Highlights
-
•
Mechanistic studies illustrate the effects of PD on systemic inflammation, platelet and endothelial function, and lipoproteins.
-
•
Trials of PD treatment have not shown reductions in cardiovascular outcomes therefore a definite causal association is lacking.
-
•
The morbidity and impact on quality of life, mutual risk factors and systemic inflammation warrant preventive efforts.
-
•
Improved screening, better collaboration, and targeted health policies could greatly improve prevention of PD and its sequelae.
Keywords: Periodontal disease, Oral health, Cardiovascular prevention, Cardiovascular Disease
Abstract
Periodontal disease (PD) is common in the US and globally. Evidence suggests that poor oral health is associated with atherosclerotic cardiovascular disease (ASCVD); however, this relationship has not been a major focus in clinical cardiology. This manuscript will review the growing evidence linking PD to ASCVD, including pathophysiologic mechanisms and coexistent risk factors. Public health considerations with a focus on disparities, social determinants, preventive strategies, and a call to action to reduce the burden of coincident ASCVD and PD are also reviewed.
Graphical abstract
1. Introduction
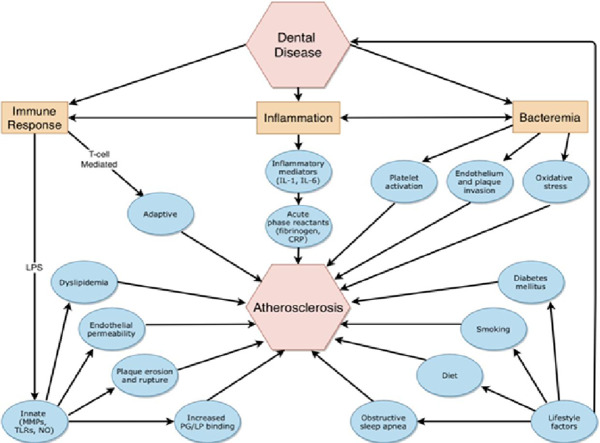
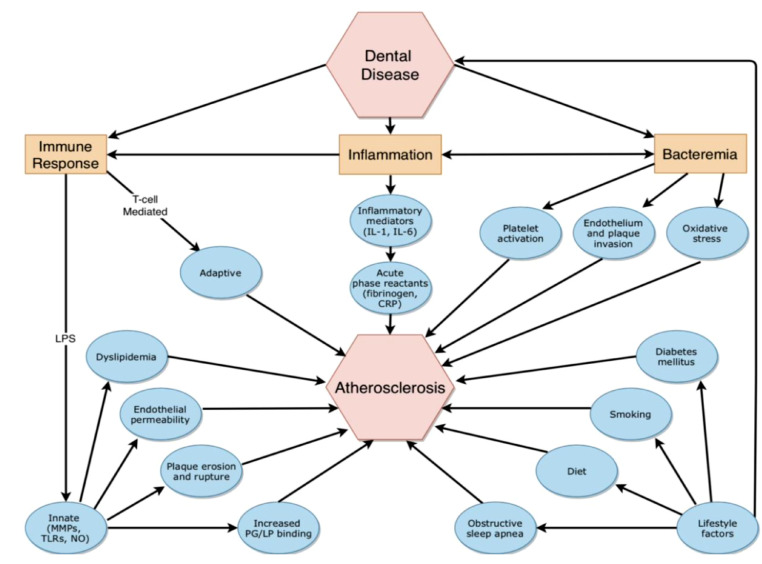
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of disability and death in the U.S. and globally [1]. It is well established that modification of established ASCVD risk factors reduces morbidity and mortality from ASCVD; however, other prevention strategies may also contribute to cardiovascular risk improvements. Periodontal disease (PD) is one of the most prevalent chronic infections, affecting 46% of US adults [2]. In the last 30 years, evidence has accumulated that links PD to ASCVD via inflammatory and immune-mediated mechanisms (Fig. 1). The objective of this narrative review is to summarize the biologic mechanisms and epidemiologic evidence linking poor oral health to ASCVD risk and the role of health inequality.
Fig. 1.
(central illustration): Proposed mechanisms linking dental disease and atherosclerosis. IL: Interleukin; CRP: C-reactive protein; MMP: Matrix metalloproteinase; TLR: Toll-like receptor; NO: Nitric oxide; PG: Proteoglycan; LP: Lipoprotein.
2. Definition and prevalence of periodontal disease
More than 700 oral microbes populate dental surfaces, and dental plaque contains a biofilm that adapts to environmental flora changes [3]. Gingivitis develops when this plaque extends to the surrounding gingiva, triggering an immune response and inflammation. Gingivitis may progress to PD when bacteria and inflammation migrate apically along the root surface and penetrate the tooth's supporting structures, including the surrounding bone. PD can lead to irreversible destruction of connective tissue fibers attached to the tooth, causing bone resorption and tooth loss. PD affects 47% of adults aged 30 years and older and increases with age, occurring in 70% of adults 65 years and older. PD is more common in men than women (56.4% vs 38.4%) and in non-Hispanic blacks and Mexican-Americans. Risk factors for PD include smoking, diabetes, poor oral hygiene, stress, heredity, crooked teeth, compromised immune function, defective fillings, mediations that cause dry mouth, bridges that no longer fit properly and female hormonal changes such as pregnancy or use of oral contraceptives [4,5].
Dysbiosis, defined as an imbalance in oral flora, may trigger bacteremia and facilitate systemic dissemination of oral bacteria [3]. Thus, PD can trigger bacteremia resulting in profound local and systemic inflammatory and immune-mediated responses.6 Risk factors for PD, including diabetes, obesity and smoking, are also risk factors for ASCVD thereby confounding the relationship between PD and ASCVD [7].
