Abstract
Objective
To summarize the association between vegetarian versus non-vegetarian diet on mortality due to ischemic heart disease, cerebrovascular disease, or all-cause mortality.
Methods
We searched PubMed, Cochrane databases, and ClinicalTrials.Gov from the inception of the databases to October 2019 with no language restriction. Randomized controlled trials or prospective observational studies comparing the association between vegetarian versus non-vegetarian diets among adults and reporting major adverse cardiovascular outcomes were selected. We used Paule-Mandel estimator for tau2 with Hartung–Knapp adjustment for random effects model to estimate risk ratio [RR] with 95% confidence interval [CI].The primary outcome of interest was all-cause mortality. The secondary outcome was ischemic heart disease mortality.
Results
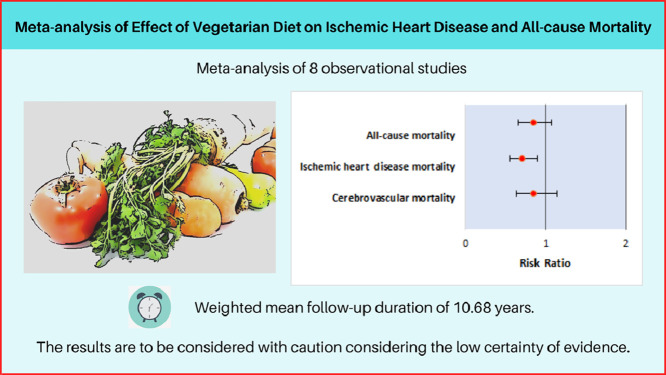
Eight observational studies (n = 131,869) were included in the analysis. Over a weighted mean follow-up of 10.68 years, very low certainty of evidence concluded that a vegetarian diet compared with a non-vegetarian diet was associated with similar risk of all-cause (RR: 0.84, 95% CI: 0.65–1.07, I2: 97%) or cerebrovascular mortality (RR: 0.84, 95% CI: 0.63–1.14, I2: 90%), but was associated with a reduced risk of ischemic heart disease mortality (RR: 0.70, 95% CI: 0.55–0.89, I2: 82%).
Conclusion
A vegetarian diet, compared with a non-vegetarian diet, was associated with a reduced risk of ischemic heart disease mortality, whereas it had no effect on all-cause and cerebrovascular mortality. However, the results are to be considered with caution considering the low certainty of evidence. Despite recent studies supporting no restriction on animal protein intake gaining wide media attention and public traction, consideration for vegetarianism amongst those with risk factors for coronary artery disease should be contemplated.
Keywords: Vegetarian, Mortality, Diet, Cardiovascular, Cerebrovascular
Graphical abstract
1. Introduction
Adverse trends in global dietary patterns have played a pivotal role in the development of chronic diseases [1]. As reported in 2017, 11 million deaths and 255 million disability-adjusted life-years (DALYs) were attributable to dietary risk factors [2]. Cardiovascular disease was the leading cause of diet-related deaths (10 million deaths) and DALYs (207 million DALYs), followed by cancer [2]. Although mortality due to ischemic heart disease (IHD) curtailed from 2005 to 2015, it remains the leading cause of death [3]. Prior studies have reported a modest increase in the incidence of the total, cardiovascular and cancer mortality with red meat consumption [[4], [5], [6], [7]]. Addressing this plight, the 2019 American College of Cardiology (ACC)/ American Heart Association (AHA) Guideline on the Primary Prevention of Cardiovascular Disease recommended a diet consisting of fruits, vegetables, legumes, nuts, whole grains and fish to reduce cardiovascular risk (Class: I, Level of Evidence: BR) [8]. However, recent studies have reported diets restricted in red meat to have no effect on all-cause, cardiovascular or cancer mortality [9].
Over the past few years, plant-based vegetarian diets have increased in popularity, and may be considered a cost-effective and low-risk intervention for many chronic diseases [10]. However, while several studies have reported a plunge in the prevalence of diabetes mellitus (DM), hypertension (HTN) and IHD with vegetarian diets [11,12], their effect on all-cause mortality remains less well-established.
In opposition to studies in the past such as the post-hoc-analysis of the PREDIMED (Prevención con Dieta Mediterránea) trial that recorded a significant mortality reduction of 41% with a provegetarian diet [13,14], most recent studies have found no evidence between following a certain diet and its effect on mortality [15]. Antecedent meta-analyses with a similar research question were either focused on red meat, included a pescatarian/semi-vegetarian diet, failed to use adjusted effect measures, or failed to report IHD or cerebrovascular mortality [9,16,17].
Given this evidence gap, we performed a meta-analysis of observational studies reporting mortality outcomes comparing a vegetarian diet with a non-vegetarian cohort.
