Abstract
Objective
The Miami Heart Study (MiHeart) at Baptist Health South Florida is an ongoing, community-based, prospective cohort study aimed at characterizing the prevalence, characteristics, and prognostic value of diverse markers of early subclinical coronary atherosclerosis and of various potential demographic, psychosocial, and metabolic risk factors. We present the study objectives, detailed research methods, and preliminary baseline results of MiHeart.
Methods
MiHeart enrolled 2,459 middle-aged male and female participants from the general population of the Greater Miami Area. Enrollment occurred between May 2015 and September 2018 and was restricted to participants aged 40–65 years free of clinical cardiovascular disease (CVD). The baseline examination included assessment of demographics, lifestyles, medical history, and a detailed evaluation of psychosocial characteristics; a comprehensive physical exam; measurement of multiple blood biomarkers including measures of inflammation, advanced lipid testing, and genomics; assessment of subclinical coronary atherosclerotic plaque and vascular function using coronary computed tomography angiography, the coronary artery calcium score, carotid intima-media thickness, pulse wave velocity, and peripheral arterial tonometry; and other tests including 12-lead electrocardiography and assessment of pulmonary function. Blood samples were biobanked to facilitate future ancillary research.
Results
MiHeart enrolled 1,261 men (51.3%) and 1,198 women (48.7%). Mean age was 53 years, 85.6% participants were White and 47.4% were of Hispanic/Latino ethnicity. The study included 7% individuals with diabetes, 33% with hypertension, and 15% used statin therapy at baseline. Overweight or obese participants comprised 72% of the population and 3% were smokers. Median 10-year estimated atherosclerotic CVD risk using the Pooled Cohort Equations was 4%.
Conclusion
MiHeart will provide important, novel insights into the pathophysiology of early subclinical atherosclerosis and further our understanding of its role in the genesis of clinical CVD. The study findings will have important implications, further refining current cardiovascular prevention paradigms and risk assessment and management approaches moving forward.
Keywords: Atherosclerosis, Cardiovascular disease, Cohort studies, Coronary computed tomography, Epidemiology, Hispanic/Latino, Populations, Primary prevention
Abbreviations: BHSF, Baptist Health South Florida; CAC, coronary artery calcium; CCTA, coronary computed tomography angiography; CIMT, carotid intima media thickness; CT, computed tomography; CVD, cardiovascular disease; EDTA, ethylenediaminetetraacetic acid; IRB, Institutional Review Board; MESA, Multi-Ethnic Study of Atherosclerosis; MiHeart, Miami Heart Study; NHW, non-Hispanic Whites
1. Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide, and accounts for a large share of total health care expenditure [1], [2], [3]. While CVD risk assessment in general has been traditionally guided by risk estimates derived from scores combining traditional risk factors, there is a growing body of evidence showing that such an approach lacks sufficient accuracy in predicting risk of future events among asymptomatic individuals [4], [5], [6], [7], [8], [9]. Consequently, in recent years there has been a considerable interest in a paradigm change in the approach to primary CVD prevention, expanding the focus from surrogate risk factors alone to also assess the disease process itself [10,11]. In this regard, detection of atherosclerosis via subclinical disease assessment provides information about the actual disease burden, and as such, performs better than surrogate biomarkers [4,[10], [11], [12], [13], [14], [15], [16], [17]].
A robust, low-radiation, relatively inexpensive marker of atherosclerotic coronary artery disease, the coronary artery calcium (CAC) score is now a guideline-endorsed test for further risk assessment [11]. CAC has consistently shown powerful predictive value beyond conventional cardiac risk factors in asymptomatic individuals in multiple population-based cohorts [4,[12], [13], [14], [15], [16], [17]]. Although CAC is an excellent marker of underlying coronary atherosclerosis, it does not capture the whole spectrum of plaque morphology and plaque burden [18,19]. Indeed, the coronary calcified plaque burden has been shown to represent only 20% of the total plaque burden, as coronary atherosclerosis is mostly lipid-laden or fibrous [20,21].
In addition, significant gaps remain in scientific knowledge with regards to the relationship between multiple lifestyle factors, genetic and metabolic risk factors and biomarkers, and early plaque biology. The analysis of these associations has so far been hampered by lack of accurate and well-defined atherosclerotic phenotypes in clinical settings in asymptomatic populations. Coronary computed tomography angiography (CCTA) is a noninvasive technique for the evaluation of the coronary anatomy, stenosis, and the characterization of atherosclerotic plaques [18,[22], [23], [24], [25], [26], [27]]. Until now, the presence, burden and differential architectural features of atherosclerotic plaque in the coronary arteries has remained unexplored in large populations of asymptomatic individuals. This is particularly true in populations of young to middle-aged asymptomatic individuals, who may at priority be considered at low risk for cardiovascular events. In such a population, CCTA may allow for the assessment of the early stages of coronary atherosclerosis, facilitating the identification of its key determinants.
