Abstract
Objective
The Pooled Cohort Equations (PCE) serve as the platform for quantitative risk assessment for atherosclerotic cardiovascular disease (ASCVD). Data are sparse regarding the benefit of adding health-related quality of life (HRQoL) measures to the PCE. We sought to estimate the association of HRQoL with ASCVD events and evaluate the potential utility of adding HRQoL to the PCE in refining quantitative risk assessment for primary prevention decisions.
Methods
Three multi-ethnic longitudinal cohorts were included in the study. HRQoL was measured using the SF-12 physical component summary (PCS) and mental component summary (MCS); higher PCS or MCS scores indicate better HRQoL. We constructed a four-level HRQoL status variable: MCS <50 and PCS <50; MCS <50 and PCS ≥50; MCS ≥50 and PCS <50; MCS ≥50 and PCS ≥50. Harrell's C statistics and net reclassification improvement (NRI) analyses were used to assess the added predictive ability of HRQoL for incident ASCVD.
Results
A total of 9,904 individuals were included in the analysis, of whom 4,743 were in the low risk subgroup (<5% predicted 10-year risk). HRQoL status, PCS and its subscale scores were independent predictors of ASCVD events. HRQoL improved both discrimination (delta C: 0.004, p = 0.05) and reclassification (cNRI: 0.15, p<0.01) modestly when added to PCE; 3% and 6% of individuals with events were correctly reclassified to higher risk in the overall sample and low risk subgroup, respectively.
Conclusion
HRQoL is an independent predictor of ASCVD events, and improves ASCVD risk prediction significantly, though modestly, overall and in low-risk individuals. HRQoL may be a cost-effective risk-enhancing factor for refining quantitative risk assessment for primary prevention decisions.
Keywords: Atherosclerotic cardiovascular disease, Pooled cohort equations, Health related quality of life, Risk-enhancing factor
Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of morbidity and mortality globally. [1] Current prevention paradigms emphasize identification of individuals’ short-term and long-term risk for ASCVD to guide decisions about intensity of lifestyle and drug therapy preventive interventions. The ACC/AHA/Multi-specialty clinical practice guidelines recommend quantitative risk assessment for all individuals without ASCVD using the US-derived Pooled Cohort Equations (PCE) in order to stratify them into low (<5%), borderline (5–7.5%), intermediate (7.5–20%), and high (≥20%) 10-year risk groups. [2], [3], [4], [5] The initial quantitative 10-year risk estimate should be followed by personalized assessment of patient-specific risk-enhancing factors that include demographic, medical history, physiologic, and biochemical factors that may modify the initial risk estimate. [2] To date, the list of risk-enhancing factors has not included psychosocial measures that have been associated with ASCVD risk in some studies. Multiple factors contribute to the lack of predictive utility demonstrated by some psychosocial factors to date, including: difficulty of measurement, precision of measurement, stability of measurement over time, relative weakness of associations with ASCVD, and correlation with strong downstream determinants of risk (e.g., obesity, hypertension, dyslipidemia, and smoking). [6], [7], [8], [9], [10]
Health-related quality of life (HRQoL) is a broad concept that includes self-reported measures of physical and mental health, is easily measured using standardized tools, and has been widely validated to correlate with prevalent health status and incident health outcomes. [11], [12], [13], [14], [15], [16], [17] Several studies have demonstrated the association of HRQoL with prevalent cardiovascular disease (CVD), and CVD is significantly associated with impaired HRQoL. [18], [19], [20] Indeed, even adverse changes in CVD risk factor levels are associated with a lower HRQoL score. [9, 21] Furthermore, the PCE tend to underestimate risk in individuals with lower socioeconomic status or with chronic inflammatory conditions, who may have lower HRQoL. [5] However, data are sparse regarding the additional utility of HRQoL measures as part of ASCVD risk estimation, in the general population or in specific risk subgroups, such as individuals without significant risk factor burden, in whom other pathways may confer ASCVD risk. [3]
Using data from the Cardiovascular Lifetime Risk Pooling Project (LRPP), we examined the association of HRQoL with ASCVD risk in the general population and the low risk subgroup (10-year ASCVD risk <5%), and addressed the potential utility of adding HRQoL to the PCE in refining quantitative risk assessment for primary prevention decisions. We hypothesized that HRQoL measures would be independently associated with ASCVD and that these measures would significantly reclassify individuals, especially those at lower predicted risk.
