Abstract
Purpose:
Prostate cancers with mutations in genes involved in homologous recombination (HR), most commonly BRCA2, respond favorably to PARP inhibition and platinum-based chemotherapy. We investigated whether other prostate tumors that do not harbor deleterious mutations in these particular genes can similarly be deficient in HR, likely rendering those sensitive to HR-directed therapies.
Experimental Design:
HRD levels can be estimated using various mutational signatures derived from next-generation sequencing data. We used this approach on whole genome (n=311) and whole exome sequencing data (n=498) of both primary and metastatic prostate adenocarcinomas (PRAD) to determine whether prostate cancer cases display clear signs of HRD in somatic tumor biopsies.
Results:
Known BRCA-deficient samples showed all previously described HR-deficiency associated mutational signatures in the whole genome sequencing data. HRD-associated mutational signatures were also detected in a subset of patients who did not harbor germline or somatic mutations in BRCA1/2 or other HR-related genes. Similar results, albeit with lower sensitivity and accuracy, were also obtained from whole exome sequencing data.
Conclusions:
These findings may expand the number of cases likely to respond to PARP inhibitor treatment. Based on the HR-associated mutational signatures, 5-8 % of localized prostate cancer cases may be good candidates for PARP-inhibitor treatment (including those with BRCA1/2 mutations).
Keywords: BRCA1, BRCA2, DNA repair genes, homologous recombination deficiency, PARP inhibitors, Prostate cancer, mutational signatures
1. Introduction
Ovarian and breast cancer are often associated with mutations in BRCA1 and BRCA2, the key enzymes of a specific DNA repair pathway, homologous recombination (HR). Inactivating mutations in these genes often render such tumors homologous recombination deficient (HRD) that lead to sensitivity to PARP inhibitors, a novel class of cancer therapy, developed for such tumors based on the principle of synthetic lethality (1). Several PARP inhibitors have been approved over the past several years for the treatment of appropriately selected ovarian and breast cancer cases (2). It was shown that PARP inhibitors are also effective in prostate cancer, which is partly due to the presence of BRCA1 and BRCA2 mutations (3,4).
HRD can be induced by germline or somatic BRCA1/2 genetic mutations; however, it can also be present in tumors with intact BRCA1/BRCA2 genes. The list of genes involved in homologous recombination is far from complete. For example, loss of CHD1 was recently implicated as a possible mechanism leading to HRD in prostate cancer (5). HRD can also be induced through the suppression of expression of key HR genes by e.g. the inactivation of SPOP in prostate cancer cells (5). Therefore, sequencing panels of HR-related genes will likely miss a subset of HR-deficient cases. Because tumors deficient in HRD are sensitive to PARPi/platinum, the consequence of not identifying tumors that harbor HRD is that treatment with these agents may be delayed or not given. Identification of such tumors is clinically relevant because translational studies indicated that likely homologous recombination deficient tumors without BRCA1 or BRCA2 mutations may also benefit from homologous recombination deficiency associated therapy (6). A particularly promising approach for functionally evaluating HRD focuses on specific DNA aberrations, or “DNA scars” that result from HRD. This approach is based on the principle that the normal function of homologous recombination ensures the error-free repair of double-strand breaks; therefore, in its absence, specific DNA scars accumulate in the genome that are the resultants of the error-prone repair of double-strand DNA breaks that occur due to e.g. replication stress. There are HRD-induced DNA aberrations ranging from single nucleotide variation-based mutations to large scale, Mb sized genomic rearrangements (7). These have been combined into predictive HRD markers both in the CLIA (8,9) and experimental (10) setting.
These methods have proven to be a robust indicator of the absence of BRCA1/BRCA2 function both in the genomic analysis of clinical samples and in direct induction experiments (10-14). A recent study suggested that germline BRCA2 mutation-associated prostate cancer possesses some of the previously described HRD-associated mutational signatures in whole genome sequencing data (15).
In the current study, we investigated three related questions: 1) whether BRCA1 or BRCA2 mutations are consistently associated with the HRD-induced mutational signatures in a large set of whole genome and whole exome sequencing data, 2) whether there are prostate cancer cases with HRD-associated mutational signatures that are not associated with germline or somatic BRCA1 or BRCA2 mutations, which would suggest that there might be prostate cancer cases with sensitivity to PARP inhibitor treatment even in the absence of mutations in key HR genes and 3) whether whole genome sequencing (WGS) and whole exome sequencing (WES) based methods are equally accurate to identify HRD deficient prostate cancer cases.
2. Methods
2.1. Patients and cohorts
In this study we analyzed 311 whole genome sequenced samples from 240 cases (215 with localized, 13 with metastatic disease, and 12 unknown status) from the following cohorts (Table 1). For the PRAD-CA, EOPC-DE, PRAD-UK cohorts processed data were used in our analyses. In case of the other 4 cohorts, we worked with the BAM files.
