Abstract
Objective
Black adults are less likely than White adults to present with adverse lipid profiles and more likely to present with low-grade inflammation. The impact of race on the association between atherogenic lipid profiles, inflammation, and coronary heart disease (CHD) is unknown.
Methods
We evaluated the association between high levels (>50th percentile) of high-sensitivity C-reactive protein (hsCRP) and of triglycerides to high density lipoprotein ratio (TG/HDL-C) and CHD events by race in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort with 30,239 Black and White participants aged 45 and older.
Results
Participants with both high hsCRP and high TG/HDL-C had highest rates of CHD (HR 1.84; 95% CI: 1.48, 2.29 vs HR 1.52; 95% CI: 1.19, 1.94 in White vs Black participants respectively). Whereas isolated high hsCRP was associated with increased CHD risk in both races (HR 1.68; 95% CI: 1.31, 2.15 and HR 1.43; 95% CI: 1.13, 1.81 for White and Black participants respectively), isolated high TG/HDL was associated with increased CHD risk only in White participants (HR 1.44; 95% CI: 1.15, 1.79 vs HR 1.01; 95% CI: 0.74, 1.38). Further, the effects of high hsCRP and high TG/HDL-C were additive, with inflammation being the driving variable for the association in both races.
Conclusion
In both races, higher inflammation combined with adverse lipid profile is associated with greater CHD risk. Therefore, inflammation increases CHD risk in both races whereas dyslipidemia alone is associated with a greater risk in White but not in Black adults. hsCRP testing should be a standard feature of CHD risk assessment, particularly in Black patients.
Keywords: TG/HDL ratio, Dyslipidemia, CAD, CHD, Vascular Inflammation, hsCRP
Graphical abstract
1. Introduction
Despite decades of research and improvements in treatment, coronary heart disease (CHD) remains the leading cause of death in the United States, accounting for 1 of every 7 deaths in 2017—more than 600,000 deaths in total [1]. Common risk factors for CHD, include dyslipidemia, diabetes mellitus, hypertension, obesity, cigarette smoking, and physical inactivity [2] and have race-specific associations. A growing body of evidence indicates higher cardiovascular disease burden among African Americans (Black adults) [3,4].
Although low-density lipoprotein cholesterol (LDL-C) remains the primary treatment target to reduce cardiovascular disease risk, several large-scale epidemiological studies have demonstrated that elevated TG levels are independently associated with increased incidence of cardiovascular events, even in patients treated effectively with statins [5], [6], [7]. Hypertriglyceridemia is an independent predictor of CHD risk and may be a stronger risk factor among women than men [8,9]. Atherogenic dyslipidemia, the joint occurrence of high triglycerides (TG) and low high-density lipoprotein cholesterol (HDL-C) in association with elevated apoprotein B and small dense LDL particle levels, is an important component of the metabolic syndrome and a strong risk factor for CHD [10], [11], [12], [13], [14]. A high TG/HDL-C ratio correlates with higher LDL-C, small HDL particles, and insulin resistance [15], [16], [17]. The ratio strongly predicts risk of myocardial infarction [18], impaired heart rate recovery after exercise [19], the extent of coronary atherosclerosis [20], [21], [22], CHD incidence [23], and cardiovascular and all-cause mortality [19,21,24].
In recent trials, such as JUPITER, CANTOS, and COLCOT, hsCRP levels have been convincingly linked to vascular events [25], [26], [27]. The CANTOS trial showed that directly reducing inflammation with canakinumab, an interleukin (IL)−1β neutralizing monoclonal antibody, reduced the hsCRP levels, rate of recurrent cardiovascular events, independent of lipid-level lowering. These studies did not examine race and sex-dependent associations of lipid and inflammation measures with CVD outcomes.
To our knowledge, no study has evaluated the presence of biological interactions (e.g., synergism) between race, inflammation and dyslipidemia on the risk of coronary events. In this paper, we will refer to synergism as the situation in which the joint effect of two risk factors is greater than the sum of their isolated effects [28]. We analyzed data from the national Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort to examine racial differences, TG/HDL-C ratios, and hsCRP levels as predictors of fatal and non-fatal CHD with the intent to identify interactions of lipid and inflammation profiles with CHD risk.
2. Methods
2.1. Study population
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a national prospective cohort of 30,239 participants established to determine the reasons for regional and racial differences in stroke mortality. 56 people dropped out and 30,183 participants with follow-up constitute the analytical cohort. Details on the design and methods of REGARDS have been previously described [29,30]. Briefly, REGARDS recruited community-dwelling Black and White women and men ≥45 years of age, identified via mail and telephone using commercially available lists of residents in the contiguous United States, and enrolled from 2003 to 2007. The sampling scheme included 30% of participants from the “stroke belt” (North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, Arkansas, and Louisiana), 20% from the “stroke buckle” (the coastal plain of North Carolina, South Carolina, and Georgia), and 50% from elsewhere in the United States. The baseline cohort was 42% Black and 55% women (goal was 50% of each). Exclusion criteria included self-identified race other than White or Black/ African American, active treatment for cancer, chronic medical conditions precluding long-term participation, cognitive impairment as judged by the interviewer, current or impending residence in a nursing home, or inability to communicate in English. An initial telephone interview was used to survey participants and establish eligibility [31]. Following verbal consent, demographic information and medical history (including risk factor evaluation) was collected by a computer-assisted telephone interview [31]. Race was self-classified by participants and included the following options defined by the investigators: White and Black/African American (referred henceforth as Black). An in-home examination was conducted to perform anthropometric measurements (e.g., height, weight, waist circumference, blood pressure), and an electrocardiogram (ECG), medication inventory, phlebotomy, and urine collection among those eligible. Follow-up telephone interviews were performed every 6 months with the participant or designated next contact in order to detect the suspect of a cardiovascular event. The study design was approved by the institutional review boards of all participating institutions. Written informed consent was obtained from each participant.
2.2. Laboratory assessments
Blood samples were obtained in the morning after a 10 to 12 hour fast [32]. Samples were centrifugated within 2 h of collection; serum and plasma were separated and sent to the University of Vermont for analysis. Upon arrival, samples were re-centrifuged at 30,000 g at 4 C and either analyzed or stored at below −80 C. CRP was analyzed in batches by particle enhanced immunonephelometry using the BNII nephelometer (N High Sensitivity CRP; Dade Behring, Deerfield, IL) with interassay coefficients of variation of 2.1–5.7%. Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides and glucose were measured by colorimetric reflectance spectrophotometry using the Ortho Vitros Clinical Chemistry System 950IRC instrument (Johnson & Johnson Clinical Diagnostics, Rochester, NY) [29,30].
