Abstract
In cell culture systems, the TCF Elk-1 represents a convergence point for extracellular signal-related kinase (ERK) and c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) subclasses of mitogen-activated protein kinase (MAPK) cascades. Its phosphorylation strongly potentiates its ability to activate transcription of the c-fos promoter through a ternary complex assembled on the c-fos serum response element. In rat brain postmitotic neurons, Elk-1 is strongly expressed (V. Sgambato, P. Vanhoutte, C. Pagès, M. Rogard, R. A. Hipskind, M. J. Besson, and J. Caboche, J. Neurosci. 18:214–226, 1998). However, its physiological role in these postmitotic neurons remains to be established. To investigate biochemically the signaling pathways targeting Elk-1 and c-fos in mature neurons, we used a semi-in vivo system composed of brain slices stimulated with the excitatory neurotransmitter glutamate. Glutamate treatment leads to a robust, progressive activation of the ERK and JNK/SAPK MAPK cascades. This corresponds kinetically to a significant increase in Ser383-phosphorylated Elk-1 and the appearance of c-fos mRNA. Glutamate also causes increased levels of Ser133-phosphorylated cyclic AMP-responsive element-binding protein (CREB) but only transiently relative to Elk-1 and c-fos. ERK and Elk-1 phosphorylation are blocked by the MAPK kinase inhibitor PD98059, indicating the primary role of the ERK cascade in mediating glutamate signaling to Elk-1 in the rat striatum in vivo. Glutamate-mediated CREB phosphorylation is also inhibited by PD98059 treatment. Interestingly, KN62, which interferes with calcium-calmodulin kinase (CaM-K) activity, leads to a reduction of glutamate-induced ERK activation and of CREB phosphorylation. These data indicate that ERK functions as a common component in two signaling pathways (ERK/Elk-1 and ERK/?/CREB) converging on the c-fos promoter in postmitotic neuronal cells and that CaM-Ks act as positive regulators of these pathways.
In many cell types, extracellular stimuli, such as serum, growth factors, phorbol esters, neurotransmitters, cytokines, Ca2+, UV light, and redox agents, regulate critical cellular events, such as growth, differentiation and apoptosis through activation of protein kinase cascades. Many of these stimuli trigger mitogen-activated protein kinase (MAPK) cascades through initial activation of their receptor-associated tyrosine kinases and subsequent phosphorylation of other intracellular substrates. In mammalian cells, three MAPK cascades, which regulate the activity of the extracellular signal-regulated kinase (ERK) subclass, the closely related c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), and p38 MAPKs are characterized at present (for a review, see reference 85). These cascades are activated by different extracellular signals, principally mitogens for ERKs (7) and various stress stimuli for JNK/SAPK and p38 MAPKs (9, 62). One of the common intracellular responses to MAPK activation is alteration of gene expression, since many MAPK substrates are transcription factors (for a review, see reference 84).
In the brain, excitatory neurotransmission elevates the calcium concentration in neuronal cells and activates the transcription of immediate-early (IE) genes, including c-fos (26, 30, 73). The c-fos promoter contains several regulatory elements that are important for its transcriptional response to calcium (for a review, see reference 31). These include the cyclic AMP (cAMP)/Ca2+-responsive element (Ca/CRE) and the serum response element (SRE), which are located approximately 60 nucleotides (CRE) and 310 nucleotides (SRE) 5′ of the initiation site of c-fos mRNA synthesis. The CRE is bound constitutively by members of the CREB/ATF family of bZIP transcription factors. CREB activation in response to increased intracellular levels of cAMP or Ca2+ involves the inducible phosphorylation of Ser133 by cAMP-dependent kinases (protein kinase A) or by calcium/calmodulin kinases (CaM-Ks) (13, 74, 75). The c-fos SRE, together with flanking DNA sequences, serves as the site of assembly of multiprotein complexes that include a dimer of serum response factor (SRF) (58, 68, 81) and ternary complex factor (TCF) (72; reviewed in reference 82 and 83). The TCF subgroup of the ETS protein family contains at least three members: Elk-1, SAP1, and SAP2/ERP/Net (12, 32, 37, 51, 64). One signature motif of the TCF proteins is a 20-amino-acid sequence which mediates protein-protein interaction with the SRF protein (76) and thus promotes TCF binding to the c-fos SRE. Although SRF is phosphorylated upon growth factor stimulation (66), no evidence directly links this phosphorylation to c-fos transcription. In contrast, phosphorylation of the TCFs by MAPKs increases the level of ternary complexes formed in vitro together with SRF on the SRE and potentiates TCF-driven activation of c-fos transcription (27–29, 36, 38, 39, 44, 45, 52, 63, 86, 87, 93).
Although TCF/Elk-1 is strongly expressed in the adult central nervous system (CNS) (32, 73) and this expression is exclusively neuronal (71), its physiological role in these postmitotic cells remains to be established. To investigate this, we developed an in vivo model system of sustained electrical stimulation of glutamatergic cortical afferents. In this model, we found a strong correlation between ERK activation, Elk-1 phosphorylation, and IE gene induction in the projection field of the stimulated cortical area, the striatum (71). Here we set out to biochemically characterize these observations. We have exploited a model system involving c-fos induction by glutamate treatment of striatal slices, which allowed us to readily analyze the kinetics of Elk-1 phosphorylation relative to those of CREB and to trace intracellular signaling pathways targeting these transcription factors. We show that glutamate generates strong phosphorylation of Elk-1 that appears progressively in a strict correspondence with ERK and JNK activation. Glutamate also causes phosphorylation of CREB but only transiently relative to Elk-1. Interestingly, the complete inhibition of ERK activity by PD98059 abolishes glutamate-induced phosphorylation of both Elk-1 and CREB, as well as the induction of c-Fos. Inhibition of CaM-K activity with KN62 also results in decreased phosphorylation of CREB indirectly via the inhibition of ERK activity. This shows that ERK plays a pivotal role in the control of calcium-induced c-fos expression via the modules ERK/Elk-1 and ERK/?/CREB, which converge on the c-fos promoter in the brain and that CaM-K is an upstream regulator of this signaling network.
MATERIALS AND METHODS
Rat striatal slices.
Rat striatal slices (300 μm thick) were prepared as previously described (65) from young adult male Sprague-Dawley rats (weighing 80 to 120 g) (Janvier, Saint Berthevir, France) with a Vibratome (Campden Instruments, London, United Kingdom) (coordinates 2.2 anterior to bregma to 0.3 posterior to bregma according to the atlas of Paxinos and Watson [59]). The slices were placed in superfusion chambers (two slices per chamber) and continuously superfused with Krebs-Ringer solution (11.1 mM glucose, 1.1 mM MgCl2, 1 mM Na2HPO4, 1.3 mM CaCl2, 25 mM NaHCO3, 1.3 mM NaCl, 4.7 mM KCl) saturated with 95% O2–5% CO2 at 37°C. Krebs superfusion was applied for 60 min before pharmacological treatment to prevent initial neuronal firing due to the slicing procedure. At the end of the experiment, striatal slices were rapidly removed from superfusion chambers and immediately lysed in solubilization buffer (10 mM Tris-Cl, 50 mM NaCl, 1% Triton X-100, 30 mM sodium pyrophosphate, 50 mM NaF, 5 μM ZnCl2, 100 μM Na3VO4, 1 mM dithiothreitol, 5 nM okadaic acid, 2.5 μg of aprotinin, 2.5 μg of pepstatin, 0.5 μM phenylmethylsulfonyl fluoride, 0.5 mM benzamidine, 2.5 μg of leupeptin). Insoluble material was removed by centrifugation (13,000 × g for 20 min at 4°C), and samples were kept at −80°C.
Western blot analysis.
