Abstract
Objective:
To understand the relationship between drug use, food insecurity (FI) and mental health among men who have sex with men (MSM).
Design:
Cohort study (2014–2019) with at least one follow-up.
Setting:
Visits at 6-month intervals included self-assessment for FI and depressive symptoms. Urine testing results confirmed drug use. Factors associated with FI were assessed using multiple logistic regression with random effects for repeated measures. General structural equation modelling tested whether FI mediates the relationship between drug use and depressive symptoms.
Participants:
Data were from HIV-positive and high-risk HIV-negative MSM in Los Angeles, CA (n 431; 1192 visits).
Results:
At baseline, FI was reported by 50·8 % of participants, depressive symptoms in 36·7 % and 52·7 % of urine screening tests were positive for drugs (i.e. marijuana, opioids, methamphetamine, cocaine and ecstasy). A positive drug test was associated with a 96 % increase in the odds of being food insecure (95 % CI 1·26, 3·07). Compared to those with high food security, individuals with very low food security have a nearly sevenfold increase in the odds of reporting depressive symptoms (95 % CI 3·71, 11·92). Findings showed 14·9 % of the association between drug use (exposure) and depressive symptoms (outcome) can be explained by FI (mediator).
Conclusion:
The prevalence of FI among this cohort of HIV-positive and high-risk HIV-negative MSM was high; the association between drug use and depressive symptoms was partially mediated by FI. Findings suggest that enhancing access to food and nutrition may improve mood in the context of drug use, especially among MSM at risk for HIV transmission.
Keywords: Food insecurity, Drug use, Depressive symptoms, Men who have sex with men, HIV/AIDS
Food insecurity (FI) is defined by the United States Department of Agriculture (USDA) as a ‘limited or uncertain availability of nutritionally adequate and safe foods or limited or uncertain ability to acquire acceptable foods in socially acceptable ways’(1). Estimates from a study in 2017 showed that 11·8 % of US households are food insecure with higher prevalence among adults living alone, non-White and low-income households(2). The National Health and Nutrition Examination Survey (NHANES) data have shown that the prevalence of FI doubled from approximately 9 % in 2005–2006 to 18 % in 2011–2012(3). This has been partially attributed to the Economic Recession (2007–2009), which has subsequently improved although not to pre-recession proportions(4,5). Prevalence estimates of FI are much higher in low-income, underserved, and substance-using individuals and communities, with estimates ranging from 42 to 71 %(6–10).
In addition to income and poverty, other factors appear to exacerbate risk of FI such as minority status or comorbid health problems. Sexual minority communities have higher proportions of FI compared to the general population(11), as do people reporting chronic health conditions (e.g. back problems and arthritis)(12). In turn, FI may increase risk of adverse health outcomes by hindering access and adherence to medical care(4). Among people living with HIV, FI is observed at nearly fivefold higher than in the general population(13) and has also been associated with prevalent drug use(14). Global HIV research has examined the bidirectionality of this association, suggesting that FI may increase HIV risk transmission behaviours (e.g. risky sexual practices), susceptibility to HIV once exposed (e.g. nutrient deficiencies leading to immunologic decline), and decreased access and adherence to treatment and care(15–20). Among low-income urban men who have sex with men (MSM), FI was reported to be a strong contributor of risky sexual practices (transactional and condom-less sex)(21).
The link between FI (and food insufficiency, defined by measures other than the USDA tool) and depressive symptoms has been well documented. A recent systematic review of 57 US studies has shown that FI is associated with a 2·74 (95 % CI 2·52, 2·97) increase in the odds of testing positive for depression (n 135 500)(22). Cohort data among those with HIV and hepatitis C virus (HCV) co-infection have shown that FI precedes depressive symptoms, and that even when FI remits, depressive symptoms can persist(23). Other studies have investigated depressive symptoms as an intermediary rather than as an outcome. Among those with HIV–HCV co-infection, depressive symptoms partially mediate the effect of severe FI on HIV viral load suppression(24). In this analysis, we conceptualise FI as a driver of depressive symptoms.
Biological pathways linking FI to depressive symptoms are likely to exist. However, this may require the assumption that FI is linked to poor dietary intake. To illustrate, within the burgeoning field of nutritional psychiatry, there is emerging evidence suggesting that the gut–brain axis may play a role in depression(25–27). For example, chronic low-fibre diets may lead to microbiome depletion, resulting in intestinal permeability, inflammation, neuroinflammation and depressive symptoms(28–30). A recent systematic review and meta-analysis concluded that diet may have a direct effect on biological processes (e.g. chronic inflammation and neurotransmitter production) involved in depression(31). Interestingly, data from the US suggest that FI is associated with obesity(32–35) which may be linked to overall poor diet quality(36). Given that obesity has been strongly correlated with depressive symptoms(37–39), we adjust for BMI in all analyses.
Individuals using substances (which includes drugs and alcohol) are more likely to be food insecure. In an urban youth population in Boston (n 400, ages 15–25), substance use was associated with a 2·5 (95 % CI 1·5, 4·3) increase in the odds of FI(40). Among people who use drugs, FI may have biological implications that link exacerbation of mental health problems to ongoing substance use. Relationships between FI and substance use may be reciprocal, related to: (1) reduced intake and absorption of food; (2) reduced access to food due to competing demands on resources; (3) stigma and lack of social support; (4) negative coping behaviour related to depression/anxiety; and (5) help-seeking and treatment adherence(41). Many investigators believe that drug use contributes to FI due to prioritisation of substances over food purchases(42). These authors have also posited that FI can be a structural driver of drug use (e.g. individuals with FI find the appetite-suppressing effect of stimulants appealing). The current study investigates the former hypothesis that drug use drives FI. This relationship may be particularly pronounced among people who inject drugs(43). Given that individuals using drugs are frequently malnourished and report preferences for nutrient-poor foods(44–47), we hypothesise that FI will partially mediate the established association between drug use and depressive symptoms(48,49).