2.3. CHD outcomes
Our study focused on CHD events defined as first definite or probable myocardial infarction (MI) or acute CHD death on/before 12/31/2016 [33]. Baseline CHD is defined as self-reported myocardial infarction (MI), coronary artery bypass grafting (CABG), angioplasty or stenting, or study baseline ECG evidence of a prior MI [33]. REGARDS study participants or proxy respondents were contacted every 6 months via telephone to assess incident CHD events. The primary study outcome was incident CHD, defined as an incident definite or probable non-fatal MI or CHD death [34]. Incident CHD events were adjudicated by a team of experts who used published guidelines [35,36]. When non-fatal events were reported, medical records were retrieved for adjudication. When fatal CHD events were reported or the participant died, interviews with next of kin or proxies, medical records in the last year of life, death certificates and autopsy reports were examined to determine if a CHD event was the main underlying cause of death. Deaths not captured by other means were captured using the national death index. A secondary study outcome was defined as definite or probable CHD death. For the current analysis, participants were followed through the date of their incident CHD event, death, last study contact or December 31, 2016, whichever occurred first.
2.4. Risk factor ascertainment
Because enrollment in REGARDS occurred between 2003 and 2007 and we have analyzed event data up to December 2016, hypertension was defined following the ACC/AHA 2003 guidelines as systolic blood pressure (SBP) greater or equal to 140 mmHg or diastolic blood pressure (DBP) greater or equal to 90 mmHg, or self-reported current medication use to control BP. Diabetes was defined as fasting glucose greater or equal to 126 mg/dL, or non-fasting glucose greater or equal to 200 mg/dL, or the self-reported use of insulin, or medications for glucose control. Smoking status was defined as a dichotomous variable where the reference category denotes the absence of the risk factor (non-current-smokers). BMI was defined as a 3-level categorical variable: normal weight (reference category, BMI < 24.9 kg/m2), overweight (BMI between 25 kg/m2 and 29.9 kg/m2), and obese (BMI > 30 kg/m2). High, low groups for TG/HDL and hsCRP were above or below median: 2.19 mg/dL for hsCRP and 2.17 for TG/HDL-C ratio. Sex, race, and smoking status were dichotomous variables with lower risk groups as reference value (woman, White, non-current-smokers). Household income was operationalized as a 5-level categorical variable describing ascending levels of the income strata (< $20k, $20k - $34k, $35k - $74k, $75k or above, or refused) where the reference category was the lowest income strata. Similarly, educational attainment was defined as a 4-level categorical variable in ascending order of educational attainment (less than high school, high school graduate, some college, college graduate or above), the reference category was less than high school education.
2.5. Statistical analysis
To create a primary predictor representing the interaction between hsCRP and TG/HDL-C ratio we dichotomized both variables into low (below median) versus high-risk (above median) categories. We performed Pearson's χ2 test to examine the association between our primary predictor (the interaction of the two dichotomized predictors hsCRP and TG/HDL-C ratio) with respect to our categorical covariates of interest. We calculated estimated proportions and 95% confidence intervals for each of the categories of our primary predictor. Similarly, we performed an F-test to examine whether age (continuous) was significantly associated with our primary predictor. Additionally, we stratified the analysis of proportions by race category to examine whether there was a differential pattern of association between our covariates, our primary predictor, and the two racial groups. We estimated Kaplan Meier (KM) survival curves for our primary predictor stratified by racial group. We used a log-rank test to determine whether the KM survival curves differed by levels of our primary predictor and by race stratification.
We fit Cox proportional hazard regression models to determine the hazard ratios (HRs) for our primary predictor. We built two hazard models: a crude model and a model adjusted for the aforementioned risk factors. These models were fit separately for each racial group in order to estimate whether the association between the interaction of hsCRP and TG/HDL-C ratio differed by race strata. Subsequently, we used the regression coefficients of our primary predictor from each of these models (adjusted and unadjusted) to calculate three different measures of interaction in the additive scale:1) the excess risk due to interaction (RERI), 2) the proportion attributable to interaction (AP), and 3) the synergy index (SI), and their respective confidence intervals (95% CI) using the delta method [37]. Derivations of measures of interactions in the additive scale using HRs are provided in the appendix. The data were analyzed using Stata 14 statistical software and R [38].
3. Results
3.1. Cohort characteristics
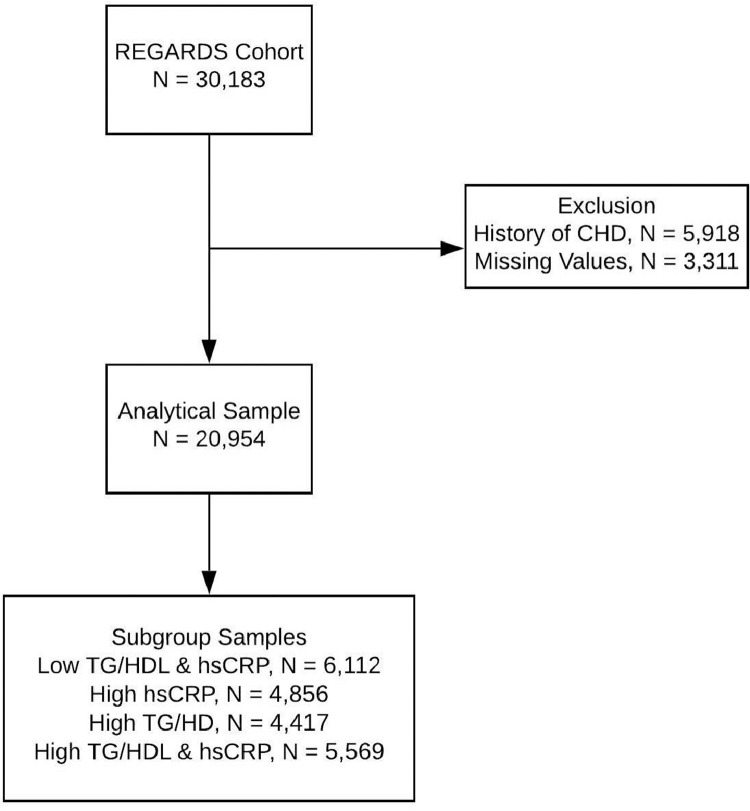
After excluding 5918 participants with baseline CHD (19.6%) and 3311 subjects with missing values in covariates (13.7%), the analytic sample included 20,954 participants (Fig. 1). Black and White participants had a comparable mean age, but Black men and women were more likely to smoke and have diabetes mellitus, to earn less than $20,000 a year, and to be overweight or obese. More than 20% of subjects in each race and sex group had abnormal levels of both TG/HDL and hsCRP at baseline (Table 1). Our primary predictor variable was formed by all four possible combinations of low/high hsCRP and low/high TG/HDLC ratio (< or >50th percentile). The HDL-C distributions were comparable for both Black and White participants, but the mean TG was lower among Black adults (Fig. 2, Supplemental Figure 1). The median TG/HDL-C cutoff effectively categorized abnormal lipid profiles: Of the men with low TG/HDL-C, 6.9% had TG>150 mg/dL or HDL-C below 40 mg/dL vs. 12.7% in women (where HDL<50 mg/dL was used) with an overall average of 10.7% (Supplemental Table S1, Supplemental Figure S2).
