strippoli R, Chiozzini C, Cereseto A, Falchi M, Nappi F, Sgadari C, Barillari G, Mainiero F, Ensoli B. 2006. HIV-1 Tat regulates endothelial cell cycle progression via activation of the Ras/ERK MAPK signaling pathway. Mol Biol Cell 17:1985–1994. 10.1091/mbc.e05-08-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pu H, Tian J, Andras IE, Hayashi K, Flora G, Hennig B, Toborek M. 2005. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J Cereb Blood Flow Metab 25:1325–1335. 10.1038/sj.jcbfm.9600125. [DOI] [PubMed] [Google Scholar]
- 55.Andras IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M. 2005. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab 25:1159–1170. 10.1038/sj.jcbfm.9600115. [DOI] [PubMed] [Google Scholar]
- 56.Bai L, Zhang Z, Zhang H, Li X, Yu Q, Lin H, Yang W. 2008. HIV-1 Tat protein alter the tight junction integrity and function of retinal pigment epithelium: an in vitro study. BMC Infect Dis 8:77. 10.1186/1471-2334-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong Y, Smart EJ, Weksler B, Couraud PO, Hennig B, Toborek M. 2008. Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J Neurosci 28:7788–7796. 10.1523/JNEUROSCI.0061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song L, Ge S, Pachter JS. 2007. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood 109:1515–1523. 10.1182/blood-2006-07-034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. 2007. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab 27:123–134. 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanmogne GD, Primeaux C, Grammas P. 2005. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol 64:498–505. 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- 61.Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. 2008. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens −1 and −2 in human brain microvascular endothelial cells. J Neurovirol 14:186–195. 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- 62.Shin K, Fogg VC, Margolis B. 2006. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22:207–235. 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 63.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. 2006. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126:741–754. 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 64.Shen L, Turner JR. 2005. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16:3919–3936. 10.1091/mbc.e04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maidji E, Somsouk M, Rivera JM, Hunt PW, Stoddart CA. 2017. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog 13:e1006202. 10.1371/journal.ppat.1006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sen R, Smale ST. 2010. Selectivity of the NF-{kappa}B response. Cold Spring Harb Perspect Biol 2:a000257. 10.1101/cshperspect.a000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayden MS, Ghosh S. 2008. Shared principles in NF-kappaB signaling. Cell 132:344–362. 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 68.McCaffrey LM, Macara IG. 2011. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol 21:727–735. 10.1016/j.tcb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 69.McCaffrey LM, Macara IG. 2012. Signaling pathways in cell polarity. Cold Spring Harb Perspect Biol 4:a009654. 10.1101/cshperspect.a009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCaffrey LM, Montalbano J, Mihai C, Macara IG. 2012. Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 22:601–614. 10.1016/j.ccr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez-Boulan E, Macara IG. 2014. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15:225–242. 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Introini A, Vanpouille C, Fitzgerald W, Broliden K, Margolis L. 2018. Ex vivo infection of human lymphoid tissue and female genital mucosa with human immunodeficiency virus 1 and histoculture. J Vis Exp 2018:57013. 10.3791/57013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sinzger C, Digel M, Jahn G. 2008. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol 325:63–83. 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- 74.Sinzger C, Jahn G. 1996. Human cytomegalovirus cell tropism and pathogenesis. Intervirology 39:302–319. 10.1159/000150502. [DOI] [PubMed] [Google Scholar]
