Abstract
Objectives:
The aim of this study was to assess the utilization of low tube potentials for coronary computed tomography angiography (CCTA) in worldwide clinical practice and its influence on radiation exposure, contrast agent volume and image quality.
Background:
CCTA is frequently employed in clinical practice. Lowering of tube potential is a potent method to reduce radiation exposure and to economize contrast agent volume.
Methods:
CCTAs of 4,006 patients from 61 international study sites were analyzed regarding very-low (≤80-kVp), low (90–100-kVp), conventional (110–120-kVp) and high (≥130-kVp) tube potentials. The impact on dose-length product (DLP) and contrast agent volume was analyzed. Image quality was determined by evaluation of the diagnostic applicability and assessment of the objective image parameters signal-to-noise-ratio (SNR) and contrast-to-noise-ratio (CNR).
Results:
When compared to conventional tube potentials, low tube potentials were used in 56% of CCTAs (≤80-kVp: 9%; 90–100-kVp: 47%), which varied among sites from 0% to 100%. Tube potential reduction was associated with low cardiovascular risk profile, low body-mass-index (BMI) and new-generation scanners. Median radiation exposure was lowered by 68% or 50%, and median contrast agent volume by 25% or 13% for tube potential protocols of ≤80-kVp or 90–100-kVp when compared to conventional tube potentials, respectively (all p<0.001). With the use of lower tube potentials, the frequency of diagnostic scans was maintained (p=0.41), while SNR and CNR significantly improved (both p<0.001). Considering BMI eligibility criteria, 58% (n=946) conventionally scanned patients would have been suitable for low tube potential protocols, and 44% (n=831) of patients scanned with 90–100-kVp would have been eligible for very-low tube potential CCTA imaging of ≤80-kVp.
Conclusions:
This large international registry confirms the feasibility of tube potential reduction in clinical practice leading to lower radiation exposure and lower contrast volumes. The current registry also demonstrates that this strategy is still underutilized in daily practice.
Keywords: Coronary Computed Tomography Angiography, CCTA, Cardiac imaging, Radiation dose, Tube potential, Dose-saving strategies
Introduction
Coronary CT angiography (CCTA) has emerged as favorable diagnostic tool with high accuracy in the detection and exclusion of obstructive coronary artery disease (1–3). Furthermore, its use has been associated with a significant reduction in mortality and non-fatal myocardial infarction (4). The downside of CCTA imaging is the adverse exposition to potentially harmful ionizing radiation (5). The safety of CCTA has improved considerably during the last decade and median radiation exposure decreased by 78% as recently described in the PROTECTION VI study (6). Reduction in tube potentials from the conventional 120 kVp is a major contributor to this dose reduction. Several studies demonstrated the feasibility of a reduced potential to 100 kVp with maintenance of image quality (7–10). Additional experimental studies with limited number of patients suggested very-low tube potentials down to 80 kVp or below with maintenance of diagnostic value and quantitative image quality parameters (11–14). The body mass index (BMI) has been acknowledged as an eligibility criterion for the application of reduced tube potentials in CCTA. In this regard, patients with a BMI below 30 kg/m2 should be selected for tube potential reduction from conventional 120 kVp to 100 kVp (10,15). The possibility of tube potential reduction in obese patients with a BMI above 30 kg/m2 has also been described, but only in small patient populations (16). An escalation of low tube potential imaging towards 80 kVp or below was proposed in several studies for patients with a BMI below 25 kg/m2 (12–14,17,18). However, the frequency in application and the magnitude of tube potential reduction in worldwide clinical practice are currently unknown. This predefined sub-analysis of the international, prospective, multicenter PROTECTION VI study has been designed to analyze the application of tube potential reduction protocols and their impact on radiation dose, contrast agent volume, and image quality in CCTA imaging.
Methods
Study protocol
The methods of the PROTECTION VI study were described previously (19). In brief, 61 international study sites provided image data and scan protocols of consecutive CCTAs performed during 1 month in 2017. Image data and CCTA study details were collected and analyzed in a central CCTA core laboratory. The selection of the CCTA scan protocol including the tube potential and the volume of iodinated contrast medium was at the discretion of the performing physician and was carried out according to local standard of clinical care. Each study site consulted the local ethics committee to evaluate the study protocol prior to patient enrolment. All patients gave written informed consent as required at the individual sites. An Executive Steering Committee composed of a group of physicians with expertise in CCTA, clinical research and statistics supervised the study. The study is registered at clinicaltrials.gov (NCT02996903).
Image quality
For standardization of quantitative image quality analysis, the axial data sets were reformatted in 1.0 mm slice thickness. Signal intensity, image noise, contrast, signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were determined in a central core laboratory as described (20). Local investigators graded the diagnostic image quality using a simplified image quality score. Each coronary artery (left main, left anterior descending, left circumflex, and right coronary artery) was determined to be of either diagnostic or non-diagnostic image quality. Non-diagnostic quality was defined by severe vessel blurring or vessel discontinuity secondary to reconstruction artifacts, which did not allow the exclusion of obstructive coronary lesions. CCTAs were considered as non-diagnostic when at least one coronary artery was of non-diagnostic image quality.
Estimation of radiation dose
The collected parameters relevant to radiation dose included the volume CT dose index (CTDIvol) and dose-length product (DLP), which were both obtained from the CT scan protocol. The DLP was the primary study outcome parameter. The calculation of the effective dose is based on the product of the DLP and an organ weighting factor for the chest k = 0.014 mSv/mGy x cm (21) or alternatively a proposed conversion factor for CCTA imaging k = 0.026 mSv/mGy x cm (22).
Statistical analysis
Variables are expressed as counts with percentages or medians with interquartile ranges. Comparison of groups was performed with the Wilcoxon-Mann-Whitney U-test or chi-square test as appropriate. Multiple comparisons of non-parametric variables were conducted with the Kruskal-Wallis chi-squared test, subsequent post-hoc analysis was performed with the Dunn’s test and p values were adjusted with the Holm method. A p-value < 0.05 was considered to be statistically significant. Statistical analysis was performed using R version 3.4.1.
Results
CCTA tube potential selection in current clinical routine
In the PROTECTION VI study, a total of 4,006 patients from 61 different study sites underwent CCTA. Among these, 377 patients (9%) were scanned with a very-low tube potential protocol of ≤ 80 kVp, and 1,889 patients (47%) were scanned with a reduced tube potential of 90–100 kVp (Figure 1). Conventional tube potential of 110–120 kVp was selected for 1,662 patients (42%) and tube potential was increased (≥ 130 kVp) in 78 cases (2%). The selection of reduced tube potential protocols varied significantly between study sites (Figure 1). Accordingly, the selection of reduced tube potential ≤ 100 kVp ranged from 0% to 100% of CCTAs in study sites. On the one end, five study sites (8% of all sites) scanned the majority of patients (> 50%) with a very-low tube potential of ≤ 80 kVp. On the other end, 28 study sites (47% of sites) exclusively scanned > 80 kVp.
Central illustration, Figure 1: Application of tube potential protocols in current clinical routine CCTA imaging.
