Abstract
Evidence from animal and human research shows that established memories can undergo changes after reactivation through a process called reconsolidation. Alterations of the level of the stress hormone cortisol may provide a way to manipulate reconsolidation in humans. Here, in a double-blind, within-subject design, we reactivated a 3-d-old memory at 3:55 A.M. in sixteen men and four women, immediately followed by oral administration of metyrapone versus placebo, to examine whether metyrapone-induced suppression of the morning cortisol rise may influence reconsolidation processes during and after early morning sleep. Crucially, reactivation followed by cortisol suppression versus placebo resulted in enhanced memory for the reactivated episode tested 4 d after reactivation. This enhancement after cortisol suppression was specific for the reactivated episode versus a non-reactivated episode. These findings suggest that when reactivation of memories is immediately followed by suppression of cortisol levels during early morning sleep in humans, reconsolidation processes change in a way that leads to the strengthening of episodic memory traces.
SIGNIFICANCE STATEMENT How can we change formed memories? Modulation of established memories has been long debated in cognitive neuroscience and remains a crucial question to address for basic and clinical research. Stress-hormone cortisol and sleep are strong candidates for changing consolidated memories. In this double-blind, placebo-controlled, within-subject pharmacological study, we investigate the role of cortisol on the modulation of reconsolidation of episodic memories in humans. Blocking cortisol synthesis (3 g metyrapone) during early morning sleep boosts memory for a reactivated but not for a non-reactivated story. This finding contributes to our understanding of the modulatory role of cortisol and its circadian variability on reconsolidation, and moreover can critically inform clinical interventions for the case of memory dysfunctions, and trauma and stress-related disorders.
Keywords: cortisol, memory, reconsolidation
Introduction
You wake up in the middle of the night. Just a hint of a past memory can be enough to change it. Several studies have reported that reactivation of a memory can make the memory malleable to subsequent change during an additional memory phase termed reconsolidation (Nader et al., 2000; Maroun and Akirav, 2008; Kindt et al., 2009; Schiller et al., 2010, 2013; Agren et al., 2012). Targeted pharmacological and behavioral manipulations following memory reactivation can modulate reconsolidation and thus critically change the reactivated memory (Hupbach et al., 2007; Schwabe et al., 2012; Kroes et al., 2014; Lee et al., 2017; Antypa et al., 2019; Galarza Vallejo et al., 2019).
Stress-hormone cortisol in humans (corticosterone in rodents) has been proposed to modulate reconsolidation, although the direction of effects remain debated (Akirav and Maroun, 2013; Meir Drexler and Wolf, 2017). If a stressor follows memory reactivation, the reactivated memory can be either disrupted or enhanced at a later test, advocating for a modulation of reconsolidation by stress-induced increases in cortisol levels (Wang et al., 2008; Marin et al., 2010; Schwabe and Wolf, 2010; Coccoz et al., 2011; Zhao et al., 2011; Yang et al., 2013; Bos et al., 2014; Hupbach and Dorskind, 2014). Pharmacological studies also support a role of cortisol in memory reconsolidation: when corticosterone or cortisol is pharmacologically administered following memory reactivation, fear memories were reported to be impaired or enhanced in both animals and humans (Akirav and Maroun, 2013; Meir Drexler and Wolf, 2017). In addition, when elevated postreactivation cortisol levels are suppressed pharmacologically with metyrapone, the stress-dependent or cortisol-dependent effects on memory reconsolidation are altered (Cai et al., 2006; Abrari et al., 2008; Yang et al., 2013; Amiri et al., 2015; Meir Drexler et al., 2016; Antypa et al., 2019). As such, the level of cortisol may critically influence reconsolidation processes.
Following a circadian rhythm, cortisol levels decrease in the evening and during early sleep, but rise again in the early morning, leading to a robust morning cortisol peak at the time of waking up (Wilhelm et al., 2007). Previous studies have shown that memory consolidation during sleep relates to this physiological early-night inhibition of cortisol release co-occurring with a distinct sleep-pattern (Plihal and Born, 1997, 1999; Plihal et al., 1999). The natural cortisol decrease during the first half of the night accompanied by long blocks of slow-wave sleep (SWS), also termed non-rapid eye movement sleep stage 3 (NREM3), has been proposed to enhance consolidation of hippocampus-dependent memories (such as memory of episodes; Plihal and Born, 1997, 1999; Payne and Nadel, 2004; Wagner and Born, 2008). By contrast, the physiological morning cortisol rise in humans, starting around 4 A.M., accompanied by key changes in sleep patterns (shorter blocks of SWS and longer blocks of REM sleep; Born et al., 1986) has been suggested to hinder the consolidation of newly encoded memories, possibly by interrupting the transfer of information between hippocampus and prefrontal cortex (Payne and Nadel, 2004; Wagner and Born, 2008). Analogously to consolidation, reconsolidation processes have been reported to be susceptible not only to cortisol (e.g., as described above, by Drexler et al., 2015; Antypa et al., 2019) but also to sleep manipulations (Kindt and Soeter, 2018), although the contribution of exact sleep stages on reconsolidation remains unclear. Thus, not only consolidation but also reconsolidation processes may be affected by the interaction of the physiological morning cortisol rise and co-occurring sleep patterns.
Given that combined effects of cortisol and sleep have been shown for consolidation of hippocampus-dependent memories, here we examined episodic memory reconsolidation taking place during the physiological morning cortisol rise compared with pharmacologically suppressed morning cortisol rise, using a within-subject crossover design. Combining metyrapone administration at 4 A.M. in the morning (Rimmele et al., 2010, 2015) with a previously established reconsolidation paradigm (Kroes et al., 2014; Antypa et al., 2019; Galarza Vallejo et al., 2019), we tested whether memory reactivation at 3:55 A.M. immediately followed by cortisol suppression changes reconsolidation, hence resulting in altered later memory of the reactivated episode. Polysomnographic (PSG) recordings were collected for the two experimental nights in a subgroup of participants. We expected that reactivation followed by a normal physiological morning cortisol rise would disrupt reconsolidation, in analogy to impairing effects of stress induction on reconsolidation and of morning cortisol rise on consolidation (Wagner et al., 2005; Cai et al., 2006; Abrari et al., 2008; Wilhelm et al., 2011; Hupbach and Dorskind, 2014; Amiri et al., 2015). Moreover, we hypothesized that cortisol suppression could block this disruptive effect of the morning rise in cortisol on reconsolidation, leading to enhanced reconsolidation when the cortisol rise is suppressed.
Materials and Methods
Participants
Sixteen male and four female healthy subjects (mean age 26 ± 4.67 years; mean body mass index 22.40 ± 2.4 kg/m2) participated in this double-blind, within-subject crossover study. They were free of neurologic, psychiatric and endocrine disorders, not receiving any medication for the period of their participation (except for two of the women taking oral contraception), non-smokers, and free of any contraindication for metyrapone administration. All participants reported having a regular sleep-wake rhythm and spent one adaptation night in the sleep lab. The study was approved by the local ethics committee. All subjects provided written informed consent and were paid for their participation. Two male participants were excluded for being outliers (i.e., deviated >2 SDs from the group mean) in memory performance in the placebo condition. An a priori power calculation, based on previous study testing metyrapone effects on memory (Rimmele et al., 2010), showed that to detect an effect of η2 = 0.482 with 95% power in a repeated measures within-subject ANOVA (one group, α = 0.05), a sample size of 17 participants will be needed (Faul et al., 2007; Rimmele et al., 2010). Because of the complicated design of the study (including in total eight sessions, three of them overnight), our total sample size was set to 20, to ensure that the final analysis will include sufficient number of participants.
