Abstract
Background
Increasing number of patients with COVID-19-associated mucormycosis have been reported, especially from India recently. We have described a patient with COVID-19-associated mucormycosis and, searched and analyzed current medical literature to delineate the characteristics of COVID-19-associated mucormycosis.
Method
We reported a patient developed mucormycosis during post-COVID period. We searched literature to describe the incidence, clinical features, and outcomes of COVID-19-associated mucormycosis. Demographic features, risk factors, clinical features, diagnostic methods, treatment and outcome were analyzed.
Results
We describe a 54-year-old male, hospitalized due to severe COVID-19 pneumonia. He was given long-term, high doses of systemic steroids. He developed maxillo-fascial mucormycosis and died of sepsis. Our literature search found 30 publications describing 100 patients including present case report. The majority (n = 68) were reported from India. 76% were male. The most commonly seen risk factors were corticosteroid use (90.5%), diabetes (79%), and hypertension (34%). Also, excessive use of broad-spectrum antibiotics were noted in cases. Most frequent involvements were rhino-orbital (50%), followed by rhino-sinusal (17%), and rhino-orbito-cerebral (15%). Death was reported as 33 out of 99 patients (33,3%).
Conclusions
Steroid use, diabetes, environmental conditions, excessive use of antibiotics, and hypoxia are main risk factors. Despite medical and surgical treatment, mortality rate is high. A multidisciplinary approach is essential to improve the conditions facilitating the emergence of COVID-19-associated mucormycosis.
Keywords: COVID-19, Coronavirus, Mucormycosis, Mucor, Fungal infections
1. Introduction
New coronavirus disease (COVID-19) continues to exhibit remarkable repercussions worldwide along with its atypical manifestations. Novel reports of SARS-CoV-2 infection underline the risk of opportunistic fungi infections, namely the pulmonary aspergillosis and mucormycosis, that accompany viral symptoms, leading to death by invading multi-organ systems [1]. Experience from SARS patients showed that incidence of fungal co-infection was 14.8–27%, and it was higher in severely ill SARS patients reaching up to 33% [2]. Furthermore, severe influenza pneumonia cases resulting in acute respiratory distress syndrome complicated by fungal infection were reported [3]. While invasive pulmonary aspergillosis was found in 83 (19%) of 432 patients with influenza, it was higher in immunocompromised patients (32%) [4].
The main reason behind invasive fungi infections is thought to be due to the impairment of innate defense mechanisms, such as ciliary clearance, and the lack of sufficient lymphatic immune response against fungal invasion during the pathophysiologic progression of deregulatory immune mechanisms in COVID-19-related acute respiratory distress syndrome (ARDS) [5]. As a matter of fact, utilization of corticosteroids, one of the widely used weapons against COVID-19 to diminish the risk of mortality, most likely causes critically ill patients in intensive care units (ICU) to be more prone to opportunistic infections, which in turn may lead to death. The exact incidence of fungal involvement is not yet known due to the incapability of common bronchoscopy diagnosis in COVID-19 patients [5]. Since the clinical and radiological findings of secondary fungal infections are not distinguishable from varying COVID-19 pneumonia and pneumonitis, the identification of pathogenic fungi is mainly dependent on the positivity in lower respiratory tract specimen tests, such as the bronchoalveolar lavage, sputum or endotracheal aspirate [[5], [6], [7]].
Specially, COVID-19-associated mucormycosis, an opportunistic fungus that invades rhinal, occipital and cerebral areas, come to light as the pandemic proceeds. Mucormycosis is caused by the fungus Mucor (class Phycomycetes, order Mucorales) that is capable of reaching craniofacial compartments such as paranasal sinuses, pharynx, orbita and intracranial cavity via the spore spread [6]. Thus, the invasion is highly lethal and rapidly progressive, requiring a multidisciplinary approach and fast actions in treatment. Mucor-derived angioinvasion presents as diverse signs and symptoms including nasal stuffiness; mucoid, purulent, bloody or black nasal discharge; epistaxis; facial, nasal or periocular edema and discoloration, speaking defects, vision impairment and excruciating headache [8]. Predisposing factors were known to be consisting of conditions such as diabetes mellitus, corticosteroid usage and immunosuppression, immunodeficiency, malignancies (especially hematologic) and cell/tissue/organ transplant treatments [8]. However, COVID-19, which requires a comprehensive and multi-organ-based treatment in varying severities, is unfortunately added to the list of risk factors for the opportunistic Mucor infection.
Increasing number of patients with COVID-19-associated mucormycosis have been reported from India recently. The association of these two critical infectious diseases is challenging not only for India but also for the rest of the World. In this systematic review, in order to delineate the characteristics of COVID-19-associated mucormycosis, we have searched current medical literature and analyzed mucormycosis infection developed in patients with COVID-19.
