Highlights
-
•
Endurance long-term high level of training induces significant cardiac remodelling involving all cardiac chambers, also known as ‘athletes-heart”.
-
•
Both left and right ventricular longitudinal strain increases significantly at exercise.
-
•
Cardiac extracellular volume is normal in master athletes and there is no evidence of cardiac fibrosis induced by long term endurance training in master athletes.
-
•
There is no evidence of cardiac damage induced by intensive endurance training in healthy asymptomatic master athletes.
Keywords: Athlete's heart, Cardiac magnetic resonance, Cardiac extracellular volume, Exercise echocardiography
Abstract
Objectives
It is under debate whether the long-term practice of intensive endurance exercise induces chronic cardiac damage such as myocardial fibrosis and ventricle contractile dysfunction. Multimodality analysis was performed to evaluate myocardial damage induced by long term intensive endurance training in master athletes.
Methods
Thirty-three asymptomatic endurance master athletes (47 ± 6 year-old, 9,6 ± 1,7 h training/week for 26 ± 6 years), were compared to 18 sedentary controls (49 ± 7 year-old). They underwent a CMR protocol including 4 chambers morphological and late gadolinium-enhancement (LGE) analysis, left (LV) and right ventricular (RV) T1 mapping and calculation of cardiac extracellular volume (ECV). A maximal exercise echocardiography with left and right ventricular longitudinal global strain (LGS) analysis was performed. Cardiac biomarkers of fibrosis (high sensitive cardiac Troponin T, N-Terminal pro brain natriuretic peptide, N-terminal propeptide of procollagen type I and N-terminal propeptide of procollagen type III) were analysed.
Results
Athletes had larger left and right atrial volume, LV and RV end diastolic volume and increased LV and RV mass compared to controls. LGE was not found in athletes. Native T1 values of LV and RV were not significantly different in athletes compared with controls. ECV was normal in both groups (21,5%± 1,6% [18.3 – 23%] in athletes, 22%± 2,2% [18.5 – 27%] in controls). LV and RV peak exercise LGS values were higher in athletes. Cardiac biomarkers levels were normal.
Conclusion
Despite significant physiological cardiac remodelling, consistent with previous descriptions of athlete's heart, there was no evidence of myocardial fibrosis or exercise left or right ventricular dysfunction or cardiac fibrosis in endurance athletes. Our results are not supporting the hypothesis of deleterious cardiac effects induced by long term and intensive endurance exercise training.
1. Introduction
High-level and long-term exercise training generally induces cardiovascular remodelling characterized by biventricular dilatation with enhanced diastolic function, bi-atrial dilation, and enhanced arterial vasomotor function, a condition called endurance athletes’ heart [1]. These adaptations help to achieve a very high level of cardiorespiratory fitness, a condition generally associated with reduced cardiovascular and all-cause mortality [2].
However, it is under debate whether the cumulative effects of intensive endurance exercise induce chronic cardiac damage [3], [4], [5], especially in master athletes. It has been proposed that long-term endurance exercise may be associated with myocardial fibrosis (MF) detected by late gadolinium-enhancement (LGE) cardiac magnetic resonance (CMR) [6], [7], [8], [9], [10], [11], and/or with right ventricle contractile dysfunction [12], creating a potential substrate for ventricular arrhythmia [13], but these aspects are not constantly found across studies [14], [15], [16], [17], [18].
Extracellular volume (ECV) assessed by CMR [19], [20], [21], circulating biomarkers of MF [22] and longitudinal global 2D strain (LGS) assessed by cardiac echography [23], [24], [25] are relevant tools to explore these potential adverse effects of exercise. Our aim was to assess the presence of diffuse and focal MF detected by CMR (ECV and LGE) and biomarkers, and to evaluate left and right ventricles function with LGS at rest and exercise, in order to investigate if a repeated exposure to intense exercise may induce cardiac damage in competitive master endurance athletes.
2. Methods
2.1. Study population
We compared a population of presumed healthy highly trained master athletes to healthy controls. Athletes were contacted through advertisement at local running, triathlon or cycling club, or at their annual competition pre-participation medical visit. Athletes were included if they were older than 35 years old, practiced endurance sport at a competition level (endurance running, triathlon, cycling), and trained for a minimum of 8 h per week in the previous 15 years. Athletes were asked not to train or compete during the week before their venue for CMR or exercise echography, to avoid any acute effect of exercise on cardiac function. The control group consisted of untrained healthy individuals who had no history of sport activity at any level. These healthy volunteers were prospectively recruited amongst military forces. Exclusion criteria for both groups included any history of chronic disease, illegal medication intake, and standard contraindications for CMR imaging. None of the included subjects had received medication within the preceding 3 weeks. The institutional ethics committee approved this study, and all subjects gave written informed consent.
2.2. Clinical and biological data
Age, type of sport, lifetime training and competition history were recorded. Body weight and height were measured, and a rest ECG was recorded.