2. Methods
We searched PubMed, Cochrane databases and ClinicalTrials.Gov from the inception of the databases until October 30, 2019, using the search terms “vegan,ee” “veggie*,” “plant,” “vegetable,” “vegetarian,” “vegetarianism,” “ovo-lacto-vegetarian*,” “non-vegetarian,” “meat,” “mortality,” and “death” [Supplementary Tables 1 and 4].The searched citations were imported into Mendeley reference manager and checked for duplicates. Additionally, we scrutinized the bibliographies of reviews and relevant studies to augment the database search. Since included individual studies had prior institutional review board clearance, no additional ethical clearance was required for this meta-analysis. The systematic review and meta-analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18] and Methodological Standards for Meta-Analyses and Qualitative Systematic Reviews of Cardiac Prevention and Treatment Studies [19].
The process of study selection was undertaken by two investigators (A.J. and E.V.), independently. The screening for relevant articles was performed at two levels. At the first level, titles and abstracts of searched citations were reviewed for relevance. At the second level, articles identified at level one of screening were subjected to a full-text review. Any disparity at the second level was solved by mutual consensus and in consultation with the senior authors. The inclusion criteria were; (a) randomized controlled trials or prospective observational studies comparing vegetarian versus non-vegetarian diets among adults; and (b) the reporting on all-cause mortality. A vegetarian diet was defined as one excluding meat, pesco or semi-vegetarian diet. The exclusion criteria were; (a) cross-sectional studies, (b) studies with undesired interventions or outcomes other than all-cause mortality; (c) studies with vegan diet forming more than 5% of the vegetarian cohort (vegan diet was combined with vegetarian diet in most of the studies, as the vegan diet formed a relatively small group); and (d) studies with more than 5% pesco or semi-vegetarian diets. A semi-vegetarian diet was defined as a meal that contains little animal protein and predominantly plant-based protein. Studies were not excluded based on language of publication or sample size.
Data extraction from included articles was carried out by two investigators (A.J. and E.V.), independently. Any disparity in extracted data was solved by mutual consensus and in consultation with the senior authors. The following data were extracted from each article; author's name, year of study, country of study, demographic information, definition of vegetarian diet, variables used for adjustment of effect size, and sample size.
To assess the quality of each included study, we used ROBINS-I (Risk of Bias in Non-randomized Studies—of Interventions) tool [20]. The ROBINS-I tool facilitates risk of bias assessment of studies that did not use randomization as a method for selecting participants, and rates studies as “low risk,” “moderate risk,” “high risk,” “ critical risk,” or “no information” based on set criteria (Supplementary Table 3). Risk of bias assessment was performed by two authors independently. To rate the certainty of evidence for each outcome, we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) approach [[21], [22], [23], [24], [25]]. Two authors independently performed GRADE assessment. Any disparity was solved by mutual consensus and in consultation with other authors.
The main outcomes of interest were all-cause mortality, mortality due to IHD or cerebrovascular disease.
We used Paule-Mandel estimator for tau [2] with Hartung–Knapp adjustment for random effects model to estimate risk ratio [RR] with 95% confidence interval [CI]. Statistical heterogeneity among included studies was computed using Higgins' I2 statistics [26], with I2 > 50% consistent with high degree of heterogeneity. Outlier analysis using the “dmetar” package was used to identify and exclude heterogeneous studies. Publication bias was not assessed if the number of studies were < 10. P-value of < 0.05 was considered statistically significant. Statistical analysis was performed with “meta” package in R version 3.6.2.
3. Results
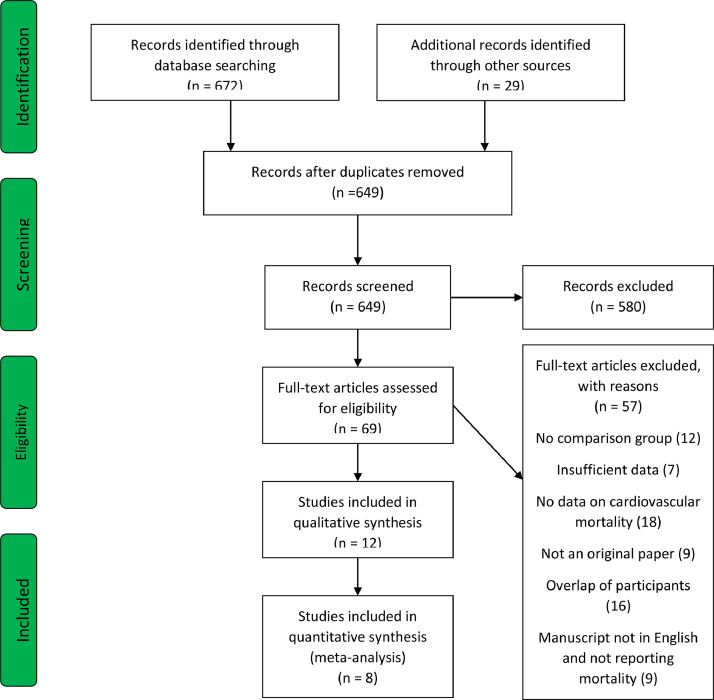
The database search identified a total of 649 articles after checking for duplicates. Twenty-nine studies were identified by manual search. There were no randomized controlled trials on the topic; eight observational studies (n = 131,869) were included in the analysis (Fig. 1) [[27], [28], [29], [30], [31], [32], [33], [34]]. Of the eight included studies, 2 studies were included from manual bibliography review [28,34]. The baseline characteristics of the included studies are presented in Table 1. The weighted mean follow-up period was 10.68 years.
