Abstract
Precisely regulated expression of oncogenes and tumor suppressor genes is essential for normal development, and deregulated expression can lead to cancer. The human N-myc gene normally is expressed in only a subset of fetal epithelial tissues, and its expression is extinguished in all adult tissues except transiently in pre-B lymphocytes. The N-myc gene is overexpressed due to genomic amplification in the childhood tumor neuroblastoma. In previous work to investigate mechanisms of regulation of human N-myc gene expression, we observed that N-myc promoter–chloramphemicol acelyltransferase reporter constructs containing sequences 5′ to exon 1 were active in all cell types examined, regardless of whether endogenous N-myc RNA was detected. In contrast, inclusion of the first exon and a portion of the first intron allowed expression only in those cell types with detectable endogenous N-myc transcripts. We investigated further the mechanisms by which this tissue-specific control of N-myc expression is achieved. Using nuclear run-on analyses, we determined that the N-myc gene is actively transcribed in all cell types examined, indicating a posttranscriptional mode of regulation. Using a series of N-myc intron 1 deletion constructs, we localized a 116-bp element (tissue-specific element [TSE]) within the first intron that directs tissue-specific N-myc expression. The TSE can function independently to regulate expression of a heterologous promoter-reporter minigene in a cell-specific pattern that mirrors the expression pattern of the endogenous N-myc gene. Surprisingly, the TSE can function in both sense and antisense orientations to regulate gene expression. Our data indicate that the human N-myc TSE functions through a posttranscriptional mechanism to regulate N-myc expression.
N-myc is a member of a small family of highly related genes encoding transcription factors critical for normal cell growth and differentiation (1, 33, 54, 60). Myc proteins form heterodimers with Max to function as transcriptional activators (2, 9, 10). Myc can also act as a transcriptional repressor (22, 38, 40, 57, 59). Individual myc family members have been independently conserved during vertebrate evolution, implying that they perform distinct functions. Several direct targets of c-Myc–Max have been identified (21, 24, 27), and N-Myc has been shown to increase the expression of a subset of these genes (41). c-myc is ubiquitously expressed, and its levels correlate with increased cell proliferation; N-myc, in contrast, is expressed only in the epithelial component of fetal tissues and transiently in pre-B lymphocytes (26, 31, 47, 62, 73).
The regulation of c-myc gene expression has been extensively studied. Its expression is controlled at multiple levels, including transcription initiation, transcription attenuation, and mRNA and protein degradation (6, 8, 11, 28, 34, 43, 63). Both the increase in transcription initiation and the loss of normal attenuation mechanisms contribute to the c-myc overexpression that occurs in human Burkitt’s lymphoma (6, 37, 63).
Precisely regulated N-myc expression is critical for normal growth and differentiation, yet the specific mechanisms controlling normal N-myc expression have not been elucidated in detail (16, 64). Given their contrasting developmental expression patterns and tissue distributions, it is likely that distinct mechanisms operate to control c-myc and N-myc expression. Deregulated N-myc expression is strongly implicated in the pathogenesis of several important human malignancies, including small-cell lung cancer (48), Wilms’ tumor (49), retinoblastoma (39), and neuroblastoma (5, 12, 61). Inappropriate overexpression of N-myc in these tumors is most often due to gene amplification, with a commensurate increase in mRNA and protein levels (5, 12, 33). N-myc gene amplification remains the single most important negative prognostic feature in the childhood tumor neuroblastoma, strongly supporting a role for deregulated N-myc expression in the pathogenesis of this malignancy (12, 56).
A limited number of studies to date have investigated the regulation of the human N-myc gene. A possible negative regulatory role for N-myc exon 1 and intron 1 sequences in the modulation of N-myc expression was first inferred from studies of c-myc regulation. Specific sequences within human c-myc exon 1 mediate transcriptional attenuation, and loss of these regions results in deregulated expression (6, 63). The presence of candidate regulatory sequences in exon 1 and/or intron 1 of N-myc is supported by the work of Xu and colleagues (71, 72), who noted that deletion of the exon 1/intron 1 region of the murine N-myc gene led to increased oncogenicity of transfected minigenes.
In previous work to define functional domains of the human N-myc promoter, we and others noted that N-myc sequences 5′ to exon 1 were active in all cells examined, while inclusion of the 3′ portion of exon 1 and a region within the first intron restored a physiologic N-myc expression pattern to promoter-reporter minigenes containing these constructs (30, 59, 68, 69). Constructs containing 3′ exon 1 and 5′ intron 1 were expressed only in cell types where endogenous N-myc transcripts accumulate, suggesting the presence of a tissue-specific regulatory element in this region.
In this study, we extended our findings by localizing more precisely the region within N-myc intron 1 that is responsible for directing expression to certain cell types. We have investigated the mechanism(s) by which this region controls N-myc expression in both neuroblastoma and N-myc-nonexpressing cells. Using nuclear run-on analyses, we have determined that the N-myc gene is actively transcribed even in those cell types that do not accumulate detectable amounts of N-myc transcript under steady-state conditions. Using deletional analysis of N-myc intron 1 in a promoter-reporter assay, we have identified a 116-bp region that directs tissue-specific expression. This N-myc tissue-specific element (TSE) can exert its effect in either orientation on transcripts initiated from the heterologous simian virus 40 (SV40) promoter when it is included within the transcribed sequence. This element bears no significant homology with known regulatory sequences and is located within an intron. Our data suggest that the N-myc intron TSE functions posttranscriptionally to regulate N-myc expression. The TSE therefore represents a novel mechanism contributing to the control of gene expression.
MATERIALS AND METHODS
Generation of N-myc promoter–reporter constructs.
N-myc promoter–chloramphenicol acetyltransferase (CAT) reporter constructs were generated as previously described (59). pE/B N-myc CAT, which includes 2,023 bp of the N-myc promoter extending from −1872 to a BamHI site in exon 1 (+151), was generated by ligation of the EcoRI-BamHI 5′ N-myc promoter fragment adjacent to CAT in pBluescript SK+ (Stratagene) (gift of Richard Wetzel, Washington University School of Medicine, St. Louis, Mo.). pE/E N-myc CAT includes an additional 907 bp of 3′ N-myc sequence including all of exon 1 and a portion of intron 1 (−872 to +1058).
To generate a series of nested 3′ deletions from pE/E N-myc CAT (see Fig. 5 and reference 59), the 2.9-kb EcoRI fragment containing exon 1 and a portion of intron 1 was cloned into pBluescript SK+ in the antisense orientation. The resulting plasmid was digested with NotI, and the Erase-a-Base system for limited exonuclease III digestion (Promega) was used according to the manufacturer’s specifications (52). Digestions were carried out at room temperature, and reactions were terminated at 1-min intervals. Constructs were religated and restricted with BstXI and BamHI to screen for the presence of inserts, and several candidates were cloned into pBluescript SK+ carrying the CAT reporter gene. Constructs were sequenced by the dideoxy termination technique (53) to determine the precise locations of deletions.
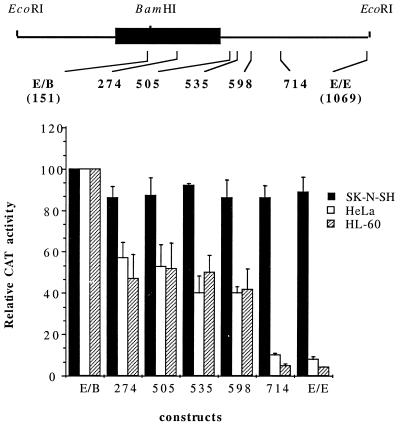
FIG. 5.
A region between nts 598 and 714 within N-myc intron 1 directs tissue-specific expression. A schematic diagram of human N-myc exon 1 is shown above a graphical representation of the promoter activity of 3′ intron deletion constructs in SK-N-SH, HeLa, and HL-60 cells. The 3′ end of each deletion construct is identified relative to the position of the major cap site. Promoter activity of each construct is expressed as CAT activity relative to that of the 5′ N-myc promoter construct pE/B N-myc CAT, normalized to the β-Gal activity of each sample, and is the mean ± standard error of the mean of at least three independent transfections.