Stimuli
During the encoding session, participants were presented with two stories per condition (metyrapone/placebo). Each story comprised 11 slides (seven neutral, four emotionally arousing) accompanied by an auditory narrative. Each slide was presented for 20 s. Participants were shown two previously used stories (Cahill et al., 1994; Kroes et al., 2014; Antypa et al., 2019; Galarza Vallejo et al., 2019), as well as two additional stories, parallel in structure and presentation from our laboratory.
Experimental design
After an adaptation night in the lab, each participant was tested in two conditions (metyrapone vs placebo), with the order of condition counterbalanced across subjects. Conditions were separated by an interval of at least 10 d. Each condition comprised an encoding session, a reactivation session, and a retrieval session (Fig. 1A). In the encoding session, participants were presented two stories. In the reactivation session, 2 d after encoding, participants slept in the lab (lights off at 11:00 P.M.) and were awakened at 3:55 A.M., when one of the two stories was reactivated (see reactivation below for more details). Directly following reactivation, at 4:00 A.M., the cortisol synthesis inhibitor metyrapone (3 g, HRA Pharma) or placebo was orally administered with a light snack (yogurt). Then, participants slept until 6:45 A.M., when they were awakened. At the retrieval session, 4 d after reactivation (7 d after encoding), participants were asked to complete a multiple-choice recognition memory questionnaire for each of the two stories in each condition (for more details, see below, Multiple-choice recognition memory task).
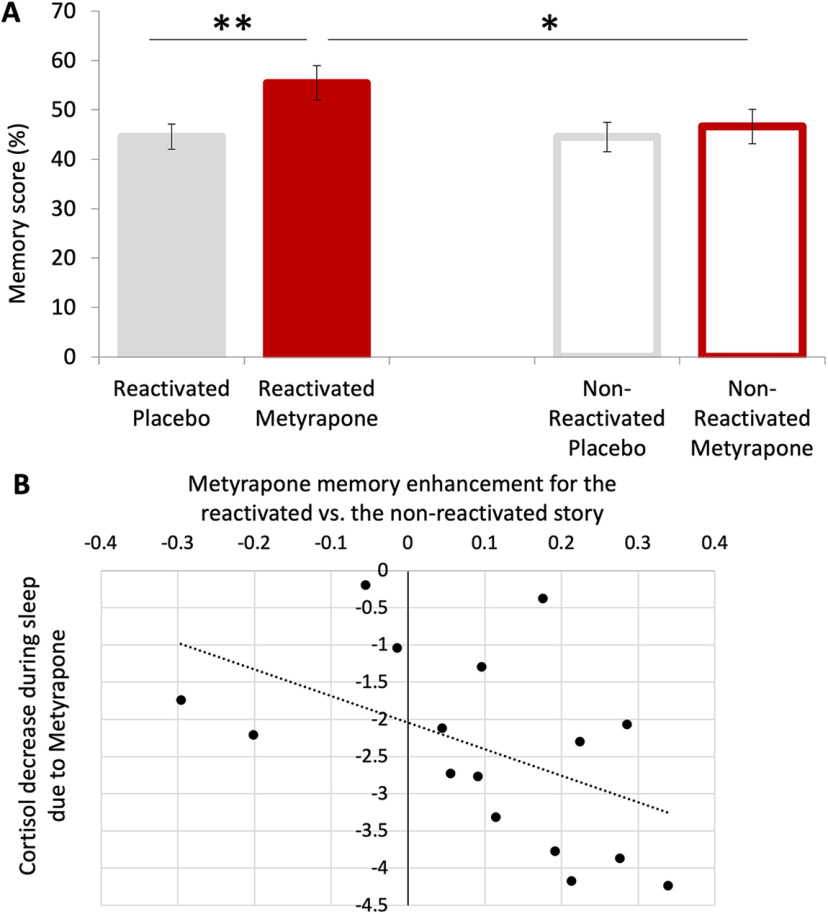
Figure 1.
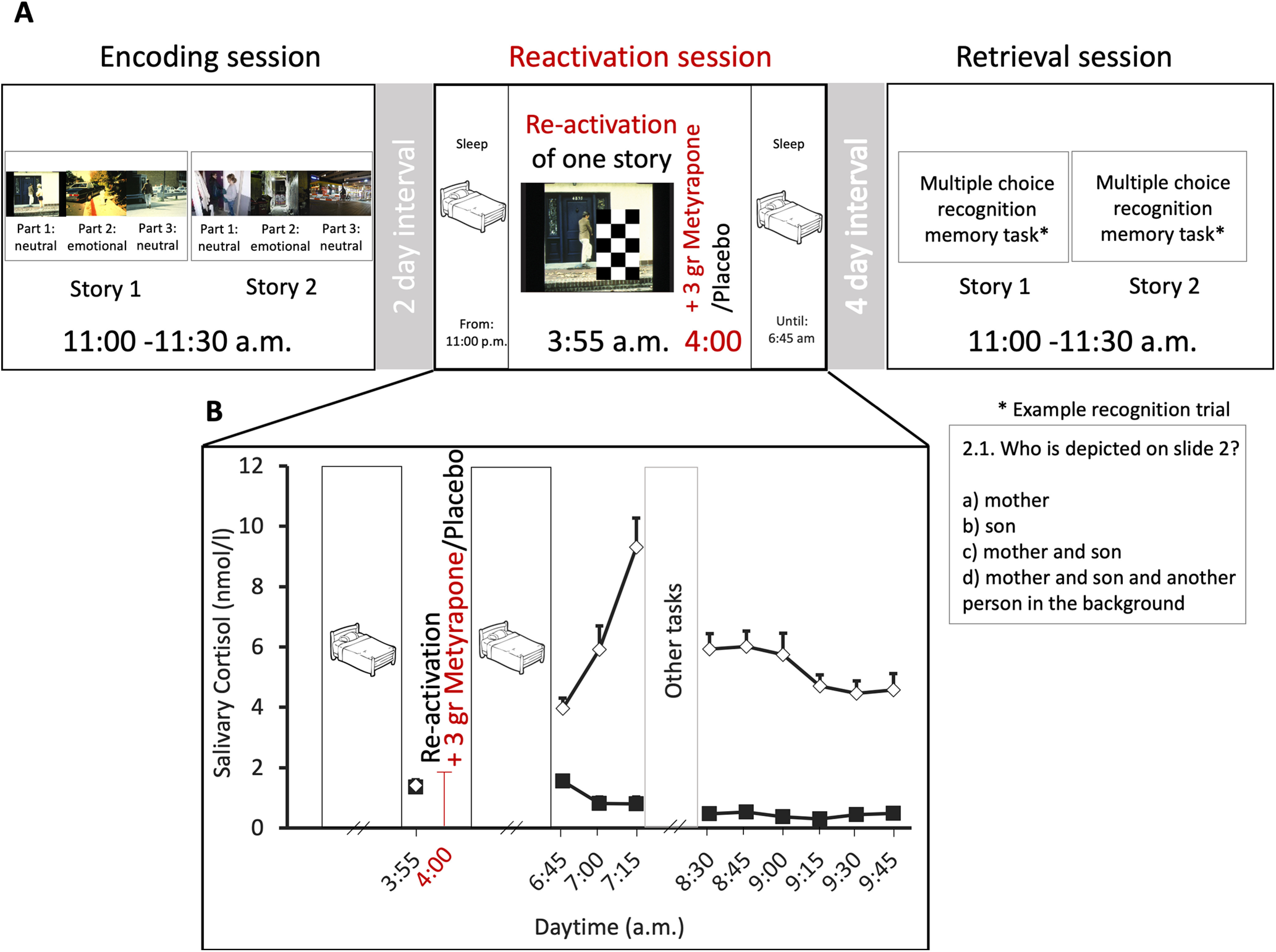
Experimental procedure (A) and cortisol levels (B). A, Using a within-subject design, each participant was tested once in a metyrapone and once in a placebo condition that both comprised three sessions. The order of conditions was counterbalanced across participants. Both conditions comprised three sessions (encoding, reactivation, retrieval). At the encoding session, participants were presented two stories. At the reactivation session (2 d after encoding), participants slept in the laboratory. After awakening at 3:55 A.M., one of the two stories was reactivated, and metyrapone or placebo was administered at 4:00 A.M. They then slept again until 6:45 A.M. At the retrieval session (7 d after encoding), memory was tested for both the reactivated and the non-reactivated story with a multiple-choice recognition memory task. B, Cortisol levels: mean ± SE salivary cortisol concentration for the reactivation session. Baseline cortisol levels before reactivation and substance administration (at 3:55 A.M.) did not differ between conditions. Metyrapone (black squares) administered at 4:00 A.M. suppressed the rise in morning cortisol seen in the placebo condition (white diamonds, salivary cortisol measured from 6:45 to 9:45).
Reactivation
At the reactivation session, one of the two encoded stories was reactivated using the procedure of previous studies (Kroes et al., 2014; Antypa et al., 2019; Galarza Vallejo et al., 2019). Participants were shown the first slide of one of the two stories, partially masked by three black-and-white checker-board patterns (Fig. 1A). Participants were asked a question about the masked part of the scene. After providing their answer, they were presented with the same scene with a smaller mask (covering a smaller part of the scene), and finally no mask, i.e., the answer to each question was progressively revealed. The other story was not reactivated.
Multiple-choice recognition memory task
At the retrieval session, participants were tested for their memory of the reactivated as well as the non-reactivated story using a multiple-choice recognition memory test (Kroes et al., 2014; Antypa et al., 2019; Galarza Vallejo et al., 2019). Participants were asked three to five questions per slide (amounting to a total of 40 questions per story) presented in the order of the original slide shows. Answers to the first slide of all stories were excluded from analysis given that the first slide had been used for reactivation for one of the two stories in each condition. Memory performance scores represent the percentage of correct answers to all questions.
Hormonal measures