Cell lysates (10 to 30 μg, depending on the protein immunodetection) were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (10% polyacrylamide) prior to electrophoretic transfer onto polyvinylidene difluoride membrane (ICN Biochemicals). The blots were blocked (1 h at room temperature) with 5% nonfat dry milk or 5% bovine serum albumin (fraction V; Sigma) for the detection of nonphosphorylated and phosphorylated proteins, respectively. Then the blots were incubated (overnight at 4°C) with primary antibodies (see below). After being rinsed, the blots were incubated for 2 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit antibodies prior to exposure to the enhanced chemiluminescence substrate. Antibodies directed against the phosphorylated form of the proteins were applied, and the detection was processed as described above. Then the blots were stripped with 0.1 M glycine-HCl (pH 2.8) twice for 20 min at 55°C followed by saturation in 5% nonfat dry milk and incubated with the antibodies corresponding to the nonactivated proteins as described above. The efficacy of the stripping step was assessed by omitting the first antibody and verifying the lack of signals on the blot. Digitized images of the immunoblots or autoradiograms were used for densitometric measurements with an image analyzer (IMSTAR). Relative enzyme or transcription factor activation was determined by normalization of the density of images from phosphorylated proteins with that of the total inactive proteins on the same blot.
Antibodies.
Commercially available antisera produced by immunizing rabbits with synthetic dually specific antiphospho-Ser218-Ser222 MAPK kinases 1 and 2 (MEK1/2) (New England Biolabs) (diluted 1:750), antiphospho-Thr183-Tyr185 ERK2 (Promega) (diluted 1:2,500), or antiphospho-Thr183-Tyr185 JNK (Promega) (diluted 1:3,000) or monospecific antiphospho-Ser133-CREB (Upstate Biotechnology Inc.) (diluted 1:750), antiphospho-Ser383–Elk-1 (New England Biolabs) (diluted 1:200), or antiphospho-Thr286-CaMKII (Promega) (diluted 1:1,000) peptides were used. Rabbit polyclonal antisera raised against synthetic peptides specific for MEK1/2 (New England Biolabs) (diluted 1:1,000), ERK2 (Santa Cruz) (diluted 1:4,000), JNK (New England Biolabs) (diluted 1:1,500), CREB (New England Biolabs) (diluted 1:1,000) Elk-1 (Santa Cruz) (diluted 1:500), or c-Fos (residues 3 to 16 of human c-Fos [Santa Cruz]) (diluted 1:500) were used to detect the inactive forms of the proteins.
RNA isolation and Northern blot analysis.
RNA isolation from whole-cell extracts was performed as previously described (39). Total RNA (10 μg per lane) was loaded on formaldehyde-agarose gels and, after electrophoresis, blotted on a nitrocellulose filter (Schleicher & Schuell, Amersham). These filters were then processed as for the Northern transfer, which was described previously along with the hybridization protocol (92). Briefly, the blot was prehybridized for 2 h at 65°C in 10 ml of hybridization buffer (50% [vol/vol] deionized formamide, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] 5× Denhardt’s solution, 0.1% SDS, 50 mM Na2HPO4–NaH2PO4 [pH 6.8]) and hybridized for 18 h at 65°C in 10 ml of the same buffer by adding 107 cpm of radiolabeled riboprobes. Hybridization against riboprobes corresponding to the fourth exon of the human c-fos cDNA and the entire rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was performed simultaneously. After removal of the hybridization solution, the blot was washed twice for 1 min in 0.2× SSC–1% SDS at room temperature and once for 20 min in the same solution at 65°C. Hybridization signals were revealed after X-ray film exposure.
Kinase assays.
The kinase activity of the B-Raf protein was determined as previously described (61) with some modifications. Striatal extracts (400 μg of proteins) were immunoprecipitated at 4°C with a specific B-Raf antiserum (IS11) (5) for 3 h and then with Pansorbin (Calbiochem) for 1 h. Immune complexes were washed three times with the lysis buffer and then once with the kinase buffer. The final pellets were resuspended in 20 μl of kinase buffer and incubated for 15 min at 30°C with 5 μCi of [γ-32P]ATP–50 μM ATP–0.5 μg of recombinant glutathione S-transferase-MAPK kinase 1 (MEK1). Incubation and separation of proteins were performed as described above.
RESULTS
Glutamate superfusion leads to Fos induction in striatal slices.
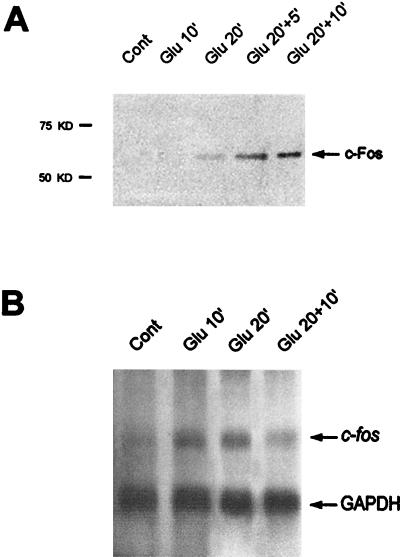
We have shown (71) that the in vivo stimulation of corticostriatal glutamatergic afferents leads to rapid changes in IE gene expression in the rat striatum. To facilitate the biochemical characterization of this gene induction, we set out to establish experimental conditions reproducing this phenomenon, namely, glutamate induction of the proto-oncogene fos in striatal slices. To avoid nonspecific induction of fos linked to slice preparation and osmotic and temperature stress, slices were continuously superfused in chambers with oxygenated Krebs buffer at 37°C for 60 min prior to and during glutamate application. Of the various glutamate concentrations and application times tested (data not shown), the following conditions best reproduced the results obtained in vivo. Glutamate (100 μM) was applied alone for 10 min (Glu10) and 20 min (Glu20) or for 20 min followed by 5 min (Glu20+5) or 10 min (Glu20+10) of Krebs buffer superfusion. Fos protein was first detectable by Western blotting (Fig. 1A) in Glu20 chambers and was then detected in increased amounts in Glu20+5 and Glu20+10 chambers. Northern blot analysis of RNA isolated from the same striatal extracts (Fig. 1B) showed an induction of c-fos mRNA at Glu10 which was maintained at Glu20 and then slightly decreased at Glu20+10. Thus, glutamate treatment rapidly and strongly activated the c-fos gene in striatal slices under our experimental conditions. This induction was transient and occurred 10 to 15 min before c-Fos protein expression.
FIG. 1.
Glutamate-induced expression of c-Fos protein (A) and c-fos mRNA (B) in striatal slices. (A) Striatal slices were superfused with Krebs buffer alone (Cont) or in the presence of glutamate (100 μM) for 10 min (Glu 10′), 20 min (Glu 20′), or 20 min followed by 5 min (Glu 20′+5′) or 10 min (Glu 20′+10′) of Krebs buffer superfusion. At the end of the experiment, striatal slices were immediately lysed and processed for extraction of proteins. c-Fos protein expression was analyzed at the various time points by Western blotting with a specific anti c-Fos antibody. Fos protein is detectable at Glu20 and then increases at Glu20+5 and Glu20+10. (B) Total RNAs were extracted from the same striatal extracts (see Materials and Methods). c-fos and GAPDH mRNAs were detected on the same Northern blot. While GAPDH hybridization signals remain identical, c-fos mRNAs are induced at Glu10 and Glu20 and then their levels decrease slightly.
Kinetics of Elk-1 and CREB phosphorylation by glutamate in striatal slices.