Methods
Study sample
Detailed methods for the inclusion criteria and harmonization of the LRPP cohort data have been published previously. [22] For this study, we selected the cohorts with HRQoL measures, ascertained using the widely validated SF-12 or SF-36 HRQoL instruments, as well as with ascertainment of hard CVD outcomes, including fatal and non-fatal coronary heart disease and stroke. We included three cohorts: the Coronary Artery Risk Development in Young Adults Study (CARDIA), [23] the Framingham Offspring Study (FOS), [24] and the Multi-Ethnic Study of Atherosclerosis (MESA). [25] CARDIA recruited 5115 black and white men and women, aged 18–30 years in 1985–1986 from Birmingham, Alabama; Chicago; Minneapolis; and Oakland, California. The last date of follow-up for this analysis was August 31, 2018. FOS enrolled 5124 white men and women, ages 5 to 70 in 1971 from Framingham, Massachusetts and the follow-up data was through 2008. MESA recruited 6814 participants aged 45–84 years in 2000–2002 at 6 field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota). The follow up data was through 2018. The Institutional Review Board at Northwestern University approved this project. Ten-year predicted ASCVD risk was calculated for each participant using the PCE, and the risk estimates for Hispanics and Chinese participants were calculated using the PCE for whites, as suggested in the guidelines. [2] We chose a priori to study all participants and to perform a subgroup analysis among low risk individuals (PCE <5%). Participants were excluded from the analysis if they had prevalent CVD history, were younger than 40 or older than 79 years of age, or were missing cardiovascular risk factor measures necessary for calculation of the 10-year ASCVD risk (blood pressure, antihypertensive treatment, total cholesterol, HDL-cholesterol, smoking and diabetes). Of the 13,329 participants at baseline, 3425 were excluded from analyses as described in Supplemental Figure 1. A total of 9904 participants were included in the main analysis and 4743 individuals were included in the low risk subgroup.
Quality of life (SF-12) measures
The 12-item Short Term Health Survey was used as a measure of self-rated health-related quality of life (HRQoL) [13, 14], including the Physical Component Summary score (PCS) and Mental Component Summary score (MCS). The PCS measures physical functioning, general health, bodily pain, and role limitations due to physical problems. The MCS assesses mental health, social functioning, vitality, role limitations due to emotional problems. The method for deriving the summary scale scores have been published in full elsewhere [11, 13]. The SF-12 was scored using the standard algorithm, [14] which assumes that the PCS and MCS scores are orthogonal, and provides norm-based standardized summary scores with a mean of 50 and a standard deviation of 10. A higher PCS or MCS score denotes better HRQoL. Thus, scores above and below 50 are above and below the average in the general US population.
The SF-12 questionnaire was administered in the year 2000 in CARDIA, 2002–2004 in MESA, and 1995 in FOS, [25], [26], [27] respectively. The SF-12 items were reversed as appropriate so that a higher score indicates better health. [23] The mean and standard deviation of SF-12 scores across these three cohorts is summarized in Supplemental Table 1.
Adjudication of outcomes
Ascertainment of ASCVD (fatal/nonfatal CHD and stroke) events used similar procedures in all three cohorts, as described elsewhere. [23], [24], [25] Briefly the FOS adjudicated CHD events via medical history, physical examinations, and electrocardiograms. All suspected CHD events were reviewed by a panel of 3 physicians, who applied established criteria for such events. [24] In CARDIA, participants were contacted annually to inquire about interim hospitalizations. For each event, medical records were obtained and adjudicated by 2 trained physician reviewers. [23] MESA collected information from death certificate, medical records from hospitalization, autopsy reports and interviews with participants, [25] and events were reviewed by two trained physician reviewers.
Statistical analysis
We stratified the study sample into 4 groups: individuals with low HRQoL scores on both (MCS and PCS < 50), individuals with scores PCS < 50 and MCS ≥ 50, individuals with scores MCS < 50 and PCS ≥ 50, and individuals with high HRQoL scores on both (MCS and PCS ≥ 50). We compared baseline characteristics across the 4 HRQoL groups, using ANOVA for continuous variables or Chi-square test for categorical variables. To assess the independent predictive effect of HRQoL for ASCVD events, we applied a Cox proportional hazard model and examined the association between HRQoL and incident ASCVD adjusting for age, race, sex and ACC/AHA Pooled Cohort Equations risk factors including total and HDL cholesterol levels, current smoking status, systolic blood pressure, use of anti-hypertensive medication, and history of diabetes. As a 5-point difference is considered a meaningful SF-12 difference clinically, we also examined hazard ratios (HR) for the association of HRQoL with outcomes for every 5-point decrement of MCS, PCS and their subscale scores.