Throughout the reactivation session, salivary cortisol samples were collected with Sarstedt salivette tubes at 3:55 A.M. (i.e., just before pill administration), at 6:45, 7:00, 7:15, 8:30, 8:45, 9:00, 9:15, 9:30, and 9:45 A.M. (Fig. 1B). Saliva samples were stored at −25°C until sent for analysis. Cortisol levels were analyzed using luminescence immunoassay (IBL) and interassay and intraassay coefficients of variations were below 5%.
PSG recordings
Whole-night PSG recording was collected for both experimental nights in a subgroup of participants (n = 11). PSG included electroencephalography (EEG; 11 electrodes were placed according to the international 10–20 system), electrooculography (EOG), and electromyography (EMG; American Academy of Sleep Medicine, 2007). The PSG signal was recorded with a V-Amp recorder (Brain Products). All recordings were sampled at 512 Hz and stored for later offline analyses. EEG recordings were referenced to contralateral mastoids (A1, A2) for the offline analyses.
Statistical analysis
Behavior
Memory performance in the multiple-choice recognition memory task was analyzed with a 2 (metyrapone/placebo) × 2 (reactivated/non-reactivated) mixed-design ANOVAs. Greenhouse–Geisser corrections of degrees of freedom were used when suitable and significant ANOVA effects were followed by pairwise t test contrasts to specify the observed effects. Order effects (placebo first vs metyrapone first) were tested by adding the order of substance administration as between subject factor in the above-mentioned ANOVA.
Using correlation analyses, we further examined whether the individual suppression of the morning cortisol peak in the second half of the night (4:05–6:45 A.M.) and after waking up (6:45–9:45 A.M.) after metyrapone compared with the placebo condition, is related to the change in memory performance for the reactivated versus non-reactivated story in the two conditions. In particular, a difference score for memory performance of the reactivated story minus memory performance for the non-reactivated story was calculated for each condition. The resulting difference score in memory performance between reactivated versus non-reactivated story in the placebo condition was then subtracted from the corresponding difference score in the metyrapone condition score. This score will be referred as metyrapone memory enhancement for the reactivated versus non-reactivated story, i.e., metyrapone memory enhancement = [(reactivated – non-reactivated memory performance)metyrapone condition – (reactivated – non-reactivated memory performance)placebo condition]. To examine the changes of cortisol levels (morning cortisol peak in placebo vs cortisol suppression after metyrapone condition) areas under the curve with respect to the increase (AUCi; Pruessner et al., 2003) were calculated for cortisol levels during sleep (cortisol levels from 3:55 to 6:45 A.M.) and cortisol levels after sleep (cortisol levels from 6:45 to 9:45 A.M.) for each condition. Then, cortisol change in the placebo condition was subtracted from the metyrapone condition (from now on referred as cortisol decrease because of metyrapone during sleep, i.e., [(AUCi3:55–6:45am)metyrapone condition – (AUCi3:55–6:45am)placebo condition] and cortisol decrease because of metyrapone after sleep [(AUCi6:45–9:45am)metyrapone condition – (AUCi6:45–9:45am)placebo condition]. We then correlated metyrapone memory enhancement with the change in cortisol decrease because of metyrapone during sleep (and after sleep, respectively). We used Kendall's tau b for these correlations, as more suitable to describe relations in smaller sample sizes (Bonett and Wright, 2000; Field, 2009).
Cortisol levels
For the analysis of cortisol levels, separate linear mixed models were used (fitlme, MATLAB), in an effort to tackle missing values of cortisol levels (because of missing saliva samples, insufficient saliva quantity for the analyses, or cortisol levels below the assay's sensitivity after metyrapone administration). Cortisol levels were log transformed to approach normal distribution of the residuals (note that untransformed cortisol levels are depicted at Fig. 1B for illustration purposes). The linear mixed model for cortisol levels was set with fixed effects of factors substance (placebo/metyrapone) and time (10 time-points of the saliva samples/condition) and random effects of the factor subject. The marginal effects of factors substance and time were assessed with a Type III F test, with the Satterthwaite approximation for the degrees of freedom, which is equivalent to omnibus repeated-measures ANOVA.