Fig. 1.
PRISMA flowchart.
Table 1.
Baseline characteristics of included studies.
| Study/ Authors name | Year | Country | Participants in Vegetarian/ Non-Vegetarian cohort | age | Percentage male | year of recruitment | ENd of follow-up | Mean length of follow-up | Definition of Vegetarian according to the study | Definition of Non-vegetarian according to the study | Outcomes adjusted for Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adventist Mortality study | 1965 | USA | 10,258/ 14,280 | NA | NA | 1959–1960 | December, 1965 | 5.6 years | Not defined | Not defined | Age, Sex and Smoking |
| Berkel | 1983 | Netherlands | 1972/1972 | NA | 33% | 1968–1977 | 1977 | 10 years | Not defined | Not defined | – |
| Japenese Zen Priest | 1984 | Japan | 2176/2176 | NA | NA | 1955–1978 | 1978 | 23 years | Not defined | Not defined | Unadjusted, however the control group was age and sex matched. |
| Adventist health study-1 | 1988 | USA | 8003/20,949 | 25- 100 years | 41% | 1976–1980 | December, 1988 | 11.1 years | Not defined | Not defined | Age, Sex and Smoking |
| Health food shoppers study | 1995 | United Kingdom | 3790/6088 | Median 45–46 | 40% | 1973–1979 | December, 1995 | 18.4 years | Based on a self-administered questionnaire. Participants who answered “Yes” to “Are you a vegetarian?” | Based on a self-administered questionnaire. Participants who answered “Yes” to “Are you a Non-vegetarian?” | Age, Sex and Smoking |
| Thorogood et al. | 1995 | United Kingdom | 4674/6373 | Median 32–34 | 38% | 1980–1984 | December, 1995 | 13.7 years | Not defined | Not defined | All-cause mortality and IHD mortality: Smoking BMI and social classCerebrovascular mortality: Age, Sex and smoking |
| Claude et al. | 2005 | Germany | 1225/679 | 39.2% over the age of 55 years | 45% | 1973–1979 | May, 1989 | 9.9 | Vegans(those who avoidmeat, fish, eggs, and dairy products), lacto-ovo vegetarian (those who avoid meat and fish but eat eggs and/or dairyProducts). Further, vegan constituted only 5% of this cohort and hence the study was included in the meta-analysis) | Those who occasionally orregularly eat meat and/or fish | All-cause mortality, IHD mortality and cardiovascular mortality: Age, gender, smoking, level of activity, alcohol consumption, BMI, educationCerebrovascular mortality: Age, Sex and smoking |
| EPIC- Oxford | 2009 | United kingdom | 16,081/31,173 | 44 years | 24% | 1993–1999 | 30 June, 2007 | ~10 years | Vegans, lacto-ovo vegetarian | Those who occasionally orregularly eat meat and/or fish | Age, sex, smoking, and alcohol consumption |
Most of the included studies had a moderate risk of bias mainly originating from either bias due to confounding or classification of intervention. Additionally, none of the included studies had any information regarding adherence to the respective diet until the end of the follow-up period, adding to the chances of deviation from intended intervention bias confounding the results. The description of bias in each domain of ROBINS-I tool across studies is provided in Supplementary Table 2.