Blood samples were obtained immediately before the CMR, to obtain concentrations of haematocrit, high-sensitivity cardiac troponin T (HS cTnT), N-terminal pro–B-type natriuretic peptide (NT-proBNP), and plasma markers of collagen biosynthesis and degradation N-terminal propeptide of procollagen type I (PINP) and N-terminal propeptide of procollagen type III (PIIINP).
2.3. Cardiac magnetic resonance
All patients received clinical CMR scans by dedicated CMR technologists with a 1.5-Tesla MR Scan (Siemens Medical Solutions, Erlangen, Germany) and a 32-channel phased array cardiovascular coil. For LV and RV functional evaluation, the protocol included a breath-hold ECG-gated cine true fast imaging with steady-state precession (FISP) sequence in the short axis plane (TR: 36 ms, TE: 1.3 ms, FOV: 340 × 290 mm, matrix 224 × 154 mm; flip angle: 65°; slice thickness: 7 mm, number of slices: 14).
Left and right ventricular dimensions, myocardial mass (indexed to body surface area), left and right ventricular volume indices, and left and right ejection fraction (EF) were calculated using manual detection of endocardial borders in end-diastole and end-systole with exclusion of trabeculae.
Left and right atrial volume was calculated using a biplane area and length method following the formula: 0.85 × A1 × A2/L, where A1 and A2 were areas measured by planimetry in 2 and 4-chamber views (atrial appendage was excluded) respectively and L was the length of left/right atrium perpendicular to the centre of mitral/tricuspid annulus in the 4-chamber plane. We acquired T1 maps in three slices representing the basal, mid, and apical slices using a shortened modified Look-Locker inversion recovery (ShMOLLI), before and 15 min after a bolus injection of 0.2 mmol/kg gadoterate meglumine (Dotarem™, Guerbet, Villepinte, France).
T1 values were calculated from source images using manual motion correction, with a region of interest for each of the 17-segment model [26]. Native T1 was calculated as the mean of T1 values of the 17 regions of interest obtained before injection. A blood sample was taken immediately prior to CMR scanning to analyse the full blood count. The haematocrit was obtained, allowing for calculation of ECV using the following equation: ECV = (∆1/T1myocardium/∆1/T1blood) (1-haematocrit) [27]. T1 value for ECV calculation was obtained 15 min after injection (∆1/T1= 1/T1 post injection – 1/native T1). T1 mapping was also obtained for right ventricle free wall, and native T1 value for right ventricle was calculated as the mean value obtain in 2 to 4 regions of interest in the basal, medial, and apical regions.
Ten minutes after a bolus injection of 0.2 mmol/kg gadoterate meglumine, end-diastolic LGE images were acquired using phase-sensitive inversion recovery (PSIR) sequences in short-axis orientation covering the entire heart and in 2-, 3-, and 4-chamber views. Distribution and pattern of LGE was visually analysed and reported by using a 17-segment model. Every parameter from the CMR data was assessed by two experienced investigators, independently and blindly.
2.4. Transthoracic echocardiography
A rest transthoracic echocardiography was first performed. Images were acquired with a Vivid S70 (General Electric, Horten, Norway) and analysed offline using EchoPAC (version 112 GE Healthcare). Resting EF was calculated by Simpson's biplane method [26]. Two-dimensional global peak-systolic strain was quantified for the left (LV) and right ventricle (RV). For LV, Longitudinal strain by speckle tracking echocardiography was obtained from three apical views. Segments that failed to track were manually adjusted. Region of interest (ROI) was adjusted to fit the average of the myocardial thickness. LV longitudinal global 2D strain (LGS) was defined as the average of peak longitudinal strains from a 16 LV segments mode. For RV, a region of interest was manually traced along the endocardial border from base to apex at the free RV wall and the interventricular septum at the end of systole, and width was set to match the wall thickness. RV longitudinal strain was measured in the basal, mid-ventricular and apical segments of the septum and the RV free wall. Global RV longitudinal strain was calculated as temporal mean of the 6 RV segments, and RV free wall longitudinal strain was calculated as mean of the 3 RV free wall segments.
The rest echocardiography was immediately followed by an exercise echocardiography on an inclinable cycle ergometer (e-bike EL, General Electric healthcare, USA), with a maximal incremental step exercise test. Depending on the subject's training history, the load was progressively increased by 20 to 40 W/min to bring the participants to the limit of tolerance within 8 to 12 min of exercise (first step from 25 to 100 W). A 12-lead electrocardiogram (ECG) and heart rate were monitored continuously, and blood pressure was automatically measured every minute. Echography acquisition was repeated, and myocardial GLS for LV and RV was analysed at the last step of exercise.
2.5. Statistical analysis
Continuous data are presented as mean±SD, and categorical data are presented as absolute numbers and percentages. Continuous variables were compared with Mann-Whitney nonparametric U test, and categorical variables were compared using chi-square or Fisher exact test as appropriate. Significance for all tests was assumed with P<0.05.
