Abstract
ACL injuries occur at a high frequency in the US with approximately 400,000 ACL reconstructions being performed each year1. While ACL reconstruction is our current gold standard of treatment, it does not restore joint motion2–4, or prevent the premature development of post-traumatic osteoarthritis (PTOA) in many patients5–9. Thus, new treatments for an ACL injury, which are less invasive and minimize patient morbidity, including cartilage damage, are highly desirable10,11. We have used a tissue engineered approach to stimulate ligament healing, to improve upon current treatment options12. In this review, we describe and discuss our work moving a tissue engineering strategy from the concept to bench, preclinical, clinical trials and ultimately FDA 510(k) de Novo approval, providing clinicians and patients with a viable alternative to ACL reconstruction.
Keywords: Anterior cruciate ligament, tissue engineering, ACL reconstruction, ACL repair, bridge-enhanced ACL repair, scaffold-enhanced ACL repair, post-traumatic osteoarthritis, platelet, clinical trial
Background
The clinical problem
Unlike other ligaments, like the medial collateral ligament of the knee, the anterior cruciate ligament (ACL) fails to heal after injury13. In addition, using sutures to repair the ACL has a high failure rate14, even with modern techniques15. When the high failure rate was noted in the 1970s, surgeons began adding a reinforcing strip of tendon or fascia to the repaired ACL to protect it16, and eventually moved to only using a graft – typically a strip of bone/patellar tendon/bone and then using two of the hamstring tendons (semitendinosus and gracilis)17,18 or part of the quadriceps19 tendon harvested from the patient. The downside of taking a graft from the patient also led to a rise in the use of allograft tendons; however, the significantly higher failure rate in active patients20 led to a dampening of the use of allograft tendons in athletes21.
The current gold standard of care, ACL reconstruction (ACLR), is performed by drilling tunnels, typically 8 mm to 10 mm in diameter, in the femur and tibia, harvesting the graft from elsewhere in the knee (extensor mechanism or hamstring tendons), passing the graft through the tunnels and securing it to the bone with screws or another fastening mechanism. This operation results in good stability of the knee and approximately 65% of patients can get back to their preinjury sport level22. Patients do, however, continue to experience weakness in the area where the graft was taken23, and the graft retear rate in teenagers is 15-20%24–26. In addition, patients who tear their ACLs have an increased risk of developing osteoarthritis of the knee – an estimated 74% will develop this on radiographs in only 14 years after the initial injury6. This is a large burden for patients who tear their ACL in their teen years.
Addressing the Clinical Problem
In thinking about this clinical problem and the current gold standard of treatment, a treatment for ACL injuries that would not require graft harvest or replacement of the torn ligament with a tendon was of interest. The ACL is one of few tissues in the body that is treated by removal and replacement, rather than with surgical repair. To determine if healing would be possible, our initial investigations focused on the biologic response of the ACL – defining the key functions required for wound healing and determining which are impaired for this ligament. Identification of the defect in wound healing biology would then enable design of a strategy to address the defect.
The Pathologic Analysis
Systematic pathologic examination of the torn ACL tissue13 and the response of the ACL cells in the torn tissue to injury, and then comparative animal models evaluating the response in the medial collateral ligament (MCL) and ACL, revealed that ACL cells and vessels were capable of proliferation, migration and collagen production after injury, much as MCL cells were13,27–31. While the MCL had the surgically created defect fill in with blood that solidified into a scaffold that could be remodeled into healing tissue, the ACL defect was filled with synovial fluid that remained liquid, and no bond between the torn ends was established32,33. With that constellation of pathologic findings, identification of a material that could be placed between the torn ligament ends to allow a protected space for healing of the ACL to occur drove the next phase of our investigation.
Preclinical Studies
After a series of in vitro studies of various materials and growth factor combinations, a hydrophilic scaffold made of collagen and other extracellular matrix proteins, which was able to soak up autologous whole blood from the patient and hold that blood in place between the torn ends of the ACL, was selected. This scaffold was combined with a suture repair of the ligament. Our studies in canine32,33 and porcine34–38 models demonstrated that the combination of the scaffold and suture repair (“scaffold-enhanced repair”) enabled the release of growth factors with spatial and temporal sequences that matched their release in healing extra-articular tissues, such as the MCL32. Focusing on the porcine model as a fit-for-purpose model for ACL injury 39,40 led to the finding that repair with the scaffold and blood resulted in a healing ligament that was more robust (Figure 1) and biomechanically superior to repair with sutures alone37, and had biomechanical properties similar to that of an ACL graft at time points up to one year after surgery (Figure 2). 34,41
Figure 1:
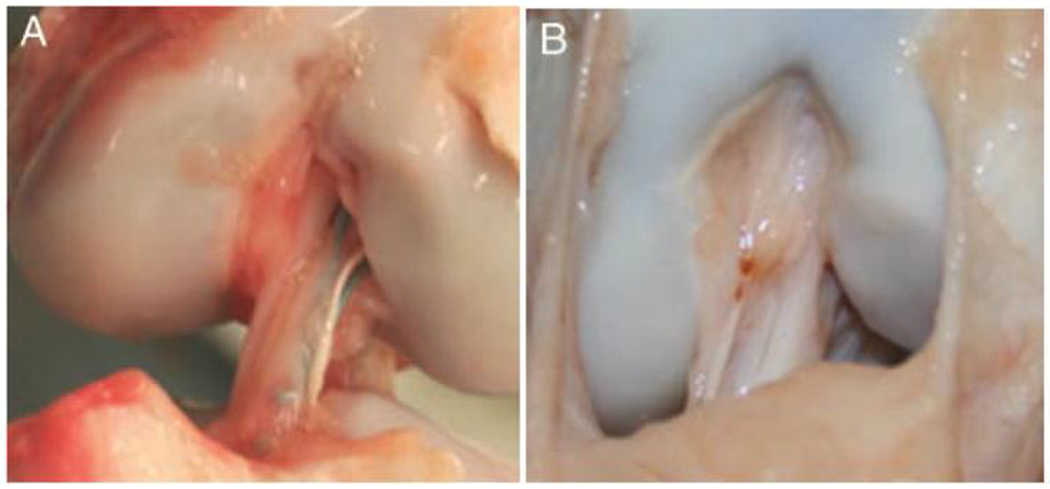
Scar mass as seen at three months after (A) simple suture repair and (B) scaffold enhanced suture repair where a protein scaffold carrying blood components was placed between the torn ligament ends at the time of repair. Ligaments treated with the scaffold enhancement had a larger and more organized scar mass at three months, with a gross appearance closer to that of an intact porcine ACL. (Figure from Joshi et al, AJSM 200937).