Plasmid pCAT3(del) was obtained by HindIII digestion and religation of the pCAT3 promoter vector (Promega). pCAT3(del) lacks the 223-bp synthetic intron sequence present in the parent pCAT3 promoter vector. SV40 promoter–CAT reporter constructs containing the TSE were generated by cloning PCR fragments carrying appropriate restriction sites into each of the above parent vectors. The sense and antisense PCR primers used to subclone the TSE were 5′ AATCTGGATCCAAGCTTCTCCAGCTTGGAG 3′ (nucleotides [nt] 576 to 605, numbering from the major transcription start site according to Kohl et al. [33]) and 5′ GCTTAATTGGAAGCTTAGCCCACCCCTG 3′ (nt 717 to 746), respectively. Both contain HindIII sites (underlined) not present in the N-myc template and amplify a 168-bp fragment containing the TSE. Following HindIII digestion, the PCR products were cloned into the HindIII site of pCAT3(del) in both orientations (constructs pSV40 CAT3-N-myc TSE-S and -AS in Fig. 7). To clone the TSE 5′ to the SV40 promoter, the HindIII TSE fragment was cloned into pBluescript SK+ and reisolated as a SacI-XhoI fragment, using restriction sites in the pBluescript polylinker. This fragment was then ligated into SacI-XhoI-digested pCAT3 and pCAT3(del) vectors [constructs pSV40 CAT3-5′N-myc TSE and pSV40 CAT(del) derivative in Fig. 7].
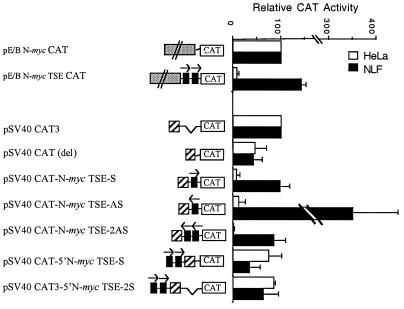
FIG. 7.
The N-myc TSE can function independently to silence expression from a heterologous promoter. The N-myc TSE was cloned into pE/B N-myc CAT under the influence of the N-myc promoter or into pSV40 CAT3 to generate the promoter constructs diagrammed at the left. Constructs were transiently transfected into the N-myc-nonexpressing line HeLa and the neuroblastoma line NLF (30 copies of N-myc). Promoter activity is expressed as CAT activity relative to that of the relevant parent construct and normalized to the β-Gal activity of each sample. Each bar represents the mean ± standard error of the mean of three independent transfections.
Cell culture and transfection conditions.
Human cell lines HL-60 (ATCC CCL 240), U937 (ATCC CRL 1593), and K-562 (ATCC CCL 243) were maintained in RPMI 1640 (Gibco, Grand Island, N.Y.) supplemented with 10% fetal calf serum (HyClone, Logan, Utah), 1 mM l-glutamine (Gibco), and 100 IU of penicillin-streptomycin per ml. Neuroblastoma lines SK-N-SH (single-copy N-myc; ATCC HTB 11), IMR-32 (amplified N-myc [25 copies]; ATCC CCL 127), NLF (30 copies), LHN (single copy), NLF (30 copies), NBL-S (single copy), and NMB (120 copies) (kind gifts of Garrett Brodeur, Children’s Hospital of Philadelphia) and HeLa (ATCC CCL 2), A293 (ATCC CRL 1573), Caco2 (ATCC HTB 37), and A431 (ATCC CRL 1555) lines were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum (HyClone) and 1 mM l-glutamine (Gibco). Cells were maintained in a humidified 5% CO2 atmosphere.
Transfections were performed by electroporation using minor modifications of previously described techniques (17, 59) or using liposome-mediated transfection (Lipofectamine [Gibco] or Superfect [Qiagen]) according to the manufacturer’s protocols. All samples were cotransfected with an equal amount of plasmid pSV40-βgal to control for variable transfection efficiency.
Assays of β-Gal and CAT enzyme activity.
Cells were harvested 48 h after transfection, and cell extracts were prepared by using reporter lysis buffer (Promega) according to the manufacturer’s recommendations. Extracts were assayed immediately or stored for up to 1 week at −80°C. β-Galactosidase (β-Gal) assays were performed in microtiter plates, using GalactoLight Plus reagent (Tropix, Bedford, Mass.). Assays of CAT activity were performed by phase extraction, using minor modifications of published techniques (55) and as previously described (59). Relative CAT activity of each sample was normalized to its β-Gal activity to control for variable transfection efficiencies among samples.
Northern and reverse transcriptase (RT)-mediated PCR (RT-PCR) analyses.
RNA was harvested from cells or isolated nuclei using Trizol (Gibco). Northern blot analysis of N-myc expression in cultured cell lines was carried out according to standard techniques (53), using 20 μg RNA per lane for all cell lines except the neuroblastoma line NMB. Two micrograms of RNA was used for N-myc-amplified NMB, which contains 120 copies of the N-myc gene. A 506-bp XhoI-BamHI N-myc exon 2 fragment, labeled by the random hexamer method (PrimeIt; Stratagene), was used as a probe to detect N-myc transcripts.
RT-PCR analysis of N-myc mRNA expression was performed with oligod(T) to prime first-strand cDNA synthesis, using SuperScriptII RT (Gibco) according to the supplied protocol. PCR primers used to detect mature N-myc mRNA were N-myc sense (5′ AGAAAAGCCAGTTCCAGCCCCGAA 3′; nt 330 to 353) and N-myc antisense (5′ GGTCTGGGTTCTTGCAGATCATGC 3′; nt 1522 to 1545) primers. Primers used to detect unprocessed N-myc RNA by nested PCRs were sense primer 5′ GGCCCAAGCTTAGACACCCGCGCAGAA 3′ (nt 114 to 140) and antisense primer 5′ CGCAACTTTGGAAACTGCCATTT 3′ (nt 1106 to 1128) (first round, primers 1 and 2) followed by sense primer 5′ ATTAGGCAGGGCGAAGCTTCCGCGGTCGCA 3′ (nt 547 to 581) and antisense primer (5′ CTGTTCCTGGCTGAAGCTTTCTAGCTCTCA 3′ (nt 1073 to 1044) (second round, primers a and b) (see Fig. 4). Primers used to detect plasmid-specific transcripts from N-myc promoter–CAT reporter transfections were pN-myc sense primer 5′ GGCCCAAGCTTAGACACCCGCGCAGAA (nt 114 to 140) 3′ and CAT antisense primer 5′ TATCAACGGTGGTATATCCAGTGA 3′ (Promega). Control sense and antisense β-actin primers were 5′ TAAGGAGAAGCTGTGGCTACGTCGC 3′ and 5′ TGCATATTTGTTTGGGGCAGG 3′, respectively. All PCR products were visualized on 0.8 or 1% agarose gels.
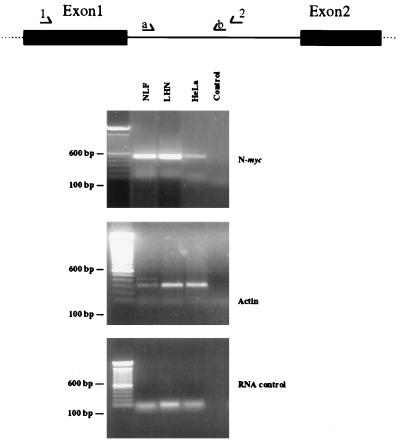
FIG. 4.
