Highlights
-
•
VIPVIZA is a pragmatic randomized controlled trial performed within the regular primary health care and targeting both individuals and physicians.
-
•
Beneficial effects on risk for cardiovascular disease regardless of sex and education level 3 years after providing pictorial information of subclinical atherosclerosis in addition to regular preventive information.
-
•
The results indicate that the intervention effect is a combined effect of pharmacological treatment and lifestyle modification.
-
•
The intervention effect was observed in the intermediate risk group, the group where most CVD events occur and in which sufficient prevention is often overlooked.
Keywords: Atherosclerosis, Cardiovascular disease, Carotid ultrasound, Prevention
Abstract
Objective
Non-adherence to guidelines and preventive measures is a major challenge, particularly so to obtain long-term adherence to lifestyle changes and recommended medication. The objective was to investigate if pictorial information regarding subclinical carotid atherosclerosis provided to individuals and physicians gave sustained effects on cardiovascular risk beyond the previously reported effect after 1 year and up to 3 years.
Methods
A Prospective Randomized Open Blinded End-point (PROBE) trial. Within a CVD prevention program in Västerbotten County, Sweden, 3532 healthy individuals aged 40, 50 or 60 years were enrolled and 1:1 randomized to intervention (n = 1749; pictorial information with additional prevention materials to participants and physicians) or control group (n = 1783; no pictorial information to participants and physicians). Preventive measures were managed within primary care. Participants were investigated at baseline during 2013–2016 and at follow-up after 1 and 3 years.
Results
A beneficial effect on cardiovascular risk was observed at 3-year follow-up; Framingham Risk Score (FRS) was 13.38 for the intervention group and 14.08 for the control group (p = 0.047) and SCORE was 1.69 vs. 1.82 (p = 0.022). The effect observed at 1-year was sustained over 3 years after adjustment for sex and education and more pronounced among participants with a severe atherosclerotic picture at baseline.
Conclusions
This study provides evidence of sustained beneficial effects on the adherence to prevention guidelines over 3 years of pictorial information about subclinical carotid atherosclerosis, resulting in lower cardiovascular risk regardless of sex and educational level. Direct visualization of the underlying still subclinical atherosclerotic disease, rather than just indirect information about risk factors and statistical risk of future myocardial infarction, stroke and death, is one way to tackle the problem of non-adherence to prevention of cardiovascular diseases.
Graphical abstract
1. Introduction
Prevention of cardiovascular disease (CVD) often fails due to lack of adherence to guidelines by both practitioners and patients. Availability of effective methods to improve adherence to evidence-based CVD prevention measures is thought to be a greater step forward than any new drug or intervention [1, 2].
An innovative method for evaluation and information about the risk of CVD among asymptomatic individuals is to visualize subclinical atherosclerosis by ultrasonography of the carotid arteries, measuring intima media thickness (cIMT) and carotid plaque [3], as a complement to traditional risk factor-based evaluation and risk information. This has the potential to improve the precision in risk stratification [4, 5], and to increase the accuracy in individuals´ risk perception by showing the atherosclerosis itself, instead of only the likelihood of CVD. This can thereby motivate patients and practitioners to preventive actions aiming to halt or reduce progression of atherosclerosis [6], [7], [8].
Pictorial information of subclinical atherosclerosis has been shown to influence physicians´ prescription of evidence-based interventions as well as patients´ motivation to exercise, implement dietary change [9], and adhere to medication and prevention-seeking behavior [10]. The VIPVIZA (Västerbotten Intervention Program - VIsualiZation of asymptomatic Atherosclerotic disease for optimum cardiovascular prevention), a pragmatic open randomized controlled trial, demonstrated after 1-year of follow-up that pictorial information about subclinical atherosclerosis combined with a nurse-led follow-up phone call improved the adherence to guidelines on primary CVD prevention and had a significant beneficial effect on the primary outcomes Framingham Risk Score (FRS) and European Systematic Coronary Risk Evaluation (SCORE) irrespective of educational level and sex [11]. However, whether these effects are sustainable over a longer period of time needs further evaluation, as recently highlighted [12]. There are, to our knowledge, no other trials similar to VIPVIZA, which are nested in a real-world setting.
The aim of this study was to investigate whether the effect of pictorial information about subclinical carotid atherosclerosis, which was observed on improved adherence to prevention guidelines in terms of levels of cardiovascular risk scores (FRS and SCORE) and individual risk factors at 1 year in the VIPVIZA trial, was sustained up to 3 years of follow-up. In addition, we investigated whether such a presumptive positive effect differs between participants with different sex, educational levels, pictorial information about the severity of subclinical atherosclerosis and level of traditionally assessed risk factor-based CVD risk.
2. Methods
VIPVIZA is a Prospective Randomized Open Blinded End-point (PROBE) trial performed within the regular primary health care setting in the county of Västerbotten, Sweden. Details on criteria for eligibility and study design have previously been published [11]. The participants were recruited by district nurses within the Västerbotten Intervention Program (VIP) [13]. VIP is a CVD prevention program in primary care since the early 1990s in Västerbotten county, in which all inhabitants are invited at ages 40, 50 and 60 years for a health survey with CVD risk factor screening and a dialog to promote healthy lifestyle habits, and when indicated pharmacological treatment.
Participants in VIP were invited to participate in VIPVIZA if they fulfilled inclusion criteria; i) aged 40 years with a first-degree relative with CVD at an age less than 60 years; ii) aged 50 years with a minimum of 1 conventional CVD risk factor or; iii) aged 60 years. Individuals with significant carotid stenosis, defined as narrowing of the lumen greater than 50%, were referred to specialized care and excluded from the study. All study participants provided written informed consent.