Sleep analysis
Sleep analyses were conducted using PRANA software (version 10.1; Phitools). An expert scorer blind to the experimental conditions determined the different sleep stages (NREM1, NREM2, NREM3, REM sleep, and wake) for each recorded night of sleep. From the scoring of the sleep architecture, we computed the duration (min) of each sleep stage, as well as the percentage of each sleep stage relative to the total sleep period (TSP; from sleep onset to wake up time) and relative to the total sleep time (TST; TSP minus intrawake epochs) for each phase of the night (i.e., sleep before the substance administration, sleep after the substance administration). Sleep efficiency (TST/time in bed × 100) for each phase was also calculated. All extracted parameters were compared between metyrapone and placebo condition with pairwise t test contrasts to identify differences in the sleep patterns between the two conditions. Sleep changes because of metyrapone were correlated with memory enhancement for the reactivated story as well as cortisol decrease during sleep because of metyrapone.
All the t tests reported were two-tailed and for all analyses the significance level was set to p ≤ 0.05.
Results
Postreactivation cortisol suppression enhances episodic memory reconsolidation
Cortisol suppression at 4:00 A.M., directly after memory reactivation, enhanced memory performance in a multiple-choice recognition memory task assessed 4 d after re-activation (main effect of substance: F(1,17) = 6.395, p = 0.022, η2 = 0.273; MMetyrapone = 0.51, SE = 0.03 vs MPL = 0.45, SE = 0.02; Fig. 2A).
Figure 2.
Memory performance in the metyrapone versus placebo condition of reactivated versus non-reactivated story (A) and relation between individual differences in memory performance and cortisol suppression during sleep (B). A, Pharmacologically suppressing cortisol at 4:00 A.M. directly after re-activation of a story at 3:55 A.M. enhanced memory performance for the re-activated story 4 d later in the metyrapone versus placebo condition. Importantly, cortisol suppression resulted in enhanced memory only for the reactivated but not the non-reactivated story (significant substance by reactivation interaction). Error bars indicate SE and *p < 0.05, **p < 0.01. B, Individual metyrapone memory enhancement for the reactivated versus non-reactivated story was negatively correlated with the individual cortisol decrease because of metyrapone during sleep (τ = –0.450, p = 0.015).
Most importantly, there was a substance by reactivation interaction (F(1,17) = 4.678, p = 0.045, η2 = 0.216): memory performance for the reactivated story was significantly higher in the metyrapone condition (MMetyrapone = 0.55, SE = 0.04) in comparison to the reactivated story in the placebo condition (MPL = 0.45, SE = 0.02; t(17) = 3.817, p = 0.001, d = 0.890). Crucially, in the metyrapone condition, memory was also higher for the reactivated (MRS = 0.55, SE = 0.04) than the non-reactivated story (MNRS = 0.47, SE = 0.03; t(17) = 2.578, p = 0.020, d = 0.608). There was no difference in memory performance for the non-reactivated stories between the metyrapone versus placebo conditions (t(17) = 0.488, p = 0.632), and no difference between reactivated and non-reactivated stories in the placebo condition (t(17) = –0.097, p = 0.924; Fig. 2A). In addition, there was no main effect of reactivation (F(1,17) = 3.019, p = 0.100). Lastly, by adding the order of substance administration as a between-subject factor, no main effect or interactions of the order of substance administration were found on memory performance (all p > 0.119).
Individual metyrapone memory enhancement for the reactivated versus non-reactivated story was negatively correlated with the individual cortisol decrease because of metyrapone during sleep (τ = –0.450, p = 0.015; Fig. 2B). In contrast, there was no correlation between metyrapone memory enhancement for the reactivated story and cortisol decrease because of metyrapone after sleep (τ = –0.015, p = 0.418). Of note, the difference between the two correlation coefficients was statistically significant (t = −3.909, p < 0.01; Field, 2009).
Metyrapone administration suppresses morning cortisol rise
Before reactivation and before substance administration (at 3:55 A.M.), baseline cortisol levels were comparable between the placebo (placebo baseline: b = 0.071, t(393) = 1.067, p = 0.287) and the metyrapone condition (metyrapone baseline: b = 0.614, t(393) = –0.504, p = 0.614). Following reactivation and substance administration, cortisol levels were lower after metyrapone versus placebo administration (main effect of substance: F(1,373) = 1321, p < 0.001; substance by time interaction: F(10,373) = 19.584, p < 0.001; main effect of time: F(10,374) = 6.988, p < 0.001) for all measurements taken between 6:45 and 10:00 A.M. (all p < 0.001). The maximum difference to baseline was observed at 7:15 (placebo: b = 0.861, t(393) = 10.263, p < 0.001; metyrapone: b = −1.161, t(393) = −9.679, p < 0.001; see Table 1; Fig. 1B).
Table 1.
Output of linear mixed model on cortisol levels with fixed effects of factors treatment (placebo/metyrapone) and time (10 time points of the saliva samples/condition) and random effects of the factor subject
| Estimate | SE | tStat | p value | 95% CI (lower, upper) | |
|---|---|---|---|---|---|
| Intercept Placebo at 3:45 A.M. (baseline for PL) |
0.071 | 0.067 | 1.067 | 0.287 | –0.060, 0.202 |
| Metyrapone at 3:45 A.M. (baseline for M) |
–0.044 | 0.090 | –0.504 | 0.614 | –0.215, 0.127 |
| Placebo at 6:45 A.M. | 0.503 | 0.086 | 5.871 | <0.001 | 0.335, 0.672 |
| Placebo at 7:00 A.M. | 0.667 | 0.084 | 7.950 | <0.001 | 0.502, 0.832 |
| Placebo at 7:15 A.M. | 0.861 | 0.084 | 10.263 | <0.001 | 0.696, 1.026 |
| Placebo at 8:30 A.M. | 0.698 | 0.084 | 8.219 | <0.001 | 0.531, 0.865 |
| Placebo at 8:45 A.M. | 0.704 | 0.087 | 8.081 | <0.001 | 0.533, 0.875 |
| Placebo at 9:00 A.M. | 0.630 | 0.085 | 7.432 | <0.001 | 0.463, 0.770 |
| Placebo at 9:15 A.M. | 0.587 | 0.087 | 6.737 | <0.001 | 0.416, 0.758 |
| Placebo at 9:30 A.M. | 0.563 | 0.086 | 6.555 | <0.001 | 0.394, 0.732 |
| Placebo at 9:45 A.M. | 0.563 | 0.088 | 6.371 | <0.001 | 0.389, 0.737 |
| Metyrapone at 6:45 A.M. | –0.430 | 0.121 | −3.547 | <0.001 | −0.669, –0.192 |
| Metyrapone at 7:00 A.M. | –0.849 | 0.120 | −7.083 | <0.001 | −1.081, –0.638 |
| Metyrapone at 7:15 A.M. | −1.161 | 0.120 | −9.679 | <0.001 | −1.370, –0.925 |
| Metyrapone at 8:30 A.M. | −1.149 | 0.125 | −9.227 | <0.001 | −1.393, –0.904 |
| Metyrapone at 8:45 A.M. | −1.142 | 0.125 | −9.161 | <0.001 | −1.387, –0.897 |
| Metyrapone at 9:00 A.M. | −1.209 | 0.121 | −10.028 | <0.001 | −1.447, –0.972 |
| Metyrapone at 9:15 A.M. | −1.253 | 0.124 | −10.128 | <0.001 | −1.496, −1.010 |
| Metyrapone at 9:30 A.M. | −1.025 | 0.121 | −8.449 | <0.001 | −1.264, –0.787 |
| Metyrapone at 9:45 A.M. | –0.992 | 0.125 | −6.827 | <0.001 | −1.237, –0.747 |
tStat: t statistic; CI: confidence interval.
