Abstract
A tripartite synapse is comprised of a neuronal presynaptic axon and a postsynaptic dendrite, which are closely ensheathed by a perisynaptic astrocyte process. Through their structural and functional association with thousands of neuronal synapses, astrocytes regulate synapse formation and function. Recent work revealed a diverse range of cell adhesion-based mechanisms that mediate astrocyte-synapse interactions at tripartite synapses. Here we will review some of these findings unveiling a highly dynamic bidirectional signaling between astrocytes and synapses, which orchestrates astrocyte morphological maturation and synapse development. Moreover, we will discuss the roles of these newly discovered molecular pathways in brain physiology and function both in health and disease.
Introduction
At its most fundamental, the brain is a highly complex web of synaptic connections. Classically, a synapse is viewed as a cell-cell adhesion between the pre-synaptic axon of a neuron and the post-synaptic dendrite of another, allowing for information to be transmitted between them. However, the intricacy, specificity and complexity of synapse formation and function is not only governed by neurons. In recent years, astrocytes, the dominant perisynaptic glial cell type in the brain, has been shown to play critical roles in the genesis, maturation, elimination, plasticity and function of synapses[1–3].
The necessity of astrocytes in synapse development were first demonstrated using purified retina ganglion cell neuron-only cultures. These neurons, despite their ability to survive and thrive under serum-free conditions, formed very few synapses when cultured alone. However, when media containing astrocyte-secreted factors were applied to these neurons, a profound increase in synapse numbers and synaptic activity were detected [4,5]. Since then, many astrocyte-released synaptogenic factors were discovered and a number of them were characterized for their roles in controlling synapse development in vivo, clearly revealing the dynamic regulation of excitatory and inhibitory synapse formation and function by astrocytes[6–8].
Another mode of signaling between astrocytes and neuronal synapses is mediated by cell adhesion molecules which will be the main focus of this review. In particular, we will focus on four recently discovered adhesion-based mechanisms (Figure 1) that control the physical and functional engagement between synapses and protoplasmic astrocytes of the grey matter. We will highlight the latest research on how these cell adhesion-based mechanisms regulate astrocyte-neuron interactions and how these findings shape our understanding of synapse function in health and disease.
Figure 1. Overview of cell adhesion mechanisms mediating astrocyte-neuron interactions.
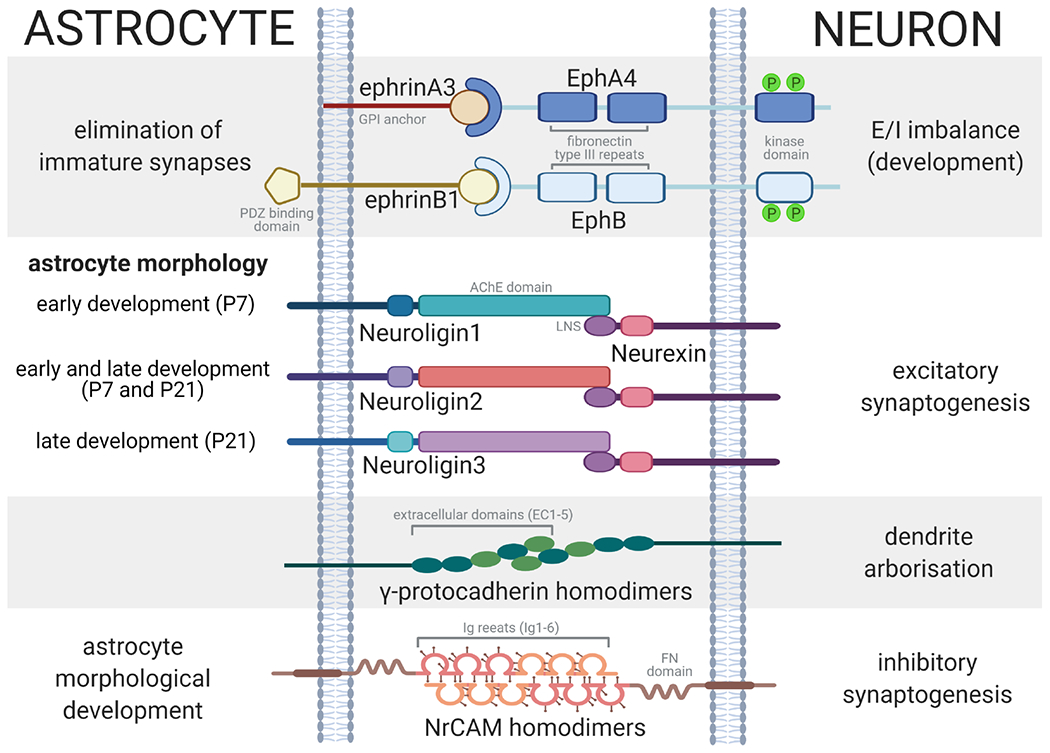
4 classes of adhesion molecules are discussed in this review. (1) Astrocytic ephrins bind to neuronal Eph receptors to negatively regulate synaptogenesis by eliminating immature synapses. ephrinAs are anchored to the astrocyte cell surface by a GPI anchor; whereas, ephrinBs have a C-terminal PDZ-binding domain that greatly augments the possiblity of astrocyte-neuron bidirectional signaling. In constrast, EphA and EphB have similar structures, comprising of extracellular fibronectin type II repeats and an intracellular kinase domain that is known to be critical for E/I balance. (2) Neuroligin-neurexin interactions are mediated by the binding of neuroligin’s acetylcholinesterase (AChE) domain and neurexin’s LNS (laminin, neurexin, sexhormone binding globulin) domain. Different neuroligins regulate astrocyte morphological complexity at different developmental timepoints. Collectively astrocytic neuroligin/neuronal neurexin interactions molecularly link astrocyte complexity with synaptogenesis during a critical window of postnatal cortical development. (3) γ-protocadherins trans-dimerize via binding of its extracellular domains at the astrocyte-neuron interface to regulate dendrite arborization. (4) The extracellular domain of NrCAM is composed of 6 immunoglobulin-like (Ig) repeats and several fibronectin (FN) domains. Astrocytic NrCAM forms homodimers with neuronal NrCAM via its Ig repeats to regulate both astrocyte morphology and inhibitory synaptogenesis.