Table 1:
Summary of analyzed WGS cohorts
To evaluate the signs of HR-deficiency, further 498 whole exome sequencing samples were analyzed from the TCGA. The normal and tumor BAM files were downloaded via the GDC Data Portal.
2.2. Mutation, Copy number, and Structural Variant Calling PRAD-CA, EOPC-DE, PRAD-UK cohorts
For the PRAD-CA, EOPC-DE, PRAD-UK cohorts, germline mutations and structural variants were obtained from the DCC Data Portal. Somatic mutation status, allele-specific copy number, and structural variant data were accessed from (16).
2.3. Mutation, Copy number, and Structural Variant Calling DFCI, CPDR, Decker, TCGA WGS and WES cohorts
In case of the other four cohorts, germline mutations were called with HaplotypeCaller, while somatic point-mutations and indels were called using Mutect2 (GATK 3.8). The high fidelity of the reported variants was ensured by the application of additional hard filters on top of the tools’ default ones. Allele-specific copy number profiles had been estimated by using Sequenza (17). Structural Variants were called using BRASS (v6.0.0 - (18)). Further details are available in the Supplementary Notes.
2.4. Genotyping – BRCA-status
The mutations were annotated using InterVar (19). Variants predicted as pathogenic or likely pathogenic were considered deleterious, while variants with unknown significance were marked differently. Copy number status of BRCA1/2 were based on Sequenza results.
2.5. Mutational Signatures
Somatic point-mutational signatures were determined with the deconstructSigs R package (20), by using the cosmic signatures as a mutational-process matrix. The extraction of rearrangement signatures was executed as described previously (21).
2.6. Genomic scar scores
The calculation of the genomics scar scores (loss-of-heterozygosity: LOH (22), large scale transitions: LST (23) and number of telomeric allelic imbalances: ntAI (24)) were determined using the scarHRD R package (25).
2.7. HRDetect
Due to the lack of sufficient numbers of bona fide HR-deficient cases within the prostate cancer cohorts, and to the dissimilarities among the cases involved in their corresponding studies, a prostate-specific HRDetect model could not be created. Instead, the weights of the original, breast cancer-specific, whole genome-based HRDetect model (10) were used to calculate the HRDetect scores of the WGS prostate samples. The scores of the WES samples were calculated by using the weights of a whole exome specific model, that was trained on 560 artificial whole exomes. The predictors were log-transformed and standardized within all prostate cancer cases (n=311 for WGS, and n=498 samples for WES). Both the scores and sample attributes are available in Supplementary Table 1-2.
3. Results
3.1. Frequency of BRCA gene aberrations in prostate cancer in the whole genome sequenced cohorts
From the 311 samples 25 samples (from 18 patients, 3 with metastatic and 15 with localized disease) had somatic or germline BRCA1/BRCA2 mutations. Two patients (DO51087, DO51965), carried BRCA1 germline mutations. One of the patients (DO51965, represented by five distinct sequenced biopsies) also had LOH, while the other (DO51087) had a somatic mutation in BRCA1. Another patient (DO52504) had a somatic structural variant aberration in BRCA1 (BRCA1-SAFB translocation).
Five patients had BRCA2 germline mutations. Of these, two had additional BRCA2 aberrations (either LOH or structural variant). Nine patients had a somatic BRCA2 mutation, out of which three also had a copy number loss and one patient had a somatic BRCA1 mutation.
Two samples had a deep deletion in BRCA2. (Supplementary Table 1.)
Both germline and somatic BRCA1/BRCA2 mutations may be present without additional loss of copy, resulting in retained homologous recombination capacity. All in all, we considered 15 biopsies from 9 cases (6 localized and 3 metastatic) as deficient either in the BRCA1 or BRCA2 function. (For summary of germline and somatic mutations see Suppl figure 10)
3.2. Genomic scars based HRD measures, the HRD score
The first scoring systems to quantify the degree of HRD were based on data derived from hybridization microarrays such as SNP arrays. The HRD-LOH score (22) is the number of LOH regions exceeding 15 Mb in size but less than the whole chromosome; the Large Scale Transitions (LST) score (23) is defined as the number of chromosomal breaks between adjacent regions of at least 10 Mb, with a distance between them not larger than 3Mb; and the Number of Telomeric Allelic Imbalances (ntAI) (24) is the number of AIs (unequal contribution of parental allele sequences) that extend to the telomeric end of a chromosome. These were later adapted to next generation sequencing data (25). The sum of these scores is referred to as HRD score, as in previous publications (11).