We assessed the discrimination and reclassification of categorical HRQoL status beyond the PCE. Harrell's C-statistic was used to estimate the discrimination of the PCE with and without HRQoL. Since the C-statistic alone is often not sensitive enough to capture a meaningful improvement from new markers, [28, 29] we also calculated the predictive utility of adding HRQoL measures using the net reclassification improvement (NRI). [30, 31] Bootstrapping was used to calculate 95% confidence intervals (CI) [32]. All analyses were performed in the entire study sample and repeated in the low-risk subgroup separately. A 2-sided P-value of 0.05 or less denoted statistical significance. All analyses were performed using SAS 9.4 (SAS Institute, Cary NC) and R 3.6.1.
Results
Sample characteristics
The baseline characteristics of all participants and the low risk subgroup are shown in Table 1 and Supplemental Table 2, separately. Overall the mean age was 57.3 (SD 11.2) years; 46.3% of participants were male, 15.2% African American, 14.7% Hispanic American, and 12.0% Chinese American. Participants in the low risk subgroup were younger and more likely to be female. Having higher HRQoL status, especially higher PCS, was associated with a more favorable CVD risk factor profile including lower rates of smoking, hypertension, obesity, and diabetes among all participants and the low risk subgroup.
Table 1.
Baseline Characteristics According to Health-Related Quality of Life Status.
| All | MCS < 50/ PCS < 50 | MCS < 50/ PCS ≥ 50 | MCS ≥ 50/ PCS < 50 | MCS ≥ 50/ PCS ≥ 50 | p value | |
|---|---|---|---|---|---|---|
| n = 9904 | n = 1285 | n = 1762 | n = 2301 | n = 4556 | ||
| Age, years | 57.3 (11.2) | 58.3 (11.8) | 53.8 (10.6) | 60.4 (10.9) | 56.7 (10.9) | < 0.01 |
| Male, (%) | 46.3 | 35.1 | 43.3 | 42.0 | 52.7 | < 0.01 |
| Race/ethnicity, (%) | < 0.01 | |||||
| White | 58.1 | 47.7 | 63.5 | 53.1 | 61.5 | |
| Black | 15.2 | 18.9 | 14.4 | 13.9 | 15.1 | |
| Hispanic | 14.7 | 16.8 | 11.2 | 18.8 | 13.2 | |
| Asian | 12.0 | 16.4 | 10.2 | 14.2 | 10.3 | |
| Bachelor's degree or higher, (%) | 67.7 | 55.3 | 72.6 | 61.9 | 72.2 | < 0.01 |
| Body mass index, kg/m2 | 28.3 (5.6) | 30.1 (6.5) | 27.8 (5.4) | 29.6 (6.2) | 27.4 (4.9) | < 0.01 |
| Systolic blood pressure, mmHg | 122 (19) | 125 (20) | 119 (18) | 126 (20) | 121 (18) | < 0.01 |
| Diastolic blood pressure, mmHg | 72 (10) | 73 (11) | 73 (10) | 72 (10) | 72 (10) | 0.06 |
| Total cholesterol, mg/dl | 194 (37) | 195 (43) | 194 (36) | 195 (39) | 194 (36) | 0.46 |
| High-density lipoprotein cholesterol, mg/dl | 51 (15) | 51 (15) | 53 (16) | 51 (15) | 52 (15) | 0.02 |
| Fasting glucose, mg/dl | 98 (28) | 102 (32) | 95 (25) | 102 (33) | 96 (24) | < 0.01 |
| Current smoker, (%) | 14.3 | 20.0 | 16.8 | 14.9 | 11.6 | < 0.01 |
| Blood pressure lowering medication, (%) | 30.0 | 38.5 | 23.3 | 41.9 | 24.3 | < 0.01 |
| Diabetesa, (%) | 10.5 | 16.3 | 7.6 | 15.1 | 7.6 | < 0.01 |
| Obesityb, (%) | 31.8 | 43.3 | 28.4 | 40.9 | 25.2 | < 0.01 |
| Hypertensionc, (%) | 39.4 | 48.5 | 31.4 | 51.8 | 33.7 | < 0.01 |
Continuous variables are shown as mean (SD); categorical variables are shown as%.
Abbreviations: ASCVD=Atherosclerotic Cardiovascular Disease; SD=standard deviation; MCS=mental health summary score; PCS=physical health summary score.