Unprocessed N-myc transcripts are detected in cells that do not accumulate N-myc mRNA under steady-state conditions. Nuclear RNA was extracted and subjected to RT-PCR using the N-myc intron-specific primer pairs diagrammed at the top. An RT-specific product was observed in both NLF (30 copies of N-myc) and LHN (1 copy of N-myc) neuroblastoma cell lines and was also detected in the nonexpressing line HeLa (top). Actin primers were used to control for RNA integrity (middle). The N-myc product is not due to DNA contamination since no product was observed when RT was deleted during cDNA synthesis (bottom). The negative PCR control was a PCR reaction without added cDNA template. The diagram shows the locations of PCR primers (arrows).
Nuclear run-on assays.
Nuclear run-on assays were performed with minor modifications of published techniques (13, 23). Nuclei were harvested from 108 logarithmically growing cells by hypotonic lysis in 10 mM Tris (pH 7.4)–10 mM NaCl–3 mM MgCl2–0.25% Nonidet P-40. Nuclei were washed once in lysis buffer, resuspended in 100 μl of glycerol storage buffer (40% glycerol, 50 mM Tris [pH 7.6], 5 M MgCl2, 0.1 mM EDTA), and used immediately or stored at −80°C or in liquid nitrogen. Nascent transcripts were labeled in a reaction with 0.25 mM rATP, rCTP, and rGTP in 5 mM Tris (pH 8.0)–2.5 mM MgCl2–25 mM KCl–2.5 mM dithiothreitol–200 μCi of [α-32P]UTP (3,000 Ci/mmol; NEN) for 30 min at 37°C. One unit of RNase-free DNase I (Worthington) or 500 U of RNase-free DNase I (Gibco-BRL) was added per reaction, and the mixtures were incubated at 37°C for 10 min. Reactions were stopped by the addition of 10× stop buffer (10% Sarkosyl or 2.5% sodium dodecyl sulfate [SDS], 1 mg of proteinase K per ml, 100 mM EDTA, 100 mM Tris [pH 7.6]) and additional incubation at 42°C for 30 min. RNA was purified either by extraction with 3 equal volumes of phenol-chloroform-isoamyl alcohol (25:24:1) and precipitation with 0.3 M sodium acetate and 100% ethanol using yeast tRNA as the carrier, or by purification through 3-ml Sephadex G-50 columns.
Nascent RNA transcripts were hybridized to both double- and single-stranded targets immobilized on nylon membranes (Duralon; Strategene). Double-stranded targets were composed of pBluescript SK+ (negative control), a 2.0-kb human β-actin cDNA in pBluescript SK+ (positive control), a 904-bp BamHI-EcoRI fragment encompassing 3′ exon 1 and part of intron 1, a 506-bp XhoI-BamHI N-myc exon 2 fragment, a 533-bp SmaI-HincII 5′ exon 3 fragment, and a 1,151-bp PstI-EcoRI 3′ exon 3 fragment. These were denatured in a final concentration of 0.1 N NaOH–10 mM EDTA at room temperature for 30 min and applied to nylon membranes at 5 μg per slot by vacuum filtration (BioDot apparatus; Bio-Rad). Single-stranded targets used included a 406-bp PvuII-BamHI 5′ exon 1 fragment, a 301-bp BamHI-BglII 3′ exon 1 fragment, a 506-bp XhoI-BamHI exon 2 fragment, and a 1,151-bp PstI-EcoRI 3′ exon 3 fragment. These were generated as antisense or sense RNA from pBluescript SK+ with either T7 or T3 RNA polymerase, using a Maxiscript kit (Ambion). Single-stranded targets, 1 μg per slot, were applied to nylon membranes in 25 mM sodium phosphate (pH 6.5). Membranes were UV cross-linked and stored for up to 2 weeks at 4°C. Hybridizations were performed in 50% (vol/vol) formamide–250 mM sodium phosphate (pH 7.2)–250 mM NaCl–0.1 mM EDTA–7% SDS–100 μg of salmon sperm DNA per ml. Membranes were prehybridized for 1 h and then hybridized with labeled RNA (0.5 × 107 to 1.0 × 107 cpm/ml per sample) at 42°C for 36 h. Membranes were then washed as follows: three times in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 57°C, once in 2× SSC–10 μg of RNase A per ml at 25°C, twice in 2× SSC–0.1% SDS at 65°C, twice in 1× SSC–0.1% SDS at 65°C, and twice in 0.1× SSC–1.0% SDS at 65°C. Membranes were exposed to film (Hyperfilm; Kodak) for 1 to 14 days or to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, Calif.) for 2 to 24 h.
RESULTS
Sequences within N-myc exon 1 and/or intron 1 direct its tissue-specific expression.
In the course of initial studies to characterize the human N-myc promoter, we and others (30, 59, 68, 69) noted that the 5′ portion of the promoter was active in all cell types examined, whereas constructs extending through all of exon 1 and a portion of intron 1 were active only in those cell types which express endogenous N-myc. Our original observation was reported for six neuroblastoma cell lines and two N-myc-nonexpressing cell lines (59). We have repeated these experiments and extended our N-myc promoter–reporter studies to two additional cell lines, U937 and A293, both of which do not accumulate significant amounts of N-myc RNA. We have confirmed that constructs containing the 3′ region of exon 1 and a portion of intron 1 are inactive in nonexpressing cell lines (Table 1). No significant difference in reporter activity was noted between neuroblastoma lines transfected with the 5′ promoter and those transfected with the construct containing the 3′ exon 1/5′ intron 1 region, implying that one or more sequence elements within this region function to modulate tissue-specific expression.
TABLE 1.
CAT activity of N-myc promoter constructs in human neuroblastoma and N-myc-nonexpressing cell lines
| Cell line | Reporter activitya
|
|
|---|---|---|
| pE/B N-myc-CAT | pE/E N-myc-CAT | |
| Neuroblastoma | 97.5 ± 18 | 87.6 ± 13 |
| NGP SK-N-SH | 88.8 ± 5 | 84.1 ± 8 |
| HeLa | 91.0 ± 25 | 1.1 ± 1 |
| HL-60 | 68.4 ± 10 | 1.5 ± 1 |
| U937 | 68.0 ± 11 | 1.3 ± 1 |
| A293 | 88.0 ± 15 | 2.0 ± 0.5 |
pE/B N-myc CAT contains 1872 bp of the 5′ promoter and extends to a BamHI site in exon 1. pE/E N-myc CAT includes an additional 907 bp of 3′ N-myc sequence including the remaining portion of exon 1 and extends to nt +1058 in intron 1. Reporter activity was measured as percent butyrylation normalized to β-Gal, and the activities of N-myc reporter genes are expressed as a percentage of the reporter activity measured for the positive control, pSV40CAT.
Human cell lines derived from multiple tissue lineages do not accumulate N-myc transcripts.
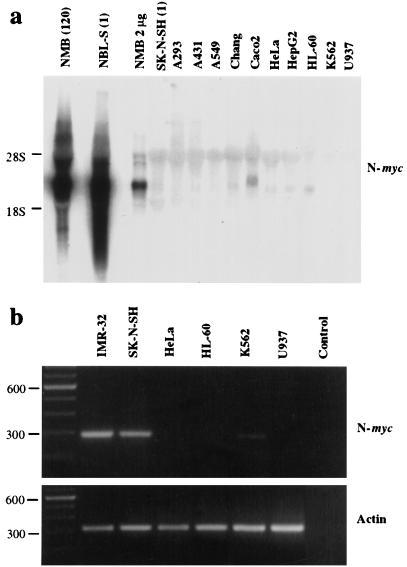
We wished to study the regulation of both the endogenous N-myc gene and a series of transfected N-myc promoter–reporter constructs in cell lines that have been previously reported to lack detectable levels of N-myc transcript. To verify the absence of N-myc mRNA in these lines, we screened an extended panel of human cell lines derived from diverse tissue lineages for N-myc expression by Northern and RT-PCR analyses. As shown in Fig. 1a, the neuroblastoma line NMB, in which the N-myc gene is amplified (120 copies), shows elevated N-myc transcript levels. In addition, the single-copy neuroblastoma line NBL-S, which has an increased N-Myc protein half-life (18), also demonstrates elevated levels of N-myc mRNA. In contrast, of many cell lines, including some derived from epithelial tissue, only the colon carcinoma line Caco2 expresses detectable N-myc transcripts. Using the more sensitive RT-PCR technique with primers derived from sequences in exons 1 and 2 of the N-myc gene, we detected N-myc transcripts only in the neuroblastoma cell lines; a faint product was also visualized in K562 cells (Fig. 1b). On the basis of these findings, we chose several of these N-myc-nonexpressing cell lines for further studies of N-myc gene regulation.