Protoplasmic astrocyte morphology reflects the complexity of astrocyte-synapse interactions
Protoplasmic astrocytes of the grey matter have highly ramified morphologies. 6-8 primary processes extend out from the astrocyte soma, branching out into secondary and tertiary processes, and finally elaborating into fine perisynaptic astrocytic processes (PAPs) that come into direct contact with the synapse[9]. Electron micrograph 3D reconstructions of rat hippocampi revealed that synapses adjacent to (<1μm) astrocytes tend to be mature (large perforated mushroom spines)[10]. This observation, coupled with evidence of activity-dependent, bidirectional signaling between astrocytes and synapses led to the concept of the tripartite synapse[11]. The tripartite synapses are abundant in the brain, accounting for 57-90% of all synapses in the hippocampus[10,12] and all excitatory synapses in the cerebellum[13,14]. In the cortex, a single astrocyte contacts up to 100 000 synapses in mice[15] and upwards of 2 million synapses in humans[16]. Indeed, these intimate astrocyte-synapse interactions are conserved through evolution[17–19] and are vital for synapse formation[18], stability and maturation[20], as well as conferring circuit specificity[21] across species. What are the molecular mechanisms that mediate astrocyte-neuron contacts at synapses? Evidently, cell adhesion emerged as major regulators of astrocyte-neuron contacts at tripartite synapses[22,23]. Here we will focus on cell surface adhesion proteins that have been identified in the past 5 years to play critical roles in the mammalian brain.
Negative regulation of synaptogenesis by ephrin/Eph signaling at the tripartite synapse
Several Eph receptors and their ephrin ligands have been identified at the astrocyte-neuron interface. Astrocytic ephrinA3 binds to EphA4 receptors on dendritic spines, resulting in spine retraction to ensure proper dendritic morphology and synapse formation during development[24]. Reverse signaling of astrocytic ephrinA3 by neuronal EphA4 limits cell surface expression of glutamate transporter in astrocytes, ensuring optimal synaptic glutamate levels that are critical for synaptic plasticity[25]. Bidirectional signaling at the tripartite synapse could be dependent on sensory experience driven synaptic activity, because ephrinA3/EphA4 signaling between suprachiasmatic nucleus (SCN) astrocytes and neurons regulates circadian plasticity in response to light[26].
Recent work identified another class of ephrins, ephrinB, at the astrocyte-synapse interface (Figure 1). Different from ephrinAs, which are bound to the cell membrane with a glycosylphophatidylinositol (GPI) anchor, ephrinBs are single-pass transmembrane proteins with a PDZ-binding domain at their cytoplasmic tails[27]. This structural difference means that in addition to tyrosine phosphorylation-dependent signaling, ephrinB can also initiate reverse signaling (outside-in) via interaction with PDZ domain proteins. This finding greatly augments the possibility of ephrinB/EphB interaction for bidirectional signaling at the astrocyte-neuron interface.
Astrocytic ephrinB1 was first shown to be a critical negative regulator of adult synaptogenesis, inhibiting exuberant excitatory synaptogenesis in the hippocampal stratum radiatum by pruning away immature spines[28,29]. Overexpression of ephrinB1 in hippocampal astrocytes results in synapse loss and reduced contextual fear memory[28]. Astrocytic ephrinB1 binds to EphB receptors on presynaptic boutons to promote the engulfment of synaptosomes in vitro, suggesting a potential role for this molecule in astrocyte-mediated synapse elimination. Loss of function mutations in the intracellular domain of ephrinB1 decrease synaptosome engulfment by 50%, indicating that astrocytic ephrinB1: neuronal EphB reverse signaling might be involved [28]. In vivo, astrocytic ephrinB1 conditional knockout mice (cKO) have significantly increased spine density and spine clustering compared to wildtype littermates, particularly on CA1 neurons[29]. Training these mice in a fear-conditioning paradigm increases AMPA receptor insertion on the surfaces of these immature spines, resulting in an increase in the number of functional excitatory synapses [28], therefore enhancing long-term contextual recall in astrocytic ephrinB1 cKO mice[28,29].
