Abstract
Objective
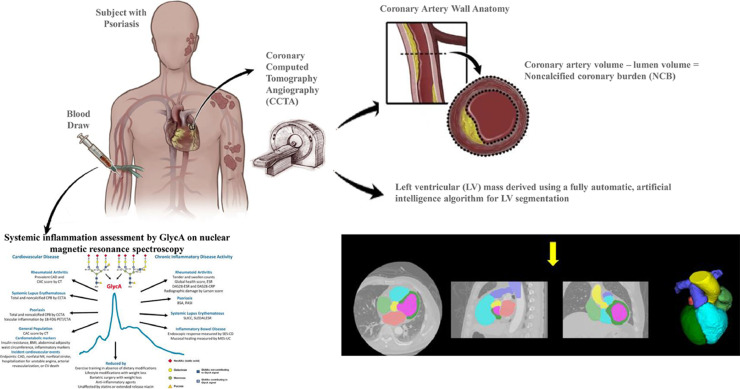
Increased left ventricular (LV) mass is an important precursor to heart failure. Inflammation plays an important role in increasing LV mass. However, the contribution of subclinical coronary artery disease (CAD) to the inflammation-LV mass relationship is unknown. In subjects with psoriasis, a chronic inflammatory skin disease, we evaluated if systemic inflammation assessed by plasma glycoprotein A (GlycA) associated with LV mass measured on coronary CT angiography (CCTA). Additionally, we analyzed whether this relationship was mediated by early CAD assessed as noncalcified coronary burden (NCB).
Methods
We performed an observational longitudinal study of 213 subjects with psoriasis free of known cardiovascular disease, 189 of whom were followed over one year. All participants had GlycA measurements by nuclear magnetic resonance spectroscopy and LV mass and NCB quantified by CCTA.
Results
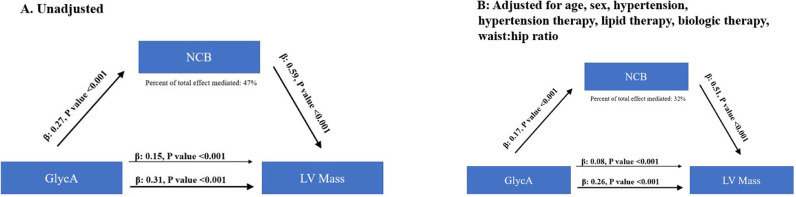
The cohort had a mean age of 50.3 (±12.9) years and 59% were male. There was moderate psoriasis severity and low cardiovascular risk. LV mass increased by GlycA tertiles [1st tertile:24.6 g/m2.7(3.8), 2nd tertile:25.5 g/m2.7(3.8), 3rd tertile:27.7 g/m2.7(5.5), p<0.001]. Both GlycA (β=0.24, p = 0.001) and NCB (β=0.50, p<0.001) associated with LV mass in models adjusted for age, sex, hypertension, hypertension therapy, lipid therapy, biologic therapy for psoriasis, waist:hip ratio, psoriasis disease duration and severity. In multivariable-adjusted mediation analyses, NCB accounted for 32% of the GlycA-LV mass relationship. Finally, over one year, change in NCB independently associated with change in LV mass (β=0.25, p = 0.002).
Conclusions
Both systemic inflammation and coronary artery NCB were associated with LV mass beyond cardiovascular risk factors in psoriasis. Furthermore, a substantial proportion of the inflammatory-LV mass relationship was mediated by NCB. These findings underscore the possible contribution of early coronary artery disease to the relationship between systemic inflammation and LV mass.
Keyterms: Inflammation, Left ventricular mass, Noncalcified coronary burden, Cardiac computed tomography angiography
Abbreviations: LV, left ventricular; CCTA, cardiac computed tomography angiography; NCB, noncalcified coronary burden; GlycA, glycoprotein A; hs-CRP, high sensitivity-C reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low- density lipoprotein cholesterol
Central Illustration: Subjects with psoriasis were prospectively enrolled to undergo plasma glycoprotein A (GlycA) measurement, coronary computed tomography angiography for assessment of coronary artery noncalcified coronary burden and left ventricular mass.
Alt-text: Unlabelled box
1. Introduction
Chronic systemic inflammation plays an important role in the pathogenesis of increased left ventricular (LV) mass and heart failure.[1,2] Psoriasis is an inflammatory skin disease associated with systemic inflammation, premature atherosclerosis, and heart failure.[3,4] Thus, psoriasis serves as a useful human disease model to investigate the relationship between chronic inflammation and LV mass, and determine whether early coronary artery disease (CAD) in the form of noncalcified coronary burden (NCB) may partly account for the inflammation-LV mass relationship.