Fig. 1.
.
Table 1.
Baseline Characteristics of the REGARDS Cohort by race and gender.
|
Men |
Women |
|||
|---|---|---|---|---|
| Black | White | Black | White | |
| (n = 3204) | (n = 5677) | (n = 5453) | (n = 6620) | |
| Mean (SD) | ||||
| Age | 63.74 (9.13) | 64.83 (9.14) | 63.42 (9.27) | 64.10 (9.49) |
| Cholesterol, mg/dL | 186 (38.6) | 187 (35.6) | 198 (39.7) | 202 (37.3) |
| LDL-C, mg/dL | 115 (34.8) | 114 (31.9) | 120 (36.4) | 117 (33.9) |
| HDL-C, mg/dL | 48.5 (14.4) | 45.3 (13.2) | 57.4 (15.9) | 58.3 (16.3) |
| Triglycerides, mg/dL | 114 (58.4) | 136 (68.5) | 114 (48.8) | 135 (65.5) |
| hsCRP, mg/dL | 4.38 (11.9) | 3.17 (6.70) | 6.08 (8.28) | 4.24 (7.44) |
| TG/HDL-C ratio | 2.91 (4.27) | 3.77 (3.48) | 2.14 (1.97) | 2.81 (2.41) |
| Median (IQR) | ||||
| hsCRP, mg/dL | 2.06 (3.47) | 1.45 (2.56) | 3.50 (6.09) | 2.17 (3.90) |
| TG/HDL-C ratio | 2.12 (1.91) | 2.84 (2.79) | 1.70 (1.42) | 2.14 (2.09) |
| Observations (Column%) | ||||
| Income | ||||
| < $20k | 568 (17.73) | 360 (6.34) | 1581 (28.99) | 923 (13.94) |
| Education | ||||
| Less than HS | 565 (17.63) | 320 (5.64) | 1000 (18.34) | 434 (6.56) |
| Body Mass Index | ||||
| Normal | 758 (23.66) | 1435 (25.28) | 816 (14.96) | 2300 (34.74) |
| Smoking Status | ||||
| Current Smoker | 628 (19.60) | 658 (11.59) | 847 (15.53) | 859 (12.98) |
| Hypertension – Yes | 2100 (65.54) | 2642 (46.54) | 3852 (70.54) | 3014 (45.53) |
| Diabetes –Yes | 912 (28.46) | 768 (13.53) | 1439 (26.39) | 730 (11.03) |
| Aspirin Use—Yes | 1140 (35.58) | 2644 (46.57) | 1808 (33.16) | 2325 (35.12) |
| Lipid lowering Medication – Yes | 828 (25.84) | 1660 (29.24) | 1509 (27.67) | 1823 (27.54) |
| hsCRP - TG/HDL-C | ||||
| Low - Low | 1001 (31.24) | 1481 (26.09) | 1512 (27.73) | 2118 (31.99) |
| High - Low | 709 (22.13) | 596 (10.50) | 2170 (39.79) | 1381 (20.86) |
| Low - High | 669 (20.88) | 2086 (36.74) | 461 (8.45) | 1201 (18.14) |
| High - High | 825 (25.75) | 1514 (26.67) | 1310 (24.02) | 1920 (29.00) |
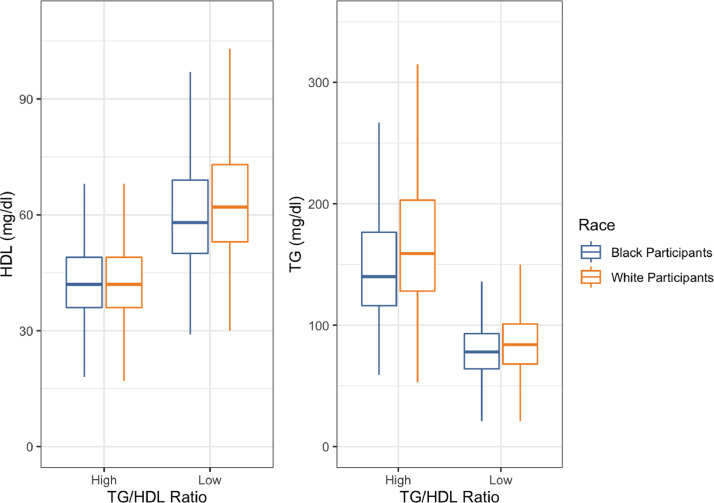
Fig. 2.
The TG and HDL-C distribution in Black and White participants. The TG/HDL-C ratio high (above median_ low (below median) plasma HDL-C (left) and TG (right) levels among Black and White adults represented as boxplots.
3.2. CHD incidence
Over a mean follow-up time of 8.91 (±3.6) years, a total of 1306 (6.23%) CHD events occurred, of which 530 (40.6%) were among Black adults and 569 (43.5%) were among women (Table S2). Black men had a higher rate of CHD and case fatality than White men (respectively, 9.48 vs 9.13 events per 1000-person years; 42.8% vs 27.3%). Similarly, CHD incidence and case fatality rates were higher for Black women vs. White women (respectively, 5.8 vs 4.9 events per 1000-person years; 41.8% vs 29.4%).