The Miami Heart Study (MiHeart) at Baptist Health South Florida (BHSF) is an ongoing, community-based, prospective cohort study of individuals from the general population free of clinical CVD. MiHeart is aimed at characterizing the presence, features, architecture as well as prognostic ability of earliest diverse markers of subclinical coronary atherosclerotic disease in a population-based cohort of young- and middle-aged men and women free of established CVD; and for this purpose, all participants underwent CCTA at the study baseline. In this article we describe the underlying rationale, study objectives, methods used, and preliminary baseline results of MiHeart.
1.1. Study objectives
The primary objectives of MiHeart are to: 1) assess and characterize the presence and severity of subclinical CVD burden among middle-aged asymptomatic individuals; and 2) determine relationships of traditional risk factors, lifestyle and behavioral factors as well and biomarkers (traditional and novel) related to presence and burden of subclinical CVD among middle-aged asymptomatic individuals.
The secondary study objectives are to: 3) determine the interplay of risk factors (traditional, lifestyle, biomarkers) and early markers of subclinical CVD with the development of clinical CVD; 4) determine relationships of traditional risk factors, lifestyle and behavioral factors as well and biomarkers (traditional and novel) related to presence and burden of subclinical CVD among middle-aged asymptomatic individuals; and 5) develop population-based methods, suitable for application in future screening and intervention studies, for characterizing cardiovascular risk among middle-aged asymptomatic persons as well cost effectiveness for such paradigms in appropriately resource allocation for early CVD management.
2. Methods
2.1. Study design and population
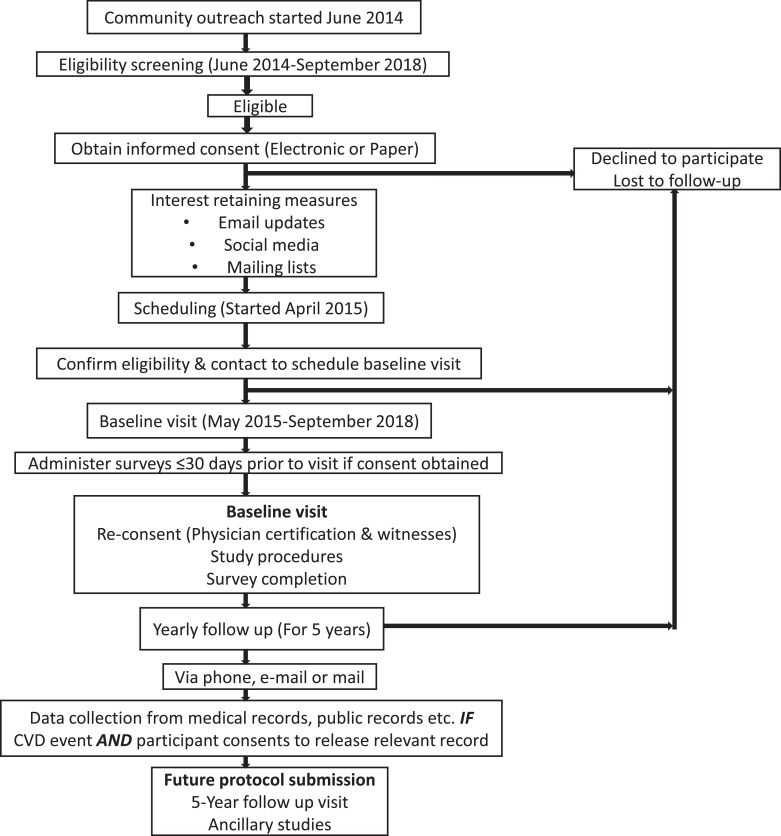
MiHeart (clinicaltrials.gov identifier NCT02508454[28]) is an observational, community-based, longitudinal, prospective cohort study that included 2459 middle-aged male and female participants from the population of the Greater Miami Area (Fig. 1). Study recruitment was based on volunteer participation. Since the study is primarily concerned with the determinants and natural history of subclinical CVD, the age range of the study population was set between 40 and 65 years, and participants with known clinical CVD were excluded from the study.
Fig. 1.
Study timeline, Miami Heart Study.
Abbreviations: CVD = cardiovascular disease.
The specific study inclusion and exclusion criteria are listed in Table 1. Besides the criteria described above, additional exclusion criteria referred to contraindications for computed tomography (CT) testing or other study procedures, active cancer, and inability to provide consent for participation. Both prevalent CVD and the rest of inclusion and exclusion criteria were determined exclusively on the basis of self-reported information provided by the study participants; no attempts were made to validate the participant's response(s) as this is the standard set by prior cohort studies.
Table 1.
