Abstract
HIV-1 infection substantially increases the risk of developing tuberculosis (TB). Mechanisms such as defects in the Th1 response to Mycobacterium tuberculosis (M.tb) in HIV-infected persons have been widely reported. However, Th1-independent mechanisms also contribute to protection against TB. To identify a broader spectrum of defects in TB immunity during HIV infection, we examined IL-17A and IL-22 production in response to mycobacterial antigens in peripheral blood of persons with latent TB infection (LTBI) and HIV co-infection. Upon stimulating with mycobacterial antigens, we observed a distinct CD4+ T helper lineage producing IL-22 in the absence of IL-17A and IFN-γ. Mycobacteria-specific Th22 cells were present at high frequencies in blood and contributed up to 50% to the CD4+ T cell response to mycobacteria, comparable in magnitude to the IFN-γ Th1 response (median 0.91% and 0.55%, respectively). Phenotypic characterization of Th22 cells revealed that their memory differentiation was similar to M.tb-specific Th1 cells (i.e. predominantly early-differentiated CD45RO+CD27+ phenotype). Moreover, CCR6 and CXCR3 expression profiles of Th22 cells were similar to Th17 cells, while their CCR4 and CCR10 expression patterns displayed an intermediate phenotype between Th1 and Th17 cells. Strikingly, mycobacterial IL-22 responses were three-fold lower in HIV-infected persons compared to uninfected persons, and the magnitude of responses correlated inversely with HIV viral load. These data provide important insights into mycobacteria-specific T helper subsets in humans and suggest a potential role for IL-22 in protection against TB during HIV infection. Further studies are needed to fully elucidate the role of IL-22 in protective TB immunity.
INTRODUCTION
Tuberculosis (TB) is the leading cause of death from an infectious agent, claiming 1.4 million lives in 2019, with 10 million new TB cases that year (1). This considerable burden of disease, along with a host of challenges in diagnosing, treating and managing TB, emphasize its significance as a global health threat. Although TB is curable and successful treatment outcomes are typically >80%, cure is achieved less frequently with drug resistant TB (56%), and outcomes during HIV co-infection are worse (2). Importantly, cure does not lead to protection from re-infection or disease reactivation. HIV-infected persons are particularly vulnerable to developing TB, with an estimated increase in risk of 20–30 fold (3). The widespread introduction of ART has coincided with only a modest decline in TB in regions most affected by HIV (4), as TB risk still remains elevated in HIV-infected persons compared to HIV-uninfected persons, despite immune reconstitution (5).
The development of an effective TB vaccine is hampered by a lack of understanding of correlates of immune protection (6), particularly the functional and phenotypic characteristics of effector T cells that mediate control of Mycobacterium tuberculosis (M.tb), and how this immune response might be balanced by immunoregulatory T cell populations to limit inflammation and avoid pathology. The recent demonstration of the first candidate TB vaccine capable of protecting adults from pulmonary TB with an efficacy of 54% (7), provides the field with an opportunity to define correlates of vaccine protection, and has the potential to uncover unique insights into immunological control of TB.
TB and HIV co-infection presents us with a further prospect to improve our understanding of the mechanisms of immune control of M.tb, by identifying how HIV renders the immune response to M.tb defective, leading to increased risk of TB disease. CD4+ T cells and specifically the Th1/IFN-γ response to M.tb are critical for protective immunity to TB (8). Most studies of HIV-TB co-infection focus on Th1 immunity, and have demonstrated depletion or dysfunction of M.tb-specific Th1 responses in both blood (9–12) and the airways (13–15) during HIV infection.
However, there is evidence of a role for IFN-γ-independent mechanisms in immune control of TB (16) that may also contribute to, or synergize with, Th1 responses to TB. Recently, we characterized the profile of Th subsets specific for M.tb using lineage-defining transcription factors, revealing the broad spectrum of Th subsets involved in mycobacterial immunity, demonstrating that the inflammatory environment associated with HIV infection skewed these profiles (17). Th17 cells form part of this spectrum of M.tb-specific Th responses, and are believed to play an important role in immune protection from TB (18). Suppression of Th17-related genes was recently shown to be associated with progression to TB disease in M.tb-infected adolescents (19). In line with this, M.tb-specific IL-17A-producing CD4+ T cells were significantly depleted in HIV-infected individuals from a TB-endemic area, compared to HIV-uninfected individuals (20).
Whilst IL-17 responses in M.tb immunity have been relatively well-studied (21–26), IL-22 responses have been overlooked in part due to their classification as a Th17 cytokine from studies in mice (27). In humans, however, IL-22 is produced by a distinct subset of CD4+ T cells (28–30), termed “Th22 cells”. IL-22 is a member of the IL-10 family of cytokines, and functions mainly to protect tissues from inflammation and infection, through stimulating proliferation and repair, and the production of antimicrobial peptides (31). Until recently, IL-22 was thought to be dispensable for control of M.tb, since deficiency or neutralization of IL-22 in mice had no effect control of M.tb using lab strains H37Rv and Erdman (32–35). However, the recent observation that IL-22 deficient mice infected with a clinical strain of M.tb (HN878) had an impaired ability to control M.tb, leading to increased bacterial burden and greater dissemination of infection (36), has triggered renewed interest in IL-22 and its role in TB control.