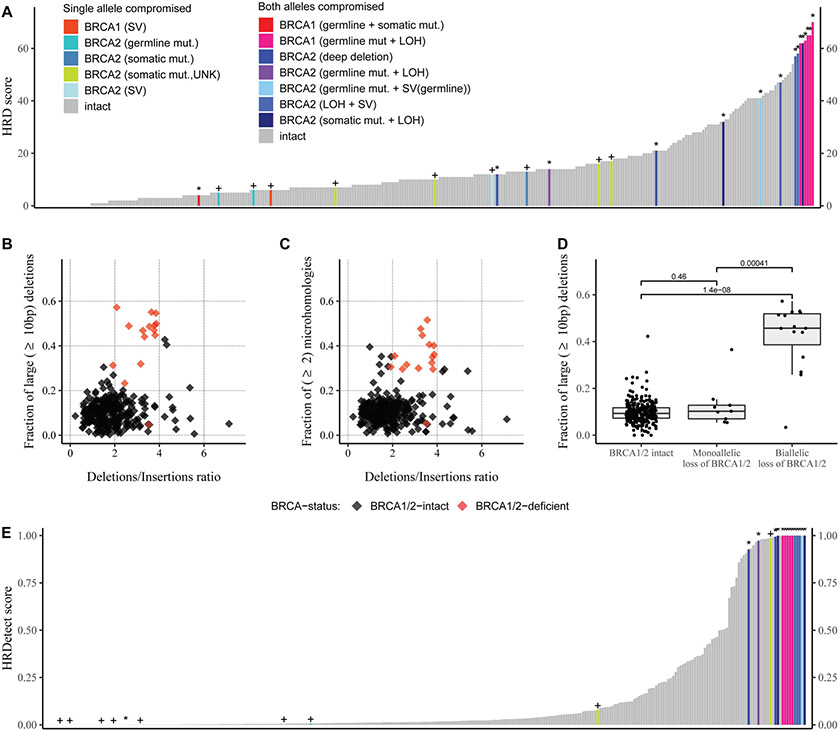
In the WGS based cohort 9 of the 15 BRCA1/2 deficient cases had an HRD score above 42, which is the threshold of HR deficiency previously published for ovarian cancer (11). However, there were 12 cases without any apparent BRCA1/2 or other HR gene mutation that also had a ≥42 HRD score. (Figure 1A) (For the individual components of the HRD score see Supplementary Figure 1).
Figure 1: Summary of the HRD-related predictors in the whole genome datasets.
A: HRD score: the sum of the three allele-specific CNV-derived genomic scars (HRD-LOH + LST + ntAI)
B: Fraction of larger than 9 bp deletions versus deletions/insertions ratio
C: Fraction of microhomology-mediated deletions with larger or equal to 10 bp in length versus deletions/insertions ratio
D: Fraction of at least 10 bp long deletions in the BRCA1/2 intact and deficient patients, the groups were compared with Mann-Whitney U-test, the p-values are represented on the plots.
E: HRDetect, the “+” signs if one allele is compromised, the “*” represent homozygous loss of BRCA1/2
3.3. Patterns of deletions and mutational signatures
Next generation sequencing revealed that loss of function of BRCA1 or BRCA2 is associated with a range of distinct mutational signatures that can be extracted from next generation sequencing data. Those include: 1) A single nucleotide variation based mutational signature (“COSMIC signature 3” or “BRCA signature” as labeled in the original publication (26)); 2) a short insertions/deletions based mutational profile, often dominated by deletions with microhomology, a sign of alternative repair mechanisms joining double-strand breaks in the absence of homologous recombination (13); 3) large scale rearrangements such as nonclustered tandem duplications of a given size range (mainly associated with BRCA1 loss of function) or deletions in the range of 1-10kb (mainly associated with BRCA2 loss of function) (7). All these can be efficiently induced by the inactivation of BRCA1, BRCA2 or several other key downstream HR genes (XRCC2, XRCC3, RAD51, etc.) (14).
Most BRCA-deficient prostate cancer cases showed higher levels of all three types of HR deficiency induced mutational aberrations (detailed results are presented in Supplementary Figures 1-5).
A set of consistent and easily detectable mutational features of HR-deficiency, especially in a BRCA2 deficient background, include the elevated Deletions/Insertions ratio, the relative increase in the number of deletions that are at least 10 bp long and the increased number of microhomology-mediated deletions. Such deletions are mechanistically explained as the result of alternative end-joining reparing double strand breaks in the absence of HR (27), therefore, we considered these aberrations as the most direct evidence of HRD. 14 of the 15 BRCA1/2 deficient cases showed elevated levels of these features (Figure 1B-D), consistent with previous observations (28). Notably, there were also thirteen cases with likely intact BRCA1 and BRCA2 function that also had a higher Deletions/Insertions ratio than the BRCA1/2 deficient cases. Three of the cases had a similar fraction of microhomology associated cases to those with BRCA2 deficiency and two of those three cases had a similar ratio of >10bp deletions to those with BRCA2 deficiency as well. This suggests that there are BRCA1/2 intact cases that display the mutational signatures usually associated with BRCA deficiency in prostate cancer.