Early human studies detected elevated plasma inflammatory cytokines in heart failure [5] while preclinical studies characterized the role of the innate immune system in the development of heart failure.[6] GlycA, a nuclear magnetic resonance (NMR) spectroscopy-derived composite biomarker of chronic inflammation, has been shown to associate with subclinical CAD assessed as NCB by coronary CT angiography (CCTA),[7] and also recently shown to promote adverse LV remodeling and heart failure.[2] Furthermore, GlycA has been shown to predict adverse cardiovascular events[8] independent of high sensitivity C-reactive protein (hs-CRP).[9] In states of chronic inflammation including psoriasis, GlycA captures cardiometabolic risk at least as much as hs-CRP,[7] making it an attractive blood systemic inflammatory biomarker for cardiovascular risk assessment.
Inflammation drives myocardial abnormalities via endothelial inflammation, which induces myocardial hypertrophy, cardiomyocyte stiffening and increased interstitial fibrosis.[10] However, few studies have directly investigated whether the association between inflammation and early myocardial abnormalities is mediated by the presence of subclinical CAD. Thus, in an ongoing cohort study to assess the association between varying degrees of psoriasis severity over time and the development of cardiometabolic diseases in subjects free of clinical cardiovascular disease (CVD), we hypothesized that circulating GlycA would associate with LV mass assessed using a fully automated, artificial intelligence (AI) software for LV segmentation on cardiac computed tomography angiography (CCTA). Furthermore, we hypothesized that NCB would partly mediate the inflammation-LV mass relationship beyond cardiovascular risk factors and that a change in NCB over one-year follow-up would associate with a favorable change in LV mass.
2. Methods
2.1. Study design and participants
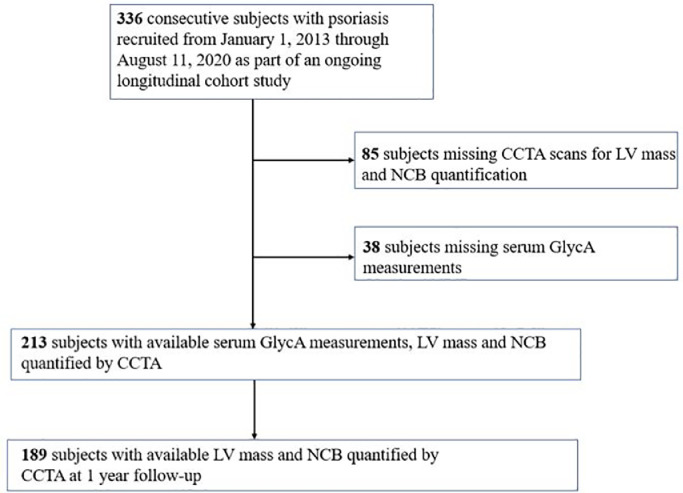
The study consisted of 336 consecutive participants recruited from January 1, 2013 to August 11, 2020 as part of the Psoriasis, Atherosclerosis, and Cardiometabolic Disease Initiative (PACI), an ongoing cohort study of participants with psoriasis. The general objective of this cohort study is to assess the association between varying degrees of psoriasis severity over time and the development of cardiometabolic diseases. A priori, as part of the CCTA analyses, our protocol stated that we would analyze the impact of inflammation on LV characteristics over time. At the time of analyses, 213 had CCTA results for LV mass, NCB, and GlycA measurements with 189 participants having one-year follow data available of these parameters (Fig. 1). PACI consists of asymptomatic, community-dwelling adults with psoriasis and without active cardiac disease at time of recruitment. Participants were excluded if they had an estimated glomerular filtration rate <30 mL/min/1.73m2, any comorbid condition known to promote CVD or systemic inflammation, such as uncontrolled hypertension, internal malignancy within 5 years, human immunodeficiency virus, active infection within the past 72 h of baseline, major surgery within the past 3 months, pregnancy or lactation. Participants underwent same day clinical assessment, blood draw, and CCTA imaging. STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines were followed.[11] The study protocol was approved by the Institutional Review Board at the National Institutes of Health (NIH). Research was conducted in accordance with the Declaration of Helsinki. Written informed consent was provided by all participants enrolled in the study.
Fig. 1.
CONSORT Diagram.