Metyrapone administration alters subsequent sleep period
As expected, before substance administration, the metyrapone and the placebo condition did not differ in sleep duration (measured by TSP and TST), or in the proportion of time spent in the different sleep stages during the first part of the night (i.e., from sleep onset to 3:55 A.M.; all p > 0.1; see Table 2). However, metyrapone intake at 4:00 A.M. significantly affected the subsequent sleep period. Compared with placebo, metyrapone increased the time spent awake between 4:05 and 6:45 A.M. by ∼15 min (from 5% to 18% of TSP) in comparison to the placebo condition (t(10) = 3.952, p = 0.003, d = 1.192). In addition, metyrapone altered the proportion of time spent in different sleep stages as revealed by an increase in N1 duration (t(10) = 4.953, p = 0.001, d = 1.493), and a decrease in N3 (t(10) = 4.238, p = 0.002, d = 1.278), and REM duration (t(10) = 4.630, p = 0.001, d = 1.396; see Table 2). Note that metyrapone intake did not affect the duration of N2 (t(10) = 0.1704, p = 0.868). The increased time spent awake after substance administration also affected TST, which was reduced by 11%, and consequently decreased sleep efficiency (MPL = 94.24 ± 5.1, MM = 81.87 ± 7.5; t(10) = 3.952, p = 0.003, d = 1.192) during the second part of the night (i.e., after substance administration; from 4:05 to 6:45 A.M.).
Table 2.
Sleep architecture in subgroup of participants (n = 11)
| Before substance administration: Sleep onset to 3:55 A.M. |
After substance administration: 4:05 to 6:45 A.M. |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Metyrapone | t (10) | p | Placebo | Metyrapone | t (10) | p | |
| TSP (min) | 271.63 ± 19 | 269.45 ± 31 | 0.216 | 0.834 | 143.31 ± 15 | 147.18 ± 4 | 0.758 | 0.466 |
| TST (min) | 262.5 ± 20 | 258.18 ± 24 | 0.486 | 0.638 | 135.36 ± 19 | 120.54 ± 12 | 1.976 | 0.076 |
| N1%TSP | 2.24 ± 1.29 | 3.16 ± 2.01 | 1.370 | 0.201 | 7.71 ± 6.71 | 29.52 ± 18.77 | 4.593 | 0.001 |
| N2%TSP | 37.38 ± 9.44 | 39.23 ± 6.82 | 0.732 | 0.481 | 41.14 ± 11.98 | 40.08 ± 18.71 | 0.170 | 0.868 |
| N3%TSP | 38.87 ± 8.16 | 38.48 ± 6.73 | 0.128 | 0.900 | 12.95 ± 10.21 | 0.13 ± 0.43 | 4.238 | 0.002 |
| REM %TSP | 18.11 ± 5.61 | 15.16 ± 4.05 | 1.471 | 0.172 | 32.44 ± 9.58 | 12.14 ± 7.83 | 4.630 | 0.001 |
TSP: total sleep period; TST: total sleep time; N1: NREM sleep stage 1; N2: NREM sleep stage 2; N3: NREM sleep stage 3; REM: rapid eye movement sleep stage. Statistically significant differences between conditions are depicted in bold.
However, individual metyrapone memory enhancement for the reactivated versus non-reactivated story was not correlated with the above-mentioned individual sleep changes because of metyrapone; while individual cortisol decrease during sleep because of metyrapone was correlated with changes in sleep efficiency and intrawake periods during sleep because of metyrapone (τ = –0.611, p = 0.022, and τ = 0.611, p = 0.022, respectively; see Table 3).
Table 3.
Non-parametric correlations between individual changes in sleep parameters because of metyrapone in subgroup (n = 11), and metyrapone memory enhancement for the reactivated versus non-reactivated story [(reactivated – non-reactivated memory performance)metyrapone condition – (reactivated – non-reactivated memory performance)placebo condition], as well as cortisol decrease (because of metyrapone during sleep) and cortisol decrease because of metyrapone during sleep [(AUCi6:45–9:45am)metyrapone condition – (AUCi6:45–9:45am)placebo condition]
| Sleep changes because of metyrapone | Memory enhancement for the reactivated story because of metyrapone | Cortisol decreases because of metyrapone during sleep |
|---|---|---|
| TSTM-PL | 0.055 (p = 0.815) | –0.444 (p = 0.095) |
| SEM-PL | –0.055 (p = 0.815) | –0.611 (p = 0.022) |
| IntrawakeM-PL | 0.055 (p = 0.815) | 0.611 (p = 0.022) |
| N1M-PL | 0.418 (p = 0.073) | 0.056 (p = 0.835) |
| N2M-PL | –0.236 (p = 0.312) | 0.389 (p = 0.144) |
| N3M-PL | 0.236 (p = 0.312) | –0.389 (p = 0.144) |
| REMM-PL | –0.127 (p = 0.586) | 0.0 (p = 1) |
TST: total sleep time; SE: sleep efficiency; N1: NREM sleep stage 1; N2: NREM sleep stage 2; N3: NREM sleep stage 3; REM: rapid eye movement sleep stage. Statistically significant differences between conditions are depicted in bold.
Discussion
This study shows that the reactivation of an episodic memory with a reminder cue at 3:55 A.M. followed by pharmacological cortisol suppression during early morning sleep enhances memory recall for the reactivated but not the non-reactivated story tested 4 d after reactivation. Crucially, memory for the reactivated story was not only enhanced compared with the non-reactivated story in the cortisol suppression condition but also in comparison to both the reactivated and the non-reactivated story in the placebo condition (reactivation by pharmacological manipulation interaction). Notably, the physiological morning cortisol rise in the placebo condition did not disrupt memory for the reactivated story, which remained at similar level as memory for the non-reactivated story. Moreover, individual memory enhancement for the reactivated versus non-reactivated story because of cortisol suppression was negatively correlated with the individual cortisol decrease during sleep after metyrapone, i.e., the more cortisol levels were suppressed during early morning sleep, the higher the increase in later memory for the reactivated versus non-reactivated story.