3. Results
3.1. Clinical, biological, and electrocardiographic data
Thirty-three competitive athletes including 2 women (all finishers of endurance / ultra-endurance events: long distance, marathon or ultra-trail running: 13, triathlon: 16, cycling: 4) and 18 controls (1 women) with a similar distribution of age and sex were enrolled between January 2017 and March 2018. All the athletes competed at national or regional level. The level of intensity in training was not monitored. No subject had cardiovascular risk factor or history of cardiovascular and non-cardiovascular disease. No subjects reported doping. Athletes trained 9.6 ± 1,7 h/week (minimum: 8 h, maximum: 12 h) for 26±6 years. We observed no sign of myocardial ischaemia and no significant arrhythmia in both groups during exercise test (Table 1). All biomarkers of fibrosis values were normal.
Table 1.
Clinical, biological, and electrocardiographic characteristics of athletes and controls.
| Athletes | Controls | p | |
|---|---|---|---|
| Clinical data | n = 33 | n = 18 | |
| Age (Years) | 47 ± 6 | 49 ± 1 | 0,27,314 |
| Weight Kg | 72,8 ± 7,8 | 83,1 ± 14,4 | 0,00,724 |
| Height (m) | 176 ± 8 | 176 ± 8 | 0,79,842 |
| BMI (Kg/m²) | 23,4 ± 2,9 | 26,4 ± 3,2 | 0,00,117 |
| Training volume (h/week) | 9,6 ± 1,7 | 0 | – |
| Training history (Years) | 26,3 ± 6,1 | 0 | – |
| Cumulative exposure to training (h) | 13,168 ± 3858 | 0 | – |
| Maximal exercise test | |||
| Peak power output (W) | 270 ± 39 | 198 ± 43 | <0,0001 |
| Double product (mmHg.bpm/min) | 30,158 ± 3953 | 30,328 ± 1935 | 0,89,417 |
| Electrocardiogram parameters | |||
| Rest HR (bpm) | 56 ± 7 | 75 ± 11 | <0,0001 |
| PR interval (ms) | 172 ± 22 | 155 ± 16 | 0,00,754 |
| QRS duration (ms) | 91 ± 15 | 89 ± 8 | 0,46,588 |
| QTc (ms) | 411 ± 24 | 407 ± 26 | 0,53,782 |
| Sokolov index (mV) | 2,1 ± 0,8 | 1,7 ± 0,5 | 0,10,479 |
| complete RBBB (subjects) | 1 | 0 | 1 |
| Incomplete RBBB (subjects) | 11 | 1 | 0,03,613 |
| Early repolarisation (subjects) | 1 | 0 | 1 |
| Ventricular extrasystoly (subjects) | 2 | 1 | 1 |
| Left axis deviation | 0 | 1 | 0,36 |
| Inverted T-waves | 2 | 0 | 0,5298 |
| Circulating cardiac biomarkers | |||
| TnT (pg/l) | 0,01 ± 0 | 0,01 ± 0 | 0,58,206 |
| NT-proBNP (ng/l) | 42,7 ± 33 | 43 ± 41 | 0,98,605 |
| Circulating biomarkers of cardiac fibrosis | |||
| PINP (ng/ml) | 51,29 ± 12,56 | 50,98 ± 17,30 | 0,47,891 |
| PIIINP (ng/ml) | 70,59 ± 13,09 | 60,50 ± 17,52 | 0,10,327 |
Abbreviations: BMI: body mass index; TnT: high-sensitivity T troponin; NT pro BNP: N-terminal pro brain natriuretic peptide; PINP: N-terminal propeptide of procollagen type I; PIIINP: N-terminal propeptide of procollagen type III; HR: heart rate; QTc: corrected QT interval (Bazett formula); RBBB: right bundle branch block.
We found differences in athletes and controls ECGs (Table 1). Rest heart rate was lower, incomplete right bundle branch block was more frequent and PR interval was longer in athletes. Despite this expected significant electrical remodelling, no abnormal ECG pattern was found in athletes, according to recommendation for ECG interpretation in athletes [28].
3.2. Cardiac magnetic resonance
There was an increase in LV and RV volumes of +24.2% and +26.6%, respectively (Fig. 1), left and right atria size (+32.0% and +54.0%, respectively), in the RV and total mass, and in stroke volume in the athletes group when compared with controls (Table 2). Left and right EF were normal in all subjects. Native T1 values for LV and RV and ECV (21,5% ± 1,6% [18.3 – 23%] in athletes, 22,0% ± 2,2% [18.5 – 27%] in controls) were normal in all cases (Fig. 2). There was no T1 elevation at and no LGE at the RV insertion points and the RV free wall. A small LGE area with a non-specific pattern was noted in the inferior wall segment of the LV in one subject of the control group (a normal ECV value of 19.8% was calculated in this subject). The presence of LGE was not observed in the athletes group.