Diabetes was defined as fasting serum glucose ≥126 mg/dL or a history of treatment for diabetes.
Obesity was defined as body mass index ≥30 kg/m2.
Hypertension was defined as systolic blood pressure ≥140 or diastolic blood pressure ≥90 mm Hg or a history of treatment for lowering blood pressure.
HRQoL measures and associations with outcomes
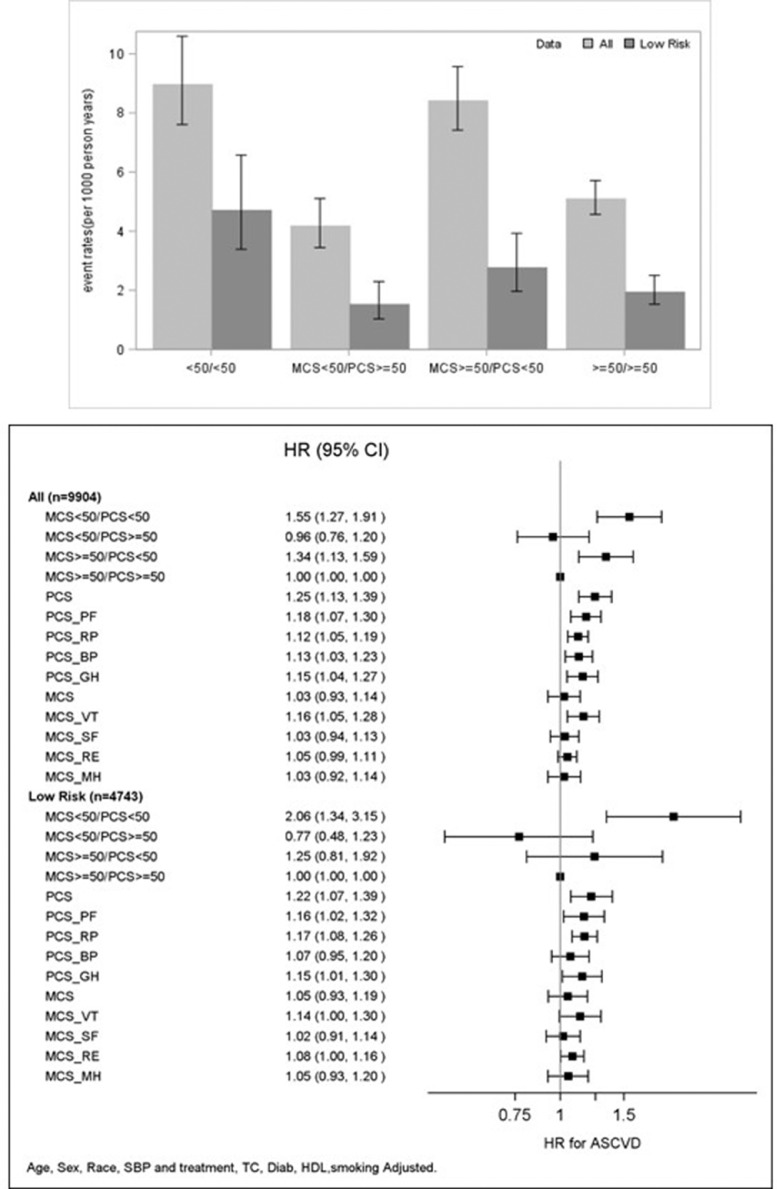
Over a median follow up of 13.1 years (interquartile range of 12.5 −15.4 years), 783 (7.9%) and 154 (3.3%) incident ASCVD events were observed in the overall sample and low risk subgroup, respectively. Participants with PCS ≥50, regardless of MCS values, had a lower ASCVD incidence rate compared to those with PCS <50 (Fig. 1).
Fig. 1.
Associations of HRQoL Measures and ASCVD The top bar graph shows the unadjusted ASCVD incidence rate and 95% confidence interval across HRQoL status in all and low risk subgroup; the bottom forest plot shows the adjusted hazard ratios (HR) and 95% confidence interval for ASCVD. Hazard Ratio per every 5-point decrement was estimated for PCS, MCS and all eight subscales. Abbreviations: ASCVD= Atherosclerotic cardiovascular disease; HRQoL= Health related quality of life; MCS=mental health summary score; PCS=physical health summary score; PCS_PF=physical functioning; PCS_RP=role limitations due to physical problems; PCS_BP=bodily pain; PCS_GH= general health; MCS_VT=vitality; MCS_SF=social functioning; MCS_RE=role limitations due to emotional problems; MCS_MH= mental health.