2.2. Clinical and laboratory assessments
Participant demographics, clinical history, physical examination, and anthropometric measurements were obtained at the time of recruitment. Participant-reported medication use was defined by use of any of the following in the three months prior to their baseline visit: systemic therapy (steroids or methotrexate), biologic therapy (adalimumab, etanercept, ustekinumab, secukinumab and ixekizumab), light therapy (psoralen plus ultraviolet or ultraviolet B), topical treatments, diabetes medications or lipid therapy. Overnight fasting blood sample were collected and analyzed for basic chemistry, fasting glucose and lipid panel, insulin levels and hs-CRP at the NIH Clinical Center clinical laboratory. GlycA was measured by NMR spectroscopy (LabCorp) as previously described.[12]
2.3. Cardiac computed tomography angiography acquisition
CCTA was performed following guidelines implemented by the National Institutes of Health Radiation Exposure Committee. All CCTA scans were acquired on a 320-detector row CT scanner (Aquilion One Genesis, Canon Medical Systems, Japan) using prospective EKG gating with similar imaging settings of 100‒120 kV tube voltage, 100‒850 mA tube current adjusted to the subject's attenuation profile, and a gantry rotation time of 275 msec. 50‒70 mL of contrast material was administered at a flow rate of 5.0‒5.5 mL/sec and adjusted for patient weight. Images were reconstructed with a square matrix size of 512, a pixel size of 0.29‒0.43 mm2, a slice thickness of 0.5 mm, and a slice spacing of 0.25 mm.[4]
2.4. Left ventricular mass characterization on cardiac computed tomography angiography
Quantitative LV mass was measured using a fully automated AI software based on a combined multi-atlas and corrective segmentation method for multi-structure CCTA image segmentation.[13,14] This automated image processing framework first uses a multi-atlas segmentation approach which includes atlas selection, atlas registration, and label fusion to label various cardiovascular structures. It then performs several computer vision algorithms to refine different cardiac labels and obtain the final segmentation. The LV mass was calculated in grams from the LV myocardium size (in mm3) multiplied by a myocardial tissue density of 1.05 g/ml. Comparison between automated segmentation and manual segmentation by expert cardiologists based on 21 randomly selected studies showed excellent correlation with an average intraclass correlation coefficient (ICC) of 0.99 and r of 0.99. LV mass was normalized to height to an allometric power of 2.7 given prior studies which show this indexing method more accurately identifies left ventricular hypertrophy in overweight and obese individuals.[15]
2.5. Coronary artery characterization on cardiac computed tomography angiography
An expert cardiologist read the scans to determine presence or absence of coronary plaque via visual assessment. Readers blinded to subject demographics and scan time evaluated coronary artery characteristics of each main coronary artery >2 mm in diameter in core lab fashion. Semi-automated contouring with manual adjustment of the inner lumen and outer wall was performed using dedicated software (QAngio CT; Medis, The Netherlands).[4] Transverse reconstructed cross-sections of the artery was reviewed at 0.5 mm increments. The adaptive threshold which considers varying Hounsfield unit intensities throughout the entire vessel was used due to increased independence of lumen contrast intensity when compared to fixed threshold methods.[16] Segmental coronary artery volume (mm3) was divided by segment length (mm) and attenuated for luminal intensity to derive noncalcified coronary burden and dense calcified coronary burden. All noncalcified coronary burden, dense calcified coronary burden and total coronary burden values indicate global values averaged from the left anterior descending artery, left circumflex artery and right coronary artery. Coronary artery burden evaluation was performed in 98% of all available coronary segments. The inter- and intrareader variations were <10%.
2.6. Statistical analysis
Continuous variables were reported as mean ± standard deviation or median [interquartile range]. Comparison of baseline characteristics between tertiles of GlycA were performed by analysis of variance or Kruskal-Wallis as appropriate. Linear regression models were used to assess univariate and multivariable-adjusted associations between GlycA and LV mass/height2.7, NCB and LV mass/height2.7, and change in NCB and LV mass/height2.7 at 1 year follow-up. Unadjusted and adjusted mediation analyses was used to assess the contribution of NCB in the relationship between GlycA and LV mass/height2.7 as previously published.[17] Covariates included age, sex, hypertension, hypertension therapy, lipid therapy, biologic therapy for psoriasis, waist:hip ratio, psoriasis disease duration and severity. Two-sided P-values <0.05 were considered statistically significant. Stata version 15 (StataCorp, College Station, TX, USA) was used for all statistical analyses.
3. Results
3.1. Characteristics of the overall psoriasis study group and stratified by GlycA
Of 336 consecutive participants recruited, 123 did not have available LV mass, NCB characterization, or circulating GlycA measurements. The clinical characteristics of 213 participants included in the present study are summarized in Table 1. The mean age of the study cohort was 50.3 (±12.9) years; 59% were male with a median Framingham cardiovascular risk score (FRS) of 2.0 [0.5–5.4] and median Atherosclerotic Cardiovascular Disease (ASCVD) 10 year risk score of 3.2 [1.6–5.7] consistent with low cardiovascular risk. When the cohort was divided into tertiles of GlycA, there was an increase in psoriasis area severity index (PASI) score, decrease in HDL-C cholesterol, and increase in hs-CRP (Table 2). Although prevalent coronary plaque did not differ between the groups, NCB increased by GlycA tertile [1st tertile:1.00 (0.32) mm2×100, 2nd tertile:1.22 (0.53) mm2×100, 3rd tertile:1.29 (0.53) mm2×100, p<0.001]. LV mass also increased by tertiles of GlycA [1st tertile:24.6 g/m2.7 (3.8), 2nd tertile:25.5 g/m2.7 (3.8), 3rd tertile:27.7 g/m2.7 (5.5), p<0.001].