BRCA1-mutations are known to be associated with a large contribution of Single Nucleotide Signature 3 (29). Based on their signature composition, several cases (such as the DO51965_M1-M5 or DO51962_M1-M3 samples) clearly demonstrate the signs of BRCA1/2 deficiency (Supplementary Figure 2).
Rearrangement Signature 3 and 5 have also been associated with BRCA1 and BRCA2-deficiency, respectively (7). These signs were also clearly detected in the investigated prostate cancer samples (Supplementary Figure 3).
3.4. HRDetect score
It was shown recently that a composite mutational signature, HRDetect, that combines the various types of mutations listed above represents a more accurate measure of HR deficiency than any of the mutational features alone (10,12).
The HRDetect score was determined as described in the methods sections (section 2.7). Fourteen out of the 15 biallelic BRCA1/2 mutants among the WGS samples had larger than 0.7 HRDetect scores (Figure 1E), which is the currently accepted threshold of HR-deficient samples on whole genome sequences (10,12). One BRCA1 mutant sample (DO51087) with both a germline and a somatic mutation acquired a low, close to zero HRDetect value, which can be explained with the likely assumption, that the somatic structural variant aberration also affected the germline allele, leaving the other allele intact, hence the true genotype being +/−. Given that BRCA1/2 biallelic mutants are in effect HR-deficient, the HRDetect model had almost perfectly identified the known samples with HRD. These samples, however, are not the only ones that have reached the higher ends of the HRDetect spectrum. 17 out of the 286 samples with a BRCA1/2 wild type background also reached above the 0.7 threshold, further strengthening the assumption, that even samples without mutations in the BRCA1/2 genes can exhibit the signs of HR-deficiency. Most of these cases had a high number of deletions with flanking microhomology, as a direct evidence of error prone correction of double strand breaks (Supplementary Figure 4).
While in general the HRD score and HRDetect values showed good correlation on the entire WGS cohort (Supplementary Figure 7), it should be noted that HRDetect was more accurate identifying the BRCA deficient cases (supplementary Figure 8).
3.5. HR deficiency associated biomarkers in whole exome sequencing data
Whole exome sequences contain approximately 1% of the sequence information contained in their whole genome-derived counterparts. In the entire set of WGS samples the average number of single nucleotide variations per sample was 3824. The same number for the entire WES data set was 64 SNVs/sample. In the WGS data the average number of deletions per sample was 256 deletions (73 with microhomologies), whereas in the WES data the same number was 4 deletions (0.75 with microhomologies)/sample. (Supplementary Figure 4-6). Despite this significantly reduced amount of information we attempted to derive predictors for HR-deficiency as previously described (30).
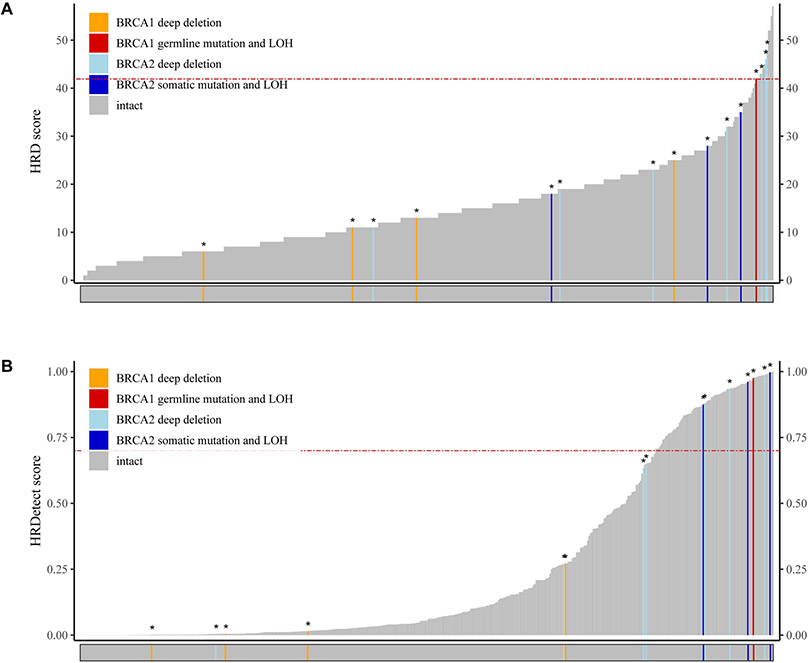
BRCA1/2 structural rearrangements, which cannot be detected by WES analysis, have been shown to underly HR deficiency in prostate cancer (28). Therefore, we expected the number of identifiable BRCA1/2 mutants might be somewhat lower in the WES cohort. In the investigated cohort (n=498) we found four clearly BRCA2-deficient and one BRCA1-deficient cases. Only one of those 4 cases had a ≥42 HRD score in the WES data set. (Figure 2A). In addition to the 4 above described BRCA1 or BRCA2 deficient cases we identified 4 cases with a deep BRCA1 deletion and 7 cases with a deep BRCA2 deletion. 3 of the BRCA2 deep deletion cases also had ≥42 HRD score. Nevertheless, the majority of the likely BRCA1 or BRCA2 deficient cases or deep deleted cases had a less than 42 HRD score. This suggests that the WES based HRD score has a significantly lower accuracy to identify HR deficient cases than its WGS based counterpart.