With MCS ≥50 and PCS ≥50 as the reference group, having MCS and PCS< 50 or MCS ≥50 and PCS<50 were both significantly associated with higher hazards for ASCVD events (HR=1.55: 95% CI 1.27–1.91 and HR=1.34: 95% CI 1.13–1.59, respectively), after adjusting for age, sex, race, systolic blood pressure and blood pressure treatment, smoking, total and high density lipoprotein cholesterol, and history of diabetes (Fig. 1). Every 5-point decrement of physical health summary score (PCS) and its four subscale scores (physical functioning, general health, bodily pain, and role limitations due to physical problems) were all significantly associated with ASCVD risk (HR=1.12–1.25). Mental health summary score (MCS) was not associated with ASCVD after covariate adjustment. Vitality was the only mental health subscale score that was significantly associated with ASCVD (HR=1.16: 95% CI 1.05–1.28). Among the low risk subgroup, the significant association of MCS <50 and PCS <50 with higher ASCVD risk remained. The associations of PCS and its subscale scores with ASCVD risk were all slightly attenuated with multivariable adjustment, and the significant association of bodily pain with ASCVD risk was abolished. We repeated the analysis for each cohort separately and findings were overall consistent across the cohorts. No significant interactions by sex and race were observed. There were no strong correlations between HRQOL score and ASCVD risk factors.
Predictive utility of HRQoL measures
The added predictive utility of HRQoL status in terms of discrimination and reclassification, beyond the PCE risk factors, is shown in Tables 2 and 3. Adding HRQoL status to the PCE model significantly though modestly increased discriminatory ability for ASCVD events (delta c = 0.004; 95% CI 0.001–0.008, p = 0.05). Table 3 shows the NRI for events and nonevents when HRQoL status was added to the model using the 5% and 20% 10-year ASCVD risk thresholds. The correct classification of events and non-events for the overall population are 3% and 0%, respectively. The addition of HRQoL to the model resulted in a significant improvement in classification (NRI =0.03: 0.001–0.05), but it was limited to an improvement in classification for events only (not non-events): 3% of those who experienced ASCVD events were correctly reclassified as having a higher risk. The continuous NRI (cNRI) was 0.15 (95% CI: 0.07–0.19, p<0.01). Among the low risk subgroup, the improvement of C statistic was modest and of borderline significance (delta C = 0.01, p = 0.07). There was a significant reclassification conferred by HRQoL with 1% and 5% ASCVD risk as the cut-off, the correct classification of events and non-events for this low risk subgroup are 6% and 1% respectively. and the improvement of classification was significant for both events and non-events: 6% of the participants with events were correctly classified in a higher category and 1% of participants with no events were correctly classified in a lower risk category, yielding a net correct reclassification of 7% (NRI 0.07, 95% CI 0.01–0.13). Category-free cNRI estimate was 0.14 (95% CI: 0.07–0.31, p< 0.01).
Table 2.
Harrell's C-statistic with adding HRQoL status to PCE in predicting ASCVD events.
| PCE | PCE+ HRQoL | Delta of C statistics (95%CI) | |
|---|---|---|---|
| All(n = 9904) | 0.752(0.734–0.771) | 0.756(0.737–0.774) | 0.004 (0.001- 0.008)P = 0.049 |
| Low risk subgroup(n = 4743) | 0.692(0.644–0.741) | 0.699(0.649–0.748) | 0.007 (−0.001–0.036)P = 0.070 |
ASCVD= Atherosclerotic cardiovascular disease; HRQoL= Health related quality of life; PCE= Pooled Cohort Equations.
Table 3.
Reclassification table for events and nonevents.