Apart from its importance in regulating excitatory synaptogenesis in adulthood, astrocytic ephrinB1 is also critical in maintaining excitation/inhibition (E/I) balance in the developing brain[30]. EphrinB1 astrocytic cKO mice have exuberant excitatory synapses, increased clustering of dendritic spines as well as a higher proportion of immature synapses during early development[30]. These observations are similar in the adult hippocampus, suggesting that astrocytic ephrinB1 also negatively regulates excitatory synaptogenesis both in development and adulthood. Interestingly, elimination of ephrinB1 from developing astrocytes during (postnatal days) P14-P19 results in a significant increase in both presynaptic and postsynaptic excitability of P28 hippocampal CA1 pyramidal neurons[30]. Also, there were significant decreases in the amplitudes of both evoked and miniature inhibitory postsynaptic currents (IPSCs). These changes were attributed to a marked decrease in parvalbumin (PV) neurons as well as a significant decrease in excitatory input onto PV neurons[30]. While the mechanism has yet to be elucidated, there is striking evidence that astrocytic ephrin B1 plays a critical role in the proper wiring of both excitatory and inhibitory circuits in the developing hippocampus[30].
Astrocytic ephrinB1 function is also relevant to our understanding on the molecular basis of neurological diseases. Knocking out ephrinB1 from astrocytes during development is sufficient to cause impairments in social memory, one aspect of social cognitive impairment observed in autism[30]. Furthermore, ephrinB1 expression increases in hippocampal astrocytes after traumatic brain injury. This suggests that astrocytic ephrinB1 can participate in synapse remodeling following injury, thus could be a therapeutic target for functional recovery after serious brain injuries[31].
Astrocytes also express ephrinB2, ephrinB3 and a full suite of Eph receptors[32], which have also been implicated in defects in astrocyte-neuron interactions. For example, elevated levels of astrocytic ephrinB3 and neuronal EphB3 (and no other Eph/ephrins) were observed in both patient and rat model of intractable temporal lobe epilepsy. This observation suggests that EphB3/ephrinB3 contact is critical in disease pathogenesis, likely through regulating synaptogenesis and synaptic plasticity[33]. Finally, astrocytes from a common mouse model of spinal muscular atrophy (SMNΔ7 mice) have a 73% reduction in ephrinB2 mRNA expression[34]. When SMNΔ7 astrocytes are co-cultured with wildtype or SMNΔ7 neurons, both synaptogenesis and synaptic function were significantly reduced. However, no synaptic changes were observed when the same experiment was repeated using a trans-well astrocyte-neuron co-culture, in which astrocytes are not in contact with neurons[34]. It is therefore hypothesized that Eph/ephrinB2-mediated astrocyte-neuron signaling is critical to the pathogenesis of early-stage spinal muscular atrophy.
Control of astrocyte morphogenesis and synaptogenesis by neurexin/neuroligin interactions
Neurexin and neuroligin families of cell adhesion molecules have been studied extensively for their heterophilic trans-synaptic interactions[35]. However, neither neuroligins nor neurexins are solely expressed by neurons. In fact, astrocytes express three neuroligins (Nlgn1, Nlgn2 and Nlgn3) as well as two neurexins (Nrxn1 and Nrxn2) at similar or higher levels compared to neurons[36].
Recent work revealed that astrocytic neuroligins bind to neuronal neurexins (Figure 1), molecularly linking astrocyte morphogenesis and synaptogenesis during the first three postnatal weeks of cortical development[37]. Indeed, astrocytes gain their morphological and functional maturity concurrently with the peak period of synaptogenesis in the cortex [38] and bidirectional signaling between astrocytes and experience-dependent synaptic activity is critical for both biological processes[39]. However, how astrocyte morphogenesis and synaptogenesis occurred in concert was unclear. Recent work revealed astrocytic Nlgn1, 2 and 3 as key players in orchestration of astrocyte and synapse development. Using purified neuron and astrocyte co-cultures, it was shown that silencing Nlgn1, Nlgn2, and Nlgn3 individually or all together in astrocytes result in significant decreases in astrocyte morphological complexity[37]. Concordantly, silencing or sequestering neuronal Nrxn1 and 2 diminished the complexity of wildtype astrocytes[37], verifying that it is neuronal neurexin/astrocytic neuroligin transcellular interactions which mediate contact-induced astrocyte morphogenesis. When individual neuroligins were silenced in astrocytes in vivo, all significantly perturbed astrocyte morphogenesis. In particular, knockdown of astrocytic Nlgn2 resulted in continued diminished neuropil infiltration volume (i.e., a quantitative measure of PAP complexity). Importantly, loss of Nlgn2 only in astrocytes was sufficient to diminish excitatory synapse numbers and function. Whereas, loss of Nlgn2 in astrocytes increased the frequency of inhibitory synaptic events without affecting the number of inhibitory synapses[37]. Taken together these findings showed that astrocytic neuroligins, such as Nlgn2, interact with neuronal neurexins to synchronize synapse formation and astrocyte morphogenesis.