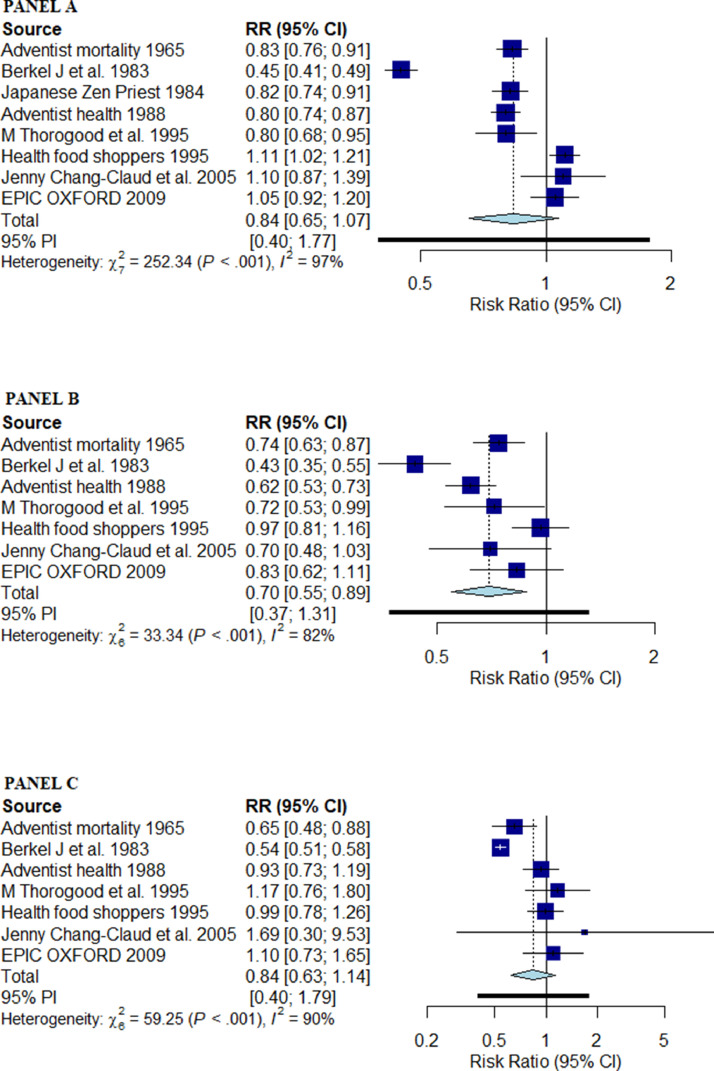
A vegetarian diet was associated with similar risk of all-cause mortality compared with a non-vegetarian diet (RR: 0.84 [95% CI: 0.65–1.07] I2: 97%) [Fig. 2, PANEL A] [Central Illustration]. Outlier analysis identified study by Berkel et al. (1965) as an outlier [33]. After excluding the study, the relative risk of all-cause mortality with a vegetarian diet compared with a non-vegetarian diet remained similar (RR: 0.91 [95% CI: 0.79–1.05], and I2 reduced to 88%. Very low certainty evidence suggested there was no association between vegetarian diet and mortality.
Fig. 2.
Forest plot for pooled estimate using Paule-Mandel estimator for tau2 with Hartung–Knapp adjustment for random effects model; PANEL A: All-cause mortality; PANEL B: Mortality due to IHD; PANEL C: Mortality due to cerebrovascular disease; RR: risk ratio; PI: prediction interval.
A vegetarian diet was associated with a significantly reduced risk of mortality due to IHD compared with a non-vegetarian diet (RR: 0.70 [95% CI: 0.55–0.89] I2: 82%) [Fig. 2, PANEL B] [Central illustration]. Outlier analysis identified studies Berkel et al. (1965) and Health Food Shoppers (1995) as outliers [[30], [33]]. After excluding the studies the relative risk of mortality due to IHD with a vegetarian diet compared with a non-vegetarian diet remained lower (RR: 0.70 [95% CI: 0.61–0.80], and I2 reduced to 2%.Very low certainty evidence suggested there was an association between vegetarian diet and mortality due to IHD.
A vegetarian diet was associated with a similar risk of mortality due to cerebrovascular disease compared with a non-vegetarian diet (RR: 0.84 [95% CI: 0.63–1.14] I2: 90%) [Fig. 2, PANEL C] [Central illustration]. Outlier analysis identified study by Berkel et al. (1965) as an outlier [33]. After excluding the study the relative risk of mortality due to cerebrovascular disease with a vegetarian diet compared with a non-vegetarian diet remained similar (RR: 0.93 [95% CI: 0.74–1.18], and I2 reduced to 35%.Very low certainty evidence suggested there was no association between vegetarian diet and mortality due to cerebrovascular disease.