| All (n = 9904) | |||||
|---|---|---|---|---|---|
| PCE | |||||
| PCE + HRQoL | <5% | 5%−20% | >=20% | Row Total (%) | % Correctly reclassified |
| Events (n = 783) | |||||
| <5% | 166 | 22 | 0 | 188 (24%) | |
| 5%−20% | 29 | 472 | 12 | 513 (66%) | |
| >=20% | 0 | 25 | 57 | 82 (10%) | |
| Column total (%) | 195 (25%) | 519 (66%) | 69 (8%) | 3% (p = 0.03) | |
| Nonevents (n = 9121) | |||||
| <5% | 5114 | 302 | 0 | 5416 (59%) | |
| 5%−20% | 299 | 3113 | 57 | 3469 (38%) | |
| >=20% | 0 | 62 | 174 | 236 (3%) | |
| Column total (%) | 5413(59%) | 3477(38%) | 231 (3%) | 0% (p = 0.94) | |
| Total NRI (95% CI) | 0.03 (0.01 - 0.05) | ||||
| Low risk Subgroup (n = 4743) | |||||
| PCE | |||||
| PCE + HRQoL | <1% | 1%−5% | >=5% | Row Total | % Correctly reclassified |
| Events (n = 154) | |||||
| <1% | 9 | 3 | 0 | 12 (8%) | |
| 1%−5% | 0 | 106 | 4 | 110 (71%) | |
| >=5% | 0 | 16 | 16 | 32 (21%) | |
| Column total (%) | 9 (6%) | 125 (81%) | 20 (13%) | 6% (p = 0.06) | |
| Nonevents (n = 4589) | |||||
| <1% | 1124 | 220 | 0 | 1344 (29%) | |
| 1%−5% | 135 | 2810 | 73 | 3018 (66%) | |
| >=5% | 0 | 101 | 126 | 227 (5%) | |
| Column total (%) | 1259 (27%) | 3131 (68%) | 199 (4%) | 1% (p = 0.01) | |
| Total NRI (95% CI) | 0.07 (0.01–0.13) | ||||
Values are n or n (%) unless otherwise indicated. Each interior cell contains the numbers of persons in the corresponding risk categories under the original PCE and HRQoL in addition to PCE.
ASCVD= Atherosclerotic cardiovascular disease; HRQoL= Health related quality of life; PCE= Pooled Cohort Equations; NRI= net reclassification improvement.
We further estimated the improvement of discrimination and reclassification with adding the continuous MCS or PCS separately to the model, and present the results as Supplemental Table 3. The addition of PCS to the PCE model resulted in significant improvement of discrimination and reclassification (delta C = 0.007 (0.001–0.01); cNRI = 0.12 (0.08–0.16)). The addition of MCS had no benefit in improving model prediction performance.
Discussion
The present analysis demonstrates that HRQoL is significantly associated with ASCVD events, and adds significantly to the 2013 Pooled Cohort Equations, providing modestly greater discrimination and reclassification power for risk stratification among a multi-ethnic sample and its low risk subgroup. In particular, it appropriately reclassified those destined to have events to higher predicted risk. This suggests that HRQoL could be an additional potential risk-enhancing factor for clinicians to consider in refining quantitative risk assessment for primary prevention decisions, as suggested by the guidelines. [2]
In primary analysis, we constructed a 4-stratum HRQoL variable using the physical and mental component scores to represent relative health related quality of life compared to the general population. We further examined the physical and mental components separately. Our study was consistent with previous research that has shown physical HRQoL was a stronger predictor for CVD events than mental HRQoL. [18, 19] One study suggested that poor physical HRQoL was significantly associated with higher risk of incident CVD events overall, and for CHD and stroke events separately, among older participants. [18] However no study has examined the added predictive ability of HRQoL for incident ASCVD events beyond the Pooled Cohort Equations. Our study extends previous findings by demonstrating that adding HRQoL status to PCE model improved ASCVD risk prediction in terms of discrimination and reclassification. Furthermore, we examined the association of HRQoL and ASCVD in a middle-aged racially diverse study sample in general as well as in the low risk subgroup.
The 2018 cholesterol guidelines identify non-traditional risk markers as risk-enhancing factors that should be considered for individual patients, including family history, metabolic syndrome, reproductive variables (in women), chronic kidney disease, inflammatory conditions, lipoprotein(a), high sensitivity C-reactive protein (hsCRP), and ankle-brachial index, among others. [33] Few studies have examined the predictive utility of adding these factors in the context of the PCE. One study showed that adding ABI and hsCRP to the PCE provided NRI of 0.027 (event NRI: 0.012; nonevent NRI: 0.015) and 0.024 (event NRI: 0.028; nonevent NRI: −0.005), respectively, in the Multi Ethnic Study of Atherosclerosis. [34] In the present study, HRQoL had an event NRI of 0.03 in the overall sample and 0.06 in the low risk subgroup. As than 50% of the US population aged 40–79 years has low predicted ASCVD risk, though a relatively large proportion of ASCVD events occur among this low risk population given the large absolute numbers in this segment of the population. [3] The average ASCVD rate among those with low PCS and MCS was 4.7 per 1000 person-years in our low risk study sample, which was significantly higher than the rates of the other low risk individuals. Of note, this low PCS and MCS subgroup accounts for a relatively large proportion of ASCVD cases in the low risk population, indicating the potential importance of assessing HRQoL. Compared to lab tests, using short, inexpensive and psychometrically-validated HRQoL instruments may be of interest in future clinical encounters. Since poor self-perception of health may occur before physiologic illness, HRQoL can incorporate patients’ perspectives into ASCVD prevention efforts and could potentially be applied to refine quantitative risk assessment for primary prevention decisions in the population, in particular in low risk individuals.