- 75.Sinzger C, Plachter B, Grefte A, The TH, Jahn G. 1996. Tissue macrophages are infected by human cytomegalovirus in vivo. J Infect Dis 173:240–245. 10.1093/infdis/173.1.240. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, Hitti J. 2011. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J Clin Microbiol 49:735–736. 10.1128/JCM.00991-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497–500. 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 78.Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. 2000. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A 97:11466–11471. 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rychert J, Strick D, Bazner S, Robinson J, Rosenberg E. 2010. Detection of HIV gp120 in plasma during early HIV infection is associated with increased proinflammatory and immunoregulatory cytokines. AIDS Res Hum Retroviruses 26:1139–1145. 10.1089/aid.2009.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poggi A, Zocchi MR. 2006. HIV-1 Tat triggers TGF-beta production and NK cell apoptosis that is prevented by pertussis toxin B. Clin Dev Immunol 13:369–372. 10.1080/17402520600645712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeMeritt IB, Milford LE, Yurochko AD. 2004. Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J Virol 78:4498–4507. 10.1128/jvi.78.9.4498-4507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan G, Bivins-Smith ER, Smith MS, Yurochko AD. 2008. Transcriptome analysis of NF-kappaB- and phosphatidylinositol 3-kinase-regulated genes in human cytomegalovirus-infected monocytes. J Virol 82:1040–1046. 10.1128/JVI.00864-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yurochko AD, Kowalik TF, Huong SM, Huang ES. 1995. Human cytomegalovirus upregulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 and p65 promoters. J Virol 69:5391–5400. 10.1128/JVI.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson RA, Ma XL, Yurochko AD, Huang ES. 2001. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J Gen Virol 82:493–497. 10.1099/0022-1317-82-3-493. [DOI] [PubMed] [Google Scholar]
- 85.Nakamura KJ, Heath L, Sobrera ER, Wilkinson TA, Semrau K, Kankasa C, Tobin NH, Webb NE, Lee B, Thea DM, Kuhn L, Mullins JI, Aldrovandi GM. 2017. Breast milk and in utero transmission of HIV-1 select for envelope variants with unique molecular signatures. Retrovirology 14:6. 10.1186/s12977-017-0331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrop HA, Coombe DR, Rider CC. 1994. Heparin specifically inhibits binding of V3 loop antibodies to HIV-1 gp120, an effect potentiated by CD4 binding. AIDS 8:183–192. 10.1097/00002030-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 87.Harrop HA, Rider CC. 1998. Heparin and its derivatives bind to HIV-1 recombinant envelope glycoproteins, rather than to recombinant HIV-1 receptor, CD4. Glycobiology 8:131–137. 10.1093/glycob/8.2.131. [DOI] [PubMed] [Google Scholar]
- 88.Connell BJ, Lortat-Jacob H. 2013. Human immunodeficiency virus and heparan sulfate: from attachment to entry inhibition. Front Immunol 4:385. 10.3389/fimmu.2013.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fantini J, Hammache D, Delezay O, Yahi N, Andre-Barres C, Rico-Lattes I, Lattes A. 1997. Synthetic soluble analogs of galactosylceramide (GalCer) bind to the V3 domain of HIV-1 gp120 and inhibit HIV-1-induced fusion and entry. J Biol Chem 272:7245–7252. 10.1074/jbc.272.11.7245. [DOI] [PubMed] [Google Scholar]
- 90.Lee C, Liu QH, Tomkowicz B, Yi Y, Freedman BD, Collman RG. 2003. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J Leukoc Biol 74:676–682. 10.1189/jlb.0503206. [DOI] [PubMed] [Google Scholar]
- 91.Del Corno M, Liu QH, Schols D, de Clercq E, Gessani S, Freedman BD, Collman RG. 2001. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood 98:2909–2916. 10.1182/blood.v98.10.2909. [DOI] [PubMed] [Google Scholar]
- 92.Dayanithi G, Yahi N, Baghdiguian S, Fantini J. 1995. Intracellular calcium release induced by human immunodeficiency virus type 1 (HIV-1) surface envelope glycoprotein in human intestinal epithelial cells: a putative mechanism for HIV-1 enteropathy. Cell Calcium 18:9–18. 10.1016/0143-4160(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 93.Sinzger C, Grefte A, Plachter B, Gouw AS, The TH, Jahn G. 1995. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol 76:741–750. 10.1099/0022-1317-76-4-741. [DOI] [PubMed] [Google Scholar]
