Abstract
Neurogenesis is a powerful mechanism for structural and functional remodeling that occurs in restricted areas of the adult brain. While different neurotransmitters regulate various aspects of the progression from neural stem cell quiescence to neuronal maturation, GABA is the main player. The developmental switch from excitation to inhibition combined with a heterogeneous population of GABAergic interneurons that target different subcellular compartments provide multiple points for the regulation of development and function of new neurons. This complexity is enhanced by feedback and feedforward networks that act as sensors and controllers of circuit activity, impinging directly or indirectly onto developing granule cells and, subsequently, on mature neurons. Newly generated granule cells ultimately connect with input and output partners in a manner that is largely sculpted by the activity of local circuits.
INTRODUCTION
In the mammalian brain, principal cells that convey excitation to carry electrical signals from one relay station to the next are mostly glutamatergic, while interneurons that control rhythms and activity of principal cells through inhibition are primarily GABAergic. The interplay between glutamatergic and GABAergic networks is critical for brain function, and subtle abnormalities in this balance may derive in neurological disorders including schizophrenia, autism, epilepsy and depression [1–4]. There is an extensive variety of GABAergic interneurons with distinctive firing properties, regional distribution, and subcellular localization of synaptic contacts in the target cell, all of which define functional specializations [5]. In addition to the rich population of interneurons, there is a growing body of evidence revealing a remarkable diversity of GABAergic projection neurons that bring extrinsic inhibition that is driven by distant networks [6]. In the hippocampus, more than 70 % of the local inhibition is carried by two types of GABAergic interneurons: those expressing the calcium buffer parvalbumin (PV-INs), and those releasing the neuropeptide somatostatin (SOM-INs) [7–9]. One distinctive feature of the dentate gyrus (DG) is that, at any given time, the majority of the GCs remain silent and information is encoded by a small fraction of active neurons [10]. The sparse activity of the granule cell layer (the region where somas of GCs are localized) is a direct result of a strong inhibitory tone exerted by GABAergic interneurons. Axons from PV-INs (fast-spiking) target the granule cell layer and provide perisomatic inhibition onto GCs, while SOM-INs (non fast spiking) extend their axons through the molecular layer and reach their dendrites. The spiking characteristics and targeting regions of these interneurons already highlight clear differences in the manner they control network activity [11]. Moreover, variations in their input connectivity confer additional alternatives to IN function, as discussed below.
Unlike most other brain regions, the DG holds the ability to generate principal cells throughout life. Neurogenesis endows the adult DG with a unique degree of circuit plasticity that is critical for its role in learning, memory and spatial navigation [10,12,13]. Initially, newborn GCs display properties that are typical of immature neurons such as high membrane resistance, weak connectivity and depolarizing responses to GABA. Once mature, they present very similar properties to those born during perinatal development. Their functional integration imposes a challenge for the regulation and control of input/output synaptogenesis and circuit activity [14]. This concept is reinforced by the fact that alterations in adult neurogenesis may result in neurological and psychiatric disease [15]. Interestingly, development, integration and function of GCs is largely determined by their interaction with GABAergic circuits [16]. The role played by GABAergic interneurons changes dynamically as GCs walk the pathway from neural progenitor cell to mature GC. Thus, interneurons alternate from activity sensors that control neural stem cell differentiation to actuators that restrict firing of mature GCs.
GABAERGIC REGULATION OF ADULT NEURAL STEM CELLS
New GCs originate from radial glia-like neural stem cells (NSCs) that reside in the subgranular zone of the adult DG. NSCs may remain quiescent or divide and become active progenitor cells that give rise to neurons [13]. As most steps in the pathway from NSC to neuron, the decision to become active is greatly influenced by circuit activity. There are several components in the hippocampal neurogenic niche that act as activity sensors. PV-INs belong to a very active neuronal population that promote quiescence of NSCs through tonic inhibition, which is likely mediated by GABA spillover from synapses formed onto neighboring GCs [17]. PV-IN activity is also influenced by long-range GABAergic projections from the medial septum, and optogenetic stimulation of those projections depolarize PV-INs in the DG, promoting quiescence of NSCs [18].