Our study had several limitations. First, we excluded participants who did not complete the HRQoL questionnaire or who were lost to follow up prior to their HRQoL administration and collection. Those individuals were more likely to have an adverse CVD risk factor profile including higher levels of blood pressure, lipid and glucose compared to the final study sample, raising the possibility of underestimation of the association between HRQoL and ASCVD risk. However, excluded individuals represented a small proportion of the study sample. Second, residual confounding may be present. We did not have the opportunity to assess other health-related factors, including healthcare access, depression scores, physical activity, diet or other health behaviors that could be potential confounders, in this pooled study sample.
We observed that HRQoL had a significant and independent association with incident ASCVD events, and that adding HRQoL to the PCE significantly, though modestly, improved ASCVD risk prediction among a multi-ethnic population and its large low-risk subgroup. If validated in other settings, self-reported health-related quality of life may be considered a potential risk enhancing factor that could be incorporated into guidelines to refine quantitative risk assessment for primary prevention decisions.
Authors’ contributions
HN and DLJ conceived the study, HN performed the statistical analysis and drafted manuscript. All authors contributed to the writing and /or editing of the manuscript and have read and approved the final of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported in part by R21 HL085375 from the National Heart, Lung, and Blood Institute, and by institutional funds from the Northwestern University Feinberg School of Medicine.
Footnotes
Supplementary data associated with this article can be found, in the online version, at 10.1016/j.ajpc.2021.100222.
Appendix A. Supplementary materials
Reference
- 1.Writing Group M. Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Writing Group M Executive Summary: Heart Disease and Stroke Statistics–2016 Update: a Report From the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019. 2019;74:1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Gibbons R. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014. 2013;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 4.Kavey R.E., Daniels S.R., Lauer R.M., Atkins D.L., Hayman L.L., Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. J Pediatr. 2003;142:368–372. doi: 10.1067/mpd.2003.205. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D.M., Braun L.T., Ndumele C.E., Smith S.C., Jr., Sperling L.S., Virani S.S. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: a Special Report From the American Heart Association and American College of Cardiology. Circulation. 2019;139:e1162–e1e77. doi: 10.1161/CIR.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 6.Whittaker K.S., Krantz D.S., Rutledge T., Johnson B.D., Wawrzyniak A.J., Bittner V. Combining psychosocial data to improve prediction of cardiovascular disease risk factors and events: the National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation study. Psychosom Med. 2012;74:263–270. doi: 10.1097/PSY.0b013e31824a58ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tillmann T., Pikhart H., Peasey A., Kubinova R., Pajak A., Tamosiunas A. Psychosocial and socioeconomic determinants of cardiovascular mortality in Eastern Europe: a multicentre prospective cohort study. Plos Med. 2017;14 doi: 10.1371/journal.pmed.1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polanka B.M., Berntson J., Vrany E.A., Stewart J.C. Are Cardiovascular Risk Factors Stronger Predictors of Incident Cardiovascular Disease in U.S. Adults With Versus Without a History of Clinical Depression? Ann Behav Med. 2018;52:1036–1045. doi: 10.1093/abm/kay007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saintila J., Lozano Lopez T.E., Ruiz Mamani P.G., White M., Huancahuire-Vega S. Health-Related Quality of Life, Blood Pressure, and Biochemical and Anthropometric Profile in Vegetarians and Nonvegetarians. J Nutr Metab. 2020;2020 doi: 10.1155/2020/3629742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song F., Bachmann M.O., Aveyard P., Barton G.R., Brown T.J., Maskrey V. Relapse to smoking and health-related quality of life: secondary analysis of data from a study of smoking relapse prevention. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0205992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware J., Jr., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Farivar S.S., Cunningham W.E., Hays R.D. Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.I. Health Qual Life Outcomes. 2007;5:54. doi: 10.1186/1477-7525-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkinson C., Layte R. Development and testing of the UK SF-12 (short form health survey) J Health Serv Res Policy. 1997;2:14–18. doi: 10.1177/135581969700200105. [DOI] [PubMed] [Google Scholar]