3. Biological mechanisms linking pd to ASCVD
3.1. Bacterial translocation
Mechanistic studies have provided evidence for dental infection as a potential mediator of ASCVD. Whether this process is initiated and advanced through direct translocation of bacteria or bacterial products or the downstream inflammatory and immune-mediated mechanisms remains unclear [6]. Bacteremia, of variable degrees, has been confirmed by multiple studies immediately following dental extraction and tooth brushing, which correlates with the systemic markers of inflammation [8]. Periodontal bacteria have also been detected in atheromatous aortic plaques,[9] and in thrombi from patients with acute myocardial infarction (MI),[10] suggesting direct effects of oral microbes at these distant sites. Periodontal bacteria, including Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis (P. gingivalis), have also been found to penetrate human vascular endothelium and cause endothelial dysfunction via lipopolysaccharide (LPS)-mediated effects [11]. LPS on the outer capsule of periodontal gram-negative bacteria, particularly P. gingivalis, participates in numerous other proatherogenic processes discussed below [11].
3.2. Inflammatory mechanisms
Inflammatory cells, including neutrophils, monocytes, and macrophages participate in all phases of atherosclerosis [12]. Similar inflammatory pathways have been shown to exist in PD. Pussinen et al. observed the inflammatory process in both atherosclerosis and periodontitis involve local macrophage activation and low-density lipoproteins (LDL)-mediated foam cell formation in the arterial intima and periodontium, respectively [13]. Pro-inflammatory cytokines and endothelial cell adhesion molecules that have been associated with PD in both human and animal studies include C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukins (IL), including IL-1β, IL-6, IL-8, and IL-17, matrix metalloproteinases (MMP), vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), E-selectin and P-selectin, most of which have been strongly implicated in the initiation and progression of ASCVD [14], [15], [16]. Studies suggest that the collagenolytic activity found at some chronic inflammation sites, such as periodontitis, derives from MMP-8 which has also been implicated in atherosclerotic plaque destabilization [17]. The PAROKRANK (Periodontitis and its Relation to Coronary Artery Disease) study, a Swedish case-control study of 805 subjects with a first MI and 805 matched controls, reported a positive association between PD and the first presentation of an MI, with a significant correlation in patients with severe periodontitis [18]. It also showed that MMPs are increased in the saliva of patients with gingivitis, but could not demonstrate an association between saliva levels of MMPs and MI incidence. A study by Kodovazenitis et al. evaluated the periodontal status and CRP levels in 87 patients with acute MI and determined that serum levels of CRP were higher in post-acute MI patients with PD versus without PD. This suggests a role for PD-mediated inflammation after controlling for confounding from shared risk factors, including smoking and diabetes [19]. Overall, common inflammatory processes seem to underlie the development of both PD and ASCVD but their specific involvement linking these two disease entities will need to be further examined in future studies.
3.3. Immune activation
Similarly, evidence supports the role of PD in triggering immune responses, including macrophage, T-cell and B cell activation [6,20]. Such immune responses are also linked to the development and progression of atherosclerosis [6]. P. gingivalis is a potent activator of macrophages and innate immune responses[11] producing Heat Shock Protein, a trigger molecule that links PD and ASCVD [15]. In animal studies, P. gingivalis tolerizes resident and infiltrating leukocytes and effectively mutes immune response against it, exacerbating PD [21].
3.4. Platelet aggregation and thrombosis
Oral pathogens have also been directly linked to platelet aggregation and thromboembolic events in human in vitro studies,[22] suggesting another mechanism by which PD may trigger ASCVD events. Streptococcus sanguis, frequently isolated from dental plaque, has been noted in animal studies to activate circulating platelets during bacteremia, forming thromboemboli, causing transient myocardial ischemia via coronary occlusion [21]. Subsequent studies in humans have reported that PD is independently associated with increased blood platelets,[23] and that periodontal treatment prevents accelerated platelet activation in patients with PD [24]. Awareness of this heightened platelet response supports the current clinical practice of continuing antiplatelet agents during routine dental procedures.
3.5. Endothelial and other direct vascular effects
There is also indirect evidence implicating endothelial dysfunction in PD. Studies in patients with severe PD have shown attenuated flow-mediated dilatation of the brachial artery compared with matched controls [25]. Tonetti et al. observed that intensive periodontal treatment resulted in improved endothelial function (i.e., increased brachial artery flow) [26]. Periodontal bacteria, Actinobacillus actinomycetemcomitans and P. gingivalis, have been found to penetrate human vascular endothelium [27] and cause endothelial dysfunction via LPS-mediated effects [28]. PD may also contribute to the formation of reactive oxygen species (ROS) within vessel walls, and periodontal treatment thereby reduces biomarkers of oxidative stress [29]. PD may enhance the number of proteoglycans in the intimal extracellular matrix and promote entrapment of remnant lipoproteins within the intima [30]. Lastly, periodontal inflammation has been associated with imbalances in the synthesis of endogenous vasodilators (e.g., nitric oxide, prostacyclin) and with changes in arterial wall distensibility [31].
4. Association between periodontal disease and ascvd risk factors
Strong associations have been noted between PD and vascular risk factors, leading to the development of the common soil hypothesis. This concept postulates that ASCVD risk factors, including physiologic and behavioral risk factors, are common in both diseases and trigger inflammation in both gingival tissue and arterial walls, leading to PD as well as ASCVD events. It is also important to keep in mind that these relationships may be bidirectional in that these disease states could also potentially worsen specific risk factors. ASCVD risk factors linked to PD are discussed below, and raise the important question of whether efforts to mitigate these shared risk factors could also reduce the risk of both PD and ASCVD.
4.1. Diabetes mellitus
There is strong evidence to support a bidirectional relationship between PD and diabetes mellitus (DM). In the PAROKRANK study, undetected dysglycemia was independently associated with both MI and severe PD, and doubled the risk of both [32]. Chronic inflammation, which is observed with PD, is also associated with insulin resistance and worsening glycemic control [33]. Aggressive treatment of PD is associated with improved glycemic control in patients with type 2 DM and improved vascular function as measured by brachial artery flow-mediated dilatation [34,35]. A single-center, parallel-group, randomized control trial by D'Aiuto et al. that randomized 264 patients with type 2 DM and moderate-to-severe periodontitis to intensive periodontal treatment (IPD) versus conventional periodontal treatment (CPT) and showed that IPD was associated with improvements in HgA1c levels at 12-months compared with community-based oral care [34].