Taken together, FI strongly correlates with poverty, BMI, depressive symptoms, drug use and HIV status. This study examines FI as a mediator between drug use and mental health outcomes. The goals of the current study are to test if: (1) drug use is an independent predictor of FI, controlling for HIV status and other known risk factors; (2) FI is an independent predictor of depressive symptoms, controlling for drug use, HIV status and other known risk factors; and (3) the relationship between drug use and depressive symptoms is partially mediated by FI.
Materials and methods
Study population
Data for the present study come from the mSTUDY(48,50), an ongoing National Institute on Drug Abuse (NIDA)-sponsored longitudinal study of HIV-positive and HIV-negative MSM with varied substance use behaviours (NIDA project U01DA036267). The mSTUDY was approved by the University of California, Los Angeles (UCLA) Institutional Review Board, and all individuals provided written informed consent at study entry. Study enrolment began in August 2014 and is ongoing. Eligible participants reported being assigned male sex at birth, aged 18–45 years; if HIV-negative, reported having sex with men in the past 12 months, and were recruited from two community clinics in Los Angeles, CA. Participants complete assessments every 6 months, including a comprehensive physical exam and medical history, urine drug panel, clinical laboratory tests and computer-assisted detailed behavioural questionnaire. By design, half of the sample is HIV-positive and the other half HIV-negative. Participants were remunerated for study participation ($75 per study visit). The current analysis uses data collected from May 2017 when the FI questions were added to the battery of behavioural data collected as part of the computer-assisted self-interviews. Data for this analysis include all visits from May 2017 (prior visits were excluded because FI measure was not yet introduced) through June 2019, totalling 1192 person-visits from 431 participants.
Social, behavioural and biological covariates
FI is operationalised using the US Household Food Security Module(51) from the Economic Research Service of the USDA. It is a three-stage design with screeners, where an affirmative answer on a question from one stage advances participants to the next. The final stage asks about not eating for an entire day because there was not enough money to buy food. The raw score (0–10) is then categorised as: high food security, marginal food security, low food security and very low food security, which can then be dichotomised into food secure (high or marginal) or food insecure (low or very low). Depressive symptoms is measured using the Center for Epidemiologic Studies Depression (CESD) scale, a 20-item validated measure for capturing depressive symptomatology in the general population(52). Scores range from 0 to 60. The standard cut-point for ‘likely depressed’ is 16; however, the cut-point of <23/23+ is used to classify clinically meaningful symptoms linked with likely diagnosis of depressive disorder(53).
Education is categorised as: didn’t finish high school (0–11 years), high school (12 years), some college (13–15 years) and college grad+ (16 years) (reference group). Ethnicity is categorised as Black, White (reference group), Other Race and Hispanic/Latino. Other Race includes categories: American Indian or Alaskan Native, Asian, Asian Indian, and Native Hawaiian Pacific Islander which were all collapsed due to small sample size. Income is categorised as: $0–19 999, $20 000–39 999 and $40 000+ (reference group). An indicator variable was created if the participant endorsed current use of cigarette or e-cig/vape (combined). Urine drug screen (Fastect® II Drug Screen Dipstick Test D, Brenan Medical Corporation, Irvine, CA) documented recent use of any of the following: methamphetamine, opiates, cocaine, ecstasy, marijuana, amphetamines and fentanyl (all drugs were tested using separate drug screen tests). A positive test for any of these drugs of misuse defined the participant as positive for current drug use. HIV status is determined positive or negative based on serology (positive antibody + viral load and negative antibody test). Age is classified as: 18–29, 30–39 and 40–49 years (reference group). Height and weight are measured at each visit to calculate BMI and was categorised using the standard US definition: underweight (below 18·5), normal weight (18·5–25) (reference group), overweight (25–29·99) and obese (30 and above).
Statistical analysis
All data analyses were performed using Stata version 16.0(54). Sample characteristics were ascertained at the first visit where FI data were collected (i.e. index visit for this analysis). Bivariate analysis of all covariates in relation to FI were conducted to examine trends and significance (set at P < 0·05). All CI were set to 95 %. Logistic regression using a random intercept with unstructured correlation was conducted using the dichotomised FI outcome. The second logistic model used the dichotomised depression score (at or above 23) as the outcome. General structural equation modelling tested whether FI mediates the relationship between drug use and depressive symptoms. Mediation analysis was conducted using linear regression (continuous variables for FI and depressive symptoms), adjusting for the same set of covariates. Because mediation analysis is less robust when using binary mediators and outcomes(55), continuous versions of the FI and CESD were used. Mediation percent was ascertained by dividing indirect effects by direct effects.
Results
Sample characteristics
The mean age of study participants at the index visit, defined as the first study visit for this analysis based on availability of FI data (n 431), was 32·9 years (sd = 6·93). In our sample, 40·4 % identified as African American/Black, 37·6 % as Hispanic/Latino and more than half had some college or were college graduates. At the index visit, FI was detected in 50·8 % of participants and depressive symptoms in 36·7 %. Table 1 summarises characteristics of the sample stratified by food security status, as well as the overall total. At this index visit, those who were food insecure were more likely to use drugs (as verified by urine drug test), endorse current use of cigarette/vape, meet criteria for likely depressed and be in the lowest income category (P < 0·001 for all comparisons).