4. Discussion
Our meta-analysis evaluated the effects of a vegetarian diet on all-cause mortality, and mortality from either IHD or cerebrovascular disease. We found a reduction in mortality due to IHD with a relative risk reduction of 30%, whereas no reduction in mortality due to cerebrovascular disease or all-cause mortality among populations consuming a vegetarian diet compared with a non-vegetarian diet. The current meta-analysis provides updated evidence regarding the potential mortality benefits of a vegetarian diet, defined as vegan or, lacto-ovo-vegetarian diet, compared with a non-vegetarian diet. A previously published meta-analysis included five studies and had conclusion similar to the present analysis, with reduction in mortality due to IHD, while no reduction in mortality due to cerebrovascular disease or all-cause mortality [35]. While these data require further validation, they support recommendations from major societies including the American Heart Association and the American Cancer Society, particularly for patients at risk for coronary artery disease. The underlying cause for a reduced risk of mortality from IHD as observed in our study among vegetarians is likely attributable to a reduction in established risk factors for IHD [36]. Though a non-vegetarian diet and red meat in particular are associated with an increased risk of stroke [37], our study reported no association between a vegetarian diet and death due to cerebrovascular disease. A previous meta-analysis analyzing red meat and mortality due to stroke reported similar conclusions, speculating the possibility of underpowered trials and type II error in the estimate [37]. Additionally, in the current meta-analysis, no benefit of a vegetarian diet on all-cause mortality was reported, despite previous studies reporting increased mortality with red meat [38]. Higher proportion of the vegetarian diet comprising carbohydrates, consumption of refined sugars and short duration of follow-up in included studies could explain the absence of mortality benefit with a vegetarian diet [39].
As pointed above, the expected benefits of adopting a vegetarian diet stem from its effects on weight loss, lipid profile, hypertension, diabetes, and other chronic diseases. A vegetarian diet is linked with reduced blood pressure [40], an improved lipid profile [41], lower postprandial blood glucose, and stabilized fasting blood sugar levels [42]. Unfavorable levels of each of these parameters have historically been linked with an increased risk of cardiovascular and all-cause mortality. In a follow-up of the participants from the Framingham Heart Study over 26 years, increased body weight was found to be a strong predictive factor in the incidence of cardiovascular disease [43]. Adopting a red meat-based diet was found to increase the risk of all-cause mortality and IHD mortality in some studies [4,5]. From a metabolomics perspective, benefits of a vegetarian diet are attributable to the presence of innate health-promoting substances in whole, plant-based foods and minimizing the exposure to harmful substances in meat products such as saturated fats, heme iron, and N-glycolylneuraminic acid (Neu5Gc) [44]. Furthermore, plant-based diets positively affect certain metabolic pathways that have been under investigation for their health benefits. Plant-based diets inhibit growth hormone (GH) and insulin-like growth factor 1 (IGF-1) axis, and activate sirtuins and adenosine monophosphate kinase (AMPK), and mammalian target of rapamycin (mTOR) pathways [[45], [46], [47], [48]]. These mechanisms may translate into lower IHD and all-cause mortality in vegetarians compared with non-vegetarians.
Studies in the past have been conducted to assess the correlation between a vegetarian diet, and overall and cause-specific mortality. Though several studies reported a mortality benefit with the incorporation of a vegetarian diet, the results have not been consistent. Several studies, including the first and second Californian Adventist Health Study (AHS) analyzing 34,198 and 73,308 participants, respectively, exhibited a significant risk reduction in all-cause and IHD-related mortality [32,49]. In a study using the data from the ARIC (Atherosclerosis Risk in Communities) study, higher adherence to a plant-based source of protein was associated with a 19% and 11% lower risk of IHD mortality and all‐cause mortality, respectively [50]. Contrary to the above findings, the British EPIC-Oxford study and the German vegetarian study with 47,254 and 1904 participants, respectively did not show any significant reduction in all-cause mortality or mortality due to IHD between the two groups [27,29]. Similar findings were reported in the 45 and Up Study, which found no evidence for following a vegetarian, semi-vegetarian or a pesco-vegetarian diet, and its independent protective effect on all-cause mortality [15]. A combined analysis of the EPIC-Oxford and Oxford Vegetarian Study further reported that United Kingdom–based vegetarians and comparable non-vegetarians have similar all-cause mortality [51].
The vegetarian diet has experienced nutritional transformation over the years [52], with higher proportions of processed food and refined sugars present in the vegetarian diets today [44]. Consequently, the attributable health benefits with the vegetarian diet have reduced. Further, the century-old idea of macronutrient-based centering of food has been replaced with quality of the overall diet in being responsible for health, further explaining the decreased mortality benefits reported by vegetarian diets in the recently published studies [44]. Also, the geographic variation in the vegetarian diet could explain the statistical heterogeneity observed across the pooled estimates.
Residual confounding may play a key role in the associations noted in the vegetarian diet studies. The motivational factor behind vegetarianism is usually underlined by the increased likelihood of adopting healthy habits. Vegetarianism usually extends beyond the scope of a diet and becomes a lifestyle with shared health practices and behaviors. For instance, vegetarians self-reported that they are more likely to abstain from smoking, alcohol, and the use of prescription drugs than the general population [53]. Additionally, 47% of participants in a study examining the lifestyle behaviors of vegans reported adopting this diet due to “health-related reasons.” Eighty-one percent of the participants in the study also self-reported no chronic disease diagnoses, with 71% exercising regularly with a reported mean BMI of 22.6 kg/m2 [54]. As a result, the healthy behaviors and lifestyle of vegetarians rather than their diet may be a strong influence and determinant of IHD risk and mortality.