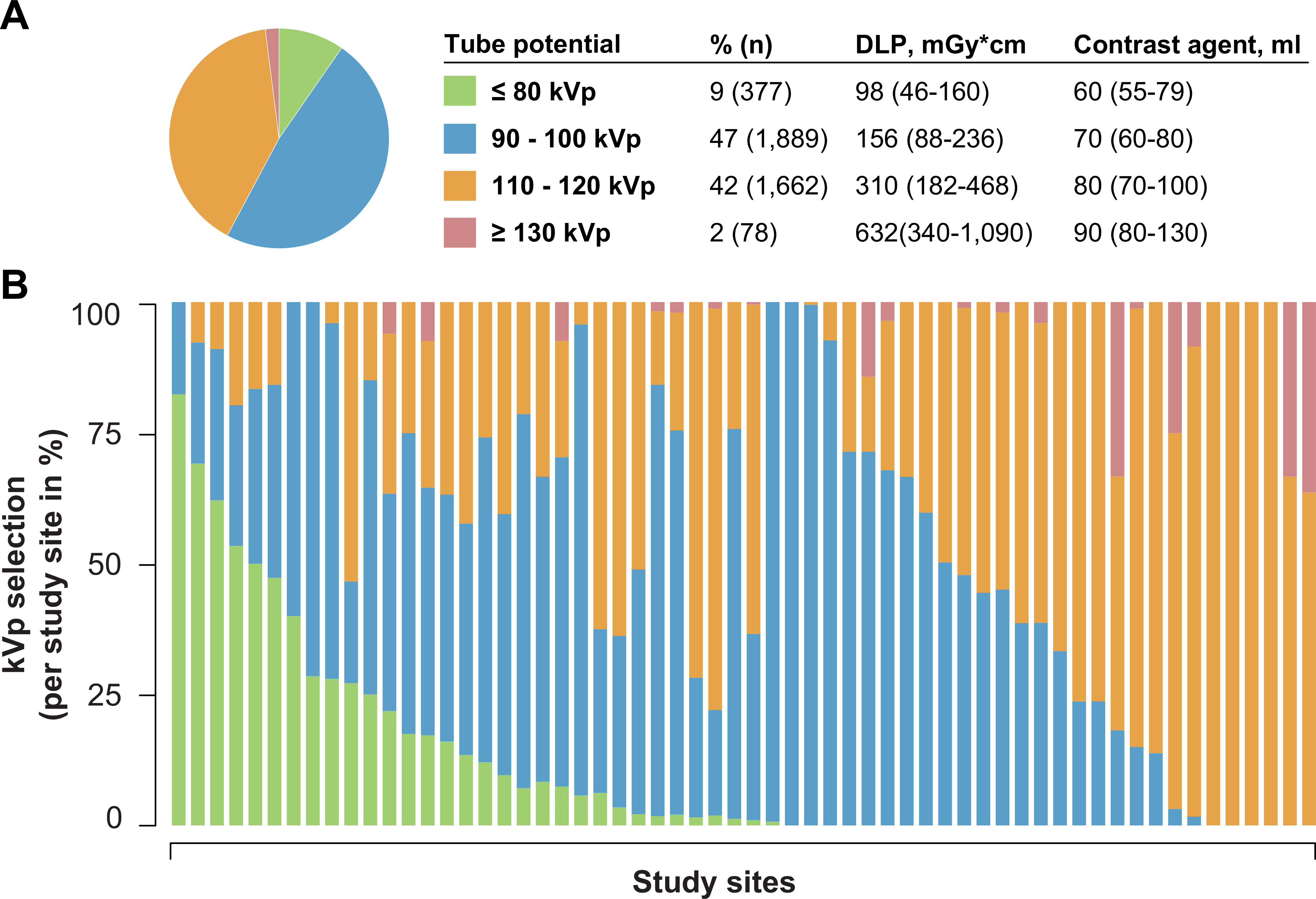
A: Frequency of different tube potential protocols aggregated to ≤ 80-kVp, 90–100-kVp, 110–120-kVp and ≥ 130-kVp. B: Variation of tube potential protocol selection per study site.
The application of tube potential reduction varied between vendors (Figure 2). Low tube potential protocols of 90–100 kVp were less frequently utilized with GE scanners (42% of CCTAs), when compared to all other vendors (Toshiba: 45%, Philips: 49%, Siemens: 50%; p < 0.05). The rate of very-low tube potential imaging of ≤ 80-kVp was significantly higher in Siemens scanners (17% of CCTAs) when compared to all other vendors (GE: 1%, Philips: 3%, Toshiba: 4%; p < 0.001). Regional differences in the application of low tube potential protocols are listed in Supplementary Table S1.
Figure 2: Tube potential protocol selection by CT vendor.
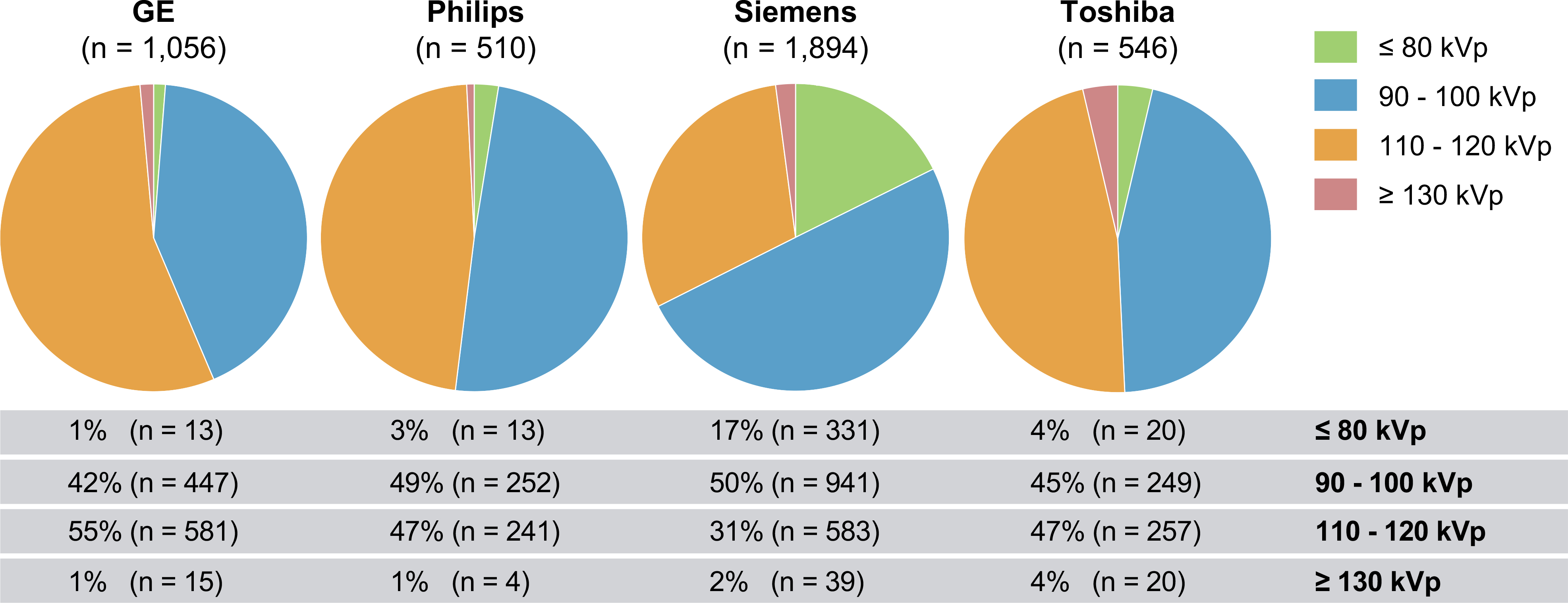
GE: General Electric Healthcare, Waukesha, Wisconsin. Philips: Philips Healthcare, Amsterdam, Netherlands. Siemens: Siemens Medical Solutions, Forchheim, Germany. Toshiba: Toshiba Medical Systems Corporation, Ōtawara, Tochigi, Japan.