FIG. 1.
Human cell lines derived from multiple lineages do not accumulate significant amounts of N-myc mRNA under steady-state conditions. (A) RNA was extracted from logarithmically growing cells, subjected to Northern electrophoresis using standard techniques, and probed with an N-myc exon 2-specific probe. One-tenth of the standard amount of total RNA, or 2 μg, was loaded for NMB, the N-myc-amplified line. Numbers in parentheses indicate N-myc copy numbers. (B) RNA from several N-myc-nonexpressing lines was subjected to RT-PCR using N-myc- and actin-specific primer pairs. Actin-specific primers were used to control for RNA integrity. Sizes are indicated in base pairs.
N-myc RNA levels in nonexpressing cell types are regulated posttranscriptionally.
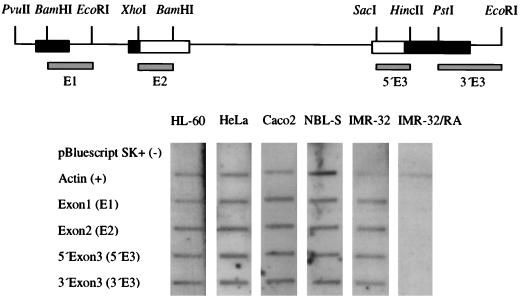
To determine whether the N-myc gene is transcriptionally silent in these nonexpressing cell lines, we performed nuclear run-on analyses. Double-stranded targets spanning the N-myc locus were used initially to detect nascent transcripts. Figure 2 shows results of nuclear run-on assays in the neuroblastoma lines IMR-32 and NBL-S, the colon carcinoma line Caco2, which demonstrates a low level of N-myc mRNA, and the N-myc-nonexpressing lines HeLa and HL-60. As expected, the N-myc gene is actively transcribed in both amplified and single-copy neuroblastoma lines as well as in Caco2. Interestingly, both HeLa and HL-60, which do not express detectable N-myc transcripts under steady-state conditions, also demonstrate active transcription of the gene, indicating that N-myc expression is controlled through a posttranscriptional mechanism. Transcription in IMR-32 is almost completely abrogated by treatment with 1 μM all-trans retinoic acid for 24 h as demonstrated by others, indicating specificity of the run-on probes for N-myc transcripts (66).
FIG. 2.
Transcription occurs across the entire N-myc gene in nonexpressing cell lines. The human N-myc locus is depicted at the top; locations of double-stranded targets used in nuclear run-on analyses are shown. Exons are boxed; nontranslated regions are shown in black. Autoradiographic signals from hybridization of targets with nascent RNAs from the single-copy neuroblastoma line NBL-S, the amplified line IMR-32 (25 copies of N-myc), and the nonexpressing lines HeLa, A293, Caco2, and HL-60 are shown. Signals were specific for human N-myc, as confirmed by the abrogation of N-myc transcription observed in IMR-32 cells treated with 1 μM all-trans retinoic acid (RA) for 24 h prior to harvest of nuclei. pBluescript (cloning vector) and human β-actin were included as negative and positive controls, respectively.
Transcription attenuation occurs in both neuroblastoma and N-myc nonexpressing cell lines.
Transcription attenuation plays an essential role in the control of c-myc expression (7, 14, 63), and attenuation has been demonstrated to control expression of the murine N-myc gene during development (19, 46, 71). To rigorously investigate the role of transcription attenuation in the regulation of human N-myc expression, we hybridized radiolabeled nascent transcripts with a series of single-stranded RNA probes to avoid quantitation of N-cym transcripts derived from a gene located on the opposite DNA strand (3) (Fig. 3).
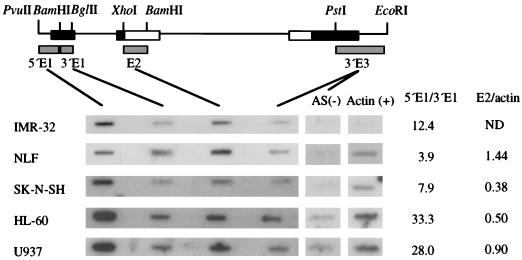
FIG. 3.
Transcriptional attenuation in N-myc exon 1 occurs in both neuroblastoma and N-myc-nonexpressing cell lines. Autoradiographic signals are shown from hybridization of nascent transcripts from IMR-32 (30 copies of N-myc), NLF (30 copies), SK-N-SH (1 copy), HL-60, and U937 cells. Actin and transcription from the antisense strand at the 3′ region of N-myc served as positive [Actin (+)] and negative [AS (−)] controls, respectively. Relative signal intensities from PhosphorImager quantitation of the data were normalized for the uridine content of each nascent transcript. Relative intensities of 3′ exon 1 (3′ E1) are shown normalized to that of 5′ exon 1 (5′ E1) in the nonexpressing lines U937 and HL-60 and in the neuroblastoma lines IMR-32 (25 copies of N-myc), NLF (30 copies), and SK-N-SH (1 copy) to give a estimate of the degree of transcriptional attenuation. Exon 2 (E2) signal intensity was normalized to actin signal to allow relative comparison of polymerase density in N-myc translated regions between cell lines.
Transcription attenuation is evident in all cell lines, as demonstrated by the significantly greater hybridization signal with the target covering the 5′ region of exon 1 compared to the signal obtained with the 3′ region of exon 1 (Fig. 3). To assess the degree of transcription attenuation within N-myc exon 1, signal intensities were quantified and corrected for the uridine content of the hybridizing RNA. Relative ratios of 5′ exon 1 signal to 3′ exon 1 ranged from 33.3:1 in HL-60 to 3.9:1 in NLF. Although attenuation is observed in both neuroblastoma and N-myc-nonexpressing cell lines, greater attenuation is evident in the two nonexpressing lines. Transcription attenuation cannot account, however, for the absence of steady-state mRNA accumulation in N-myc-nonexpressing cell lines, since considerable polymerase density is observed across exons 2 and 3 of the gene in these cell lines. Quantitation of exon 2 signal normalized to actin to allow comparison among lines shows similar polymerase densities between neuroblastoma (NLF, 1.4:1; SK-N-SH, 0.38:1) and nonexpressing (HL-60, 0.5:1; U937, 0.9:1) lines (Fig. 3).
Unprocessed N-myc transcripts are detected in nonexpressing cell lines.
To corroborate the findings of our nuclear run-on experiments, we used RT-PCR of nuclear RNA with intron-specific primer pairs to investigate whether unprocessed N-myc transcripts are detectable in HeLa cells, where stable N-myc message cannot be detected by Northern or RT-PCR analysis of total RNA. We extracted RNA from nuclei isolated identically as for nuclear run-ons and subjected it to RT-PCR using N-myc intron-specific primers. As predicted from nuclear run-on experiments, Fig. 4 demonstrates that unprocessed transcripts are found in the nonexpressing line HeLa in a qualitatively lower amount than in either the amplified neuroblastoma line NLF (30 copies of N-myc) or the single-copy line LHN. These results support the results of our nuclear run-on analyses in showing that the N-myc gene is actively transcribed in all cell lines examined, but unprocessed N-myc RNA is apparently rapidly degraded in cell types that do not accumulate significant amounts of N-myc mRNA.