Strengths of our review included a comprehensive search strategy complemented with a manual search, risk of bias assessment, and GRADE certainty of evidence assessment for each outcome independently by two authors. Additionally, the use of adjusted effect measures and Paule-Mandel estimator for tau [2] estimation with Hartung–Knapp adjustment provided a conservative pooled estimate with a wide confidence interval.
Our study has several limitations. The first limitation stems from the nature of the cohort studies involved in our analysis. As with most vegetarian diet studies, the information was gathered by questionnaires and surveys, and relied heavily on participant answers and subjective recall, without clear definition of diet in few studies. Further compliance to the specified diet could not be reported considering the observational nature of the studies. Second, the non-randomized nature of the studies involved and the different use of adjustments limited confounding bias reduction. Furthermore, the generalizability of our results is mainly limited by geographic distribution, along with other factors like diet profile. Few studies in this meta-analysis included a vegan/ pesco or semi-vegetarian diets in vegetarian/non-vegetarian subheading, respectively. However, the inclusion of studies was limited to those with less than 5% of these diet groups. This is a study-level meta-analysis, limited in its ability to examine the source of heterogeneity. Lastly, considering the high heterogeneity of all-cause mortality pooled estimate even after adjusted analysis, the results should be interpreted with caution. We did not find any randomized clinical trials that met our search criteria, although they would generate the strongest evidence base. Unfortunately, conducting randomized clinical trials comparing vegetarians with non-vegetarians is challenging due to the numerous types of vegetarian diets and their elements, latency periods requiring long-term follow-up, and strict diet adherence. Nonetheless, even within the limitations of observational data, developing a standardized approach to measure the accuracy of vegetarian diet assessment, and degrees of adherence to a vegetarian diet and lifestyle become paramount for future research. Further, considering a major lifestyle modification in implementing a vegetarian diet, the role of vegetarian diet in specific subgroups like in obesity, patients with heart disease, high-blood pressure etc., is to be determined, with previous studies reporting beneficial results [55,56].
In conclusion, the current meta-analysis reported a statistically significant reduction in mortality due to IHD, while there was no effect on cerebrovascular and all-cause mortality among participants with vegetarian dietary habits. Results from randomized clinical trials like Vegetarian Diet in Patients With Ischemic Heart Disease (VERDI) and Impact of MEditerranean Diet, Inflammation and Microbiome After an Acute Coronary Syndrome (MEDIMACS) trials will provide further evidence on the possible role of a vegetarian diet as a cost-effective intervention for mitigating mortality and morbidity due to IHD.
CRediT authorship contribution statement
Ahmad Jabri: Data curation, Writing – original draft. Ashish Kumar: Methodology, Software, Visualization, Writing – original draft. Elizabeth Verghese: Data curation, Writing – original draft. Anas Alameh: Writing – original draft. Anirudh Kumar: Writing – original draft. Muhammad Shahzeb Khan: Methodology, Writing – original draft. Safi U. Khan: Methodology, Writing – original draft. Erin D. Michos: Supervision, Writing – review & editing. Samir R. Kapadia: Supervision, Writing – review & editing. Grant W. Reed: Supervision, Writing – review & editing. Ankur Kalra: Conceptualization, Methodology, Supervision, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
The study was funded by makeadent.org's Ram and Sanjita Kalra Aavishqaar Fund at Heart, Vascular, and Thoracic Department at Cleveland Clinic Akron General in Akron, Ohio.
Disclosure
Dr. Kalra is the Chief Executive Officer and Creative Director of makeadent.org.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2021.100182.