Patient and scan characteristics for all 4 groups of tube potential protocols (≤ 80-kVp, 90–100-kVp, 110–120-kVp and ≥ 130-kVp) are summarized in Table 1. While patient age did not vary between groups, very-low kVp was favored in female patients and patients with lower BMI. A reduced cardiovascular risk was significantly associated with the selection of lower tube potential, as observed by reduced frequency of hypertension, diabetes, dyslipidemia and smoking history in CCTAs performed with ≤ 80 kVp protocols. Site experience expressed as the median duration of CCTA scanning in years varied between 11 to 12 years. The proportion of modern CT scanners (≥ 128-slices) was significantly increased with low tube potential scanning. Iterative image reconstruction as an additional dose reduction strategy was more frequently used with very-low tube potential scanning (98% vs. 82% for ≤ 80-kVp vs. 110–120-kVp; p < 0.001), while the proportion of prospective scan techniques was similar (86% vs. 88% for ≤ 80-kVp vs. 110–120-kVp; p = 0.39).
Table 1:
Patient and CCTA scanning characteristics
| ≤ 80 kVp (n = 377) | 90–100 kVp (n = 1889) | 110–120 kVp (n = 1662) | ≥ 130 kVp (n = 78) | p value | |
|---|---|---|---|---|---|
|
| |||||
| Patient characteristics | |||||
| Age, years | 61 (50–70) | 59 (50–68) | 59 (51–68) | 59 (52–68) | 0.71 |
| Male sex, % | 45 (166) | 54 (1027) | 63 (1048) | 70 (55) | < 0.001 |
| Height, cm | 167 (160 – 173) | 168 (160 – 175) | 171 (163 – 178) | 175 (168 – 181) | < 0.001 |
| Weight, kg | 65 (59 – 74) | 73 (64 – 83) | 85 (75 – 98) | 108 (91 – 124) | < 0.001 |
| BMI, kg/m2 | 24 (22 – 26) | 26 (23 – 28) | 29 (26 – 32) | 35 (31 – 40) | < 0.001 |
| Hypertension, % | 39 (149) | 54 (1026) | 50 (825) | 67 (52) | < 0.001 |
| Diabetes, % | 10 (37) | 14 (262) | 18 (296) | 22 (17) | < 0.001 |
| Dyslipidemia, % | 36 (135) | 32 (603) | 46 (757) | 47 (37) | < 0.001 |
| Smoker, % | 11 (42) | 18 (339) | 16 (265) | 19 (15) | 0.0084 |
| CCTA scanning characteristics | |||||
| Site experience, years | 11 (10–13) | 11 (8–13) | 12 (8–14) | 12 (8–14) | 0.049 |
| Modern CT (≥ 128-slices), % | 99 (373) | 94 (1783) | 88 (1460) | 73 (57) | < 0.001 |
| Scan length, mm | 130 (119 – 140) | 137 (124 – 144) | 138 (130 – 142) | 137 (124 – 142) | < 0.001 |
| Iterative reconstruction, % | 98 (368) | 81 (1526) | 82 (1359) | 68 (53) | < 0.001 |
| Scan technique, % | < 0.001 | ||||
| prospective | 86 (322) | 91 (1717) | 88 (1457) | 60 (47) | |
| retrospective | 14 (53) | 9 (167) | 12 (196) | 40 (31) | |
Data are presented as % (n) or median (interquartile range). Body mass index (BMI).
Reduction of radiation dose and contrast agent in low tube potential CCTA
When using conventional tube potential (110–120 kVp), the median CTDIvol added up to 22.8 [13.2 to 34.4] mGy. Application of low tube potential protocols significantly lowered the median CTDIvol to 11.1 [6.3 to 16.6] mGy or 6.9 [2.8 to 10.6] mGy using 90–100 kVp or ≤ 80 kVp protocols, respectively (p < 0.001). The median DLP for conventional 110–120-kVp scanning resulted in 310 [182 to 468] mGy x cm and was reduced by 50% (156 [88 to 236] mGy x cm) or even 68% (98 [46 to 160] mGy x cm) with the use of 90–100 kVp or ≤ 80 kVp tube potential protocols (both p < 0.001; Figure 3A). Consequently, median radiation dose for conventional tube potential imaging was estimated to 4.3 (8.1) mSv when using the dose conversion factor k = 0.014 (or alternatively k = 0.026). The estimated median radiation dose was lowered to 2.2 (4.1) mSv or even 1.4 (2.5) mSv with the application of 90–100 kVp or ≤ 80 kVp tube potential protocols (both p < 0.001). In addition to the reduction of radiation dose, significantly less contrast agent volume was used with tube potential reduction. For CCTAs with a conventional tube potential of 110–120 kVp, median contrast agent volume added up to 80 [70 to 100] ml and could be reduced by 13% (70 [60 to 80] ml) or even 25% (60 [55 to 79] ml) using 90–100 kVp or ≤ 80 kVp protocols, respectively (p < 0.001; Figure 3B).
Figure 3: Impact of tube potential reduction on dose-length product and contrast agent volume in CCTA imaging.