Table 1.
Sample characteristics of MSM from two clinics in Los Angeles, California, at baseline visit (n 431), stratified by food security status
| Food secure (n 212) mean (sd), n | % | Food insecure (n 219) mean (sd), n | % | P-value | Total (n 431) mean (sd), n | % | |
|---|---|---|---|---|---|---|---|
| Age (range 18–45 years) | 33·2 | 6·86 | 32·6 | 6·99 | 0·883 | 32·9 | 6·93 |
| HIV-positive | 114 | 53·8 | 125 | 57·1 | 0·490 | 239 | 55·5 |
| Drug use | 93 | 43·9 | 134 | 61·2 | <0·001 | 227 | 52·7 |
| Cigarette/vape use | 47 | 22·2 | 85 | 38·8 | <0·001 | 132 | 30·6 |
| Likely depressed* | 43 | 20·9 | 112 | 51·9 | <0·001 | 155 | 36·7 |
| Ethnicity | 0·401 | ||||||
| Black | 77 | 36·3 | 97 | 44·3 | 174 | 40·4 | |
| Hispanic/Latino | 86 | 40·6 | 76 | 34·7 | 162 | 37·6 | |
| White | 34 | 16·0 | 31 | 14·2 | 65 | 15·1 | |
| Other | 15 | 7·1 | 15 | 6·9 | 30 | 7·0 | |
| Education | 0·169 | ||||||
| Less than high school | 17 | 8·1 | 26 | 12·1 | 43 | 10·1 | |
| Finished high school | 66 | 31·6 | 76 | 35·4 | 142 | 33·5 | |
| Some college | 66 | 31·6 | 69 | 32·1 | 135 | 31·8 | |
| College grad+ | 60 | 28·7 | 44 | 20·5 | 104 | 24·5 | |
| Income | <0·001 | ||||||
| $0–19 999 | 107 | 50·7 | 161 | 74·2 | 268 | 62·6 | |
| $20 k–39,999 | 51 | 24·2 | 45 | 20·7 | 96 | 22·4 | |
| $40 000+ | 53 | 25·1 | 11 | 5·1 | 64 | 15·0 | |
| BMI† | 0·075 | ||||||
| Underweight | 4 | 1·9 | 6 | 2·9 | 10 | 2·4 | |
| Normal | 68 | 33·0 | 87 | 41·6 | 155 | 37·4 | |
| Overweight | 70 | 34·0 | 73 | 34·9 | 143 | 34·5 | |
| Obesity | 64 | 31·1 | 43 | 20·6 | 107 | 25·8 |
MSM, men who have sex with men; CESD, Center for Epidemiologic Studies Depression.
Based on CESD cut-point 22 (likely depressed is 23 or greater).
Underweight <18·5; normal 18·5–24·9; overweight 25–29·9; obese ≥ 30.
Association between drug use and food insecurity
Table 2 summarises the logistic model with FI as the outcome, with n 426 across 1147 person-visits. As hypothesised, drug use is associated with 96 % increase in the odds of being food insecure (95 % CI 1·26, 3·07), after adjustment for covariates.
Table 2.
Adjusted mixed logistic random intercept model of drug use in food insecurity among MSM from two clinics in Los Angeles, California (n 426; 1147 person-visits)
| Food insecurity | aOR | 95 % CI | P-value |
|---|---|---|---|
| HIV-positive | 1·06 | 0·62, 1·81 | 0·834 |
| Drug use | 1·96 | 1·26, 3·07 | 0·003 |
| Cigarette/vape use | 1·60 | 0·98, 2·60 | 0·060 |
| Age (years) | |||
| 18–29 | 1·74 | 0·86, 3·49 | 0·121 |
| 30–39 | 2·24 | 1·20, 4·20 | 0·011 |
| 40–49 | – | – | – |
| Ethnicity | |||
| Black | 0·70 | 0·34, 1·41 | 0·317 |
| Hispanic/Latino | 0·55 | 0·28, 1·08 | 0·086 |
| White | – | – | – |
| Other | 0·54 | 0·22, 1·34 | 0·184 |
| Education | |||
| Less than high school | 1·88 | 0·81, 4·37 | 0·144 |
| Finished high school | 1·24 | 0·65, 2·38 | 0·512 |
| Some college | 1·72 | 0·92, 3·21 | 0·091 |
| College grad+ | – | – | – |
| Income | |||
| $0–19 999 | 7·42 | 3·53, 15·62 | <0·001 |
| $20 k–39,999 | 4·21 | 1·93, 9·16 | <0·001 |
| $40 000+ | – | – | – |
| BMI* | |||
| Underweight | 0·48 | 0·11, 2·14 | 0·333 |
| Normal | – | – | – |
| Overweight | 0·73 | 0·43, 1·23 | 0·239 |
| Obesity | 0·56 | 0·30, 1·03 | 0·062 |
| Constant | 0·71 | 0·02, 0·23 | <0·001 |
MSM, men who have sex with men; aOR: adjusted OR.
Underweight <18·5; normal 18·5–24·9; overweight 25–29·9; obese ≥ 30.