Figure 2: Summary of the HRD-related predictors in the whole exome datasets.
A: HRD score per sample: the sum of the three allele-specific CNV-derived genomic scars (HRD-LOH + LST + ntAI)
B: HRDetect the “+” signs if one allele is compromised, the “*” represent homozygous loss of BRCA1/2
A modified, WES-based HRDetect score was also determined for these cases (Figure 2B). Since a subset of the analyzed samples in the TCGA dataset had both WES and WGS data (n = 20), we performed a comparative analysis on the HRD-related attributes of the corresponding WES-WGS pairs (Supplementary Figure 9). We found, that the sum of the three genomic scars had a strong linear correlation (r = 0.79 +/− 0.14) between the sets, however this correlation was mainly driven by the number of large-scale transitions (rLST = 0.92 +/− 0.09), while both the HRD-LOH and TAI scores exhibited weak positive correlation (rHRD-LOH = 0.22 +/− 0.23 and rTAI = 0.24 +/− 0.23 respectively). Similar weak correlations were observed between the WES and WGS-derived SNV signature 3 ratios (r = 0.21 +/− 0.23) and microhomology-mediated deletion ratios (r = 0.23 +/− 0.23) (Supplementary Figure 9). The WES based HRDetect score was more accurate to reidentify the likely HR deficient cases than the WES based HRD score because all four BRCA1 and BRCA2 deficient cases and three BRCA2 deep deleted cases had a >0.7 HRDetect score (Figure 2B). However, there were also 78 BRCA 1/2 wild type samples above the same HRDetect threshold we used for the WGS measurement (>0.7). Unlike in the WGS HRDetet data set, most of the WES based cases with >0.7 HRDetect score had no or low number of deletions (and in most cases no microhomology flanked deletions) detected ( Supplementary Figure 6).
This lower accuracy of WES based methods to identify HR deficient cases is similar to the results of a recent publication (31), in which whole exome sequencing was performed on BRCA deficient and BRCA wild type prostate cancer cases and the large scale transition (23) and signature 3 values and their combined score as a measure of HR deficiency were determined. In this cohort, about one-third of the verified BRCA deficient prostate cancer cases had a WES based HR deficiency measure in the range of the BRCA wild type biopsies suggesting a significant rate of false negative cases.
3.6. Mutational analysis of other HR-related genes
We showed recently that in addition to eliminating BRCA1 or BRCA2, deletion of any of a number of other key HR genes (RAD51C, XRCC2, XRCC3, PALB2, RAD54) also induces the same mutational signatures usually associated with HR deficiency (14). Therefore, we investigated whether the high HRDetect scores in BRCA 1/2 wild type cases could be explained by the presence of biallelic aberration in other HR-related genes (such as BLM, PALB2, RAD50, RAD51 gene family, RAD52, RAD54B, and RAD54L). None of the WGS cases with higher than 0.7 HRDetect scores presented biallelic aberration in these genes. From the 55 WES cases that had higher than 0.7 HRDetect scores, 7 had biallelic BRCA1/2 mutations and additional 3 cases could be explained by biallelic mutations in PALB2, RAD51, or RAD54B. (Supplementary Figure 10-11)
3.6. Intratumoral heterogeneity of HR-deficiency
Ten patients had multiple tumor samples sequenced. Eight of them had HRDetect values less than 0.3 in all of their specimens. For one patient, although the HRDetect scores varied between the tumor samples, they remained below 0.7. In case of a tumor without biallelic loss of BRCA1/2 (EOPC-035), five out of the six tumor samples showed signs of HR-deficiency (Supplementary Figure 12).