The fate of NSCs is also shaped by glutamatergic activity. Hilar mossy cells (MCs) project ipsi- and contralaterally, establishing monosynaptic excitatory synapses and disynaptic inhibition onto GCs via local GABAergic interneurons. Interestingly, moderate chemogenetic activation of MCs can promote quiescence of NSCs through activation of PV-INs, while strong MC activation may trigger NSC differentiation through a direct action [19]. Moreover, GABAergic INs releasing cholecystokinin (CCK) can depolarize NSCs indirectly, as CCK elicits glutamate release from astrocytes and, in turn, promotes proliferation [20]. Therefore, the decision of NSCs to maintain quiescence or enter a neurogenic differentiation pathway relies on the integration of glutamate- and GABA-mediated signals, with an outcome that can be influenced by the state of the niche, patterns of activity, relative position of NSCs, and their metabolic status (Fig. 1).
Figure 1. Timeline of integration of newborn GCs in the adult dentate gyrus.
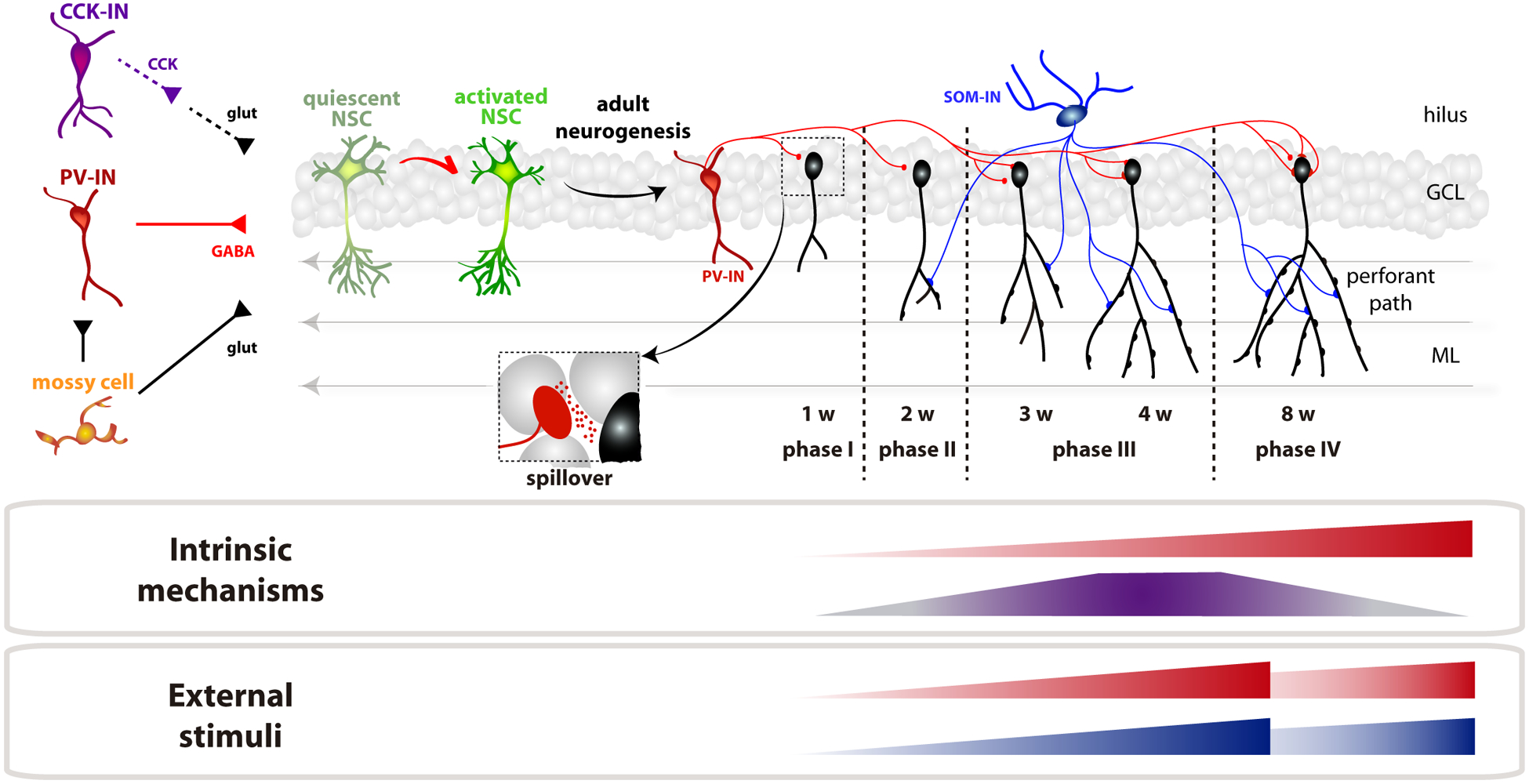
NSCs integrate inputs from PV-INs (depolarizing GABA), mossy cells (glutamate) and CCK-INs (indirect via astrocytic glutamate release) to determine quiescence vs. neurogenic activation. Neuronal maturation requires 8 weeks (w), that we propose to be divided in 4 phases. Phase I: GCs receive depolarizing GABA through spillover from PV-INs. Phase II: SOM-INs (blue) establish depolarizing dendritic inputs, glutamatergic synaptogenesis begins. Phase III: increased density of glutamatergic synapses, onset of direct PV-IN inhibition, and high excitation/inhibition ratio. GABA becomes inhibitory. Phase IV: all synaptic inputs already mature. There is also a time-dependent maturation of GABAB-mediated inhibition through GIRK channels that reduces intrinsic excitability. Synaptogenesis of PV- and SOM-INs is accelerated by activity, such as exposure to an enriched environment or voluntary running.