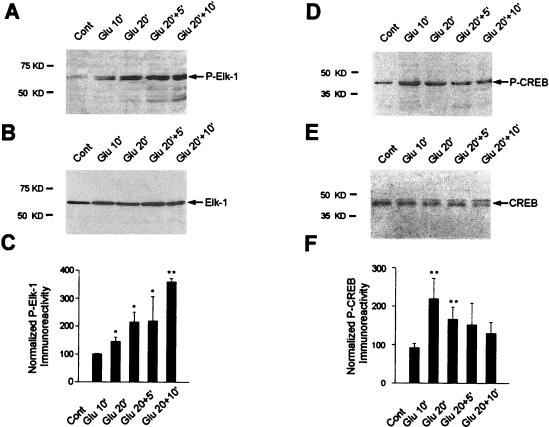
The TCF-2SRF ternary complex assembled on the c-fos SRE is important in transcriptional induction by many signals. The TCF protein Elk-1 is strongly expressed in the adult CNS, more particularly in striatal neurons (71). Given its role as an important mediator of transcriptional induction by intracellular signaling, we tested whether it is activated by glutamate in our model. Phosphorylation of Elk-1 is associated with activated, SRE-dependent gene expression (27–29, 36, 38, 39, 44, 45, 52, 63, 86, 87, 93). Multiple studies have indicated that phosphorylation on serine 383, although not sufficient for full transcriptional activation, is a prerequisite for Elk-1 function (29, 42, 52). We tested the effect of 100 μM glutamate on the phosphorylation status of Elk-1 in the same striatal extracts analyzed above for c-fos mRNA induction. Western blots were incubated with an antibody that specifically recognizes the Ser383-phosphorylated form of Elk-1 (antiphospho-Ser383–Elk-1). Glutamate increased the phosphorylation of Elk-1. This was first visible at Glu10 (Fig. 2A) and was followed by a strong increase at later time points (Fig. 2A). Western blots probed with a control Elk-1 antiserum revealed that comparable levels of Elk-1 were present at each time point (Fig. 2B). Figure 2C represents quantitation of four independent experiments (representing 12 striatal slices for each time point) and shows a significant increase in the phosphorylation of Elk-1 at Glu10 relative to control striatal slices (+50%; P < 0.05, unpaired Student’s t test). This effect increased to +260% at Glu20+10 (P < 0.005, unpaired Student’s t test). Thus, on striatal slices, Elk-1 phosphorylation is regulated by glutamate and reaches maximal levels after a sustained application of glutamate followed by Krebs buffer superfusion.
FIG. 2.
Kinetics of Ser383 Elk-1 and Ser133 CREB phosphorylation after glutamate application. The activation of Elk-1 and CREB was studied by Western blotting from the same striatal extracts as those used for the experiment in Fig. 1. (A) Immunolabeling obtained with an anti-active Elk-1 antibody (antiphospho- Ser383–Elk-1). (B) Detection of Elk-1 proteins on the same blot after a stripping procedure. Note the marked increase of phosphorylated 62-kDa proteins after glutamate application and the equal amount of Elk-1 proteins in all extracts. (C) Densitometric measurements of digitized images of autoradiograms were performed in four independent experiments (representing 12 striatal slices for each time point). Relative Elk-1 activation was determined by normalization of the density of images from phosphorylated Elk-1 to that of the total Elk-1 from parallel experiments in the same samples. (D) Immunolabeling obtained with an anti-active CREB antibody (antiphospho-Ser133–CREB). (E) Detection of CREB proteins on the same blot after a stripping procedure. Note the marked increase of phosphorylated 43-kDa proteins after glutamate application and the equal amount of CREB proteins in all extracts. (F) Determination of relative CREB activation. This was performed as specified for panel C. Statistical analysis: *, P < 0.05; **, P < 0.005 (unpaired Student’s t test) when comparing Glu chambers with control chambers.
Glutamate receptor stimulation leads to increases in intracellular levels of calcium that activate signaling pathways targeting the SRE or Ca/CRE promoter elements (4, 31). Therefore, we analyzed the kinetics of CREB activation by phosphorylation on Ser133, since CREB can account for activation of c-fos via the Ca/CRE (40, 74, 75). As above, Western blots representing striatal extracts prepared at various times of glutamate application were incubated with an antibody which specifically recognizes Ser133-phosphorylated CREB. Levels of phospho-CREB were detectable at Glu10 (Fig. 2D). Then phospho-CREB gradually returned to basal levels between Glu 20+5 and Glu20+10 (Fig. 2D). Testing the same Western blot with control CREB antibody showed the presence of comparable levels of CREB in each lane (Fig. 2E). Quantitation (Fig. 2F) confirmed that the maximal activation of CREB occurred at Glu10 (+120%; P < 0.005, unpaired Student’s t test) and then decreased to basal levels between Glu 20+5 and Glu20+10.
Kinetics of MAPK cascade induction by glutamate.
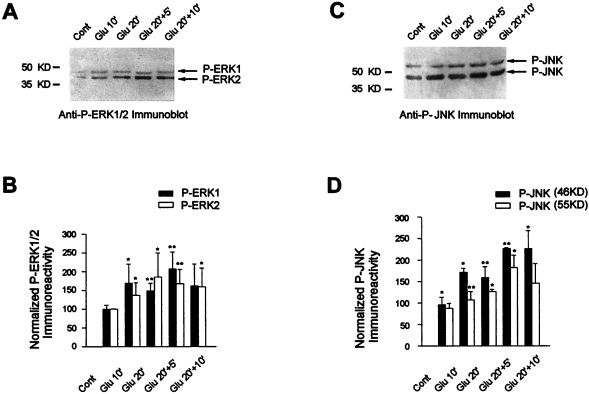
Elk-1 represents a convergence point for mammalian MAPK cascades, since ERK, JNK, and p38MAPK can phosphorylate this protein and drive Elk-1-dependent transcriptional activation in transfection assays. It is unclear which of these cascades might be important in signaling downstream of glutamate. Therefore, we used Western blots of crude striatal extracts together with antibodies directed against the activated forms of the two enzymes. ERKs are activated by MEK1 and MEK2 through dual phosphorylation on Thr183 and Tyr185 of ERK2 and Thr202 and Tyr204 of ERK1, while JNKs are activated by dual phosphorylation on Thr183 and Tyr185. Despite these similar activation sites, antibodies directed against the dually phosphorylated forms of ERKs and JNKs specifically recognize the appropriate kinases due to the different neighboring amino acid sequences (Fig. 3 and supplier’s information).
FIG. 3.
ERK and JNK proteins are activated by glutamate. (A and C) The activation of ERK (A) and JNK (C) proteins was studied by Western blotting with specific anti-active ERK and anti-active JNK antibodies, respectively. (B) Relative ERK activation was determined by normalization of the density of images from phosphorylated ERK1 and ERK2 to that of total ERK1 and ERK2. (D) Relative JNK activation after normalization from phosphorylated JNK-p46 and JNK-p55 to that of total JNK-p46 and JNK-p55. Statistical analysis: *, P < 0.05; **, P < 0.005 (unpaired Student’s t test) when comparing Glu chambers with control chambers.
With the phospho-ERK specific antibody, we detected substantial levels of phosphorylated p42ERK2 and p44ERK1 appearing at Glu10 with slight increases variably appearing at later time points (Fig. 3A and B). The control Western blot shows identical amounts of ERK1 and ERK2 in all lanes (data not shown).
JNK proteins are encoded by three different genes (JNK1, JNK2, and JNK3), giving rise to alternatively spliced isoforms (33). In vitro translation of these various isoforms encodes two major proteins of 46 and 55 kDa. Incubation of the Western blots with the phospho-JNK specific antibody shows that p46 is activated with kinetics similar to those of ERKs whereas p55 phosphorylation seems to occur more slowly (Fig. 3C and D). Similar levels of p46 and p55 were present at each time point (data not shown). In some experiments, we also tested p38MAPK activation on glutamate stimulation with an anti-active antibody (anti-phospho-Thr180/Tyr182-p38MAPK). We found a slight and nonreproducible increase of p38MAPK phosphorylation in our model (data not shown).
Thus, glutamate treatment of striatal slices leads to strong activation of both the ERK and JNK cascades, and either or both could account for Elk-1 phosphorylation and c-fos activation.
Phosphorylation of Elk-1 and CREB is dependent on ERK after glutamate stimulation.
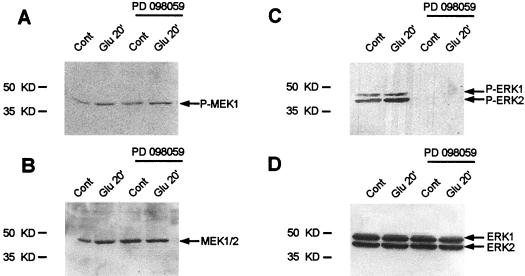
To identify the roles played by these two MAPK cascades in Elk-1 phosphorylation, we tested the effects of a specific inhibitor of the ERK cascade, the MEK inhibitor PD98059 (16). Striatal slices were superfused with this compound for 30 min prior to and during the glutamate application. PD98059 did not affect the phosphorylation status of MEKs at Glu20 (Fig. 4A and B), which is consistent with previous observations that the inhibitor targets the catalytic activity of MEK (16). Nevertheless, the inhibitor was effective, since it completely blocked the phosphorylation and therefore the activation of ERK1 and ERK2 (Fig. 4C and D). Full inhibition was also observed at the different time points of glutamate superfusion (data not shown). In direct contrast to these results, PD98059 did not inhibit the activation of JNK (data not shown).