In total, 3532 participants were recruited to VIPVIZA between April 29, 2013 and June 7, 2016, and consecutively assigned by research nurses (1:1) to the intervention or control arm based on a computerized randomization list, which was generated by a statistician before enrolment, but concealed for the participants and the biomedical technicians performing the ultrasound examination. At baseline, conventional risk factors for CVD were measured and participants answered a questionnaire covering health, medication, health behaviors, family history on premature CVD, diabetes and education level. After VIP, at a separate visit [11], a carotid ultrasound examination was performed by biomedical technicians according to a standardized protocol using portable carotid ultrasound equipment [14], [15], [16]. The end-points were blinded until after completion of all 3-year follow-up visits.
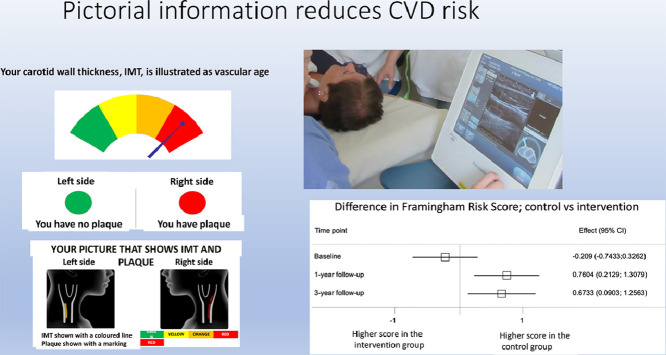
The intervention; i) The individuals in the intervention group and their family physician were mailed the result of the carotid ultrasound examination in a pictorial format. Presence of plaque was illustrated with a red circle while a green circle demonstrated that no plaque was identified. Intima-media thickness was illustrated with a color gage representing vascular age and ranging from green over yellow and orange to red (Supplementary Figure 1). The dynamic and modifiable nature of atherosclerosis was explained in text. A follow-up phone call to the participants was carried out 2–4 weeks after sending the result to them. To physicians, current guideline-based information about the clinical significance of carotid ultrasound results was enclosed. ii) At six months, the pictorial information from the ultrasound examination was sent once again to participants in the intervention group with a reminder of preventive measures. iii) At nine months, 2 and 2.5 years, a letter was sent to the participants in the intervention group with information regarding the next follow-up visit. The letters contained general information about proceedings in the study, the importance of a healthy lifestyle to prevent progression of atherosclerosis, and a reminder of the upcoming follow-up visits. No individualized information was given in the letters.
In the control group, no information was given either to participants or their family physician regarding the baseline carotid ultrasound result, and no information letters were sent at 6 and 9 months, 2 or 2.5 years to the participants. The intervention and follow-up visits for both groups are presented in the Supplementary Figure 2.
Identical content of follow up for the intervention and control groups: At 1 year, the intervention and the control groups were assessed on the same CVD risk factors and the same questions regarding physical activity, smoking, alcohol consumption, and self-reported pharmacological treatment as at baseline. All participants, in both the control and intervention groups, and their family physician were informed about the results in a standardized written form. After three years a follow-up was conducted between September 5, 2016 and May 28, 2019. All participants had the same CVD risk factors assessed and enquiries as at the 1- year follow-up. In addition, a carotid ultrasound examination was similarly conducted as at baseline. Both the control and the intervention groups were treated according to clinical guidelines on CVD risk factor control throughout the study by nurses and physicians within regular health care.
The primary outcome were the levels of FRS and SCORE in the intervention and control group 3 years after the first ultrasound examination. Secondary outcomes were levels of individual risk factors, i.e. cholesterol measures, systolic blood pressure, weight, waist circumference, smoking and diabetes.
There were 3532 participants at baseline, and the number of participants recruited to the study was based on power calculations done a priori at the VIPVIZA design stage [11]. Independent t-tests were used to assess significant differences between groups on continuous variables, and χ2 tests for categorical variables at the 3-year follow-up. We used all available observations for each analysis resulting in slightly different numbers of individuals in different analyses.
The multilevel regression analysis using panel data analysis included 3529 individuals (out of 3532 total individuals) who had FRS or SCORE measurement in any of the waves (baseline, 1-year or 3-year). We built stepwise 2-level multilevel linear regression models separately for FRS and SCORE with time of measurement at level 1 (t = 3 including baseline, 1-year, 3-year) and individuals at level 2 (n = 3529). We analyzed differences in FRS and SCORE (primary outcome variables) between the intervention and control groups, controlling for sex (1=male; 2=female) and education level (1=low/mid-level; 2=high level).
Model 0: Null random intercept model assuming each individual had different intercepts, i.e. they had different outcome levels at baseline.
Model 1: Random intercept model with inclusion of only a randomization group variable.
Model 2: Random intercept model with the interaction term between randomization group and wave to assess if the differences in FRS and SCORE between the 2 groups differ by wave.
Model 3: Model 2 with adjustment for the sex variable (random intercept model).
Model 4: Model 3 with additional adjustment for the education variable (full random intercept model).
Model 5: Random slope model with inclusion of all variables in Model 4, and a random slope with regression slopes allowed to vary in different individuals across different waves. In this model, we assumed that the trajectories of FRS and SCORE differ between individuals.
We used the likelihood-ratio test to compare a model with its preceding model. The LR-test follows χ2 distribution, and a significant p-value of <0.05 indicates that the subsequent model is a better fit than its preceding model.