Apart from neuroligins, transcriptomic analyses have long suggested that astrocytes also express neurexins, amongst which Nrxn1 expression is profoundly higher in mouse and human astrocytes than neurons[36,40]. Profiling of neurexin isoforms in the brain by in-situ hybridization confirmed that Nrxn1α and Nrx1β mRNA expression is abundant in hippocampal and cortical astrocytes[41]. A recent preprint suggests a role for astrocytic Nrxn1α in synaptic plasticity[42]. In the proposed model, astrocytic Nrxn1α is spliced differently from the neuronal variant and is more heavily modified by heparan sulfate and hence has different binding partners[42]. Comparison of neuronal and astrocytic Nrxn1α cKO suggests distinct transcriptional profiles, defects in synaptic function and behavioral impairments[42]. However, whether astrocytic neurexins can bind to neuronal neuroligins to mediate astrocyte-neuron contact dependent adhesion is not known.
These studies yield exciting new questions to be explored. How are cell adhesion molecules compartmentalized on astrocyte surfaces? In neurons, compartmentalization of neurexins to axons and neuroligins to dendrites is documented, though several studies also showed their presence in the opposing compartments[43–45]. Is there a connection between astrocytic neuroligins and neurexins and the proper polarity of these molecules in neurons? Are these molecules targeted to specific astrocyte domains? Answering these basic cell biological questions would have important disease implications, as neurexins and neuroligins have been strongly implicated in neurodevelopmental diseases such as autism[46,47] and schizophrenia[48,49].
Promotion of dendrite arborization by γ-protocadherin homophilic interactions
The cadherin superfamily is a large group of cell adhesion proteins characterized by calcium-dependent intercellular adhesions. Protocadherins, the largest subfamily, are predominantly expressed in the central nervous system. These proteins play critical roles in neurodevelopment, particularly in generating functional diversity within neurons and promoting synaptogenesis[50]. Interestingly, homophilic γ-protocadherin interactions between astrocytes and neurons are required for proper excitatory and inhibitory synaptogenesis[51]. The initial experiments were conducted in the spinal cord[51], and now homophilic γ-protocadherin astrocyte-neuron interactions have also been shown to be critical in the developing mouse cortex[52]. Elimination of astrocytic γ-protocadherins in the cortex reduces dendritic complexity, non-cell autonomously. Specifically, astrocytic manipulation results in a decrease in the number of branchpoints and total dendrite length of layer V pyramidal neurons[52]. Interestingly, loss of γ-protocadherin in astrocytic processes does not appear to adversely impact astrocyte survival or complexity[52]. Notably, alternative splicing of γ-protocadherin gives rise to 22 different isoforms. A mismatch of isoforms disrupts homophilic interactions between the astrocyte and the growing dendrite[52]. Taken together, these studies show that transcellular γ-protocadherin interactions can confer specificity and significant diversity in astrocyte-dendrite interactions.
Apart from homophilic trans-interactions, γ-protocadherins can make cis-interactions with Nlgn1[53], functioning as a competitive inhibitor of transcellular neurexin-neuroligin signaling[53]. In a heterologous cell culture system, cis-interactions between γ-protocadherin and Nlgn1 in non-neuronal cells, such as Cos7 cells, prevented both presynaptic differentiation and dendritic spines formation[53]. Similarly, inhibitory cis-interactions between γ-protocadherins and Nlgn2 were also identified recently[54]. Whether γ-protocadherins co-localize with Nlgn1 or Nlgn2 on PAPs is yet to be determined. However, it is highly plausible that astrocytic γ-protocadherins through their trans and cis binding partners control important aspects of astrocyte-neuron interactions and provide a dynamic specificity and diversity to the contacts between cells.
Regulation of inhibitory synaptogenesis by NrCAM
In the past decade, several astrocyte-neuron signaling mechanisms regulating excitatory synaptogenesis have been elucidated. However, our understanding of how cell adhesion between astrocytes and neurons impact inhibitory synapse formation and function has been severely lacking. A recent study characterized the proteome of the astrocyte-synapse junctions as they exist in vivo using a targeted biotinylation-based approach. Among the 118 proteins which were identified as high confidence tripartite synaptic proteins, Neuronal Cell Adhesion Molecule (NrCAM) was shown to be a critical regulator of transcellular interactions between astrocytes and inhibitory post-synapses[55].