4.2. Cigarette smoking and vaping
Cigarette smoking and electronic cigarettes are both associated with significant increases in the risk of CVD events. Both are also associated with PD, oral bone loss, gingivitis, and tooth loss [36]. Presumed mechanisms are increases in oxidative stress and inflammatory cytokines [37]. In large retrospective studies, smoking 15 or more cigarettes per day was associated with an OR of 3.64 (95% CI, 3.00 - 4.42) for tooth loss in men and 2.47 (95% CI, 2.11 - 2.89) in women after adjustment for multiple potential confounders [38]. Similarly, based on data from 12,325 US adults in the NHANES, a strong risk of PD was observed among young smokers (adjusted OR 18.55; 95% CI 9.44 - 36.45) compared to non-smokers [39]. For smokers 50 years or older, the PD risk was even higher (adjusted OR 25.64; 13.04 - 50.40). Oral microflora, and the inflammatory response to dental plaque biofilm, appear to be affected by smoking.36, 38\
4.3. Dyslipidemia
PD is associated with pro-atherogenic changes in lipoprotein metabolism, as reflected in increased plasma levels of non-esterified fatty acids, cholesterol, and triglycerides (TGs) [15]. A 2017 meta-analysis noted a significant positive association between PD and elevated blood levels of low-density lipoprotein cholesterol (LDL-C) and TGs, and lower levels of high-density lipoprotein cholesterol (HDL-C), after adjusting for potential confounders, including diabetes and smoking [40]. LDL particles in patients with PD are reported to be smaller, denser, and more atherogenic than patients without PD [41]. Nishimura et al. notes human inflammatory cytokines released in response to P. gingivalis increase the expression of 3‑hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, thereby increasing hepatic cholesterol synthesis and circulating levels of very low-density lipoprotein (VLDL) and LDL [42]. In observational studies, statin treatment has been associated with a reduced incidence of PD [43]. These findings may relate to the anti-inflammatory effects of statins; however, the potential for confounding due to better access to health care cannot be excluded [43]. It should also be noted that periodontal therapy has not been found to have an impact on lipid parameters [44].
4.4. Hypertension
Data from the National Health and Nutrition Examination Survey (NHANES) from 2009 to 2014 supports a positive association between elevated blood pressure (BP) and severe PD [45]. A recent systematic review of 40 publications, including three cohort studies, showed that the odds of having hypertension were significantly higher in patients with periodontitis than without periodontitis [46]. Furthermore, there is a positive linear association between increasing severity of periodontitis and hypertension. A 2018 study of 3600 participants with hypertension found that those with PD were less likely to respond to anti-hypertensive medications and 20% less likely to achieve BP control [45]. In the Korea National Health and Nutrition Examination Survey (KNHANES), data from approximately 20,000 participants from Korea between 2008 and 2010 were analyzed to determine the relationship of the prevalence and control rate of hypertension and numerous variables, including oral hygiene behavior assessed by questionnaires. Using a multivariate analysis and adjusting for various factors, they found that systolic BP was lower in those who reported an increase in tooth brushing frequency and the use of secondary oral hygiene products. The adjusted odds ratio (OR) of hypertension prevalence was 1.195 (95% CI, 1.033 - 1.383) for subjects who brushed their teeth hardly ever or once daily versus those who brushed after every meal. They concluded that poor oral hygiene may be an independent risk factor for hypertension and implementing good oral hygiene behaviours may help prevent and control hypertension [47]. A biologically plausible mechanism may be an improvement in inflammation and, therefore, endothelial function.
4.5. Obesity
Obesity is associated with chronic inflammatory diseases such as arthritis, diabetes and cardiovascular disease and similarly, is associated with higher rates of PD. In one study, obesity was associated with two times the rate of PD than people with a normal BMI [48]. An early study looking at NHANES III data (n = 13,664) found a correlation between BMI and PD in younger adults, but not middle-age or olders adults [49]. The OR of having PD for BMI <18.5 kg/m2, 25−29.9 kg/m2, and ≥30 kg/m2 were 0.21, 1.00 and 1.76 respectively. Younger patients with high waist circumference had an OR of 2.27 for having PD. Overall obesity and abdominal obesity were linked to an increased prevalence of PD, especially in patients aged 18 to 34, and may represent a critical population that would benefit from the promotion of healthy nutrition and adequate physical activity.
The links between obesity and PD may lie in common risk factors including specific dietary components, associated diabetes or suboptimal lifestyle behaviors overall. However, it is also feasible that the systemic inflammation associated with obesity contributes to PD [44]. Adipocytes contain activated macrophages and as adipocytes become more abundant and larger, these activated macrophages trigger production of cytokines, such as interleukins and tumor necrosis factors [50,51]. It is these inflammatory cytokines which may increase a host's risk for periodontal breakdown.
4.6. Dietary quality
Dietary patterns are associated with both oral and cardiovascular health, with similar diets linked to benefits in both conditions. Compared to a Western dietary pattern, diets high in fruit and vegetable intake have been associated with a lower risk of PD [52]. Fiber intake is associated with lower prevalence and severity of PD [53]. Whole grain intake is also inversely associated with incident PD [54]. This relationship was postulated to be, in part, due to the fiber content of whole grains leading to decreased systemic inflammation [54]. In contrast, refined carbohydrates are positively associated with dental caries, and PD [55]. Saturated fats are positively associated with PD in a dose-dependent manner, possibly mediated through increased inflammation [56].
5. Association between periodontal disease and ASCVD
Studies in humans have observed a positive association between PD and ASCVD since the late 1980s [57]. PD has been associated with an increased overall ASVCD risk of an estimated 3.5 fold,[58] and a 24–35% increased risk of acute CHD events [59]. In a meta-analysis of 22 studies that included 129,630 participants, PD increased the risk of MI by 2-fold (pooled OR 2.02, 95% CI, 1.59–2.57) after adjusting for cardiovascular risk factors [60]. Likewise, a meta-analysis of 7 studies that individually adjusted for a variety of risk factors, found that PD was a significant risk factor for developing peripheral arterial disease [61]. A positive association between PD and incident ischemic stroke was observed among 10,362 adults after controlling for age, cardiovascular risk factors, and socioeconomic characteristics [62]. PD severity has been observed to be associated with an increased risk for ischemic stroke [62].