Association between food insecurity and depressive symptoms
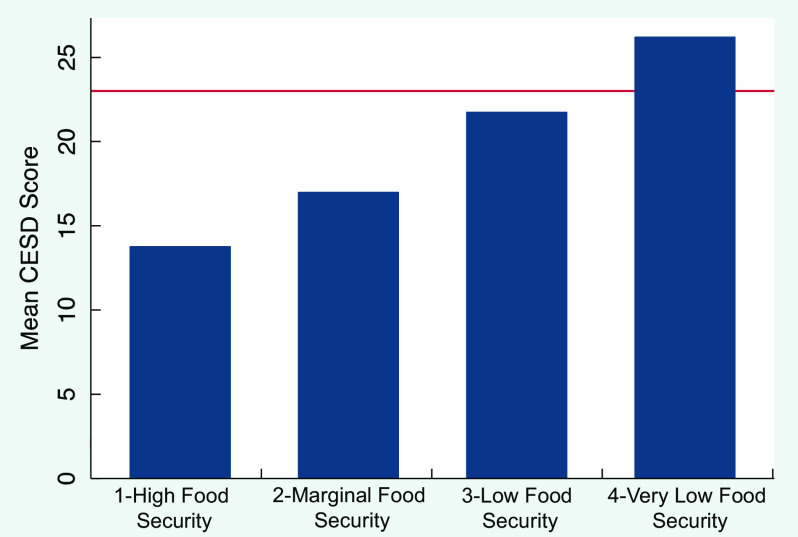
Figure 1 displays the relationship between FI and depressive symptoms, demonstrating a graded relationship between FI and CESD score, with a line indicating our cut-point for likely depressed. The gradient remained after adjusting for HIV status, drug use, current cigarette/vape use, age, ethnicity, education, income and BMI in our final model. Table 3 shows the signal increases between FI and likelihood of depression (i.e. CESD score ≥ 23) in a significant and stepwise manner, after adjusting for HIV status, drug use, current cigarette/vape use, age, ethnicity, education, income and BMI. The final adjusted model showed that drug use associated with 2·22 times increase in the odds of depressive symptoms (95 % CI 1·36, 3·63), compared to those who did not screen positive for drugs.
Fig. 1.
Relationship between food security and mean depressive symptoms (CESD) score in an MSM cohort at two clinics in Los Angeles (n 430; 1170 person-visits). Red Line (Y = 23) represents cut-point for likely depressed. CESD, Center for Epidemiologic Studies Depression; MSM, men who have sex with men
Table 3.
Adjusted mixed logistic random intercept model of food security on depressive symptoms among MSM from two clinics in Los Angeles, California (n 425; 1126 person-visits)
| Depressive symptoms | aOR | 95 % CI | P-value |
|---|---|---|---|
| HIV-positive | 1·49 | 0·83, 2·68 | 0·177 |
| Drug use | 2·22 | 1·36, 3·63 | 0·002 |
| Cigarette/vape use | 1·42 | 0·85, 2·38 | 0·181 |
| Food security | |||
| High food security | – | – | – |
| Marginal food security | 2·50 | 1·39, 4·50 | 0·002 |
| Low food security | 4·61 | 2·64, 8·07 | <0·001 |
| Very low food security | 6·65 | 3·71, 11·92 | <0·001 |
| Age (years) | |||
| 18–29 | 1·22 | 0·57, 2·59 | 0·612 |
| 30–39 | 1·2 | 0·61, 2·36 | 0·589 |
| 40–49 | – | – | – |
| Ethnicity | |||
| Black | 0·81 | 0·38, 1·72 | 0·589 |
| Hispanic/Latino | 0·81 | 0·40, 1·66 | 0·571 |
| White | – | – | – |
| Other | 1·07 | 0·40, 2·83 | 0·894 |
| Education | |||
| Less than high school | 1·87 | 0·76, 4·60 | 0·174 |
| Finished high school | 1·17 | 0·59, 2·31 | 0·657 |
| Some college | 0·71 | 0·37, 1·39 | 0·323 |
| College grad+ | – | – | – |
| Income | |||
| $0–19 999 | 1·17 | 0·55, 2·52 | 0·681 |
| $20 k–39,999 | 0·71 | 0·31, 1·60 | 0·406 |
| $40 000+ | – | – | – |
| BMI* | |||
| Underweight | 1·63 | 0·34, 7·81 | 0·541 |
| Normal | – | – | – |
| Overweight | 91 | 0·63, 1·90 | 0·751 |
| Obesity | 0·84 | 0·44, 1·62 | 0·595 |
| Constant | 0·06 | 0·02, 0·21 | <0·001 |
MSM, men who have sex with men; aOR: adjusted OR.
Underweight <18·5; normal 18·5–24·9; overweight 25–29·9; obese ≥ 30.
Mediation of drug use on depressive symptoms by food insecurity
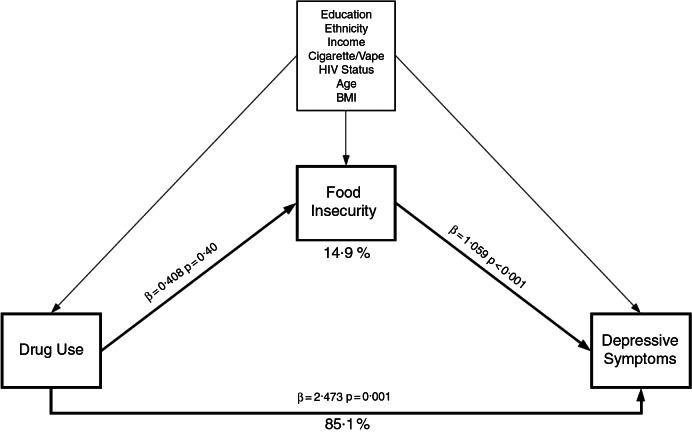
Figure 2 shows that after adjusting for covariates, indirect effects equal 0·432 (product of the mediating pathways) and direct effects equal 2·905 (sum of main effect and indirect effect) using linear regression models. Our model suggests that 14·9 % of the relationship between drug use and depressive symptoms can be explained by FI, after adjusting for covariates. By using continuous variables for FI and CESD, we have generated the most robust and conservative mediation estimate relative to other approaches.