Figure 2:
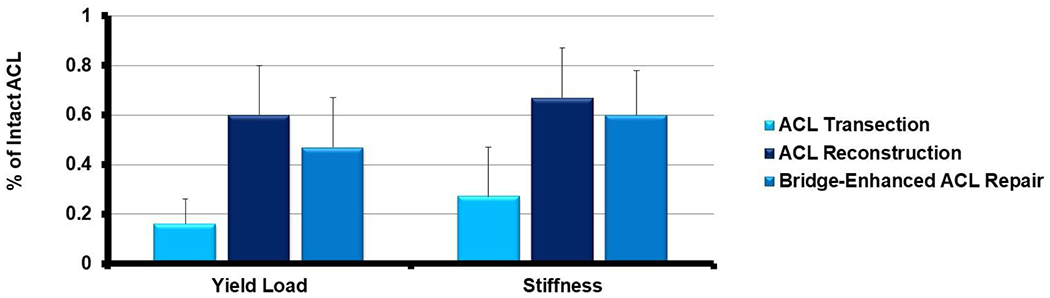
The yield load and stiffness of the ACL at one year after ACL transection (light blue bar), ACL reconstruction (dark blue bar) and repair enhanced with a scaffold (medium blue, “bridge-enhanced ACL repair”) at one year after surgery. There was no significant difference in mechanical properties between the ACL reconstruction group and the scaffold enhanced repair group34. Both reconstruction and scaffold enhanced repair had significantly improved mechanical properties when compared to ACL transection34.
We also found that skeletally immature animals had a healing ACL with a 50% higher maximum load and stiffness at 15 weeks after scaffold enhanced repair42,43 than adult animals, and that male and female animals had healing ACLs with different biomechanical properties when absorbable sutures were used, but not when nonabsorbable sutures were employed for the scaffold enhanced repair44. Lastly, we found that the animals treated with scaffold enhanced repair did not develop as much osteoarthritis as those treated with a tendon graft34 (Figure 3). We have since repeated that study in a second group of animals and found the same result45.
Figure 3:
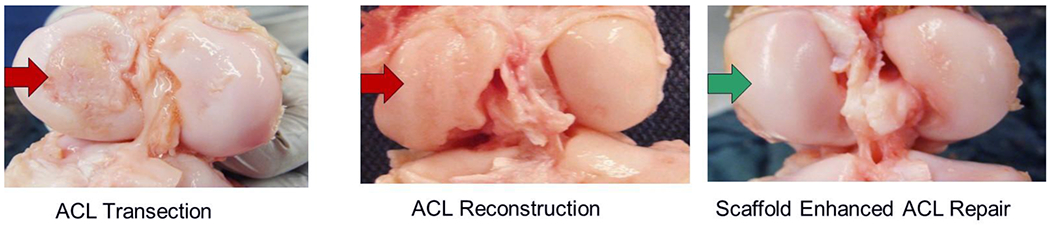
Porcine knees at one year after ACL transection with no further treatment (left panel), ACL reconstruction (center panel) and scaffold-enhanced ACL repair (right panel). In the ACL transection and ACL reconstruction knees, breakdown of the cartilage in the medial femoral condyle is noted (red arrows), while in the scaffold enhanced repair group, there was no significant loss of cartilage integrity (green arrow; adapted from Murray and Fleming, AJSM 201334).
Clinical Studies
Safety Study (cohort study)
With reasonable results in the preclinical “fit-for-purpose” model, additional studies were completed to obtain Institutional Review Board (IRB) and US Food and Drug Administration approvals for a first-in-human study, the Bridge-Enhanced® ACL Repair (BEAR) Trial (NCT02292004)46–48. This first study was primarily designed to assess patient safety and had two 10 patient cohorts followed in parallel and prospectively recruited. Patients with a complete, midsubstance ACL tear were enrolled into two groups, one group had scaffold enhanced suture repair and one group had autograft hamstring reconstruction. All patients were over 18, and the average Marx activity score at the time of injury was 14 (out of a maximum score of 16), which correlates with performing cutting and pivoting sports a few times a week during the prior year. Patients selected the procedure they wished to have, and the post-operative examiners were blinded as to procedure and side of surgery during the follow-up examinations. The early results of that study at three months demonstrated that there were no joint infections or signs of significant inflammation in either group. There were no differences between groups in effusion or pain, and no failures by Lachman examination criteria. Magnetic resonance images from the scaffold enhanced repair and ACL-reconstruction knees all demonstrated a continuous ACL or intact graft (Figures 4 and 5).