We also conducted difference-in-difference (DID) analysis on changes from baseline to 3-year-follow-up in the intervention and the control-group. Moreover, this was performed for different subgroups of sexes, education groups (basic and mid-level education defined as compulsory nine years of schooling or senior high school less than 13 years, and high level of education defined as 13 years or more of schooling), baseline risk score, and atherosclerosis severity (based on the presence of plaques and intima-media thickness presented as vascular age at baseline). We also performed a dropout analysis to assess whether the baseline characteristics differed between individuals with 3-year follow-up and those who dropped out.
As a sensitivity test, we conducted an intention-to-treat analysis on the primary outcomes, both in the whole sample and in sub-groups, using imputed data. Details of the imputation following the procedures proposed by Rubins [17] are described in the Supplementary material.
The statistical analyses were done in SPSS version 26, and Stata Version 16. All graphs were generated in Stata Version 16.
This study is registered at ClinicalTrials.gov, number NCT01849575. www.clinicaltrials.gov
3. Results
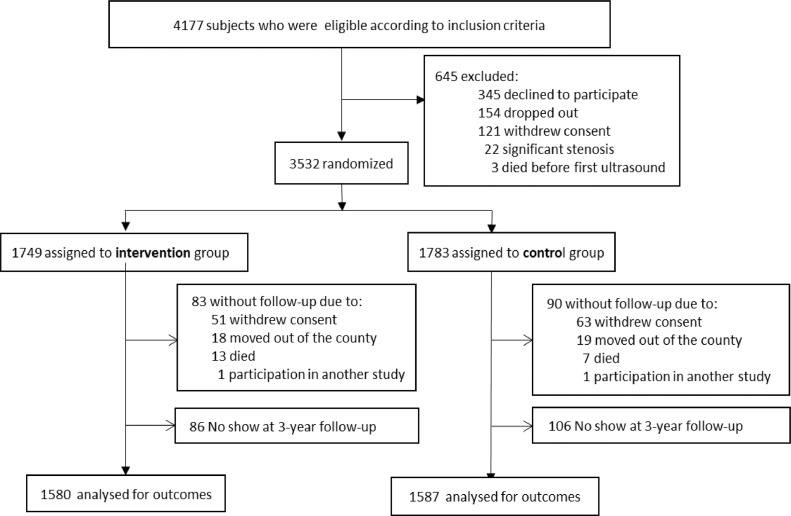
3532 participants were enrolled, 1738 were randomized to the control arm and 1749 to the intervention arm. Those 3167 participants who completed the 3-year follow-up are described in Fig. 1. The follow-up rate was 89.7%. There were no differences between the intervention and control groups at baseline on FRS, SCORE, individual components of the risk scores or other cardiovascular risk factors as previously presented [11].
Fig. 1.
flowchart of the VIPVIZA trial.
Regarding the primary outcomes at the 3-year follow-up, the mean level of FRS was 13.38 for the intervention group and 14.08 for the control group (p = 0.047), and mean levels for SCORE was 1.69 vs 1.82 (p = 0.022), respectively (Table 1).
Table 1.
Characteristics of the intervention and the control group at 3-year follow-up on primary and secondary outcome variables. Mean levels are shown for continuous variables, and number of participants and percentages for categorical variables.
| Variable | Male |
Female |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention n = 736 | Control n = 741 | p-value | Intervention n = 844 | Control n = 846 | p-value | Intervention n = 1580 | Control n = 1587 | p-value | |
| FRS | 19.09 | 19.72 | 0.261 | 8.42 | 9.15 | 0.011 | 13.38 | 14.08 | 0.047 |
| SCORE | 2.58 | 2.72 | 0.134 | 0.93 | 1.03 | 0.002 | 1.69 | 1.82 | 0.022 |
| P-Total Cholesterol (mmol/l) | 5.11 | 5.34 | <0.001 | 5.44 | 5.66 | <0.001 | 5.29 | 5.51 | <0.001 |
| P-LDL-Cholesterol (mmol/l) | 3.10 | 3.31 | <0.001 | 3.16 | 3.38 | <0.001 | 3.13 | 3.35 | <0.001 |
| P-HDL-Cholesterol (mmol/l) | 1.35 | 1.35 | 0.731 | 1.70 | 1.69 | 0.492 | 1.54 | 1.53 | 0.456 |
| P-Triglycerides (mmol/l) | 1.50 | 1.52 | 0.763 | 1.26 | 1.30 | 0.204 | 1.37 | 1.40 | 0.302 |
| Syst BP (mmHg) | 133.7 | 134.5 | 0.319 | 127.4 | 127.3 | 0.910 | 130.3 | 130.7 | 0.572 |
| Diast BP (mmHg) | 87.6 | 87.8 | 0.641 | 84.7 | 84.0 | 0.163 | 86.0 | 85.8 | 0.489 |
| Body weight (kg) | 90.1 | 90.4 | 0.718 | 75.1 | 76.2 | 0.095 | 82.0 | 82.8 | 0.181 |
| Waist (cm) | 102.3 | 103.1 | 0.204 | 93.9 | 95.1 | 0.063 | 97.8 | 98.9 | 0.032 |
| Smokers N (%) | 69 (9.4) | 77 (10.5) | 0.502 | 71 (8.5) | 95 (11.4) | 0.046 | 140 (8.9) | 172 (11.0) | 0.055 |
A significant difference between the intervention and control groups was observed in the FRS and SCORE levels at 3-year follow-up, similar to the 1-year follow-up (Table 2). This sustained effect included adjustment for sex and educational level (model 5).