NrCAM is enriched at astrocyte processes. Super resolution imaging of PAPs and synaptic markers revealed that NrCAM is localized to astrocyte-inhibitory synapse contacts in the cortex. Nrcam deletion in cortical astrocytes results in increased astrocyte territory and neuropil infiltration volume[55], opposite of the phenotype observed with the loss of astrocytic neuroligins. These phenotypes are rescued by wildtype NcCAM but not by NrCAM lacking the extracellular immunoglobulin domains. These results indicate that astrocytic NrCAM mediates a transcellular interaction through its extracellular region[55]. In agreement, a homophilic interaction mediated by NrCAM between astrocytes and neurons were found to be critical, because knockout of Nrcam in neurons phenocopied the loss of astrocytic Nrcam[55]. These results indicate that NrCAM from both neurons and astrocytes interact transcellularly to negatively regulate astrocyte morphogenesis[55].
Importantly, homophilic NrCAM interaction between astrocytes and neurons also regulates inhibitory synapse formation, which was revealed by the comparison of inhibitory synapse numbers within the domains of NrCAM-depleted astrocytes and their wildtype neighbors[55]. Loss of NrCAM in astrocytes or neurons both result in a significant decrease in inhibitory synapses[55]. Interestingly, in neurons NrCAM is localized to the inhibitory postsynaptic specializations, because NrCAM directly binds to the postsynaptic scaffold protein, Gephyrin. In summary, through a homophilic transcellular interaction, NrCAM bridges growing astrocyte processes to inhibitory synapse development. When this interaction is impaired, astrocytes overgrow into the neuropil and disrupt inhibitory synapse formation[55].
NRCAM gene in humans has been implicated in polysubstance abuse[56]. A recent study showed that NrCAM regulates addiction-related neural circuits both during development and in the mature brain[57]. The decline in inhibitory synapses due to NrCAM loss of function is likely to profoundly impact E/I balance, which drive congenital preference for addictive substances. The exact pathogenic mechanisms are yet unknown; however, loss-of-function mutations in NRCAM impact both astrocytes and neurons by disrupting transcellular interactions. There are many other neurological diseases in which inhibitory circuits and E/I balance are disrupted. Therefore, a deeper knowledge of the molecular players at the inhibitory tripartite synapses would provide a better understanding of the pathophysiology of these diseases.
Modes of contact-mediated astrocyte-synapse interactions
Initial characterization studies in the early 2000s about protoplasmic astrocyte morphological complexity and functional proximity to axons and dendrites[10,11,15] highlighted the potential importance of cell adhesion molecules at the tripartite synapse. New knowledge about these contact-mediated mechanisms have demonstrated the diversity and complexity of bidirectional signaling between astrocytes and neurons in development, plasticity, and disease. Importantly, there is newfound appreciation for how astrocyte morphological maturation directly regulates both excitatory and inhibitory synaptogenesis during development[37,55].
How do astrocyte-neuron interactions coordinate astrocyte process arborization and synaptic circuit development? As we mentioned above, astrocytic adhesions could boost[37] or inhibit[55] synapse formation and function. Thus, there could be at least two distinct modes of cell-cell interactions at the tripartite synapse: cooperation and competition (Figure 2)[58].
Figure 2. Modes of astrocyte-neuron contact-contact mediated interactions.
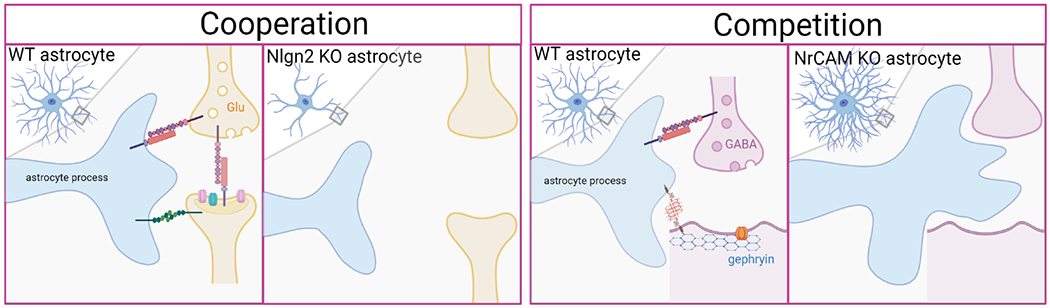
(Left) Astrocytes mature morphologically concurrently with synapse development. Cooperation between developing astrocytes and neurites are critical in stabilizing trans-synaptic connections, resulting in a functional tripartite synapse. (Right) On the other hand, astrocyte-neuron contacts may act as a negative regulator of astrocyte overgrowth that would otherwise encroach into the forming synapse and prevent synaptogenesis.