- 14.Jakobsson U. Using the 12-item Short Form health survey (SF-12) to measure quality of life among older people. Aging Clin Exp Res. 2007;19:457–464. doi: 10.1007/BF03324731. [DOI] [PubMed] [Google Scholar]
- 15.Pei M., Aguiar R., Pagels A.A., Heimburger O., Stenvinkel P., Barany P. Health-related quality of life as predictor of mortality in end-stage renal disease patients: an observational study. BMC Nephrol. 2019;20:144. doi: 10.1186/s12882-019-1318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen T.B., Thygesen L.C., Zwisler A.D., Helmark L., Hoogwegt M., Versteeg H. Self-reported health-related quality of life predicts 5-year mortality and hospital readmissions in patients with ischaemic heart disease. Eur J Prev Cardiol. 2015;22:882–889. doi: 10.1177/2047487314535682. [DOI] [PubMed] [Google Scholar]
- 17.Hartog L.C., Landman G.W., Cimzar-Sweelssen M., Knipscheer A., Groenier K.H., Kleefstra N. Health-related quality of life, rehabilitation and mortality in a nursing home population. Neth J Med. 2016;74:247–256. [PubMed] [Google Scholar]
- 18.Pinheiro L.C., Reshetnyak E., Sterling M.R., Richman J.S., Kern L.M., Safford M.M. Using health-related quality of life to predict cardiovascular disease events. Qual Life Res. 2019;28:1465–1475. doi: 10.1007/s11136-019-02103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ul-Haq Z., Mackay D.F., Pell J.P. Association between physical and mental health-related quality of life and adverse outcomes; a retrospective cohort study of 5,272 Scottish adults. BMC Public Health. 2014;14:1197. doi: 10.1186/1471-2458-14-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myint P.K., Surtees P.G., Wainwright N.W., Luben R.N., Welch A.A., Bingham S.A. Physical health-related quality of life predicts stroke in the EPIC-Norfolk. Neurology. 2007;69:2243–2248. doi: 10.1212/01.wnl.0000296010.21252.78. [DOI] [PubMed] [Google Scholar]
- 21.Amiri P., Jalali-Farahani S., Rezaei M., Cheraghi L., Hosseinpanah F., Azizi F. Which obesity phenotypes predict poor health-related quality of life in adult men and women? Tehran Lipid and Glucose Study. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0203028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkins J.T., Karmali K.N., Huffman M.D., Allen N.B., Ning H., Berry J.D. Data Resource Profile: the Cardiovascular Disease Lifetime Risk Pooling Project. Int J Epidemiol. 2015;44:1557–1564. doi: 10.1093/ije/dyv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman G.D., Cutter G.R., Donahue R.P., Hughes G.H., Hulley S.B., Jacobs D.R. Cardia - Study Design, Recruitment, and Some Characteristics of the Examined Subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 24.Feinleib M., Kannel W.B., Garrison R.J., McNamara P.M., Castelli W.P. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 25.Bild D.E., Bluemke D.A., Burke G.L., Detrano R., Diez Roux A.V., Folsom A.R. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 26.Pletcher M.J., Vittinghoff E., Thanataveerat A., Bibbins-Domingo K., Moran A.E. Young Adult Exposure to Cardiovascular Risk Factors and Risk of Events Later in Life: the Framingham Offspring Study. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman G.D., Cutter G.R., Donahue R.P., Hughes G.H., Hulley S.B., Jacobs D.R., Jr. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 28.Pepe M.S., Janes H., Longton G., Leisenring W., Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 29.Ware J.H. Statistics and medicine - The limitations of risk factors as prognostic tools. New Engl J Med. 2006;355:2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
- 30.Uno H., Tian L., Cai T., Kohane I.S., Wei L.J. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pencina M.J., D'Agostino R.B., Sr., Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr K.F., Wang Z., Janes H., McClelland R.L., Psaty B.M., Pepe M.S. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25:114–121. doi: 10.1097/EDE.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grundy S.M., Stone N.J. American Heart Association/American College of Cardiology/Multisociety Guideline on the Management of Blood Cholesterol-Secondary Prevention. JAMA Cardiol. 2019. 2018;4:589–591. doi: 10.1001/jamacardio.2019.0911. [DOI] [PubMed] [Google Scholar]
- 34.Yeboah J., Young R., McClelland R.L., Delaney J.C., Polonsky T.S., Dawood F.Z. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J Am Coll Cardiol. 2016;67:139–147. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.