Given the paucity of data on M.tb-specific IL-22 CD4+ responses, and the knowledge that HIV infection results in the preferential targeting and depletion of Th22 cells (37), we sought to characterize HIV-induced defects in adaptive immunity to M.tb, with a focus on Th22 cells. Our findings highlight the large contribution IL-22 makes to the human CD4+ T cell response to TB (equivalent in magnitude to the IFN-γ response), with M.tb-specific Th22 cells being entirely distinct from Th1 and Th17 cells. Moreover, we show for the first time that M.tb-specific Th22 cells are depleted during HIV co-infection to a similar extent as Th1 responses. These findings emphasize the potential importance of this understudied CD4+ Th subset in TB immunity, and suggest that the loss of M.tb-specific Th22 cells may contribute to the increased risk of TB during HIV infection.
MATERIALS AND METHODS
Study Participants
Volunteers were recruited from Cape Town, South Africa, and fell within the following groups: ART naive HIV-seropositive persons with CD4 counts >400 cells/mm3 (n=25; median age 31; 96% female) and HIV-seronegative persons (n=25; median age 23; 60% female). HIV RNA levels were determined using an Abbott m2000 RealTime HIV-1 assay and blood CD4 counts by the Flow-CARE™ PLG CD4 test. All volunteers were TB sensitized based on a positive IFN-γ release assay (IGRA; Quantiferon, Cellestis), and active TB was excluded, based on symptoms and radiological evidence.
Healthy donors were recruited from the University of Cape Town, South Africa. Participants were >18 years of age, weighed >55 kg, did not have any chronic disease, did not use immunosuppressive medication and were not pregnant or lactating. These studies were approved by the Research Ethics Committee of the University of Cape Town (158/2010, 279/2012). All participants provided written, informed consent.
Whole blood stimulation assays
Venous blood was collected and processed within 4 hours. Whole blood stimulation was performed as previously described (38) with the following antigens: Bacillus Calmette-Guerin (BCG; MOI of 4; SSI), Purified Protein Derivative (PPD) of M. tuberculosis (20μg/ml; Statens Serum Institute), ESAT-6 and CFP-10 peptide pools (4μg/ml), M. tuberculosis whole cell lysate (10μg/ml; BEI Resources) or PMA and Ionomycin (0.01μg/ml and 1μg/ml, respectively, Sigma), in the presence of anti-CD28 and anti-CD49d (1μg /ml each). Optimal antigen concentrations were determined experimentally. Unstimulated cells were incubated with co-stimulatory antibodies only. Brefeldin A (BFA, 10μg/ml; Sigma) was added 7 hours after the onset of stimulation, and 5 hours after BFA addition, cells were either stained immediately, or red blood cells were lysed, the cell pellet stained with a violet viability dye, ViViD (Molecular Probes), fixed with FACS Lyse (BD Biosciences) and cryopreserved in 10% DMSO in FCS. For peripheral blood mononuclear cells (PBMC) and whole blood co-culture experiments, PBMC were isolated by density gradient centrifugation. Freshly isolated PBMC were stained with the fluorescent dye Oregon Green (0.5 mg/ml; Invitrogen) for 4 min at room temperature. After washing in PBS, 2×106 cells were combined with 500μl blood from the same donor and stimulated as described above.
Antibody Staining and Flow Cytometry
Cryopreserved or freshly stimulated whole blood was stained as previously described (15). For intracellular markers, cells were permeabilized with Perm/Wash buffer (BD Biosciences) and then stained intracellularly. The following antibodies were used for characterizing cytokine expression and memory phenotype: CD3 APC-H7 (SK7; BD Biosciences), CD4 PE-Cy5.5 (S3.5), CD8 QDot705 (3B5; both Invitrogen), CD45RO ECD (UCLH1), CD27 PE-Cy5 (1A4CD27; both Beckman Coulter), IFN-γ Alexa700 (B27), IL-17A Alexa488 (N49–653; both BD Biosciences) and IL-22 PE (22URTI; e-Bioscience). To characterise chemokine receptor expression on Th1, Th17 and Th22 cells, whole blood was stained directly after stimulation, without red blood cell lysis, with CD14-Pacific blue, CCR4-BV510 (L291H4; Biolegend), CCR6-BV605 (11A9); CCR10-PE (1B5) and CXCR3-PECy7 (1C6/CXCR3; all BD Biosciences). The cells were then permeabilized and stained intracellularly with CD3-APC-H7 (SK7), IFN-γ-Alexafluor 700 (B27; both BD Biosciences), IL-17A-FITC (BL-168; Biolegend) and IL-22-eFluor450 (22URTI; e-Bioscience). An additional panel included KLRG1 PE-vio770 (REA261; Miltenyi Biotec) and CD26 FITC (M-A261; BD Biosciences). Cells were acquired on a BD Fortessa using FACSDiva software and data analysed using FlowJo (TreeStar) and Pestle and Spice (39). Fluorescent minus one (FMO) controls were run to optimize each antibody panel and ensure that there was no spillover from other channels and to set gates for positive populations (Supplemental Figure S1A). The gating strategy is indicated in Supplemental Figure S1B. Cells were gated first on time, followed by singlets, lymphocytes, live CD3+, total CD4+ IFN-γ+ cells and then CD4+ CD8- cells. After that, cytokine and memory gates were drawn. A similar gating strategy was employed for the analysis of chemokine receptor expression on cytokine positive populations. A positive cytokine response was defined as twice background (unstimulated sample) and a net response >0.025%, and all data are reported after background subtraction. The backgrounds were low overall, namely for IFN-γ: median 0.007% (IQR, 0.002–0.01%), IL-22: median 0.02% (IQR, 0.014–0.036%) and IL-17A: median 0.012% (IQR, 0.007–0.027%). A minimum of 30 cytokine-positive events was required for memory or chemokine receptor phenotyping.