FIG. 4.
The MEK inhibitor PD98059 abolishes ERK activation by glutamate. Striatal slices were treated with PD98059 (100 μM) for 30 min prior to and during glutamate application (Glu10, Glu20, and Glu20+10). Shown are Western blots obtained with striatal slices (Glu 20′) with anti-active antibodies (A and C) or antibodies labeling the total proteins (B and D). Similar results were observed at the various time points (data not shown). Note that PD98059 treatment completely abolishes ERK activation in the presence or absence of glutamate (C). Glutamate-induced activation of MEK (A) remained unchanged after PD98059 treatment. Total levels of proteins remain unchanged whatever the treatment (B and D).
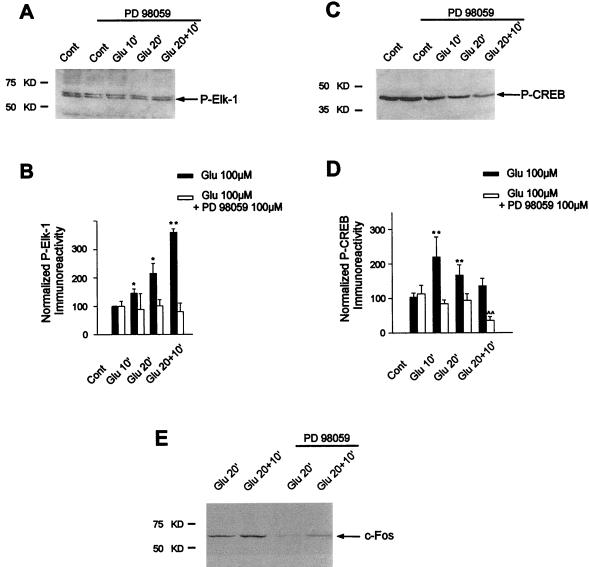
Given the specific inhibition of glutamate-induced ERK cascade activation by PD98059 in our system, we then analyzed the effect of ERK inhibition on Elk-1 phosphorylation. The Western blots (Fig. 5A) and their quantitation (Fig. 5B) very clearly show that PD98059 completely abolished the increase in Elk-1 phosphorylation on Ser383 after glutamate superfusion. These data indicate that Elk-1 is targeted by ERK and not JNK signaling pathways in our model.
FIG. 5.
PD98059 abolishes glutamate-induced Elk-1, CREB phosphorylation and c-Fos induction by glutamate. The phosphorylation of Elk-1 and CREB and the induction of c-Fos proteins were studied by Western blotting of the same striatal extracts as used in the experiment in Fig. 4. For each time point, the efficacy of glutamate superfusion was verified (data not shown). (A) Immunolabeling obtained with antiphospho-Ser383–Elk-1 from striatal extracts activated by glutamate (Glu 10′, Glu 20′, and Glu 20+10′) in presence of PD98059 (100 μM). (B) Densitometric measurements were performed in five independent experiments (representing 15 striatal slices) in the presence or absence of PD98059. They show a complete inhibition of glutamate-induced phosphorylation of Elk-1 after PD98059 treatment. (C) Immunolabeling obtained with antiphospho-Ser133–CREB from striatal extracts activated by glutamate (Glu 10′, Glu 20′, Glu 20+10′) in the presence of PD98059 (100 μM). (D) Densitometric measurements performed in five independent experiments (representing 15 striatal slices) show the complete inhibition of glutamate-induced phosphorylation of CREB after PD98059 treatment at Glu10 and Glu20 and a decreased level of CREB phosphorylation at Glu20+10 compared to control slices. Statistical analysis: *, P < 0.05; **, P < 0.005 (unpaired Student’s t test) when comparing glutamate alone with control chambers; ∧∧, P < 0.005 when comparing glutamate plus PD98059 with control chambers. (E) c-Fos protein expression was analyzed by Western blotting at Glu 20′ and Glu 20+10′ in the presence or absence of PD98059.
Calcium-induced CREB phosphorylation classically depends on the activation of the CaM-K signaling pathway. However, recent evidence showed that in neurotrophin-treated PC12 cells or cortical neurons in culture, CREB phosphorylation can also occur via an ERK-dependent signaling pathway targeting p90RSK proteins, which in turn can activate CREB (22, 90). Thus, it seemed reasonable to postulate that glutamate-induced CREB activation could occur, at least in part, via an ERK/p90RSK module. To address this question, we analyzed the effect of PD98059 on CREB modification in our model. Indeed, we found an inhibition of glutamate-induced CREB phosphorylation on Ser133 at Glu10 and Glu20 (Fig. 5C and D). At Glu20+10, levels of CREB phosphorylation by glutamate were even lower than in control slices (Fig. 5D), a result discussed below. In conclusion, CREB is also targeted by the ERK cascade after glutamate receptor stimulation in striatal slices.
PD98059 completely abolished glutamate-induced Elk-1 and CREB phosphorylation. These data strongly suggested that ERK signaling cascade played a key role in glutamate signaling to c-fos. We thus analyzed c-Fos protein induction at Glu20 and Glu20+10, which represent the time points for Fos induction in our model (Fig. 1A), in the presence or not of PD98059. The Western blot (Fig. 5E) shows a strong inhibition of glutamate-induced c-Fos expression upon PD98059 treatment.
MEK1 and B-Raf activation on glutamate stimulation.
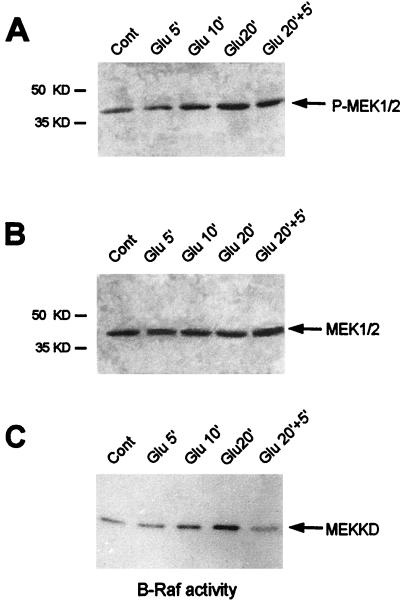
The above data demonstrate that ERK plays a critical role in the striatal response to glutamate. The components of the ERK cascade are well defined in culture cell systems but less so in semi-in vivo systems. The effect of PD98059 strongly indicates that MEK1 is the upstream activator of ERK in the striatum. Nevertheless we investigated this cascade in more detail by analyzing the kinetics of MEK and Raf activation in the striatal slices by glutamate superfusion. Since ERK was activated at Glu10, we began measuring these upstream events at an earlier time point (Glu5). Western blots obtained with an anti-active MEK1/2 antibody showed a stronger signal at Glu10 than at Glu5. The signal persisted at Glu20 and Glu20+5 (Fig. 6A). This reflected increased MEK activation, since comparable levels of MEK were present in each lane (Fig. 6B).
FIG. 6.
Kinetics of MEK1 and B-Raf activation on glutamate stimulation of striatal slices. (A) Western blot analysis of MEK1 and MEK2 phosphorylation with a specific antiphospho-Ser217-Ser221 MEK1/2 antibody. This antibody stained a single band (43 kDa) on immunoblots, consistent with the molecular masses of the MEK1 and MEK2 proteins. (B) The same blot was stripped and rehybridized with the antibody corresponding to the inactive MEK1 and MEK2 proteins. This step allowed us to detect the total amounts of MEK proteins in the immunoblot. (C) B-Raf protein kinases were isolated by immunoprecipitation, and B-Raf protein kinase activity was detected in the immune complex by using [γ-32P]ATP and the MEK-kinase dead (MEKKD) fusion protein as the substrate. Note the increase of B-Raf activity at Glu 10′ and Glu 20′.