2. Material and methods
2.1. Search strategies and study selection
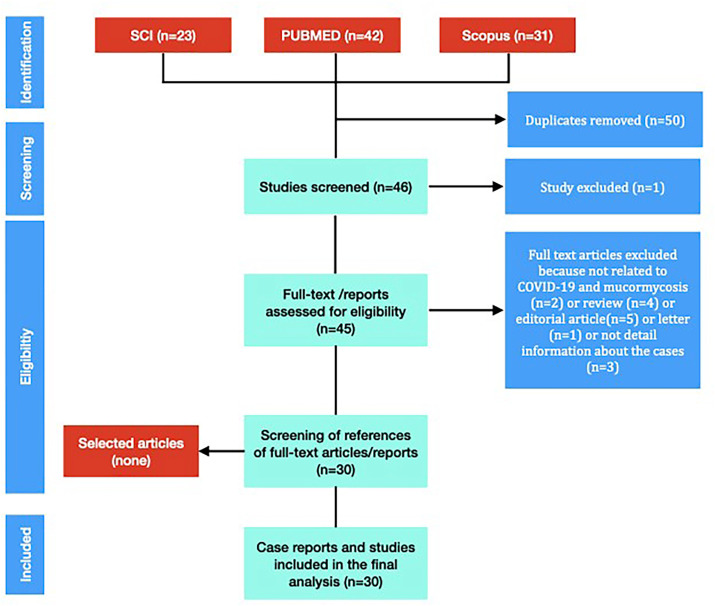
Literature search was performed in PubMed, PUBMED, Web of Science, and Scopus according to the PRISMA guidelines [9]. Papers published in any language between December 1, 2019, to June 1, 2021, were included. The literature was searched using keywords of [(COVID 19 OR Coronavirus OR corona) AND (mucormycosis OR mucor)]. The EndNote database was used from importing and managing abstracts and full texts. After first evaluation of the paper, duplicates were removed. Full text papers were evaluated and selected by two independent authors (D.A., M.S.) (Fig. 1 ). All the authors approved this selection process.
Fig. 1.
Review process of medical literature about COVID-19 and mucormycosis.
2.2. Inclusion criteria
Case reports, case series, and observational studies describing the incidence, clinical features, and outcomes of mucormycosis developed in COVID-19 patients were included in the systematic review. Case reports without clinical and laboratory features were excluded. Demographic features, risk factors, clinical features, diagnostic methods, treatment and outcome were analyzed.
2.3. Case definition
Mucormycosis cases were classified as “possible”, “probable” or “proven” according to the recently published guideline of “Code Mucor: Guidelines for the Diagnosis, Staging and Management of Rhino-Orbito-Cerebral Mucormycosis in the Setting of COVID-19” [8]. This guideline describes rhino-orbito-cerebral mucormycosis. For the patients with involvement other than these, the criterion of “probable” was modified to include organ-specific endoscopic procedures and MRI or CT imaging studies.
In brief, among patients with proven COVID-19 infections, presence of typical clinical mucormycosis findings is described as “possible”; in addition to these, presence of nasal/pulmonary/gastrointestinal endoscopic or MRI/CT findings compatible with mucormycosis is described as “probable”; and in addition to these, if mucormycosis is proven by microbiologic, histologic, and molecular methods, it is classified as “proven”. All “probable” and “proven” cases were included into the review.
2.4. Statistical analysis
Data were analyzed using SPSS software, version 20.0 (IBM Corp, Armonk, NY). Descriptive data were presented as mean ± standard deviation or percent. Continuous and categorical variables were compared by Student's t-test and chi-square respectively. p < 0.05 was taken statistically significant.
3. Results
3.1. Case report
A 54-year-old male patient was hospitalized 45 days ago due to severe COVID-19 pneumonia. During the hospitalization and especially in the intensive care unit, systemic steroid was administered parenterally. Daily 1 gr methylprednisolone was given at first 3 days of respiratory failure. He was discharged with oral methylprednisolone and it was ceased in the 15th day of treatment (Fig. 2 ). Two days later, he was admitted to the hospital with severe headache, imbalance, visual impairment and edema evident in right side of the face. He was conscious yet not fully cooperating. Intermittent loss of consciousness was observed. In the MRI images, an opacity which occupy the right maxillary sinus was detected (Fig. 3 ). Following the deterioration in general status, the patient was intubated. During the intubation, dark-colored necrotizing plaques were seen in the roof of the oral cavity. The biopsy taken from these plaques established the diagnosis of mucormycosis. He died due to mucormycosis two months after the COVID-19 infection.
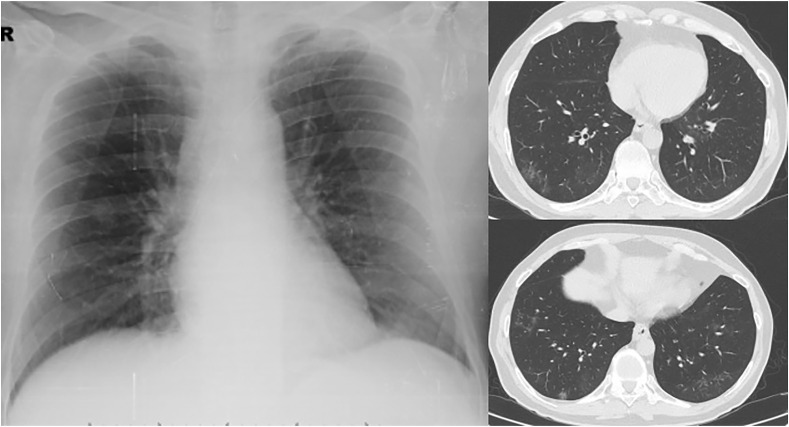
Fig. 2.