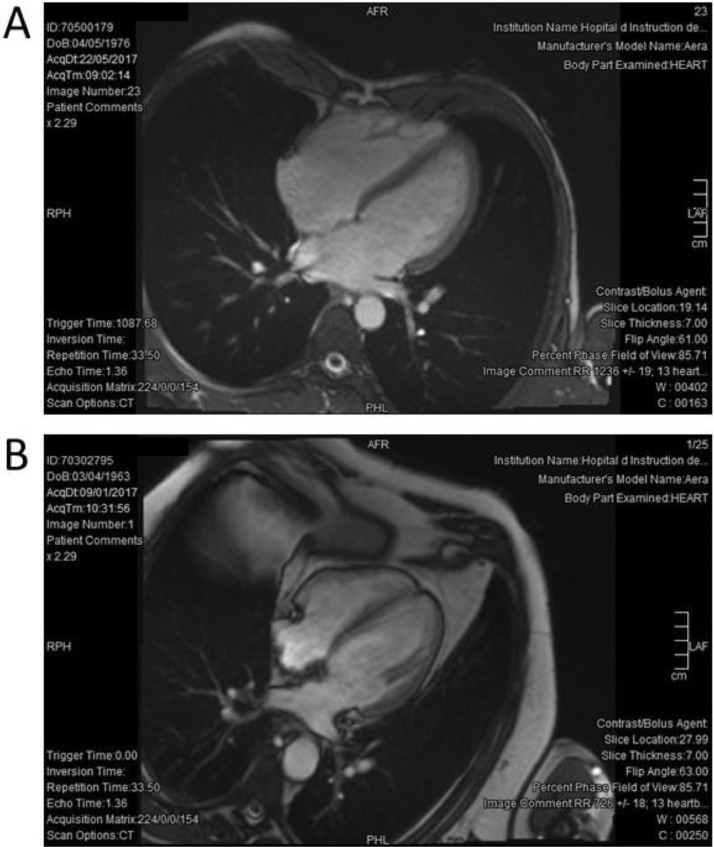
Fig. 1.
Typical four-chamber view in cardiac magnetic resonance in athlete and control. A: harmonious dilatation of the 2 atria and ventricles (LVEDV 140 ml/m²) in a 41 years old triathlete. B: Normal aspect of the same view in a 53 years old control subject (LVEDV 62 ml/m²).
Table 2.
Exercise transthoracic echography (TTE) and cardiac magnetic resonance (CMR) parameters in athletes and controls.
| Athletes | Controls | p | ||
|---|---|---|---|---|
| TTE parameters | n = 33 | n = 18 | ||
| Rest LV EF (%) | 60,8 ± 3,82 | 59,4 ± 4,53 | 0,25,591 | |
| Rest LV LGS (%) | 18,23 ± 2,16 | 16,53 ± 1,32 | 0,00,401 | |
| Peak exercise LV LGS (%) | 23,19 ± 2,86 | 20,82 ± 2,05 | 0,00,335 | |
| Peak exercise LV LGS (%) (variation vs. rest) | 4,99 ± 2,9 | 4,47 ± 2,29 | 0,51,564 | |
| Rest RV LGS (%) | 23,09 ± 2,85 | 22,75 ± 2,87 | 0,69,033 | |
| Peak exercise RV LGS (%) | 29,25 ± 3,47 | 26,48 ± 3,22 | 0,00,777 | |
| Peak exercise RV LGS (%) (variation vs. rest) | 6,13 ± 3,48 | 3,73 ± 3,1 | 0,51,564 | |
| Rest RVFW LGS (%) | 26,23 ± 3,07 | 26,06 ± 3,04 | 0,84,904 | |
| Peak exercise RVFW LGS (%) | 33,75 ± 3,65 | 30,64 ± 3,28 | 0,00,431 | |
| Peak exercise RVFW LGS (%) (variation vs. rest) | 7,59 ± 4,05 | 4,64 ± 2,53 | 0,00,756 | |
| CMR parameters | ||||
| LV EF, (%) | 60,15 ± 3,96 | 60,84 ± 4,87 | 0,59,755 | |
| RV EF (%) | 49,55 ± 3,76 | 49,06 ± 4,52 | 0,69,627 | |
| LV mass (g/m²) | 77,72 ± 11,03 | 72,69 ± 12,25 | 0,15,786 | |
| RV mass (g/m²) | 31,83 ± 5,73 | 25,97 ± 5,47 | 0,00,145 | |
| total mass (g/m²) | 109,61 ± 14,18 | 98,66 ± 16,25 | 0,02,038 | |
| LV EDD (mm/m²) | 27,8 ± 3,1 | 23,6 ± 2,8 | <0,0001 | |
| LV EDV (ml/m²) | 84,0 ± 20,8 | 67,62 ± 12,4 | 0,00,593 | |
| LV ESV (ml/m²) | 33,5 ± 9,6 | 27,5 ± 7,87 | 0,03,484 | |
| RV EDV (ml/m²) | 82,4 ± 17,0 | 65,1 ± 10,2 | <0,0001 | |
| RV ESV(ml/m²) | 41,3 ± 9,5 | 32,9 ± 6,0 | 0,00,228 | |
| LAV (ml/m²) | 37,1 ± 5,0 | 28,2 ± 7,03 | <0,0001 | |
| RAV (ml/m²) | 51,4 ± 11,6 | 33,0 ± 7,5 | <0,0001 | |
| Presence of LGE (subjects) | 0 | 1 | 0,33,333 | |
| LV native T1 (ms) | 938 ± 21 | 940 ± 34 | 0,80,987 | |
| RV native T1 (ms) | 1027 ± 53 | 1026 ± 114 | 0,92,983 | |
| ECV (%) | 21,5 ± 1,6 | 22,0 ± 2,2 | 0,40,868 | |
Abbreviations: LV: left ventricle; EF: ejection fraction; LGS: longitudinal global 2D strain absolute value; RV: right ventricle; FW: free wall; CMR: cardiac magnetic resonance; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; EDD: end-diastolic diameter; EDV: end-diastolic volume; ESV: end-systolic volume; LAV: left atrial volume; RAV: right atrial volume; LGE: late gadolinium enhancement; ECV: extracellular volume.