Figure 4:
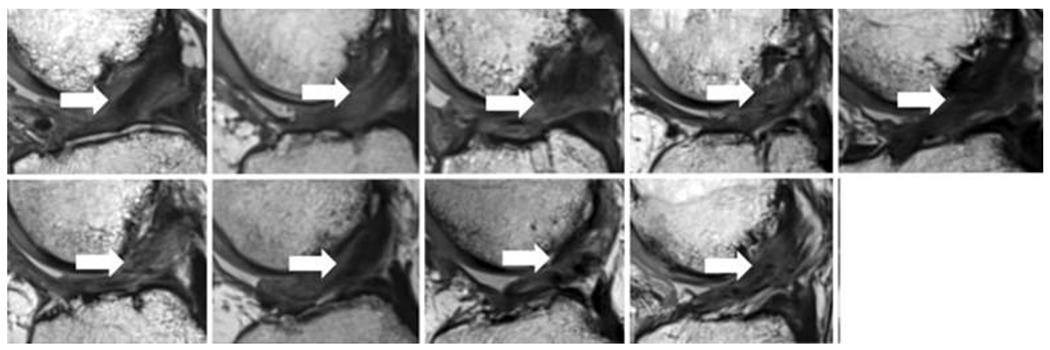
Magnetic resonance imaging from 9 of the 10 patients in the scaffold enhanced repair group in the first-in-human study (sagittal view, 24 months after scaffold enhanced repair). All subjects had intact anterior cruciate ligament (ACL) fibers from the femoral to tibial attachment sites (arrows). The intact fibers have low signal intensity (black) reflecting highly organized tissue with little free water. (Used from Murray et al, OJSM, 201949).
Figure 5:
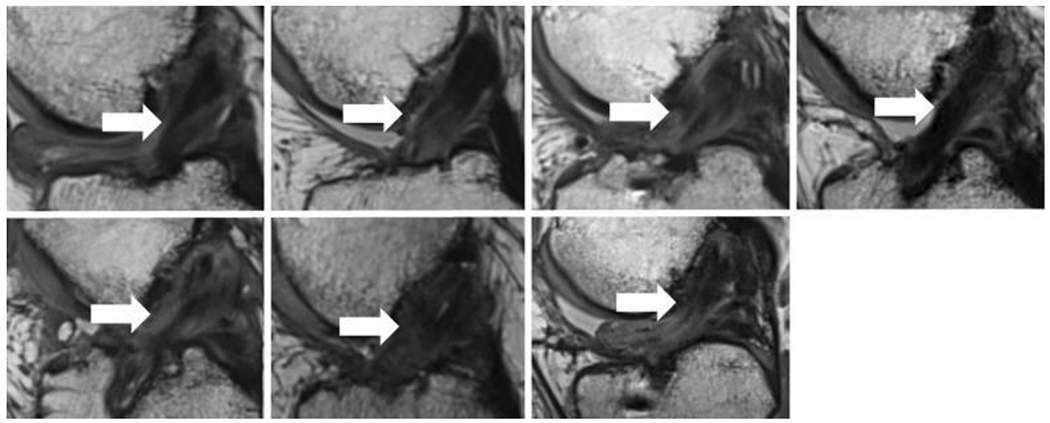
Magnetic resonance imaging from 7 of the 10 patients in the autograft ACL reconstruction group in the first-in-human study (sagittal view, 24 months after ACL reconstruction). All subjects had intact grafts coursing from the femoral to tibial tunnels (arrows). The intact fibers have low signal intensity (black) reflecting highly organized tissue with little free water. (Used from Murray et al, OJSM, 201949), with some variability among patients in the amount of highly organized tissue and less organized tissue in the region of the graft.
In addition, hamstring strength at 3 months was significantly better in the scaffold enhanced repair group than in the hamstring autograft group (mean ± SD: 77.9% ± 14.6% vs 55.9% ± 7.8% of the contralateral side; P < .001).50 We continued to follow these patients out to two years, and found that there were no graft or repair failures in either group. The IKDC subjective scores in both groups improved significantly from baseline (P < .0001) at 12 and 24 months, to 84.6 ± 17.2 in the ACL reconstruction group and to 91.7 ± 11.7 in the scaffold enhanced repair group. Arthrometer testing demonstrated mean side-to-side differences in AP laxity that were similar in the 2 groups at 24 months (scaffold enhanced repair, 1.94 ± 2.08 mm; ACL reconstruction, 3.14 ± 2.66 mm). Functional hop testing results were similar in the 2 groups at 12 and 24 months after surgery. Hamstring strength indices were significantly higher in the scaffold enhanced repair group compared with the ACL reconstruction group (P = .0001). In this small, first-in-human study, scaffold enhanced ACL repair produced equivalent or better outcomes to ACL reconstruction with a hamstring autograft49.
Randomized Control Trial of Scaffold Enhanced Repair vs ACL reconstruction with Autograft Tendon
The results of the first-in-human study led to IRB and US Food and Drug Administration approval for a second trial of this technique – a larger randomized controlled trial (NCT02664545; BEAR II Trial), where the examiners, patients, and physical therapists were blinded to the surgical procedure patient until after completion of the two year visit.51 One hundred young and active patients (median age, 17 years; median Marx activity score at the time of injury, 16, the highest score on the scale) who had sustained a complete midsubstance ACL tear were randomly assigned to receive either scaffold enhanced ACL repair (n = 65) or autograft ACLR (n = 35). All patients had surgical treatment within 45 days of injury. In this study, 96% of the patients returned for 2-year follow-up and another 3 were contacted by phone at two years to establish the incidence of additional surgery on the surgical or contralateral knee in 99% of the patients. The patients who had scaffold enhanced ACL repair had similar IKDC score and instrumented AP laxity when compared to the patients who had an ACL reconstruction (Figure 6). In addition, the patients who had a scaffold enhanced repair had a significantly higher mean hamstring muscle strength index than the ACL reconstructed group at 2 years. In addition, 14% of the scaffold enhanced repair group and 6% of the ACLR group had a re-injury that required a second ipsilateral ACL surgical procedure, a difference that was not statistically significant (P = .32). Additional studies with larger numbers of patients are needed to determine if this is a true difference. Interestingly, the 8 patients who converted from scaffold enhanced repair to ACLR in the study period and returned for the 2-year postoperative visit had similar primary outcomes to patients who had a single ipsilateral ACL procedure, which bodes well for those who undergo ACLR after scaffold enhanced repair.51
Figure 6:
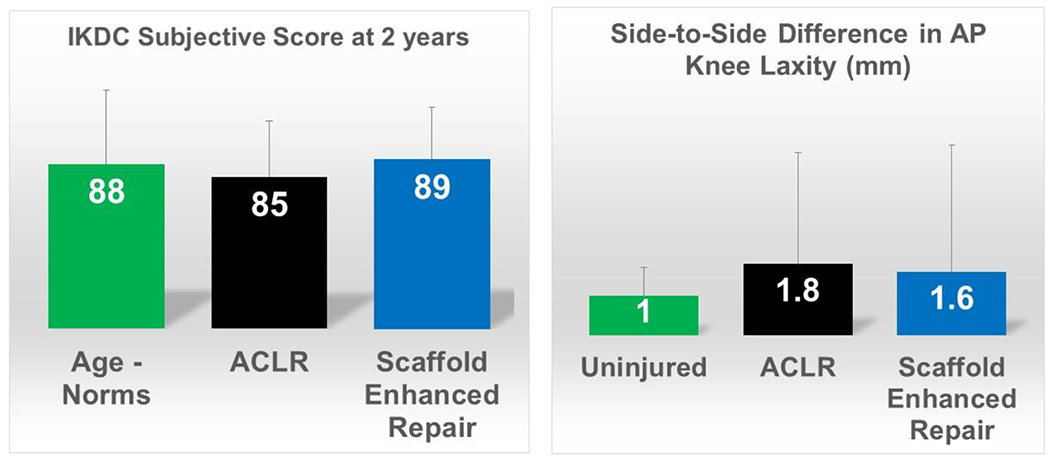
Primary outcomes from the 100-patient randomized control trial of autograft ACL reconstruction vs scaffold enhanced repair in young, active patients, including the IKDC score and side-to-side difference in instrumented AP knee laxity measurements at 24 months after surgery. The age-matched norms for IKDC score have a mean of 88 points52 and the mean difference in AP knee laxity for uninjured patients has been previously reported to be 1 mm.53
To put these results in perspective relative to prior, larger studies of ACL reconstruction, the mean 2 year IKDC Subjective score was 85 points in the ACL reconstruction group in this trial, which is consistent with the 2 year post-ACL reconstruction scores reported by the Multicenter Orthopedic Outcomes Network (MOON) group (81 points)54 and others (86 points).55 The scaffold enhanced ACL repair patients had mean scores at 2 years (89 points) more similar to that previously reported for an uninjured age-matched cohort (89 points for men and 86 points for women).56 The clinically important difference in IKDC score is thought to be over 11 points, so the differences between the scaffold enhanced repair results in this early trial and the established IKDC scores in larger cohorts (a difference of 8 points) may not be clinically significant. Similarly, prior studies of ACL reconstruction have reported mean side-to-side differences in AP knee laxity after ACL reconstruction ranging from (1.1 to 2.5mm) two years post-operatively,57,58 similar to those seen in both groups of the randomized control trial.