Study participant enrollment criteria, Miami Heart Study.
| Inclusion Criteria |
|---|
| Current full time/part-time employees of BHSF (occupational cohort) or resident of Greater Miami Area for at least six months (community cohort)Age 40 to 65 yearsAsymptomatic individuals free of any known clinically overt cardiovascular diseaseAble to comprehend and sign an informed consent form |
| Exclusion Criteria |
| Prior history of major cardiovascular events (angina, myocardial infarction, prior coronary revascularization)History of cerebrovascular disease including stroke and transient ischemic attackHistory of peripheral arterial diseaseHistory of either diagnosis or surgery for abdominal aortic aneurysmHistory of heart failureWeight greater than 350 lbsAny contraindication for computed tomography scanning or non-iodinated contrast (BHSF West Kendall computed tomography angiography Imaging Screening/Prerequisites/Methods)Active treatment for cancerCurrently pregnant, breastfeeding, seeking to become pregnant, or suspect they may be pregnant.Patients who do not agree to provide informed consent |
Potential participants who responded “Don't know” to questions about medical conditions were not be considered ineligible.
Abbreviations: BHSF = Baptist Health South Florida.
2.2. Research ethics
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of BHSF. All study procedures adhered to the principles included in the Declaration of Helsinki [29]. All participants provided written informed consent prior to study entry, and potential participants unable to comprehend and sign an informed consent form were excluded. The same was true for potential participants who failed to provide written consent for whichever reason. As BHSF is a sponsor of this study, additional strategies with IRB guidance were implemented to minimize potential ethics risks (e.g., privacy) associated with employee-participants.
2.3. Recruitment strategy
The principal investigator and research staff were responsible for creating recruitment strategies which complied with both BHSF and federal regulations. The purpose, rationale, and design of the study was publicized to targeted groups in Baptist Health and community members of South Florida (table 2). The efforts included mailings of letters and brochures, posters, newspaper and radio advertisement as well as detailed on the study website (MiamiHeartStudy.net) followed by personal contacts via telephone, email, or in person. The marketing and advertising materials tailored to meet unique aspects of the source population and recruitment strategy were submitted to the IRB for approval prior to implementation tailored to meet unique aspects of the source population and recruitment strategy.
Table 2.
Recruitment strategies, Miami Heart Study.
| BHSF Employees |
|---|
|
| Non BHSF employees/Community members |
|
Abbreviations: BHSF = Baptist Health South Florida; CME = continued medical education.
2.4. Baseline data collection
The baseline study examination took place between May 2015 and September 2018. As shown in Table 3, the baseline study examination included 1) a detailed assessment of self-reported cardiovascular risk factors, including demographic characteristics, medical history, medication use, lifestyle characteristics, and psychosocial factors; 2) a detailed physical exam, which included vitals and anthropometric measurements; 3) blood sampling for determination of biomarker assessment, advanced “omics” and biobanking; 4) assessment of subclinical atherosclerotic plaque, and vascular dysfunction; and 5) other tests, such as 12-lead electrocardiography, and assessment of pulmonary function.
Table 3.
Schedule of procedures and data collection in the Miami Heart Study.
| Procedure | Baseline | Yearly Follow-up |
|---|---|---|
| Enrollment and informed consent | X | |
| Lifestyle, diet and behavior questionnaires | X | |
| Vitals | X | |
| Anthropometric measurements | X | |
| 12-lead ECG | X | |
| Spirometry | X | |
| Endothelial function assessment | X | |
| Carotid ultrasound/Intima-media thickness measurement | X | |
| CT coronary calcium scoring/CT angiography | X | |
| Laboratory analysis | X | |
| Biobanking | X | |
| Pulse wave analysis | X | |
| Follow-up for clinical events | X |
Abbreviations: CT = computed tomography; ECG = electrocardiogram.
Study survey and questionnaires were administered via RedCap survey after online consent prior to clinic visit (Table 4). For participants unable to fill the questionnaires prior to their study visit, access to computers and to a paper version of the survey was provided at their clinic visit. Both English and Spanish versions were available
Table 4.
Baseline questionnaires and surveys, Miami Heart Study.