Chest roentgenography is showing as normal but HRCT images showing the fibrotic changes after the COVID 19 infection.
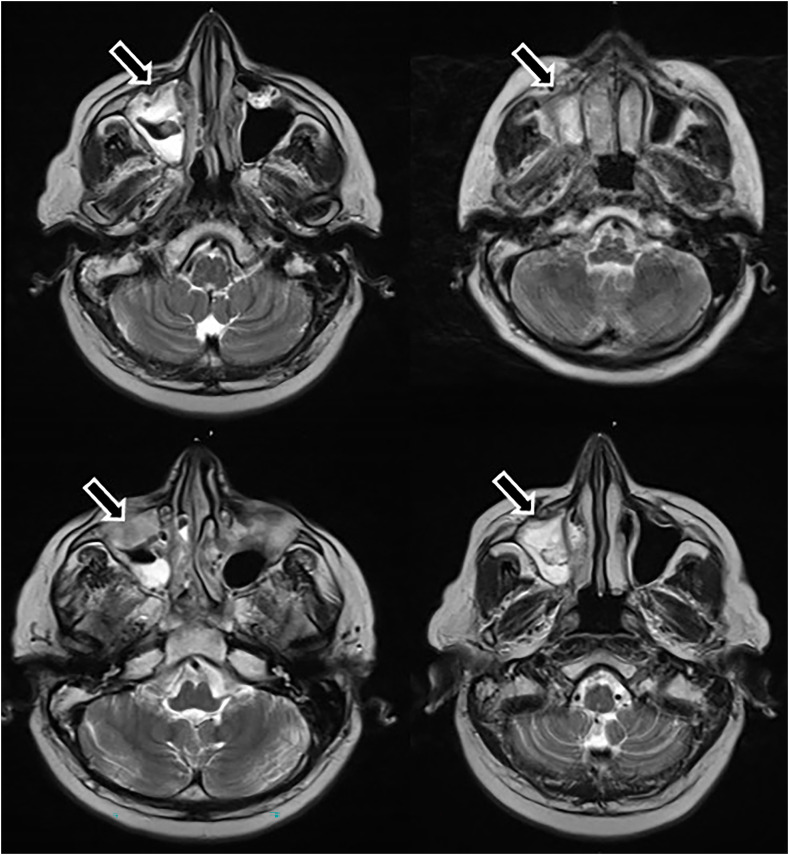
Fig. 3.
Cranial MRI images showing the irregular opacities in the right maxillary sinus (arrows) and just adjacent to orbital & oral cavity which consisted with mucormycosis.
3.2. Review on published cases of COVID 19 and mucormycosis
Our literature search of databases (PUBMED, Web of Science, and Scopus) yielded 44 reports. Unrelated publications were omitted and remaining 30 publications (24 case reports and 6 case series) were evaluated. (Fig. 1). All case reports have been reported after 2020 following COVID-19 pandemic. Papers were published in 2020 (n = 7) and in 2021 (n = 23).
The papers (case reports and case series) reported 100 patients including present case report (Table 1 ) [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]]. The majority of the patients were presented from India (7 case reports and 5 case series, a total of 68, 68%), followed by Turkey (12), USA (9), Iran (3), Spain (2), United Kingdom (1), Brasil (1), Italy (1), France (1), Mexico (1), and Austria (1). Among case series, five were retrospective and one was prospective. Range of age was 22 years–86 years. Seventy six percent were male. At least one risk factor was noted in 94% of reported cases.
Table 1.
Studies and clinical characteristics, treatment and outcomes in patients with COVID-19-associated mucormycosis.