GABA ACTIONS ON DEVELOPING GCs
Newborn neurons in the adult mouse DG reach functional maturation about 8 weeks after differentiation (Fig1). Through this period, GCs undergo distinct phases presenting qualitative differences in terms of morphology, connectivity and function [14]. During the first phase (GC age < 1.5 weeks), neurons display active radial migration, as dendrites lacking spines extend towards the molecular layer. Initially, few or no synapses are present and GCs are sensitive to ambient GABA. Towards the end of the first week, axodendritic GABAergic contacts begin to assemble, eliciting slow responses that are depolarizing due to the reverse chloride gradient [21,22]. In agreement with this notion, initial functional contacts by PV-INs and SOM-INs become detected between the first and the second week of GC development, as revealed by optogenetic activation in slices [23].
Glutamatergic synaptogenesis begins at two weeks post-mitosis (phase II, >2 weeks), which results in dendritic spines forming along the molecular layer. GCs cease to migrate and dendrites continue to develop in extension and complexity (branching). GABAergic postsynaptic responses exhibit immature features such as small amplitude and slow kinetics. They display substantial developmental growth for both PV-INs and SOM-INs that relies on a time-dependent increase in quantal size and in the number of individual synapses [23,24]. An alternative mechanism for early GABAergic transmission has been proposed, whereby responses may arise from GABA spillover rather than direct synaptic contacts [25]. In this view, time-dependent growth of synaptic responses might be explained by changes in the density and subunit composition of extrasynaptic GABAA receptors. Interestingly, when coincident with subthreshold glutamatergic activity, slow depolarizing GABAergic transmission may elicit spiking of the postsynaptic GC, which might be critical for synapse formation and remodeling [26].
After ~3–4 weeks (phase III), GCs reach maximal levels of dendritic arborization, while the density of afferent glutamatergic inputs continue to increase rapidly. GABA has already switched from depolarizing to hyperpolarizing due to the delayed expression of the potassium-chloride cotransporter KCC2, which results in a decrease of the intracellular chloride concentration. Still, new GCs display a high excitation/inhibition balance due to the delayed maturation of inhibition. In particular, perisomatic inhibition by PV-INs displays slow kinetics and exerts poor control of GC firing at this time, as if synapses would be maintained functionally immature for prolonged periods [24,27]. Interestingly, voluntary running and exposure to an enriched environment were shown to accelerate the maturation of this synapse, which reveals a phase of adult GC development that is tailored by activity [23,24]. Even though mature morphological properties are reached during this phase, GCs remain functionally immature, exhibiting heightened excitability conferred by their weak coupling to inhibition, and enhanced synaptic plasticity.
The inhibitory network is a key determinant for controlling development and activity of new GCs, but changes in intrinsic excitability also play a crucial role. Mechanisms that control excitability through G protein-activated inwardly rectifying potassium (GIRK) channels are absent in immature GCs, which contributes to both higher membrane resistance and weak responsiveness of GABAB signaling mediated by GIRK channels [28]. GIRK signaling becomes entirely functional after several weeks of maturation, decreasing GC excitability and allowing GABAB-mediated inhibition to take place. GABAB transmission is activated by SOM-INs and, most prominently, by interneurons expressing nNOS, both of them targeting the GC dendrites.