Although the association between PD and ASCVD was noted to hold true after adjustment for demographic and cardiovascular risk factors in the above studies, several studies have noted attenuation in the association between PD and ASCVD after adjustment for demographic and cardiovascular risk factors [63]. Park et al. observed an increased risk of ASCVD events (a composite of CV mortality, non-fatal MI, HF, and non-fatal stroke) among 247,696 Korean adults with PD compared to those without PD over 10-years of follow-up [64]. Although this association was no longer statistically significant after adjustment for age and cardiovascular risk factors, the number of missing teeth, dental caries, daily tooth brushings, and professional cleanings all remained significantly associated with cardiovascular outcomes [64].
6. Association between periodontal disease and subclinical ASCVD
In an effort to understand the increased ASCVD rates noted, it is also important to understand whether PD is associated with subclinical disease. A recent large prospective cohort study showed that, during a median follow-up of 27 years, there was a positive link between childhood oral infections and subsequent subclinical atherosclerosis [65]. The presence of periodontitis in a cohort of 755 children was associated with a RR of 1.69 (95% CI 1.21 - 2.36) for the development of increased carotid artery intima-media thickness (IMT) in adulthood. In addition, a recent study of 304 patients who underwent PET imaging for cancer screening and had no clinical CVD at the time of the PET scan showed that PD was associated with a 2.25-fold increased risk (95% CI 1.47 - 3.44, p < 0.001) of developing a major adverse cardiovascular event, which was believed to be secondary to arterial inflammation in 80% of cases [66]. Although these observations suggest a causal mechanism, it has yet to be confirmed.
7. Treatment of pd to reduce cvd risk
Periodontal therapy may include dental hygiene, surgical approaches (e.g., deep scaling and root planning), and in some cases, antibiotic management. In a study in which dental hygiene was held for 21 days in young adults, improvements in markers of systemic inflammation (hsCRP, IL-6, and MCP-1) were observed when dental hygiene was resumed [67]. A recent review also noted that nonsurgical periodontal treatment may reduce levels of systemic inflammatory markers in the short-term, potentially influencing atherogenesis and resolving inflammatory oral foci [68].
Despite the observational data that links PD to the risk of ASCVD, evidence that treatment of PD reduces hard cardiovascular events is limited. A recent Cochrane review noted a single RCT of patients with ASCVD and PD that examined whether surgical intervention for PD (i.e., deep scaling and root planning) reduced inflammation and ASCVD events compared to maintenance treatment (oral hygiene instruction with recommendations to see a dentist) [69]. The use of low-dose antibiotics such as doxycycline can decrease PD by inhibiting MMPs, suggesting a mechanism that could reduce ASCVD events [70]. However, data examining the use of doxycycline for the prevention of ASCVD progression is not conclusive and routine use of antibiotics without a clear indication should be avoided due to the risks of side effects, drug-resistance and changes in the microbiome [70]. Other agents targeting inflammation such as aspirin, statins, and omega-3 fatty acids in treating PD show promise;[43,71] however, additional data is warranted.
8. A call to action: leveraging public awareness, literacy and health care reform to improve the risk of pd and future CVD
The observed relationship between PD and ASCVD has important public health implications. Both are highly prevalent and disproportionately affect those with lower socioeconomic status (SES). In a report by the Institute of Medicine in 2011, they recommended that interprofessional, team-based dental care has the potential to improve care-coordination, patient oucomes and produce cost savings [72].
The noted link between poor oral health and SES appears to be due to both low patient awareness and greater barriers to regular dental care, with low to middle income families affected the most [73]. A systematic review of 41 predominantly cross-sectional studies found that the number of dental caries was associated with education, income, and occupation [74] as well as low health literacy [75,76], yet data also suggest these disparities are not reduced when barriers to oral care are eliminated [76]. Therefore, patient education by clinicians and the public health sector are likely needed to raise awareness of the importance of dental health for reducing future CV disease (Table 1). Infographics and tailored print information via patient portals from dental and cardiology professionals (e.g., like the American College of Cardiology CardioSmart portal), as well as digital education and messaging, and mobile device and applictaions, could play important roles.
Table 1.
A call to action.
| Action Item | Description | Potential Impact |
|---|---|---|
| Clinician Education | Increase clinician awareness of the association between PD and CVD to facilitate screening practices, education of patients, and early referrals. | Early implementation of preventative care strategies to reduce PD and CVD risk. |
| Screening for CVD in Dental Practices | Recognition of CV Risk factors in PD patients (including hypertension, obesity and smoking screening) accomplished with BP checks and weights with BMI calculation in the dental office. | Increase preventive and treatment strategies for undiagnosed CVD |
| Screening for PD in Medical Offices (including cardiology) | Recognition of PD risk factors in CVD patients with reminders to visit twice annually for dental checkups, periodic questioning about oral health with a focus on oral health during the physical exam. | Increase the preventive and treatment strategies for undiagnosed PD |
| Improved Communication/ Collaboration | Enhance communication between dentists, physicians, and patients in a multidisciplinary model by obtaining the name of the dental and physician providers at check in, sharing office / progress notes and holding interdisciplinary conferences. | Improved quality of care and opportunity for collaborative health care solutions as well as research investigation. |
| Shared Data/EMR | Use of shared EMR to facilitate information exchange. The future may allow for increased interoperability between dental and medical EMRs. | Increase the recognition of risk factors that might otherwise be missed with more automated alerts. |
| Lifestyle Programs | Increased availability of accessible programs for lifestyle modification (smoking cessation, healthier eating, weight loss, blood pressure management) | Improved oral and CV health attainment. |
| Health Care Reform | Advocacy by clinicians, medical and dental organizations for increased patient coverage through Medicaid and Medicare plans. | Expand coverage for preventive care and treatment of PD to lessen the downstream associated disease |
Improving clinical awareness of patient populations at risk for PD-associated CV events is also needed, and could lead to better screening, prevention and treatment. As noted, oral health has not been a major focus of clinical cardiologists or CV team members, except for patients planning valve surgery [73]. Routine screening, identification and treatment of PD in post-MI patients, triggered by cardiology referrals to dentists, could potentially reduce the risk of recurrent ischemic events. Better education of dental clinicians about the link between PD and CVD could provide opportunities for screening of CVD risk factors among underserved dental patients with reduced access to other medical care, and could potentially improve health and economic outcomes. Such educational efforts should start within medical and dental school curricula. Recent modeling data have shown that dental practice-based screening for high BP, cholesterol and glucose in adults age 40 and older who have not seen a physician in more than 12 months could save the health care system between $42.4 million ($13.51 saved per person screened) and $102.6 million ($32.72 saved per person screened) over one year [77].