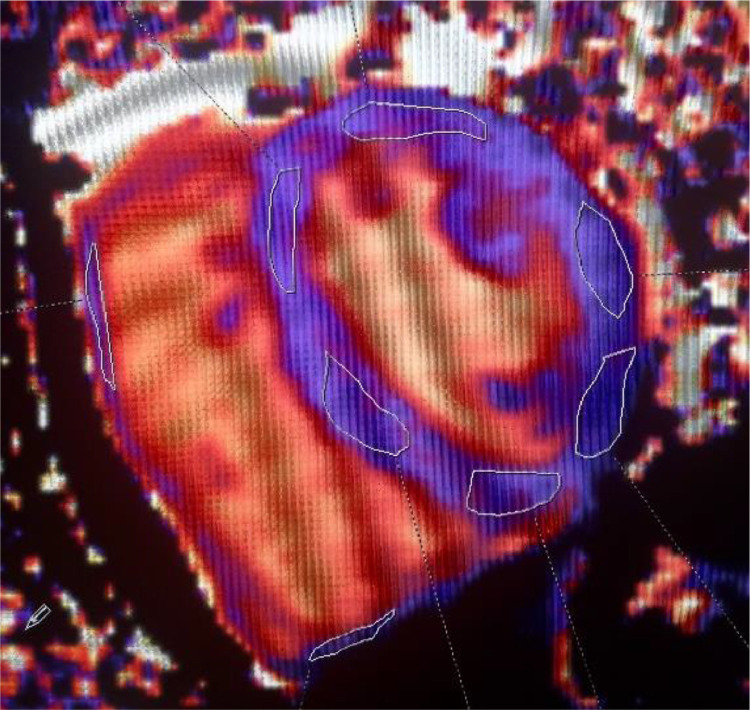
Fig. 2.
Example of normal native T1 mapping in athletes. Left and right ventricles shortened MOLLI T1 map in a 43 years old marathon runner. Mid-ventricular apical short-axis view with 6 regions of interest in the left ventricle myocardium (mean native T1 value 980 ms) and 2 regions of interest in the right ventricle myocardium (mean native T1 value 1019 ms).
3.3. Transthoracic echocardiography
LVEF assessed by TTE was similar in both groups (Table 2), GLS was significantly higher in athletes compared to controls at rest (+10.3%) and at the last step of exercise (+11.4%) for the left ventricle. For the right ventricle, LGS was significantly higher only at peak exercise (+10.5%).
4. Discussion
Our aim was to evaluate cardiac damage in endurance competitive master athletes with multimodality imaging at rest and exercise, analysis of electrical remodelling and biomarkers of fibrosis and cardiac dysfunction. First, we found that master athletes showed signs of physiological cardiac remodelling, consistent with previous descriptions of athletes’ heart. Second, despite this important remodelling, there was no evidence of myocardial fibrosis or exercise left or right ventricular dysfunction in athletes. Our results are not supporting the hypothesis of cardiac damage induced by long term and intensive endurance exercise training.
4.1. Athlete's heart in master athletes
Athlete's heart is well described in young subject (typically 20–25 yrs) [1], but less data are available for senior or master athletes (i.e. typically > 35 yrs). We found a harmonious dilatation of the 2 ventricles, and a moderate increase in total ventricular mass, in accordance with previous description of athlete's heart syndrome in master athletes [17]. Values for these parameters did not exceed the pathological thresholds hypertrophic cardiomyopathy [29]. Pronounced LV and RV enlargement (up to 140 ml/m² for LV and RV end diastolic volume) was shown in particularly highly trained triathletes. An important atrial dilatation was observed in senior athletes (32% and 54% larger for left and right atria respectively, compared to controls). This was quantitatively consistent with published data for the left atrium [30]. For the right atrium volume, echocardiographic quantitative assessment is difficult because of the complex anatomy of the right chambers [30], and only sparse data have been published using CMR. In this study, we found a larger enlargement compared to others [31]. Thus, right atrium pronounced enlargement may be a specific sign of athlete's heart in senior/master athletes. Atrial dilatation observed in athletes may represent an adaptive mechanism to the increased volume overload induced by training, but provide a substrate potentially explaining the higher prevalence of atrial arrhythmia in older athletes [32]. Atrial function was not studied here, but it may have helped distinguish adaptive from pathologic remodelling [30].