3.7. Evolution of HR-deficiency
Ten patients from the investigated cohorts with metastatic prostate cancer had multiple metastatic sites sequenced. For two of the patients (A17 and A34) the primary prostate cancer and all of the metastatic sites showed signs of HR-deficiency and had higher than 0.9 HRDetect scores. All of these samples showed biallelic BRCA-loss (A17: BRCA1 germline mutation and LOH, A34: BRCA2 structural variant and LOH). For six of the patients (A10, A12, A21, A22, A24, A31) the primary tumor and all of the metastatic sites had low HRDetect scores, although in some samples (A21, A22, A31) slightly elevated HRDetect scores could be observed. Two patients (A29 and A32) showed heterogeneity in HR-deficiency, where although the primary prostate cancer was HR-competent, the metastases showed signs of HR-deficiency. (Supplementary Figure 13)
4. Discussion
Personalized therapy of prostate cancer has entered a new phase since the demonstration of the clinical efficacy of PARP inhibitors and platinum in cases with mutations in the DNA repair pathway, especially homologous recombination (3,32). However, some of the DNA damage checkpoint gene germline mutants do not benefit from PARP inhibitor therapy (33) and some cases without such mutations are sensitive to platinum-based therapy (32,34). Therefore, the optimal use of these therapeutic approaches will require more accurate identification of HR-deficient cases. A similar diagnostic problem was addressed in the case of breast and ovarian cancer using HR-deficiency induced mutational signatures (6,11,24). We decided to apply the same approach here. First, we demonstrated that bona fide, key HR gene (BRCA1/2) deficient cases show all three major types of HR deficiency-induced mutational signatures. Second, we also found that there is a significant number of cases without germline or somatic mutations in these genes that also demonstrate the same HR deficiency-induced mutational signatures. This strongly suggests that there is an additional, identifiable set of prostate cancer cases that will likely benefit from PARP inhibitor-based therapy. In support of this assumption, a recent clinical report found at least one case without germline mutations of HR genes with exceptionally good response to platinum-based therapy showing HRD+ score based on WES analysis (34). A clinical trial, prioritizing patients based on such molecular signatures will be needed to further validate this hypothesis.
We could explain the presence of HRD-induced mutational signatures with mutations in other HR-related genes, such as PALB2, RAD51, or RAD54B, in a few cases. HRD can also be caused by the low expression of HR-related genes (5). However, detecting correlations between low expression of HR genes and HRD-induced mutational signatures is severely limited by factors such as normal tissue contamination. For example, significant expression deficiency or LOH of the BRCA1 or BRCA2 genes can often be masked by the presence of these genes in the normal cells in the tumor biopsy. These results are highly consistent with a recent publication that determined HRDetect values from 254 triple-negative breast cancer cases and only a subset of the high HRDetect cases could be explained by direct mutational analysis of HR-related genes (12).
Mutational signatures can be most accurately determined from whole genome sequencing (WGS) data since, for example, large scale rearrangements can be detected only in such data (12). Furthermore, structural rearrangements in and around the BRCA1/2 genes, detectable only by WGS, also contribute to HRD in prostate cancer (28). Since WGS data are not always available, we investigated whether whole exome sequencing could also serve as a reliable detector of HR deficiency. WES data was also able to provide a measure of HR deficiency of clinical cases but the correlation between WGS and WES based HRD measures was rather limited. This is likely due to the fact that the total number of HRD induced mutations per sample is about 100-fold lower in WES data with the number of deletions near or below the detection threshold. Therefore, one of the mechanistically strongly linked mutational signatures to HRD, microhomology mediated deletions, cannot be reliable quantified in WES data. The limited accuracy of WES based HRD measures was also seen in a recent publication that WES sequenced a large number of BRCA1/2 mutant and BRCA1/2 wild type cases (31). The HRD measures of about 30% of bona fide BRCA1/2 mutant prostate cases were in the range of the BRCA1/2 wild type, likely HR proficient cases. The higher accuracy to identify truly HR-deficient cases by WGS data was also demonstrated recently for breast cancer (12).
Despite the wide availability of next generation sequencing (NGS), no CLIA certified WGS or WES based methods are available for determining HR deficiency in the diagnostic setting. Currently only targeted-sequencing based estimation of the genomic scar scores are available in CLIA-certified setting such as the myChoice assay (Myriad Genetics) for breast and ovarian cancer. With this method, tumors are considered HRD+ if they have a high myChoice HRD score (≥42) or a tumor BRCA1/2 mutation and HRD− if they have a low myChoice HRD score (<42) and wild-type BRCA1/2 (11). Unfortunately, our analysis didn’t have the sufficient number of BRCA-mutant cases to determine a prostate cancer-specific cutoff value, as in the case of ovarian cancer (11). Nevertheless, the BRCA-deficient cases have clearly higher genomic scar scores both in the WES and the WGS cohorts. (Supplementary Figure 5-7). A significantly higher number of cases will be needed to determine the clinically useful threshold value. Using the ovarian cancer specific cut-off value (≥42) the WGS based HRD score was reasonably accurate to identify HR deficient cases but it was outperformed by WGS based HRDetect.
It has been previously demonstrated that (35) that metastasis to metastasis spread of prostate tumor clones is common. This may explain the small heterogeneity seen in HR-status of multiple metastases.
5. Conclusions
In summary, we show that the presence of BRCA1/2 mutations is associated with the presence of HR-deficiency associated mutational signatures in prostate adenocarcinoma. HRD-patterns were also detected in a subset of patients who did not harbor germline or somatic mutations in BRCA1/2 or other HR-related genes. This likely defines a subset of prostate cancer patients, most accurately identified by WGS data, that will likely benefit from PARP inhibitor or platinum-based therapy.
Supplementary Material
Translational relevance.