Appendix. Supplementary materials
References
- 1.Schulze M.B., Martínez-González M.A., Fung T.T., Lichtenstein A.H., Forouhi N.G. Food based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396. doi: 10.1136/bmj.k2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afshin A., Sur P.J., Fay K.A. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393(10184) doi: 10.1016/S0140-6736(19)30041-8. 1958-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NA N., Mauro G., HJ P., FD P., Rasha A.L. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6) doi: 10.1161/CIRCOUTCOMES.118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha R., Cross A.J., Graubard B.I., Leitzmann M.F., Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169(6):562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan A., Sun Q., Bernstein A.M. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555–563. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhubi-Bakija F., Bajraktari G., Bytyçi I. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: a position paper from the international lipid expert panel (ILEP) Clin Nutr. 2021;40(1):255–276. doi: 10.1016/j.clnu.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Mazidi M., Katsiki N., Mikhailidis D.P., Bartłomiejczyk M.A., Banach M. Association of empirical dietary atherogenic indices with all-cause and cause-specific mortality in a multi-ethnic adult population of the United States. Nutrients. 2019;11(10):2323. doi: 10.3390/nu11102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AD K., BR S., AM A. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeraatkar D., Johnston B.C., Bartoszko J. Effect of lower versus higher red meat intake on cardiometabolic and cancer outcomes: a systematic review of randomized trials. Ann Intern Med. 2019;171(10):721–731. doi: 10.7326/M19-0622. [DOI] [PubMed] [Google Scholar]
- 10.Hever J. Plant-based diets: a physician's guide. Perm J. 2016;20(3):15–82. doi: 10.7812/TPP/15-082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satija A., Bhupathiraju S.N., Spiegelman D. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411–422. doi: 10.1016/j.jacc.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsh K., Zeuschner C., Saunders A. Health implications of a vegetarian diet: a review. Am J Lifestyle Med. 2011;6(3):250–267. doi: 10.1177/1559827611425762. [DOI] [Google Scholar]
- 13.Martínez-González M.A., Sánchez-Tainta A., Corella D. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. 2014;100(suppl_1):320S–328S. doi: 10.3945/ajcn.113.071431. [DOI] [PubMed] [Google Scholar]
- 14.Estruch R., Ros E., Salas-Salvadó J. Primary Prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 15.Mihrshahi S., Ding D., Gale J., Allman-Farinelli M., Banks E., Bauman A.E. Vegetarian diet and all-cause mortality: evidence from a large population-based Australian cohort - the 45 and Up Study. Prev Med Lifestyle. 2017;97:1–7. doi: 10.1016/j.ypmed.2016.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Kwok C.S., Umar S., Myint P.K., Mamas M.A., Loke Y.K. Vegetarian diet, seventh day adventists and risk of cardiovascular mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;176(3):680–686. doi: 10.1016/j.ijcard.2014.07.080. [DOI] [PubMed] [Google Scholar]
- 17.Huang T., Yang B., Zheng J., Li G., Wahlqvist M.L., Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: a meta-analysis and systematic review. Ann Nutr Metab. 2012;60(4):233–240. doi: 10.1159/000337301. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goutham R., Francisco L-J, Jack B. Methodological standards for meta-analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American heart association. Circulation. 2017;136(10):e172–e194. doi: 10.1161/CIR.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 20.Sterne J.A.C., Hernán M.A., Reeves B.C. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt G.H., Oxman A.D., Vist G. GRADE guidelines: 4. Rating the quality of evidence–2014;study limitations (risk of bias) J Clin Epidemiol. 2011;64(4):407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt G.H., Oxman A.D., Kunz R. GRADE guidelines 6. Rating the quality of evidence–2014;imprecision. J Clin Epidemiol. 2011;64(12):1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Guyatt G.H., Oxman A.D., Kunz R. GRADE guidelines: 7. Rating the quality of evidence–2014;inconsistency. J Clin Epidemiol. 2011;64(12):1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt G.H., Oxman A.D., Kunz R. GRADE guidelines: 8. Rating the quality of evidence–2014;indirectness. J Clin Epidemiol. 2011;64(12):1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt G.H., Oxman A.D., Montori V. GRADE guidelines: 5. Rating the quality of evidence–2014;publication bias. J Clin Epidemiol. 2011;64(12):1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414) doi: 10.1136/bmj.327.7414.557. 557 LP - 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang-Claude J., Hermann S., Eilber U., Steindorf K. Lifestyle determinants and mortality in german vegetarians and health-conscious persons: results of a 21-year follow-up. Cancer Epidemiol Biomarker Prev. 2005;14(4) doi: 10.1158/1055-9965.EPI-04-0696. 963 LP - 968. [DOI] [PubMed] [Google Scholar]
- 28.Thorogood M., Mann J., Appleby P., McPherson K. Risk of death from cancer and ischaemic heart disease in meat and non-meat eaters. BMJ. 1994;308(6945) doi: 10.1136/bmj.308.6945.1667. 1667 LP - 1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Key T.J., Appleby P.N., Spencer E.A., Travis R.C., Roddam A.W., NE Allen. Mortality in British vegetarians: results from the European prospective investigation into cancer and nutrition (EPIC-Oxford) Am J Clin Nutr. 2009;89(5):1613S–1619S. doi: 10.3945/ajcn.2009.26736L. [DOI] [PubMed] [Google Scholar]