| Author(s) | References | Year | Country | Publication type | Case numbers | Age (years) | Gender | Systemic diseases | Pulmonary involvement of COVID-19 | Type of Mucormycosis | Diagnosis | Probable or Proven | Post COVID | Steroid use | Antibiotic use | Tocilizumab use | Medical Treatment |
Surgical treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hanley B et al. | [10] | 2020 | UK | Case | 1 | 22 | M | No | Yes ARDS |
Disseminated | Post mortem | Proven | No | NA | NA | NA | NA | NA | Death |
| 2 | Mehta S et al. | [11] | 2020 | India | Case | 1 | 60 | M | DM | Yes ARDS |
Rhino-orbital | Nasal biopsy and culture | Proven | No | Yes | Meropenem, oseltamivir | Yes (400 mg) | Amphotericin B | No | Death |
| 3 | Mekonnen ZK et al. | [12] | 2020 | USA | Case | 1 | 60 | M | DM, asthma, HT, hyperlipidemia | Yes ARDS |
Rhino-orbital | Biopsy and culture: Rhizopus spp. |
Proven | No | Yes | Vancomycin, cefepime | NA | Amphotericin B, caspofungin added, then switched from amphotericin B to posaconazole | Surgical debridement | Death |
| 4 | Monte Junior ESD et al. | [13] | 2020 | Brasil | Case | 1 | 86 | M | HT | Yes | Gastrointestinal | Pathological | Proven | No | Yes | Ceftriaxone, azithromycin, oseltamivir | NA | No | No | Death |
| 5 | Pasero D et al. | [14] | 2020 | Italy | Case | 1 | 66 | M | HT | Yes | Pulmonary | Bronchial aspirate, BAL: Rhizopus spp. |
Proven | No | No | Piperacillin/tazobactam, levofloxacin | No | Amphotericin B, then isavuconazole | No | Death |
| 6 | Placik DA et al. | [15] | 2020 | USA | Case | 1 | 49 | M | No | Yes | Pulmonary | Microbiological analysis: Rhizopus spp. |
Proven | No | Yes | Ceftriaxone, azithromycin | Yes | Amphotericin B | Thoracotomy: the affected area was repaired and resected | Death |
| 7 | Werthman-Ehrenreich A | [16] | 2020 | USA | Case | 1 | 33 | F | HT, asthma | Yes | Rhino-orbito-cerebral | Sinus cultures | Proven | No | No | Piperacillin/tazobactam, vancomycin | No | Amphotericin B | Sinus debridement | Death |
| 8 | Alekseyev K et al. | [17] | 2021 | USA | Case | 1 | 41 | M | DM | Yes | Rhino-cerebral | Radiological | Probable | No | Yes | NA | No | Amphotericin B | Surgical debridement | Alive |
| 9 | Arana C et al. | [18] | 2021 | Spain | Two cases | 2 | 48, 62 |
2 M | DM (n = 1), HT (n = 2), kidney transplantation (n = 1), CRF (n = 1) | Yes | Rhino-sinusal (n = 1) Musculoskeletal (n = 1) |
Culture from the necrotic tissue | Proven (n = 2) | Yes (n = 1) No (n = 1) |
Yes (n = 2) | Ceftriaxone, azithromycin (n = 1) Azithromycin (n = 1) |
Yes (n = 1) 400 mg No (n = 1) |
Amphotericin B (n = 2) and initially isavuconazole and subsequently posaconazole (n = 1) | Surgical debridement (n = 2) | Alive (n = 2) |
| 10 | Bayram N et al. | [19] | 2021 | Turkey | Case series | 11 | 73.1 (61–88) | M (n = 9) F (n = 2) |
DM (n = 8), HT (n = 7), renal failure (n = 5) | Yes ARDS (n = 11) |
Rhino-orbital (n = 8) Rhino-orbito-cerebral (n = 3) |
Culture (n = 11) | Proven (n = 11) | No (n = 11) | Yes (n = 11) | NA | Yes (n = 2) | Amphotericin B (n = 11) | Radical debridement (n = 11) | Death (n = 7) Alive (n = 4) |
| 11 | Bellanger AP et al. | [20] | 2021 | France | Case | 1 | 55 | M | Lymphoma | ARDS | Pulmonary | A. fumigatus, Rhizopus microsporus | Proven | No | NA | NA | No | Amphotericin B | No | Death |
| 12 | Dallalzadeh LO et al. | [21] | 2021 | USA | Case | 2 | 36, 48 |
M (n = 2) | DM (type 2) (n = 2) | Yes (n = 2) | Rhino-orbito-cerebral (n = 2) | 1 radiologic, 1 culture | Probable (n = 1) Proven (n = 1) |
No | Yes (n = 1) No (n = 1) |
Oral antibiotics (n = 1) NM (n = 1) |
No | Amphotericin B (n = 2), isovuconazole (n = 2), and micafungin (n = 1) | Surgical debridement was deferred | Alive (n = 2) |
| 13 | Garg D et al. | [22] | 2021 | India | Case and systematic review | 1 | 55 | M | DM, HT, ischemic cardiomyopathy, end-stage kidney disease | Yes | Pulmonary | Mycological: Rhizopus microsporus | Proven | Yes | Yes | Meropenem | No | Amphotericin B | No | Alive |
| 14 | Johnson AK et al. | [23] | 2021 | USA | Case | 1 | 79 | M | DM HT |
Yes | Pulmonary | BAL culture: Rhizopus arrhizus |
Proven | Yes | Yes | Ceftriaxone, azithromycin | No | Voriconazole, then amphotericin B | No | Alive |
| 15 | Kanwar A et al. | [24] | 2021 | USA | Case | 1 | 56 | M | End-stage kidney disease | Yes ARDS |
Pulmonary | Culture | Proven | Yes | Yes | Piperacillin/tazobactam, vancomycin | Yes (single dose) | Amphotericin B | No | Death |
| 16 | Karimi-Galougahi M et al. | [25] | 2021 | Iran | Case | 1 | 61 | F | DM | Yes | Rhino-orbital | Histopathology | Proven | Yes | Yes | NA | No | Systemic antifungal | Debridement | Alive |
| 17 | Khatri A et al. | [26] | 2021 | USA | Case and review of literature | 1 | 68 | M | Orthotopic heart transplantation, CAD |