Fig. 2.
Mediation analysis using general structural equation modelling with linear regression, examining food insecurity as an intermediate between drug use and depressive symptoms among MSM in Los Angeles, California. Mediation analysis using general structural equation modeling with linear regression, examining food insecurity (continuous) as an intermediate between drug use (binary) and depressive symptoms (continuous), adjusting for covariates in a longitudinal sample of MSM at two clinics in Los Angeles, California (n=431; 1,190 visits). Dark lines represent indirect and direct effects, lighter lines represent model adjustment. MSM, men who have sex with men
Discussion
The current study showed that our community-based sample of diverse MSM in Los Angeles have approximately four times higher prevalence of FI than the general US population from 2017(2). Our observed prevalence of FI (51 %) was similar to other studies of at-risk groups such as Hispanic adults (n 10 966) living in poverty in California (43 %)(9), HIV-positive participants in San Francisco, CA, (49 %)(10) and HIV-positive persons using intravenous drugs (71 %)(7). When stratifying by individuals who screened positive for drug use, 59 % were FI at the index visit and 54 % reported FI over the entire study period. While FI decreased over time, depressive symptoms were relatively stable (36·7 % at index visit and 36 % overall).
Consistent with our hypothesis, testing positive for recent drug use almost doubled the odds of being food insecure. This compares with a study of people who inject drugs that showed being food insecure was associated with sharing of needles, increasing the likelihood of HIV transmission(56). In conjunction with the findings of the current study, addressing food-related needs among persons who use drugs is timely and warranted. A simple, actionable effort to provide assistance with food access is one avenue to reducing suffering and towards improving mood in marginalised groups with substance use disorders. Meanwhile, provision of food assistance does not necessarily address the upstream determinants of FI; therefore, greater attention is needed to address the occurrence of FI in the first place(57). Notwithstanding, findings provide evidence for including FI measures in programmes and efforts to address health conditions faced by those with comorbidities of HIV, HIV-risk, mental health, substance use and poverty.
The signal strength that increased in a linear stepwise fashion between increasing levels of FI and increasing scores on the CESD suggest a strong relationship between these variables, even after adjustment for social, behavioural and biological factors. A similar finding of increasing signal strength between FI and likelihood of depression is reported in studies of nationally representative samples of Supplemental Nutrition Assistance Program (SNAP) participants(58). Future research might aim to determine if improving FI decreases depressive symptoms, in both drug-using and non-using populations.
Our hypothesis for mediation analysis of drug use on depressive symptoms by FI was generated to explore the questions: (1) what happens when individuals spend their money on drugs instead of food? and (2) does chronic malnourishment lead to depression? Importantly, FI is not the same thing as malnourishment, or even hunger(59). Although these concepts overlap, they are not synonymous. However, for the sake of this discussion, we are assuming that individuals with FI have worse nutritional status than those who are food secure, particularly in the context of drug use. This assumption is used to triangulate findings from the field of nutritional psychiatry which describes links between nutrition and depressive symptoms(26–31). Given the emerging evidence on the relationship between nutrition and mental health (i.e. the gut–brain axis), it is biologically plausible that limited or compromised eating patterns can exacerbate mood disorders, particularly in those who are using drugs.
Using general equation structural modelling, our data suggest that nearly 15 % of the relationship between drug use and depressive symptoms can be explained by FI, adjusting for covariates. While it has been established that FI is associated with poor diet quality (e.g. processed foods) known to increase chronic disease risk(60), less is known about the connection between FI and mental health in people who use drugs. However, one recent study examined effect modification between drug use and mood disorders with FI as the outcome(61). Novel findings linking nutrition to mental health have led to new conceptual models which identify ecological (e.g. food availability), economic (e.g. income, education) and social (e.g. gender, stigma) factors in the FI–mood–substance intersection. Our work provides additional support for the explanatory pathways suggested in their model, but there are many questions in the domain of nutritional psychiatry that remain unanswered. For example, there is a need to understand how drugs impact gut health in humans, and whether gut-based nutrition interventions can improve mental health during abstinence(62,63) or if/when the individual continues to use drugs. Future research should investigate if efforts to address FI lead to improved nutritional status, and whether or not improved nutritional status can improve mood. It has been established that anti-inflammatory diets (high in dietary fibre and n-3 fats) may reduce risk for depressive symptoms(64–66), but this remains less clear among disadvantaged groups.
There are multiple potential modes by which FI might exacerbate risk of depressive symptoms. In addition to the potential for nutrient deficiencies/imbalances and altered gastrointestinal function leading to inflammatory conditions, there is a potential pathway through the psychological impact of experiencing FI. Deprivation and socio-economic disadvantage will be stressful independent of nutritional factors. Meanwhile, we are interested in highlighting potential mechanisms explaining the association between FI and mental health, because it may inform novel intervention targets. For example, there is substantial literature suggesting that probiotics significantly reduce depressive symptoms(67–69), such as through the production of butyrate (a short chain fatty acid) in the large intestine(70). Other authors have proposed that the microbial production of and delivery of neuroactive substances such as serotonin and gamma-aminobutyric acid might explain links to depression(25). Emerging evidence suggests that substances (e.g. methamphetamine) alter the microbiome, promote inflammation and may even mediate behavioural responses to drugs(71–73). Taken together, FI may partially explain the impact of drug use on depression, with a cumulative impact over the life course. Intervening on FI through targeted nutrition interventions including life skills such as grocery shopping and cooking(74,75) might have potential to alleviate depressive symptoms among those dependent on drugs, which should be investigated in prospective cohorts.