Tissue-specific N-myc expression is mediated by a 116-bp element within intron 1.
We sought to localize more precisely the domain within 3′ exon 1/5′ intron 1 that directs tissue-specific expression. To accomplish this, we generated a series of N-myc promoter–CAT reporter constructs bearing 3′ nested deletions, using the construct pE/E N-myc CAT (Fig. 5), by limited exonuclease digestion. These were transfected into N-myc-expressing neuroblastoma lines and two N-myc-nonexpressing lines. As illustrated in Fig. 5, restriction of promoter activity to N-myc-expressing cells requires the inclusion of a 116-bp segment located between nt 598 and 714 within N-myc intron 1. Deletion of this element has no effect on the level of N-myc promoter activity in neuroblastoma cells, suggesting that a negative regulatory activity, rather than that of an enhancer element, is involved.
This functional assay also suggests that a second negative regulatory region is located between nt 151 and 274. Reporter activity of constructs containing this region was decreased consistently in nonexpressing cell lines, to 50% of the activity of the reporter construct containing only the 5′ N-myc promoter region (pE/B N-myc CAT). These findings imply that at least two regulatory regions exist within the first exon and/or first intron of the human N-myc gene. One, located between nt 598 and 714 (TSE), is necessary to impart tissue-specific expression on reporter constructs. A second element, between nt 151 and 274, is associated with a partial decrease in reporter expression in those cells where endogenous N-myc RNA is not detectable. Interestingly, it is within this second functionally defined region that transcription attenuation occurs (Fig. 3).
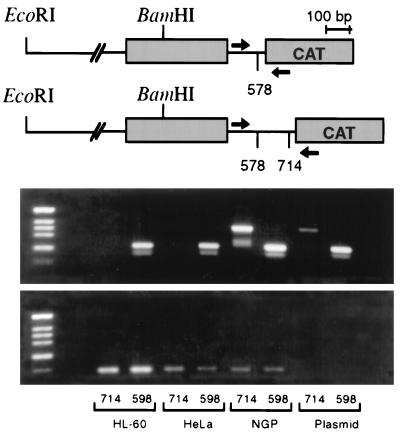
Expression of N-myc promoter–CAT constructs are regulated by similar mechanisms that control expression of the endogenous N-myc gene.
We next asked whether N-myc promoter–CAT reporter constructs, like the endogenous N-myc gene, were subject to posttranscriptional regulation in nonexpressing cell types. We transfected both neuroblastoma and N-myc-nonexpressing cell lines with the two reporter plasmids containing intron regions that flank the TSE and analyzed plasmid transcript expression by RT-PCR in parallel with CAT reporter assays. As shown in Fig. 6, NGP (150 copies of N-myc) neuroblastoma cells express transcripts from both constructs. Consistent with the reporter data shown in Fig. 5 is the finding that neither transfected HL-60 nor transfected HeLa cells contain detectable transcript from the +714 construct, which contains the 116-bp TSE. This element appears sufficient to extinguish reporter expression in nonexpressing cells, and reporter constructs containing the TSE do not generate stable transcripts in the same cells that do not accumulate detectable N-myc transcript. This finding implies that the transfected N-myc promoter–CAT reporter constructs are subject to regulation of expression that parallels that of the endogenous N-myc gene.
FIG. 6.
No transcripts are detected from N-myc promoter–CAT reporter constructs containing the N-myc TSE in N-myc-nonexpressing cell lines. The relevant N-myc promoter–CAT reporter constructs are diagrammed at the top (see Fig. 5 also). The two constructs both contain N-myc exon 1 (grey boxes) and extend 1,872 bp 5′ to the major cap site. They differ from one another by the inclusion of the 116-bp TSE region. RNA was harvested from transiently transfected cells in parallel with preparation for CAT reporter assays, and expression of plasmid-specific transcripts was determined by RT-PCR. RT-PCR for actin transcripts was used as a control for RNA integrity. Arrows show the locations of PCR primers.
The N-myc intron element can function to abrogate expression from a heterologous promoter.
We also questioned whether the N-myc TSE could function independently and whether it would be sufficient to silence transcript expression in the context of the heterologous SV40 promoter. To address this issue, we used a PCR-based approach to clone the 116-bp TSE adjacent to the promiscuous 5′ N-myc promoter region in pE/B N-myc CAT, as well as into the pCAT3 (SV40 promoter) vector, within the transcribed region of the construct. We also cloned the TSE 5′ to the transcriptional unit in the pCAT3 vector. Based on the results of nuclear run-on experiments showing active transcription of N-myc in all cell types examined, we predicted that the intron element would not silence reporter expression unless it was incorporated into the transcript.
Figure 7 shows results of transient transfection experiments with this set of promoter-reporter constructs. The TSE, independent of other 3′ exon 1/5′ intron 1 elements, was able to regulate the 5′ N-myc promoter region appropriately. When placed within the transcribed unit of pCAT3, the N-myc TSE was also sufficient to abrogate reporter expression in HeLa cells. Furthermore, consistent with the results of nuclear run-on analyses in N-myc-nonexpressing cell types, the TSE did not decrease reporter activity when positioned 5′ to the SV40 promoter. This finding demonstrates again that the element does not act as a transcriptional silencer and further supports the interpretation that it functions posttranscriptionally. Interestingly, the TSE also functioned appropriately in the antisense orientation, as either one or two copies. The presence of a single copy of the TSE in the antisense orientation resulted in a marked increase in reporter activity in NLF cells (Fig. 7).
DISCUSSION
In this study we investigated the mechanism by which human N-myc expression is restricted to particular cell types. We have determined that a major component of this regulation occurs posttranscriptionally. N-myc transcripts are synthesized in all cell types examined but are rapidly degraded in some prior to completion of their processing. We have found within the first intron of the human N-myc gene a 116-bp sequence, the TSE, that appears to be necessary and sufficient for destabilization of the nascent transcripts into which it is incorporated. Our results show excellent concordance between the regulation of the endogenous gene and promoter-reporter minigenes with respect to the measurable effects of the TSE.
Other published studies have suggested that sequences in human N-myc exon 1 and/or intron 1 play a critical regulatory role. Xu and colleagues (71, 72) first observed that a candidate regulatory element(s) within exon 1 and/or intron 1 of the murine N-myc gene appeared responsible for directing its expression to a limited subset of cell types and for decreasing its oncogenicity in ras cotransformation assays. Sequences within exon 1 of the human c-myc gene mediate transcription attenuation, and loss of these domains contributes to the deregulated c-myc expression characteristic of Burkitt’s lymphoma (7, 42, 63). We and others have previously determined that sequences within the 3′ portion of human N-myc exon 1 and/or the 5′ portion of intron 1 appear to serve a regulatory function, based on their influence on linked reporter activity in a variety of murine and human cell lines (30, 59, 67).
The results presented here extend our previous findings and further emphasize the complexity of human N-myc regulation. Using a deletional analysis similar to ours, Woodruff and colleagues identified a 533-bp candidate regulatory region within human N-myc intron 1 and noted that this region contains a consensus sequence for the chicken lysozyme gene transcriptional silencer (69). The 116-bp N-myc intron TSE identified in our work lies within this larger region. We have observed active N-myc transcription even in cell types that do not accumulate N-myc mRNA, indicating that the TSE does not function as a transcriptional silencer. The reasons for discordance between our results and those reported by Woodruff and colleagues (69) attributing transcriptional silencer activity to this region of the human N-myc gene are not immediately clear. Our results regarding the activities of both the endogenous N-myc gene and transfected promoter-reporter minigenes are fully internally consistent, and we have extended our studies to multiple cell lines to avoid potential artifacts. In agreement with our results, Babiss and Friedman also observed posttranscriptional control of N-myc expression in cell lines where N-myc RNA does not accumulate (4). Another candidate regulatory element in N-myc intron 1 was recently identified based on sequence homology to the consensus binding site for nuclear retinoid receptors (20). This element lies just 5′ to the TSE, and the relative contribution of this region to N-myc expression remains to be investigated.