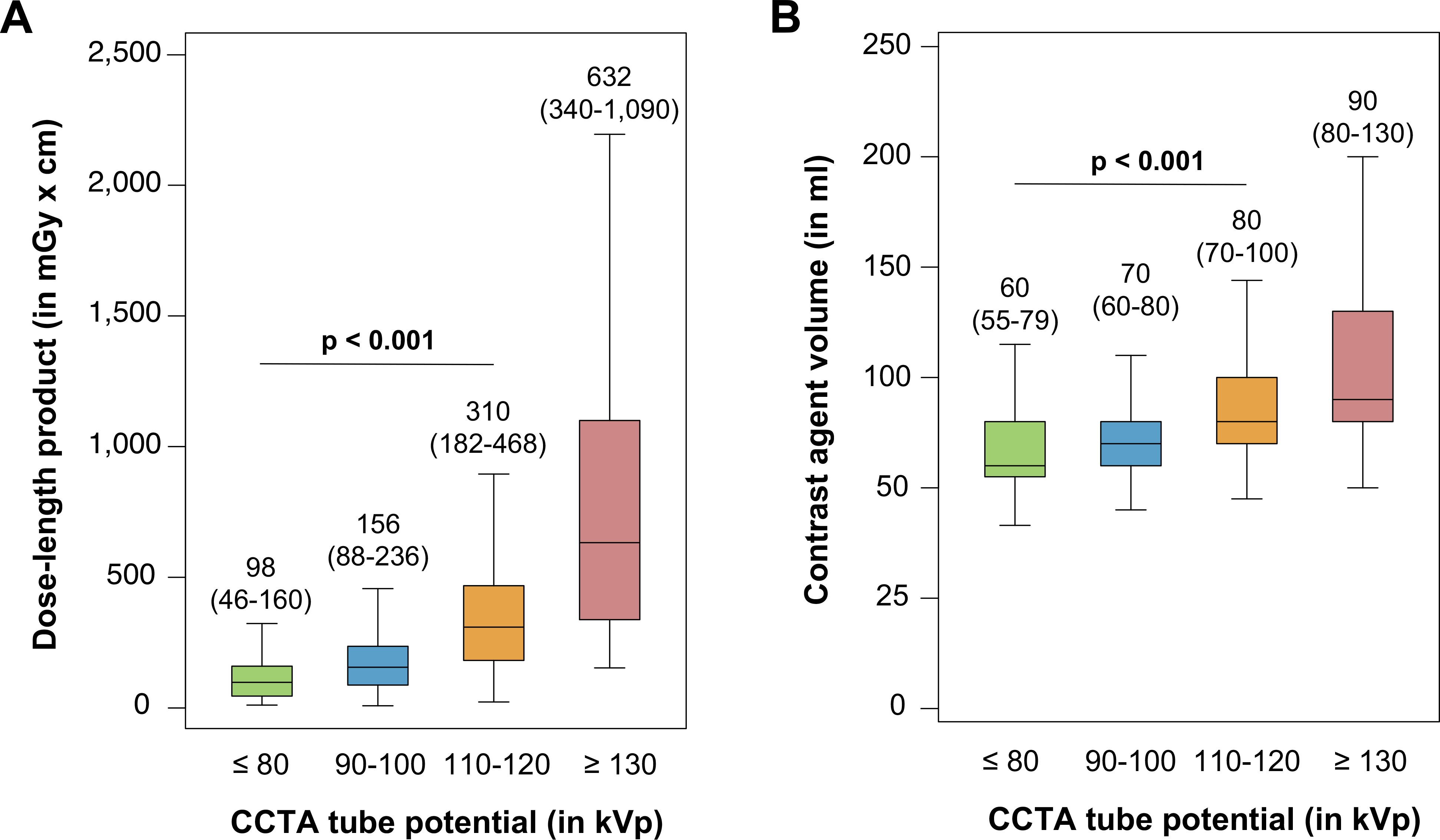
Median dose-length product (A) and median contrast agent volume (B) per tube potential protocol. The middle horizontal line represents the median, the box shows the interquartile range (IQR), and error bars show the range of non-outlying data points (whiskers). The lower whisker shows the lowest data point within the 25th percentile minus 1.5 times IQR and the upper whisker shows the highest data point within the 75th percentile plus 1.5 times IQR.
Maintenance of image quality in low tube potential CCTA
Quantitative image quality parameters including median image noise, SNR, and CNR in reference to the selected tube potential are displayed in Table 2. Reduction of tube potential to 90–100 kVp or ≤ 80 kVp increased image noise by 6% or 23%, when compared to conventional 110–120 kVp CCTAs (both p < 0.001). However, the median SNR improved with tube potential reduction by 20% or 31% for 90–100 kVp or ≤ 80 kVp compared to 110–120 kVp scanning (both p < 0.001). Similarly, CNR improved by 25% or 39% with the utilization of 90–100-kVp or ≤ 80-kVp protocols (both p < 0.001). Importantly, the frequency of diagnostic scans was similar between all groups (97.6%, 98.4%, 97.8% and 97.4% for ≤ 80-kVp, 90–100-kVp, 110–120-kVp and ≥ 130-kVp; p = 0.41).
Table 2:
CCTA image quality by tube potential protocol
| ≤ 80 kVp | 90–100 kVp | 110–120 kVp | ≥ 130 kVp | p value | |
|---|---|---|---|---|---|
|
| |||||
| Quantitative Image Quality | |||||
| Image noise, HU | 39.6 (31.3–52.6) | 34.3 (27.5–43.3) | 32.3 (25.1–40.5) | 28.0 (20.7–36.5) | < 0.001 |
| Signal intensity, HU | 687 (566–796) | 540 (450–624) | 414 (356–473) | 320 (294–357) | < 0.001 |
| Contrast-to-noise ratio (CNR) | 13.3 (10.2–17.5) | 12.0 (9.2–15.6) | 9.6 (7.4–12.8) | 8.5 (5.9–10.6) | < 0.001 |
| Signal-to-noise ratio (SNR) | 16.8 (12.9–21.8) | 15.3 (12.0–19.4) | 12.8 (10.0–16.6) | 11.4 (8.4–15.2) | < 0.001 |
| Qualitative Image Quality | |||||
| Diagnostic scans, % | 97.6 (360) | 98.4 (1840) | 97.8 (1524) | 97.4 (76) | 0.41 |
Data are presented as % (n) or median (interquartile range). HU = Hounsfield units.
Capacity for tube potential reduction in clinical practice using BMI eligibility criteria
To assess the capacity for further tube potential reduction in clinical practice, we analyzed the application of the different tube potential protocols by BMI eligibility criteria (Figure 4A). The majority of patients had a BMI < 30 kg/m2 (n = 2,940) and was eligible for the application of tube potential reduction. In fact, 67% of these patients were scanned with low tube potential protocols (90–100 kVp: 55%; ≤ 80 kVp: 12%). Patients with a BMI ≥ 30 kg/m2 (n = 1,030) were mostly scanned with conventional tube potentials between 110 and 120 kVp (68% of patients), however, 26% of these patients were also scanned with low tube potentials (90–100 kVp: 24%; ≤ 80 kVp: 2%). A considerable subpopulation of patients had a BMI < 25 kg/m2 (n = 1,363). Only 19% of these patients (n = 256) were selected for eligible very-low tube potential protocols of ≤ 80 kVp. In an additional analysis, we calculated the BMI distribution by tube potential protocol (Figure 4B). In the conventional group without tube potential reduction (110–120 kVp), we identified 58% of patients (n = 946) with a BMI < 30 kg/m2 that would have been eligible for tube potential reduction to at least 100 kVp. In the group of CCTAs performed with 90–100-kVp, we identified 44% of patients (n = 831) with a BMI < 25 kg/m2 that would have qualified for additional escalation of tube potential ≤ 80 kVp.
Figure 4: Capacity for low tube potential CCTA imaging by BMI eligibility criteria.