Plasma cytokine measurement
Whole blood stimulation was performed as described above (but without the addition of BFA), and plasma was harvested 12 and 24 hours after stimulation with M.tb lysate. Plasma was assayed using a commercial sandwich ELISA (Invitrogen) to quantify soluble IFN-γ, IL-22 and IL-17A, according to manufacturer’s instructions. The sensitivity of the kits ranged from 4 pg/ml (IFN-γ and IL-17A) to 8 pg/ml (IL-22).
Statistical Analysis
Statistical analyses were performed using Prism 7 (GraphPad). Non-parametric tests (Mann-Whitney U test, Wilcoxon matched pairs test and Spearman Rank test) were used for all comparisons. Kruskal-Wallis and Friedman tests with Dunn’s post-test was used for multiple comparisons. A p value of <0.05 was considered significant.
RESULTS
IL-22 responses are a major component of the CD4+ mycobacterial response
We examined CD4+ T cell cytokine profiles in response to a range of mycobacterial antigens in 25 healthy, HIV-uninfected persons sensitized by M. tuberculosis (M.tb IGRA+; Table 1). Figure 1A shows representative flow cytometry plots of IFN-γ, IL-22 and IL-17A CD4+ responses to M. bovis BCG, M.tb PPD and a pool of ESAT-6 and CFP-10 peptides from M.tb. As expected, CD4+ T cell IFN-γ responses to BCG were detected in all donors (median 0.55%, IQR: 0.28–1.46%; Figure 1B). Remarkably, IL-22 accounted for the greatest proportion of the CD4+ response to BCG (median 0.91%, IQR: 0.52–1.24%). In fact, the frequency of IL-22+ cells was greater than IFN-γ in 75% of participants. IL-17A CD4+ responses to BCG were significantly lower (median 0.11%, IQR: 0.06–1.66%) than both IFN-γ (p=0.007) and IL-22 (p=0.0008). Stimulation with M.tb PPD led to the detection of a similar IFN-γ response as BCG (median 0.74%), with comparatively lower frequencies of PPD-specific IL-22+ CD4+ T cells (median 0.21%; p=0.02) and IL-17A+ cells (median 0%; p<0.0001) (Figure 1B). The ESAT-6/CFP-10 response was dominated by IFN-γ (median 0.07%), with low to undetectable IL-17A and IL-22 responses (medians of 0%). Taken together, these data demonstrate that different mycobacterial antigen preparations result in detection of different CD4+ T cell cytokine profiles. Of note, IL-22 made a substantial contribution to the anti-mycobacterial CD4+ response, with responses equivalent to or greater than the IFN-γ response to BCG.
Table 1:
Characteristics of study participants
| HIV-uninfected (n=25) | HIV-infected (n=25) | ||||
|---|---|---|---|---|---|
| PID | CD4 count (cells/mm3) | PID | CD4 count (cells/mm3) | Viral Load (RNA copies/ml) | |
| 1032 | ND | 1086 | 1449 | 4250 | |
| 1035 | 1459 | 1151 | 988 | 1848 | |
| 1052 | 1412 | 1075 | 965 | 5922 | |
| 1031 | 1169 | 1150 | 894 | 311 | |
| 1023 | 1120 | 1006 | 802 | 4141 | |
| 1070 | 1028 | 1039 | 790 | 4521 | |
| 1024 | 939 | 1080 | 774 | 14100 | |
| 1025 | 915 | 1152 | 749 | <40 | |
| 1057 | 871 | 1154 | 714 | 2954 | |
| 1058 | 866 | 1018 | 681 | 4614 | |
| 1054 | 832 | 1143 | 656 | 618 | |
| 1094 | 827 | 1073 | 632 | 12274 | |
| 1028 | 814 | 1084 | 619 | 9192 | |
| 1033 | 813 | 1134 | 599 | 6383 | |
| 1011 | 801 | 1079 | 591 | 32485 | |
| 1095 | 760 | 1153 | 571 | 9697 | |
| 1038 | 743 | 1137 | 560 | 18797 | |
| 1072 | 741 | 1141 | 552 | 9826 | |
| 1047 | 680 | 1076 | 543 | 908 | |
| 1061 | 674 | 1045 | 522 | 59125 | |
| 1015 | 659 | 1129 | 510 | 4559 | |
| 1049 | 655 | 1074 | 478 | 10093 | |
| 1001 | 631 | 1142 | 441 | 544849 | |
| 1066 | 621 | 1126 | 433 | 32994 | |
| 1010 | 580 | 1020 | 406 | 31145 | |
| Median | 813 | 619 | 6383 | ||
| IQR | 675.5–933 | 532.5–782 | 3548–16449 | ||
Figure 1: CD4+ T cell cytokine responses to mycobacterial antigens in latent TB infection.