- 30.Key T.J., Thorogood M., Appleby P.N., Burr M.L. Dietary habits and mortality in 11,000 vegetarians and health conscious people: results of a 17 year follow up. BMJ. 1996;313(7060):775–779. doi: 10.1136/bmj.313.7060.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Key T.J., Fraser G.E., Thorogood M. Mortality in vegetarians and non-vegetarians: a collaborative analysis of 8300 deaths among 76,000 men and women in five prospective studies. Public Health Nutr. 1998;1(1):33–41. doi: 10.1079/PHN19980006. [DOI] [PubMed] [Google Scholar]
- 32.Fraser G.E. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr. 1999;70(3):532s–538s. doi: 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- 33.Berkel J., de Waard F. Mortality pattern and life expectancy of seventh-day adventists in the Netherlands. Int J Epidemiol. 1983;12(4):455–459. doi: 10.1093/ije/12.4.455. [DOI] [PubMed] [Google Scholar]
- 34.Ogata M., Ikeda M., Kuratsune M. Mortality among Japanese Zen priests. J Epidemiol Community Health. 1984;38(2):161–166. doi: 10.1136/jech.38.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinu M., Abbate R., Gensini G.F., Casini A., Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–3649. doi: 10.1080/10408398.2016.1138447. [DOI] [PubMed] [Google Scholar]
- 36.Micha R., Wallace S.K., Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121(21):2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim K., Hyeon J., Lee S.A. Role of total, red, processed, and white meat consumption in stroke incidence and mortality: a systematic review and meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9) doi: 10.1161/JAHA.117.005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alshahrani S.M., Fraser G.E., Sabaté J. Red and processed meat and mortality in a low meat intake population. Nutrients. 2019;11(3):622. doi: 10.3390/nu11030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarys P., Deliens T., Huybrechts I. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients. 2014;6(3):1318–1332. doi: 10.3390/nu6031318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokoyama Y., Nishimura K., Barnard N.D. Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med. 2014;174(4):577–587. doi: 10.1001/jamainternmed.2013.14547. [DOI] [PubMed] [Google Scholar]
- 41.Ferdowsian H.R., Barnard N.D. Effects of plant-based diets on plasma lipids. Am J Cardiol. 2009;104(7):947–956. doi: 10.1016/j.amjcard.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 42.Kim M.S., Hwang S.S., Park E.J., Bae J.W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ Microbiol Rep. 2013;5(5):765–775. doi: 10.1111/1758-2229.12079. [DOI] [PubMed] [Google Scholar]
- 43.Kannel W.B. Framingham study insights into hypertensive risk of cardiovascular disease. Hypertens Res. 1995;18(3):181–196. doi: 10.1291/hypres.18.181. [DOI] [PubMed] [Google Scholar]
- 44.Hever J., Cronise R.J. Plant-based nutrition for healthcare professionals: implementing diet as a primary modality in the prevention and treatment of chronic disease. J Geriatr Cardiol. 2017;14(5):355–368. doi: 10.11909/j.issn.1671-5411.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hubbard B.P., Sinclair D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35(3):146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercken E.M., Carboneau B.A., Krzysik-Walker S.M., de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11(3):390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longo V.D., Fasting Panda S. Circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi I.Y., Lee C., Longo V.D. Nutrition and fasting mimicking diets in the prevention and treatment of autoimmune diseases and immunosenescence. Mol Cell Endocrinol. 2017;455:4–12. doi: 10.1016/j.mce.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orlich M.J., Fraser G.E. Vegetarian diets in the adventist health study 2: a review of initial published findings. Am J Clin Nutr. 2014;100(Suppl(1)) doi: 10.3945/ajcn.113.071233. 353S-8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyunju K., CL E., Vanessa G., SL M., Josef C., RC M. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J Am Heart Assoc. 2019;8(16) doi: 10.1161/JAHA.119.012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Appleby P.N., Crowe F.L., Bradbury K.E., Travis R.C., Key T.J. Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am J Clin Nutr. 2016;103(1):218–230. doi: 10.3945/ajcn.115.119461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borude S. Which is a good diet—veg or non-veg? faith-based vegetarianism for protection from obesity—a myth or actuality? Obes Surg. 2019;29(4):1276–1280. doi: 10.1007/s11695-018-03658-7. [DOI] [PubMed] [Google Scholar]
- 53.Wirnitzer K., Boldt P., Lechleitner C. Health status of female and male vegetarian and vegan endurance runners compared to omnivores-results from the NURMI study (Step 2) Nutrients. 2018;11(1):29. doi: 10.3390/nu11010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dyett P.A., Sabaté J., Haddad E., Rajaram S., Shavlik D. Vegan lifestyle behaviors. An exploration of congruence with health-related beliefs and assessed health indices. Appetite. 2013;67:119–124. doi: 10.1016/j.appet.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Tuso P.J., Ismail M.H., Ha B.P., Bartolotto C. Nutritional update for physicians: plant-based diets. Perm J. 2013;17(2):61–66. doi: 10.7812/TPP/12-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kahleova H., Levin S., Barnard N.D. Vegetarian dietary patterns and cardiovascular disease. Prog Cardiovasc Dis. 2018;61(1):54–61. doi: 10.1016/j.pcad.2018.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.