Table 2.
Differences in primary and secondary outcome between intervention and control groups at baseline, 1-year and 3-year follow-up analyzed with random slope multilevel analysis models. A positive value means a lower level in the intervention group compared to the control group.
| Outcomes | Baseline Difference (95%CI) nI=1735 / nC=1762 | 1-year follow-up Difference (95%CI) nI=1586 / nC=1558 | 3-year follow-up Difference (95%CI) nI=1566 / nC=1570 |
|---|---|---|---|
| Primary outcomes | |||
| FRS | −0.209 (−0.7443; 0.3262) | 0.7604 (0.2129; 1.3079) | 0.6733 (0.0903; 1.2563) |
| SCORE | −0.0093 (−0.0788; 0.0602) | 0.1217 (0.0447; 0.1986) | 0.1258 (0.0299; 0.2217) |
| Secondary outcomes | |||
| P-Total-Cholesterol (mmol/L) | 0.0021 (−0.0686; 0.0729) | 0.1896 (0.1170; 0.2621) | 0.2423 (0.1650; 0.3196) |
| Systolic blood pressure (mmHg) | −0.286 (−1.3342; 0.7621) | 1.4411 (0.3750; 2.5073) | 0.5463 (−0.5697; 1.6624) |
| P-HDL-Cholesterol (mmol/L) | 0.0031 (−0.0242; 0.0304) | −0.0346 (−0.0628; −0.0063) | −0.0086 (−0.0377; 0.0205) |
| P-LDL-Cholesterol (mmol/L) | −0.0194 (−0.0841; 0.0453) | 0.1729 (0.1067; 0.2391) | 0.2305 (0.1605; 0.3005) |
| Weight (kg) | 0.6624 (−0.3343; 1.6592) | 1.3953 (0.3885; 2.4021) | 0.8227 (−0.2186; 1.8640) |
| Waist (cm) | 0.6403 (−0.1711; 1.4517) | 1.0212 (0.1962; 1.8462) | 0.8681 (0.0072; 1.7290) |
| Smoking (%) | 0.1106 (−0.7236; 0.9448) | 0.2924 (−0.5670; 1.1518) | 0.2409 (−0.019; 0.9836) |
| Diabetes (%) | −0.0727 (−0.3725; 0.227) | 0.0679 (−0.2161; 0.3518) | 0.0089 (−0.2501; 0.2678) |
Note: The difference between the intervention and the control groups at the three time points was analyzed using multilevel random slope model. All analyses were adjusted for sex and educational level. We used the command xtmixed for the continuous outcomes and meqrlogit for the categorical outcomes. For diabetes, we present the estimate derived from single-level analysis using logit command in Stata, since the multilevel analysis using meqrlogit resulted in the error “Hessian is not negative semidefinite”.
Analyses of the secondary outcomes showed a difference between the intervention and control groups in total and LDL-cholesterol levels (p<0.001) and waist circumference (p = 0.032) (Table 1). Analysis of the levels of HDL-cholesterol, triglycerides, current smoking, body weight, and systolic blood pressure showed favorable results in the intervention group compared with the controls, although not statistically significant (Table 1). When the analysis was adjusted for sex and education level the intervention effect was sustained over 3-years, which was notable also in differences between groups in total and LDL-cholesterol, as well as in waist circumference (Table 2).
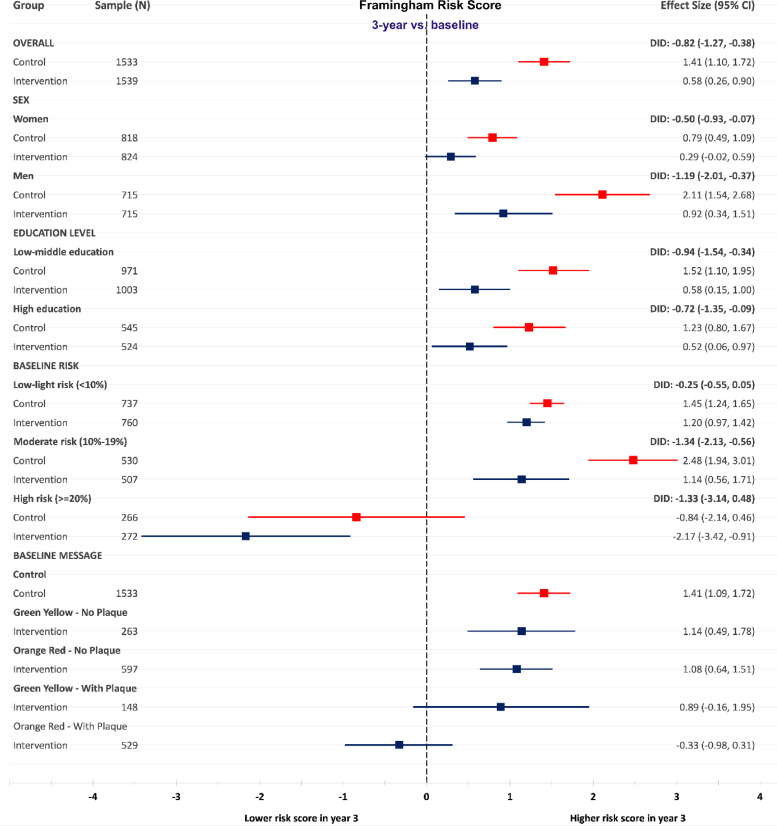
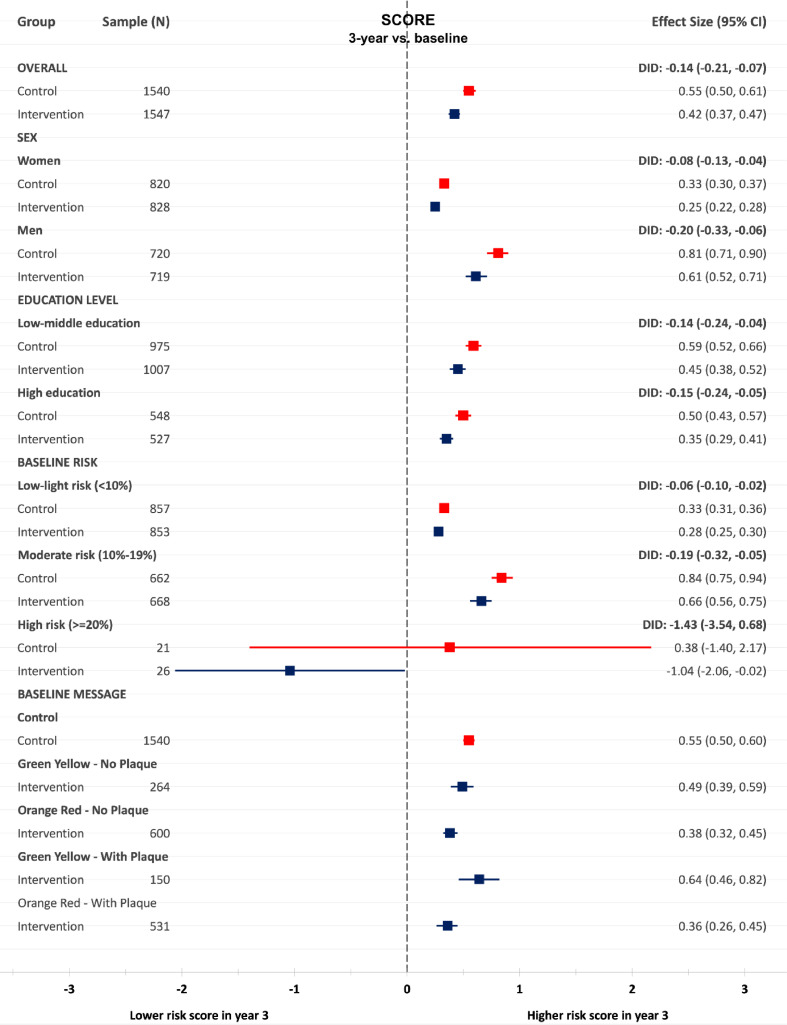
Sub-group analyses of FRS and SCORE by sex showed a significant difference in difference (DID) between the control and intervention groups over 3 years for men and women in FRS and SCORE in favor of the intervention group (Fig. 2, Fig. 3).