MEK1 and MEK2 are activated through phosphorylation by kinases of the Raf family, serine/threonine kinases whose activation is linked to the small GTP-binding proteins of the Ras family (3, 41, 55). In the Raf protein kinase family, B-Raf is the strongest MEK activator (41) and phosphorylates Ser218 and Ser222 residues of MEK proteins (61). Furthermore, B-Raf is highly enriched in the brain and more particularly in the striatum (5). Therefore, we purified B-Raf proteins by immunoprecipitation (5) from striatal extracts prepared at various times after glutamate superfusion. B-Raf showed increased activity at Glu10 and Glu20, which then returned to basal levels at Glu20+5 (Fig. 6C). Thus, there was a temporal correspondence between the activity of B-Raf, MEK, and ERK, suggesting that these are the components of the ERK cascade induced by glutamate in the striatum.
CaM-Ks exert a positive control on the ERK signaling pathway.
ERK activation was linked to Ca2+ influx into the cells, since it was sensitive to chelation of extracellular Ca2+ by EGTA (data not shown). Well-characterized mediators of Ca2+ signaling events are the multifunctional CaM-K proteins. They are implicated in transcriptional regulation, since Ca2+-dependent transcription of c-fos is blocked in PC12 cells by the CaM-K inhibitor KN62 (20). CaM-KIV has been linked to CREB phosphorylation on Ser133 (53, 78). However, both CaM-KIV (21) and CaM-KII (57) have recently been described to mediate Ca2+-induced ERK activation. Thus, CREB phosphorylation might be due, in our model, to an indirect effect of CaM-KII or CaM-KIV via the ERK pathway.
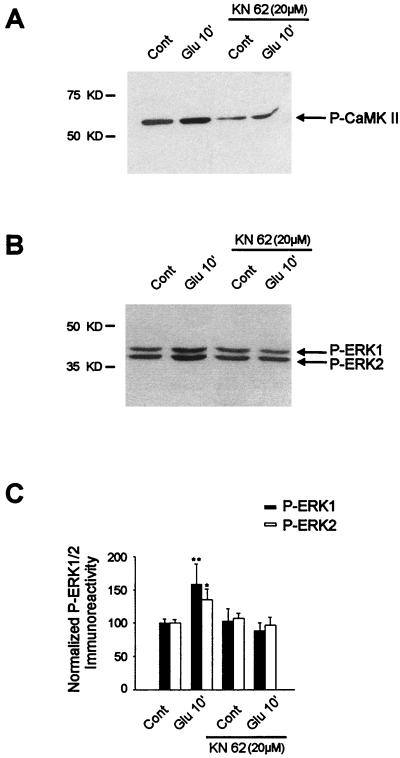
To address this, KN62 (20 μM) was superfused for 30 min prior to and during glutamate application. This compound is competitive with calmodulin binding and inhibits both CaM-KII (80) and CaM-KIV activity with similar 50% inhibitory concentrations (19). With regard to CaM-KII, KN62 inhibits both kinase activity and autophosphorylation on Thr286 (80). We thus tested for its efficacy by using an antiphospho-Thr286–CaM-KII antibody. Figure 7A shows that glutamate induced an increase of phospho-Thr286–CaM-KII signals as soon as Glu10, which was blocked by KN62 treatment (20 μM).
FIG. 7.
Role of the CaM-K inhibitor KN62 in glutamate-induced ERK activation. Striatal slices were superfused with KN62 (20 μM) for 30 min prior to and during glutamate application. (A) The efficacy of this compound was analyzed by Western blotting with an antiphospho-Thr286–CaM-KII antibody. Note that KN62 strongly decreases both basal levels and glutamate-induced phospho-Thr286-CaM-KII levels. (B) The same striatal extracts were analyzed with an anti-active ERK antibody. Note the inhibition of glutamate-induced ERK activation by KN62. (C) Densitometric measurements were performed in three independent experiments (representing nine striatal slices) in the presence or absence of KN62 (for each experiment, the inhibition of CaM-K activity by KN62 was verified as specified for panel A). Statistical analysis: *, P < 0.05; **, P < 0.005 (unpaired Student’s t test) when comparing glutamate alone with control chambers.
We then analyzed the effect of KN62 on glutamate-induced ERK activation. Western blots (Fig. 7B) and their quantitation (Fig. 7C) clearly show that KN62 completely abolished the increase in ERK1 and ERK2 phosphorylation and therefore their activation by glutamate.
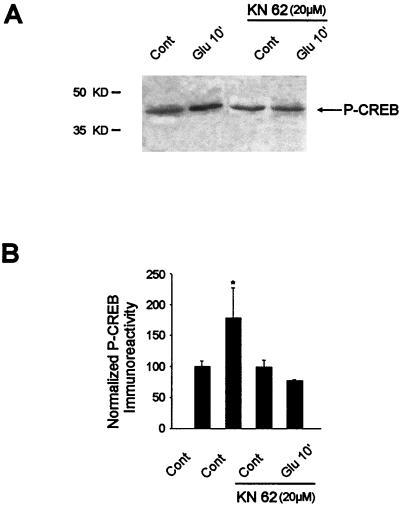
Given the role of KN62 in glutamate-induced ERK activity, we then analyzed the effect of CaM-K inhibition on CREB phosphorylation at Glu10. Consistent with the results above, KN62 abolished glutamate-induced Ser133-CREB phosphorylation (Fig. 8).
FIG. 8.
Ser133-CREB phosphorylation by glutamate is inhibited by KN62 treatment. (A) Glutamate-induced Ser133-CREB phosphorylation in the presence or absence of KN62 was analyzed by Western blotting from the same striatal extracts as used in the experiment in Fig. 7. (B) Densitometric measurements were performed in three independent experiments (representing nine striatal slices) in the presence or absence of KN62. Statistical analysis: *, P < 0.05 (unpaired Student’s t test) when comparing glutamate alone with control chambers.
The fact that inhibiting CaM-K abrogates ERK activation suggests that CaM-K plays a key role in transmitting a Ca2+ signal to the ERK signaling pathway.
DISCUSSION
In the CNS, glutamate receptor stimulation leads to increases in intracellular calcium levels, which are critically involved in gene regulation and long-term adaptive responses implicated in synaptic plasticity (26, 30, 31, 73). We have developed this semi-in vivo system of striatal slices to dissect out biochemical steps of early glutamate-induced signaling events underlying c-fos gene regulation in this region of the brain. We found a tight kinetic link between MAPK cascade induction, phosphorylation of Elk-1, and the expression of c-fos mRNAs upon treatment with glutamate. Although both ERK and JNK cascades were induced, Elk-1 phosphorylation was blocked with the specific MEK inhibitor PD98059, thus uncoupling its activation from JNK signaling pathways. Interestingly, glutamate-induced CREB phosphorylation, which classically depends on CaM-K pathways (13, 74), was also blocked after PD98059 treatment. Instead, inhibition of CaM-K activity by KN62 abolished glutamate-induced ERK activation along with TCF/Elk-1 (data not shown) and CREB phosphorylation. We thus establish the first in vivo evidence that ERK is the principal mediator of Ca2+-dependent c-fos induction via two different modules, (i) ERK/Elk-1 and (ii) ERK/?/CREB, a pathway positively controlled by CaM-Ks.
Concomitant phosphorylation of Elk-1 and CREB coincides with c-fos mRNA induction.