3.3. Lipids, inflammation and CHD risk
All baseline characteristics and covariates were significantly associated with the primary predictors even when stratified by race (Table 2). White (61.7%) and women (58.0%) participants were more likely to have high levels of both hsCRP and TG/HDL-C ratio. Similarly, individuals with lower education, lower income, higher BMI, higher blood pressure, higher blood glucose and currently smoking were more likely to have elevated levels of both biomarkers. Individuals with high hsCRP and high TG/HDL-C were 4 times more likely to be obese (Table 2).
Table 2.
Covariate Distributions for Primary Predictor Groups.
|
hsCRP - TG/HDL-C |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Low - Low |
High - Low |
Low - High |
High - High |
Total |
|||||||||
| Obs | % | Obs | % | Obs | % | Obs | % | Obs | % | ||||
| Age -Mean | 6112 | 64.19 | 4856 | 64.17 | 4417 | 64.15 | 5569 | 63.72 | 20,954 | 64.10 | |||
| Race | |||||||||||||
| Blacks | 2513 | 41.1 | 2879 | 59.3 | 1130 | 25.6 | 2135 | 38.3 | 8657 | 41.3 | |||
| Whites | 3599 | 58.9 | 1977 | 40.7 | 3287 | 74.4 | 3434 | 61.7 | 12,297 | 58.7 | |||
| Sex | |||||||||||||
| Female | 3630 | 59.4 | 3551 | 73.1 | 1662 | 37.6 | 3230 | 58.0 | 12,073 | 57.6 | |||
| Male | 2482 | 40.6 | 1305 | 26.9 | 2755 | 62.4 | 2339 | 42.0 | 8881 | 42.4 | |||
| Income | |||||||||||||
| < $20k | 824 | 13.5 | 981 | 20.2 | 561 | 12.7 | 1066 | 19.1 | 3432 | 16.4 | |||
| $20k - $34k | 1308 | 21.4 | 1227 | 25.3 | 983 | 22.3 | 1447 | 26.0 | 4965 | 23.7 | |||
| $35k - $74k | 1901 | 31.1 | 1383 | 28.5 | 1471 | 33.3 | 1669 | 30.0 | 6424 | 30.6 | |||
| > = $75 | 1279 | 21.0 | 679 | 14.0 | 916 | 20.7 | 742 | 13.3 | 3616 | 17.3 | |||
| Refused | 807 | 13.1 | 586 | 12.1 | 486 | 11.0 | 645 | 11.6 | 2517 | 12.0 | |||
| Education | |||||||||||||
| Less than HS | 527 | 8.6 | 667 | 14.0 | 395 | 8.9 | 730 | 13.1 | 2319 | 11.1 | |||
| HS Graduate | 1366 | 22.4 | 1278 | 26.5 | 1092 | 24.7 | 1547 | 27.8 | 5283 | 25.2 | |||
| Some College | 1581 | 25.9 | 1351 | 28.0 | 1157 | 26.2 | 1590 | 28.6 | 5679 | 27.1 | |||
| College Graduate | 2638 | 43.1 | 1560 | 31.5 | 1773 | 40.2 | 1702 | 30.5 | 7673 | 36.6 | |||
| Body Mass Index | |||||||||||||
| Normal | 2609 | 42.6 | 1002 | 20.6 | 945 | 21.4 | 753 | 13.5 | 5309 | 25.3 | |||
| Overweight | 2316 | 38.0 | 1609 | 33.1 | 2052 | 46.4 | 1771 | 31.8 | 7748 | 37.0 | |||
| Obese | 1187 | 19.4 | 2245 | 46.3 | 1420 | 32.2 | 3045 | 54.7 | 7897 | 37.7 | |||
| Smoking Status | |||||||||||||
| Current Smoker | 673 | 11.0 | 696 | 14.3 | 537 | 12.2 | 1086 | 19.5 | 2992 | 14.3 | |||
| Non-Smoker | 5439 | 89.0 | 4160 | 85.7 | 3880 | 87.8 | 4483 | 80.5 | 17,962 | 85.8 | |||
| Hypertension | |||||||||||||
| Yes | 2787 | 45.6 | 2891 | 59.5 | 2329 | 52.7 | 3601 | 64.7 | 11,608 | 55.4 | |||
| No | 3325 | 54.4 | 1965 | 40.5 | 2088 | 47.3 | 1968 | 35.3 | 9346 | 44.6 | |||
| Diabetes | |||||||||||||
| Yes | 715 | 11.7 | 847 | 17.4 | 829 | 18.8 | 1458 | 26.2 | 3849 | 18.4 | |||
| No | 5397 | 88.3 | 4009 | 82.6 | 3588 | 81.2 | 4111 | 73.8 | 17,105 | 81.6 | |||
| Aspirin Use | |||||||||||||
| Yes | 2254 | 36.9 | 1645 | 33.9 | 1876 | 42.5 | 2142 | 38.5 | 7917 | 37.8 | |||
| No | 3858 | 63.1 | 3211 | 66.1 | 2541 | 57.5 | 3427 | 61.5 | 13,037 | 62.2 | |||
| Lipid Lowering Medication | |||||||||||||
| Yes | 1547 | 25.3 | 1159 | 23.9 | 1496 | 33.9 | 1618 | 29.1 | 5820 | 27.8 | |||
| No Missing |
4512 53 |
73.8 0.9 |
3643 54 |
75.0 1.1 |
2873 48 |
65.0 1.1 |
3887 64 |
69.8 1.2 |
14,915 219 |
71.2 1.1 |
|||
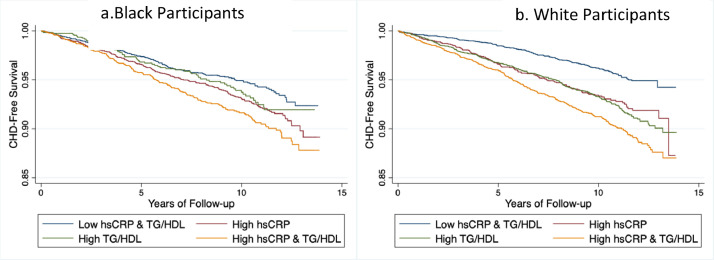
CHD-free survival for individuals with combined high levels of hsCRP and TG/HDL-C was lower than for individuals with either isolated high hsCRP or high TG/HDL-C or with low levels of both markers (Figs. 3a & 3b), these findings were significant for both Black (log-rank test, χ2 =1 8.4, p<0.001) and White participants (log-rank test, χ2 = 71.3, p<0.001)
Fig. 3.