Fig. 2.
Changes between baseline and 3-year follow-up in Framingham Risk Score in the intervention and control group, in total, by sex, education, baseline risk categories and atherosclerosis severity category.
Fig. 3.
Changes between baseline and 3-year follow-up in SCORE in the intervention and control group, in total, sex, education, baseline risk categories and atherosclerosis severity category.
Further stratification by education showed a statistically significant DID between the control and intervention groups regardless of education level (Fig. 2 and Fig. 3).
Stratification by baseline FRS and SCORE risk category showed a beneficial pattern of the intervention in all risk groups. We observed significant DID at 3-year follow-up on FRS and SCORE between the intervention and control groups with moderate risk at baseline. Individuals categorized as high risk based on FRS at baseline lowered their FRS over 3 years in both the control and intervention group. No significant DID of FRS and SCORE was observed between the control and intervention groups in the high-risk categories (Fig. 2 and Fig. 3).
Those in the intervention group with the most severe ultrasound results regarding atherosclerosis development (shown in the pictorial ultrasound report as Orange-Red on the IMT gage, illustrating vascular age higher than chronological age, and presence of plaque), displayed a reduction in FRS over 3 years. This change differed significantly from the increase shown in the control group and among those in the intervention group without any plaques (Fig. 2 and Fig. 3).
FRS and SCORE increased from the 1-year follow-up to the 3-year follow up to the same extent in both the control and intervention groups. Furthermore, there were no statistically significant DID regardless of sex, educational level or baseline risk stratification (Supplementary Figure 3 and Figure 4).
Drop-out analysis and missing values: The dropout analysis showed no differences in the FRS and SCORE at baseline between those who dropped-out and those who participated in the 3-year follow-up, among both the control and the intervention groups. Participants in the control group who dropped out had lower age, lower education level, higher blood pressure and higher smoking prevalence compared to those who participated in the follow-up. In the intervention group, the dropouts were younger, had higher waist circumference, and the prevalence of diabetes and smoking were higher (Supplementary Table 1). Percentage of missing values on variables included in the 3-year follow-up varied from 0.7% for daily/occasional smoking in the intervention group to 3.3% on LDL-cholesterol in the control group (Supplementary Table 2).
The intention-to-treat analysis based on imputed data showed the same effect size as the complete case analyses in the overall analyses, as well as in subgroup analyses by sex, risk categories and education (Supplementary Table 3).