Based on cell culture models with a transiently introduced c-fos reporter gene, it appears that two DNA regulatory elements are implicated in c-fos transcriptional regulation by glutamate: the Ca/CRE and SRE sites (4). We set out to determine the upstream events underlying c-fos mRNA induction by glutamate in our semi-in vivo model, starting at the level of modification of transcription factors targeting these DNA regulatory elements, namely, Elk-1 and CREB. Phosphorylation of both Elk-1 and CREB was slightly detectable in control slices, a result consistent with the constitutive expression of these activated proteins in immunocytochemical studies (reference 71 and unpublished data). However, the phosphorylation of both transcription factors increased within 10 min of glutamate application, which corresponds kinetically to the induction of c-fos mRNA and subsequent appearance of c-Fos protein. The role of CREB phosphorylation in c-fos regulation by glutamate is now well established (2, 31). Such a role for Elk-1 is still controversial. Based on in vitro studies, the culture cell context and mode of calcium entry determine whether Elk-1 can activate a transiently introduced c-fos reporter gene (46, 54, 89). In our model with striatal slices, i.e., the whole-neuron context, it appears that Elk-1 is phosphorylated on glutamate receptor stimulation and is thus a good candidate for activating the SRE site. The concomitant activation of CREB and Elk-1, together with the induction of c-fos mRNA at early time points, is consistent with the results of an elegant study showing that the entire gamut of c-fos regulatory sequences is required for its expression in various tissues, particularly the CNS (67). In cell culture systems, the phosphorylation of CREB or Elk-1 strongly increases transactivation via interactions with the coactivator CREB-binding protein (43, 49), which facilitates much more efficient transcription through multiple contacts with the basal transcriptional machinery. Our data would be consistent with a similar mechanism occurring in organized neuronal circuits. The cooperative effect of glutamate-driven phosphorylation of multiple protein-DNA complexes bound to the promoter (35) ensure recruitment of the coactivator. It will be exciting to test this model in the appropriate mutant contexts.
By extrapolation from culture cell systems, the decrease in the level of c-fos mRNA probably reflects the postinduction repression of the c-fos promoter (1). We note that CREB phosphorylation also diminishes at this point while hyperphosphorylated Elk-1 persists. This would appear to uncouple the dephosphorylation of Elk-1 from c-fos transcription repression, while suggesting that CREB dephosphorylation may play a role in this still-uncharacterized process. In addition, it is possible that glutamate-induced Elk-1 phosphorylation observed at a late time point is a determinant for genes containing SRE but not CRE sites in their promoters (84).
The ERK module mediates glutamate signaling to Elk-1 independently of JNK.
Both ERK and JNK were activated with similar kinetics by glutamate in the striatal slices. The tight temporal correlation between these kinetics and that of phosphorylation of Elk-1 indicates that either one or both could transduce the signal to the SRE site of the c-fos promoter. However, experiments with the MEK inhibitor clearly showed an uncoupling of Elk-1 phosphorylation from JNK activation, a result in agreement with data showing that glutamate-induced c-fos expression is maintained in JNK3 knockout mice (91). In culture cells, the ERK cascade is strongly activated by proliferative signals (70) and the JNK cascade is activated by a wide variety of stresses (15, 47). More relevant to our system, withdrawal of nerve growth factor from neuronally differentiated PC12 cells leads to apoptosis, which is preceded by a decrease of ERK activity and an increase of JNK activity (88). These results suggest that ERK and JNK have different and possibly opposing functions in culture cell systems. In the CNS, glutamate receptor stimulation can activate both ERK (23, 48) and JNK (69) signaling pathways. While ERK appears to play a critical role in intracellular mechanisms leading to long-term plasticity, as has been shown in the rat hippocampus (17, 18), JNK proteins show a high constitutive activity in the brain, and one isoform, JNK-3, is critically involved in glutamate-induced excitotoxicity in the hippocampus (91). Perhaps these two MAPK pathways play complementary roles in glutamate-signaling in the striatum via different components of the transcription factor AP1.
The ERK cascade plays a primary role in glutamate signaling to c-fos.
The different kinetics of Elk-1 and CREB phosphorylation observed in the present work suggest that these transcription factors are targeted by distinct signaling pathways. However, the strong reduction of Elk-1 and CREB upon inhibition of ERK induction, after PD98059 superfusion, indicates that they are both targeted by the ERK signaling pathway. This reduction was associated with decreased levels of c-Fos expression, indicating that ERK plays a primary role in glutamate signaling to c-Fos in our model. Elk-1 is well documented to be a major nuclear substrate of the MAPK cascades, and Elk-1 phosphorylation strictly followed ERK activation in our model. In the case of CREB, CaM-KIV but not CaM-KII can activate transcription though the direct phosphorylation of CREB (53, 78). Activation of CaM-KIV can occur, depending on the cell line model, after nuclear translocation of Ca2+ (34) or calmodulin (14). However, MAPKs of ERK and p38MAPK subclasses can also target CREB on cell culture models via intermediate kinases: p90RSK for ERK activation (22, 90) and MAPK-activated protein kinase 2 (79) for p38MAPK. The reduction of CREB phosphorylation observed after PD98059 superfusion in the present study indicates that glutamate-induced CREB phosphorylation results from ERK activation of p90RSK. In this scenario, the decrease in CREB phosphorylation in the presence of increasing ERK activity would suggest the activation of a CREB-specific phosphatase activity. Such activation of phosphatase could also explain the strong dephosphorylation of CREB observed at late time points after PD98059. In fact, steady-state levels of activated transcription factors depend critically on the dynamics of their phosphorylation and dephosphorylation. One candidate for CREB dephosphorylation is protein phosphate PP-1, which is activated in a calcium-dependent manner (8).
CaM-Ks act as positive regulators of the ERK signaling cascade.
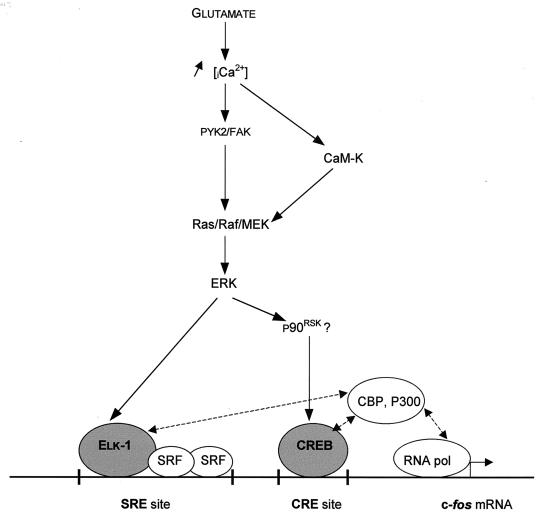
ERK activation in this study was linked to glutamate-induced Ca2+ influx into neurons, as indicated by its sensitivity to EGTA treatment. A key intermediate downstream of the glutamate receptor stimulation appears to be the Ca2+-dependent activation of the nonreceptor tyrosine kinase pp125FAK or PYK2 (50, 77), which in turn can activate the Ras/Raf/MEK/ERK pathway (50) (Fig. 9). Increases in intracellular Ca2+ levels can also modate CaM-K activity. While CaM-Ks can directly target c-fos regulatory factors, recent evidence suggests that CaM-Ks can also mediate MAPK induction. Transient transfection of constitutively activated forms of CaM-KIV or CaM-K kinase into PC12 cells induced all three MAPKs (21). Similarly, CaM-KII has been associated with ERK activation in rabbit aortic smooth muscle cells (57). In the brain, both CaM-KII and CaM-KIV are strongly expressed, and either of them could be responsible for the effect we observed after superfusion of the CaM-K inhibitor, KN62. Since B-Raf and MEK were activated by glutamate and since transcription induction of c-fos was sensitive to PD98059, these data suggest that CaM-Ks lie upstream of ERK (Fig. 9), and it will be interesting to determine the mechanisms by which these two signaling mediators are linked. In this context, it is interesting that the components of the ERK machinery, including B-Raf (56) and MEK and ERK proteins (24, 60), are enriched in the dendritic processes of striatal neuronal cells, where glutamate receptors are localized (6). While CaM-KII is also enriched in these cytoplasmic compartments (25), Cam-KIV is expressed exclusively in the nucleus (8). Thus, we propose that calcium entry into neurons produces, locally near the glutamate receptor, activation of CaM-KII, which can in turn regulate the ERK signaling pathway (57). This pathway appears to be linked to c-fos induction, via two different modules: ERK/Elk-1 and ERK/?/CREB, as demonstrated after PD98059 treatment. The link between ERK and CREB activation could be the cytoplasmic substrate of ERK, p90RSK, which in turn could translocate to the nucleus (11) to activate CREB. The concomitant activation of both transcription factors at early time points could be the initial event in c-fos transcriptional regulation via CREB-binding protein (43, 49).
FIG. 9.