| Questionnaire | Description/Items Assessed |
|---|---|
| Anxiety | General Anxiety Disorder7-item scale (GAD-7) |
| Demographics | Age, Gender, Race, Ethnicity, Language, Education, Employment, Marital Status, socioeconomic status |
| Depression | Modified Patient Health Questionnaire (PHQ-9) |
| Diet | Food Frequency Questionnaire, Food Access Survey |
| Lung Function | Assessment of Coughing, Wheezing, Shortness of Breath, Inhibition of ADL's |
| Medical & Family History | Conditions related to heart and vascular disorders, lung and breathing related disorders, gastrointestinal disorders, endocrine, hematologic, mental health, eye, rheumatologic & dermatologic disorders, reproductive history, cancer, family medical history |
| Medication History | Prescription and non-prescription medication use, vitamin/dietary supplements and alternative medications |
| Mental Health Continuum | Feelings of happiness, confidence, satisfaction with life, and belonging |
| Mindfulness | Assessment of spiritual and mental awareness and presence |
| Physical Activity | Current physical activity including work, household, leisure, sport related activity, sedentary behavior |
| Sleep | Snoring, Tiredness (Berlin Questionnaire), Daytime sleepiness (Epworth Sleepiness), Sleep quality (Pittsburgh Sleep Quality Index) |
| Smoking, Caffeine Consumption | Past and current cigarette use, ever use of cigars and pipes, use of modified harm-reduction tobacco products, exposure to secondhand smoke. Current consumption of caffeinated products on a daily basis |
| Alcohol Use | Usual intake and drinking patterns. |
| Social Support | Available and perceived social support, emotional support, help with chores, someone who will listen, etc |
| Spirituality | Feelings of peace, purpose, finding strength and comfort in faith (FACIT-12) |
| Stress | Perceived stress scale, stress in the workplace |
Vitals: All participants had their temperature, heart rate, systolic and diastolic blood pressure, and respirations per minute taken and recorded using standard procedures. Specifically, for blood pressure, subjects were asked to sit quietly and rest for 5 min with their legs uncrossed, and an OMRON automatic blood pressure monitor (model BP710) was used. Three separate readings were taken at intervals of at least 1 min
Anthropometric measurements: Participants were weighed on a calibrated scale, without shoes and with all pockets emptied. The results were recorded to the nearest 0.2 kgs. Height was measured in meters using the SECA 213 measuring system. The body mass index was computed as weight (kg) / (height [m] x height [m]). Waist circumference was measured at the top of the iliac crest using a K-E anthropometric tape positioned in a horizontal plane around the abdomen, applying tension to the tape to ensure it was snug yet without causing indentation to the skin. The measurement was performed at the end of a normal expiration. The hip circumference was measured at the level of the greatest girth—typically the level of the trochanters. Both measurements were made to the nearest 0.5 cm. The waist to hip ratio was then computed as waist circumference (cm) / hip circumference (cm). Body fat percentage was measured using a hand-held body fat analyzer (OMRON Fat Loss Monitor Model HBF-306C). Subjects held the monitor with their arms straight out at a 90° angle to their body. The device provided a percent fat estimate on the basis of measurements using the bioelectrical impedance method combined with inputs on the subjects’ age, sex, weight and height.
ECG: For electrocardiography, three 12-lead recordings were obtained using Welch Allyn standard digital 12-lead ECG.
Spirometry: Spirometry measurements were obtained using the Welch Allyn spirometry machine. Welch Allyn disposable flow transducers were used and three measurements were made during rapid and forceful exhalation.
Endothelial function: Peripheral arterial tone signal was used to measure, non-invasively, arterial tone changes in peripheral arterial beds, using the Itamar EndoPat 2000 system. Both the Reactive Hyperemia Index (RHI), a measure of endothelial function, and the Augmentation Index (AI), a measure of arterial stiffness, were measured. The test involved baseline measurement with the EndoPat probe on the index finger for 5 min. A blood pressure cuff was then applied on the non-dominant arm for next 5 min to occlude blood supply, and a recording is done during this phase. After 5 min of occlusion to the non-dominant arm, the pressure is released. Once the blood flow is restored resulting in blood vessels to dilate, the EndoPat machine calculates the stiffness in the arterial walls. The vessel wall reactivity is the comparison of the pre-occlusion and post-occlusion blood flow and is reported as reactive hyperemia index.
Carotid intima-media thickness (CIMT) was measured using the Panasonic CardioHealth Station automated carotid ultrasound device. Using a single transducer angle, fully automated CIMT measurements were recorded. The mean CIMT measurement is defined as the mean of 24 spatial measurements performed over a 1-cm region in the far wall of the common carotid artery. In addition, presence of any atherosclerotic coronary plaque was defined as any obvious focal luminal encroachment >1.00 mm.
Pulse Wave Analysis: Pulse wave analysis and pulse wave velocity measurements were obtained using AtCor Medical SphygmoCor XCEL machine. It used a standard brachial cuff to measure brachial systolic and diastolic pressures and capture a brachial waveform. The brachial waveform was then analyzed by SphygmoCor to provide a central aortic waveform. Noninvasive central blood pressure measurements such as central aortic systolic blood pressure, central pulse pressure, and an aortic augmentation index (AIx) were also reported. Pulse wave velocity was measured using a carotid tonometer simultaneously with a leg cuff to capture blood pressure waveforms at the carotid and femoral sites.