Table 1.
Characteristics of the cohort.
| N = 213 | |
|---|---|
| Clinical characteristics | |
| Age | 50.3 (12.9) |
| Male, n (%) | 125 (59) |
| Hypertension, n (%) | 59 (28) |
| Dyslipidemia, n (%) | 84 (39) |
| Diabetes mellitus, n (%) | 17 (8) |
| Current smoker, n (%) | 23 (11) |
| Framingham risk score | 2 [0.5–5.4] |
| ASCVD 10 year risk score | 3.2 [1.6–5.7] |
| Waist: hip ratio | 0.95 (0.08) |
| Psoriasis area severity index score | 7.7 (7.5) |
| Medications | |
| Cardiovascular | |
| Anti- hypertensives | 48 (23) |
| Lipid- lowering medications | 62 (29) |
| Diabetes medications | 14 (7) |
| Psoriasis | |
| Biologic therapy | 72 (34) |
| Topical therapy | 128 (60) |
| Light therapy | 22 (10) |
| Blood markers | |
| Total cholesterol, mg/dL | 185.6 (40.5) |
| HDL-C cholesterol, mg/dL | 57.4 (19.5) |
| LDL-C cholesterol, mg/dL | 104.8 (32.5) |
| Triglycerides, mg/dL | 118.2 (66.9) |
| Hs-CRP, mg/ L | 3.9 (6.8) |
| GlycA, µmol/L | 406.4 (69.5) |
| Coronary artery characterization on CCTA | |
| Plaque present, n (%) | 65 (31) |
| Total coronary burden (mm2×100) | 1.22 (0.48) |
| Noncalcified coronary burden (mm2×100) | 1.16 (0.47) |
| Dense calcified coronary burden (mm2×100) | 0.05 (0.08) |
| LV characteristics on CCTA | |
| LV mass, g | 111.4 (25.2) |
| LV mass/height2.7, g/m2.7 | 25.9 (4.6) |
Values are mean (±SD), median [Q1, Q3], or number (%).
ASCVD indicates Atherosclerotic Cardiovascular Disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low- density lipoprotein cholesterol; hs-CRP, high sensitivity-C reactive protein; LV, left ventricular.
Table 2.
Characteristics of the cohort stratified by tertiles of GlycA.
| GlycA 1st tertile (n = 73) | GlycA 2nd tertile (n = 73) | GlycA 3rd tertile (n = 73) | P value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age | 49.7 (12.6) | 49.1 (13.2) | 52.1 (12.8) | 0.36 |
| Male, n (%) | 42 (58) | 46 (63) | 39 (62) | 0.50 |
| Hypertension, n (%) | 15 (21) | 21 (29) | 23 (32) | 0.24 |
| Dyslipidemia, n (%) | 31 (42) | 27 (63) | 26 (36) | 0.80 |
| Diabetes mellitus, n (%) | 7 (10) | 4 (5) | 6 (8) | 0.70 |
| Current smoker, n (%) | 6 (8) | 10 (14) | 7 (10) | 0.55 |
| Framingham risk score | 1.5 [0.4–4.4] | 3.3 [0.5–7.3] | 2.0 [1.0–5.5] | 0.25 |
| ASCVD 10 year risk score | 2.3 [1.2–4.5] | 4.6 [2.6–6.8] | 3 [1.4–5.7] | 0.012 |
| Waist: hip ratio | 0.94 (0.07) | 0.95 (0.08) | 0.96 (0.09) | 0.37 |
| Psoriasis area severity index score | 5.9 (6.0) | 6.6 (5.4) | 10.7 (9.5) | <0.001 |
| Medications | ||||
| Cardiovascular | ||||
| Anti- hypertensives | 9 (12) | 17 (23) | 22 (30) | 0.02 |
| Lipid- lowering medications | 20 (27) | 21 (29) | 21 (29) | 0.95 |
| Diabetes medications | 6 (8) | 3 (4) | 5 (7) | 0.66 |
| Psoriasis | ||||
| Biologic therapy | 29 (40) | 23 (32) | 20 (27) | 0.45 |
| Topical therapy | 40 (55) | 45 (62) | 43 (59) | 0.55 |
| Light therapy | 7 (10) | 5 (7) | 10 (14) | 0.36 |
| Blood markers | ||||
| Total cholesterol, mg/dL | 186.9 (42.6) | 189.3 (40.2) | 181.0 (35.6) | 0.46 |
| HDL-C cholesterol, mg/dL | 62.2 (23.2) | 56.1 (18.4) | 53.4 (15.0) | 0.02 |
| LDL-C cholesterol, mg/dL | 100.9 (33.7) | 108.8 (32.5) | 105.1 (31.0) | 0.35 |
| Triglycerides, mg/dL | 114.5 (75.7) | 121.7 (61.7) | 120.3 (63.8) | 0.79 |
| Hs-CRP, mg/ L | 1.4 (0.3) | 2.5 (4.1) | 7.9 (9.9) | <0.001 |
| GlycA, µmol/L | 338.3 (26.9) | 398.8 (17.4) | 484.6 (51.4) | <0.001 |
| Coronary artery characterization on CCTA | ||||
| Plaque present, n (%) | 22 (30) | 22 (30) | 21 (29) | 0.91 |
| Total coronary burden (mm2×100) | 1.05 (0.32) | 1.28 (0.55) | 1.34 (0.53) | <0.001 |
| Noncalcified coronary burden (mm2×100) | 1.00 (0.32) | 1.22 (0.53) | 1.29 (0.53) | <0.001 |
| Dense calcified coronary burden (mm2×100) | 0.05 (0.06) | 0.06 (0.10) | 0.04 (0.07) | 0.40 |
| LV characteristics on CCTA | ||||
| LV mass, g | 104.9 (21.6) | 113.0 (34.6) | 116.8 (28.1) | 0.015 |
| LV mass/height2.7, g/m2.7 | 24.6 (3.8) | 25.5 (3.8) | 27.7 (5.5) | <0.001 |
Values are mean (±SD), median [Q1, Q3], or number (%).