Kaplan Meier curves indicating survival free of CHD or death by levels of hsCRP and TG/HDL-C ratio among Black adults (a) and White adults (b), REGARDS using baseline (2003–2007) samples. The low and high groups were determined by median thresholds for their respective phenotypes.
In the unadjusted analysis, individuals with elevated hsCRP or TG/HDL-C ratio (when compared to individuals with low levels) had increased risk of CHD (HR 1.56; 95% CI 1.33, 1.85 vs HR 1.55; 95% CI 1.31, 1.83). Having both biomarkers elevated (compared with neither) increased the risk for CHD further (HR 2.03; 95% CI 1.74, 2.37). The association was maintained after adjusting for age, race, sex, income, education, hypertension, BMI, diabetes, and aspirin use (HR 1.69; 95% CI: 1.44, 1.99). The adjusted CHD risk was significantly increased for participants who had high hsCRP (HR 1.57 95% CI: 1.33, 1.86) or high TG/HDL-C ratio (HR: 1.28, 95% CI: 1.08, 1.52). To understand the synergistic contribution of both hsCRP and TG/HDL-C ratio to CHD risk we calculated the measures of interaction for unadjusted and adjusted models on the additive scale using the Relative Excess risk due to Interaction (RERI), the Attributable Proportion (AP), and the Synergy Index (SI). In the unadjusted and adjusted analysis there was no evidence of a synergistic interaction, thus suggesting that the two phenotypes influence CHD risk in an additive manner (Table 3).
Table 3.
Fatal and nonfatal acute coronary heart disease events.
| hsCRP | TG:HDL-C Ratio | Number of individuals (%) | Hazard RatioUn-adjusted | Hazard RatioAdjusted* |
|---|---|---|---|---|
| Low | Low | 6112 (29.2%) 4856 (23.2%) 4417 (21.0%) 5569 (26.6%) |
1.00 (Reference) | 1.00 (Reference) |
| High | Low | 1.56 (1.33, 1.85) | 1.57 (1.33, 1.86) | |
| Low | High | 1.55 (1.31, 1.83) | 1.28 (1.08, 1.52) | |
| High | High | 2.03 (1.74, 2.37) | 1.69 (1.44, 1.99) | |
| Observations | 20,954 | 20,954 | 20,954 | |
| Measures of Interaction in the Additive Scale | Un-adjusted | Adjusted | ||
| Excess Risk due to Interaction (RERI) Attributable Proportion (AP) Synergy Index (SI) |
−0.08 (−0.42, 0.26) | −0.18 (−0.50, 0.13) | ||
| −0.04 (−0.21, 0.13) | −0.11 (−0.30, 0.08) | |||
| 0.93 (0.68, 1.23) | 0.77 (0.53, 1.14) | |||
| hsCRP | TG:HDL-C Ratio | Number of individuals (%) | Hazard RatioUn-adjusted | Hazard RatioAdjusted* |
|---|---|---|---|---|
| Low | Low | 6112 (29.2%) 4856 (23.2%) 4417 (21.0%) 5569 (26.6%) |
1.00 (Reference) | 1.00 (Reference) |
| High | Low | 1.56 (1.33, 1.85) | 1.57 (1.33, 1.86) | |
| Low | High | 1.55 (1.31, 1.83) | 1.28 (1.08, 1.52) | |
| High | High | 2.03 (1.74, 2.37) | 1.69 (1.44, 1.99) | |
| Observations | 20,954 | 20,954 | 20,954 | |
| Measures of Interaction in the Additive Scale | Un-adjusted | Adjusted | ||
| Excess Risk due to Interaction (RERI) Attributable Proportion (AP) Synergy Index (SI) |
−0.08 (−0.42, 0.26) | −0.18 (−0.50, 0.13) | ||
| −0.04 (−0.21, 0.13) | −0.11 (−0.30, 0.08) | |||
| 0.93 (0.68, 1.23) | 0.77 (0.53, 1.14) | |||
*Model adjusted for race, sex, age, income, education, smoking status, hypertension, use of aspirin, BMI category, and diabetes status. Total events include fatal, and nonfatal Acute Coronary Heart Disease (CHD) events.
The race stratified analysis recapitulated the patterns observed in the entire cohort for hsCRP but not for TG/HDL-C ratio (Table 4). White participants with elevated levels of both biomarkers had adjusted risk (HR 1.84; 95% CI 1.48, 2.27) greater than those with either isolated high hsCRP (HR 1.68; 95% CI 1.31, 2.15) or isolated high TG/HDL ratio (HR 1.44; 95% CI 1.15, 1.79). Black participants with high hsCRP and high TG/HDL-C ratio had an increased risk of CHD (HR 1.52; 95% CI 1.19, 1.94) relative to those with both parameters in the low range. However, while isolated elevated hsCRP was associated with increased CHD risk (HR 1.43, 95% CI 1.13, 1.81), isolated elevated TG/HDL-C ratio was not (HR 1.01; 95% CI 0.74, 1.38). The measures of interactions between hsCRP and TG/HDL-C did not reveal greater than additive interactions, with the caveat that isolated high TG/HDL-C ratio was not associated with CHD in Black REGARDS participants (Table 4).
Table 4.
Cox regression models stratified by race.
|
Models for Blacks |
Models for Whites |
||||
|---|---|---|---|---|---|
| hsCRP | TG:HDLRatio | Hazard RatioUn-adjusted | Hazard RatioAdjusted+ | Hazard RatioUn-adjusted | Hazard RatioAdjusted+ |
| Low | Low | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| High | Low | 1.34 (1.07, 1.68) | 1.43 (1.13, 1.81) | 1.72 (1.35, 2.20) | 1.68 (1.31, 2.15) |
| Low | High | 1.16 (0.86, 1.57) | 1.01 (0.74, 1.38) | 1.84 (1.49, 2.27) | 1.44 (1.15, 1.79) |
| High | High | 1.64 (1.29, 2.07) | 1.52 (1.19, 1.94) | 2.38 (1.94, 2.91) | 1.84 (1.48, 2.29) |
| Observations | 8657 | 8657 | 12,297 | 12,297 | |
| Measures of Interaction in the Additive Scale | |||||
| RERI | 0.14 (−0.32, 0.59) |
0.06 (−0.37, 0.48) |
−0.19 (−0.70, 0.33) |
−0.30 (−0.76, 0.17) |
|
| AP | 0.08 (−0.19, 0.36) | 0.04 (−0.25, 0.33) | −0.08 (−0.29, 0.14) | −0.16 (−0.42, 0.91) | |
| SI | 1.28 (0.51, 3.16) | 1.14 (0.40, 3.29) | 0.88 (0.63, 1.23) | 0.73 (0.48, 1.12) | |
Total including fatal, and nonfatal CHD events.