CoronaryCT: In all participants, a non-contrast cardiac-gated CT scan for CAC testing was performed prior to conducting a contrast-enhanced, cardiac-gated CCTA. Both contrast and non-contrast scans were performed using GE Revolution (GE Healthcare) scans. Before the test, study participants received oral and/or intravenous β-blocker (metoprolol) as needed to achieve a heart rate of <75 beats per minute. Additionally, 0.4 mg of sublingual nitroglycerine was given immediately before angiographic image acquisition. The radiation dose was estimated from the dose-length product multiplied by the conversion factor (0.014 mSv/mGy • cm). Assessment of 17 segment plaque presence/burden and classification was performed at a central core imaging lab.
Laboratoryanalysesandbiobanking: In each participant, 97.5 ml of fasting blood were collected at baseline (approximately ~53 aliquots per participant) for serum, plasma and whole blood. Samples were tested for a wide range of biomarkers (Table 5). In addition, blood aliquots were bio-banked and maintained at −80 °C for future ancillary analyses. One 7.5 ml SST vacutainer drawn is left at room temperature for 20–30 min for clotting before centrifugation at 3600xg for 15 min. After centrifugation, serum is removed from SST vacutainer. It is aliquoted in 2 ml Starstedt microtubes creating four aliquots. Six 10 ml red top vaccutainers are drawn and allowed to clot at room temperature before centrifugation. If a patient opted out of biobanking then only five red top vaccutainers are drawn. They are also centrifuged at 3600xg for 15 min. The serum is then aliquoted into 40 2 ml Starstedt tubes with each tube containing 0.75 ml serum. One 4 ml Na+ Heparin vacutainer is drawn and then centrifuged at 3600xg for 15 min within one hour of blood draw. It is then aliquoted in 0.75 ml volumes into 2 ml Starstedt tubes creating 2 aliquot tubes. Two 10 ml ethylenediaminetetraacetic acid (EDTA) vacutainers are drawn which are also centrifuged at 3600xg for 15 min within one hour of blood draw. The serum is then aliquoted into 12 2 ml Starstedt tubes with each tube containing 0.75 ml serum. Additionally, three 2 ml K2 EDTA tubes are drawn for every participant. If the subject opted out of genetic testing, then only one 2 ml K2 EDTA tube is drawn. These K2 EDTA tubes are not centrifuged and are stored as whole blood. All blood specimens are then stored at −80 C.
Table 5.
Baseline laboratory analyses, Miami Heart Study.
| Group of measurements | Specific measurements |
|---|---|
| Cardiac Enzymes |
|
| Hemostasis and Fibrinolysis |
|
| Inflammation |
|
| Diabetes |
|
| Endothelial Cell Function |
|
| Lipids |
|
| Nutrition |
|
| Genetics |
|
| Obesity/Metabolism |
|
| Renal Function |
|
| Sex Hormones |
|
| Vitamins and Minerals |
|
2.5. Data management
All MiHeart data are collected and stored using the Research Electronic Data Capture (REDCap) electronic data capture software, which is widely used for research purposes at Baptist Health South Florida and elsewhere. REDCap is a secure, web-based application designed to support data collection for research studies. Only authorized study personnel have access to the study data, and the study datasets undergo periodic quality control checks by the database administrator and study coordinators. Additionally, all study data undergo periodic audits by the IRB at Baptist Health South Florida.
3. Results
MiHeart enrolled 2459 participants free of clinical CVD at baseline, including 1261 (51.3%) men and 1198 women (48.7%). Mean age was 53 years, the majority of study participants were White (85.6%), and 1166 (47.4%) were of Hispanic/Latino ethnicity.
The baseline sociodemographic and clinical characteristics of the study population are summarized in Table 6. More than 72% participants were either overweight or obese, while only 3% were current smokers. Approximately 7% had diabetes, 33% hypertension, and 15% used statin therapy at baseline. Median 10-year estimated atherosclerotic CVD risk using the Pooled Cohort Equations was 4%. Compared with 2010 US Census data for the population of Miami (Table 1), the MiHeart study population had a similar proportion of women (50.2% in Miami vs. 48.7% in MiHeart), and a lower proportion of Hispanic/Latino participants (65% vs. 47.4%). Compared with the demographics of the BHSF workforce, the proportion of women was markedly lower in MiHeart, while the proportion of Hispanic/Latinos was identical.
Table 6.