By 6–8 weeks, optogenetic activation of PV-INs and SOM-INs results in maximal levels of inhibition, with both fast kinetics and large conductance [24]. At this stage, input resistance decreases, GABAergic perisomatic inhibition becomes fast and strong, excitability is diminished, and synaptic plasticity is reduced [14]. This is the time at which adult-born GCs become physiologically indistinguishable from mature neurons born during perinatal development.
With regard to the output from GCs onto GABAergic interneurons, axons begin to establish functional connections by the 4th week and reach mature connectivity by 6 weeks (onto PV-INs) and 11 weeks (SOM-INs) [24]. Thus, new GCs become able to recruit feedback inhibition rather late in development.
NEURONAL MATURATION AND SURVIVAL
In addition to the complex regulation of NSCs, depolarizing GABA shapes neuronal maturation during the early phases of GC development. GABA signaling controls growth and integration of new GCs at several levels, promoting synapse formation for both GABAergic and glutamatergic afferents as well as dendritic growth [29]. Initially, glutamatergic synapses do not participate in basal synaptic transmission because they lack AMPA receptors and require depolarization to release the Mg2+ block of NMDA receptors. GABA can provide this depolarization, resulting in the incorporation of AMPA receptors (synapse unsilencing) and subsequent spine formation [21]. In addition, other mechanisms triggered by GABA are thought to involve Ca2+ influx through voltage-dependent Ca2+ channels, which activates the mTOR pathway to stimulate cell growth [30,31]. Overactivation of mTOR signaling may result in abnormal development. Downregulation of the disrupted-in-schizophrenia 1 (DISC1) gene, a negative modulator of mTOR, accelerates growth, synaptogenesis and migration, resulting in abnormal neuronal size and ectopic localization. Chronic activation of PV-INs in a context of DISC1 downregulation results in an excessive density of both GABAergic and glutamatergic synapses within the depolarizing phase of GABA [32]. In the hyperpolarizing phase of GABA, PV-IN activity renders excessive inhibition and loss of glutamatergic synapses, resulting in a marked imbalance between excitation and inhibition.
Activity of PV-INs is also relevant for mediating network remodeling triggered by certain behaviors. For example, a brief exposure to enriched environment (EE) was found to accelerate growth and integration of immature GCs [33]. In this paradigm, PV-INs transduce exploratory behavior into local changes in the neurogenic niche, to a point in which silencing PV-INs can completely abolish circuit remodeling. PV networks can also regulate the survival of new GCs. EE exposure was found to increase blood flow in the DG microvasculature [34]. Pharmacological blockade of nitric oxide (NO) synthase prevented hyperemia and abolished the EE-induced increase in neurogenesis. NO production by PV-INs was both necessary and sufficient to increase blood flow and promote neurogenesis. In a different study, exploration of an EE was shown to reduce spiking of GCs while increasing the firing rate of fast-spiking interneurons, producing an overall reduction in the excitation/inhibition ratio in the DG [35]. This effect was mediated by hippocampal sphingolipids, whose production was promoted by EE. Sphingolipids increased IN spiking, reduced overall DG activity, and increased survival of adult-born neurons. Together, these studies offered a novel view on the complexity of the pathways involved in bridging behavioral inputs with local changes in the neurogenic niche, with direct actions in new GCs.