Better communication and integration between medical and dental specialists could also improve CVD outcomes linked to PD. Medical and dental care are currently fragmented and in need of better integration nationally and locally, with shared Electronic Health Records (EHR's) potentially playing a larger role. Lastly, interdisciplinary conferences are needed to determine the best strategies for clinical collaborations and to identify needs for future research on the association between oral health and ASCVD.
Better health policies that impact dental health are also needed. In the US, few dental providers participate in Medicaid; traditional Medicare Part A and B plans provide no dental coverage except for that performed in a hospital; and dental care “deserts” exist in many rural areas [78]. Broader use of Medicare Advantage (Part C) plans, which now cover most preventive dental services, and increased Medicaid coverage for low income individuals (currently only in 17 states) could reduce disparities in dental care among the 50 million elderly Medicare beneficiaries in the U.S, underserved populations and those purchasing individual health insurance plans [78].
9. Future research
The current review is limited by the inability to establish causality between PD and CVD in prior studies due to confounding factors from multiple shared risk factors and pathophysiologic mechanisms. The existing data is predominantly observational, with limited sample size and very little randomized controlled trial data. In addition, the criteria and evaluation methods for PD appear to differ among studies potentially impacting outcomes.
Studies that investigate genetic susceptibility and less studied molecular mechanisms are needed to improve our understanding of the link between PD and ASCVD. It would be interesting to conduct a prospective cohort study following patients with a new diagnosis of PD and screen them for the development of clinical and subclinical ASCVD, and conversely, screen those with clinical ASCVD for the development of PD. There is also a need for high quality data from randomized controlled trials to evaluate whether PD contributes to the development of ASCVD after controlling for confounding factors.
10. Conclusions
Despite the lack of causality, PD and ASCVD are inflammatory diseases that share many common pathophysiological mechanisms and risk factors. However, efforts aimed at improving awareness and prevention of PD as a risk factor for ASCVD should not be delayed while awaiting greater proof of. Increased public and provider awareness, and interdisciplinary research and clinical collaborations (related to screening, prevention and referrals), combined with ongoing dental health care policy reforms may greatly reduce the high prevalence of PD and the associated burden of CVD in the U.S. population.
Statement of authorship
Eugenia Gianos MD, Elizabeth A. Jackson MD MPH, Andrew M. Freeman MD, Kenneth E. Fleisher DDS, Astha Tejpal MD, Karen Aspry MD MS worked together on manuscript design, writing and editing. James O'Keefe MD, Monica Aggarwal MD, Ankur Jain MD, Dipti Itchhaporia MD,6Kim Williams MD, Travis Batts MD, Clark Yarber MD, Robert Ostfeld MD MS [11], Michael Miller MD [12], Koushik Reddy MD [13], each wrote sections of the manuscript. Kathleen E. Allen MS RD- assisted with writing and managing the references for the manuscript.
Declaration of Competing Interest
EAJ Research funding: (NIH [R01AG045136, UL1TR001417, UOA-212,513, P30CA013148-47S6], Amgen; Consulting: ACC, McKesson; Roylaties: UpToDate; Editorial Board: American Heart Association; JOK major ownership interest: CardioTabs; DI Research Funding Amgen; RO Consulting: Better Therapeutics; AMF: Speaker Board Boehringer-Ingleheim; All other authors have nothing to disclose.
Funding and disclosures
EAJ Research funding: (NIH [R01AG045136, UL1TR001417, UOA-212513, P30CA013148-47S6], Amgen; Consulting: ACC, McKesson; Royalties: UpToDate; Editorial Board: American Heart Association; JOK major ownership interest: CardioTabs; DI Research Funding Amgen; RO Consulting: Better Therapeutics; AMF: Speaker Board Boehringer-Ingleheim; RO received research grants from the Purjes and Greenbaum Foundations and consulted for Better Therapeutics (2018). He is also a speaker for Main Street Vegan Academy and the International Plant-Based Nutrition Healthcare Conference. He is on the Board of Directors, (uncompensated) for T. Colin Campbell Center for Nutrition Studies, sits on the Scientific Advisory Board (uncompensated) for Physician Committee for Responsible Medicine, and consults for Brightplate. All other authors have nothing to disclose.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics-2020 update: A report from the American heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Eke P.I., Dye B.A., Wei L., Slade G.D., Thornton-Evans G.O., Borgnakke W.S. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilian M., Chapple I.L., Hannig M., Marsh P.D., Meuric V., Pedersen A.M. The oral microbiome - an update for oral healthcare professionals. Br Dent J. 2016;221(10):657–666. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 4.Division of oral health NCfCDPaHP. Periodontal disease. July 10, 2013 March 4, 2021]; Available from: https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html
- 5.Thornton-Evans G., Eke P., Wei L., Palmer A., Moeti R., Hutchins S. Periodontitis among adults aged ≥30 years - United States, 2009-2010. MMWR Suppl. 2013;62(3):129–135. [PubMed] [Google Scholar]