- 94.Scrivano L, Sinzger C, Nitschko H, Koszinowski UH, Adler B. 2011. HCMV spread and cell tropism are determined by distinct virus populations. PLoS Pathog 7:e1001256. 10.1371/journal.ppat.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456–461. 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 96.Soroceanu L, Akhavan A, Cobbs CS. 2008. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 455:391–395. 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- 97.Feire AL, Roy RM, Manley K, Compton T. 2010. The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry. J Virol 84:10026–10037. 10.1128/JVI.00710-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feire AL, Koss H, Compton T. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci U S A 101:15470–15475. 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kabanova A, Marcandalli J, Zhou T, Bianchi S, Baxa U, Tsybovsky Y, Lilleri D, Silacci-Fregni C, Foglierini M, Fernandez-Rodriguez BM, Druz A, Zhang B, Geiger R, Pagani M, Sallusto F, Kwong PD, Corti D, Lanzavecchia A, Perez L. 2016. Platelet-derived growth factor-alpha receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat Microbiol 1:16082. 10.1038/nmicrobiol.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu Y, Prager A, Boos S, Resch M, Brizic I, Mach M, Wildner S, Scrivano L, Adler B. 2017. Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-alpha as a key for entry. PLoS Pathog 13:e1006281. 10.1371/journal.ppat.1006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stegmann C, Hochdorfer D, Lieber D, Subramanian N, Stohr D, Laib Sampaio K, Sinzger C. 2017. A derivative of platelet-derived growth factor receptor alpha binds to the trimer of human cytomegalovirus and inhibits entry into fibroblasts and endothelial cells. PLoS Pathog 13:e1006273. 10.1371/journal.ppat.1006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martinez-Martin N, Marcandalli J, Huang CS, Arthur CP, Perotti M, Foglierini M, Ho H, Dosey AM, Shriver S, Payandeh J, Leitner A, Lanzavecchia A, Perez L, Ciferri C. 2018. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 174:1158–1171.E19. 10.1016/j.cell.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 103.Del Corno M, Donninelli G, Varano B, Da Sacco L, Masotti A, Gessani S. 2014. HIV-1 gp120 activates the STAT3/interleukin-6 axis in primary human monocyte-derived dendritic cells. J Virol 88:11045–11055. 10.1128/JVI.00307-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Planes R, Ben Haij N, Leghmari K, Serrero M, BenMohamed L, Bahraoui E. 2016. HIV-1 Tat protein activates both the MyD88 and TRIF pathways to induce tumor necrosis factor alpha and interleukin-10 in human monocytes. J Virol 90:5886–5898. 10.1128/JVI.00262-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ben Haij N, Planes R, Leghmari K, Serrero M, Delobel P, Izopet J, BenMohamed L, Bahraoui E. 2015. HIV-1 Tat protein induces production of proinflammatory cytokines by human dendritic cells and monocytes/macrophages through engagement of TLR4-MD2-CD14 complex and activation of NF-kappaB pathway. PLoS One 10:e0129425. 10.1371/journal.pone.0129425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soderberg-Naucler C, Fish KN, Nelson JA. 1998. Growth of human cytomegalovirus in primary macrophages. Methods 16:126–138. 10.1006/meth.1998.0650. [DOI] [PubMed] [Google Scholar]
- 107.Sinzger C, Eberhardt K, Cavignac Y, Weinstock C, Kessler T, Jahn G, Davignon JL. 2006. Macrophage cultures are susceptible to lytic productive infection by endothelial-cell-propagated human cytomegalovirus strains and present viral IE1 protein to CD4+ T cells despite late downregulation of MHC class II molecules. J Gen Virol 87:1853–1862. 10.1099/vir.0.81595-0. [DOI] [PubMed] [Google Scholar]
- 108.Gredmark-Russ S, Soderberg-Naucler C. 2012. Dendritic cell biology in human cytomegalovirus infection and the clinical consequences for host immunity and pathology. Virulence 3:621–634. 10.4161/viru.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Riegler S, Hebart H, Einsele H, Brossart P, Jahn G, Sinzger C. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J Gen Virol 81:393–399. 10.1099/0022-1317-81-2-393. [DOI] [PubMed] [Google Scholar]