4.2. Myocardial fibrosis and long-term endurance exercise
It is still unclear and a matter of controversy if repeated endurance exercise induces MF. In endurance athletes, focal MF has been reported in several cohort studies, based on LGE analysis, with a prevalence ranging from 2.2% to 50% [11]. This high variability is probably explained by the small sample sizes. Various LGE pattern has been found in these studies and may be the consequence of other factors than repeated exercise, e.g. coronary artery disease or silent myocarditis [10], and recent data emphasize the heterogeneous phenotype of MF in athletes [33]. In parallel, LGE has also been found in up to 40% of control subjects in these studies [11], concerning not only the LV, but also the RV insertion points [17]. The later point is important because it has been proposed that the RV overload during exercise may result in structural myocardial changes in the septal points of insertion of the RV wall. Several other CMR studies in endurance athletes did not identify focal MF [[14], [15], [16], [17],[34], [35], [36], [37], [38]]. Our results are consistent with these observations.
Also, to our knowledge, there is no evidence of diffuse MF in endurance athletes, evaluated by ECV. ECV is considered a useful tools for differentiating between athlete's heart and patients with early cardiomyopathy [15]. Studies using quantitative ECV analysis have reported identical ECV [15,17,37] or even lower values in younger athletes [16] than in controls. Lower ECV values could suggest that collagen content decreases with chronic exercise, as shown in trained healthy rats [39], but this may only apply to young but not senior athletes. Interestingly, long-term excessive exercise training has been shown to induce MF in sedentary hypertensive rats [40], and MF seems to be associated with exercise hypertension in triathletes [10]. The absence of MF in our study may be linked to the fact that none of the subjects involved in our study had history of hypertension.
Global 2D strain might provide useful information on myocardial fibrosis because it generally worsens with the presence myocardial fibrosis [24,41]. We can reasonably consider that the absence of alteration of strain in our study, both at rest and during exercise, is a reassuring finding. This finding is consistent with the absence of myocardial fibrosis detected with CMR. In the literature, strain values observed in healthy athletes are often normal [33], but increased longitudinal contractility for left ventricle at rest was also described in well-trained amateur Marathon runners [42], in agreement with our results.
Overall, results of the present study are not indicating that long-term intense endurance is associated with MF in healthy asymptomatic athletes. Normal values of biological markers of MF, despite limited number of available data, reinforce this finding. It seems reasonable to propose that long-term exercise alone may not induce MF in healthy athletes, but that a precipitating factor such as hypertension [10], or other factors remaining to identify, may increase the risk of presenting fibrosis in athletes. Our results are in agreement with the idea that the overall risks of very intense exercise are low [43], and that cardiorespiratory fitness is associated with improvements in cardiovascular health and longevity [44]. Thus, healthy athletes should not be discouraged from very intense and repetitive exercise training.
4.3. Right ventricle dysfunction and long-term endurance exercise
It is also debated if intense endurance exercise could be responsible for exercise RV dysfunction. This arises from the observation of ventricular arrhythmia in high-level athletes presenting RV dilatation and exercise systolic dysfunction [13], with the hypothesis that repeated volume overload cause excessive RV solicitation [12]. We did not observe any systolic RV alteration in athletes, and LGS was even improved during exercise for both ventricles. In parallel, we report normal native T1 values for RV in senior athletes, whereas T1 values generally increase in the myocardium in pressure overload RV diseases [45]. Thus, our data did not support the hypothesis of exercise-induced RV cardiomyopathy in healthy master athletes.
4.4. Limitations
Thirty-three athletes were enrolled, which may be insufficient to detect MF. However, the majority of studies reporting MF in athletes included less subjects [11]. Larger studies are nevertheless required to confirm our findings. In particular, sex differences could not be analysed because of the low number of female athletes in our study. Histological confirmation of the absence of elevated levels of fibrosis with endomyocardial biopsy was not possible for ethical reasons. Our cross-sectional study targeted healthy master athletes. Further research such as longitudinal follow-up with serial CMRs, echocardiograms and cardiovascular outcomes should be carried out in healthy master athletes or more liberalized subject population.