Elevated re-injury rates in active adolescents are a well-acknowledged problem after ACL reconstruction59,60, with revision rates for ACL reconstruction reported to range from 10% to 28%.20,61–65 For adolescents undergoing simple repair (no augmentation with a scaffold), the risk for reinjury, even in carefully selected patients and using modern techniques, is 49% at 2 years.66 Thus, the revision rate of 14% seen in the scaffold enhanced repair group in the randomized control trial is 35% lower than the rate reported ed for suture repair without a scaffold in this patient population,66 and is similar to that previously reported for autograft ACLR for this age group. Lowering the risk of reinjury after ACL surgery is an important topic, particularly as revision ACL surgery is known to result in lower patient reported outcomes67. Advances in rehabilitation strategies and return-to-sport evaluations, potentially including imaging prior to return68–72, are likely to play a role in this, in addition to improvements in surgical techniques, patient selection and patient education.
In an effort to identify a non-invasive measure to predict healing ligament strength, imaging was performed in both the preclinical and clinical studies of scaffold enhanced repair. In those studies, magnetic resonance imaging (MRI) was useful in measuring the healing ligament volume and the quality of the tissue when specific sequences were utilized69,73. Both cross sectional area of the healing ligament and the tissue quality as measured by signal intensity correlate with the maximum load and stiffness of the healing ligament in preclinical models70,73 and have been used to evaluate the healing ACL in the clinical trials71,72. In human patients, a larger cross-sectional area of the repaired ligament at six months was associated with having a notchplasty performed during the surgical procedure, as well as older age and male sex72. A lower signal intensity (indicative of tissue closer to normal ligament) was found for patients with a smaller posterior tibial slope and less strength recovery in the quadriceps muscle on the surgical leg at three months after surgery (possibly an indicator of less aggressive strengthening of that leg or greater protection in the first few months after scaffold enhanced repair). While some factors are not modifiable (sex, age), others may be surgically modifiable (tibial slope, notch size) and some may be addressed by altering the post-operative rehabilitation protocol (quadriceps strength deficit).
The use of platelet-containing autologous products, including platelet-rich plasma and whole blood, for musculoskeletal tissue repair has become more popular in recent years. 74–78. In our preclinical work, we found that increasing the concentration of platelets did not enhance either scaffold enhanced ACL repair or ACL reconstruction enhanced with a sleeve scaffold containing platelets 79,80. Mesenchymal stem cells, either obtained from peripheral blood, fat or bone marrow, were also not found to improve ACL repair in preclinical models81. In the scaffold enhanced repair randomized control trial, while platelets were not concentrated prior to delivery, the complete blood count of the blood added to the implant was recorded. The number of platelets delivered to different patients in that trial ranged from 144 to 336 K cells/ul (a two-fold difference between the lowest and highest values) and the range of white blood cell concentrations ranged from 3.9 to 19.2 K cells/ul (a five-fold difference between the lowest and highest values). The six-month MR images were analyzed for cross sectional area and signal intensity as noted above, and associations between those findings (as a surrogate for the healing strength of the ligament) were sought. The results were stratified by sex, and univariate and multivariate regression analyses determined significant correlations between blood cell concentrations on these 2 magnetic resonance imaging parameters. Adjusted multivariable analyses indicated that total platelet concentration and total white blood cell concentration had no significant effect on either magnetic resonance imaging parameter. This led us to conclude that for this range of physiologic platelet and white blood cell concentration, any significant effect on cross-sectional area or signal intensity of the healing ACL at 6 months after scaffold enhanced ACL repair could not be detected. Given these findings, factors other than the physiologic platelet concentration and total WBC concentration may be more important in the rate and amount of ACL healing after scaffold enhanced ACL repair82. These factors may include the time from injury to surgery, the rehabilitation program after surgery, intrinsic healing ability of the individual or the neurovascular status of the ACL remnants at the time of scaffold enhanced repair.