Baseline characteristics of the study population, Miami Heart Study.
| MiHeart Study (N = 2459) | |
|---|---|
| Demographics | |
| Age, years | 53.35 (6.78) |
| Women | 1198 (48.7%) |
| Race | |
| White | 2104 (85.6%) |
| Black | 94 (3.8%) |
| Asian | 74 (3%) |
| Other | 186 (7.6%) |
| Ethnicity | |
| Hispanic/Latino | 1166 (47.4%) |
| Not Hispanic/Latino | 1255 (51%) |
| Other cardiovascular risk factors | |
| Body mass index, kg/m2 | 28.45 (5.29) |
| Body mass index categories | |
| Normal weight | 679 (27.6%) |
| Overweight | 991 (40.3%) |
| Obesity | 789 (32.1%) |
| Family history of CVD | 707 (28.8%) |
| Family history of premature CVD | 185 (7.5%) |
| Tobacco use | |
| Current smoker | 75 (3.1%) |
| Former smoker | 602 (24.5%) |
| Never smoker | 1782 (72.5%) |
| Diabetes | 175 (7.1%) |
| Hypertension | 808 (32.9%) |
| Hyperlipidemia | 331 (13.5%) |
| Dyslipidemia | 731 (29.7%) |
| Baseline medication use | |
| Statin use | 371 (15.1%) |
| Aspirin use | 569 (23.1%) |
| Estimated ASCVD risk | |
| 10-Year Estimated ASCVD risk,*% | 3.96 (4.16) |
*Using the Pooled Cohort Equations.
Results are presented as either number (%) or mean (standard deviation).
Abbreviations: ASCVD = atherosclerotic cardiovascular disease; CVD = cardiovascular disease.
4. Discussion
MiHeart is a large, ongoing, community-based, prospective cohort study of men and women aged 40 to 65 years who underwent a comprehensive phenotyping of traditional and novel cardiovascular risk factors, and diverse measurements of subclinical CVD. So far, to the best of our knowledge no large US prospective study has conducted a baseline CCTA assessment of the study population. MiHeart will provide a comprehensive evaluation of coronary plaque burden, subtypes, morphology, and vulnerability features in a community-based primary prevention cohort. These unique data from MiHeart will generate invaluable opportunities for groundbreaking research, enhancing our understanding of the natural history of CVD, the early stages of subclinical atherosclerosis, and the key underlying social, lifestyle and metabolic determinants.
Prior landmark multi-ethnic cardiovascular cohorts such as the Multi-Ethnic Study of Atherosclerosis (MESA) or the Dallas Heart Study restricted their baseline assessment of coronary atherosclerosis to CAC [30], [31], [32], [33], [34]. While CAC has demonstrated an excellent performance as a tool for further risk assessment in middle-aged individuals, whether the inability of non-contrast CT to detect non-calcified plaque may limit its performance for risk prediction in younger adults, or whether a CAC score of zero may be less reassuring in this age group, remain unclear. It must be noted, however, that as of 2021 the Agatston score, which was developed in 1990 [35], has not been replaced by more modern measurements of plaque morphology or high-risk plaque features that can be measured with CCTA [18,[22], [23], [24], [25], [26], [27]].
MiHeart is a natural extension of existing cohorts, like MESA, which with more than 2000 publications has provided significant insights into the role of atherosclerosis imaging with CAC in guiding risk assessment and subsequent management mostly in middle-aged individuals [[13], [14], [15],[30], [31], [32], [33], [34]]. In spite of notable improvements in our understanding of the significance of atherosclerotic processes to date, in studies such as MESA, the role of non-invasive atherosclerotic plaque imaging tools has been restricted to an older subset of middle-aged individuals (usually aged 60 and above) and limited to CIMT and CAC [30], [31], [32], [33], [34]. With advances in contrast enhanced multi-detector CT we have now significantly improved our ability to non-invasively obtain comprehensive information not only based on presence and burden, but also accounting for characteristics of coronary atherosclerotic plaques to form the basis of more advanced and contemporary indices of atherosclerotic disease assessment. MiHeart will be the first prospective multi-ethnic population-based cohort study of a younger population free of clinical CVD undergoing CCTA testing. This information will provide detailed insights on burden and plaque characteristics in earlier stages of atherosclerosis, along with the characterization of biomarkers that can identify high-risk individuals at an early stage. This has been relatively unexplored, particularly in populations of (relatively) younger asymptomatic individuals (age 40–65 years) who may initially be considered at low-risk for cardiovascular events.
Furthermore, with robust core lab based plaque assessment protocols, strict quality control measures and leveraging our stored biobank, MiHeart will further our understanding of the pathogenesis of atherosclerosis and other CVDs by (1) providing more accurate and quantifiable measures of subclinical CVD among (younger) middle-aged asymptomatic individuals; (2) characterizing earliest changes of atherosclerosis (when it may be subjected to interventions that disrupt the natural history and progression of the disease); prior to clinical manifestation; (3) allowing for discovery and validation of accurate and reliable biomarkers for early atherosclerotic disease so that they can be rapidly translated into everyday clinical practices and (4) allowing comparisons among groups at different levels of risk that may provide clues to pathogenesis.