Based on analysis of N-myc intron 1 deletion constructs in a functional reporter assay, we defined a second negative regulatory region, between nts 151 and 274 relative to the major cap site, that appears to function as a transcriptional attenuation site. In our functional assays, the inclusion of this site was associated with a 40 to 50% decrease in promoter activity in nonexpressing lines compared to activity observed in the neuroblastoma line, SK-N-SH. The results of nuclear run-on analyses using single-stranded targets demonstrated a significant decrease in RNA polymerase density in 3′ exon 1 relative to 5′ exon 1 as defined by the BamHI site at nt 151. Attenuation was seen in all cell lines but to a greater extent in nonexpressing lines. This site of attenuation detected by run-on analysis correlated qualitatively with the partial decrease in promoter activity measured in promoter deletion experiments. This region maps to a site of transcription attenuation previously noted in the murine N-myc gene (70, 72). Attenuation cannot account, however, for the total absence of N-myc transcript in nonexpressing cells, since similar polymerase densities are observed across translated exons 2 and 3 in both nonexpressing HeLa and HL-60 cells and neuroblastoma lines. The equal processivity of RNA polymerase II across translated exons 3′ to the TSE also indicates that the TSE does not function through transcription attenuation.
The TSE of the human N-myc gene is unique in its ability to function posttranscriptionally from its location within an intron. RNA instability determinants, including those characterized in the human and murine c-myc genes, are often found in the 3′ coding or untranslated regions (8, 11, 25, 28, 51). In the case of the human c-myc gene, each of the defined instability determinants can also destabilize a heterologous message, and the two appear to synergize to fully destabilize c-myc mRNA (8, 28). Similar instability elements have also been identified in the 3′ untranslated region of the human N-myc gene. A member of the ELAV-like family recognizes two distinct AU-rich sequences in the 3′ untranslated region of the human N-myc transcript, and its binding is associated with increased stability (15).
The data summarized above indicate that the TSE functions posttranscriptionally. This property is dependent on cell type, indicating the presence of a trans-acting factor, most likely a protein, that is differentially expressed. Based on the data presented here, we have considered four possible models for the effect of the TSE on RNA stability: (i) inhibition of normal splicing, (ii) activation of alternative splicing, (iii) regulation by antisense transcription, and (iv) direct regulation of pre-mRNA stability.
The tissue-specific gene regulation that we have observed does not appear to depend on the integrity of native splice acceptor and donor sites since many of our constructs lack such elements. This suggests that inhibition of normal splicing, possibly through inhibition of splicing enhancers with the generation of an aberrant transcript, does not account for the absence of N-myc mRNA in nonexpressing cell types (65). Alternative splicing could lead to the inclusion of a cryptic exon in the mature message that could similarly act to promote rapid degradation. The fact that the element operates in the antisense orientation (with inversion of any splice acceptor or donor sites) also argues against a model involving a specific effect of the TSE on splicing. In addition, to date we have not detected abnormally spliced N-myc products by PCR in nonexpressing cells (data not shown).
In a third model, antisense transcription from the N-cym gene could lead to the formation of RNA duplexes that could be targeted for degradation by cellular RNases (3). Although extensively documented in prokaryotes, gene regulation through endogenous antisense transcription has rarely been found in eukaryotes (reviewed in references 29, 50, and 58). Interestingly, Krystal et al. showed the presence of stable nonpolyadenylated antisense N-myc transcripts in small-cell lung cancer lines and also demonstrated RNA-RNA duplexes (35). However, a direct antisense mechanism would predict an inverse relationship between N-cym and N-myc expression. Previous work and preliminary experiments in our laboratory have shown that N-cym expression occurs in every cell line where N-myc transcripts have been detected (3, 35, 44). It is still possible that a differentially expressed trans-acting protein or RNase acts to (de)stabilize primary N-myc transcripts through RNA duplex recognition motifs.
Our data are also consistent with a model in which the TSE leads to rapid degradation of primary N-myc transcripts in selected cell types. The transcript may be targeted for rapid degradation by a trans-acting factor in N-myc-nonexpressing cells, or the naturally short-lived transcript could be stabilized in cells that are programmed to express N-myc. Whatever the mechanism, degradation in nonexpressing cells must be extremely efficient, since transcription and processing of the primary transcript, with removal of regulatory intron sequences, occur coordinately (32, 45).
The fact that the TSE functions when placed in the antisense orientation is puzzling, and current models must account for the observation. An antisense mechanism is consistent with this observation, and primary transcripts generated from transiently transfected reporter genes could complex with endogenous antisense transcripts, thus accounting for our results. The fourth model, a direct effect on pre-mRNA stability, is more difficult to reconcile with this observation. However, based on a model of a protein-RNA interaction that mediates rapid degradation of N-myc pre-mRNA, two predictions can be made: (i) the RNA sequence motif responsible for tissue-specific regulation is preserved in both the sense and antisense orientations; and (ii) the N-cym gene located on the opposite DNA strand is subject to the same type of regulation, since the TSE is contained within the first intron of the N-cym gene. Modeling of RNA structure does indicate possible conservation of a predicted stable loop in both orientations (data not shown). Previous work also demonstrates coordinate expression of both N-myc and N-cym RNAs, and our preliminary results with nuclear run-on analysis show that the N-cym gene, like the N-myc gene, is regulated posttranscriptionally in nonexpressing cells (3, 36, 44). Further investigation will clarify the mechanism of TSE mediated N-myc gene regulation.
We consistently saw greater expression from constructs containing a single TSE cloned in the antisense orientation in neuroblastoma cells but not when two antisense TSE units are contained within the transcribed region. We cannot account for this observation, but it could be related to relatively greater affinity of a protein for an RNA structure contained within the TSE imparted by flanking sequences unique to the antisense orientation. Such an increase in affinity could conceivably be disrupted by changes in secondary and/or tertiary structure that occur when two TSE units are juxtaposed.
In summary, our laboratory has identified an element within the first intron of N-myc, the TSE, that functions posttranscriptionally to regulate N-myc transcripts levels in selected cell types. The role that the TSE plays in regulating N-myc expression during normal development and how this regulation occurs is the subject of ongoing work.
ACKNOWLEDGMENTS
We thank Trong Le and Kurt R. Schibler for technical assistance with nuclear run-on experiments. We thank Elizabeth Leibold, Steve Prescott, Don Ayer, and Ray White for many helpful suggestions and for critical reading of the manuscript.
This work was partially supported by institutional training grant 5T32CA09602 from the National Cancer Institute (L.E.S.) and generous support from Steven and Kallen Lund.