First, astrocytes and synapses may depend on each other to develop and mature cooperatively. Astrocyte morphological complexity brings the fine PAPs into close contact with the developing synapse. Transcellular adhesions between astrocytes and neurons help anchor axons and dendrites close to each other and stabilize interactions between the pre-and post-synaptic compartments, resulting in a functional tripartite synapse. When astrocytes fail to mature, for example in the case of Nlgn2 knockout astrocytes[37], they are no longer in close proximity to the developing axon/dendrite and are unable to mediate excitatory synapse formation and maturation. Secondly, astrocyte-neuron interactions might be competitive, limiting astrocyte growth into neuronal synapses. For instance, NrCAM homophillic interactions between astrocytes and neurons negatively regulate astrocyte complexity and when this interaction is lost, PAPs overgrow. This overgrowth occurs concurrently with loss of inhibitory synapse formation suggesting that astrocytic PAP overgrowth may compete off forming inhibitory synapses by encroaching into the nascent synaptic adhesions[55].
Conclusions
In this review, we highlight some of the latest advances in our understanding of how adhesion-based mechanisms control astrocyte-neuron interactions at the tripartite synapse. Furthermore, we discuss how these molecular interactions regulate concordant development of astrocytes and synapses. The findings emerging from these studies clearly demonstrate that synaptic cell adhesions cannot be solely viewed as made between neurons. Astrocytes use similar cell adhesion molecules to infiltrate into the developing neuropil and interact with synapses.
In the future, synaptic functions of cell adhesion molecules should be evaluated not just in neurons but also in astrocytes, because both cell types are integral to the structure and function of excitatory and inhibitory circuits. Another critical question that is yet to be resolved is how do cell adhesion molecules cross-talk at the interface of astrocytes and neurons? Do different molecular players, such as protocadherins and neuroligins, dynamically regulate each other’s functions at the tripartite synapse? Do astrocyte contacts specialize in tune with the needs of the neighboring synapse? It would be important to answer these questions not only because astrocytes have been implicated in synaptic pathologies of a wide range of neurological diseases, but mutations in the cell adhesion molecules discussed in this review are also strongly associated with neuropathology. This suggests that aberrant bidirectional communication between astrocytes and neurons may be an underlying mechanism in the pathogenesis of many neurological diseases and could be an excellent target for therapeutics.
Highlights.
Astrocytes directly contact axons and dendrites via cell adhesion molecules (CAMs)
Transcellular contact control synaptogenesis and astrocyte maturation concurrently
Dysfunction in astrocytic CAMs contribute to brain pathology and disease
Acknowledgments
Relevant work by the Eroglu laboratory mentioned in this review is supported by NIH RO1 DA031833, NIH ROI1 DA047258 and a Holland Trice Brain Research Award which are awarded to C.E. The authors declare no competing conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no competing conflicts of interest.
Bibliography
- 1.Farhy-Tselnicker I, Allen NJ: Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev 2018, 13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papouin T, Dunphy J, Tolman M, Foley JC, Haydon PG: Astrocytic control of synaptic function. Philos Trans R Soc B Biol Sci 2017, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santello M, Toni N, Volterra A: Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci 2019, 22:154–166. [DOI] [PubMed] [Google Scholar]
- 4.Pfrieger FW, Barres BA: Synaptic efficacy enhanced by glial cells in vitro. Science 1997, 277:1684–1687. [DOI] [PubMed] [Google Scholar]
- 5.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA: Control of synapse number by glia. Science 2001, 291:657–661. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin KT, Eroglu C: Molecular mechanisms of astrocyte-induced synaptogenesis. Curr Opin Neurobiol 2017, 45:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen NJ, Eroglu C: Cell biology of astrocyte-synapse Interactions. Neuron 2017, 96:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan CX, Burrus Lane CJ, Eroglu C: Role of astrocytes in synapse formation and maturation. Academic Press; 2021. [DOI] [PubMed] [Google Scholar]
- 9.Zhou B, Zuo YX, Jiang RT: Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci Ther 2019, 25:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventura R, Harris KM: Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 1999, 19:6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araque A, Parpura V, Sanzgiri RP, Haydon PG: Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci 1999, 22:208–215. [DOI] [PubMed] [Google Scholar]
- 12.Arizono M, Inavalli VVGK, Panatier A, Pfeiffer T, Angibaud J, Levet F, Ter Veer MJT, Stobart J, Bellocchio L, Mikoshiba K, et al. : Structural basis of astrocytic Ca2+ signals at tripartite synapses. Nat Commun 2020, 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu-Friedman MA, Harris KM, Regehr WG: Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci 2001, 21:6666–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.lino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, et al. : Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 2001, 292:926–929. [DOI] [PubMed] [Google Scholar]