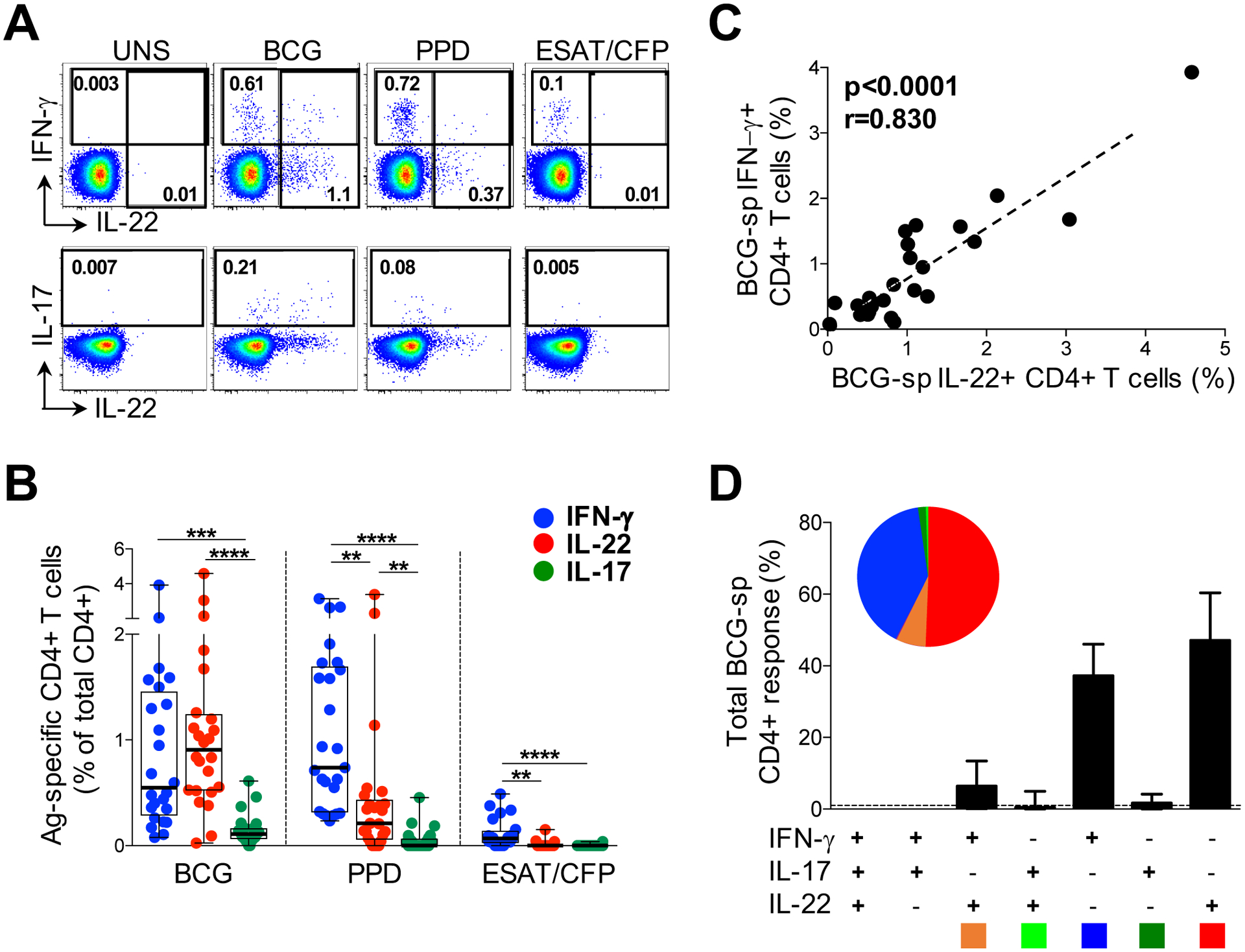
(A) Representative flow cytometry plots of the production of IFN-γ, IL-22 and IL-17A from CD4+ T cells after stimulation with M. bovis BCG, M.tb PPD and ESAT-6/CFP-10 peptides, in one study participant. UNS corresponds to the unstimulated control. The frequency of cytokine-producing cells is shown as a percentage of the total CD4+ T cell population, after gating on live, CD3+ lymphocytes. (B) Individual IFN-γ (blue), IL-22 (red) or IL-17A (green) responses to BCG, PPD or ESAT-6/CFP-10 in individuals with latent TB infection (LTBI; n=25). The frequency of cytokine-producing cells is shown as a percentage of the total CD4+ T cell population, after gating on live, CD3+ lymphocytes. (C) The relationship between the frequency of CD4+ T cells producing IFN-γ and IL-22 in response to BCG (n=24). (D) Populations of CD4+ T cells producing different combinations of IFN-γ, IL-22 and IL-17A in response to BCG. The pie charts indicate the proportion of cytokine combinations that makes up the BCG response. Each slice of the pie represents a specific subset of cells, defined by a combination of cytokines shown by the color at the bottom of the graphs. Data are shown as box and whisker (interquartile range) plots and horizontal bars represent the median. Each dot represents one individual. Statistical comparisons were performed using Friedman’s test with Dunn’s multiple comparison, and a non-parametric Spearman rank test for the correlation. **p≤0.01, ***p≤0.001, ****p≤0.0001.