REFERENCES
- 1.Alt F W, DePinho R, Zimmerman K, Legouy E, Hatton K, Ferrier P, Tesfaye A, Yancopoulos G, Nisen P. The human myc gene family. Cold Spring Harbor Symp Quant Biol. 1986;51:931–941. doi: 10.1101/sqb.1986.051.01.106. [DOI] [PubMed] [Google Scholar]
- 2.Amanti B, Land H. Myc-Max-Mad: a transcription factor network controlling cell cycle progression, differentiation and death. Curr Opin Genet Dev. 1994;4:102–108. doi: 10.1016/0959-437x(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, B. C., and G. W. Krystal. 1992. Isolation and characterization of complementary DNA for N-cym, a gene encoded by the DNA strand opposite to N-myc. 3:385–390. [PubMed]
- 4.Babiss L E, Friedman J M. Regulation of N-myc gene expression: use of an adenovirus vector to demonstrate posttranscriptional control. Mol Cell Biol. 1990;10:6700–6708. doi: 10.1128/mcb.10.12.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartram C R, Bethold F. Amplification and expression of the N-myc gene in neuroblastoma. Eur J Pediatr. 1987;146:162–165. doi: 10.1007/BF02343225. [DOI] [PubMed] [Google Scholar]
- 6.Bentley D L, Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986;321:702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- 7.Bentley D L, Groudine M. Sequence requirements for premature termination of transcription in the human c-myc gene. Cell. 1988;53:245–256. doi: 10.1016/0092-8674(88)90386-8. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein P L, Herrick D J, Prokipcak R D, Ross J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev. 1992;6:642–654. doi: 10.1101/gad.6.4.642. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell T K, Kretzner L, Blackwood E M, Eisenman R N, Weintraub H. Sequence-specific DNA binding by the c-myc protein. Science. 1990;250:1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- 10.Blackwood E M, Eisenman R N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with myc. Science. 1991;251:1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 11.Brewer G. An A+U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991;11:2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodeur G C, Seeger R C, Schwab M, Varmus H E, Bishop J M. Amplification of N-myc in untreated human neuroblastoma correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 13.Cairo M S, Suen Y, Knoppel E, van de Ven C, Nguyen A, Sender L. Decreased stimulated GM-CSF production and GM-CSF gene expression but normal numbers of GM-CSF receptors in human term newborns compared with adults. Pediatr Res. 1991;30:362–367. doi: 10.1203/00006450-199110000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Cesarman E, Dalla-Favera R, Bentley D, Groudine M. Mutations in the first exon are associated with altered transcription of c-myc in Burkitt lymphoma. Science. 1987;238:1272–1274. doi: 10.1126/science.3685977. [DOI] [PubMed] [Google Scholar]
- 15.Chagnovich D, Cohn S L. Binding of a 40-kDa protein to the N-myc 3′-untranslated region correlates with enhanced N-myc expression in human neuroblastoma. J Biol Chem. 1996;271:33580–33586. doi: 10.1074/jbc.271.52.33580. [DOI] [PubMed] [Google Scholar]
- 16.Charron J, Malynn B A, Fisher P, Stewart V, Jeanotte L, Goff S P, Robertson E J, Alt F W. Embryomic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 1992;6:2248–2257. doi: 10.1101/gad.6.12a.2248. [DOI] [PubMed] [Google Scholar]
- 17.Chu G, Hayakawa H, Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987;15:1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohn S L, Salwen H, Quasney M W, Ikegaki N, Cowan J M, Herst C V, Kennett R H, Rosen S T, DiGiuseppe J A, Brodeur G M. Prolonged N-myc protein half-life in a neuroblastoma cell line lacking N-myc amplification. Oncogene. 1990;5:1821–1827. [PubMed] [Google Scholar]
- 19.DePinho R A, Legouy E, Feldman L B, Kohl N E, Yancopoulos G D, Alt F W. Structure and expression of the murine N-myc gene. Proc Natl Acad Sci USA. 1986;83:1827–1831. doi: 10.1073/pnas.83.6.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dussault I, Giguere V. Differential regulation of the N-myc proto-oncogene by ROR and RVR, two orphan members of the superfamily of nuclear hormone receptors. Mol Cell Biol. 1997;17:1860–1867. doi: 10.1128/mcb.17.4.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eilers M, Schirm S, Bishop J M. The myc protein activates transcription of the α-prothymosin gene. EMBO J. 1991;10:133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Facchini L M, Chen S, Marhin W W, Lear J N, Penn L Z. The Myc negative autoregulation mechanism requires Myc-Max association and involves the c-myc P2 minimal promoter. Mol Cell Biol. 1997;17:100–114. doi: 10.1128/mcb.17.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaspohler J A, Milcarek C. Myelomas and lymphomas expressing the Igγ2a H chain gene have similar transcription termination regions. J Immunol. 1990;144:2802–2810. [PubMed] [Google Scholar]
- 24.Gaubatz S, Meichle A, Eilers M. An E-box element localized in first intron mediates regulation of the prothymosin x gene by c-myc. Mol Cell Biol. 1994;14:3853–3862. doi: 10.1128/mcb.14.6.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillis P, Malter J S. The adenosine-uridine binding factor recognizes the AU-rich elements of cytokine, lymphokine, and oncogene mRNAs. J Biol Chem. 1991;266:3172–3177. [PubMed] [Google Scholar]
- 26.Grady E, Schwab M, Rosenau W. Expression of NMYC and c-src during the development of fetal human brain. Cancer Res. 1987;47:2931–2936. [PubMed] [Google Scholar]
- 27.Grandori C, Mac J, Siebelt F, Ayer D E, Eisenman R N. Myc-Max heterodimers activate a DEAD box gene and interact with multiple E box-related sites in vivo. EMBO J. 1996;15:4344–4357. [PMC free article] [PubMed] [Google Scholar]
- 28.Herrick D J, Ross J. The half-life of c-myc mRNA in growing and serum-stimulated cells: influence of the coding and 3′ untranslated regions and role of ribosome translocation. Mol Cell Biol. 1994;14:2119–2128. doi: 10.1128/mcb.14.3.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildebrandt M, Nellen W. Differential antisense transcription from the dictyostelium EB4 gene locus: implications on antisense-mediated regulation of mRNA stability. Cell. 1992;69:197–204. doi: 10.1016/0092-8674(92)90130-5. [DOI] [PubMed] [Google Scholar]
- 30.Hiller S, Breit S, Wang Z-Q, Wagner E F, Schwab M. Localization of regulatory elements controlling human MYCN expression. Oncogene. 1991;6:969–977. [PubMed] [Google Scholar]
- 31.Jakobovits A, Schwab M, Bishop J M, Marin G R. Expression of N-myc in teratocarcinoma stem cells and mouse embryos. Nature. 1985;318:188–191. doi: 10.1038/318188a0. [DOI] [PubMed] [Google Scholar]
- 32.Jimenez-Garcia L, Spector D. In vivo evidence that transcription and splicing are coordinated by a recruiting mechanism. Cell. 1993;73:47–59. doi: 10.1016/0092-8674(93)90159-n. [DOI] [PubMed] [Google Scholar]
- 33.Kohl N E, Gee C E, Alt F W. Activated expression of the N-myc gene in human neuroblastoma and related tumors. Science. 1984;226:1335–1337. doi: 10.1126/science.6505694. [DOI] [PubMed] [Google Scholar]
- 34.Krumm A, Meulia T, Brunvand M, Groudine M. The block to transcriptional elongation within the human c-myc gene is determined in the promoter-proximal region. Genes Dev. 1992;6:2201–2213. doi: 10.1101/gad.6.11.2201. [DOI] [PubMed] [Google Scholar]
- 35.Krystal G W, Armstrong B C, Battey J F. N-myc mRNA forms an RNA-RNA duplex with endogenous antisense transcripts. Mol Cell Biol. 1990;10:4180–4191. doi: 10.1128/mcb.10.8.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krystal G W, Armstrong B C, Battey J F. N-myc mRNA forms an RNA-RNA duplex with endogenous antisense transcripts. Mol Cell Biol. 1990;10:4180–4191. doi: 10.1128/mcb.10.8.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leder P, Battey J, Lenoir G, Moulding C, Murphy W, Potter H, Stewart T, Taub R. Translocations among antibody genes in human cancer. Science. 1983;222:765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- 38.Lee L A, Dolde C, Barrett J, Wu C, Dang C V. A link between c-Myc-mediated transcriptional repression and neoplastic transformation. J Clin Investig. 1996;97:1687–1695. doi: 10.1172/JCI118595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee W, Murphee A, Benedict A. Expression and amplification of the N-myc gene in primary retinoblastoma. Nature. 1984;309:458–460. doi: 10.1038/309458a0. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Nerlov C, Prendergast G, MacGregor D, Ziff E B. C-Myc represses transcription in vivo by a novel mechanism dependent on the initiator element and Myc box II. EMBO J. 1994;13:4070–4079. doi: 10.1002/j.1460-2075.1994.tb06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lutz W, Stohr M, Schurmann J, Wenzel A, Lohr A, Schwab M. Conditional expression of N-myc in human neuroblastoma cells increases expression of α-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene. 1996;13:803–812. [PubMed] [Google Scholar]
- 42.Madison L, Groudine M. Identification of a locus-control region in the immunoglobulin heavy-chain locus that deregulates c-myc expression in plasmacytoma and Burkitt’s lymphoma cells. Genes Dev. 1994;8:2212–2226. doi: 10.1101/gad.8.18.2212. [DOI] [PubMed] [Google Scholar]
- 43.Marcu K B, Bossone S A, Patel A J. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- 44.Mayr, G., G. Pont-Kingdon, and W. Carroll. Unpublished data.