ASCVD indicates Atherosclerotic Cardiovascular Disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low- density lipoprotein cholesterol; hs-CRP, high sensitivity-C reactive protein; LV, left ventricular.
3.2. The relationship between chronic inflammation, left ventricular mass, and noncalcified coronary burden
On univariate analysis, GlycA was associated with LV mass (β=0.31, p<0.001). In multivariable analyses, the association between GlycA with LV mass persisted after adjusting for age, sex, hypertension, hypertensin therapy, lipid therapy, biologic therapy for psoriasis, waist: hip ratio, psoriasis disease duration, and PASI score (β=0.24, p = 0.001). Additionally, we found that NCB was associated with LV mass on univariate analysis (β=0.59, p<0.001) which remained significant in multivariable-adjusted models (β=0.50, p<0.001).
In mediation analyses, the contribution of NCB to the relationship between GlycA and LV mass demonstrated that NCB accounted for 47% of the association between GlycA and LV mass in unadjusted analyses (Fig. 2A). When age, sex, hypertension, hypertension treatment, lipid therapy, biologic therapy for psoriasis, and waist: hip ratio were added to the model, NCB accounted for 32% of the relationship between GlycA and LV mass (Fig. 2B).
Fig. 2.
Noncalcified coronary burden (NCB) partly mediates the relationship between GlycA and LV mass in unadjusted (A) and adjusted analysis (B).
3.3. A change in noncalcified coronary burden is associated with a favorable change in LV mass over one year
Finally, to understand if a change in NCB associated with LV mass over one year, 189 subjects who had repeat CCTA with available NCB and LV mass measurement at one year were included. At one-year, important markers of inflammation including GlycA (406.4 ± 5.8 to 386.6 ± 5.4, p = 0.005) and hs-CRP (3.7 ± 6.7 to 2.8 ± 4.1, p = 0.004) decreased. There was concordance in change in NCB and change in LV mass in both unadjusted models (β=0.18, p = 0.013) and in models adjusted for age, sex, hypertension, hypertension therapy, lipid therapy, biologic therapy, baseline NCB, and baseline LV mass (β=0.25, p = 0.002).
4. Discussion
In this study, we demonstrated that GlycA, a measure of chronic inflammation, was associated with LV mass, which remained significant after adjusting for cardiovascular risk factors. Early, subclinical CAD in the form of NCB also significantly associated with LV mass, and accounted for approximately 32% of the inflammation-LV mass relationship beyond important cardiovascular risk factors. Finally, there was an association between a change in NCB and a favorable change in LV mass over a one-year study period. Collectively, our findings support that inflammation-driven myocardial abnormalities occur incrementally beyond the effect of traditional cardiovascular risk factors, and that subclinical CAD may account for a substantial portion of this relationship.