In addition to the primary outcomes, imaging and blood cell work, we also studied the effect of sex on overall outcomes after scaffold enhanced suture repair. That analysis found no significant effect of sex on the either the IKDC or KOOS scores at time points up to two years after surgery83. There was also no significant effect of sex on instrumented AP laxity testing at 2 years, nor on rates of secondary ACL injury rates. Six months after surgery, however, males had a seven times larger deficit in hamstring strength on the operated leg and a five times larger deficit in quadriceps strength on the operated leg (p<0.03 for both comparisons), while females had significantly better single leg hop testing 6 and 12 months after surgery (p<0.01 for both comparisons). There were no differences in either measure by the two-year time point, suggesting the males had caught up in the ensuing months. Our findings are in contrast to previous literature evaluating the effect of sex after ACL reconstruction where women have been reported to have greater AP laxity of the knee84,85 and larger deficits in both quadriceps and/or hamstring strength after surgery. 86,87
Conclusions
Scaffold enhanced ACL repair, where a suture repair is supplemented with a scaffold that can hold a patient’s blood between the torn ends of the ACL and provide a protected space for healing, has moved from bench to clinical trials. The initial results of the first two trials, including Level 1 evidence of similar performance of scaffold enhanced repair to the current gold standard of ACL reconstruction with autograft, are promising and have resulted in FDA Marketing Approval of the first implant labeled for use in augmenting ACL healing. The preclinical model and clinical study results justify continuing on this new avenue for research into improving outcomes of patients with ACL injuries. Future studies to optimize the surgical technique and rehabilitation strategies are likely to further improve outcomes, with scaffold enhanced ACL repair providing a less invasive, equally effective, option for treatment of mid-substance ACL tears.
ACKNOWLEDGEMENTS
We would like to acknowledge the significant contributions of the preclinical research teams at both Boston Children’s Hospital and Rhode Island Hospital that made the studies reviewed here possible, including Braden C Fleming, Leslie Kalish, Gary Badger, Benedikt Proffen, Ata Kiapour, Kirsten Ecklund, Gabe Perrone, Gordon Roberts, Doris Peterkin, Jakob Sieker, Nicholas Sant, Brett Flutie, Laura Thurber, Christina Freiberger, Rachael Henderson, Samuel Barnett, Ryan Sanborn, Bethany Trainor, Elizabeth Carew, Lyle Micheli, Dennis Kramer and Yi-Meng Yen, as well as the myriad of other research coordinators, research assistants, and clinical staff in the Department of Orthopedics at both Boston Children’s Hospital and Rhode Island Hospital.
We would like to acknowledge funding support from the Translational Research Program at Boston Children’s Hospital, the Children’s Hospital Orthopaedic Surgery Foundation, the Children’s Hospital Sports Medicine Foundation and the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases through grant numbers R01-AR065462 and R01-AR056834. This research was also conducted with support from the Football Players Health Study at Harvard University. The Football Players Health Study is funded by a grant from the National Football League Players Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Medical School, Harvard University or its affiliated academic health care centers, the National Football League Players Association, Boston Children’s Hospital, Rhode Island Hospital or the National Institutes of Health.
Disclosures:
Funding was received from the Translational Research Program at Boston Children’s Hospital, the Boston Children’s Hospital Orthopaedic Surgery Foundation, the Boston Children’s Hospital Sports Medicine Foundation, and the NFLPA through the Harvard Catalyst’s Football Players Health Study, as well as the NIH and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR065462 and R01-AR056834). M.M.M. and Boston Children’s Hospital have equity interests in MIACH Orthopaedics, a company that has licensed the BEAR® scaffolding technology from Boston Children’s Hospital. M.M.M. also receives royalties from Springer Publishing and research grants from the NIH, Department of Defense, and NFLPA through the Harvard Football Players Health Study.