- 45.Misteli T, Caceres J, Spector D. The dynamics of a pre-mRNA splicing factor in living cells. Nature. 1997;387:523–527. doi: 10.1038/387523a0. [DOI] [PubMed] [Google Scholar]
- 46.Morrow M A, Lee G, Gillis S, Yancopoulos G D, Alt F W. Interleukin-7 induces N-myc and c-myc expression in normal precursor B lymphocytes. Genes Dev. 1992;6:61–70. doi: 10.1101/gad.6.1.61. [DOI] [PubMed] [Google Scholar]
- 47.Mugrauer G, Alt F W, Ekblom P. N-myc proto-oncogene expression during organogenesis in the developing mouse as revealed by in situ hybridization. J Cell Biol. 1988;107:1325–1335. doi: 10.1083/jcb.107.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nau M, Brooks B, Carney D, Gazdar A, Battey J, Sausville E, Minna J. Human small-cell lung cancers show amplification and expression of the N-myc gene. Proc Natl Acad Sci USA. 1986;83:1092–1094. doi: 10.1073/pnas.83.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nisen P D, Zimmerman K A, Cotter S V, Gilbert F, Alt F W. Enhanced expression of the N-myc gene in Wilms’ tumors. Cancer Res. 1986;46:6217–6222. [PubMed] [Google Scholar]
- 50.Noguchi M, Miyamoto S, Silverman T A, Safer B. Characterization of an antisense Inr element in the eIF-2α gene. J Biol Chem. 1994;269:29161–29167. [PubMed] [Google Scholar]
- 51.Prokipcak R D, Herrick D J, Ross J. Purification and properties of a protein that binds to the c-terminal coding region of human c-myc mRNA. J Biol Chem. 1994;269:9261–9269. [PubMed] [Google Scholar]
- 52.Putney S D, Benkovic S J, Schimmel P R. A DNA fragment with an alpha-phosphorothionate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci USA. 1981;78:7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 54.Schwab M, Aitalo K, Klempnauer K H, Varmus H E, Bishop J M, Gilbert F, Brodeur G, Goldstein M, Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumor. Nature. 1984;305:245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- 55.Seed B, Sheen J Y. A simple phase extraction assay for chloramphenicol acetyltransferase activity. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 56.Seeger R C, Brodeur G M, Sather H, Dalton A, Siegel S E, Wong K Y, Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 57.Shrivastava A, Saleque S, Kalpana G V, Artandi S, Goff S P, Calame K. Inhibition of transcriptional regulator Yin-Yang-1 by association with c-Myc. Science. 1993;262:1889–1892. doi: 10.1126/science.8266081. [DOI] [PubMed] [Google Scholar]
- 58.Simons R W, Kleckner N. Biological regulation by antisense RNA in prokaryotes. Annu Rev Genet. 1988;22:567–600. doi: 10.1146/annurev.ge.22.120188.003031. [DOI] [PubMed] [Google Scholar]
- 59.Sivak L, E, Tai K-F, Smith R S, Dillon P A, Brodeur G M, Carroll W L. Autoregulation of the human N-myc oncogene is disrupted in amplified but not single-copy neuroblastoma cell lines. Oncogene. 1997;15:1937–1946. doi: 10.1038/sj.onc.1201363. [DOI] [PubMed] [Google Scholar]
- 60.Slamon D J, Boone T C, Seeger R C, Keith D E, Chazin V, Lee H C, Souza L M. Identification and characterization of the protein encoded by the human N-myc oncogene. Science. 1986;232:768–772. doi: 10.1126/science.3008339. [DOI] [PubMed] [Google Scholar]
- 61.Slavc I, Ellenbogen R, Jung W-H, Vawter G F, Kretschmar C, Grier H, Korf B R. myc gene amplification and expression in primary human neuroblastoma. Cancer Res. 1990;50:1459–1463. [PubMed] [Google Scholar]
- 62.Smith R K, Zimmerman K, Yancopoulos G D, Ma A, Alt F W. Transcriptional down-regulation of N-myc expression during B-cell development. Mol Cell Biol. 1992;12:1578–1584. doi: 10.1128/mcb.12.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spencer C A, LeStrange R C, Novak U, Hayward W S, Groudine M. The block to transcription elongation is promoter dependent in normal and Burkitt’s lymphoma c-myc alleles. Genes Dev. 1990;4:75–88. doi: 10.1101/gad.4.1.75. [DOI] [PubMed] [Google Scholar]
- 64.Stanton B R, Perkins A S, Tessarollo L, Sassoon D A, Parada L F. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 1992;6:2235–2247. doi: 10.1101/gad.6.12a.2235. [DOI] [PubMed] [Google Scholar]
- 65.Tacke R, Tohyama M, Ogawa S, Manley J L. Human Tra2 proteins are sequence-specific activators of pre-mRNA splicing. Cell. 1998;93:139–148. doi: 10.1016/s0092-8674(00)81153-8. [DOI] [PubMed] [Google Scholar]
- 66.Thiele C J, Reynolds C P, Israel M A. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature. 1985;313:404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- 67.Wada R K, Seeger R C, Brodeur G M, Einhorn P A, Rayner S A, Tomayko M M, Reynolds C P. Human neuroblastoma cell lines that express N-myc without gene amplification. Cancer. 1993;72:3346–3354. doi: 10.1002/1097-0142(19931201)72:11<3346::aid-cncr2820721134>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 68.Wada R K, Seeger R C, Reynolds C P, Alloggiamento T, Yamashiro J M, Ruland C, Black A C, Rosenblatt J D. Cell type-specific expression and negative regulation by retinoic acid of the human N-myc promoter in neuroblastoma cells. Oncogene. 1992;7:711–717. [PubMed] [Google Scholar]
- 69.Woodruff K A, Rosenblatt J D, Moore T B, Medzoyan R H, Pai D M, Noland J L, Yamashiro J M, Wada R K. Cell type-specific activity of the N-myc promoter in human neuroblastoma cells is mediated by a downstream silencer. Oncogene. 1995;10:1335–1341. [PubMed] [Google Scholar]
- 70.Xu L, Meng Y, Wallen R, DePinho R. Loss of transcriptional attenuation in N-myc is associated with progression towards a more malignant phenotype. Oncogene. 1995;10:1865–1872. [PubMed] [Google Scholar]
- 71.Xu L, Morgenbesser S D, DePinho R A. Complex transcriptional regulation of myc family gene expression in the developing mouse brain and liver. Mol Cell Biol. 1991;11:6007–6015. doi: 10.1128/mcb.11.12.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu L, Wallen R, Patel V, DePinho R. Role of first exon/intron sequences in the regulation of myc family oncogenic potency. Oncogene. 1993;8:2547–2553. [PubMed] [Google Scholar]
- 73.Zimmerman K A, Yancopoulos G D, Collum R G, Smith R K, Kohl N E, Denis K A, Nau M M, Witte O N, Toran-Allerand D, Gee C E, Minna J D, Alt F W. Differential expression of myc family genes during murine development. Nature. 1986;319:780–783. doi: 10.1038/319780a0. [DOI] [PubMed] [Google Scholar]