GABA SIGNALING IN MATURE GCs
Mature GCs integrate excitation from different cortical and subcortical areas, including glutamatergic inputs from the medial and lateral entorhinal cortex, commissural fibers from mossy cells, cholinergic projections from the medial septum, and the supramammilary nucleus that releases both GABA and glutamate resulting in a net excitatory effect [7,36,37]. GABAergic interneurons participate in feedback and feedforward loops that control activity in the granule cell layer [38]. Action potentials reaching the DG through the perforant path produce direct activation of mature GCs but also recruit fast feedforward loops that attenuate or preclude GC spiking [27]. These loops are predominantly driven by PV-INs that extend dendrites over the molecular layer and project their axons back to the GC somas, providing substantial inhibition to the layer (Fig. 2) [24,39,40]. PV-INs also extend dendrites to the hilus, where they become targets of GCs. Interestingly, the same individual feedforward PV-INs recruited by entorhinal axons are also activated by GCs to create a feedback loop [24]. As a result, PV-INs integrate excitatory dentate inputs and GC activity generated by those same inputs. In contrast, SOM-INs extend dendrites to the hilus and are poorly recruited by entorhinal axons. While they do not participate in feedforward loops, they become important players in feedback circuits that limit activity of the granule cell layer through dendritic inhibition of the GCs. In addition, recent evidence suggested that contralateral projections from SOM-INs might contribute to some extent to dendritic inhibition in the dentate gyrus, building a loop that might synchronize activity over both hemispheres [41]. Mossy fibers can activate SOM-INs that target GC dendrites and PV-INs that impinge on their soma, constituting two parallel feedback pathways that set the brake in the activity of the granule cell layer. In addition, the PV-IN loop might be strengthened by semilunar GCs, multipolar neurons located within the inner molecular layer that were shown to contact PV-INs through perisomatic excitatory synapses [42]. These multiple loops of inhibition result in sparse activation of the granule cell layer, with PV-INs dominating inhibition at a global level. An elegant study using multiple simultaneous whole-cell recoding in slices has measured the probability of direct connections between GCs, PV-INs, SOM-INs and CCK-INs [43]. This work showed that PV-INs project onto GCs with much higher probability than CCK- or SST-INs and, in turn, GCs contact PV-INs with much higher frequency than any other interneuron. GCs are 10 times more likely to recruit PV-IN-mediated lateral inhibition onto neighboring GCs than recurrent inhibition onto itself. Finally, GABAergic projections from the medial septum were shown to depolarize PV-INs in the DG [18]. This finding opens the intriguing possibility that PV-INs may be in general depolarized rather than inhibited by GABA. If this were the case, then most inputs (GABA and glutamate) would tend to increment the activity of PV-INs, resulting in a continuous brake on the activity of the dentate gyrus.