Stratified model by race adjusted for sex, age, income, education, smoking status, hypertension, use of aspirin, BMI category, and diabetes status. 95% CI in parenthesis.
3.4. Sensitivity analyses
To understand if the median cutoff is a viable strategy we performed sensitivity analyses with 20,735 participants. The sensitivity model contains use of lipid lowering medication in addition to main covariates. Coronary events were more prevalent among participants with both elevated biomarkers (n = 471 or 36.4%), hazard ratios and measures of interaction in the additive scale were comparable to main models (Supplementary material, Table S2). Race stratified sensitivity analysis showed that the risk of CHD is greater for Black and White adults with high levels of both biomarkers. However, high TG/HDL-C ratio associated with CHD positively in White participants only, not in Black participants (Supplementary material, Table S3).
In our stratified analysis, point estimates of the measures of interaction in the additive scale were positive for Black individuals (RERI = 0.18, AP = 0.11, SI = 1.46) suggesting biological synergism (Supplementary material, Table S4). Because, systemic inflammation and dyslipidemia can widely differ by race and sex, we built two additional Cox models with cutoff points for hsCRP and TG/HDL-C ratio based on the median and 75th percentile of the race-sex distribution of each biomarker (Supplementary material, Table S5). These models showed comparable patterns to the main model, highlighting the double biomarker exposure as the highest risk group, followed by the high hsCRP group (Supplementary material, Table S6). Measures of interaction in the additive scale were not different between the cutoff specific models and our main model (Supplementary material, Table S6). The median cutoff point model yield point estimates with larger effect size than the 75th cutoff point model suggesting that dichotomizing our biomarkers at the median has more predictive value than at a higher percentile point.
To evaluate the effect of LDL-C, we further adjusted the Cox model in Table 4 and found that adjustment by LDL-C did not change the point estimates and the overall results (Supplementary material, Table S7). We also further explored the role of using lipid lowering medication in our main association of interest by fitting the Cox regression model among those who used lowering lipid medications and stratifying by race (Supplementary material, Table S8 and S9)
4. Discussion
We evaluated the associations of the TG/HDL-C ratio and hsCRP with incident CHD over a mean follow-up of 8.91 years in 20,954 participants from the biracial REGARDS cohort. Independent of race, participants with high hsCRP had increased CHD risk only in White adults, no association was seen in Black adults. Overall, the combined effect of both risk factors (e.g., TG/HDL-C ratio and hsCRP) is no more than what is expected when adding the effect of each risk factor alone.
The TG/HDL-C ratio has been shown to be a viable surrogate of the atherogenic lipid phenotype characterized by elevated levels of remnant lipoproteins (included in non-HDL-C), low HDL-C, and reduced LDL particle size [39]. In many cohorts the biologically linked pair of high triglycerides and low HDL-C was found strongly associated with cardiovascular risk [40], [41], [42], [43]. Although one might argue that creating a ratio of dichotomized risk factors at their median could result in the loss of statistical power and loss of efficiency relative to an ungroup analysis [44], in our REGARDs cohort the median TG/HDL-C ratio cutoff only missed 10.7% of participants with either isolated low HDL-C (<40 mg/dL for males and <50 mg/dL for females), isolated high TG (>150 mg/dL), or combination of both.
Currently, there is no agreement on the normal cut-off threshold for the TG/HDL-C ratio. For example, the dyslipidemia of metabolic syndrome as defined by ATP-III criteria uses thresholds of TG <150 mg/dL and HDL-C >40 mg/dL (men) and >50 mg/dL (women) [2] corresponding to TG/HDL-C ratios of 3.75 and 3.0, respectively. Studies by Sumner et al. using the threshold of 3.0 showed that TG/HDL-C ratio may be useful to predict insulin resistance among Whites but not Blacks [45]. Lower and race specific thresholds of 2.5 for men and 2.0 for women have also been recommended [41,46]. Studies by McLaughlin et al. indicated that a level of 3.5 may be useful in identifying individuals at increased risk of developing CVD [15,47].
In REGARDs the median TG/HDL-C ratio was 2.45 for White adults, 1.85 for Black adults, and 2.17 for the full cohort. The lower TG/HDL-C ratio for Black participants is contributed by lower TG (in men and women) and higher HDL (in men only). A TG/HDL-C ratio above the median was associated with 1.44 higher risk of CHD in White but not in Black participants. The lack of association among Black individuals suggests that the TG/HDL-C ratio alone may not be a marker of CHD risk in this group, or that the predictive value is triggered after a specific threshold rather than showing linear association with risk.
A recent study has surprisingly shown that very low HDL-C levels (<30 mg/dL) are associated with reduced risk of incident CHD in Black adults [48]. In our analysis these cases are captured with the high TG/HDL-C ratio group which showed no association with CHD risk. It is possible that the strength of the association between high TG/HDL-C ratio and CHD risk is tempered by an unusual association between very low HDL-C and CHD risk in Black adults.
In REGARDS, high hsCRP levels were not only independently associated with CHD risk in both Black and White adults, but also were the driving variable in the joint association of elevated TG/HDL-C ratio and elevated hsCRP with CHD risk. For example, the difference in risk between participants with elevation of both biomarkers vs. participants with only high levels of hsCRP was estimated to be 1.08 (95% CI: 0.87, 1.34) and 1.03 (95%CI: 0.83, 1.27) among White and Black individuals, respectively. However, the difference in risk between participants with elevation of both biomarkers vs. participants with only high levels of TG/HDL-C was estimated to be 1.26 (95%CI: 1.06, 1.51) and 1.49 (95%CI: 1.11, 2.00) among White and Black individuals, respectively. Our findings are consistent with results from the CANTOS trial [49]. Canakinumab significantly reduced hsCRP levels from baseline, without reducing the LDL cholesterol level, and the 150-mg dose decreased the incidence of recurrent cardiovascular events. While this trial was not designed to look at race-specific effects (only around 3% of participants were Black), these findings and ours support the idea that strategies targeting inflammation may reduce ASCVD risk irrespective of race.