Yes | Pulmonary | Culture: Rhizopus microsporus | Proven | Yes | Yes | Vancomycin, meropenem | No | Amphotericin B, posaconazole | Surgical debridement | Death |
| 18 | Maini A et al. | [27] | 2021 | India | Case | 1 | 38 | M | No | Yes | Rhino-orbital | Culture: Rhizopus oryzae | Proven | Yes | Yes | Piperacillin/tazobactam, metronidazole | No | Amphotericin B | Surgical debridement | Alive |
| 19 | Moorthy A et al. | [28] | 2021 | India | Cases series | 17 | 54.6 (35–73) | M (n = 15) F (n = 2) |
DM (n = 15) | Yes | Rhino-orbital (n = 6), rhino-orbito-cerebral (n = 5), rhino-cerebral (n = 3), Rhino-sinusal (n = 3) | KOH test and culture (n = 17) | Proven (n = 17) | Yes (n = 14), no (n = 3) | Yes (n = 15) No (n = 2) |
NA | No | Amphotericin B (n = 17) | Surgical (n = 17) | Alive (n = 10), death (n = 6), NA (n = 1) |
| 20 | Nehara HR et al. | [29] | 2021 | India | Case series | 5 | 62.2 (52–70) | M (n = 1) F (n = 4) |
DM (type 2) (n = 5), HT (n = 2) | Yes | Rhino-orbito-cerebral (n = 5) | LCB & KOH Mount of Nasal Culture (n = 5) |
Proven (n = 5) | No (n = 5) | Yes (n = 4), NA (n = 1) | Antibiotics (n = 5) | No | Amphotericin B (n = 5) | Debridement (n = 2), no (n = 3) | Death (n = 2), alive (n = 3) |
| 21 | Rao R et al. | [30] | 2021 | India | Case | 1 | 66 | M | DM | Yes | Rhino-orbital | Nasal swab confirmed (KOH) | Proven | No | Yes | NA | No | Amphotericin B | Orbital exenteration | Alive |
| 22 | Revannavar SM et al. | [31] | 2021 | India | Case | 1 | Middle-aged | F | DM | No | Rhino-orbito-cerebral | FESS culture: Rhizopus spp. |
Proven | No | No | NA | No | Amphotericin B | FESS | Alive |
| 23 | Sai Krishna D et al. | [32] | 2021 | India | Two cases | 2 | 34, 50 |
M (n = 2) | DM (type 2) (n = 2), HT (n = 1) | Yes | Maxillo-facial (n = 2) | Maxillary biopsy + histopathological examination (n = 2) | Proven (n = 2) | Yes (n = 2) | No (n = 2) | Antibiotics (n = 2) | No | Amphotericin B (n = 2) + then itraconazole (n = 1), posaconazole (n = 1) | Surgical resection (n = 2) | Alive (n = 2) |
| 24 | Saldanha M et al. | [33] | 2021 | India | Case | 1 | 32 | F | DM | NA | Rhino-orbital | Histopathological examination | Proven | No | NA | NA | No | Conventional amphotericin B | Endoscopic sinus surgery | Alive |
| 25 | Sarkar S et al. | [34] | 2021 | India | Cases series | 10 | 45.5 (23–67) | M (n = 8) F (n = 2) |
DM (n = 10) | Yes | Rhino-orbital (n = 10) | Radiological (n = 4), tissue biopsy (n = 4), nasal swab (n = 2) | Probable (n = 4), proven (n = 6) | No | Yes (n = 10) | NA | No | Amphotericin B (n = 10) | Debridement and/or surgery (n = 7), no surgery (n = 3) | Death (n = 4), alive n = 6) |
| 26 | Sen M et al. | [35] | 2021 | India | Case Series | 5 | 58 (46–73) | M (n = 5) | DM (n = 5), HT (n = 2), CAD (n = 1) | Yes | Rhino-orbito-cerebral (n = 1) Rhino-orbital (n = 4) |
Histopathology | Proven (n = 5) | Yes (n = 4), no (n = 1) | Yes (n = 4), no (n = 1) | Cefoperazone/sulbactam (n = 1) Systemic antibiotics (n = 4) |
NA | Amphotericin B | FESS | Alive (n = 5) |
| 27 | Sharma S et al. | [36] | 2021 | India | Case series | 23 | NA | M (n = 15) F (n = 8) |
DM (n = 21), HT (n = 14), renal failure (n = 1) | No | Rhino-orbito-cerebral (n = 2) Rhino-orbital (n = 8) Rhino-sinusal (n = 13) |
Radiological | Probable (n = 23) | Yes (n = 19) No (n = 4) |
Yes (n = 23) | NA | No | Amphotericin B (n = 23) | 23 surgical debridement | Alive (n = 23) |
| 28 | Veisi A et al. | [37] | 2021 | Iran | Case | 2 | 54, 40 |
M (n = 1) F (n = 1) |
DM (n = 1) | Yes | Rhino-orbital (n = 1) Rhino-orbito-cerebral (n = 1) |
Histopathologic and radiologic (n = 2) | Proven (n = 2) | No (n = 2) | Yes (n = 2) | Meropenem, vancomycin (n = 1) Levofloxacin then piperacillin/tazobactam, vancomycin (n = 1) |
No | Amphotericin B | Endoscopic debridement | Death (n = 1), alive (n = 1) |
| 29 | Waizel-Haiat S et al. | [38] | 2021 | Mexico | Case | 1 | 24 | F | Obesity | Yes | Rhino-orbital | Culture: Lichtheimia (Absidia) spp |
Proven | No | NA | Amoxicillin-clavulanate (n = 1) | NA | Amphotericin B | No | Death |
| 30 | Zurl C et al. | [39] | 2021 | Austria | Case | 1 | 53 | M | MDS, obesity and depression | Yes ARDS |
Pulmonary | Autopsy: Rhizopus microsporus | Proven | No | Yes | Piperacillin/tazobactam, linezolid (n = 1) | Yes | Intravenous voriconazole | No | Death |
| 31 | Current report | 2021 | Turkey | Case | 1 | 54 | M | No | Yes | Maxillo-fascial | Histopathologic and radiologic | Proven | Yes | Yes | Ampicillin/sulbactam, clindamycin (n = 1) | No | No | No | Death |
HT: hypertension, UK: United Kingdom, ARDS: acute respiratory distress syndrome, NA: not available, DM: diabetes mellitus, USA: United States of America, BAL: bronchoalveolar lavage, CAD: coronary artery disease, FESS: functional endoscopic sinus surgery, CRF: chronic renal failure, KOH: potassium hydroxide, LCB: lactophenol cotton blue, MSD: myelodysplastic syndrome.