Figure 2. Local networks of the dentate gyrus.
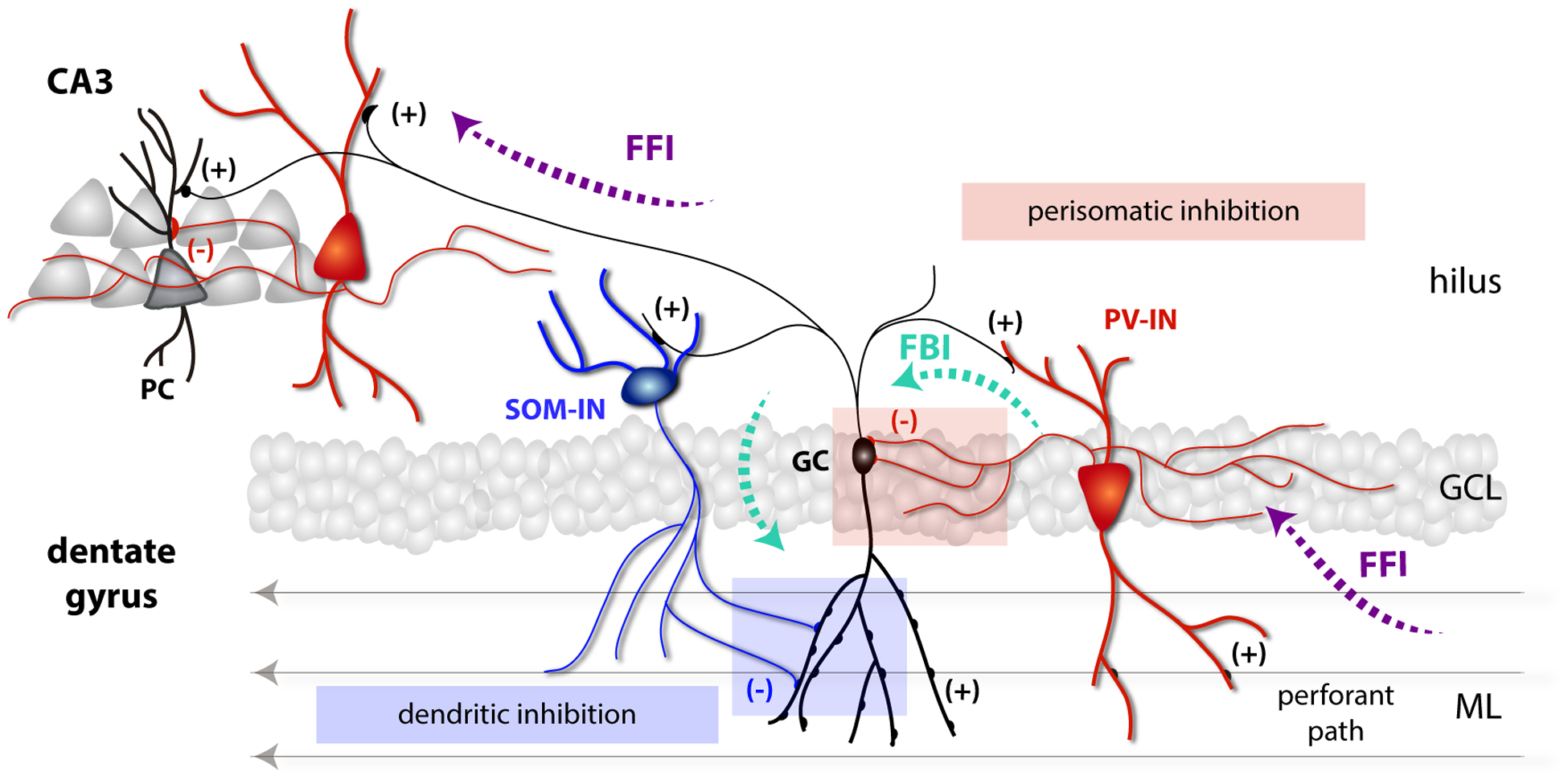
Mature GCs (black) residing the granule cell layer (GCL) receive glutamatergic inputs from the perforant path through dendritic spines in the molecular layer (ML). Axons from GCs project through the hilus toward CA3 pyramidal cells. Entorhinal axons from the performant path also activate PV-INs (red) that control the activity of GCs through feedforward inhibition (FFI). GCs recruit feedback inhibition (FBI) by activating PV and SOM-INs (blue) and also generate FFI onto pyramidal cells (PC) in CA3.
CONCLUSIONS
In adult neurogenesis, new excitatory neurons incorporate in a working circuit without altering its function, responding to cellular and molecular signals that follow intrinsic and extrinsic homeostatic rules. New neurons and their host network combine strategies that are shared with brain development and circuit plasticity in the adult brain. GABAergic interneurons play fundamental roles along this pathway, and PV-INs appear to be central to convey GABA signals onto the different cellular stages from NSCs to mature GCs. The developmental switch of GABA from excitation to inhibition is central to this self-regulated process; the postsynaptic effect of GABAergic transmission is determined by the intracellular chloride concentration that decreases with time and, when hyperpolarizing, it limits neuronal activity. This negative loop is reinforced because the GABA switch is accelerated by depolarizing GABAergic transmission [44]. Hence, GABA excitation drives neuronal and synapse formation during early development, while GABA inhibition controls the activity of large groups of principal neurons and the transition is driven by activity. Mature GCs become strongly coupled to dendritic and somatic inhibition that restrict neuronal excitability and synaptic plasticity, contributing to sparse coding in the DG. The interplay between adult neurogenesis and GABA interneurons emerges as a robust candidate to confer processing flexibility to the DG network. Why PV-INs seem more efficient in driving developmental and plasticity programs than other GABAergic interneurons, and how new neurons integrate a space of complementary signals to decide how far to grow, how to connect, and when to fire, remains poorly understood. Those fundamental rules are there, waiting to be revealed.
HIGHLIGHTS.
Neural stem cells integrate glutamate- and GABA-mediated signals to define their fate
GABAergic networks control development and function of adult-born granule cells
The GABA switch from excitation to inhibition is crucial to adult neurogenesis
PV interneurons convert behavioral inputs into trophic signals for new neurons
ACKNOWLEDGMENTS
MFT, DG and AFS were supported by the Argentine National Research Council (CONICET). This work was supported by grants from the Argentine Agency for the Promotion of Science and Technology (PICT2015–3814 and PICT2016–0675), and the National Institute Of Neurological Disorders And Stroke (NINDS) and Fogarty International Center (FIC) (R01NS103758) to A.F.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
Nothing declared.