REFERENCES
- 1.Junkin DM, Johnson DL, Fu FH, et al. 2009. Knee Ligament Injuries. In: Kibler WB editor. Orthopaedic Knowledge Update:Sports Medicine. Rosemont, IL: American Academy of Orthopaedic Surgeons; p. 136. [Google Scholar]
- 2.Beynnon BD, Uh BS, Johnson RJ, et al. 2005. Rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind comparison of programs administered over 2 different time intervals. Am J Sports Med 33:347–359. [DOI] [PubMed] [Google Scholar]
- 3.Fleming BC, Brattbakk B, Peura GD, et al. 2002. Measurement of anterior-posterior knee laxity: a comparison of three techniques. J Orthop Res 20:421–426. [DOI] [PubMed] [Google Scholar]
- 4.Tashman S, Kolowich P, Collon D, et al. 2007. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res 454:66–73. [DOI] [PubMed] [Google Scholar]
- 5.Lohmander LS, Ostenberg A, Englund M, et al. 2004. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum 50:3145–3152. [DOI] [PubMed] [Google Scholar]
- 6.Von Porat A, Roos EM, Roos H. 2004. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Br J Sports Med 38:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holm I, Oiestad BE, Risberg MA, et al. 2010. No difference in knee function or prevalence of osteoarthritis after reconstruction of the anterior cruciate ligament with 4-strand hamstring autograft versus patellar tendon-bone autograft: a randomized study with 10-year follow-up. The American journal of sports medicine 38:448–454. [DOI] [PubMed] [Google Scholar]
- 8.Oiestad BE, Holm I, Aune AK, et al. 2010. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. The American journal of sports medicine 38:2201–2210. [DOI] [PubMed] [Google Scholar]
- 9.Potter HG, Jain SK, Ma Y, et al. 2012. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. The American journal of sports medicine 40:276–285. [DOI] [PubMed] [Google Scholar]
- 10.Steiner ME, Murray MM, Rodeo SA. 2008. Strategies to improve anterior cruciate ligament healing and graft placement. Am J Sports Med 36:176–189. [DOI] [PubMed] [Google Scholar]
- 11.Murray MM. 2009. Current status and potential of primary ACL repair. Clinics in sports medicine 28:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beynnon BD, Fleming BC, Churchill DL, et al. 2003. The effect of anterior cruciate ligament deficiency and functional bracing on translation of the tibia relative to the femur during nonweightbearing and weightbearing. American Journal of Sports Medicine 31:99–105. [DOI] [PubMed] [Google Scholar]
- 13.Murray MM, Martin SD, Martin TL, et al. 2000. Histological Changes in the Human Anterior Cruciate Ligament After Rupture*. The Journal of Bone & Joint Surgery 82:1387–1387. [DOI] [PubMed] [Google Scholar]
- 14.Feagin JA Jr., Curl WW. 1976. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med 4:95–100. [DOI] [PubMed] [Google Scholar]
- 15.Gagliardi AG, Carry PM, Parikh HB, et al. 2019. ACL Repair With Suture Ligament Augmentation Is Associated With a High Failure Rate Among Adolescent Patients. Am J Sports Med 47:560–566. [DOI] [PubMed] [Google Scholar]
- 16.Meunier A, Odensten M, Good L. 2007. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15-year follow-up. Scand J Med Sci Sports 17:230–237. [DOI] [PubMed] [Google Scholar]
- 17.Aune AK, Holm I, Risberg MA, et al. 2001. Four-strand hamstring tendon autograft compared with patellar tendon-bone autograft for anterior cruciate ligament reconstruction. A randomized study with two-year follow-up. Am J Sports Med 29:722–728. [DOI] [PubMed] [Google Scholar]
- 18.Feller JA, Webster KE. 2003. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med 31:564–573. [DOI] [PubMed] [Google Scholar]
- 19.Belk JW, Kraeutler MJ, Marshall HA, et al. 2018. Quadriceps Tendon Autograft for Primary Anterior Cruciate Ligament Reconstruction: A Systematic Review of Comparative Studies With Minimum 2-Year Follow-Up. Arthroscopy 34:1699–1707. [DOI] [PubMed] [Google Scholar]
- 20.Kaeding CC, Aros B, Pedroza A, et al. 2011. Allograft Versus Autograft Anterior Cruciate Ligament Reconstruction: Predictors of Failure From a MOON Prospective Longitudinal Cohort. Sports Health 3:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch TS, Parker RD, Patel RM, et al. 2015. The Impact of the Multicenter Orthopaedic Outcomes Network (MOON) Research on Anterior Cruciate Ligament Reconstruction and Orthopaedic Practice. J Am Acad Orthop Surg 23:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ardern CL, Webster KE, Taylor NF, et al. 2011. Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. Am J Sports Med 39:538–543. [DOI] [PubMed] [Google Scholar]
- 23.Ardern CL, Webster KE, Taylor NF, et al. 2010. Hamstring strength recovery after hamstring tendon harvest for anterior cruciate ligament reconstruction: a comparison between graft types. Arthroscopy 26:462–469. [DOI] [PubMed] [Google Scholar]
- 24.Cordasco FA, Black SR, Price M, et al. 2019. Return to Sport and Reoperation Rates in Patients Under the Age of 20 After Primary Anterior Cruciate Ligament Reconstruction: Risk Profile Comparing 3 Patient Groups Predicated Upon Skeletal Age. Am J Sports Med 47:628–639. [DOI] [PubMed] [Google Scholar]
- 25.Mariscalco MW, Flanigan DC, Mitchell J, et al. 2013. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) Cohort Study. Arthroscopy 29:1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber-Westin S, Noyes FR. 2020. One in 5 Athletes Sustain Reinjury Upon Return to High-Risk Sports After ACL Reconstruction: A Systematic Review in 1239 Athletes Younger Than 20 Years. Sports Health 12:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray MM, Martin SD, Spector M. 2000. Migration of cells from human anterior cruciate ligament explants into collagen-glycosaminoglycan scaffolds. J Orthop Res 18:557–564. [DOI] [PubMed] [Google Scholar]
- 28.Murray MM, Bennett R, Zhang X, et al. 2002. Cell outgrowth from the human ACL in vitro: regional variation and response to TGF-beta1. J Orthop Res 20:875–880. [DOI] [PubMed] [Google Scholar]
- 29.Frank C, Amiel D, Akeson WH. 1983. Healing of the medial collateral ligament of the knee. A morphological and biochemical assessment in rabbits. Acta Orthop Scand 54:917–923. [DOI] [PubMed] [Google Scholar]
- 30.Frank C, Schachar N, Dittrich D. 1983. Natural history of healing in the repaired medial collateral ligament. J Orthop Res 1:179–188. [DOI] [PubMed] [Google Scholar]