The findings from this study should be interpreted in light of its limitations. While the data come from a longitudinal cohort and were analysed with repeated measures analysis, there remains challenges with ascertaining temporal precedence between all study variables. The current analysis was underpowered for lagged analysis across multiple time points. Therefore, it is possible that FI can increase risk of drug use, creating a reverse causation effect. Since many drugs decrease appetite, this is not implausible and has been proposed(42). The role of alcohol consumption could also contribute to FI as well as depressive symptoms and was not included in our analysis. Furthermore, the current study did not directly address dietary intake, which would be required to make any conclusions about the link between nutrition and mental health. It is also possible that FI is merely a proxy for other qualities of low socioeconomic status which can exacerbate risk for drug use and comorbid mental health problems. Finally, medication status was not included in our analysis and will be important to consider when making future inferences linking nutrition to mental health, particularly for those on HIV medications (antiretroviral therapy), anti-depressants or medication-assisted treatment.
Conclusion
In this cohort of HIV-positive and high-risk HIV-negative MSM, drug use almost doubled the odds of being food insecure. Increasing levels of FI was associated with a graded increase in the odds of depressive symptoms. Our models found that drug use more than doubled the odds of meeting criteria for being likely depressed. Mediation analysis adjusting for covariates found that FI partially explained the association between drug use and depressive symptoms. Public health efforts to enhance access to food and nutrition may be one way to improve depressive symptoms among MSM who use drugs and are at risk for HIV infection. Efforts to address the structural drivers of FI including social factors that predispose individuals to use drugs may prove beneficial for long-term mental health outcomes among MSM.
Acknowledgements
Acknowledgements: None. Financial support: Data for the present study come from the mSTUDY, an ongoing NIDA-sponsored longitudinal study of HIV-positive and HIV-negative MSM with varied substance use behaviours (NIDA project U01DA036267). Conflict of interest: There are no conflicts of interest. Authorship: All authors participated in the conception of the study and contributed to the final manuscript. D.A.W. generated hypotheses, conducted statistical analysis and wrote the first draft. M.J. and M.J.L. assisted with conceptualisation, statistical analysis and contributed to each draft revision. R.B. was involved in data collection and draft revision. M.P., S.S. and P.M.G. were involved along the entire process including multiple revisions of the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the University of California, Los Angeles (UCLA) Institutional Review Board (IRB). Written informed consent was obtained from all subjects/patients.
References
- 1. Bickel G, Nord M, Price C et al. (2000) Guide to Measuring Household Food Security. Alexandria, VA: U.S. Department of Agriculture, Food and Nutrition Science. [Google Scholar]
- 2. Coleman-Jensen A, Rabbit MP, Gregory CA et al. (2018) Household Food Security in the United States in 2017. United States Department of Agriculture. [Google Scholar]
- 3. Berkowitz SA, Berkowitz TS, Meigs JB et al. (2017) Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005–2012. PLoS One 12, e0179172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gundersen C & Ziliak JP (2017) Food insecurity and health outcomes. Health Aff 34, 1830–1839. [DOI] [PubMed] [Google Scholar]
- 5. Seligman HK & Berkowitz SA (2018) Aligning programs and policies to support food security and public health goals in the United States. Annu Rev Public Health 40, 319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kushel MB, Gupta R, Gee L et al. (2006) Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med 21, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shannon K, Kerr T, Milloy M-J et al. (2011) Severe food insecurity is associated with elevated unprotected sex among HIV-seropositive injection drug users independent of HAART use. AIDS 25, 2037–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silverman J, Krieger J, Kiefer M et al. (2015) The relationship between food insecurity and depression, diabetes distress and medication adherence among low-income patients with poorly-controlled diabetes. J Gen Intern Med 30, 1476–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becerra BJ, Sis-Medina R, Reyes A et al. (2015) Association between food insecurity and serious psychological distress among hispanic adults living in poverty. Prev Chronic Dis 12, E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weiser SD, Frongillo EA, Ragland K et al. (2009) Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. J Gen Intern Med 24, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patterson JG, Russomanno J & Tree JMJ (2020) Sexual orientation disparities in food insecurity and food assistance use in U.S. adult women: national health and nutrition examination survey, 2005–2014. BMC Public Health 20, 1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tarasuk V, Mitchell A, McLaren L et al. (2013) Chronic physical and mental health conditions among adults may increase vulnerability to household food insecurity. J Nutr 143, 1785–1793. [DOI] [PubMed] [Google Scholar]
- 13. Normén L, Chan K, Braitstein P et al. (2005) Food insecurity and hunger are prevalent among HIV-positive individuals in British Columbia, Canada. J Nutr 135, 820–825. [DOI] [PubMed] [Google Scholar]
- 14. Palar K, Laraia B, Tsai AC et al. (2016) Food insecurity is associated with HIV, sexually transmitted infections and drug use among men in the United States. AIDS 30, 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rollins N (2007) Food insecurity—a risk factor for HIV infection. PLoS Med 4, e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anema A, Vogenthaler N, Frongillo EA et al. (2009) Food insecurity and HIV/AIDS: current knowledge, gaps, and research priorities. Curr HIV AIDS Rep 6, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gwatirisa P & Manderson L (2009) Food insecurity and HIV/AIDS in low-income households in Urban Zimbabwe. Hum Organ 68, 103–112. [Google Scholar]