- 110.Hamilton ST, Scott GM, Naing Z, Rawlinson WD. 2013. Human cytomegalovirus directly modulates expression of chemokine CCL2 (MCP-1) during viral replication. J Gen Virol 94:2495–2503. 10.1099/vir.0.052878-0. [DOI] [PubMed] [Google Scholar]
- 111.Luganini A, Terlizzi ME, Gribaudo G. 2016. Bioactive molecules released from cells infected with the human cytomegalovirus. Front Microbiol 7:715. 10.3389/fmicb.2016.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christensen-Quick A, Vanpouille C, Lisco A, Gianella S. 2017. Cytomegalovirus and HIV persistence: pouring gas on the fire. AIDS Res Hum Retroviruses 33:S23–S30. 10.1089/aid.2017.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Redman TK, Britt WJ, Wilcox CM, Graham MF, Smith PD. 2002. Human cytomegalovirus enhances chemokine production by lipopolysaccharide-stimulated lamina propria macrophages. J Infect Dis 185:584–590. 10.1086/339007. [DOI] [PubMed] [Google Scholar]
- 114.Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, Arbuckle S, Craig ME, Rawlinson WD. 2012. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One 7:e52899. 10.1371/journal.pone.0052899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnson EL, Boggavarapu S, Johnson ES, Lal AA, Agrawal P, Bhaumik SK, Murali-Krishna K, Chakraborty R. 2018. Human cytomegalovirus enhances placental susceptibility and replication of human immunodeficiency virus type 1 (HIV-1), which may facilitate in utero HIV-1 transmission. J Infect Dis 218:1464–1473. 10.1093/infdis/jiy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson EL, Howard CL, Thurman J, Pontiff K, Johnson ES, Chakraborty R. 2015. Cytomegalovirus upregulates expression of CCR5 in central memory cord blood mononuclear cells, which may facilitate in utero HIV type 1 transmission. J Infect Dis 211:187–196. 10.1093/infdis/jiu424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang WL, Baumgarth N, Yu D, Barry PA. 2004. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J Virol 78:8720–8731. 10.1128/JVI.78.16.8720-8731.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc Natl Acad Sci U S A 97:1695–1700. 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McSharry BP, Avdic S, Slobedman B. 2012. Human cytomegalovirus encoded homologs of cytokines, chemokines and their receptors: roles in immunomodulation. Viruses 4:2448–2470. 10.3390/v4112448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xiao J, Palefsky JM, Herrera R, Berline J, Tugizov SM. 2009. EBV BMRF-2 facilitates cell-to-cell spread of virus within polarized oral epithelial cells. Virology 388:335–343. 10.1016/j.virol.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gulino D, Delachanal E, Concord E, Genoux Y, Morand B, Valiron MO, Sulpice E, Scaife R, Alemany M, Vernet T. 1998. Alteration of endothelial cell monolayer integrity triggers resynthesis of vascular endothelium cadherin. J Biol Chem 273:29786–29793. 10.1074/jbc.273.45.29786. [DOI] [PubMed] [Google Scholar]
- 122.Ngiam J, Cistulli PA. 2015. Dental treatment for paediatric obstructive sleep apnea. Paediatr Respir Rev 16:174–181. 10.1016/j.prrv.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 123.Chan AS, Cistulli PA. 2009. Oral appliance treatment of obstructive sleep apnea: an update. Curr Opin Pulm Med 15:591–596. 10.1097/MCP.0b013e3283319b12. [DOI] [PubMed] [Google Scholar]
- 124.Ng A, Gotsopoulos H, Darendeliler AM, Cistulli PA. 2005. Oral appliance therapy for obstructive sleep apnea. Treat Respir Med 4:409–422. 10.2165/00151829-200504060-00005. [DOI] [PubMed] [Google Scholar]
- 125.Sutherland K, Lee RW, Cistulli PA. 2012. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology 17:213–222. 10.1111/j.1440-1843.2011.02082.x. [DOI] [PubMed] [Google Scholar]
- 126.Pereira L. 1984. Glycoproteins specified by human cytomegalovirus, p 383–404. In Roizman B (ed), The herpesviruses, vol. 3. Plenum Press, New York, NY. [Google Scholar]
- 127.Pereira L, Hoffman M. 1986. Immunology of human cytomegalovirus glycoproteins, p 69–92. In Lopez C, Roizman B (ed), Human herpesvirus infections: pathogenesis, diagnosis, and treatment. Raven Press, New York, NY. [Google Scholar]
- 128.Tugizov S, Maidji E, Pereira L. 1996. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J Gen Virol 77:61–74. 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]