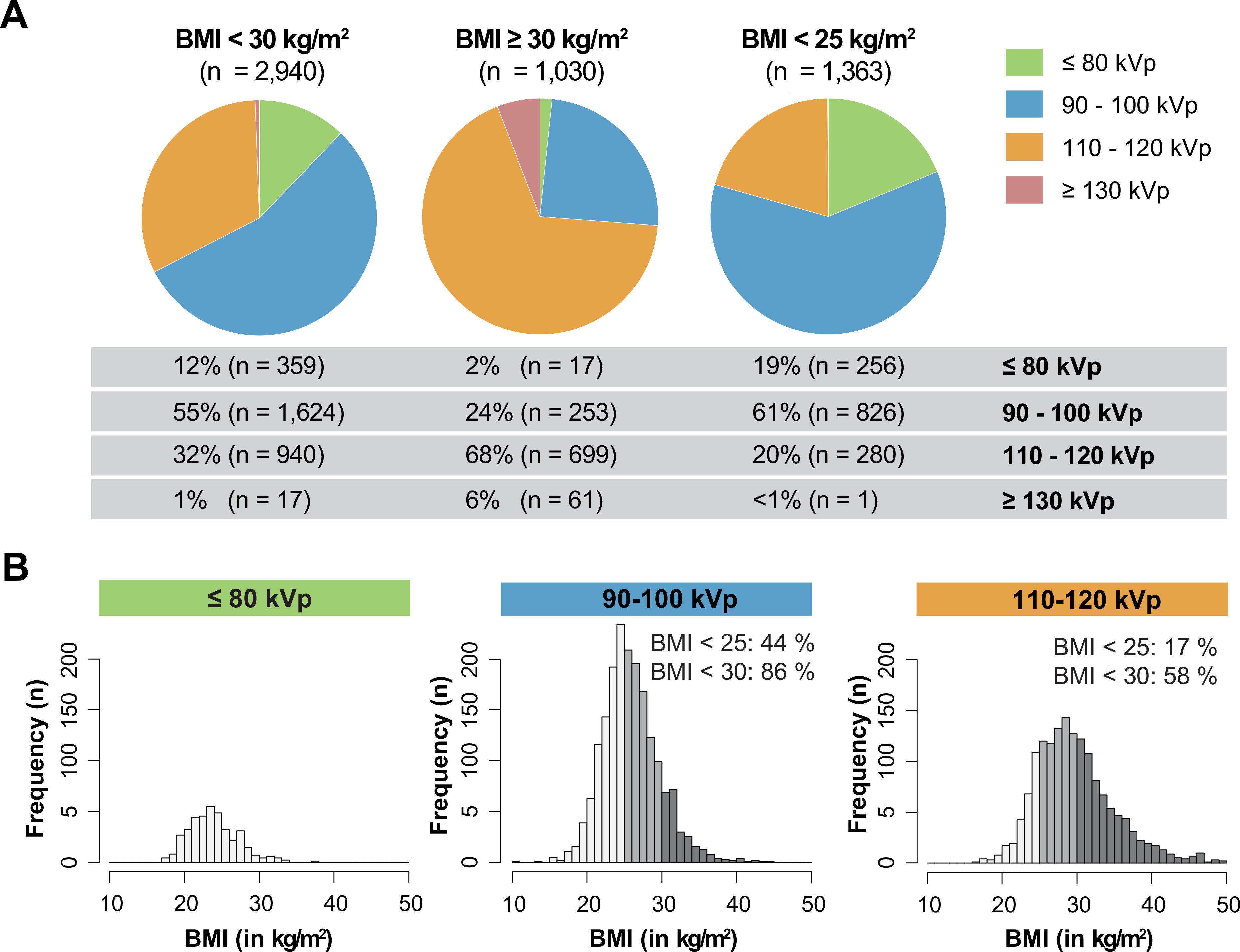
A: The application of respective tube potential protocols is demonstrated for different categories of body mass index (BMI) < 30 kg/m2 (left), ≥ 30 kg/m2 (middle) or < 25 kg/m2 (right). B: BMI distribution of scanned patients ranging from 10 to 50 kg/m2 for the respective tube potential protocols of ≤ 80-kVp (left), 90–100-kVp (middle) and 110–120-kVp (right).
Discussion
CCTA has evolved to an important non-invasive tool for the evaluation of coronary artery disease in clinical routine. However, safety concerns remain in terms of exposure to ionizing radiation and the need for iodinated contrast agent with potential kidney damage. Consequently, several techniques to reduce radiation exposure and contrast agent volume have been developed during the last decade. The recently finalized international PROTECTION VI study revealed a median DLP of 195 mGy x cm for CCTA in current clinical practice with a considerable variation in dose between study sites (6). The PROTECTION VI study evaluated predictors for the magnitude of CCTA radiation exposure and identified body weight, heart rate and rhythm, iterative image reconstruction, high-pitch helical scan technique, and tube potential reduction as independent predictors (6). The reduction of tube potential is extremely effective due to the exponential reduction of radiation dose. Additionally, iodine absorption is increased at lower tube potential settings, giving rise to advantages in iodinated contrast enhanced CCTA imaging (23).
The current analysis of the PROTECTION VI study assessed the utilization of tube potential reduction in worldwide clinical practice and analyzed the impact of different tube potential reduction protocols on radiation dose, contrast agent volume and image quality. Specifically, this study is the first report analyzing the worldwide feasibility and efficacy of very-low tube potential protocols down to 80 kVp and below in clinical routine imaging. The utilization of very-low tube potentials of ≤ 80 kVp was associated with a reduction of the mean DLP by 68% compared to conventional scanning with 120 kVp tube potential protocols. Low tube potential protocols between 90 and 100 kVp still lowered the mean DLP by 50%. Likewise, tube potential reduction lowered median contrast agent volume by 25% or 13% with the application of ≤ 80-kVp or 90–100-kVp protocols. The reduction of iodinated contrast agent during CCTA helps to protect kidney function on the one hand. On the other hand, iodinated contrast has been demonstrated to amplify radiation induced DNA-damage, and thus the reduced contrast volumes add to the protection from radiation exposure (24).
Importantly, the feasibility of tube potential reduction regarding image quality was demonstrated in this study. As described in previous studies, low tube potential CCTA imaging leads to an increase of image noise, because of the reduced penetration of photons at lower energy (14). This increase in image noise is a potential disadvantage because of a decline of the overall image quality. However, tube potential reduction was associated with significant improvement of SNR and CNR due to the concomitant increase in signal and contrast, despite of the reduced volume of contrast agent applied in low tube potential imaging. Hence, the reduction of tube potential may add to improved delineation of coronary lumen stenosis. Additionally, the rate of non-diagnostic CCTAs was unaffected by the selection of low tube potential protocols. However, low tube potential imaging with increased image noise might enhance blooming of coronary calcifications. Therefore, diagnostic accuracy of severely calcified coronary arteries with low tube potential imaging, particularly in combination with iterative image reconstruction, remains to be investigated.