- 31.Spindler KP, Clark SW, Nanney LB, et al. 1996. Expression of collagen and matrix metalloproteinases in ruptured human anterior cruciate ligament: an in situ hybridization study. J Orthop Res 14:857–861. [DOI] [PubMed] [Google Scholar]
- 32.Murray MM, Spindler KP, Ballard P, et al. 2007. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res 25:1007–1017. [DOI] [PubMed] [Google Scholar]
- 33.Murray MM, Spindler KP, Devin C, et al. 2006. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res 24:820–830. [DOI] [PubMed] [Google Scholar]
- 34.Murray MM, Fleming BC. 2013. Use of a Bioactive Scaffold to Stimulate Anterior Cruciate Ligament Healing also Minimizes Posttraumatic Osteoarthritis After Surgery. Am J Sports Med 41:1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray MM, Magarian E, Harrison SL, et al. 2010. The Effect of Skeletal Maturity on Functional Healing of the Anterior Cruciate Ligament. J Bone Joint Surg 92:2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magarian EM, Fleming BC, Harrison SL, et al. 2010. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med 38:2528–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joshi SM, Mastrangelo AN, Magarian EM, et al. 2009. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med 37:2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray MM, Spindler KP, Abreu E, et al. 2007. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res 25:81–91. [DOI] [PubMed] [Google Scholar]
- 39.Proffen BL, McElfresh M, Fleming BC, et al. 2012. A comparative anatomical study of the human knee and six animal species. Knee 19:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiapour AM, Shalvoy MR, Murray MM, et al. 2015. Validation of porcine knee as a sex-specific model to study human anterior cruciate ligament disorders. Clin Orthop Relat Res 473:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vavken P, Fleming BC, Mastrangelo AN, et al. 2012. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy 28:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray MM, Magarian EM, Harrison SL, et al. 2010. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am 92:2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mastrangelo AN, Haus BM, Vavken P, et al. 2010. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res 28:1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vavken P, Proffen B, Peterson C, et al. 2013. Effects of suture choice on biomechanics and physeal status after bioenhanced anterior cruciate ligament repair in skeletally immature patients: a large-animal study. Arthroscopy 29:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karamchedu NP, Murray MM, Sieker JT, et al. 2021. Bridge-Enhanced Anterior Cruciate Ligament Repair Leads to Greater Limb Asymmetry and Less Cartilage Damage Than Untreated ACL Transection or ACL Reconstruction in the Porcine Model. Am J Sports Med 49:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perrone GS, Proffen BL, Kiapour AM, et al. 2017. Bench-to-Bedside: Bridge-Enhanced Anterior Cruciate Ligament Repair. J Orthop Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proffen BL, Perrone GS, Fleming BC, et al. 2015. Effect of low-temperature ethylene oxide and electron beam sterilization on the in vitro and in vivo function of reconstituted extracellular matrix-derived scaffolds. Journal of biomaterials applications 30:435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proffen BL, Perrone GS, Fleming BC, et al. 2015. Electron beam sterilization does not have a detrimental effect on the ability of extracellular matrix scaffolds to support in vivo ligament healing. J Orthop Res 33:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray MM, Kalish LA, Fleming BC, et al. 2019. Bridge-Enhanced Anterior Cruciate Ligament Repair: Two-Year Results of a First-in-Human Study. Orthop J Sports Med 7:2325967118824356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murray MM, Flutie BM, Kalish LA, et al. 2016. The Bridge-Enhanced Anterior Cruciate Ligament Repair (BEAR) Procedure: An Early Feasibility Cohort Study. Orthop J Sports Med 4:2325967116672176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray MM, Fleming BC, Badger GJ, et al. 2020. Bridge-Enhanced Anterior Cruciate Ligament Repair Is Not Inferior to Autograft Anterior Cruciate Ligament Reconstruction at 2 Years: Results of a Prospective Randomized Clinical Trial. Am J Sports Med 48:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson AF, Irrgang JJ, Kocher MS, et al. 2006. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med 34:128–135. [DOI] [PubMed] [Google Scholar]
- 53.Akelman MR, Fadale PD, Hulstyn MJ, et al. 2016. Effect of Matching or Overconstraining Knee Laxity During Anterior Cruciate Ligament Reconstruction on Knee Osteoarthritis and Clinical Outcomes: A Randomized Controlled Trial With 84-Month Follow-up. Am J Sports Med 44:1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariscalco MW, Magnussen RA, Mitchell J, et al. 2015. How much hamstring graft needs to be in the femoral tunnel? A MOON cohort study. Eur Orthop Traumatol 6:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Razi M, Moradi A, Safarcherati A, et al. 2019. Allograft or autograft in skeletally immature anterior cruciate ligament reconstruction: a prospective evaluation using both partial and complete transphyseal techniques. J Orthop Surg Res 14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson AF, Irrgang JJ, Kocher MS, et al. 2006. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med 34:128–135. [DOI] [PubMed] [Google Scholar]
- 57.Heijne A, Werner S. 2010. A 2-year follow-up of rehabilitation after ACL reconstruction using patellar tendon or hamstring tendon grafts: a prospective randomised outcome study. Knee Surg Sports Traumatol Arthrosc 18:805–813. [DOI] [PubMed] [Google Scholar]
- 58.Feller JA, Webster KE. 2003. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med 31:564–573. [DOI] [PubMed] [Google Scholar]
- 59.Andernord D, Desai N, Bjornsson H, et al. 2015. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med 43:121–127. [DOI] [PubMed] [Google Scholar]
- 60.Parkinson B, Robb C, Thomas M, et al. 2017. Factors That Predict Failure in Anatomic Single-Bundle Anterior Cruciate Ligament Reconstruction. Am J Sports Med 45:1529–1536. [DOI] [PubMed] [Google Scholar]