The point estimates for the measures of additive interaction seemed to differ by race. Calculated measures of interaction between inflammation and dyslipidemia for White participants showed an additive rather than synergistic interaction. However, these measures were weaker in Black individuals, probably due to the lack of association between high TG/HDL-C ratio and CHD risk. Due to the wide confidence intervals for the additive measures of interaction, we do not interpret these apparent differences as effect modification by race. Rather, our results show that, for both racial groups the effect of increased levels of inflammation on CHD risk is greater than the effect of an adverse lipid profile.
Due to the triglyceride-lowering effect of statins, statins use may have had an impact on our findings. In REGARDS, the medication use is self-reported and statin use is categorized as lipid lowering medication which likely includes other medications. The analysis of the subcohort with only participants that use a lipid lowering medication revealed significant disparities among White and Black adults: Among 5728 adults on medication, 2315 (~40%) were Black participants. In addition, the CHD event was 410 (169 for Black adults and 241 for White adults) –much lower than the entire cohort, 1306 (530 for Black adults and 776 for White adults) (Supplemental Table S8). Despite the lower power, the associations between higher inflammation, adverse lipid profiles and greater CHD risk were maintained for White adults. However, since the sample size for Black adults was smaller than for White adults –in particular with regard to CHD events, the model for Black adults should be interpreted with caution.
Hypertension was defined according to blood pressure guidelines that were in effect for the majority of the study period —2002–2016— as above 140 and 90 mmHg for diastolic and systolic blood pressure (ACC/AHA 2003).
The time between inflammation and cholesterol measures and the CHD event, the self-reporting of some risk factors and co-morbidities, and the use of BMI as an obesity measure are limitations of our study. Smoking status was self-reported at the telephone interview and classified as dichotomous variable (smoker or non- smoker) which misses information on former smokers. The risk models in clinical practice use 10-year predicted risk to determine interventions to reduce CHD risk, so while a limitation our approach reflects clinical practice [50]. The observational design limits the ascertained CHD incidence, which might result in spuriously lower rates.
Collectively, our data suggest that dyslipidemia expressed as elevated ratio of TG/HDL-C and inflammation assessed as elevated hsCRP have additive power in predicting CHD risk in White adults but not in Black adults. While hsCRP was comparably associated with CHD risk in both races, the TG/HDL ratio did not predict CHD risk in Black individuals. Further studies are needed to elucidate the mechanisms responsible for the lack of association between the ratio of TG/HDL-C in Black adults and to determine if incorporating inflammation in CHD risk prediction especially in Black patients improves clinical outcomes. In the meantime, providers should be aware that the TG/HDL-C ratio is not an informative proxy for increased atherogenicity in Black patients.
Author contributions
CH: Data analysis, initial draft
JM: Study design, Supervising the data analysis, data interpretation
SF, MMS, LDC, MRI, VH: Critical review and editing
NAZ: Data interpretation, study design, critical review and editing
NP: Study design, data interpretation, manuscript writing
Declaration of Competing Interest
There are no conflicts to disclose for all authors.
Acknowledgments
Acknowledgments
We would like to thank Dr. Bart Duell for critical review of the manuscript.
Funding
NP, NAZ, and JM were supported by R01HL136373. SF was supported by NIH grant R01HL132985. This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at: https://www.uab.edu/soph/regardsstudy/
Footnotes
Subject Codes: Lipids and Cholesterol, race, inflammation, CHD risk
Disclosure summary: All authors have nothing to disclose
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2021.100198.
Appendix. Supplementary materials
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Detection T., on of in. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25) 3143–3143. [PubMed] [Google Scholar]
- 3.Frank A.T.H., Zhao B., Jose P.O., Azar K.M.J., Fortmann S.P., Palaniappan L.P. Racial/ ethnic differences in dyslipidemia patterns. Circulation. 2014;129(5):570–579. doi: 10.1161/CIRCULATIONAHA.113.005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2020;139(10):e56–e66. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 5.Chapman J.M., Ginsberg H.N., Amarenco P., Andreotti F., Borén J., Catapano A.L. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collaborators C. Mihaylova B., Emberson J., Blackwell L., Keech A., Simes J. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet Lond Engl. 2012;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborators C. Baigent C., Blackwell L., Emberson J., Holland L., Reith C. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hokanson J., Austin M. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–219. [PubMed] [Google Scholar]
- 9.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S. Triglycerides and the risk of coronary heart disease. Circulation. 2007;115(4):450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 10.Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 11.Ballantyne C., Olsson A., Cook T., Mercuri M., Pedersen T., Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104(25):3046–3051. doi: 10.1161/hc5001.100624. [DOI] [PubMed] [Google Scholar]
- 12.Manninen V., Tenkanen L., Koskinen P., Huttunen J., Mänttäri M., Heinonen O. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 2018;85(1):37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 13.Assmann G., Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience) Am J Cardiol. 1992;70(7):733–737. doi: 10.1016/0002-9149(92)90550-i. [DOI] [PubMed] [Google Scholar]
- 14.Dobiásová M., Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin Biochem. 2001;34(7):583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin T., Reaven G., Abbasi F., Lamendola C., Saad M., Waters D. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96(3):399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 16.Hanak V., Munoz J., Teague J., Stanley A., Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94(2):219–222. doi: 10.1016/j.amjcard.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 17.Jia L., Long S., Fu M., Yan B., Tian Y., Xu Y. Relationship between total cholesterol/high-density lipoprotein cholesterol ratio, triglyceride/high-density lipoprotein cholesterol ratio, and high-density lipoprotein subclasses. Metabolis. 2006;55(9):1141–1148. doi: 10.1016/j.metabol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Gaziano M.J., Hennekens C.H., O'Donnell C.J., Breslow J.L., Buring J.E. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96(8):2520–2525. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 19.Shishehbor M., Hoogwerf B. Lauer. Association of triglyceride-to-HDL cholesterol ratio with heart rate recovery. Diabetes Care. 2004;27(4):936–941. doi: 10.2337/diacare.27.4.936. [DOI] [PubMed] [Google Scholar]