This large registry revealed that a reduction of tube potential to 100 kVp or below was applied in 56% of CCTAs in current clinical routine. The majority of these patients were scanned with moderately reduced tube potential protocols between 90 to 100 kVp. In a way this can be interpreted as a success of the promotion of tube potential reduction strategies during recent years (7–10). However, we observed great variability in the application of low tube potential protocols ranging from 0% to 100%. Several sites followed a “one protocol fits all” strategy and excluded any variation in tube potential. In this regard, four participating sites in this study exclusively scanned using 120-kVp tube potential imaging. Very-low tube potential protocols of ≤ 80 kVp were applied in only 9% of CCTAs in clinical practice and are thus underrepresented in current clinical routine. Several contributing factors have been determined for the preference of very-low tube potential protocols of ≤ 80 kVp. First, the selection of very-low tube potential protocols was significantly influenced by the vendor of the installed CT system. The results of this study were obtained by the use of multiple vendors with a variety of commercially available CT scanners and are thus representative for real-world clinical routine imaging. Nevertheless, some hospitals might not have access to modern CT systems that are a prerequisite for very-low tube potential imaging. These technical circumstances might have also affected the results of this study. Secondly, the education and conventions at the individual study sites seem to contribute to the magnitude of tube voltage reduction. Regional differences in the selection of tube potential protocols have been identified. Many study sites rarely consider the application of very-low tube potential protocols, while on the other hand several study sites demonstrated that the majority of patients could be scanned with protocols of 80 kVp and below in clinical practice. Finally, the study demonstrated that clinicians select individual patients for very-low tube potential imaging. Practitioners seem to prefer both women and healthier patients as expressed by lower cardiovascular risk profiles for very-low tube potential imaging. Additionally, low BMI was significantly associated with the reduction of tube voltage. However, analysis of the BMI distribution revealed that a significant portion of patients would have been eligible for application and escalation of low tube voltage protocols. In this perspective, only 19% of patients that would have been eligible for very-low tube potential protocols (BMI below 25 kg/m2) were actually considered for tube potentials ≤ 80 kVp. A strict implementation of BMI eligibility criteria with ≤ 80-kVp protocols for patients with a BMI below 25 kg/m2 and 90–100-kVp protocols for patients with a BMI between 25 and 30 kg/m2 would have lowered the median DLP of the PROTECTION VI population by 23% to 150 mGy x cm (interquartile range 84 to 275 mGy x cm). A clinical practice guideline for the selection of tube potential protocols that includes these BMI eligibly criteria is illustrated in Figure 5.
Figure 5: Clinical practice guideline for the selection of tube potential protocols in CCTA imaging.
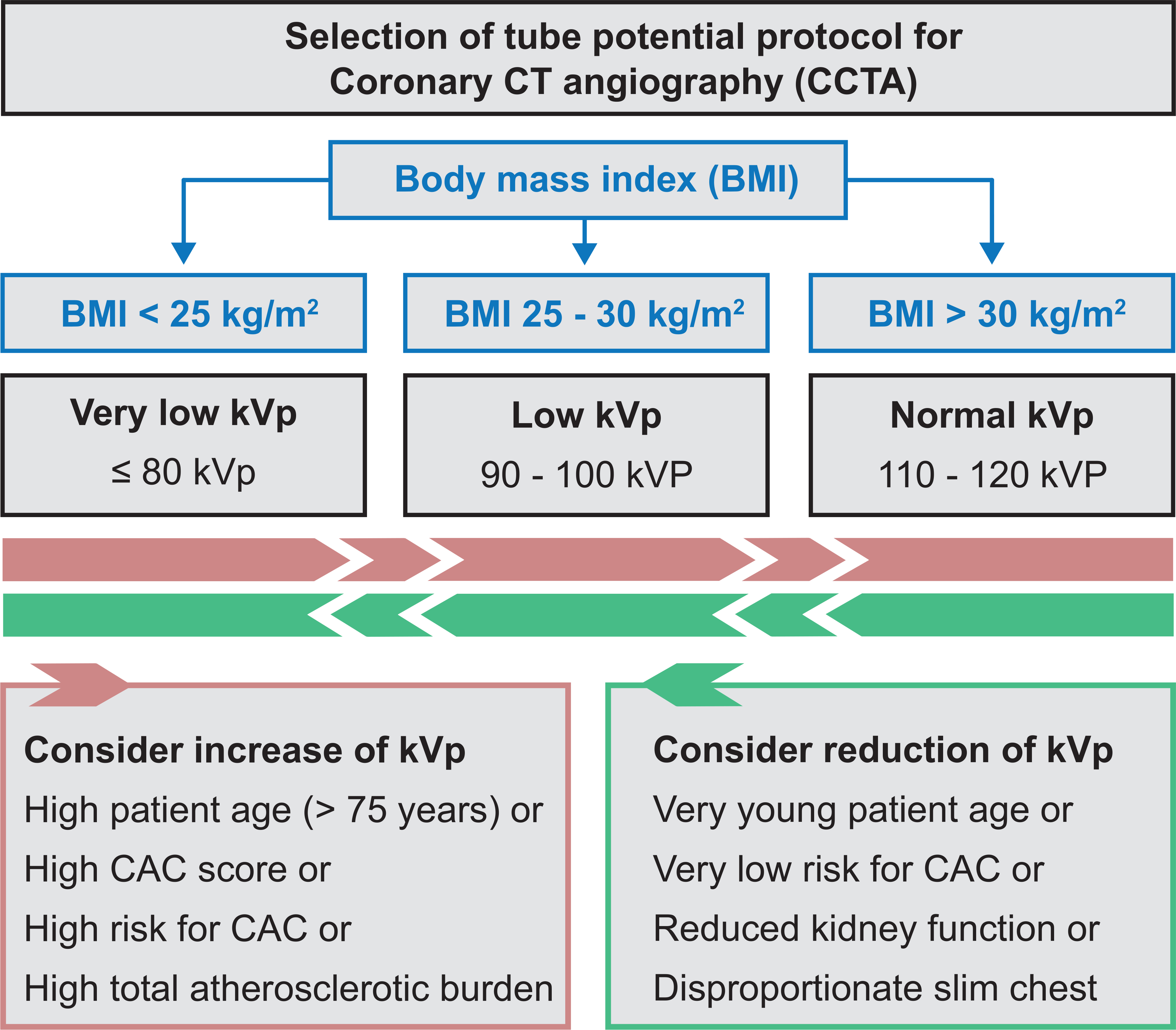
Body mass index (BMI) eligibility criteria categorize the tube potential protocol. Additional factors may increase (red box) or reduce (green box) the ideal tube potential category. Coronary artery calcification (CAC).
Limitations
Although the diagnostic accuracy of CCTA with low and very-low tube potentials is high (25), the use of too strong iterative image reconstruction algorithms for counterbalancing image noise might result in too smoothened vessel delineation, which might result in concealing circumscriptive coronary stenosis. While the delineation of the coronary artery lumen still appears feasible at 100 kVp tube potentials in patients with extensive coronary calcifications, the increased blooming artifacts of large calcified plaques might impair the diagnostic reading at very-low tube potentials. Thus, in elderly patients with a higher probability of extensive coronary calcifications, it might be helpful to estimate the burden of coronary calcification calcium scoring before CCTA with very-low tube potentials. Finally, the impact of very-low kVp scan protocols on coronary plaque quantification, on assessment of plaque composition and on new CT technologies including CT-derived fractional flow reserve is currently unknown. Additional iterative reconstruction could have influenced the outcome parameters of the current study, however, only a very small fraction of low kVp scans was performed without iterative reconstruction. Statistical limitations are the lack of adjustments in patient characteristics for clustered observations between study sites.