- 61.Cordasco FA, Black SR, Price M, et al. 2019. Return to sport and reoperation rates in patients under the age of 20 after primary anterior cruciate ligament reconstruction: Risk profile comparing 3 patient groups predicated upon skeletal age. Am J Sports Med:363546518819217. [DOI] [PubMed] [Google Scholar]
- 62.Ho B, Edmonds EW, Chambers HG, et al. 2018. Risk Factors for Early ACL Reconstruction Failure in Pediatric and Adolescent Patients: A Review of 561 Cases. J Pediatr Orthop 38:388–392. [DOI] [PubMed] [Google Scholar]
- 63.Perkins CA, Busch MT, Christino M, et al. 2019. Allograft Augmentation of Hamstring Anterior Cruciate Ligament Autografts Is Associated With Increased Graft Failure in Children and Adolescents. Am J Sports Med 47:1576–1582. [DOI] [PubMed] [Google Scholar]
- 64.Astur DC, Cachoeira CM, da Silva Vieira T, et al. 2018. Increased incidence of anterior cruciate ligament revision surgery in paediatric verses adult population. Knee Surg Sports Traumatol Arthrosc 26:1362–1366. [DOI] [PubMed] [Google Scholar]
- 65.Jacobs CA, Burnham JM, Makhni E, et al. 2017. Allograft Augmentation of Hamstring Autograft for Younger Patients Undergoing Anterior Cruciate Ligament Reconstruction. Am J Sports Med 45:892–899. [DOI] [PubMed] [Google Scholar]
- 66.Gagliardi AG, Carry PM, Parikh HB, et al. 2019. ACL repair with suture ligament augmentation is associated with a high failure rate among adolescent patients. Am J Sports Med:363546518825255. [DOI] [PubMed] [Google Scholar]
- 67.Group M, Wright RW, Huston LJ, et al. 2019. Predictors of Patient-Reported Outcomes at 2 Years After Revision Anterior Cruciate Ligament Reconstruction. Am J Sports Med 47:2394–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biercevicz AM, Akelman MR, Fadale PD, et al. 2015. MRI volume and signal intensity of ACL graft predict clinical, functional, and patient-oriented outcome measures after ACL reconstruction. Am J Sports Med 43:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biercevicz AM, Miranda DL, Machan JT, et al. 2013. In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am J Sports Med 41:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biercevicz AM, Murray MM, Walsh EG, et al. 2014. T2 * MR relaxometry and ligament volume are associated with the structural properties of the healing ACL. J Orthop Res 32:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiapour AM, Ecklund K, Murray MM, et al. 2019. Changes in Cross-sectional Area and Signal Intensity of Healing Anterior Cruciate Ligaments and Grafts in the First 2 Years After Surgery. Am J Sports Med 47:1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murray MM, Kiapour AM, Kalish LA, et al. 2019. Predictors of Healing Ligament Size and Magnetic Resonance Signal Intensity at 6 Months After Bridge-Enhanced Anterior Cruciate Ligament Repair. Am J Sports Med 47:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beveridge JE, Machan JT, Walsh EG, et al. 2018. Magnetic resonance measurements of tissue quantity and quality using T2 * relaxometry predict temporal changes in the biomechanical properties of the healing ACL. J Orthop Res 36:1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Etulain J 2018. Platelets in wound healing and regenerative medicine. Platelets 29:556–568. [DOI] [PubMed] [Google Scholar]
- 75.Yoshida M, Marumo K. 2017. An Autologous Leukocyte-Reduced Platelet-Rich Plasma Therapy for Chronic Injury of the Medial Collateral Ligament in the Knee: A Report of 3 Successful Cases. Clin J Sport Med. [DOI] [PubMed] [Google Scholar]
- 76.Eirale C, Mauri E, Hamilton B. 2013. Use of Platelet Rich Plasma in an Isolated Complete Medial Collateral Ligament Lesion in a Professional Football (Soccer) Player: A Case Report. Asian J Sports Med 4:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bagwell MS, Wilk KE, Colberg RE, et al. 2018. The Use of Serial Platelet Rich Plasma Injections with Early Rehabilitation to Expedite Grade Iii Medial Collateral Ligament Injury in a Professional Athlete: A Case Report. Int J Sports Phys Ther 13:520–525. [PMC free article] [PubMed] [Google Scholar]
- 78.M.W.W. L, R.W.S. S 2018. Healing of Complete Tear of the Anterior Talofibular Cruciate Ligament and Early Ankle Stabilization after Autologous Platelet Rich Plasma: a Case Report Literature Review. Arch Bone Jt Surg 6:146–149. [PMC free article] [PubMed] [Google Scholar]
- 79.Mastrangelo AN, Vavken P, Fleming BC, et al. 2011. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res 29:1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fleming BC, Proffen BL, Vavken P, et al. 2015. Increased platelet concentration does not improve functional graft healing in bio-enhanced ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 23:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Proffen BL, Vavken P, Haslauer CM, et al. 2015. Addition of autologous mesenchymal stem cells to whole blood for bioenhanced ACL repair has no benefit in the porcine model. Am J Sports Med 43:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freiberger C, Kiapour AM, Liu S, et al. 2020. Higher Physiologic Platelet Counts in Whole Blood Are Not Associated With Improved ACL Cross-sectional Area or Signal Intensity 6 Months After Bridge-Enhanced ACL Repair. Orthop J Sports Med 8:2325967120927655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barnett S, Badger GJ, Kiapour A, et al. 2020. Females Have Earlier Muscle Strength and Functional Recovery After Bridge-Enhanced Anterior Cruciate Ligament Repair. Tissue Eng Part A 26:702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salmon LJ, Refshauge KM, Russell VJ, et al. 2006. Gender differences in outcome after anterior cruciate ligament reconstruction with hamstring tendon autograft. Am J Sports Med 34:621–629. [DOI] [PubMed] [Google Scholar]
- 85.Ahlden M, Sernert N, Karlsson J, et al. 2012. Outcome of anterior cruciate ligament reconstruction with emphasis on sex-related differences. Scand J Med Sci Sports 22:618–626. [DOI] [PubMed] [Google Scholar]
- 86.Keays SL, Bullock-Saxton JE, Newcombe P, et al. 2003. The relationship between knee strength and functional stability before and after anterior cruciate ligament reconstruction. J Orthop Res 21:231–237. [DOI] [PubMed] [Google Scholar]
- 87.Yasuda K, Ohkoshi Y, Tanabe Y, et al. 1992. Quantitative evaluation of knee instability and muscle strength after anterior cruciate ligament reconstruction using patellar and quadriceps tendon. Am J Sports Med 20:471–475. [DOI] [PubMed] [Google Scholar]