It is well known that inflammation is critical for the development of CAD.[18] There is also increasing evidence that inflammation is integral in the pathogenesis of heart failure.[6] For example, inflammatory cytokines such as IL-6 and acute phase reactants such as hs-CRP are directly linked to both myocardial mechanical function[1] and myocardial structural changes and also are associated with increased incidence of heart failure and adverse clinical outcomes.[19] Furthermore, inflammatory markers including GlycA,[2] have served as important prognostic markers for disease progression and clinical outcomes in those with established heart failure.[20] However, despite intense interest in assessing targeted anti-inflammatory therapies for both prevention and treatment for heart failure, phase III clinical trials in treating inflammation in heart failure have mostly been disappointing.[6] A potential explanation for failure to translate biological observations to positive clinical trial outcomes may be explained by imprecise patient selection based solely on symptoms (NYHA class) to identify those patients who may be most likely to derive benefit from therapy. Indeed, in an exploratory analysis of the CANTOS trial in which elevated hs-CRP (>2 mg/L) was used as an inclusion criteria, treatment with canakinumab lead to a reduction in heart failure hospitalizations.[21] Such use of inflammatory biomarkers to optimize patient selection may be particularly relevant in heart failure with preserved ejection fraction (HFpEF), a heterogenous condition where comorbidities such as obesity and hypertension induce systemic chronic proinflammatory states with associated metabolic derangements that eventually lead to myocardial structural and functional alterations.[10]
Identification of intermediate heart failure phenotypes is important for prevention of progression to clinical heart failure. Our results add to prior studies which attempt to identify the determinants of subclinical LV abnormalities. Large epidemiological studies have identified increased LV mass as a potent risk factor for increased cardiovascular and all-cause mortality.[22] Additional studies have related LV remodeling, defined as alterations in ventricular architecture, with myocardial functional impairment such as decreased regional systolic function and increased myocardial dyssynchrony by myocardial strain imaging.[23] Recent findings from our group showing the relationship between NCB and high-sensitivity troponin in a cohort of subjects with psoriasis but no known CVD support the notion that early vascular changes may drive myocardial injury.[24] The results from the current study showing the close relationship between subclinical CAD and LV mass in psoriasis further support the concept that chronic inflammation induces both coronary and myocardial abnormalities which may have additive risk for future outcomes. This may be especially relevant in the populations of obesity, hypertension, or diabetes mellitus where there is an increase in pro-inflammatory cytokines which may increase LV mass. Together, these data substantiate the need for additional studies focused on earlier, subclinical LV changes prior to development of clinical heart failure.
In this study, we showed a strong relationship between subclinical CAD assessed as NCB on CCTA and increased LV mass in the setting of chronic inflammation. This observation may be particularly relevant in HFpEF which is more associated with inflammatory markers, compared to heart failure with reduced ejection fraction (HFrEF), which often results from direct cardiac injury from ischemia or myocarditis.[25] Chronic inflammation is known to trigger coronary microvascular endothelial inflammation and promote oxidative stress, leading to reduced nitric oxide availability, cyclic guanosine monophosphate content and protein kinase G activity in cardiomyocytes.[10] Subsequent myocardial hypertrophy and injury characterizes the development of clinical heart failure and promotes a vicious cycle of pro-inflammatory cell signaling.[26] Thus, identification of early, subclinical CAD may aid in identification of those at highest risk of development of heart failure in the future. In this context, our results of observing a change in NCB which was associated with a favorable change in LV mass further highlights the importance that early coronary artery disease may play in the risk of future heart failure in chronic inflammation.
In this study, LV mass was measured on CCTA using a fully automated AI segmentation algorithm. Although LV mass measurements have traditionally been performed using echocardiography and cardiac magnetic resonance (CMR) imaging, CCTA-derived LV mass measurements have been shown to be reliable and confer prognostic value.[27] To account for body size differences, we normalized LV mass for height to an allometric power (height[2,7]). Prior studies comparing different indices for normalizing LV mass found that LV mass/height2.7, when compared to LV mass/BSA, identified a higher percent of LVH in patients who have hypertension and are overweight or obese.[15] Additionally, LV mass/height2.7 when compared to LV mass/BSA, was associated with higher incidence of cardiovascular events linked to obesity- related LVH,[28] an especially relevant consideration in this study given the high prevalence of obesity in our cohort.
5. Study limitations
This was an observational study and thus subject to residual confounding even after adjustments in our statistical modeling. Furthermore, the observational nature precludes conclusions about causality and further studies designed for this purpose are needed to assess the role of early CAD in the pathway from inflammation to LV mass. Additionally, this was a relatively small sample size compared to other epidemiological studies investigating LV mass using CCTA or CMR. However, because the same CT scanner was used to obtain all CCTA scans and an automated AI algorithm was used for LV segmentation, our measurements of LV mass are highly objective and reproducible. The ability to obtain precise measurements will be especially useful in evaluating LV mass changes over a longer follow-up period, an important consideration given the long natural history of progression from LV dysfunction to clinical heart failure. Furthermore, we do not yet have enough clinical heart failure outcomes to correlate with LV mass characterization. However, the goal of this study was to understand the relationship between systemic inflammation with subclinical vascular and myocardial abnormalities. Future studies should also focus on earlier steps in the LV remodeling process prior to development of increased LV mass including understanding whether myocardial perfusion is deranged, refined tissue characterization using CMR, and translational studies to uncover cellular pathways which link systemic inflammation-driven cardiomyocyte dysfunction.