Conclusions
This large international study demonstrates that a decrease of tube potential gradually improves patient safety by significantly reducing radiation dose and contrast agent volume. Low tube potential protocols are feasible in clinical routine and are non-inferior regarding image quality, when compared to conventional tube potential imaging. This study also demonstrates that tube potential reduction has been increasingly implemented during the last decade. Especially moderate tube potential reduction between 90 kVp and 100 kVp has gained popularity in clinical routine. However, the current study also demonstrates the need for additional and escalated tube potential reduction. In particular, the application of very-low tube potential protocols ≤ 80 kVp should be considered in clinical practice.
Supplementary Material
Supplementary Table S1: Application of tube potential protocols in CCTA imaging by region
Perspectives.
Clinical competencies (in Patient Care and Procedural Skills)
The clinical indications for CCTA imaging have significantly expanded during recent years and, likewise, the proportion of younger patients has increased. In this context, safety concerns in CCTA imaging and especially the exposure to potentially harmful radiation are increasingly recognized. Low tube potential imaging is a powerful method to lower radiation dose in eligible patients. Tube potential in CCTA imaging should be set as low as technically possible and reasonably achievable considering BMI eligibility criteria.
Translational outlook
Training programs for CT operators are necessary to improve CCTA image protocols and increase the proportion of low tube potential scans. Further improvements of software with implementation of automated tube potential selection on the basis of patient characteristics including height and body weight may add to dose reduction. The application of quality control programs should be recommended to guide patients in the identification of expert centers following recommendations for dose reduction in CCTA imaging.
Relationship with industry (conflict of interest statement and financial disclosure statement):
TJ Stocker, M Hadamitzky, R Rubinshtein, S Deseive, M Heckner, K Kitagawa, H Marques, C Silva, J Mahmarian, JW Kang, J Lesser, and S Massberg report no relationship with the industry. J Leipsic is a consultant to and holds stock options in Circle CVI and HeartFlow and receives fellow support from GE Healthcare outside the submitted work. M Chen reports non-financial support from Canon Medical systems outside the submitted work. JJ Bax reports grants from Biotronik, Medtronic, Boston Scientific, GE Healthcare, and Edwards Lifesciences outside the submitted work. A Schmermund reports grants from Siemens outside the submitted work. EL Grove has received speaker honoraria or consultancy fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, MSD, Pfizer and Roche outside the submitted work. J Hausleiter received speaker honoraria and research support of Abbott Vascular and Edwards Lifesciences outside the submitted work.
Abbreviations list
- CT
Computed tomography
- CCTA
Coronary CT angiography
- DLP
Dose-length product
- BMI
Body mass index
- SNR
Signal-to-noise ratio
- CNR
Contrast-to-noise ratio
Appendix
PROTECTION VI Investigators (sorted by country)
Patricia Carrascosa and Alejandro Deviggiano, Diagnóstico Maipú, Buenos Aires, Argentina
Christopher Naoum and John Magnussen, Macquarie University Hospital, Sydney, Australia
James Otton and Anthony Kaplan, Spectrum Radiology Liverpool, Sydney, Australia
Gudrun Feuchtner and Fabian Plank, Medizinische Universität, Innsbruck, Austria
Kristof De Smet and Nico Buls, Universitair Ziekenhuis, Brussel, Belgium
Roberto Caldeira Cury and Marcio Sommer Bittencourt, Delboni / DASA, Sao Paulo, Brazil
Cesar Higa Nomura and Roberto Nery Dantas Junior, Heart Institute – InCor, Sao Paulo, Brazil
Jonathon Leipsic and Philipp Blanke, University of British Columbia, Vancouver, Canada
Carl Chartrand-Lefebvre and Anne Chin, University of Montreal, Montreal, Canada
Gary Small and Benjamin Chow, University of Ottawa Heart Institute, Ottawa, Canada
Claudio Silva F, Clinica Alemana de Santiago, Santiago, Chile
Marcelo Godoy Z. and Claudio Silva F., Clinica Alemana de Temuco, Temuco, Chile
Xiang-Ming Fang and Wang Jie, Wuxi People’s Hospital, Wuxi, China
Alberto Cadena, Clínica de la Costa, Barranquilla, Colombia
Theodor Adla and Vojtech Suchanek, Motol University Hospital, Prague, Czech Republic
Erik Lerkevang Grove and Kamilla Bech Pedersen, Aarhus University Hospital, Aarhus, Denmark
Jess Lambrechtsen and Mirza Husic, OUH-Svendborg, Svendborg, Denmark
Juhani Knuuti and Teemu Maaniitty, Turku University Hospital, Turku, Finnland
Bernhard Bischoff and Elisabeth Arnoldi, Klinikum der Universität München, München, Germany
Axel Schmermund and Joachim Eckert, Cardioangiologisches Centrum Bethanien, Frankfurt, Germany
Martin Hadamitzky and Tom Finck, Deutsches Herzzentrum München, München, Germany
Michaela Hell and Mohamed Marwan, Universitätsklinikum Erlangen-Nürnberg, Erlangen, Germany
Fabian Bamberg and Stefanie Mangold, Universitätsklinikum Tübingen, Tübingen, Germany
Thomas Schlosser and Johannes Ludwig, Universitätsklinikum Essen, Essen, Germany
Maria Mylona and Spyros Skiadopoulos, Olympion Hospital, Patras, Greece
Pál Maurovich-Horvat and Bálint Szilveszter, MTA-SE Cardiovascular Imaging Research Group, Heart and Vascular Center, Budapest, Hungary
Uday Jadav and Brian V. Pinto, MGM New Bombay Hospital, Vashi New Mumbai, India
28. Ronen Rubinshtein and Essam Hussein, Lady Davis Carmel Medical Center, Haifa, Israel
Daniele Andreini and Gianluca Pontone, Centro Cardiologico Monzino, Milan, Italy
Eugenio Martuscelli and Massimiliano Sperandio, Policlinico di Tor Vergata, Rome, Italy
Kakuya Kitagawa and Naoki Nagasawa, Mie University Hospital, Tsu, Japan
Ramzi Tabbalat and Rami A. Farhan, Khalidi Hospital, Amman, Jordan
Lilia M. Sierra Galán and Leovigildo A. Delgado, American British Cowdray Medical Center Observatory Campus, Mexico City, Mexico
Lilia M. Sierra Galán and Marco A. Reza Orozco, American British Cowdray Medical Center Santa Fe Campus, Mexico City, Mexico
Francisco C. Castellón and Mariana D. Zamudio, Instituto Ignacio Chavez, Mexico City, Mexico
Andres Preciado-Anaya and Rafael P. Gómez, Hospital Siena, Leon, Mexico
Signe Helene Forsdahl and Grete Anita Hansen, University Hospital North Norway, Tromsø, Norway
Anne Günther and Joanna F. Kristiansen, Oslo University Hospital Rikshospitalet, Oslo, Norway
Edith Chavez Huapalla and Percy Teran Chavez, Complejo Hospitalario San-Pablo, Surco, Peru
Hugo M. Rodrigues Marques and Pedro de A. Goncalves, UNICA (Cardiovascular imaging Unit), Hospital da Luz, Lisbon, Portugal
Subramaniyan Ramanathan, Al Wakra Hospital, Doha, Qatar
Valentin Sinitsyn and Maria Glazkova, Federal Center of Medicine and Rehabilitation, Moscow, Russia