REFERENCES
- [1].Duman RS, Sanacora G, Krystal JH: Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Righes Marafiga J, Vendramin Pasquetti M, Calcagnotto ME: GABAergic interneurons in epilepsy: More than a simple change in inhibition. Epilepsy Behav 2020:106935. [DOI] [PubMed] [Google Scholar]
- [3].Sohal VS, Rubenstein JLR: Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry 2019, 24:1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang XJ: Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nat Rev Neurosci 2020, 21:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huang ZJ, Paul A: The diversity of GABAergic neurons and neural communication elements. Nat Rev Neurosci 2019, 20:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Melzer S, Monyer H: Diversity and function of corticopetal and corticofugal GABAergic projection neurons. Nat Rev Neurosci 2020, 21:499–515. [DOI] [PubMed] [Google Scholar]
- [7].Hainmueller T, Bartos M: Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat Rev Neurosci 2020, 21:153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hosp JA, Struber M, Yanagawa Y, Obata K, Vida I, Jonas P, Bartos M: Morpho-physiological criteria divide dentate gyrus interneurons into classes. Hippocampus 2014, 24:189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yuan M, Meyer T, Benkowitz C, Savanthrapadian S, Ansel-Bollepalli L, Foggetti A, Wulff P, Alcami P, Elgueta C, Bartos M: Somatostatin-positive interneurons in the dentate gyrus of mice provide local- and long-range septal synaptic inhibition. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Piatti VC, Ewell LA, Leutgeb JK: Neurogenesis in the dentate gyrus: carrying the message or dictating the tone. Front Neurosci 2013, 7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ: Hippocampal GABAergic Inhibitory Interneurons. Physiol Rev 2017, 97:1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lodge M, Bischofberger J: Synaptic properties of newly generated granule cells support sparse coding in the adult hippocampus. Behav Brain Res 2019, 372:112036. [DOI] [PubMed] [Google Scholar]
- [13].Vicidomini C, Guo N, Sahay A: Communication, Cross Talk, and Signal Integration in the Adult Hippocampal Neurogenic Niche. Neuron 2020, 105:220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schinder AF, Trinchero MF, Groisman AI: Synaptogenesis in the adult CNS—Hippocampus. In Synapse Development and Maturation, edn 2nd edition. Edited by Rubenstein J, Chen B, Kwan KY, Cline HT, Cardin J. Academic Press; 2020: 235–253. [Google Scholar]
- [15].Toda T, Parylak SL, Linker SB, Gage FH: The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry 2019, 24:67–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Catavero C, Bao H, Song J: Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res 2018, 371:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al. : Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 2012, 489:150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bao H, Asrican B, Li W, Gu B, Wen Z, Lim SA, Haniff I, Ramakrishnan C, Deisseroth K, Philpot B, et al. : Long-Range GABAergic Inputs Regulate Neural Stem Cell Quiescence and Control Adult Hippocampal Neurogenesis. Cell Stem Cell 2017, 21:604–617 e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yeh CY, Asrican B, Moss J, Quintanilla LJ, He T, Mao X, Casse F, Gebara E, Bao H, Lu W, et al. : Mossy Cells Control Adult Neural Stem Cell Quiescence and Maintenance through a Dynamic Balance between Direct and Indirect Pathways. Neuron 2018, 99:493–510 e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Asrican B, Wooten J, Li YD, Quintanilla L, Zhang F, Wander C, Bao H, Yeh CY, Luo YJ, Olsen R, et al. : Neuropeptides Modulate Local Astrocytes to Regulate Adult Hippocampal Neural Stem Cells. Neuron 2020, 108:349–366 e346. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study reveals a novel effect whereby CCK released by local interneurons elicit calcium spikes in astrocytes, which depolarize NSCs through glutamate signaling and promote neurogenic differentiation.
- [21].Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS: GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci 2013, 33:6614–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF: Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 2005, 25:10074–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Remmers CL, Castillon CCM, Armstrong JN, Contractor A: Recruitment of parvalbumin and somatostatin interneuron inputs to adult born dentate granule neurons. Sci Rep 2020, 10:17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Groisman AI, Yang SM, Schinder AF: Differential Coupling of Adult-Born Granule Cells to Parvalbumin and Somatostatin Interneurons. Cell Rep 2020, 30:202–214 e204. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors used optogenetics and slice electrophysiology to establish a detailed spatial and temporal map of GC integration to the preexisting inhibitory network of the dentate gyrus.
- [25].Vaden RJ, Gonzalez JC, Tsai MC, Niver AJ, Fusilier AR, Griffith CM, Kramer RH, Wadiche JI, Overstreet-Wadiche L: Parvalbumin interneurons provide spillover to newborn and mature dentate granule cells. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• By combining optogenetics and electrophysiological recordings, the authors demonstrate that synaptic responses from PV-IN onto immature GCs arise from GABA spillover from nearby mature synapses.