6. Conclusions
In conclusion, chronic inflammation was associated with LV mass in subjects with psoriasis after adjusting for important cardiovascular risk factors. Subclinical CAD in the form of NCB partly accounted for the relationship between inflammation and LV mass. Finally, there was a significant relationship between change in NCB and change in LV mass over one year suggesting subclinical atherosclerosis may play a role in increasing LV mass in chronic inflammation.
Contributorship statement
Design of work: WZ, NNM
Conduct of work and data acquisition: WZ, MT VB, PK, GAM, NP, JEA, JAR, AK, SMB, MYC, LYH, NNM
Data analysis and interpretation: WZ, MT, VB, GAM, AKD, AVS, NP, HLT, MPP, LYH, DAB, MC, NNM
Drafting the work: WZ, NNM
Review the work and providing input: all authors
Final approval: all authors
Guarantors overall content: WZ, NNM.
Declaration of Competing Interest
Dr. Mehta is a full-time US government employee and has served as a consultant for Amgen, Eli Lilly, and Leo Pharma receiving grants/other payments; as a principal investigator and/or investigator for AbbVie, Celgene, Janssen Pharmaceuticals, Inc., and Novartis receiving grants and/or research funding; and as a principal investigator for the National Institutes of Health receiving grants and/or research funding.
All other authors declare no conflicts of interests in relation to the work presented in this manuscript.
Acknowledgments
Acknowledgements
We would like to acknowledge and thank NIH Clinical Center outpatient clinic-7 nurses for their invaluable contribution to the process of patient recruitment.
Funding
This study was supported by the National Heart, Lung and Blood Institute (NHLBI) Intramural Research Program (HL006193- 05). This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors.
References
- 1.Rosen B.D., Cushman M., Nasir K., Bluemke D.A., Edvardsen T., Fernandes V., Lai S., Tracy R.P., Lima J.A. Relationship between C-reactive protein levels and regional left ventricular function in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2007;49:594–600. doi: 10.1016/j.jacc.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 2.Jang S., Ogunmoroti O., Ndumele Chiadi E., Zhao D., Rao Vishal N., Fashanu Oluwaseun E., Tibuakuu M., Otvos James D., Benson E.-.M., Ouyang P., Michos Erin D. Association of the novel inflammatory marker Glyca and incident heart failure and its subtypes of preserved and reduced ejection fraction. Circulation. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koppikar S., Colaco K., Harvey P., Akhtari S., Chandran V., Gladman D.D., Cook R., Eder L. Incidence of and risk factors for heart failure in patients with psoriatic disease - a cohort study. Arthritis Care Res (Hoboken) 2021 doi: 10.1002/acr.24578. [DOI] [PubMed] [Google Scholar]
- 4.Lerman J.B., Joshi A.A., Chaturvedi A., Aberra T.M., Dey A.K., Rodante J.A., Salahuddin T., Chung J.H., Rana A., Teague H.L., Wu J.J., Playford M.P., Lockshin B.A., Chen M.Y., Sandfort V., Bluemke D.A., Mehta N.N. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263–276. doi: 10.1161/CIRCULATIONAHA.116.026859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine B., Kalman J., Mayer L., Fillit H.M., Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. New Eng J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 6.Adamo L., Rocha-Resende C., SD Prabhu, Mann D.L. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–285. doi: 10.1038/s41569-019-0315-x. [DOI] [PubMed] [Google Scholar]
- 7.Joshi A.A., Lerman J.B., Aberra T.M., Afshar M., Teague H.L., Rodante J.A., Krishnamoorthy P., Ng Q., Aridi T.Z., Salahuddin T., Natarajan B., Lockshin B.N., Ahlman M.A., Chen M.Y., Rader D.J., Reilly M.P., Remaley A.T., Bluemke D.A., Playford M.P., Gelfand J.M., Mehta N.N. GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circ Res. 2016;119:1242–1253. doi: 10.1161/CIRCRESAHA.116.309637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akinkuolie A.O., Buring J.E., Ridker P.M., Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruppen E.G., Riphagen I.J., Connelly M.A., Otvos J.D., Bakker S.J., Dullaart R.P. GlycA, a pro-inflammatory glycoprotein biomarker, and incident cardiovascular disease: relationship with c-reactive protein and renal function. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0139057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., Initiative S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 12.Otvos J.D., Shalaurova I., Wolak-Dinsmore J., Connelly M.A., Mackey R.H., Stein J.H., Tracy R.P. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015;61:714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 13.Bui V., Shanbhag S.M., Levine O., Jacobs M., Bandettini W.P., Chang L.