The novelty of this study is to uncover a specific enhancement of a briefly reactivated episodic memory in humans likely as a consequence of altered reconsolidation processes because of cortisol suppression applied immediately after memory reactivation. Our study closely followed set criteria to test for pharmacologically-induced changes of reconsolidation (Schiller and Phelps, 2011; Kroes et al., 2014): (1) a consolidated memory was reactivated by a reminder cue; (2) the substance (manipulation) was administered after reactivation; and (3) memory was tested >24 h later. Of note, for criterion 3, Schiller and Phelps (2011) additionally mention that short-term memory should be intact, and memory only altered in the long-term, as reconsolidation is a process affecting long-term memory storage. Considering criterion 1, here, a 3-d-old and therefore consolidated memory was reactivated with a reminder cue (presentation of the first photograph of the story and photo-related questions). As expected, at the time of memory reactivation (3:55 A.M.), before substance administration, baseline cortisol levels did not differ between the placebo versus metyrapone condition. As set out in criterion 2, following memory reactivation, 3 g of metyrapone were administered at 4 A.M., i.e., the pharmacological manipulation aimed at altering reconsolidation was applied postreactivation. This pharmacological intervention suppressed cortisol levels, in particular, the morning cortisol peak, as depicted by significantly lower salivary cortisol levels in the metyrapone versus placebo condition at all measurement points from 6:45 to 9:45 A.M., replicating previous findings (Rimmele et al., 2010, 2015). To allow a sufficiently large time window for reconsolidation to take place, in accordance with criterion 3, memory for both the re-activated and the non-reactivated story were tested after four additional days (Agren, 2014; Kindt and Soeter, 2018). However, we did not test whether short-term memory was intact (see limitations). Having observed these criteria, our finding that cortisol suppression specifically boosts memory for the reactivated story is consistent with our interpretation that changing cortisol levels critically modulates memory reconsolidation of episodic memories.
This finding adds to our knowledge on episodic memory reconsolidation in humans. Previous studies using the same stimulus material showed memory to be impaired for the reactivated versus non-reactivated story if propofol (medication that induces general anesthesia) or electroconvulsive shock therapy followed memory reactivation and memory was tested 24 h later. Possibly, both manipulations led to a physiological blockade of episodic memory reconsolidation resulting in later memory impairment (Kroes et al., 2014; Galarza Vallejo et al., 2019). In contrast, here we show the opposite effect: cortisol suppression boosted memory for the reactivated story, i.e., our pharmacological change in cortisol levels likely enhanced reconsolidation processes. Moreover, here, individual metyrapone-induced memory enhancement for the reactivated (vs the non-reactivated) story, i.e., the source of the reactivation by manipulation effect, was negatively correlated to the individual cortisol decrease because of the pharmacological manipulation during sleep, indicating a direct relation of the two measures.
The fact that the reconsolidation window in our study took place in the early morning, with changes in both cortisol levels as well as changes in sleep, makes our findings difficult to compare to previous studies examining stress effects on reconsolidation. In humans, a stressor applied in the afternoon after reactivation of 1- to 6-d-old memory enhanced later memory (Marin et al., 2010; Coccoz et al., 2011, 2013; Bos et al., 2014), an effect not found for reactivation of older memories (Schwabe and Wolf, 2010; Coccoz et al., 2013). By contrast, stress after reactivation in the morning impaired reconsolidation process (Zhao et al., 2009; Hupbach and Dorskind, 2014). Therefore, the time passed after memory encoding, as well as the time of day when reactivated seem to be important parameters influencing how stress and associated cortisol changes modulate memory reconsolidation.
Interestingly, the main finding of this study contrasts with previous literature on cortisol suppression effects on memory retrieval (Rimmele et al., 2010; Marin et al., 2011). When asked to recall their memories at a time when cortisol levels are acutely suppressed, i.e., metyrapone is already active, participants showed impaired memory recall (Rimmele et al., 2010, 2015; Marin et al., 2011). This recall impairment persists when tested a week later when cortisol levels are back to normal levels (Rimmele et al., 2015). These findings together with the current data suggest that it is crucial whether a memory is retrieved under normal or under suppressed cortisol levels to influence later memory recall. If cortisol levels are low at the time of recall, acute and later memory recall are impaired, with metyrapone potentially altering acute memory recall as well as subsequent reconsolidation processes. In contrast, if metyrapone is inactive at the time of reactivation and only administered thereafter as in the present study, metyrapone would possibly only affect reconsolidation processes, which may lead to a different outcome such as enhanced memory, as we found. Alternatively, the observed memory enhancement may be attributed to retrieval practice and/or context-dependent effects in the cortisol suppression condition.
In addition, the effect of cortisol suppression altering reconsolidation might depend on whether reconsolidation takes place during sleep or awake state. In a previous study, in which we administered half the dose of metyrapone (1.5 g instead of 3 g as in the present study) at 9 A.M. (vs 4 A.M. in the present study) after reactivation of one of the stories (as in the present study), we found memory to be decreased in the metyrapone versus placebo condition independent of memory reactivation (Antypa et al., 2019). This finding accords with studies on memory consolidation reporting that pharmacological administration of cortisol or cortisol synthesis inhibitor during sleep versus wakefulness results in opposite effects on memory (Wagner et al., 2005; Wilhelm et al., 2011; Feld and Born, 2020). In sum, these findings highlight the importance of circadian modulations of cortisol on memory processes. In addition, in our study metyrapone-induced cortisol suppression during early-morning sleep critically altered sleep architecture and quality/efficiency (increase in N1 and wake duration, and decrease in time spent in N3 and REM). Similarly to the described influences on the co-occurring changes in cortisol and sleep on memory consolidation, thought to be mediated by the interplay between hippocampus and prefrontal cortex, the changes in cortisol and sleep observed in the current study may exert important effects on reconsolidation (Payne and Nadel, 2004; Wagner and Born, 2008). Although we report a correlation between cortisol suppression during the sleep period and memory enhancement, we found no direct relations between individual changes in sleep or time spent awake because of metyrapone and memory enhancement because of metyrapone. However, given that our sleep analyses were performed on a sub-sample of participants, they may lack statistical power. Taken together, future studies are necessary to identify the mechanisms underlying the circadian role of cortisol on memory reconsolidation and the role that sleep may have therein.
A limitation of our study is that we included only a small sample of women (n = 4). Future studies should include a representative female sample to allow the generalization across genders of the reconsolidation effects of cortisol suppression. This is particularly important, as of now, female participants have not yet been tested in most of the studies examining metyrapone effects on memory (Maheu et al., 2004, 2005; Rimmele et al., 2010, 2015; Marin et al., 2011). Another limitation of our study is that we did not assess memory performance immediately after encoding, which would provide a baseline to which memory after the pharmacological intervention could have been adjusted. We omitted an immediate memory test following the design of past reconsolidation studies to allow direct comparison of our data to these studies (Kroes et al., 2014; Antypa et al., 2019; Galarza Vallejo et al., 2019). Moreover, the present study did not include a control condition testing short-term memory effects immediately after reactivation and/or substance administration. Because memory encoding and reactivation was performed before any pharmacological manipulation (i.e., similar for both experimental conditions) in the present study, we presumed the differences in long-term memory effects were primarily attributable to cortisol suppression affecting reconsolidation processes. Yet, future studies may additionally test memory directly after reactivation to further dissociate the effects of morning cortisol rise and metyrapone-induced cortisol suppression (Elsey and Kindt, 2017).