4. Discussion
This randomized controlled trial provides evidence that pictorial information in addition to conventional risk-factor based information is beneficial over 3 years as assessed as estimated CVD risk. The pictorial information described the degree of the individual's subclinical carotid atherosclerosis, and was combined with a follow up dialog with a trained nurse and repeated after 6 months. Three letters about CVD prevention in general (not individualized) were given over 3 years to mimic a real-life situation in which patients most likely would have had some contact with their health care provider. There were no additional changes in FRS and SCORE between years 1 and 3, suggesting that it was the initial intervention, and not these later contacts that impacted the results. The findings of the VIPVIZA trial emphasize the potential of visualization of actual atherosclerosis to increase adherence for recommended therapy.
A similar beneficial effect on FRS and SCORE as at 1-year follow-up was observed at 3 years. Importantly, we also found sex- and education-adjusted differences between the intervention and control groups. Most other interventions have showed favorable effects mainly in high-income and high-educated groups. The subgroup analyses pointed to the same direction. After 1-year follow-up, there was no significant difference in change of FRS and SCORE between the control and intervention group until the 3-year follow-up. There was a similar difference in levels in the risk scores at 1-year and at 3-year follow-up, to the benefit of the intervention group, thus, showing a sustained intervention effect. In both the control and intervention group, the risk scores increased to the same extent from 1-year follow-up to 3-year follow up. This could be largely explained by the increase in age over two years.
The difference between groups in LDL-cholesterol, taken together with the difference in waist circumference, suggests that the intervention effect is a combined effect of pharmacological treatment and lifestyle modification. The pattern of changes in other risk factors, even though not statistically significant, all point in a more favorable direction in the intervention group. Moreover, the effect was greater for participants with demonstrated more severe atherosclerosis.
Previous research has shown that the effect of interventions on lifestyle and pharmacological treatment with statins usually decreases after 1 year, and sustained effects after 2 years are scarce in the literature [18], [19], [20]. Therefore, the sustained effect in this study is highly relevant with regard to the major problem of non-adherence to CVD prevention by individuals and health professionals. Furthermore, the increased intervention effect noticed between 1 and 3 years with a further reduction in total cholesterol and LDL-cholesterol (mmol/L) is important. This may partly be explained by the increased awareness of handling the VIPVIZA result over time, by both physicians and participants [21]. This phenomenon may be explained by a gradual implementation and acceptance of VIPVIZA intervention by physicians as motivated by the diffusion of innovation model in which different categories of adopters are identified including the early adopters and late laggards [22].
The dual effect of targeting patients and physicians with pictorial information was previously shown in a RCT that used CT-scanning for coronary artery calcium [23], showing a significant change in FRS and cardiovascular risk factors after four years. This supports our findings, but, to the best of our knowledge, a similar intervention has not been tested in a pragmatic setting. Moreover, 91% of their study population was highly educated as compared with 35% in our trial. Other studies on the effect of pictorial information to motivate patients for behavior change in different CVD conditions have been contradictory [24], [25], [26]. A recent population-based study, the Swedish CardioPulmonary bioImage Study (SCAPIS), shows that 84% of the participants with plaque on both the right and left carotid artery were in the intermediate risk group according to SCORE [27]. Furthermore, most CVD events occur in this risk group, in which sufficient prevention is often overlooked [28], [29], [30]. In the present study, a significant intervention effect measured as change of FRS and SCORE was observed in the intermediate risk group, indicating that the VIPVIZA intervention may strengthen public health strategies for primary prevention, since even small improvements in this large group may have significant effect on the public health level [31]. This has further been shown for a reduction in LDL-levels in which a small decrease has shown to have a long-term benefit on a population level [32]. In addition, the life expectancy in this study population is long and even small effects can be important on the individual level.
The size of the intervention effect should be considered with regard to four circumstances. First, the intervention was given to a low to moderate risk population group with somewhat limited potential for individual improvement. Second, the intervention was nested within the VIP - an effective prevention program provided to all inhabitants in the county [33]. The benefit of VIPVIZA was thereby obtained on top of the VIP. Third, the study was performed within ordinary primary care, which during the study period was under stress due to many vacant positions, and may have ceased preventive actions. Fourth, the intervention is a low-intensity intervention as compared to a new potent drug or a surgical or catheter-based intervention.
The pragmatic design of VIPVIZA renders a high external validity to the study. Its simple design can easily be implemented in a regular health care setting. The dropout rate of 10.3% might be a limitation. However, considering 3 years of follow-up and compared to other public health interventions, this is rather a strength of the study. Sensitivity and intention-to-treat analyses with imputed data on missing values on primary outcomes showed very similar and corresponding results. The results in this study cannot be translated into effects on hard clinical events such as myocardial infarction, stroke and CVD death. This requires prolonged follow-up for ten years or more. Further studies within VIPVIZA to investigate the potential effects from pharmacotherapy versus life-style changes on the overall reduction of CVD risk can give more in depth knowledge on underlaying processes of the intervention.
We found that the pictorial risk communication engaged peoples’ minds among both physicians and participants in the intervention arm (unpublished results). This will encourage further studies in which pictorial information can be tailored to the individual's psychological and social profile and health behaviors in order to improve adherence and to provide an effective person-centered prevention.