- 18. Ivers LC, Cullen KA, Freedberg KA et al. (2009) HIV/AIDS, undernutrition, and food insecurity. Clin Infect Dis 49, 1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frega R, Duffy F, Rawat R et al. (2010) Food insecurity in the context of HIV/AIDS: a framework for a new era of programming. Food Nutr Bull 31, S292–S312. [PubMed] [Google Scholar]
- 20. Weiser SD, Young SL, Cohen CR et al. (2011) Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr 94, 1729S–1739S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whittle HJ, Palar K, Napoles T et al. (2015) Experiences with food insecurity and risky sex among low-income people living with HIV/AIDS in a resource-rich setting. J Int AIDS Soc 18, 20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arenas DJ, Thomas A, Wang J et al. (2019) A systematic review and meta-analysis of depression, anxiety, and sleep disorders in US adults with food insecurity. J Gen Intern Med 34, 2874–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canadian Co-infection Cohort Investigators, Aibibula W, Cox J et al. (2017) Impact of food insecurity on depressive symptoms among HIV–HCV co-infected people. AIDS Behav 21, 3464–3472. [DOI] [PubMed] [Google Scholar]
- 24. Aibibula W, Cox J, Hamelin A-M et al. (2020) The mediating role of depressive symptoms in the association between food insecurity and HIV related health outcomes among HIV–HCV co-infected people. AIDS Behav 24, 2188–2194. [DOI] [PubMed] [Google Scholar]
- 25. Evrensel A & Ceylan ME (2015) The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci 13, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacka FN (2017) Nutritional psychiatry: where to next? EBioMedicine 17, 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adan RAH, van der Beek EM, Buitelaar JK et al. (2019) Nutritional psychiatry: towards improving mental health by what you eat. Eur Neuropsychopharm 29, 1321–1332. [DOI] [PubMed] [Google Scholar]
- 28. Deehan EC & Walter J (2016) The fiber gap and the disappearing gut microbiome: implications for human nutrition. Trends Endocrinol Metabol 27, 239–242. [DOI] [PubMed] [Google Scholar]
- 29. Melo HM, Santos L & Ferreira ST (2019) Diet-derived fatty acids, brain inflammation, and mental health. Front Neurosci 13, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swann OG, Kilpatrick M, Breslin M et al. (2019) Dietary fiber and its associations with depression and inflammation. Nutr Rev 78, 394–411. [DOI] [PubMed] [Google Scholar]
- 31. Wu P-Y, Lin M-Y & Tsai P-S (2018) Alternate healthy eating index and risk of depression: a meta-analysis and systemematic review. Nutr Neurosci 23, 101–109. [DOI] [PubMed] [Google Scholar]
- 32. Pan L, Sherry B, Njai R et al. (2012) Food insecurity is associated with obesity among US adults in 12 states. J Acad Nutr Diet 112, 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen NT, Nguyen X-MT, Lane J et al. (2011) Relationship between obesity and diabetes in a US adult population: findings from the national health and nutrition examination survey, 1999–2006. Obes Surg 21, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dinour LM, Bergen D & Yeh M-C (2007) The food insecurity–obesity paradox: a review of the literature and the role food stamps may play. J Am Diet Assoc 107, 1952–1961. [DOI] [PubMed] [Google Scholar]
- 35. Dhurandhar EJ (2016) The food-insecurity obesity paradox: a resource scarcity hypothesis. Physiol Behav 162, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hanson KL & Connor LM (2014) Food insecurity and dietary quality in US adults and children: a systematic review. Am J Clin Nutr 100, 684–692. [DOI] [PubMed] [Google Scholar]
- 37. Milano W, Ambrosio P, Carizzone F et al. (2020) Depression and obesity: analysis of common biomarkers. Diseases 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rao W-W, Zong Q-Q, Zhang J-W et al. (2020) Obesity increases the risk of depression in children and adolescents: results from a systematic review and meta-analysis. J Affect Disord 267, 78–85. [DOI] [PubMed] [Google Scholar]
- 39. Gowey MA, Khodneva Y, Tison SE et al. (2019) Depressive symptoms, perceived stress, and metabolic health: the REGARDS study. Int J Obes 43, 615–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baer TE, Scherer EA, Fleegler EW et al. (2015) Food insecurity and the burden of health-related social problems in an urban youth population. J Adolesc Health 57, 601–607. [DOI] [PubMed] [Google Scholar]
- 41. Anema A, Mehra D, Weiser S et al. (2015) Drivers and consequences of food insecurity among illicit drug users. In Health of HIV Infected People, pp. 359–385 [Watson RR, editor]. Cambridge, MA: Academic Press. [Google Scholar]
- 42. Whittle HJ, Sheira LA, Frongillo EA et al. (2019) Longitudinal associations between food insecurity and substance use in a cohort of women with or at risk for HIV in the United States. Addiction 114, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McLinden T, Moodie EEM, Harper S et al. (2018) Injection drug use, food insecurity, and HIV–HCV co-infection: a longitudinal cohort analysis. AIDS Care 30, 1–7. [DOI] [PubMed] [Google Scholar]
- 44. Baptiste F & Hamelin A-M (2009) Drugs and diet among women street sex workers and injection drug users in Quebec City. Can J Urban Res 18, 78–95. [Google Scholar]
- 45. Noble C & McCombie L (1997) Nutritional considerations in intravenous drug misusers: a review of the literature and current issues for dietitians. J Hum Nutr Diet 10, 181–191. [Google Scholar]
- 46. Tang AM, Bhatnagar T, Ramachandran R et al. (2011) Malnutrition in a population of HIV-positive and HIV-negative drug users living in Chennai, South India. Drug Alcohol Depend 118, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saeland M, Haugen M, Eriksen F-L et al. (2010) High sugar consumption and poor nutrient intake among drug addicts in Oslo, Norway. Br J Nutr 105, 618–624. [DOI] [PubMed] [Google Scholar]
- 48. Javanbakht M, Shoptaw S, Ragsdale A et al. (2019) Depressive symptoms and substance use: changes overtime among a cohort of HIV-positive and HIV-negative MSM. Drug Alcohol Depend 207, 107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Davis L, Uezato A, Newell JM et al. (2008) Major depression and comorbid substance use disorders. Curr Opin Psychiatr 21, 14–18. [DOI] [PubMed] [Google Scholar]
- 50. Javanbakht M, Ragsdale A, Shoptaw S et al. (2019) Transactional sex among men who have sex with men: differences by substance use and HIV status. J Urban Health 96, 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. USDA ERS (2012) U.S. adult food security survey module: three-stage design, with screeners. undefined. https://www.ers.usda.gov/media/8279/ad2012.pdf (accessed February 2021).