- 15.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG: Synaptic islands defined by the territory of a single astrocyte. J Neurosci 2007, 27:6473–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberheim NA, Takano T, Han X, He W, Lin JHC, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, et al. : Uniquely hominid features of adult human astrocytes. J Neurosci 2009, 29:3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto M, Ueda R, Takahashi K, Saigo K, Uemura T: Control of Axonal Sprouting and Dendrite Branching by the Nrg-Ank Complex at the Neuron-Glia Interface. Curr Biol 2006, 16:1678–1683. [DOI] [PubMed] [Google Scholar]
- 18.Colón-Ramos DA, Margeta MA, Shen K: Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 2007, 318:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doherty J, Logan MA, Tafdemir ÖE, Freeman MR: Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci 2009, 29:4768–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishida H, Okabe S: Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci 2007, 27:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ango F, Wu C, Van der Want JJ, Wu P, Schachner M, Huang ZJ: Bergmann Glia and the Recognition Molecule CHL1 Organize GABAergic Axons and Direct Innervation of Purkinje Cell Dendrites. PLoS Biol 2008, 6:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng SP, Schachner M, Boddeke E, Copray S: Effect of Cell Adhesion Molecules on the Neurite Outgrowth of Induced Pluripotent Stem Cell-Derived Dopaminergic Neurons. Cell Reprogram 2016, 18:55–66. [DOI] [PubMed] [Google Scholar]
- 23.Cho S, Muthukumar AK, Stork T, Coutinho-Budd JC, Freeman MR: Focal adhesion molecules regulate astrocyte morphology and glutamate transporters to suppress seizure-like behavior. Proc Natl Acad Sci U S A 2018, 115:11316–11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murai KK, Nguyen LN, Irie F, Yu Y, Pasquale EB: Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci 2003, 6:153–160. [DOI] [PubMed] [Google Scholar]
- 25.Filosa A, Paixo S, Honsek SD, Carmona MA, Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R, Klopstock T, et al. : Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci 2009, 12:1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiessling S, O’Callaghan EK, Freyburger M, Cermakian N, Mongrain V: The cell adhesion molecule EphA4 is involved in circadian clock functions. Genes, Brain Behav 2018, 17:82–92. [DOI] [PubMed] [Google Scholar]
- 27.Park I, Lee HS: EphB/ephrinB signaling in cell adhesion and migration. Mol Cells 2015, 38:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **28.Koeppen J, Nguyen AQ, Nikolakopoulou AM, Garcia M, Hanna S, Woodruff S, Figueroa Z, Obenaus A, Ethell IM: Functional consequences of synapse remodeling following astrocyte-specific regulation of ephrin-B1 in the adult hippocampus. J Neurosci 2018, 38:5710–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study presents a novel mechanism of astrocyte-mediated synaptic plasticity and establishes astrocytic ephrin-B1 as a critical regulator of learning and memory. The authors propose a mechanism whereby astrocyte-neuron contact mediated by astrocytic ephrinB1/neuronal EphB results promotes engulfment of immature synapses.
- 29.Nguyen AQ, Koeppen J, Woodruff S, Mina K, Figueroa Z, Ethell IM: Astrocytic Ephrin-B1 Controls Synapse Formation in the Hippocampus During Learning and Memory. Front Synaptic Neurosci 2020, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **30.Nguyen AQ, Sutley S, Koeppen J, Mina K, Woodruff S, Hanna S, Vengala A, Hickmott PW, Obenaus A, Ethell IM: Astrocytic ephrin-B1 controls excitatory-inhibitory balance in developing hippocampus. J Neurosci 2020, 40:6854–6871. [DOI] [PMC free article] [PubMed] [Google Scholar]; Following their study from 2018 (ref [28]), the authors identified novel functions for astrocytic ephrinB1 during development. Altered expression of astrocytic ephrinB1 affects excitation/inhibition balance and impairs socialibility.
- 31.Nikolakopoulou AM, Koeppen J, Garcia M, Leish J, Obenaus A, Ethell IM: Astrocytic ephrin-BI regulates synapse remodeling following traumatic brain injury. ASN Neuro 2016, 8:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuang Z, Yang B, Theus MH, Sick JT, Bethea JR, Sick TJ, Liebl DJ: EphrinBs regulate D-serine synthesis and release in astrocytes. J Neurosci 2010, 30:16015–16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H, Li R, Yuan J, Zhou X, Liu X, Ou S, Xu T, Chen Y: Up-regulated ephrinB3/EphB3 expression in intractable temporal lobe epilepsy patients and pilocarpine induced experimental epilepsy rat model. Brain Res 2016, 1639:1–12. [DOI] [PubMed] [Google Scholar]
- 34.Zhou C, Feng Z, Ko CP: Defects in motoneuron—astrocyte interactions in spinal muscular atrophy. J Neurosci 2016, 36:2543–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Südhof TC: Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Cell 2017, 171:745–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. : An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014, 34:11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **37.Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji RR, Eroglu C: Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 2017, 551:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is the first to demonstrate a function for neuroligins in astrocytes in vitro and in vivo. The authors also link astrocyte morphological maturation and synaptogenesis, two concurrent processes vital during cortical development.