- 20.Frohlich J., Dobiášová M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49(11):1873–1880. doi: 10.1373/clinchem.2003.022558. [DOI] [PubMed] [Google Scholar]
- 21.Drexel H., Aczel S., Marte T., Benzer W., Langer P., Moll W. Is atherosclerosis in diabetes and impaired fasting glucose driven by elevated LDL cholesterol or by decreased HDL cholesterol? Diabetes Care. 2004;28(1):101–107. doi: 10.2337/diacare.28.1.101. [DOI] [PubMed] [Google Scholar]
- 22.Bittner V., Johnson D.B., Zineh I., Rogers W.J., Vido D., Marroquin O.C. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia. Am Heart J. 2009;157(3):548–555. doi: 10.1016/j.ahj.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeppesen J., Hein H.O., Suadicani P., Gyntelberg F. Low triglycerides–high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Intern Med. 2001;161(3):361. doi: 10.1001/archinte.161.3.361. [DOI] [PubMed] [Google Scholar]
- 24.Barzi F., Patel A., Woodward M., Lawes C.M.M., Ohkubo T., Gu D. A comparison of lipid variables as predictors of cardiovascular disease in the Asia Pacific region. Ann Epidemiol. 2005;15(5):405–413. doi: 10.1016/j.annepidem.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Ridker P.M. The JUPITER trial: results, controversies, and implications for prevention. Circul Cardiovasc Qual Outcomes. 2009;2(3):279–285. doi: 10.1161/CIRCOUTCOMES.109.868299. [DOI] [PubMed] [Google Scholar]
- 26.Tardif J.-.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P. Efficacy and safety of low-dose colchicine after myocardial infarction. New Engl J Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 27.Ridker P.M., MacFadyen J.G., Everett B.M., Libby P., Thuren T., Glynn R.J. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 28.Darroch J. Biologic synergism and parallelism. Am J Epidemiol. 1997;145(7):661–668. doi: 10.1093/oxfordjournals.aje.a009164. [DOI] [PubMed] [Google Scholar]
- 29.Howard V.J., Woolson R.F., Egan B.M., Nicholas J.S., Adams R.J., Howard G. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens. 2010;4(1):32–41. doi: 10.1016/j.jash.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard V.J., Cushman M., Pulley L., Gomez C.R., Go R.C., Prineas R.J. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 31.Colantonio L.D., Gamboa C.M., Kleindorfer D.O., Carson A.P., Howard V.J., Muntner P. Stroke symptoms and risk for incident coronary heart disease in the reasons for geographic and racial differences in stroke (REGARDS) study. International Journal Cardiology. 2016;220(Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:122–128. doi: 10.1016/j.ijcard.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillett S.R., Boyle R.H., Zakai N.A., McClure L.A., Jenny N.S., Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47(16–17):243–246. doi: 10.1016/j.clinbiochem.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safford M.M., Brown T.M., Muntner P.M., Durant R.W., Glasser S., Halanych J.H. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safford M.M., Gamboa C.M., Durant R.W., Brown T.M., Glasser S.P., Shikany J.M. Race-sex differences in the management of hyperlipidemia: the reasons for geographic and racial differences in stroke study. Am J Prev Med. 2020;48(5):520–527. doi: 10.1016/j.amepre.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luepker R.V., Apple F.S., Christenson R.H., Crow R.S., Fortmann S.P., Goff D. Case definitions for acute coronary heart disease in epidemiology and clinical research studies. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 36.Thygesen K., Alpert J.S., White H.D., Jaffe A.S., Apple F.S. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. Infarction J for the of. [DOI] [PubMed] [Google Scholar]
- 37.Assmann S.F., Hosmer D.W., Lemeshow S., Mundt K.A. Confidence intervals for measures of interaction. Epidemiology. 1996;7(3):286–290. doi: 10.1097/00001648-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Team C.R. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. null.
- 39.Quispe R., Manalac R.J., Faridi K.F., Blaha M.J., Toth P.P., Kulkarni K.R. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: the Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis. 2015;242(1):243–250. doi: 10.1016/j.atherosclerosis.2015.06.057. [DOI] [PubMed] [Google Scholar]
- 40.Jeppesen J., Hein H., Suadicani P., Gyntelberg F. Relation of high TG–low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease: an 8-year follow-up in the Copenhagen male study. Arterioscler Thrombosis Vasc Biol. 1997;17(6):1114–1120. doi: 10.1161/01.atv.17.6.1114. [DOI] [PubMed] [Google Scholar]
- 41.Miller M., Stone N.J., Ballantyne C., Bittner V., Criqui M.H., Ginsberg H.N. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 42.da Luz P, D Favarato, Junior J., Lemos P., Chagas A. High ratio of triglycerides to hdl-cholesterol predicts extensive coronary disease. Clinics. 2008;63(4):427–432. doi: 10.1590/S1807-59322008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sampson U.K., Fazio S., Linton M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2011;14(1):1–10. doi: 10.1007/s11883-011-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Royston P., Altman D.G., Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2005;25(1):127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 45.Sumner A.E., Finley K.B., Genovese D.J., Criqui M.H., Boston R.C. Fasting triglyceride and the triglyceride–HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med. 2005;165(12):1395. doi: 10.1001/archinte.165.12.1395. [DOI] [PubMed] [Google Scholar]
- 46.Sumner A.E., Zhou J., Doumatey A., Imoisili O.E., Amoah A., Acheampong J. Low HDL-cholesterol with normal triglyceride levels is the most common lipid pattern in West Africans and African Americans with Metabolic Syndrome: implications for cardiovascular disease prevention. Cvd Prev Control. 2010;5(3):75–80. doi: 10.1016/j.cvdpc.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaughlin T., Abbasi F., Cheal K., Chu J., Lamendola C., Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 48.Penson P., Long L.D., Howard G., Howard V.J., Jones S.R., Martin S.S. Associations between cardiovascular disease, cancer, and very low high-density lipoprotein cholesterol in the reasons for geographical and racial differences in stroke (REGARDS) study. Cardiovasc Res. 2018;115(1):204–212. doi: 10.1093/cvr/cvy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 50.Damen J.A., Pajouheshnia R., Heus P., Moons K.G., Reitsma J.B., Scholten R. Performance of the Framingham risk models and pooled cohort equations for predicting 10-year risk of cardiovascular disease: a systematic review and meta-analysis. Bmc Med. 2019;17(1):109. doi: 10.1186/s12916-019-1340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.