5. Conclusion
Significant physiological cardiac remodelling was found in healthy asymptomatic master athletes as shown in previous descriptions of athlete's heart in young athletes. Despite this major remodelling, there was no evidence of myocardial fibrosis or exercise ventricular dysfunction. Our results are not supporting the hypothesis of deleterious cardiac effects induced by long term and intensive endurance exercise training.
Declaration of Competing Interest
All authors have no relevant conflict of interest.
Acknowledgments
Funding sources
None.
Acknowledgements
None.
CRediT authorship contribution statement
Olivier Missenard: Conceptualization, Methodology, Project administration, Validation, Visualization, Writing - review & editing.
Charline Gabaudan: Data curation, Formal analysis.
Helene Astier: Data curation Formal analysis.
Florian Desmots: data curation, Formal analysis.
Eric Garnotel: Conceptualization, Data curation, Formal analysis.
Pierre-Laurent Massoure: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing - review & editing.
References
- 1.Fagard R. Athlete's heart. Heart. 2003;89:1455–1461. doi: 10.1136/heart.89.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R., Blair S.N., Arena R. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe J.H., Patil H.R., Lavie C.J. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin Proc. 2012;87:587–595. doi: 10.1016/j.mayocp.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S., Merghani A., Mont L. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J. 2015;36:1445–1453. doi: 10.1093/eurheartj/ehv090. [DOI] [PubMed] [Google Scholar]
- 5.Eijsvogels T.M.H., Fernandez A.B., Thompson P.D. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol Rev. 2016;96:99–125. doi: 10.1152/physrev.00029.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson M., O'Hanlon R., Prasad S. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol. 2011;110:1622–1626. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breuckmann F., Möhlenkamp S., Nassenstein K. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50–57. doi: 10.1148/radiol.2511081118. [DOI] [PubMed] [Google Scholar]
- 8.Karlstedt E., Chelvanathan A., Da Silva M. The impact of repeated marathon running on cardiovascular function in the aging population. J Cardiovasc Magn Reson. 2012;14:58. doi: 10.1186/1532-429X-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Gerche A., Burns A.T., Mooney D.J. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 10.Tahir E., Starekova J., Muellerleile K. Myocardial fibrosis in competitive triathletes detected by contrast-enhanced CMR correlates with exercise-induced hypertension and competition history. JACC Cardiovasc Imaging. 2018;11:1260–1270. doi: 10.1016/j.jcmg.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 11.van de Schoor F.R., Aengevaeren V.L., Hopman M.T.E. Myocardial Fibrosis in Athletes. Mayo Clin Proc. 2016;91:1617–1631. doi: 10.1016/j.mayocp.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 12.La Gerche A., Heidbüchel H., Burns A.T. Disproportionate exercise load and remodeling of the athlete's right ventricle. Med Sci Sports Exerc. 2011;43:974–981. doi: 10.1249/MSS.0b013e31820607a3. [DOI] [PubMed] [Google Scholar]
- 13.La Gerche A., Claessen G., Dymarkowski S. Exercise-induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J. 2015;36:1998–2010. doi: 10.1093/eurheartj/ehv202. [DOI] [PubMed] [Google Scholar]
- 14.O'Hanlon R., Wilson M., Wage R. Troponin release following endurance exercise: is inflammation the cause? A cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2010;12:38. doi: 10.1186/1532-429X-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mordi I., Carrick D., Bezerra H. T1 and T2 mapping for early diagnosis of dilated non-ischaemic cardiomyopathy in middle-aged patients and differentiation from normal physiological adaptation. Eur Heart J Cardiovasc Imaging. 2016;17:797–803. doi: 10.1093/ehjci/jev216. [DOI] [PubMed] [Google Scholar]
- 16.McDiarmid A.K., Swoboda P.P., Erhayiem B. Athletic cardiac adaptation in males is a consequence of elevated myocyte mass. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pujadas S., Doñate M., Li C.-H. Myocardial remodelling and tissue characterisation by cardiovascular magnetic resonance (CMR) in endurance athletes. BMJ Open Sport Exerc Med. 2018;4 doi: 10.1136/bmjsem-2018-000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Małek Ł.A., Barczuk-Falęcka M., Werys K. Cardiovascular magnetic resonance with parametric mapping in long-term ultra-marathon runners. Eur J Radiol. 2019;117:89–94. doi: 10.1016/j.ejrad.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Haaf P., Garg P., Messroghli D.R. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 30 November 2016;18 doi: 10.1186/s12968-016-0308-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterhouse D.F., Ismail T.F., Prasad S.K. Imaging focal and interstitial fibrosis with cardiovascular magnetic resonance in athletes with left ventricular hypertrophy: implications for sporting participation. Br J Sports Med. 2012;46(Suppl 1):i69–i77. doi: 10.1136/bjsports-2012-091482. [DOI] [PubMed] [Google Scholar]