We next focused on the high magnitude IL-22 response detected to BCG, to further characterize IL-22 CD4+ responses and their relationship with IFN-γ and IL-17A. There was a highly significant positive correlation between IFN-γ and IL-22 responses to BCG (p<0.0001, r=0.830; Figure 1C). The frequency of IL-17A+ CD4+ T cells also correlated with both IFN-γ and IL-22 production (p=0.039, r=0.424 and p=0.005, r=0.559, respectively; data not shown). Given these associations between IFN-γ, IL-22 and IL-17A, we examined the co-expression patterns of the cytokines following BCG stimulation (Figure 1D). The majority of BCG-responding CD4+ T cells produced only IL-22 (median 47%; IQR: 36.6–59.6), whilst CD4+ cells secreting IFN-γ-alone made up a median of 37% (IQR: 27.1–47.4). There was minimal co-expression of IL-22 with both IL-17A (median 0.5%) and with IFN-γ (median 6.4%). When examining all CD4+ T cells producing IL-22, a median of 78% produced IL-22 alone (IQR: 71.1–89.2%), while 14% and 1.5% co-expressed IFN-γ or IL-17A, respectively (data not shown). We also investigated IL-22 production in combination with other cytokines and found low or negligible co-expression with TNF-α, IL-2 and IL-21 (medians 0.3%, 0.5% and 3%, respectively, data not shown). Our data reveal that the large proportion of BCG-specific IL-22 was produced predominantly by CD4+ T cells secreting IL-22 in the absence of either IL-17A or IFN-γ, consistent with being a distinct ‘Th22’ lineage (28–30).
We next sought to determine whether we could detect soluble IL-22 in response to mycobacterial stimulation. For these studies, we stimulated whole blood with M.tb whole cell lysate. To ensure that we were measuring similar cytokine responses to BCG, we compared IFN-γ, IL-22 and IL-17A induced by each antigen and found highly comparable frequencies of CD4+ T cell responses in the same donors, using optimal antigen concentrations determined by titration (Supplemental Figure S2). Then, using a direct ELISA, we quantified soluble cytokine secretion in plasma of whole blood stimulated with M.tb lysate for 12 and 24 hours in 8 healthy donors with LTBI (Figure 2). IFN-γ was readily detectable in stimulated plasma at 12 hours (median 722pg/ml; range: 542–1640pg/ml). Of note, soluble IL-22 was also abundantly detectable in plasma, at a median of 3232pg/ml (range 2600–7489pg/ml), five-fold higher than IFN-γ concentrations. Levels of both IFN-γ and IL-22 more than doubled at 24 hours compared to 12 hours (p=0.004). In contrast, low concentrations of IL-17 were detectable at 12 hours and decreased significantly by 24 hours of stimulation (p=0.02). These data support the cytokine production demonstrated by flow cytometry, indicating robust secretion of IL-22 in response to mycobacteria.
Figure 2: Cytokine secretion in M.tb-stimulated whole blood.
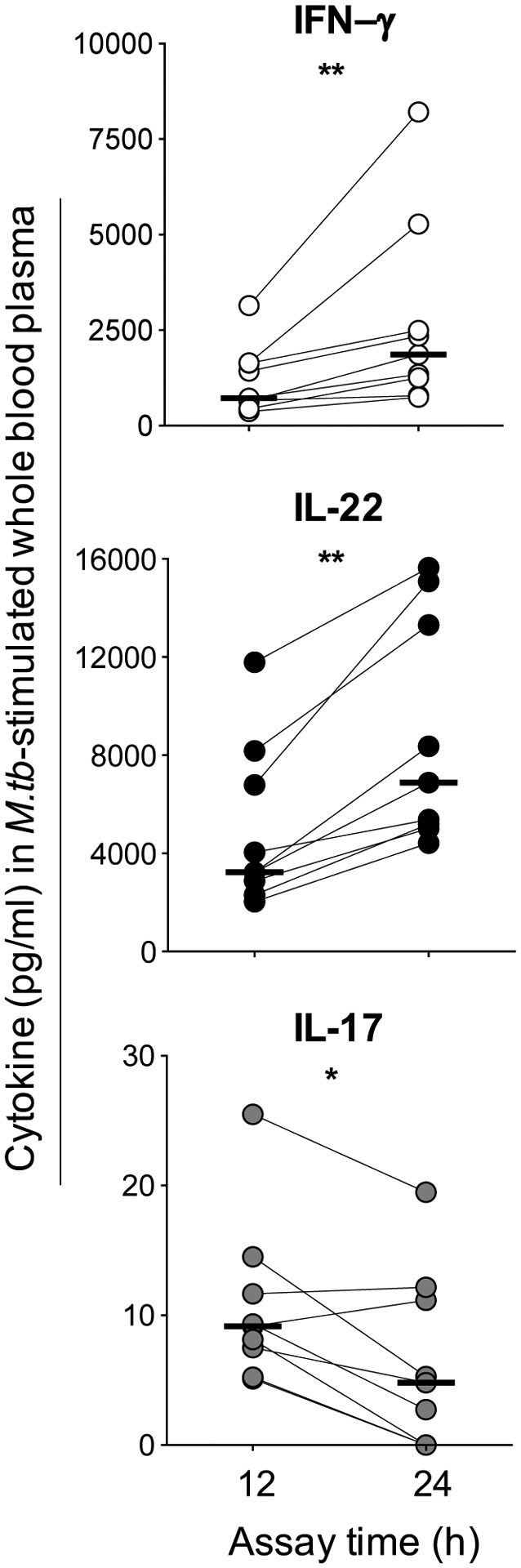
The concentration of soluble IFN-γ (white), IL-22 (black) and IL-17A (gray) was measured by ELISA (pg/ml) in plasma from whole blood stimulated with M.tb lysate in individuals with LTBI (n=9). Plasma was harvested at 12 and 24 hours after stimulation. Horizontal bars represent the median. Statistical comparisons were performed using a Wilcoxon test. *p≤0.05, **p≤0.01.