CaM-Ks act as positive regulators of the ERK signaling cascade.
In conclusion, our data provide new insights into mechanisms that can account for the integration of different intracellular signaling pathways to yield distinct biological responses. In particular, they support the idea of a crucial role of calcium signaling to ERK signaling modules in gene regulation underlying long-term potentiation in the striatum (10).
ACKNOWLEDGMENTS
We thank Parke-Davis for the generous gift of PD98059. We also thank N. Kayadjanian, E. Valjent, and M. Leonhard for helpful technical assistance.
This work was supported by the University Pierre and Marie Curie, the CNRS, the Fondation pour la Recherche Medicale (R.A.H.), Institut Lilly, and Biomed Program (BMHY-CT-97-2215).
REFERENCES
- 1.Abate C, Luck D, Gagne E, Roeder R G, Curran T. Fos and Jun cooperate in transcriptional regulation via heterologous activation domains. Mol Cell Biol. 1990;10:5532–5535. doi: 10.1128/mcb.10.10.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn S, Olive M, Aggarwal S, Krylov D, Ginty D D, Vinson C. A dominant-negative inhibitor of CREB levels that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi D R, Saito Y, Campbell D G, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall C J, Cowley S. Identification of the sites in MAP kinase kinase 1 phosphorylated by p74raf1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bading H, Ginty D D, Greenberg M E. Regulation of gene expression in hippocampal neurons by distinct signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 5.Barnier J V, Papin C, Eychène A, Lecoq O, Calothy G. The mouse B-raf gene encodes multiple protein isoforms with tissue-specific expression. J Biol Chem. 1995;270:23381–23389. doi: 10.1074/jbc.270.40.23381. [DOI] [PubMed] [Google Scholar]
- 6.Bernard V, Somogyi P, Bolam J P. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulton T G, Nye S H, Robbins D J, Ip N Y, Radziejewska E, Morgenbesser S D, DePinho R A, Panayotatos N, Cobb M H, Yancopoulos G D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 8.Bito H, Deisseroth K, Tsien R W. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 9.Cahill M A, Janknecht R, Nordheim A. Signalling pathways: Jack of cascades. Curr Biol. 1996;6:16–19. doi: 10.1016/s0960-9822(02)00410-4. [DOI] [PubMed] [Google Scholar]
- 10.Charpier S, Deniau J M. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci USA. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen R H, Sarneki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton S, Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum element. Cell. 1992;68:597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- 13.Dash P K, Karl K A, Prywes R, Kandel E R. cAMP response element-binding protein is activated by Ca2+/calmodulin as well as cAMP dependent protein kinase. Proc Natl Acad Sci USA. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deisseroth K, Bito H, Tsien R W. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 15.Dérijeard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 16.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7668–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.English J D, Sweatt J D. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- 18.English J D, Sweatt J D. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- 19.Enslen H, Sun P, Brickey D, Soderling S H, Klamo E, Soderling T R. Characterization of Ca2+/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J Biol Chem. 1994;269:15520–15527. [PubMed] [Google Scholar]
- 20.Enslen H, Soderling T R. Role of calmodulin-dependent protein kinases and phosphatase in calcium-dependent transcription of immediate early genes. J Biol Chem. 1994;269:20872–20877. [PubMed] [Google Scholar]
- 21.Enslen H, Tokumitsu H, Stork P J S, Davis R J, Soderling T R. Regulation of mitogen-activated protein kinases by a calcium/calmodulin-dependent protein kinase cascade. Proc Natl Acad Sci USA. 1996;93:10803–10808. doi: 10.1073/pnas.93.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkbeiner S, Tavazoie S F, Maloratsky A, Jacobs K M, Harris K M, Greenberg M E. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 23.Fiore R S, Murphy T H, Sanghera J S, Pelech S L, Baraban J M. Activation of p42 mitogen-activated protein kinase by glutamate receptor stimulation in rat primary cortical cultures. J Neurochem. 1993;61:1626–1633. doi: 10.1111/j.1471-4159.1993.tb09796.x. [DOI] [PubMed] [Google Scholar]
- 24.Fiore R S, Bayer V E, Pelech S L, Posada J, Cooper J A, Baraban J M. p42 mitogen-activated protein kinase in brain: prominent localization in neuronal cell bodies and dendrites. Neuroscience. 1993;55:463–472. doi: 10.1016/0306-4522(93)90516-i. [DOI] [PubMed] [Google Scholar]
- 25.Fukunaga K, Goto S, Miyamoto E. Immunohistochemical localization of calcium-calmodulin dependent protein kinase II in rat brain and various tissues. J Neurochem. 1988;51:1070–1078. doi: 10.1111/j.1471-4159.1988.tb03070.x. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh A, Greenberg M E. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 27.Gille H, Sharrocks A D, Shaw P E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature (London) 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 28.Gille H, Kortenjann M, Thoma O, Moomaw C, Slaughter C, Cobb M, Shaw P E. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gille H, Strahl T, Shaw P E. Activation of ternary complex factor Elk-1 by stress-activated protein kinases. Curr Biol. 1995;5:1191–1200. doi: 10.1016/s0960-9822(95)00235-1. [DOI] [PubMed] [Google Scholar]
- 30.Ginty D D, Bading H, Greenberg M E. Trans-synaptic regulation of gene expression. Curr Opin Neurobiol. 1992;2:312–316. doi: 10.1016/0959-4388(92)90121-z. [DOI] [PubMed] [Google Scholar]
- 31.Ginty D D. Calcium regulation of gene expression: isn’t that spatial? Neuron. 1997;18:183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 32.Giovane A, Pintzas A, Maira S M, Sobieszczuk P, Wazylyk B. Net, a new ets transcription factor that is activated by ras. Genes Dev. 1994;8:1502–1513. doi: 10.1101/gad.8.13.1502. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss H K, Dérijard B, Davis R J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 34.Hardingham G E, Chawla S, Johnson C M, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 35.Herrera R E, Shaw P E, Nordheim A. Occupation of the c-fos response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature (London) 1989;340:68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- 36.Hill C S, Marais R, John S, Wynne J, Dalton S, Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993;73:395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- 37.Hipskind R A, Rao V N, Mueller C G F, Reddy E S P, Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature (London) 1991;354:531–534. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- 38.Hipskind R A, Baccarini M, Nordheim A. Transient activation of RAF-1, MEK, and ERK2 coincides kinetically with ternary complex factor phosphorylation and immediate-early gene promoter activity in vivo. Mol Cell Biol. 1994;14:6219–6231. doi: 10.1128/mcb.14.9.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hipskind R A, Büscher D, Nordheim A, Baccarini M. Ras/MAP kinase-dependent and-independent signaling pathways target distinct ternary complex factors. Genes Dev. 1994;8:1803–1816. doi: 10.1101/gad.8.15.1803. [DOI] [PubMed] [Google Scholar]
- 40.Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 41.Jaiswal R K, Moodie S A, Wolfman A, Landreth G E. The mitogen-activated protein kinase cascade is activated by B-Raf in response to nerve growth factor through interaction with p21ras. Mol Cell Biol. 1994;14:6944–6953. doi: 10.1128/mcb.14.10.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janknecht R, Ernst W H, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janknecht R, Nordheim A. MAP kinase-dependent transcriptional coactivation by ELK-1 and its cofactor CBP. Biochem Biophys Res Commun. 1996;228:831–837. doi: 10.1006/bbrc.1996.1740. [DOI] [PubMed] [Google Scholar]
- 44.Janknecht R, Hunter T. Activation of the Sap-1A transcription factor by the c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase. J Biol Chem. 1997;272:4219–4224. doi: 10.1074/jbc.272.7.4219. [DOI] [PubMed] [Google Scholar]