Another distinguishing feature is that MiHeart enrolled participants from most racial/ethnic groups in the US, including a large representation of the two largest groups—non-Hispanic Whites (NHWs) and Hispanics/Latinos [36]. While NHWs have been included in numerous American cardiovascular studies since the landmark Framingham study [37], prospective cardiovascular research in Hispanics/Latinos has been more limited. With a population of 60.6 million in 2019, Hispanics/Latinos have become the largest minority group in the United States [36,38]. With evidence of increasing burden of ASCVD risk factors among Hispanics/Latinos in the US [39,40], there is a need to better understand the key determinants of disease in this large, rapidly growing demographic group. MiHeart will generate very important insights on the early stages of atherosclerosis in a large, rapidly growing group in the US.
5. Limitations
Some potential challenges are worth discussing. First, compared to the racial/ethnic distribution of the US population, NHWs are underrepresented in MiHeart. Although this is consistent with the specific demographic characteristics of the Greater Miami Area (according to the 2010 US Census [41], only 12% were NHWs, and researchers will have to account for the large proportion of Hispanic/Latino participants in MiHeart when drawing conclusions to the general US population from analyses not stratified by race/ethnicity. This may have implications for risk factor and plaque burden findings, as well as for their prognostic implications. Second, follow-up is initially planned to last only 5 years. However, there are ongoing efforts to secure additional funding to further extend the follow-up to 10 or more years. Also, efforts are also ongoing to fund a second, repeat CCTA measurement over time, which will allow to evaluate changes in plaque characteristics over time and define the key baseline determinants.
6. Conclusion
MiHeart at BHSF will provide important new information about the pathophysiology of early subclinical disease development and its role in clinical CVD. The study has the potential to identify new risk factors, discover/validate novel biomarkers (metabolic and genetic) for the earliest and highest risk plaques in younger asymptomatic individuals and, therefore, increase the ability to predict CVD and, ultimately, to design new interventions to prevent CVD. The multiethnic composition of the study population is a major strength of the study, allowing comparisons that may provide unique insights about new risk factors and subclinical disease, and opening the possibility of tailoring prevention efforts according to ethnic differences in disease processes. The results of the study will be applicable to clinical practice by identifying noninvasive subclinical disease measures that best predict risk and by suggesting new approaches to interventions that prevent progression of subclinical disease and its transformation to clinical disease. Findings may be directly applicable to clinical practice, may be used to design clinical trials or optimize interventions, and/or may lead to research resulting in new methods of intervention. Wellness programs may be able to utilize the study findings to better target and optimize CVD prevention efforts.
Central Illustration, Study design, Miami Heart Study.

Author disclosures
The authors declare that they have no conflicts of interest relevant to the content of this manuscript.
Authors’ contributions
KN, MCA, SSA drafted the manuscript and revised subsequent versions critically for important intellectual content. JZ, DF, LA, AS, TF, RC, MB, JF made substantial contributions to the design of the study, participated in data collection and study coordination and/or management, and revised the manuscript for content accuracy. All authors read and approved the final version of the manuscript.
Funding
The Miami Heart Study is funded by Baptist Health South Florida.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Khurram Nasir, Email: knasir@houstonmethodist.org.
Jonathan Fialkow, Email: JonathanFi@baptisthealth.net.
References
- 1.Virani S.S., Alonso A., Benjamin E.J. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.European Heart Network. European Cardiovascular Disease Statistics 2017. Available online at: http://www.ehnheart.org/cvd-statistics.html (Accessed January 10, 2021)
- 3.Mensah G.A., Roth G.A., Fuster V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J Am Coll Cardiol. 2019;74:2529–2532. doi: 10.1016/j.jacc.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Nasir K., Bittencourt M.S., Blaha M.J. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2015;66:1657–1668. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 5.DeFilippis A.P., Young R., Carrubba C.J. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–275. doi: 10.7326/M14-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh A., Collins B.L., Gupta A. Cardiovascular Risk and Statin Eligibility of Young Adults After an MI: Partners YOUNG-MI Registry. J Am Coll Cardiol. 2018;71:292–302. doi: 10.1016/j.jacc.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeitouni M., Nanna M.G., Sun J.L., Chiswell K., Peterson E.D., Navar A.M. Performance of Guideline Recommendations for Prevention of Myocardial Infarction in Young Adults. J Am Coll Cardiol. 2020;76:653–664. doi: 10.1016/j.jacc.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasir K., Michos E.D., Blumenthal R.S., Raggi P. Detection of high-risk young adults and women by coronary calcium and National Cholesterol Education Program Panel III guidelines. J Am Coll Cardiol. 2005;46:1931–1936. doi: 10.1016/j.jacc.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 9.Akosah K.O., Schaper A., Cogbill C., Schoenfeld P. Preventing myocardial infarction in the young adult in the first place: how do the National Cholesterol Education Panel III guidelines perform? J Am Coll Cardiol. 2003;41:1475–1479. doi: 10.1016/s0735-1097(03)00187-6. [DOI] [PubMed] [Google Scholar]