Prostate adenocarcinoma cases with homologous recombination deficiency (HRD) benefit from PARP inhibitor therapy. Currently, direct sequencing of a handful of key homologous recombination genes serves as an indicator of HRD in prostate cancer. It is well known, however, that other mechanisms, such as suppression of expression of HR-related genes can also cause HRD, but the detection of those in biological samples is often difficult due to e.g. normal tissue contamination. Here we show that in 5-10% of localized prostate adenocarcinoma cases whole genome sequencing data display homologous recombination deficiency associated mutational signatures even in the absence of loss of function mutations in BRCA1/2 or other canonical homologous recombination genes. These cases may also be sensitive to PARP inhibitor or platinum-based therapy. Extending PARP inhibitor therapy to all cases with HRD-associated mutational signatures, even to those without BRCA1/2 mutations, may increase the efficacy of this treatment.
Acknowledgment
This work was supported by the Research and Technology Innovation Fund (KTIA_NAP_13-2014-0021 and NAP2-2017-1.2.1-NKP-0002 to Z.S.); Breast Cancer Research Foundation (BCRF-17-156 to Z.S.) and the Novo Nordisk Foundation Interdisciplinary Synergy Programme Grant (NNF15OC0016584 to Z.S.), Det Fri Forskningsrad (award number #7016-00345B) to Z.S; Department of Defense through the Prostate Cancer Research Program (award number is W81XWH-18-2-0056) to Z.S. and M.F. The Danish Cancer Society grant (R90-A6213 to MK). Z.S. and J.B. were supported by Velux Foundation 00018310 grant. The results shown here are based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/ and the International Cancer Genome Consortium (ICGC): https://icgc.org/.
Footnotes
Availability of data and material
Data analysis was carried out using R, version 3.5.0. Sequencing data that support the findings of this study have been deposited in the dbGaP with the accession code phs000661.v1.p and the previously published datasets repositories are listed in the supplementary material.
Conflict of Interest
Z. Szallasi is an inventor on a patent used in the myChoice HRD assay.
References:
- 1.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. American Association for the Advancement of Science; 2017;355:1152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstantinopoulos PA, Matulonis UA. PARP Inhibitors in Ovarian Cancer: A Trailblazing and Transformative Journey. Clin Cancer Res. 2018;24:4062–5. [DOI] [PubMed] [Google Scholar]
- 3.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. Massachusetts Medical Society; 2015;373:1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quigley DA, Dang HX, Zhao SG, Lloyd P, Aggarwal R, Alumkal JJ, et al. Genomic Hallmarks and Structural Variation in Metastatic Prostate Cancer. Cell. 2018;174:758–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjorth-Jensen K, Maya-Mendoza A, Dalgaard N, Sigurðsson JO, Bartek J, Iglesias-Gato D, et al. SPOP promotes transcriptional expression of DNA repair and replication factors to prevent replication stress and genomic instability. Nucleic Acids Res. 2018;46:9484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. The Lancet Oncology. 2019;20:636–48. [DOI] [PubMed] [Google Scholar]
- 7.Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seiden MV, Gordon AN, Bodurka DC, Matulonis UA, Penson RT, Reed E, et al. A phase II study of irofulven in women with recurrent and heavily pretreated ovarian cancer. Gynecol Oncol. 2006;101:55–61. [DOI] [PubMed] [Google Scholar]
- 9.Schilder RJ, Blessing JA, Shahin MS, Miller DS, Tewari KS, Muller CY, et al. A phase 2 evaluation of irofulven as second-line treatment of recurrent or persistent intermediately platinum-sensitive ovarian or primary peritoneal cancer: a Gynecologic Oncology Group trial. Int J Gynecol Cancer. NIH Public Access; 2010;20:1137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies H, Glodzik D, Morganella S, Yates LR, Staaf J, Zou X, et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat Med. Nature Research; 2017;23:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin Cancer Res. American Association for Cancer Research; 2016;22:3764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staaf J, Glodzik D, Bosch A, Vallon-Christersson J, Reuterswärd C, Häkkinen J, et al. Whole-genome sequencing of triple-negative breast cancers in a population-based clinical study. Nat Med. 2019;456:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zámborszky J, Szikriszt B, Gervai JZ, Pipek O, Póti Á, Krzystanek M, et al. Loss of BRCA1 or BRCA2 markedly increases the rate of base substitution mutagenesis and has distinct effects on genomic deletions. Oncogene. Nature Publishing Group; 2017;36:5085–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Póti Á, Gyergyák H, Németh E, Rusz O, Tóth S, Kovácsházi C, et al. Correlation of homologous recombination deficiency induced mutational signatures with sensitivity to PARP inhibitors and cytotoxic agents. Genome Biol. BioMed Central; 2019;20:240–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. Nature Publishing Group; 2011;43:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favero F, Joshi T, Marquard AM, Birkbak NJ, Krzystanek M, Li Q, et al. Sequenza: allele-specific copy number and mutation profiles from tumor sequencing data. Ann Oncol. 