Altogether, this study suggests that suppressing cortisol during early morning sleep may alter reconsolidation processes and enhances memory for the material reactivated before the manipulation. This finding indicates that metyrapone-induced cortisol suppression acts on what may be a physiological function and effect of normal early-morning cortisol peak and respective sleep patterns to memory processing. That is, reactivation of past memories in early morning hours physiologically followed by cortisol increase and REM sleep may hinder memory enhancement because of their reconsolidation (i.e., memory for the reactivated material remains at the levels of the non-reactivated material), in accordance to the described memory pruning function of sleep (Tononi and Cirelli, 2003; Payne and Nadel, 2004; Wagner and Born, 2008; Hardt et al., 2013), yet without disrupting the reactivated memory (below the non-reactivated material levels), as it would be expected based on the reconsolidation literature. By contrast, reactivating past memories in early morning hours in a context of cortisol depletion (because of metyrapone) and sleep alterations (increase in time spent awake in N1 sleep, decrease of N3 and REM sleep), may enhance their reconsolidation, as shown in the present study. This finding may help to better understand the persistence of emotional memories in posttraumatic stress disorder (PTSD). Indeed, PTSD is associated with reduced morning cortisol levels and sleep disturbances, i.e., increase in time spent awake and in N1 sleep, decrease in N3 (Pitman, 1989; Yehuda et al., 1996; Wagner et al., 2005; Kobayashi et al., 2007; Wagner and Born, 2008). These changes in sleep patterns in PTSD are similar to the sleep changes reported in the present study because of metyrapone. Therefore, it is plausible that the reactivation of past memories in these physiological conditions may exacerbate the reconsolidation of traumatic memories in PTSD patients.
Footnotes
This work was supported by grants from the Pierre Mercier foundation (U.R.); Swiss National Science Foundation Grants PZ00P1_137126, PZ00P1_160861 and PCEFP1_186911, 320030_159862, and 320030_182589; the European Community Seventh Framework Program (FP7/2007-2013) under Grant Agreement 334360; and the National Center of Competence in Research (NCCR) Affective Sciences and the Academic Society of Geneva (Foremane Fund). D.A. was supported by the Ernst and Lucie Schmidheiny Foundation. We thank Virginie Sterpenich for training experimenters on the set-up of polysomnographic recordings and Megan Mulligan, Florian Ruiz, Laura Riontino, Emalie McMahon, Anais Chevalley, Shannon Wilfred, Vassilis Kehayas, and Kristoffer Aberg for help with data acquisition and analysis.
The authors declare no competing financial interests.
References
- Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y (2008) Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: dependence upon training intensity. Neurobiol Learn Mem 89:178–184. 10.1016/j.nlm.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Agren T (2014) Human reconsolidation: a reactivation and update. Brain Res Bull 105:70–82. 10.1016/j.brainresbull.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Agren T, Engman J, Frick A, Björkstrand J, Larsson EM, Furmark T, Fredrikson M (2012) Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science 337:1550–1552. 10.1126/science.1223006 [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M (2013) Stress modulation of reconsolidation. Psychopharmacology (Berl) 226:747–761. 10.1007/s00213-012-2887-6 [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. Darien: American Academy of Sleep Medicine. [Google Scholar]
- Amiri S, Jafarian Z, Vafaei AA, Motaghed-Larijani Z, Samaei SA, Rashidy-Pour A (2015) Glucocorticoids interact with cholinergic system in impairing memory reconsolidation of an inhibitory avoidance task in mice. Basic Clin Neurosci 6:155–162. [PMC free article] [PubMed] [Google Scholar]
- Antypa D, Rodrigues Cabrita D, Vuilleumier P, Rimmele U (2019) Cortisol suppression after memory reactivation impairs later memory performance. Psychoneuroendocrinology 106:226–232. 10.1016/j.psyneuen.2019.03.035 [DOI] [PubMed] [Google Scholar]
- Bonett DG, Wright TA (2000) Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika 65:23–28. 10.1007/BF02294183 [DOI] [Google Scholar]
- Born J, Kern W, Bieber K, Fehm-Wolfsdorf G, Schiebe M, Fehm HL (1986) Night-time plasma cortisol secretion is associated with specific sleep stages. Biol Psychiatry 21:1415–1424. 10.1016/0006-3223(86)90333-1 [DOI] [PubMed] [Google Scholar]
- Bos MG, Schuijer J, Lodestijn F, Beckers T, Kindt M (2014) Stress enhances reconsolidation of declarative memory. Psychoneuroendocrinology 46:102–113. 10.1016/j.psyneuen.2014.04.011 [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL (1994) Beta-adrenergic activation and memory for emotional events. Nature 371:702–704. 10.1038/371702a0 [DOI] [PubMed] [Google Scholar]
- Cai WH, Blundell J, Han J, Greene RW, Powell CM (2006) Postreactivation glucocorticoids impair recall of established fear memory. J Neurosci 26:9560–9566. 10.1523/JNEUROSCI.2397-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccoz V, Maldonado H, Delorenzi A (2011) The enhancement of reconsolidation with a naturalistic mild stressor improves the expression of a declarative memory in humans. Neuroscience 185:61–72. 10.1016/j.neuroscience.2011.04.023 [DOI] [PubMed] [Google Scholar]
- Coccoz V, Sandoval AV, Stehberg J, Delorenzi A (2013) The temporal dynamics of enhancing a human declarative memory during reconsolidation. Neuroscience 246:397–408. 10.1016/j.neuroscience.2013.04.033 [DOI] [PubMed] [Google Scholar]
- Drexler SM, Merz CJ, Hamacher-Dang TC, Tegenthoff M, Wolf OT (2015) Effects of cortisol on reconsolidation of reactivated fear memories. Neuropsychopharmacology 40:3036–3043. 10.1038/npp.2015.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsey JWB, Kindt M (2017) Tackling maladaptive memories through reconsolidation: from neural to clinical science. Neurobiol Learn Mem 142:108–117. 10.1016/j.nlm.2017.03.007 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- Feld GB, Born J (2020) Neurochemical mechanisms for memory processing during sleep: basic findings in humans and neuropsychiatric implications. Neuropsychopharmacology 45:31–44. 10.1038/s41386-019-0490-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A (2009) Discovering statistics using SPSS. London: SAGE Publications Ltd. [Google Scholar]