The most commonly seen comorbidities were diabetes (n = 79, 79%), followed by hypertension (n = 34, 34%), and chronic kidney disease (n = 8, 8%). Obesity was noted in 2 cases (2%). Corticosteroid use for COVID-19 diseases was not defined for 5 patients; not used for 9 patients and was used for 86 patients (90.5%). However, the doses were not clearly defined. Antibiotic data were available for 32 cases: 21 were given broad-spectrum antibiotics, in 11 cases, the type of antibiotic was not mentioned. Mucormycosis developed during COVID-19 infection in 53%, while it developed in post-COVID-19 period in remaining 47%. Among these patients, 16 and 1 patients had severe COVID-19 or ARDS in the former and the latter groups respectively. Most frequent involvement sites of mucormycosis were rhino-orbital in 50 (50%), rhino-sinusal in 17 (17%), and rhino-orbito-cerebral in 15 (15%) (Table 2 ).
Table 2.
Involvement sites of mucormycosis in COVID-19 co-infected patients.
| Involment site | Number of patients |
|---|---|
| Rhino-orbital | 50 |
| Rhino-sinusal | 17 |
| Rhino-orbito-cerebral | 15 |
| Pulmonary | 8 |
| Rhino-cerebral | 4 |
| Maxilllo-facial | 3 |
| Disseminated | 1 |
| Gastrointestinal | 1 |
| Musculoskeletal | 1 |
The diagnosis was established postmortem in two. The diagnoses were classified proven in 71 (71%) and probable in 29 (29%). Death was reported 33 out of 99 patients (33,3%); one patient's outcome was not described.
4. Discussion
Our literature search found 99 patients with COVID-19-associated mucormycosis. Patients were given steroid and had diabetes in the majority. The reports were mainly from India. The eye and/or brain involvement was seen in 72%. This deadly combination of COVID-19 and mucormycosis caused death of nearly one third of the patients.
Mucormycosis is extremely rare in otherwise healthy individuals, while it is seen patients with predisposing conditions including uncontrolled diabetes (with or without diabetic ketoacidosis), hematological and other malignancies, organ transplantation, prolonged neutropenia, immunosuppressive and corticosteroid use, iron overload or hemochromatosis, deferoxamine therapy, severe burns, acquired immunodeficiency syndrome (AIDS), intravenous drug abusers, and open wound following trauma [40]. Recent accumulating reports suggested an increasing prevalence of mucormycosis in COVID-19 patients. In the pathophysiology of mucormycosis in COVID-19 patients, beside the evident roles of ketoacidosis, high blood sugar levels, iron metabolism, long-term use of antibiotics, steroid use and mechanical ventilation of the host, some other factors were suggested to play a role: the role of ferritin which is high in most of the COVID-19 cases, high serum iron, endothelitis induced by free radicals, hepcidin activation through viral mimicry, and upregulation of glucose receptor protein (GRP78) [41].
Increasing mucormycosis cases may be partially explained by increasing steroid use in COVID-19 patients. Steroid use was accelerated after publication of randomized-controlled trial of RECOVERY study [40]. The study showed that for patients hospitalized with COVID-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone. Although steroids have no benefit in patients who do not require respiratory support in the trial, many patients with COVID-19 not requiring mechanical ventilation have been treated with glucocorticoids, even with higher doses and longer durations than recommended in the trial [42]. In the current analysis, 90.5% of patients with mucormycosis and COVID-19 were given steroids (Table 1). Diabetes is another risk factor for mucormycosis. Beside already high or uncontrolled glucose levels in diabetics, steroid use induced high blood glucose levels in persons with prediabetic and diabetic patients. In the reviewed patients, 79% had diabetes at presentation with fungal infection (Table 1).
The vast majority of COVID-19-induced mucormycosis cases was reported from India. Mucormycosis cases were already increasing in India before the pandemic [43]. Many Indian centres have published series of mucormysis in patients with varying risk factors. This high incidence has been primarily linked to increase in patient population with uncontrolled diabetes [43]. India has the second largest diabetic population of the world (65.1 million), and nearly 70% of these diabetics are uncontrolled [43].