Rami Abazid and Osama A. Smettei, Prince Sultan Cardiac Center, Burydah, Saudi Arabia
Ahmed Dawood and Salama Hussain Omar, Dr Erfan and Bagedo Hospital, Jeddah, Saudi Arabia
Joon-Won Kang and Dong Hyun Yang, Asan Medical Center, Seoul, South Korea
Tae Hoon Kim and Chul Hwan Park, Gangnam Severance Hospital, Seoul, South Korea
Sanghoon Shin and Seok Jong Ryu, Ilsan Hospital, Goyang-si, South Korea
Jose R. Palomares and Hug Cuellar, Hospital Val d’Hebron, Barcelona, Spain
Jeroen J. Bax and Alexander van Rosendael, Leiden University Medical Center, Leiden, Netherlands
Michelle C. Williams, David Newby and Edwin J. R. van Beek, University of Edinburgh, Edinburgh, United Kingdom
Russell Bull and Kavin Jayawardhana, Royal Bournemouth Hospital, Bournemouth, United Kingdom
Patricia Dickson and Jennifer Espey, Capital Cardiology Associates, Albany, United States
John Lesser, Minneapolis Heart Institute, Minneapolis, United States
Renée Bullock-Palmer, Deborah Heart and Lung Center, Browns Mills, United States
Mark Rabbat and Nancy Schoenecker, Loyola University Medical Center, Maywood, United States
Dustin M. Thomas and Rosco S. Gore, San Antonio Military Medical, San Antonio, United States
Melany Atkins, Fairfax Radiological Consultants, Fairfax, United States
Marcus Y. Chen and Sujata M. Shanbhag, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, United States
John Mahmarian, Houston Methodist Hospital, Houston, United States
Jeannie Yu, Long Beach VA Healthcare System, Long Beach, United States
Todd C. Villines and Binh Nguyen, Walter Reed National Military Medical Center, Bethesda, USA
Footnotes
Clinical Trial Information: clinicaltrials.gov: NCT02996903
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams MC, Hunter A, Shah ASV et al. Use of Coronary Computed Tomographic Angiography to Guide Management of Patients With Coronary Disease. J Am Coll Cardiol 2016;67:1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budoff MJ, Dowe D, Jollis JG et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–32. [DOI] [PubMed] [Google Scholar]
- 3.CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 4.Newby DE, Adamson PD, Berry C et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. The New England journal of medicine 2018;379:924–933. [DOI] [PubMed] [Google Scholar]
- 5.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317–23. [DOI] [PubMed] [Google Scholar]
- 6.Stocker TJ, Deseive S, Leipsic J et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur Heart J 2018;39:3715–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff B, Hein F, Meyer T et al. Impact of a reduced tube voltage on CT angiography and radiation dose: results of the PROTECTION I study. JACC Cardiovasc Imaging 2009;2:940–6. [DOI] [PubMed] [Google Scholar]
- 8.Leschka S, Stolzmann P, Schmid FT et al. Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol 2008;18:1809–17. [DOI] [PubMed] [Google Scholar]
- 9.Hausleiter J, Meyer T, Hadamitzky M et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation 2006;113:1305–10. [DOI] [PubMed] [Google Scholar]
- 10.Sun G, Hou YB, Zhang B et al. Application of low tube voltage coronary CT angiography with low-dose iodine contrast agent in patients with a BMI of 26–30 kg/m2. Clin Radiol 2015;70:138–45. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Wang Y, Kai H et al. Application of 80-kVp tube voltage, low-concentration contrast agent and iterative reconstruction in coronary CT angiography: evaluation of image quality and radiation dose. Int J Clin Pract 2016;70Suppl 9B:B50–5. [DOI] [PubMed] [Google Scholar]
- 12.Mangold S, Wichmann JL, Schoepf UJ et al. Automated tube voltage selection for radiation dose and contrast medium reduction at coronary CT angiography using 3(rd) generation dual-source CT. Eur Radiol 2016;26:3608–16. [DOI] [PubMed] [Google Scholar]
- 13.Jun BR, Yong HS, Kang EY, Woo OH, Choi EJ. 64-slice coronary computed tomography angiography using low tube voltage of 80 kV in subjects with normal body mass indices: comparative study using 120 kV. Acta Radiol 2012;53:1099–106. [DOI] [PubMed] [Google Scholar]
- 14.LaBounty TM, Leipsic J, Poulter R et al. Coronary CT angiography of patients with a normal body mass index using 80 kVp versus 100 kVp: a prospective, multicenter, multivendor randomized trial. AJR Am J Roentgenol 2011;197:W860–7. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Fu Q, Yu H et al. Evaluate of the effect of low tube voltage on the radiation dosage using 640-slice coronary CT angiography. J Xray Sci Technol 2018;26:463–471. [DOI] [PubMed] [Google Scholar]
- 16.Pan YN, Li AJ, Chen XM, Wang J, Ren DW, Huang QL. Coronary Computed Tomographic Angiography at Low Concentration of Contrast Agent and Low Tube Voltage in Patients with Obesity:: A Feasibility Study. Acad Radiol 2016;23:438–45. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Yang L, Song X et al. Feasibility study of low tube voltage (80 kVp) coronary CT angiography combined with contrast medium reduction using iterative model reconstruction (IMR) on standard BMI patients. Br J Radiol 2016;89:20150766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Zhao YE, Qi L et al. Prospectively ECG-triggered high-pitch coronary CT angiography at 70 kVp with 30mL contrast agent: An intraindividual comparison with sequential scanning at 120 kVp with 60mL contrast agent. Eur J Radiol 2017;90:97–105. [DOI] [PubMed] [Google Scholar]
- 19.Stocker TJ, Deseive S, Chen M et al. Rationale and design of the worldwide prospective multicenter registry on radiation dose estimates of cardiac CT angiography in daily practice in 2017 (PROTECTION VI). J Cardiovasc Comput Tomogr 2018;12:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hausleiter J, Meyer T, Hermann F et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500–7. [DOI] [PubMed] [Google Scholar]
- 21.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK: 2003. Br J Radiol 2006;79:968–80. [DOI] [PubMed] [Google Scholar]
- 22.Trattner S, Halliburton S, Thompson CM et al. Cardiac-Specific Conversion Factors to Estimate Radiation Effective Dose From Dose-Length Product in Computed Tomography. JACC Cardiovasc Imaging 2018;11:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigal-Cinqualbre AB, Hennequin R, Abada HT, Chen X, Paul JF. Low-kilovoltage multi-detector row chest CT in adults: feasibility and effect on image quality and iodine dose. Radiology 2004;231:169–74. [DOI] [PubMed] [Google Scholar]
- 24.Piechowiak EI, Peter JF, Kleb B, Klose KJ, Heverhagen JT. Intravenous Iodinated Contrast Agents Amplify DNA Radiation Damage at CT. Radiology 2015;275:692–7. [DOI] [PubMed] [Google Scholar]
- 25.Andreini D, Mushtaq S, Conte E et al. Coronary CT angiography with 80 kV tube voltage and low iodine concentration contrast agent in patients with low body weight. J Cardiovasc Comput Tomogr 2016;10:322–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Application of tube potential protocols in CCTA imaging by region