- 52. Radloff LS (1977) The CES-D scale. Appl Psych Meas 1, 385–401. [Google Scholar]
- 53. Henry SK, Grant MM & Cropsey KL (2018) Determining the optimal clinical cutoff on the CES-D for depression in a community corrections sample. J Affect Disord 234, 270–275. [DOI] [PubMed] [Google Scholar]
- 54. StataCorp. (2017) Stata Statistical Software. College station, TX: StataCorp., LLC. [Google Scholar]
- 55. VanderWeele TJ (2015) Mediation analysis: a practitioner’s guide. Annu Rev Public Health 37, 1–16. [DOI] [PubMed] [Google Scholar]
- 56. Strike C, Rudzinski K, Patterson J et al. (2012) Frequent food insecurity among injection drug users: correlates and concerns. BMC Public Health 12, 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tarasuk V (2001) A critical examination of community-based responses to household food insecurity in Canada. Health Educ Behav 28, 487–499. [DOI] [PubMed] [Google Scholar]
- 58. Leung CW, Epel ES, Willett WC et al. (2015) Household food insecurity is positively associated with depression among low-income supplemental nutrition assistance program participants and income-eligible nonparticipants. J Nutr 145, 622–627. [DOI] [PubMed] [Google Scholar]
- 59. Jones AD, Ngure FM, Pelto G et al. (2013) What are we assessing when we measure food security? A compendium and review of current metrics. Adv Nutr Int Rev J 4, 481–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leung CW, Epel ES, Ritchie LD et al. (2014) Food insecurity is inversely associated with diet quality of lower-income adults. J Acad Nutr Diet 114, 1943.e2–1953.e2. [DOI] [PubMed] [Google Scholar]
- 61. Davison KM, Holloway C, Gondara L et al. (2018) Independent associations and effect modification between lifetime substance use and recent mood disorder diagnosis with household food insecurity. PLoS One 13, e0191072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wiss DA (2019) A biopsychosocial overview of the opioid crisis: considering nutrition and gastrointestinal health. Front Public Health 7, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wiss DA (2019) The Role of Nutrition in Addiction Recovery: What We Know and What We Don’t. Amsterdam, Netherlands: Elsevier. [Google Scholar]
- 64. Taylor AM & Holscher HD (2018) A review of dietary and microbial connections to depression, anxiety, and stress. Nutr Neurosci 23, 237–250. [DOI] [PubMed] [Google Scholar]
- 65. Firth J, Marx W, Dash S et al. (2019) The effects of dietary improvement on symptoms of depression and anxiety: a meta-analysis of randomized controlled trials. Psychosom Med 81, 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spencer SJ, Korosi A, Layé S et al. (2017) Food for thought: how nutrition impacts cognition and emotion. NPJ Sci Food 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Huang R, Wang K & Hu J (2016) Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients 8, 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wallace CJ & Milev R (2017) The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatr 16, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith KS, Greene MW, Babu JR et al. (2019) Psychobiotics as treatment for anxiety, depression, and related symptoms: a systematic review. Nutr Neurosci, 1–15 (online ahead of print). [DOI] [PubMed]
- 70. Knudsen KB, Lærke H, Hedemann M et al. (2018) Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 10, 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cook RR, Fulcher JA, Tobin NH et al. (2019) Alterations to the gastrointestinal microbiome associated with methamphetamine use among young men who have sex with men. Sci Rep 9, 14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meckel KR & Kiraly DD (2019) A potential role for the gut microbiome in substance use disorders. Psychopharmacology 236, 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee K, Vuong HE, Nusbaum DJ et al. (2018) The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43, 2606–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moore K, Gray V, Wiss D et al. (2016) Hands-on nutrition and culinary intervention within a substance use disorder residential treatment facility. J Acad Nutr Diet 116, Supplement A20.
- 75. Wiss DA, Schellenberger M & Prelip ML (2018) Registered dietitian nutritionists in substance use disorder treatment centers. J Acad Nutr Diet 118, 2217–2221. [DOI] [PubMed] [Google Scholar]