- 45.Janknecht R, Hunter T. Convergence of MAP kinase pathways on the ternary complex factor Sap-1A. EMBO J. 1997;16:1620–1627. doi: 10.1093/emboj/16.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson C M, Hill C S, Chawla S, Treisman R, Bading H. Calcium controls gene expression via three distinct pathways that can function independently of the Ras/mitogen-activated protein kinase (ERKs) signaling cascade. J Neurosci. 1997;17:6189–6202. doi: 10.1523/JNEUROSCI.17-16-06189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubi E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 48.Kurino M, Fukunaga K, Ushio Y, Miyamoto E. Activation of mitogen activated protein kinase in cultured rat hippocampal neurons by stimulation of glutamate receptors. J Neurochem. 1995;65:1282–1289. doi: 10.1046/j.1471-4159.1995.65031282.x. [DOI] [PubMed] [Google Scholar]
- 49.Kwok R P S, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature (London) 1994;370:223. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 50.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 51.Lopez M, Oettgen P, Akbarali Y, Dendorfer U, Liberman T A. ERP, a new member of the ets transcription factor/oncoprotein family: cloning, characterization and differential expression during B-cell development. Mol Cell Biol. 1994;14:3292–3309. doi: 10.1128/mcb.14.5.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 53.Matthews R P, Guthrie C R, Wailes L M, Zhao X, Means A R, McKnight G S. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miranti C K, Ginty D D, Huang G, Chatila T, Greenberg M E. Calcium activated serum response factor-dependent transcription by a Ras- and Elk-1-independent mechanims that involves a Ca2+/calmodulin-dependent kinase. Mol Cell Biol. 1995;15:3672–3684. doi: 10.1128/mcb.15.7.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moodie S, Willumsen B, Weber M, Wolfman A. Complexes of ras-GTP with raf-1 and mitogen activated protein kinase kinase. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 56.Morice, C., F. Nothias, S. Köenig, P. Vernier, M. Baccarini, J. D. Vincent, and J. V. Barnier. Submitted for publication. [DOI] [PubMed]
- 57.Muthalif M M, Benter I F, Uddin M R, Malik K U. Calcium/calmodulin-dependent protein kinase II α mediates activation of mitogen-activated protein kinase and cytosolic phospholipase A2 in norepinephrine-induced arachidonic acid release in rabbit aortic smooth muscle cells. J Biol Chem. 1996;271:30149–30157. doi: 10.1074/jbc.271.47.30149. [DOI] [PubMed] [Google Scholar]
- 58.Norman C, Runswick M, Pollok R, Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988;55:989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 59.Paxinos G, Watson C. The rat brain stereotaxic coordinates. 2nd ed. New York, N.Y: Academic Press; 1986. [Google Scholar]
- 60.Ortiz J, Harris H W, Guitart X, Terwilliger R Z, Haycock J W, Nestler E J. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papin C, Eychène A, Brunet A, Pagès G, Pouysségur J, Calothy G, Barnier J V. B-Raf protein isoforms interact with and phosphorylate Mek-1 on serine residues 218 and 222. Oncogene. 1995;10:1647–1651. [PubMed] [Google Scholar]
- 62.Pelech S L. Signalling pathways: kinase connections on the cellular intranet. Curr Biol. 1996;6:551–554. doi: 10.1016/s0960-9822(02)00540-7. [DOI] [PubMed] [Google Scholar]
- 63.Price M A, Cruzalegui F H, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- 64.Price M A, Rogers A E, Treisman R. Comparative analysis of ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET) EMBO J. 1995;14:2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Retaux S, Besson M J, Pénit-Soria J. Opposing effects of the dopamine D2 receptor stimulation on the spontaneous and the electrically evoked release of 3H-GABA on rat prefrontal cortex slices. Neuroscience. 1991;42:61–71. doi: 10.1016/0306-4522(91)90150-m. [DOI] [PubMed] [Google Scholar]
- 66.Rivera V M, Miranti R P, Misra D D, Ginty D D, Chen R H, Blenis J, Greenberg M E. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA binding activity. Mol Cell Biol. 1993;13:6260–6273. doi: 10.1128/mcb.13.10.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robertson L M, Kerpolla T K, Vendrell M, Luk D, Smeyne R J, Bocchiara C, Morgan J I, Curran T. Regulation of c-fos expression in transgenic mice requires multiple interdependent transcription control elements. Neuron. 1995;14:214–252. doi: 10.1016/0896-6273(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 68.Schröter H, Mueller C G F, Meese K, Nordheim A. Synergism in ternary complex formation between the dimeric glycoprotein p67SRF, polypeptide p62TCF and the c-fos serum response element. EMBO J. 1990;9:1123–1130. doi: 10.1002/j.1460-2075.1990.tb08218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwarzschild M A, Col R L, Hyman S E. Glutamate, but not dopamine, stimulates stress-activated protein kinase and AP-1-mediated transcription in striatal neurons. J Neurosci. 1997;17:3455–3466. doi: 10.1523/JNEUROSCI.17-10-03455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seger R, Krebs E G. The MAPK signaling pathway. FASEB J. 1995;9:6–735. [PubMed] [Google Scholar]
- 71.Sgambato V, Vanhoutte P, Pagès C, Rogard M, Hipskind R A, Besson M J, Caboche J. In vivo expression and regulation of Elk-1, a target of extracellular regulated kinase, in the adult rat brain. J Neurosci. 1998;18:214–226. doi: 10.1523/JNEUROSCI.18-01-00214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw P E, Schröter H, Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989;56:563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- 73.Sheng M, Greenberg M E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 74.Sheng M, Thompson M A, Greenberg M E. CREB: a Ca2+ regulated transcription factor. Science. 1990;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 75.Sheng M, McFadden G, Greenberg M E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 76.Shore P, Sharrocks A D. The transcription factor Elk-1 and serum response factor interact by direct protein-protein contacts mediated by a short region of Elk-1. Mol Cell Biol. 1994;14:3283–3291. doi: 10.1128/mcb.14.5.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siciliano J C, Toutant M, Derkinderen P, Sasaki T, Girault J A. Differential regulation of proline-rich tyrosine kinase 2/cell adhesion kinase b (PYK2/CAKb) and pp125FAK by glutamate and depolarization in rat hippocampus. J Biol Chem. 1996;271:28942–28946. doi: 10.1074/jbc.271.46.28942. [DOI] [PubMed] [Google Scholar]
- 78.Sun P, Lou L, Maurer R A. Regulation of activating transcription factor-1 and the cAMP response element-binding protein by Ca2+/calmodulin dependent protein kinases type I, II, and IV. J Biol Chem. 1996;271:3066–3073. doi: 10.1074/jbc.271.6.3066. [DOI] [PubMed] [Google Scholar]
- 79.Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb M J. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996;15:4629–4642. [PMC free article] [PubMed] [Google Scholar]
- 80.Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Kikada H. KN-62, 1-[N, O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990;265:4315–4320. [PubMed] [Google Scholar]
- 81.Treisman R. Identification of a protein-binding site that mediates the transcriptional response of the c-fos gene to serum factors. Cell. 1986;46:657–674. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- 82.Treisman R. The serum response element. Trends Biochem Sci. 1992;17:423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- 83.Treisman R. Journey to the surface of the cell: fos regulation and the SRE. EMBO J. 1995;14:4905–4913. doi: 10.1002/j.1460-2075.1995.tb00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Treisman R. Regulation of transcription by MAP kinase cascade. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 85.Waskiewicz A J, Cooper J A. Mitogen and stress response pathways: MAP kinase cascade and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 86.Whittmarsh A J, Shore P, Sharroks A D, Davis R J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 87.Whittmarsh A J, Yang S-H, Su M S-S, Sharrocks A D, Davis R J. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 89.Xia Z, Dudek H, Miranti C K, Greenberg M E. Calcium influx via the NMDA receptor induces immediate-early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xing J, Ginty D D, Greenberg M E. Coupling of the ras-MAPK pathway to gene activation by RSK2, a growth-factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 91.Yang D D, Kuan C-Y, Whitmarsh A J, Rinçon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature (London) 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 92.Zinck R, Hipskind R A, Pingoud V, Nordheim A. c-fos transcriptional activation and repression correlate temporally with the phosphorylation status of TCF. EMBO J. 1993;12:2377–2387. doi: 10.1002/j.1460-2075.1993.tb05892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zinck R, Cahill M A, Kracht M, Sachsenmaier C, Hipskind R A, Nordheim A. Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol Cell Biol. 1995;15:4930–4938. doi: 10.1128/mcb.15.9.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]