The findings of this 3-year follow-up of the VIPVIZA pragmatic RCT provides evidence of a sustained beneficial effect of pictorial information about subclinical atherosclerosis on the development of CVD risk, irrespective of sex and educational level. Stratified sub-analyses of sex, age, education level, level of traditional risk factor-based CVD risk, and pictorial information about the severity of subclinical atherosclerosis show results pointing in the same direction. Direct visualization of the underlying still subclinical atherosclerotic disease, rather than just indirect information about risk factors and statistical risk of future myocardial infarction, stroke and death, is one way to tackle the non-adherence problem in prevention of cardiovascular diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank the study participants for taking their time and effort to participate in the VIPVIZA trial. We would also like to thank the VIP nurses and physicians at health care centers in the county of Västerbotten for their engagement in the VIPVIZA trial. Carola Sundholm and Maria Backlund, research nurses, and ultrasound technicians at the Department of Clinical Physiology, Heart centre, are acknowledged for their great work throughout the study. Wolfgang Lohr, database manager, is acknowledged for valuable contributions, and Rachel Nicholl, PhD, for language review.
Statement of authorship
AB, MN, NN, PWes, BC, CG, JH, BerntL, BertilL, SN, EN, PWen and UN designed and planned the study. AB, MN, NN, PWes and UN drafted the manuscript, AB and NN did the statistical analysis. All authors revised the manuscript, provided important content and approved the final manuscript before publication.
Funding
Region Västerbotten (Central ALF, Dnr ALFVLL-298001 and ALFVLL-643391), the Swedish Research Council (Dnr 521-2013-2708, 2016-01891, 2017-02246), the Heart and Lung Foundation (Dnr 20150369, 20170481), SKANDIA Risk & Health, and an unconditional donation from Carl Bennet Ltd, Sweden. In addition to major grants, VIPVIZA was also funded by the Swedish Society of Medicine, the Heart Foundation in Northern Sweden, STROKE – the national association, The Swedish Insurance Society, Visare Norr (the four Northern Regions), and the Swedish and the Västerbotten Heart and Lung Associations. The funders of the study had no role in the study design, data collection, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decisions to submit for publication.
Disclosures
Nothing to declare.
Footnotes
Grant support: Region Västerbotten (Central ALF, Dnr ALFVLL-298001 and ALFVLL-643391), the Swedish Research Council (Dnr 521-2013-2708, 2016-01891, 2017-02246), the Heart and Lung Foundation (Dnr 20150369, 20170481), SKANDIA Risk & Health, and an unconditional donation from Carl Bennet Ltd, Sweden. In addition to major grants, VIPVIZA was also funded by the Swedish Society of Medicine, the Heart Foundation in Northern Sweden, STROKE – the national association, The Swedish Insurance Society, Visare Norr (the four Northern Regions), and the Swedish and the Västerbotten Heart and Lung Associations. The funders of the study had no role in the study design, data collection, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decisions to submit for publication.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2021.100199.
Appendix. Supplementary materials
References
- 1.Kones R. Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag. 2013;9:617–670. doi: 10.2147/VHRM.S37119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotseva K., De Bacquer D., De Backer G., Ryden L., Jennings C., Gyberg V. Lifestyle and risk factor management in people at high risk of cardiovascular disease. A report from the European society of cardiology european action on secondary and primary prevention by intervention to reduce events (EUROASPIRE) IV cross-sectional survey in 14 European regions. Eur J Prev Cardiol. 2016;23:2007–2018. doi: 10.1177/2047487316667784. [DOI] [PubMed] [Google Scholar]
- 3.Novo S., Carita P., Lo Voi A., Muratori I., Tantillo R., Corrado E. Impact of preclinical carotid atherosclerosis on global cardiovascular risk stratification and events in a 10-year follow-up: comparison between the algorithms of the Framingham Heart Study, the European SCORE and the Italian 'Progetto Cuore'. J Cardiovasc Med. 2019;20:91–96. doi: 10.2459/JCM.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 4.Baber U., Mehran R., Sartori S., Schoos M.M., Sillesen H., Muntendam P. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015;65:1065–1074. doi: 10.1016/j.jacc.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Gepner A.D., Young R., Delaney J.A., Tattersall M.C., Blaha M.J., Post W.S. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the multi-ethnic study of atherosclerosis. Circul Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadi A., Argulian E., Leipsic J., Newby D.E., Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1608–1617. doi: 10.1016/j.jacc.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Sala-Vila A., Romero-Mamani E.S., Gilabert R., Nunez I., de la Torre R., Corella D. Changes in ultrasound-assessed carotid intima-media thickness and plaque with a Mediterranean diet: a substudy of the PREDIMED trial. Arterioscler Thromb Vasc Biol. 2014;34:439–445. doi: 10.1161/ATVBAHA.113.302327. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadi A., Narula J. Primary and Secondary Prevention, or Subclinical and Clinical Atherosclerosis. JACC Cardiovasc Imaging. 2017;10:447–450. doi: 10.1016/j.jcmg.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Korcarz C.E., DeCara J.M., Hirsch A.T., Mohler E.R., Pogue B., Postley J. Ultrasound detection of increased carotid intima-media thickness and carotid plaque in an office practice setting: does it affect physician behavior or patient motivation? J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2008;21:1156–1162. doi: 10.1016/j.echo.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denissen S.J., van der Aalst C.M., Vonder M., Oudkerk M., de Koning H.J. Impact of a cardiovascular disease risk screening result on preventive behaviour in asymptomatic participants of the ROBINSCA trial. Eur J Prev Cardiol. 2019;26:1313–1322. doi: 10.1177/2047487319843396. [DOI] [PubMed] [Google Scholar]