2015;26:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Wang K. InterVar: Clinical Interpretation of Genetic Variants by the 2015 ACMG-AMP Guidelines. Am J Hum Genet. 2017;100:267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal R, McGranahan N, Herrero J, Taylor BS, Swanton C. DeconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol. BioMed Central; 2016;17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boot A, Huang MN, Ng AWT, Ho S-C, Lim JQ, Kawakami Y, et al. In-depth characterization of the cisplatin mutational signature in human cell lines and in esophageal and liver tumors. Genome Res. 2018;28:654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Ng AW, Wu Y, et al. The Repertoire of Mutational Signatures in Human Cancer. bioRxiv [Internet]. Cold Spring Harbor Laboratory; 2019;24:322859. Available from: https://www.biorxiv.org/content/10.1101/322859v2 [Google Scholar]
- 21.Blokzijl F, Janssen R, van Boxtel R, Cuppen E. MutationalPatterns: comprehensive genome-wide analysis of mutational processes. Genome Med. BioMed Central; 2018;10:33–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. Nature Publishing Group; 2012;107:1776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popova T, Manié E, Rieunier G, Caux-Moncoutier V, Tirapo C, Dubois T, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72:5454–62. [DOI] [PubMed] [Google Scholar]
- 24.Birkbak NJ, Wang ZC, Kim J-Y, Eklund AC, Li Q, Tian R, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. American Association for Cancer Research; 2012;2:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sztupinszki Z, Diossy M, Krzystanek M, Reiniger L, Csabai I, Favero F, et al. Migrating the SNP array-based homologous recombination deficiency measures to next generation sequencing data of breast cancer. NPJ Breast Cancer. Nature Publishing Group; 2018;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceccaldi R, Liu JC, Amunugama R, Hajdu I, Primack B, Petalcorin MIR, et al. Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature. Nature Publishing Group; 2015;518:258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decker B, Karyadi DM, Davis BW, Karlins E, Tillmans LS, Stanford JL, et al. Biallelic BRCA2 Mutations Shape the Somatic Mutational Landscape of Aggressive Prostate Tumors. Am J Hum Genet. 2016;98:818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexandrov LB, Nik-Zainal S, Siu HC, Leung SY, Stratton MR. A mutational signature in gastric cancer suggests therapeutic strategies. Nat Commun. Nature Publishing Group; 2015;6:8683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diossy M, Reiniger L, Sztupinszki Z, Krzystanek M, Timms KM, Neff C, et al. Breast cancer brain metastases show increased levels of genomic aberration based homologous recombination deficiency scores relative to their corresponding primary tumors. Ann Oncol. 2018;22:3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsson P, Bandlamudi C, Cheng ML, Srinivasan P, Chavan SS, Friedman ND, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. Nature Publishing Group; 2019;571:576–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pomerantz MM, Spisák S, Jia L, Cronin AM, Csabai I, Ledet E, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer. 2017;123:3532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall CH, Sokolova AO, McNatty AL, Cheng HH, Eisenberger MA, Bryce AH, et al. Differential Response to Olaparib Treatment Among Men with Metastatic Castration-resistant Prostate Cancer Harboring BRCA1 or BRCA2 Versus ATM Mutations. Eur Urol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zafeiriou Z, Bianchini D, Chandler R, Rescigno P, Yuan W, Carreira S, et al. Genomic Analysis of Three Metastatic Prostate Cancer Patients with Exceptional Responses to Carboplatin Indicating Different Types of DNA Repair Deficiency. Eur Urol. 2019;75:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bova GS, Kallio HML, Annala M, Kivinummi K, Högnäs G, Häyiynen S, et al. Integrated clinical, whole-genome, and transcriptome analysis of multisampled lethal metastatic prostate cancer. Cold Spring Harb Mol Case Stud. Cold Spring Harbor Laboratory Press; 2016;2:a000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards SM, Evans DGR, Hope Q, Norman AR, Barbachano Y, Bullock S, et al. Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br J Cancer. Nature Publishing Group; 2010;103:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraser M, Sabelnykova VY, Yamaguchi TN, Heisler LE, Livingstone J, Huang V, et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature. Nature Publishing Group; 2017;541:359–64. [DOI] [PubMed] [Google Scholar]
- 38.Gerhauser C, Favero F, Risch T, Simon R, Feuerbach L, Assenov Y, et al. Molecular Evolution of Early-Onset Prostate Cancer Identifies Molecular Risk Markers and Clinical Trajectories. Cancer Cell. 2018;34:996–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. Nature Publishing Group; 2015;520:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163:1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrovics G, Li H, Stümpel T, Tan S-H, Young D, Katta S, et al. A novel genomic alteration of LSAMP associates with aggressive prostate cancer in African American men. EBioMedicine. 2015;2:1957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.