IL-22 detection differs between whole blood and PBMC
The finding of high frequencies of Th22 cells specific for mycobacteria in LTBI contributing so substantially to the overall T cell response that we report here was surprising, given that Th1/IFN-γ responses are widely reported to be the dominant response to mycobacteria. PBMC are most often used in studies analyzing human cytokine expression, so we compared our assay format, direct stimulation of whole blood with mycobacterial antigens, with cytokine responses from stimulated PBMC. Figure 3A (bottom left panel) shows representative flow cytometry plots of the CD4+ T cell IFN-γ and IL-22 responses to M.tb in whole blood and PBMC. We noted that whilst IFN-γ production by CD4+ T cells was readily detected in both assays, IL-22 was barely detectable, and this was confirmed in 8 healthy donors with LTBI (Figure 3B). Based on these poor CD4+ IL-22 responses we observed in PBMC, we investigated whether CD4+ T cells from PBMC retained the ability to produce IL-22 under optimal conditions. To determine whether the CD4+ IL-22 response from PBMC could be recovered, a mixed reaction of autologous PBMC added to whole blood was stimulated (Figure 3A). Strikingly, the IL-22 response was detectable in PBMC from the mixed reaction to such an extent that it was similar to IL-22 from both whole blood and whole blood from the mixed culture (medians 0.65%, 0.73% and 0.98%, respectively). The PBMC IFN-γ response was also higher in the mixed reaction compared to PBMC alone, and comparable to whole blood from the co-culture (medians 0.72% and 0.98%). Our results demonstrate that CD4+ T cells from PBMC retain the ability to produce IL-22 and this response can be restored under specific culture conditions.
Figure 3: Detection of the CD4+ T cell IL-22 responses in PBMC.
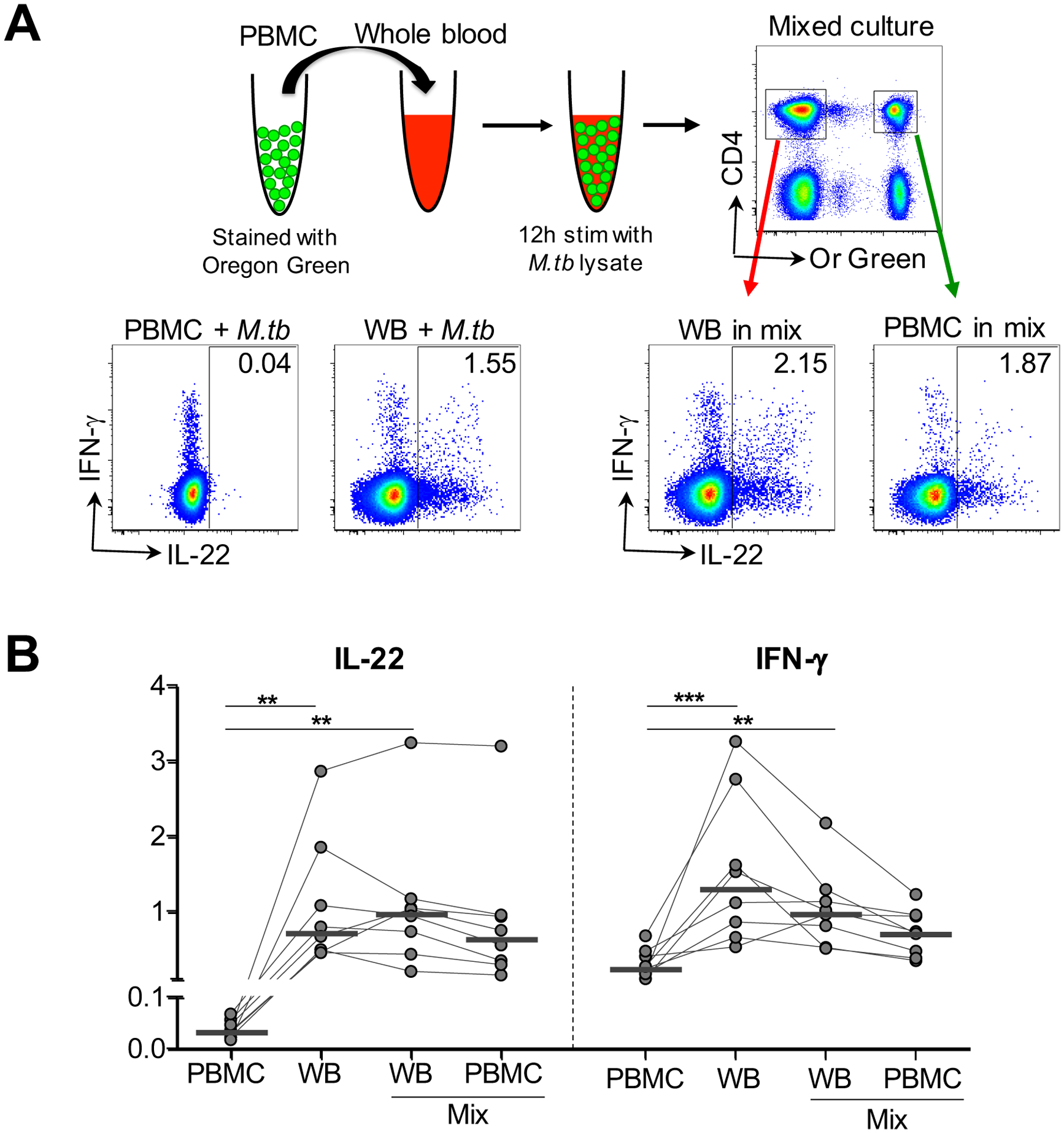