- 10.Greenland P., LaBree L., Azen S.P., Doherty T.M., Detrano R.C. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 11.Arnett D.K., Blumenthal R.S., Albert M.A. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Detrano R., Guerci A.D., Carr J.J. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 13.Silverman M.G., Blaha M.J., Krumholz H.M. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35:2232–2241. doi: 10.1093/eurheartj/eht508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin S.S., Blaha M.J., Blankstein R. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaha M.J., Budoff M.J., DeFilippis A.P. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tota-Maharaj R., Blaha M.J., McEvoy J.W. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33:2955–2962. doi: 10.1093/eurheartj/ehs230. [DOI] [PubMed] [Google Scholar]
- 17.Nasir K., Rubin J., Blaha M.J. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5:467–473. doi: 10.1161/CIRCIMAGING.111.964528. [DOI] [PubMed] [Google Scholar]
- 18.Williams M.C., Kwiecinski J., Doris M. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART) Circulation. 2020;141:1452–1462. doi: 10.1161/CIRCULATIONAHA.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki K., Matsumoto T., Aono H., Furukawa H., Samukawa M. Prevalence of non-calcified coronary plaque on 64-slice computed tomography in asymptomatic patients with zero and low coronary artery calcium. Can J Cardiol. 2010;26:377–380. doi: 10.1016/s0828-282x(10)70419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rumberger J.A., Simons D.B., Fitzpatrick L.A., Sheedy P.F., Schwartz R.S. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 21.Sangiorgi G., Rumberger J.A., Severson A. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 22.Knuuti J., Wijns W., Saraste A. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 23.Abdelrahman K.M., Chen M.Y., Dey A.K. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76(10):1226–1243. doi: 10.1016/j.jacc.2020.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurovich-Horvat P., Ferencik M., Voros S. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;11(7):390–402. doi: 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- 25.Schaar J.A., Muller J.E., Falk E. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur Heart J. 2004;25(12):1077–1082. doi: 10.1016/j.ehj.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Nasir K., Budoff M.J., Shaw L.J., Blumenthal R.S. Value of multislice computed tomography coronary angiography in suspected coronary artery disease. J Am Coll Cardiol. 2007;49(20):2070–2071. doi: 10.1016/j.jacc.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Choi E.K., Choi S.I., Rivera J.J. Coronary computed tomography angiography as a screening tool for the detection of occult coronary artery disease in asymptomatic individuals. J Am Coll Cardiol. 2008;52:357–365. doi: 10.1016/j.jacc.2008.02.086. [DOI] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov. The Miami Heart Study at Baptist Health South Florida (MiHEART). Available online at: https://clinicaltrials.gov/ct2/show/NCT02508454 (Accessed January 10, 2021)
- 29.World Medical Association. Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. Available online at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (Accessed January 10, 2021)
- 30.Bild D.E., Bluemke D.A., Burke G.L. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 31.Paixao A.R., Ayers C.R., El Sabbagh A. Coronary Artery Calcium Improves Risk Classification in Younger Populations. JACC Cardiovasc Imaging. 2015;8:1285–1293. doi: 10.1016/j.jcmg.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Carr J.J., Jacobs D.R., Jr, Terry J.G. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol. 2017;2:391–399. doi: 10.1001/jamacardio.2016.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavousi M., Elias-Smale S., Rutten J.H. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. 2012;156:438–444. doi: 10.7326/0003-4819-156-6-201203200-00006. [DOI] [PubMed] [Google Scholar]
- 34.Mortensen M.B., Fuster V., Muntendam P. Negative Risk Markers for Cardiovascular Events in the Elderly. J Am Coll Cardiol. 2019;74:1–11. doi: 10.1016/j.jacc.2019.04.049. [DOI] [PubMed] [Google Scholar]
- 35.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 36.United States Census Bureau. Quick Facts. Available online at: https://www.census.gov/quickfacts/fact/table/US/PST045219 (Accessed January 10, 2021)
- 37.Dawber T.R., Kannel W.B. The Framingham study. An epidemiological approach to coronary heart disease. Circulation. 1966;34:553–555. doi: 10.1161/01.cir.34.4.553. [DOI] [PubMed] [Google Scholar]
- 38.Pew Research Center. U.S. Hispanic population surpassed 60 million in 2019, but growth has slowed. Available online at: https://www.pewresearch.org/fact-tank/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/ (Accessed January 10, 2021)
- 39.Cheng Y.J., Kanaya A.M., Araneta M.R.G. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011-2016. JAMA. 2019;322:2389–2398. doi: 10.1001/jama.2019.19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Health of Hispanic or Latino Population. Available online at: https://www.cdc.gov/nchs/fastats/hispanic-health.htm (Accessed January 10, 2021)
- 41.Florida Population: Census Summary 2010. April 2011. Available online at: https://www.bebr.ufl.edu/sites/default/files/Research%20Reports/census_summary_2010.pdf (Accessed January 10, 2021)