- [26].Heigele S, Sultan S, Toni N, Bischofberger J: Bidirectional GABAergic control of action potential firing in newborn hippocampal granule cells. Nat Neurosci 2016, 19:263–270. [DOI] [PubMed] [Google Scholar]
- [27].Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF: Unique Processing During a Period of High Excitation/Inhibition Balance in Adult-Born Neurons. Science 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gonzalez JC, Epps SA, Markwardt SJ, Wadiche JI, Overstreet-Wadiche L: Constitutive and Synaptic Activation of GIRK Channels Differentiates Mature and Newborn Dentate Granule Cells. J Neurosci 2018, 38:6513–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H: GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 2006, 439:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kang E, Wen Z, Song H, Christian KM, Ming GL: Adult Neurogenesis and Psychiatric Disorders. Cold Spring Harb Perspect Biol 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, Feighery E, Lu B, Rujescu D, St Clair D, et al. : Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell 2012, 148:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kang E, Song J, Lin Y, Park J, Lee JH, Hussani Q, Gu Y, Ge S, Li W, Hsu KS, et al. : Interplay between a Mental Disorder Risk Gene and Developmental Polarity Switch of GABA Action Leads to Excitation-Inhibition Imbalance. Cell Rep 2019, 28:1419–1428 e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alvarez DD, Giacomini D, Yang SM, Trinchero MF, Temprana SG, Buttner KA, Beltramone N, Schinder AF: A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science 2016, 354:459–465. [DOI] [PubMed] [Google Scholar]
- [34].Shen J, Wang D, Wang X, Gupta S, Ayloo B, Wu S, Prasad P, Xiong Q, Xia J, Ge S: Neurovascular Coupling in the Dentate Gyrus Regulates Adult Hippocampal Neurogenesis. Neuron 2019, 103:878–890 e873. [DOI] [PMC free article] [PubMed] [Google Scholar]; • By imaging the neurovascular niche the authors unmasked new metabolic mechanisms controlling adult neurogenesis. Exploration elevates blood flow in the dentate gyrus, activating a metabolic pathway mediated by PV-INs that promotes GC survival.
- [35].Wang X, Liu H, Morstein J, Novak AJE, Trauner D, Xiong Q, Yu Y, Ge S: Metabolic tuning of inhibition regulates hippocampal neurogenesis in the adult brain. Proc Natl Acad Sci U S A 2020, 117:25818–25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Amaral DG, Scharfman HE, Lavenex P: The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 2007, 163:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hashimotodani Y, Karube F, Yanagawa Y, Fujiyama F, Kano M: Supramammillary Nucleus Afferents to the Dentate Gyrus Co-release Glutamate and GABA and Potentiate Granule Cell Output. Cell Rep 2018, 25:2704–2715 e2704. [DOI] [PubMed] [Google Scholar]
- [38].Elgueta C, Bartos M: Dendritic inhibition differentially regulates excitability of dentate gyrus parvalbumin-expressing interneurons and granule cells. Nat Commun 2019, 10:5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee CT, Kao MH, Hou WH, Wei YT, Chen CL, Lien CC: Causal Evidence for the Role of Specific GABAergic Interneuron Types in Entorhinal Recruitment of Dentate Granule Cells. Sci Rep 2016, 6:36885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, Giacomini D, Beltramone N, Lanuza GM, Schinder AF: Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cells. Neuron 2015, 85:116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Eyre MD, Bartos M: Somatostatin-Expressing Interneurons Form Axonal Projections to the Contralateral Hippocampus. Front Neural Circuits 2019, 13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rovira-Esteban L, Hajos N, Nagy GA, Crespo C, Nacher J, Varea E, Blasco-Ibanez JM: Semilunar Granule Cells Are the Primary Source of the Perisomatic Excitatory Innervation onto Parvalbumin-Expressing Interneurons in the Dentate Gyrus. eNeuro 2020, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Espinoza C, Guzman SJ, Zhang X, Jonas P: Parvalbumin(+) interneurons obey unique connectivity rules and establish a powerful lateral-inhibition microcircuit in dentate gyrus. Nat Commun 2018, 9:4605. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This very elegant work uses simultaneous whole-cell recordings in multiple neurons to provide an accurate assessment of dentate gyrus connectivity among principal cells and interneurons, concluding that PV-INs confer powerful lateral inhibition to the granule cell network.
- [44].Ganguly K, Schinder AF, Wong ST, Poo M: GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 2001, 105:521–532. [DOI] [PubMed] [Google Scholar]