(A) Schematic of mixed stimulation assay with representative flow cytometry data. PBMC were stained with a fluorescent dye, Oregon Green (Or Green), and added to autologous whole blood, and subsequently stimulated with M.tb lysate. Representative flow cytometry plots show the production of IFN-γ and IL-22 from CD4+ T cells in fresh PBMC (PBMC+ M.tb) alone, whole blood (WB + M.tb) alone, and the mixed stimulation assay (WB in mix, PBMC in mix), after stimulation with M.tb lysate in one participant. (B) Comparison of the frequency of CD4+ T cells producing IL-22 and IFN-γ in each experimental condition in the same individuals (n=8). Horizontal bars represent the median. Statistical comparisons were performed using Friedman’s test with Dunn’s multiple comparison. **p≤0.01, ***p≤0.001.
Phenotypic characteristics of mycobacteria-specific IL-22-producing CD4+ T cells
In order to characterize the Th22 subset in more detail, we determined the memory differentiation profile of mycobacteria-specific Th22 cells (i.e. those producing IL-22 alone) compared to cells producing only IFN-γ or IL-17A. Figure 4A shows representative flow cytometry plots of CD45RO and CD27 expression on total CD4+ cells with overlays of BCG-specific cytokine-producing CD4+ T cells (IFN-γ, IL-22 or IL-17 alone). The memory profile of BCG-specific CD4+ T cells was comparable, regardless of their cytokine secretion profile, with approximately 79% having an early differentiated phenotype (ED: CD45RO+CD27+, comprising central and transitional memory cells). Of the remaining cells, a median of ~17% were late differentiated (LD: CD45RO+CD27-, comprising effector memory and intermediate cells), with few terminally differentiated (TD: CD45RO-CD27-; ~0.3%) or naïve-like (CD45RO-CD27+; ~2 %) cells (Figure 4B). Thus, CD4+ T cells producing IFN-γ, IL-22 or IL-17A shared a similar memory differentiation phenotype.
Figure 4: Memory profiles of CD4+ T cells producing IFN-γ, IL-22 or IL-17A in response to BCG.