- 6.Libby P., Loscalzo J., Ridker P.M., Farkouh M.E., Hsue P.Y., Fuster V. Inflammation, immunity, and infection in atherothrombosis: jacc review topic of the week. J Am Coll Cardiol. 2018;72(17):2071–2081. doi: 10.1016/j.jacc.2018.08.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AlJehani Y.A. Risk factors of periodontal disease: review of the literature. Int J Dent. 2014;2014 doi: 10.1155/2014/182513. 182513-182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Forner L., Larsen T., Kilian M., Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33(6):401–407. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 9.Reyes L., Herrera D., Kozarov E., Roldan S. Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Clin Periodontol. 2013;40(14):S30–S50. doi: 10.1111/jcpe.12079. Suppl. [DOI] [PubMed] [Google Scholar]
- 10.Ohki T., Itabashi Y., Kohno T., Yoshizawa A., Nishikubo S., Watanabe S. Detection of periodontal bacteria in thrombi of patients with acute myocardial infarction by polymerase chain reaction. Am Heart J. 2012;163(2):164–167. doi: 10.1016/j.ahj.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Stoll Lynn L., Denning Gerene M., Weintraub Neal L. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(12):2227–2236. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 12.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pussinen P.J., Vilkuna-Rautiainen T., Alfthan G., Palosuo T., Jauhiainen M., Sundvall J. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2004;24(11):2174–2180. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- 14.Talvan E., Mohor C., Chisnoiu D., Cristea V., Campian R. Expression of interleukin (IL)-1β, IL-8, IL-10 and IL-13 in chronic adult periodontitis progression. Arch Med. 2017;9 [Google Scholar]
- 15.Schenkein H.A., Loos B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol. 2013;40(14):S51–S69. doi: 10.1111/jcpe.12060. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrizales-Sepulveda E.F., Ordaz-Farias A., Vera-Pineda R., Flores-Ramirez R. Periodontal Disease Systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018;27(11):1327–1334. doi: 10.1016/j.hlc.2018.05.102. [DOI] [PubMed] [Google Scholar]
- 17.Herman M.P., Sukhova G.K., Libby P., Gerdes N., Tang N., Horton D.B. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104(16):1899–1904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 18.Ryden L., Buhlin K., Ekstrand E., de Faire U., Gustafsson A., Holmer J. Periodontitis increases the risk of a first myocardial infarction: a report from the PAROKRANK study. Circulation. 2016;133(6):576–583. doi: 10.1161/CIRCULATIONAHA.115.020324. [DOI] [PubMed] [Google Scholar]
- 19.Kodovazenitis G., Pitsavos C., Papadimitriou L., Deliargyris E.N., Vrotsos I., Stefanadis C. Periodontal disease is associated with higher levels of C-reactive protein in non-diabetic, non-smoking acute myocardial infarction patients. J Dent. 2011;39(12):849–854. doi: 10.1016/j.jdent.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Qi J., Zihang Z., Zhang J., Park Y.M., Shrestha D., Jianling B. Periodontal antibodies and all-cause and cardiovascular disease mortality. J. Dent. Res. 2020;99(1):51–59. doi: 10.1177/0022034519884012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzberg M.C., Weyer M.W. Dental plaque, platelets, and cardiovascular diseases. Ann Periodontol. 1998;3(1):151–160. doi: 10.1902/annals.1998.3.1.151. [DOI] [PubMed] [Google Scholar]
- 22.Herzberg M.C., Nobbs A., Tao L., Kilic A., Beckman E., Khammanivong A. Oral streptococci and cardiovascular disease: searching for the platelet aggregation-associated protein gene and mechanisms of Streptococcus sanguis-induced thrombosis. J Periodontol. 2005;76(11):2101–2105. doi: 10.1902/jop.2005.76.11-S.2101. Suppl. [DOI] [PubMed] [Google Scholar]
- 23.Romandini M., Lafori A., Romandini P., Baima G., Cordaro M. Periodontitis and platelet count: a new potential link with cardiovascular and other systemic inflammatory diseases. J Clin Periodontol. 2018;45(11):1299–1310. doi: 10.1111/jcpe.13004. [DOI] [PubMed] [Google Scholar]
- 24.Laky M., Anscheringer I., Wolschner L., Heber S., Haririan H., Schrottmaier W.C. Periodontal treatment limits platelet activation in patients with periodontitis-a controlled-randomized intervention trial. J Clin Periodontol. 2018;45(9):1090–1097. doi: 10.1111/jcpe.12980. [DOI] [PubMed] [Google Scholar]
- 25.Amar S., Gokce N., Morgan S., Loukideli M., Van Dyke T.E., Vita J.A. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol. 2003;23(7):1245–1249. doi: 10.1161/01.ATV.0000078603.90302.4A. [DOI] [PubMed] [Google Scholar]
- 26.Tonetti M.S., D'Aiuto F., Nibali L., Donald A., Storry C., Parkar M. Treatment of Periodontitis and Endothelial Function. New Eng J Med. 2007;356(9):911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 27.Kozarov E.V., Dorn B.R., Shelburne C.E., Dunn W.A., Jr. Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. 2005;25(3):e17–e18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 28.Kebschull M., Demmer R.T., Papapanou P.N. Gum bug, leave my heart alone!"–epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010;89(9):879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Silva J.C., Muniz F., Oballe H.J.R., Andrades M., Rosing C.K., Cavagni J. The effect of periodontal therapy on oxidative stress biomarkers: a systematic review. J Clin Periodontol. 2018;45(10):1222–1237. doi: 10.1111/jcpe.12993. [DOI] [PubMed] [Google Scholar]
- 30.Bale B.F., Doneen A.L., Vigerust D.J. High-risk periodontal pathogens contribute to the pathogenesis of atherosclerosis. BMJ. 2017;93(1098):215–220. doi: 10.1136/postgradmedj-2016-134279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolosi L.N., Lewin P.G., Rudzinski J.J., Pompeo M., Guanca F., Rodríguez P. Relation between periodontal disease and arterial stiffness. J. Periodont. Res. 2017;52(1):122–126. doi: 10.1111/jre.12376. [DOI] [PubMed] [Google Scholar]