- 11.Naslund U., Ng N., Lundgren A., Fharm E., Gronlund C., Johansson H. Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (VIPVIZA): a pragmatic, open-label, randomised controlled trial. Lancet. 2019;393:133–142. doi: 10.1016/S0140-6736(18)32818-6. [DOI] [PubMed] [Google Scholar]
- 12.Heiss C., Pitcher A., Belch J.J.F., De Carlo M., Reinecke H., Baumgartner I. The year in cardiology: aorta and peripheral circulation. Eur Heart J. 2020;41:501. doi: 10.1093/eurheartj/ehz939. -8b. [DOI] [PubMed] [Google Scholar]
- 13.Norberg M., Wall S., Boman K., Weinehall L. The Vasterbotten Intervention Programme: background, design and implications. Glob Health Action. 2010;3 doi: 10.3402/gha.v3i0.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein J.H., Korcarz C.E., Hurst R.T., Lonn E., Kendall C.B., Mohler E.R. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 89-90. [DOI] [PubMed] [Google Scholar]
- 15.Vanoli D., Lindqvist P., Wiklund U., Henein M., Naslund U. Fully automated on-screen carotid intima-media thickness measurement: a screening tool for subclinical atherosclerosis. J Clin Ultrasound: JCU. 2013;41:333–339. doi: 10.1002/jcu.22041. [DOI] [PubMed] [Google Scholar]
- 16.Den Ruijter H.M., Peters S.A., Anderson T.J., Britton A.R., Dekker J.M., Eijkemans M.J. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 17.Rubin D.B. Multiple imputation after 18+ Years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 18.Zhang X., Imperatore G., Thomas W., Cheng Y.J., Lobelo F., Norris K. Effect of lifestyle interventions on glucose regulation among adults without impaired glucose tolerance or diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2017;123:149–164. doi: 10.1016/j.diabres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBlanc E.S., Patnode C.D., Webber E.M., Redmond N., Rushkin M., O'Connor E.A. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;320:1172–1191. doi: 10.1001/jama.2018.7777. [DOI] [PubMed] [Google Scholar]
- 20.Drexel H., Coats A.J.S., Spoletini I., Bilato C., Mollace V., Perrone Filardi P. ESC position paper on statins adherence and implementation of new lipid-lowering medications: barriers to be overcome. Eur Heart J Cardiovasc Pharmacother. 2020;6:115–121. doi: 10.1093/ehjcvp/pvz079. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson A., Lindvall K., Norberg M., Fharm E. Increased knowledge makes a difference! - general practitioners' experiences of pictorial information about subclinical atherosclerosis for primary prevention: an interview study from the VIPVIZA trial. Scand J Prim Health Care. 2021;39:77–84. doi: 10.1080/02813432.2021.1882083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers E.M. Diffusion of preventive innovations. Addict Behav. 2002;27:989–993. doi: 10.1016/s0306-4603(02)00300-3. [DOI] [PubMed] [Google Scholar]
- 23.Rozanski A., Gransar H., Shaw L.J., Kim J., Miranda-Peats L., Wong N.D. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (early identification of subclinical atherosclerosis by noninvasive imaging research) prospective randomized trial. J. Am. Coll. Cardiol. 2011;57:1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones A.S., Ellis C.J., Nash M., Stanfield B., Broadbent E. Using animation to improve recovery from acute coronary syndrome: a randomized trial. Ann Behav Med. 2016;50:108–118. doi: 10.1007/s12160-015-9736-x. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz J., Allison M., Wright C.M. Health behavior modification after electron beam computed tomography and physician consultation. J Behav Med. 2011;34:148–155. doi: 10.1007/s10865-010-9294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodondi N., Collet T.H., Nanchen D., Locatelli I., Depairon M., Aujesky D. Impact of carotid plaque screening on smoking cessation and other cardiovascular risk factors: a randomized controlled trial. Arch Intern Med. 2012;172:344–352. doi: 10.1001/archinternmed.2011.1326. [DOI] [PubMed] [Google Scholar]
- 27.Ostgren C.J., Soderberg S., Festin K., Angeras O., Bergstrom G., Blomberg A. Systematic Coronary Risk Evaluation estimated risk and prevalent subclinical atherosclerosis in coronary and carotid arteries: a population-based cohort analysis from the Swedish Cardiopulmonary Bioimage Study. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320909300. [DOI] [PubMed] [Google Scholar]
- 28.Polonsky T.S., Greenland P. CVD screening in low-risk, asymptomatic adults: clinical trials needed. Nat Rev Cardiol. 2012;9:599–604. doi: 10.1038/nrcardio.2012.114. [DOI] [PubMed] [Google Scholar]
- 29.Baldassarre D., Hamsten A., Veglia F., de Faire U., Humphries S.E., Smit A.J. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (carotid intima media thickness [IMT] and IMT-progression as predictors of vascular events in a high risk European population) study. J Am Coll Cardiol. 2012;60:1489–1499. doi: 10.1016/j.jacc.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 30.Turner G.M., Calvert M., Feltham M.G., Ryan R., Fitzmaurice D., Cheng K.K. Under-prescribing of prevention drugs and primary prevention of stroke and transient Ischaemic attack in UK general practice: a retrospective analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose G. Strategy of prevention: lessons from cardiovascular disease. Br Med J (Clin Res Ed) 1981;282:1847–1851. doi: 10.1136/bmj.282.6279.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman M.G., Ference B.A., Im K., Wiviott S.D., Giugliano R.P., Grundy S.M. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 33.Blomstedt Y., Norberg M., Stenlund H., Nystrom L., Lonnberg G., Boman K. Impact of a combined community and primary care prevention strategy on all-cause and cardiovascular mortality: a cohort analysis based on 1 million person-years of follow-up in Vasterbotten County, Sweden, during 1990-2006. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-009651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.