-C, Chen M.Y., Hsu L.-Y. Simultaneous multi-structure segmentation of the heart and peripheral tissues in contrast enhanced cardiac computed tomography angiography. IEEE Access. 2020;8:16187–16202. doi: 10.1109/access.2020.2966985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bui V., Hsu L.Y., Shanbhag S.M., Tran L., Bandettini W.P., LC Chang, Chen M.Y. Improving multi-atlas cardiac structure segmentation of computed tomography angiography: a performance evaluation based on a heterogeneous dataset. Comput Biol Med. 2020;125 doi: 10.1016/j.compbiomed.2020.104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuspidi C., Meani S., Negri F., Giudici V., Valerio C., Sala C., Zanchetti A., Mancia G. Indexation of left ventricular mass to body surface area and height to allometric power of 2.7: is the difference limited to obese hypertensives? J Hum Hypertens. 2009;23:728–734. doi: 10.1038/jhh.2009.16. [DOI] [PubMed] [Google Scholar]
- 16.de Knegt M.C., Haugen M., Jensen A.K., Linde J.J., Kühl J.T., Hove J.D., Kofoed K.F. Coronary plaque composition assessed by cardiac computed tomography using adaptive Hounsfield unit thresholds. Clin Imaging. 2019;57:7–14. doi: 10.1016/j.clinimag.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Teague H.L., Aksentijevich M., Stansky E., Silverman J.I., Varghese N.J., Dey A.K., Elnabawi Y., Goyal A., Dagur P.K., Chen M.Y., McCoy J.P., Playford M.P., Hourigan C., Gelfand J.M., Mehta N.N. Cells of myeloid origin partly mediate the association between psoriasis severity and coronary plaque. J Invest Dermatol. 2020;140:912–915. doi: 10.1016/j.jid.2019.07.724. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby P., Ridker P.M., Hansson GK, Leducq Transatlantic Network on A Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasan R.S., Sullivan L.M., Roubenoff R., Dinarello C.A., Harris T., Benjamin E.J., Sawyer D.B., Levy D., Wilson P.W., D'Agostino R.B., Framingham Heart S. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 20.Testa M., Yeh M., Lee P., Berman J.W., Lejemtel T.H., Fanelli R., Loperfido F. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J. Am. Coll. Cardiol. 1996;28:964–971. doi: 10.1016/s0735-1097(96)00268-9. [DOI] [PubMed] [Google Scholar]
- 21.Everett Brendan M., Cornel Jan H., Lainscak M., Anker Stefan D., Abbate A., Thuren T., Libby P., Glynn Robert J., Ridker Paul M. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. doi: 10.1161/CIRCULATIONAHA.118.038010. [DOI] [PubMed] [Google Scholar]
- 22.Levy D., Garrison R.J., Savage D.D., Kannel W.B., Castelli W.P. Prognostic implications of Echocardiographically determined left ventricular mass in the Framingham heart study. New England Journal of Medicine. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 23.Rosen B.D., Fernandes V.R., Nasir K., Helle-Valle T., Jerosch-Herold M., DA Bluemke, Lima J.A. Age, increased left ventricular mass, and lower regional myocardial perfusion are related to greater extent of myocardial dyssynchrony in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:859–866. doi: 10.1161/CIRCULATIONAHA.108.787408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou W., Abdelrahman K.M., Dey A.K., Reddy A., Uceda D.E., Lateef S.S., Elnabawi Y.A., Anzenberg P., Al Najafi M., Rodante J.A., Keel A., Ortiz J., Teague H.L., Erb-Alvarez J., Singh D., Joshi A.A., Playford M.P., Chen M.Y., Gelfand J.M., Remaley A.T., Bluemke D.A., Mehta N.N. Association among noncalcified coronary burden, fractional flow reserve, and myocardial injury in psoriasis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders-van Wijk S., van Empel V., Davarzani N., Maeder M.T., Handschin R., Pfisterer M.E., Brunner-La Rocca H.P., investigators T-C Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail. 2015;17:1006–1014. doi: 10.1002/ejhf.414. [DOI] [PubMed] [Google Scholar]
- 26.Castillo E.C., Vazquez-Garza E., Yee-Trejo D., Garcia-Rivas G., Torre-Amione G. What is the role of the inflammation in the pathogenesis of heart failure? Curr Cardiol Rep. 2020;22:139. doi: 10.1007/s11886-020-01382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein R., Ametepe E.S., Yam Y., Dwivedi G., Chow B.J. Cardiac CT assessment of left ventricular mass in mid-diastasis and its prognostic value. Eur Heart J Cardiovasc Imaging. 2017;18:95–102. doi: 10.1093/ehjci/jev357. [DOI] [PubMed] [Google Scholar]
- 28.de Simone G., Kizer J.R., Chinali M., Roman M.J., Bella J.N., Best L.G., Lee E.T., Devereux R.B. Normalization for body size and population-attributable risk of left ventricular hypertrophy: the Strong Heart Study. Am J Hypertens. 2005;18:191–196. doi: 10.1016/j.amjhyper.2004.08.032. [DOI] [PubMed] [Google Scholar]