- 32.Norhammar A., Kjellström B., Habib N., Gustafsson A., Klinge B., Nygren Å. Undetected dysglycemia is an important risk factor for two common diseases, myocardial infarction and periodontitis: a report from the PAROKRANK study. Diabetes Care. 2019;42(8):1504–1511. doi: 10.2337/dc19-0018. [DOI] [PubMed] [Google Scholar]
- 33.Santos Tunes R., Foss-Freitas M.C., Nogueira-Filho Gda R. Impact of periodontitis on the diabetes-related inflammatory status. J Can Dent Assoc. 2010;76:a35. [PubMed] [Google Scholar]
- 34.D'Aiuto F., Gkranias N., Bhowruth D., Khan T., Orlandi M., Suvan J. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018;6(12):954–965. doi: 10.1016/S2213-8587(18)30038-X. [DOI] [PubMed] [Google Scholar]
- 35.Grossi S.G., Skrepcinski F.B., DeCaro T., Robertson D.C., Ho A.W., Dunford R.G. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68(8):713–719. doi: 10.1902/jop.1997.68.8.713. [DOI] [PubMed] [Google Scholar]
- 36.Chaffee B.W., Couch E.T., Ryder M.I. The tobacco-using periodontal patient: role of the dental practitioner in tobacco cessation and periodontal disease management. Periodontol. 2016;71(1):52–64. doi: 10.1111/prd.12120. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karaaslan F., Dikilitaş A., Yiğit U. The effects of vaping electronic cigarettes on periodontitis. Aust Dent J. 2020;65(2):143–149. doi: 10.1111/adj.12747. [DOI] [PubMed] [Google Scholar]
- 38.Dietrich T., Walter C., Oluwagbemigun K., Bergmann M., Pischon T., Pischon N. Smoking, smoking cessation, and risk of tooth loss: the EPIC-potsdam study. J Dent Res. 2015;94(10):1369–1375. doi: 10.1177/0022034515598961. [DOI] [PubMed] [Google Scholar]
- 39.Hyman J.J., Reid B.C. Epidemiologic risk factors for periodontal attachment loss among adults in the United States. J Clin Periodontol. 2003;30(3):230–237. doi: 10.1034/j.1600-051x.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 40.Nepomuceno R., Pigossi S.C., Finoti L.S., Orrico S.R.P., Cirelli J.A., Barros S.P. Serum lipid levels in patients with periodontal disease: a meta-analysis and meta-regression. J Clin Periodontol. 2017;44(12):1192–1207. doi: 10.1111/jcpe.12792. [DOI] [PubMed] [Google Scholar]
- 41.Griffiths R., Barbour S. Lipoproteins and lipoprotein metabolism in periodontal disease. Clin Lipidol. 2010;5(3):397–411. doi: 10.2217/clp.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura F., Taniguchi A., Yamaguchi-Morimoto M., Soga Y., Iwamoto Y., Kokeguchi S. Periodontal infection and dyslipidemia in type 2 diabetics: association with increased HMG-CoA reductase expression. Horm Metab Res. 2006;38(8):530–535. doi: 10.1055/s-2006-949525. [DOI] [PubMed] [Google Scholar]
- 43.Muniz F., Taminski K., Cavagni J., Celeste R.K., Weidlich P., Rosing C.K. The effect of statins on periodontal treatment-a systematic review with meta-analyses and meta-regression. Clin Oral Investig. 2018;22(2):671–687. doi: 10.1007/s00784-018-2354-9. [DOI] [PubMed] [Google Scholar]
- 44.Kamil W., Al Habashneh R., Khader Y., Al Bayati L., Taani D. Effects of nonsurgical periodontal therapy on C-reactive protein and serum lipids in Jordanian adults with advanced periodontitis. J Periodontal Res. 2011;46(5):616–621. doi: 10.1111/j.1600-0765.2011.01380.x. [DOI] [PubMed] [Google Scholar]
- 45.Pietropaoli D., Pinto R.D., Ferri C., Wright J.T., Giannoni M., Ortu E. Poor oral health and blood pressure control among US hypertensive adults. Hypertension. 2018;72(6):1365–1373. doi: 10.1161/HYPERTENSIONAHA.118.11528. [DOI] [PubMed] [Google Scholar]
- 46.Muñoz Aguilera E., Suvan J., Buti J., Czesnikiewicz-Guzik M., Barbosa Ribeiro A., Orlandi M. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. 2020;116(1):28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 47.Choi H.M., Han K., Park Y.-.G., Park J.-.B. Associations among oral hygiene behavior and hypertension prevalence and control: the 2008 to 2010 Korea national health and nutrition examination survey. J Periodontol. 2015;86(7):866–873. doi: 10.1902/jop.2015.150025. [DOI] [PubMed] [Google Scholar]
- 48.Khader Y.S., Bawadi H.A., Haroun T.F., Alomari M., Tayyem R.F. The association between periodontal disease and obesity among adults in Jordan. J Clin Periodontol. 2009;36(1):18–24. doi: 10.1111/j.1600-051X.2008.01345.x. [DOI] [PubMed] [Google Scholar]
- 49.Al-Zahrani M.S., Bissada N.F., Borawskit E.A. Obesity and periodontal disease in young, middle-aged, and older adults. J Periodontol. 2003;74(5):610–615. doi: 10.1902/jop.2003.74.5.610. [DOI] [PubMed] [Google Scholar]
- 50.Kwon H., Pessin J.E. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne) 2013;4:71. doi: 10.3389/fendo.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dodington D.W., Fritz P.C., Sullivan P.J., Ward W.E. Higher intakes of fruits and vegetables, beta-Carotene, Vitamin C, alpha-Tocopherol, EPA, and DHA are positively associated with periodontal healing after nonsurgical periodontal therapy in nonsmokers but not in smokers. J Nutr. 2015;145(11):2512–2519. doi: 10.3945/jn.115.211524. [DOI] [PubMed] [Google Scholar]
- 53.Nielsen S.J., Trak-Fellermeier M.A., Joshipura K., Dye B.A. Dietary fiber intake is inversely associated with periodontal disease among US adults. J Nutr. 2016;146(12):2530–2536. doi: 10.3945/jn.116.237065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merchant A.T., Pitiphat W., Franz M., Joshipura K.J. Whole-grain and fiber intakes and periodontitis risk in men. Am J Clin Nutr. 2006;83(6):1395–1400. doi: 10.1093/ajcn/83.6.1395. [DOI] [PubMed] [Google Scholar]
- 55.Lula E.C., Ribeiro C.C., Hugo F.N., Alves C.M., Silva A.A. Added sugars and periodontal disease in young adults: an analysis of NHANES III data. Am J Clin Nutr. 2014;100(4):1182–1187. doi: 10.3945/ajcn.114.089656. [DOI] [PubMed] [Google Scholar]