Environmental factors of tropical and sub-tropical humid climate and high environmental temperature in most parts of India also appeared to contribute to the disease prevalence [44]. COVID-19 pandemic added new risk factors to increasing mucormycosis pandemic. India is currently experiencing another wave of the COVID-19 and it challenged the health care system of the country. During the COVID-19 pandemic, India experienced another pandemic of mucormycosis. The significantly higher prevalence of COVID-19-induced mucormycosis in India may be multifactiorial, including the roles of poorly controlled diabetes, excessive use of corticosteroids and possibly antibiotics, and environmental exposure [45]. The hot and humid environment India may have promoted growth of Mucorales species [42].
Hypoxia of the tissues in COVID-19 disease can be another contributing factor. Low oxygen levels in the tissues in addition to the partial infraction of fungal angioinvasion deepens the tissue damage. Also, overuse of antibiotics which is common in COVID-19 management suppresses the normal bacterial flora and facilitates establishment and invasion of fungi. In this systematic review it is shown that broad-spectrum antibiotic use is common in cases of COVID-19 with mucormycosis. Langford et al. [46] found that the prevalence of antibiotics use was 74.6% in COVID cases. Analysis of the registry, SEMI-COVID showed that 78.1% of COVID patients were prescribed antibiotics whereas 34% of antibiotic prescriptions were inappropriate [47]. Although use of antibiotics has been shown ineffective, an estimated 216 million excess doses antibiotics and 6.2 million azithromycin treatment courses were attributed to COVID-19 during the first wave of COVID-19 in India [42].
The treatment of COVID-19-associated mucormycosis includes a timely combination of surgery and antifungal therapy. Surgery is sinonasal debridement in most of the cases. Pal et al. [48] compared deceased and survived COVID-19 mucormycosis patients in their systematic review and showed that surgery combined with antifungal therapy was associated with higher survival rates.
5. Conclusion
Current literature review showed that mucormycosis in COVID-19 context is a growing challenge. The majority of the patients are reported from India. Beside ongoing risks of mucormycosis including high incidence of uncontrolled diabetes and environmental conditions, COVID-19 pandemic added new factors such as steroid use, excessive use of antibiotics, and hypoxia. Despite medical and surgical treatment, mortality rate is high. Therefore, clinical guidelines should be implanted for appropriate use of antibiotics in COVID-19 cases. A multidisciplinary approach is essential to improve the conditions facilitating the emergence of mucormycosis among COVID-19 patients.
Funding
There was no specific funding for this project.
Conflict of interest/funding/role of the funding source
All authors declare no conflict of interest.
CRediT authorship contribution statement
Ahmet Dilek and Mustafa Sunbul: Screened all papers, compiled the tables, revising the manuscript critically for important intellectual content. All co-authors contributed to, and endorsed, the final version of the manuscript.
Resat Ozaras and Elif Itir Sen: Writing - original draft, revising the manuscript critically for important intellectual content. All co-authors contributed to, and endorsed, the final version of the manuscript.
Sevket Ozkaya: The patient's pulmonology physician. Writing - original draft, revising the manuscript critically for important intellectual content. All co-authors contributed to, and endorsed, the final version of the manuscript.
Hakan Leblebicioglu: Senior author. Writing - original draft, designed the study, conducted the literature searches, revising the manuscript critically for important intellectual content All co-authors contributed to, and endorsed, the final version of the manuscript.
References
- 1.Bhatt K., Agolli A., Patel M.H., et al. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries. 2021;9:e126. doi: 10.15190/d.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song G., Liang G., Liu W. Fungal Co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185:599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thevissen K., Jacobs C., Holtappels M., Toda M., Verweij P., Wauters J. International survey on influenza-associated pulmonary aspergillosis (IAPA) in intensive care units: responses suggest low awareness and potential underdiagnosis outside Europe. Crit Care. 2020;24:84. doi: 10.1186/s13054-020-2808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauwvlieghe A., Rijnders B.J.A., Philips N., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 5.Mitaka H., Kuno T., Takagi H., Patrawalla P. Incidence and mortality of COVID-19-associated pulmonary aspergillosis: a systematic review and meta-analysis. Mycoses. 2021;64(9):993–1001. doi: 10.1111/myc.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravani S.A., Agrawal G.A., Leuva P.A., Modi P.H., Amin K.D. Rise of the phoenix: mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69:1563–1568. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong W.H., Neu K.P. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): a systematic review. J Hosp Infect. 2021;113:115–129. doi: 10.1016/j.jhin.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honavar S.G. Code mucor: guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69:1361–1365. doi: 10.4103/ijo.IJO_1165_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanley B., Naresh K.N., Roufosse C., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mekonnen Z.K., Ashraf D.C., Jankowski T., et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021;37:e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monte Junior E.S.D., Santos M., Ribeiro I.B., et al. Rare and fatal gastrointestinal mucormycosis (zygomycosis) in a COVID-19 patient: a case report. Clin Endosc. 2020;53:746–749. doi: 10.5946/ce.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasero D., Sanna S., Liperi C., et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020:1–6. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Placik D.A., Taylor W.L., Wnuk N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol Case Rep. 2020;15:2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264 e5–e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alekseyev K., Didenko L., Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases. 2021;12:85–89. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arana C., Cuevas Ramirez R.E., Xipell M., et al. Mucormycosis associated with covid19 in two kidney transplant patients. Transpl Infect Dis. 2021 doi: 10.1111/tid.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayram N., Ozsaygili C., Sav H., et al. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65(4):515–525. doi: 10.1007/s10384-021-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellanger A.P., Navellou J.C., Lepiller Q., et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect Dis News. 2021:S2666–9919–0. doi: 10.1016/j.idnow.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallalzadeh L.O., Ozzello D.J., Liu C.Y., Kikkawa D.O., Korn B.S. Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit. 2021:1–4. doi: 10.1080/01676830.2021.1903044. [DOI] [PubMed] [Google Scholar]
- 22.Garg D., Muthu V., Sehgal I.S., et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186:289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson A.K., Ghazarian Z., Cendrowski K.D., Persichino J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanwar A., Jordan A., Olewiler S., Wehberg K., Cortes M., Jackson B.R. A fatal case of rhizopus azygosporus pneumonia following COVID-19. J Fungi (Basel) 2021;7 doi: 10.3390/jof7030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimi-Galougahi M., Arastou S., Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19) Int Forum Allergy Rhinol. 2021;11:1029–1030. doi: 10.1002/alr.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khatri A., Chang K.M., Berlinrut I., Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient - case report and review of literature. J Mycol Med. 2021;31:101125. doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maini A., Tomar G., Khanna D., Kini Y., Mehta H., Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep. 2021;82:105957. doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorthy A., Gaikwad R., Krishna S., et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021:1–8. doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nehara H.R., Puri I., Singhal V., Ih S., Bishnoi B.R., Sirohi P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: case series from the north-western part of India. Indian J Med Microbiol. 2021;39(3):380–383. doi: 10.1016/j.ijmmb.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao R., Shetty A.P., Nagesh C.P. Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of COVID-19: clinico-radiological features. Indian J Ophthalmol. 2021;69:1627–1630. doi: 10.4103/ijo.IJO_1053_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Revannavar S.M., P S.S., Samaga L., V K.V. COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sai Krishna D., Raj H., Kurup P., Juneja M. Maxillofacial infections in covid-19 era-actuality or the unforeseen: 2 case reports. Indian J Otolaryngol Head Neck Surg. 2021:1–4. doi: 10.1007/s12070-021-02618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saldanha M., Reddy R., Vincent M.J. Title of the article: paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg. 2021:1–4. doi: 10.1007/s12070-021-02574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarkar S., Gokhale T., Choudhury S.S., Deb A.K. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002–1004. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen M., Lahane S., Lahane T.P., Parekh R., Honavar S.G. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69:244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021:1–6. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veisi A., Bagheri A., Eshaghi M., Rikhtehgar M.H., Rezaei Kanavi M., Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur J Ophthalmol. 2021 doi: 10.1177/11206721211009450. 11206721211009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waizel-Haiat S., Guerrero-Paz J.A., Sanchez-Hurtado L., Calleja-Alarcon S., Romero-Gutierrez L. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021;13 doi: 10.7759/cureus.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zurl C., Hoenigl M., Schulz E., et al. Autopsy proven pulmonary mucormycosis due to rhizopus microsporus in a critically ill COVID-19 patient with underlying hematological malignancy. J Fungi (Basel) 2021;7 doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugar A.M. Mucormycosis. Clin Infect Dis. 1992;14(Suppl 1):S126–S129. doi: 10.1093/clinids/14.supplement_1.s126. [DOI] [PubMed] [Google Scholar]
- 41.Jose A., Singh S., Roychoudhury A., Kholakiya Y., Arya S., Roychoudhury S. Current understanding in the pathophysiology of SARS-CoV-2-associated rhino-orbito-cerebral mucormycosis: a comprehensive review. J Maxillofac Oral Surg. 2021:1–8. doi: 10.1007/s12663-021-01604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandra S., Ram S., Levitz S.M. The “black fungus” in India: the emerging syndemic of COVID-19-associated mucormycosis. Ann Intern Med. 2021:M21–2354. doi: 10.7326/m21-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakrabarti A., Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57(Suppl 3):85–90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- 44.Chakrabarti A., Singh R. The emerging epidemiology of mould infections in developing countries. Curr Opin Infect Dis. 2011;24:521–526. doi: 10.1097/QCO.0b013e32834ab21e. [DOI] [PubMed] [Google Scholar]
- 45.Singh A.A., Singh R., Joshi S.R., Misrac A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4):102146. doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langford B.J., So M., Raybardhan S., et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calderon-Parra J., Muino-Miguez A., Bendala-Estrada A.D., et al. Inappropriate antibiotic use in the COVID-19 era: factors associated with inappropriate prescribing and secondary complications. Analysis of the registry SEMI-COVID. PloS One. 2021;16 doi: 10.1371/journal.pone.0251340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal R., Singh B., Bhadada S.K., et al. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021 doi: 10.1111/myc.13338. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]