- 21.Sibley C.T., Noureldin R.A., Gai N. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265:724–732. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López B., González A., Ravassa S. Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. J Am Coll Cardiol. 2015;65:2449–2456. doi: 10.1016/j.jacc.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Di Franco A., Kim J., Rodriguez-Diego S. Multiplanar strain quantification for assessment of right ventricular dysfunction and non-ischemic fibrosis among patients with ischemic mitral regurgitation. PLoS ONE. 29 September 2017;12 doi: 10.1371/journal.pone.0185657. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haland T.F., Almaas V.M., Hasselberg N.E. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2016;17:613–621. doi: 10.1093/ehjci/jew005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Gerche A., Jurcut R., Voigt J.-U. Right ventricular function by strain echocardiography. Curr. Opin. Cardiol. 2010;25:430. doi: 10.1097/HCO.0b013e32833b5f94. [DOI] [PubMed] [Google Scholar]
- 26.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. e14. [DOI] [PubMed] [Google Scholar]
- 27.Moon J.C., Messroghli D.R., Kellman P. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S., Drezner J.A., Baggish A. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol. 2017;69:1057–1075. doi: 10.1016/j.jacc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Kawel-Boehm N., Maceira A., Valsangiacomo-Buechel E.R. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Ascenzi F., Anselmi F., Focardi M. Atrial Enlargement in the Athlete's Heart: assessment of atrial function may help distinguish adaptive from pathologic remodeling. J Am Soc Echocardiogr. 2018;31:148–157. doi: 10.1016/j.echo.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Mosén H., Steding-Ehrenborg K. Atrial remodelling is less pronounced in female endurance-trained athletes compared with that in male athletes. Scand Cardiovasc J. 2014;48:20–26. doi: 10.3109/14017431.2013.860234. [DOI] [PubMed] [Google Scholar]
- 32.Abdulla J., Nielsen J.R. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–1159. doi: 10.1093/europace/eup197. [DOI] [PubMed] [Google Scholar]
- 33.Eijsvogels T.M.H., Oxborough D.L., O'Hanlon R. Global and regional cardiac function in lifelong endurance athletes with and without myocardial fibrosis. Eur J Sport Sci. 2017;17:1297–1303. doi: 10.1080/17461391.2017.1373864. [DOI] [PubMed] [Google Scholar]
- 34.Mousavi N., Czarnecki A., Kumar K. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol. 2009;103:1467–1472. doi: 10.1016/j.amjcard.2009.01.294. [DOI] [PubMed] [Google Scholar]
- 35.Hanssen H., Keithahn A., Hertel G. Magnetic resonance imaging of myocardial injury and ventricular torsion after marathon running. Clin Sci. 2011;120:143–152. doi: 10.1042/CS20100206. [DOI] [PubMed] [Google Scholar]
- 36.Heidbüchel H., Hoogsteen J., Fagard R. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Role of an electrophysiologic study in risk stratification. Eur Heart J. 2003;24:1473–1480. doi: 10.1016/s0195-668x(03)00282-3. [DOI] [PubMed] [Google Scholar]
- 37.Małek Ł.A., Barczuk-Falęcka M., Werys K. Cardiovascular magnetic resonance with parametric mapping in long-term ultra-marathon runners. Eur J Radiol. 2019;117:89–94. doi: 10.1016/j.ejrad.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Abdullah S.M., Barkley K.W., Bhella P.S. Lifelong physical activity regardless of dose is not associated with myocardial fibrosis. Circ Cardiovasc Imaging. November 2016;9 doi: 10.1161/CIRCIMAGING.116.005511. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verboven M., Cuypers A., Deluyker D. High intensity training improves cardiac function in healthy rats. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-42023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz Rebecca L., Swallow John G., Waters Robert P. Effects of excessive long-term exercise on cardiac function and myocyte remodeling in hypertensive heart failure rats. Hypertension. 2007;50:410–416. doi: 10.1161/HYPERTENSIONAHA.106.086371. [DOI] [PubMed] [Google Scholar]
- 41.Saito M., Okayama H., Yoshii T. Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2012;13:617–623. doi: 10.1093/ejechocard/jer318. [DOI] [PubMed] [Google Scholar]
- 42.Schattke S., Xing Y., Lock J. Increased longitudinal contractility and diastolic function at rest in well-trained amateur Marathon runners: a speckle tracking echocardiography study. Cardiovasc Ultrasound. 2014;12:11. doi: 10.1186/1476-7120-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zubin Maslov P., Schulman A., Lavie C.J. Personalized exercise dose prescription. Eur Heart J. 2018;39:2346–2355. doi: 10.1093/eurheartj/ehx686. [DOI] [PubMed] [Google Scholar]
- 44.Lavie C.J., Ozemek C., Carbone S. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124:799–815. doi: 10.1161/CIRCRESAHA.118.312669. [DOI] [PubMed] [Google Scholar]
- 45.Wang J., Zhao H., Wang Y. Native T1 and T2 mapping by cardiovascular magnetic resonance imaging in pressure overloaded left and right heart diseases. J Thorac Dis. 2018;10:2968–2975. doi: 10.21037/jtd.2018.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]