- 56.Iwasaki M., Manz M.C., Moynihan P., Yoshihara A., Muramatsu K., Watanabe R. Relationship between saturated fatty acids and periodontal disease. J Dent Res. 2011;90(7):861–867. doi: 10.1177/0022034511405384. [DOI] [PubMed] [Google Scholar]
- 57.Yu Y.H., Chasman D.I., Buring J.E., Rose L., Ridker P.M. Cardiovascular risks associated with incident and prevalent periodontal disease. J Clin Periodontol. 2015;42(1):21–28. doi: 10.1111/jcpe.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Genco R., Offenbacher S., Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc. 2002;(133):14s–22s. doi: 10.14219/jada.archive.2002.0375. Suppl. [DOI] [PubMed] [Google Scholar]
- 59.Sanz M., D'Aiuto F., Deanfield J., Fernandez-Avilés F. European workshop in periodontal health and cardiovascular disease—Scientific evidence on the association between periodontal and cardiovascular diseases: a review of the literature. Eur Heart J Suppl. 2010;12(suppl_B):B3–B12. [Google Scholar]
- 60.Xu S., Song M., Xiong Y., Liu X., He Y., Qin Z. The association between periodontal disease and the risk of myocardial infarction: a pooled analysis of observational studies. BMC Cardiovasc Disord. 2017;17(1):50. doi: 10.1186/s12872-017-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang S., Zhao L.S., Cai C., Shi Q., Wen N., Xu J. Association between periodontitis and peripheral artery disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2018;18(1):141. doi: 10.1186/s12872-018-0879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sen S., Giamberardino L.D., Moss K., Morelli T., Rosamond W.D., Gottesman R.F. Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke. 2018;49(2):355–362. doi: 10.1161/STROKEAHA.117.018990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lockhart P.B., Bolger A.F., Papapanou P.N., Osinbowale O., Trevisan M., Levison M.E. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125(20):2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 64.Park S.-.Y., Kim S.-.H., Kang S.-.H., Yoon C.-.H., Lee H.-.J., Yun P.-.Y. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur. Heart J. 2018;40(14):1138–1145. doi: 10.1093/eurheartj/ehy836. [DOI] [PubMed] [Google Scholar]
- 65.Pussinen P.J., Paju S., Koponen J., Viikari J.S.A., Taittonen L., Laitinen T. Association of childhood oral infections with cardiovascular risk factors and subclinical atherosclerosis in adulthood. JAMA Network Open. 2019;2(4) doi: 10.1001/jamanetworkopen.2019.2523. e192523-e192523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Dyke T.E., Kholy K.E., Ishai A., Takx R.A.P., Mezue K., Abohashem S.M. Inflammation of the periodontium associates with risk of future cardiovascular events. J Periodontol. 2021:1–11. doi: 10.1002/JPER.19-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eberhard J., Grote K., Luchtefeld M., Heuer W., Schuett H., Divchev D. Experimental gingivitis induces systemic inflammatory markers in young healthy individuals: a single-subject interventional study. PLoS ONE. 2013;8(2):e55265. doi: 10.1371/journal.pone.0055265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D'Isidoro O., Perrotti V., Hui W.L., Piattelli A., Iaculli F., Quaranta A. The impact of non-surgical therapy of periodontal disease on surrogate markers for cardiovascular disease: a literature review. Am J Dent. 2019;32(4):191–200. [PubMed] [Google Scholar]
- 69.Li C., Lv Z., Shi Z., Zhu Y., Wu Y., Li L. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. 2014;(8) doi: 10.1002/14651858.CD009197.pub2. Cd009197. [DOI] [PubMed] [Google Scholar]
- 70.Tilakaratne A., Soory M. Anti-inflammatory actions of adjunctive tetracyclines and other agents in periodontitis and associated comorbidities. Open Dent J. 2014;8:109–124. doi: 10.2174/1874210601408010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chee B., Park B., Fitzsimmons T., Coates A.M., Bartold P.M. Omega-3 fatty acids as an adjunct for periodontal therapy-a review. Clin Oral Investig. 2016;20(5):879–894. doi: 10.1007/s00784-016-1750-2. [DOI] [PubMed] [Google Scholar]
- 72.Institute of Medicine . The National Academies Press; Washington, DC: 2011. Improving access to oral health care for vulnerable and underserved populations. [Google Scholar]
- 73.Sanchez P., Everett B., Salamonson Y., Ajwani S., Bhole S., Bishop J. Oral health and cardiovascular care: perceptions of people with cardiovascular disease. PLoS ONE. 2017;12(7) doi: 10.1371/journal.pone.0181189. e0181189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costa S.M., Bonfim M., Zina L.G., Paiva S.M., Pordeus I.A., Abreu M.H.N.G. A systematic review of socioeconomic indicators and dental caries in adults. Int J Environ Res Public Health. 2012;9(10):3540–3574. doi: 10.3390/ijerph9103540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batista M.J., Lawrence H.P., Sousa M.D.L.R. Oral health literacy and oral health outcomes in an adult population in Brazil. BMC Public Health. 2017;18(1):60. doi: 10.1186/s12889-017-4443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J.Y., Divaris K. The ethical imperative of addressing oral health disparities: a unifying framework. J Dent Res. 2014;93(3):224–230. doi: 10.1177/0022034513511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nasseh K., Greenberg B., Vujicic M., Glick M. The effect of chairside chronic disease screenings by oral health professionals on health care costs. Am J Public Health. 2014;104(4):744–750. doi: 10.2105/AJPH.2013.301644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bersell C.H. Access to oral health care: a national crisis and call for reform. JDH. 2017;91(1):6–14. [PubMed] [Google Scholar]