- 38.Freeman MR: Specification and morphogenesis of astrocytes. Science (80- ) 2010, 330:774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morel L, Higashimori H, Tolman M, Yang Y: VGluT1+ Neuronal Glutamatergic signaling regulates postnatal developmental maturation of cortical protoplasmic astroglia. J Neurosci 2014, 34:10950–10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MSB, Li G, et al. : Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchigashima M, Cheung A, Suh J, Watanabe M, Futai K: Differential expression of neurexin genes in the mouse brain. J Comp Neurol 2019, 527:1940–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trotter JH, Dargaei Z, Wöhr M, Liakath-Ali K, Raju K, Essayan-Perez S, Nabet A, Liu X, Südhof TC: Astrocytic Neurexin-1 orchestrates functional synapse assembly. bioRxiv 2020, doi: 10.1101/2020.08.21.262097. [DOI] [Google Scholar]
- 43.Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P: Silencing of neuroligin function by postsynaptic neurexins. J Neurosci 2007, 27:2815–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Z, Hom S, Kudze T, Tong XJ, Choi S, Aramuni G, Zhang W, Kaplan JM: Neurexin and neuroligin mediate retrograde synaptic inhibition in C. elegans. Science (80- ) 2012, 337:980–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen YC, Lin YQ, Banerjee S, Venken K, Li J, Ismat A, Chen K, Duraine L, Bellen HJ, Bhat MA: Drosophila neuroligin 2 is required presynaptically and postsynaptically for proper synaptic differentiation and synaptic transmission. J Neurosci 2012, 32:16018–16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao X, Tabuchi K: Functions of synapse adhesion molecules neurexin/neuroligins and neurodevelopmental disorders. Neurosci Res 2017, 116:3–9. [DOI] [PubMed] [Google Scholar]
- 47.Matta SM, Moore Z, Walker FR, Hill-Yardin EL, Crack PJ: An altered glial phenotype in the NL3R451C mouse model of autism. Sci Rep 2020, 10:14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owczarek S, Bang ML, Berezin V: Neurexin-neuroligin synaptic complex regulates schizophrenia-related DISC1/Kal-7/Rac1 “signalosome”. Neural Plast 2015, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tromp A, Mowry B, Giacomotto J: Neurexins in autism and schizophrenia—a review of patient mutations, mouse models and potential future directions. Mol Psychiatry 2020, doi: 10.1038/s41380-020-00944-8. [DOI] [PubMed] [Google Scholar]
- 50.Pancho A, Aerts T, Mitsogiannis MD, Seuntjens E: Protocadherins at the Crossroad of Signaling Pathways. Front Mol Neurosci 2020, 13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrett AM, Weiner JA: Control of CNS synapse development by γ-protocadherin-mediated astrocyte-neuron contact. J Neurosci 2009, 29:11723–11731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Molumby MJ, Keeler AB, Weiner JA: Homophilic Protocadherin Cell-Cell Interactions Promote Dendrite Complexity. Cell Rep 2016, 15:1037–1050. Building on their previous study in 2009 (ref [51]), the authors demonstrate that γ-protocadherin-mediated astrocyte-neuron interactions regulate dendrite complexity. They also propose a model where homophillic γ-protocadherin interactions are isoform specific, suggesting that the diversity of protocadherin-based interactions that could in turn regulate specific local circuits.
- 53.Molumby MJ, Anderson RM, Newbold DJ, Koblesky NK, Garrett AM, Schreiner D, Radley JJ, Weiner JA: γ-Protocadherins Interact with Neuroligin-1 and Negatively Regulate Dendritic Spine Morphogenesis. Cell Rep 2017, 18:2702–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steffen DM, Ferri SL, Marcucci CG, Blocklinger KL, Molumby MJ, Abel T, Weiner JA: The γ-Protocadherins Interact Physically and Functionally with Neuroligin-2 to Negatively Regulate Inhibitory Synapse Density and Are Required for Normal Social Interaction. Mol Neurobiol 2021, doi : 10.1007/s12035-020-02263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **55.Takano T, Wallace JT, Baldwin KT, Purkey AM, Uezu A, Courtland JL, Soderblom EJ, Shimogori T, Maness PF, Eroglu C, et al. : Chemico-genetic discovery of astrocytic control of inhibition in vivo. Nature 2020, 588:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is the first to characterize astrocyte-neuron interactions at the inhibitory synapse. It identified a novel role for NrCAM in astrocytes and also functionally links astrocyte morphogenesis and inhibitory synaptogenesis during development.
- 56.Ishiguro H, Liu QR, Gong JP, Hall FS, Ujike H, Morales M, Sakurai T, Grumet M, Uhl GR: NrCAM in addiction vulnerability: Positional cloning, drug-regulation, haplotype-specific expression, and altered drug reward in knockout mice. Neuropsychopharmacology 2006, 31:572–584. [DOI] [PubMed] [Google Scholar]
- 57.Ishiguro H, Miyake K, Tabata K, Mochizuki C, Sakurai T, Onaivi ES: Neuronal cell adhesion molecule regulating neural systems underlying addiction. Neuropsychopharmacol Reports 2019, 39:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakers K, Eroglu C: Control of neural development and function by glial neuroligins. Curr Opin Neurobiol 2019, 57:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]