- Galarza Vallejo A, Kroes MCW, Rey E, Acedo MV, Moratti S, Fernández G, Strange BA (2019) Propofol-induced deep sedation reduces emotional episodic memory reconsolidation in humans. Sci Adv 5:eaav3801. 10.1126/sciadv.aav3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt O, Nader K, Nadel L (2013) Decay happens: the role of active forgetting in memory. Trends Cogn Sci 17:111–120. 10.1016/j.tics.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Hupbach A, Dorskind JM (2014) Stress selectively affects the reactivated components of a declarative memory. Behav Neurosci 128:614–620. 10.1037/bne0000006 [DOI] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L (2007) Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn Mem 14:47–53. 10.1101/lm.365707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M (2018) Pharmacologically induced amnesia for learned fear is time and sleep dependent. Nat Commun 9:1316. 10.1038/s41467-018-03659-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B (2009) Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci 12:256–258. 10.1038/nn.2271 [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Boarts JM, Delahanty DL (2007) Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology 44:660–669. 10.1111/j.1469-8986.2007.537.x [DOI] [PubMed] [Google Scholar]
- Kroes MCW, Tendolkar I, van Wingen GA, van Waarde JA, Strange BA, Fernández G (2014) An electroconvulsive therapy procedure impairs reconsolidation of episodic memories in humans. Nat Neurosci 17:204–206. 10.1038/nn.3609 [DOI] [PubMed] [Google Scholar]
- Lee JLC, Nader K, Schiller D (2017) An update on memory reconsolidation updating. Trends Cogn Sci 21:531–545. 10.1016/j.tics.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Joober R, Beaulieu S, Lupien SJ (2004) Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behav Neurosci 118:420–428. 10.1037/0735-7044.118.2.420 [DOI] [PubMed] [Google Scholar]
- Maheu FS, Joober R, Lupien SJ (2005) Declarative memory after stress in humans: differential involvement of the beta-adrenergic and corticosteroid systems. J Clin Endocrinol Metab 90:1697–1704. 10.1210/jc.2004-0009 [DOI] [PubMed] [Google Scholar]
- Marin MF, Pilgrim K, Lupien SJ (2010) Modulatory effects of stress on reactivated emotional memories. Psychoneuroendocrinology 35:1388–1396. 10.1016/j.psyneuen.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Marin MF, Hupbach A, Maheu FS, Nader K, Lupien SJ (2011) Metyrapone administration reduces the strength of an emotional memory trace in a long-lasting manner. J Clin Endocrinol Metab 96:E1221–E1227. 10.1210/jc.2011-0226 [DOI] [PubMed] [Google Scholar]
- Maroun M, Akirav I (2008) Arousal and stress effects on consolidation and reconsolidation of recognition memory. Neuropsychopharmacology 33:394–405. 10.1038/sj.npp.1301401 [DOI] [PubMed] [Google Scholar]
- Meir Drexler S, Wolf OT (2017) The role of glucocorticoids in emotional memory reconsolidation. Neurobiol Learn Mem 142:126–134. 10.1016/j.nlm.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Meir Drexler S, Merz CJ, Hamacher-Dang TC, Wolf OT (2016) Cortisol effects on fear memory reconsolidation in women. Psychopharmacology (Berl) 233:2687–2697. 10.1007/s00213-016-4314-x [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726. 10.1038/35021052 [DOI] [PubMed] [Google Scholar]
- Payne JD, Nadel L (2004) Sleep, dreams, and memory consolidation: the role of the stress hormone cortisol. Learn Mem 11:671–678. 10.1101/lm.77104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK (1989) Post-traumatic stress disorder, hormones, and memory. Biol Psychiatry 26:221–223. 10.1016/0006-3223(89)90033-4 [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J (1997) Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 9:534–547. 10.1162/jocn.1997.9.4.534 [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J (1999) Memory consolidation in human sleep depends on inhibition of glucocorticoid release. Neuroreport 10:2741–2747. 10.1097/00001756-199909090-00009 [DOI] [PubMed] [Google Scholar]
- Plihal W, Pietrowsky R, Born J (1999) Dexamethasone blocks sleep induced improvement of declarative memory. Psychoneuroendocrinology 24:313–331. 10.1016/s0306-4530(98)00080-8 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH (2003) Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–931. 10.1016/s0306-4530(02)00108-7 [DOI] [PubMed] [Google Scholar]
- Rimmele U, Meier F, Lange T, Born J (2010) Suppressing the morning rise in cortisol impairs free recall. Learn Mem 17:186–190. 10.1101/lm.1728510 [DOI] [PubMed] [Google Scholar]
- Rimmele U, Besedovsky L, Lange T, Born J (2015) Emotional memory can be persistently weakened by suppressing cortisol during retrieval. Neurobiol Learn Mem 119:102–107. 10.1016/j.nlm.2015.01.010 [DOI] [PubMed] [Google Scholar]
- Schiller D, Phelps EA (2011) Does reconsolidation occur in humans? Front Behav Neurosci 5:24. 10.3389/fnbeh.2011.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA (2010) Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463:49–53. 10.1038/nature08637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils MH, Phelps EA (2013) Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci USA 110:20040–20045. 10.1073/pnas.1320322110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT (2010) Stress impairs the reconsolidation of autobiographical memories. Neurobiol Learn Mem 94:153–157. 10.1016/j.nlm.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS (2012) Stress effects on memory: an update and integration. Neurosci Biobehav Rev 36:1740–1749. 10.1016/j.neubiorev.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C (2003) Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull 62:143–150. 10.1016/j.brainresbull.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Wagner U, Born J (2008) Memory consolidation during sleep: interactive effects of sleep stages and HPA regulation. Stress 11:28–41. 10.1080/10253890701408822 [DOI] [PubMed] [Google Scholar]
- Wagner U, Degirmenci M, Drosopoulos S, Perras B, Born J (2005) Effects of cortisol suppression on sleep-associated consolidation of neutral and emotional memory. Biol Psychiatry 58:885–893. 10.1016/j.biopsych.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L (2008) Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci 28:5602–5610. 10.1523/JNEUROSCI.0750-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S (2007) Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology 32:358–366. 10.1016/j.psyneuen.2007.01.008 [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Wagner U, Born J (2011) Opposite effects of cortisol on consolidation of temporal sequence memory during waking and sleep. J Cogn Neurosci 23:3703–3712. 10.1162/jocn_a_00093 [DOI] [PubMed] [Google Scholar]
- Yang C, Liu JF, Chai BS, Fang Q, Chai N, Zhao LY, Xue YX, Luo YX, Jian M, Han Y, Shi HS, Lu L, Wu P, Wang JS (2013) Stress within a restricted time window selectively affects the persistence of long-term memory. PLoS One 8:e59075. 10.1371/journal.pone.0059075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ (1996) Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry 40:79–88. 10.1016/0006-3223(95)00451-3 [DOI] [PubMed] [Google Scholar]
- Zhao X, Li Y, Peng T, Seese RR, Wang Z (2011) Stress impairs consolidation of recognition memory after blocking drug memory reconsolidation. Neurosci Lett 501:50–54. 10.1016/j.neulet.2011.06.050 [DOI] [PubMed] [Google Scholar]
- Zhao LY, Zhang XL, Shi J, Epstein DH, Lu L (2009) Psychosocial stress after reactivation of drug-related memory impairs later recall in abstinent heroin addicts. Psychopharmacology (Berl) 203:599–608. 10.1007/s00213-008-1406-2 [DOI] [PMC free article] [PubMed] [Google Scholar]