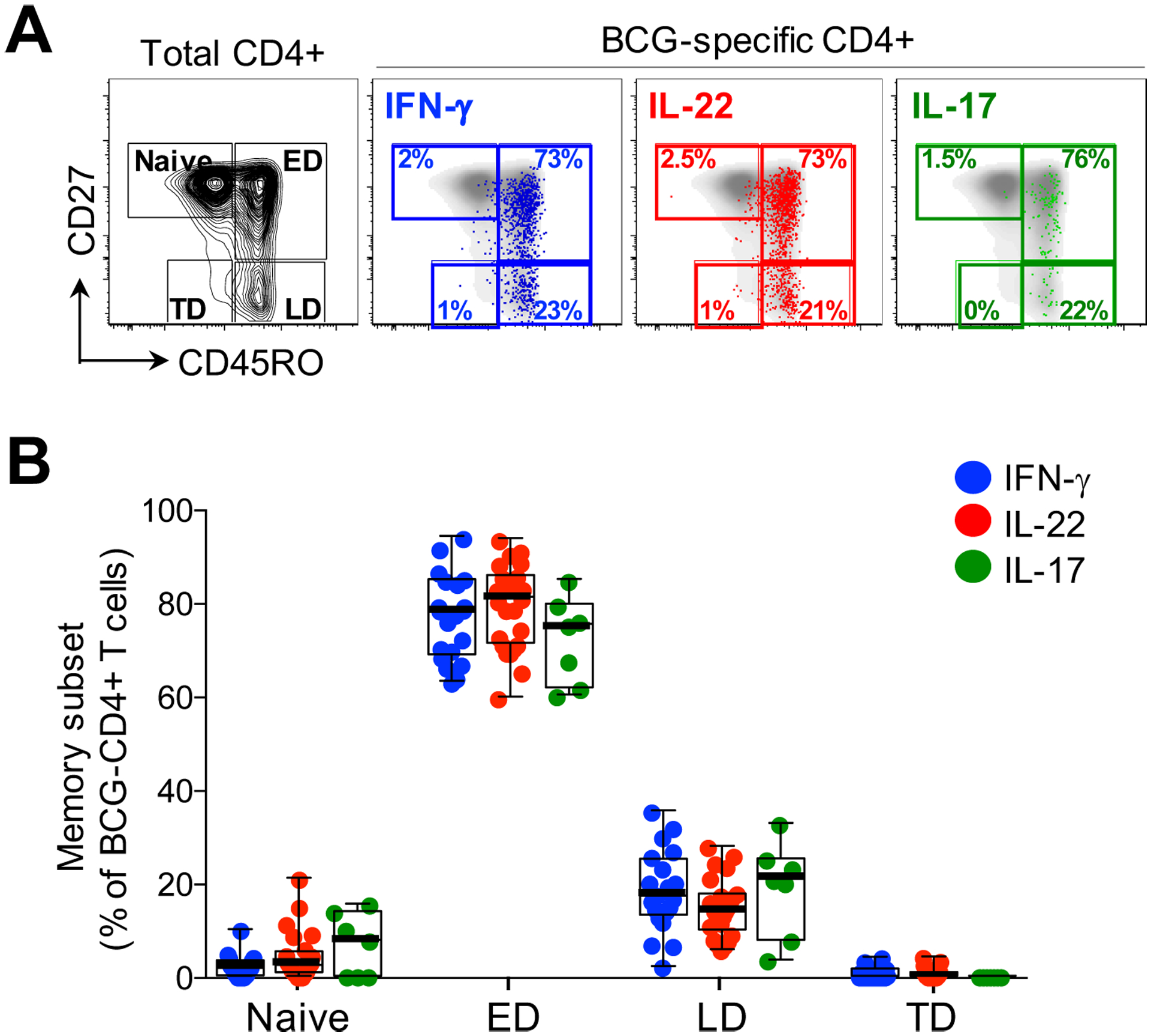
(A) Representative flow cytometry plots of total CD4+ memory subset distribution in one individual based on CD45RO and CD27 staining. Naïve: CD45RO-CD27+, early differentiated (ED: CD45RO+CD27+), late differentiated (LD: CD45RO+CD27-) and terminally differentiated (TD: CD45RO-CD27-). The overlays indicate the antigen specific CD4+ T cells producing IFN-γ (blue), IL-22 (red) or IL-17A (green). The frequencies of each subset are indicated. (B) The memory distribution of cells producing IFN-γ (blue), IL-22 (red) or IL-17A (green) in response to BCG (n=20, 25 and 7, respectively). Only individuals with a positive cytokine response and more than 30 cytokine events were included in the phenotyping. Each dot represents one individual. Data are shown as box and whisker (interquartile range) plots and horizontal bars represent the median. Statistical comparisons were performed using Kruskal-Wallis and Dunn’s multiple comparison test.
To further characterize the phenotype of the different cytokine-producing subsets, we examined chemokine receptor expression profiles on CD4+ cells producing IFN-γ, IL-22 or IL-17A. For these studies, we stimulated whole blood with M.tb whole cell lysate. Figure 5A shows representative flow cytometry plots of M.tb-specific CD4+ T cell production of IFN-γ, IL-22 and IL-17A overlaid onto chemokine receptor expression profiles (CXCR3, CCR6, CCR4 and CCR10). Whilst a majority of IFN-γ-producing cells expressed CXCR3 (median 61.5%), a sizable fraction also expressed CCR6 (median 54.5%), with a low proportion expressing CCR4 (median 4.1%) and negligible CCR10 (median 0.5%; Figure 5B). In contrast, CD4+ cells producing IL-22 were almost all CCR6 positive (median 94.7%), and compared to cells producing IFN-γ, significantly fewer expressed CXCR3 (median 27.7%), and significantly more expressed CCR4 and CCR10 (median 23.3% and 2.7%, respectively). Th17 cells (IL-17A+) shared comparable expression profiles for CCR6 and CXCR3 (medians 96.1% and 17.2%, respectively), but a higher proportion expressed CCR4 (median 50.8%) and CCR10 (median 5.8%) compared to Th22 cells. Of note, cells co-producing IFN-γ and IL-22 had a similarly high expression of CCR6 as Th22 and Th17 cells, but were otherwise intermediate between IFN-γ+ and Th22 for the remaining chemokine receptors (data not shown). These findings demonstrate distinct patterns of chemokine receptor expression on different cytokine-producing subsets. These data are consistent with previous descriptions (28, 30), but also serve to highlight the substantial overlap in chemokine receptor expression between T helper subsets producing distinct cytokines.
Figure 5: Chemokine receptor expression of CD4+ T cells producing IFN-γ, IL-22 or IL-17A in response to M.tb whole cell lysate.
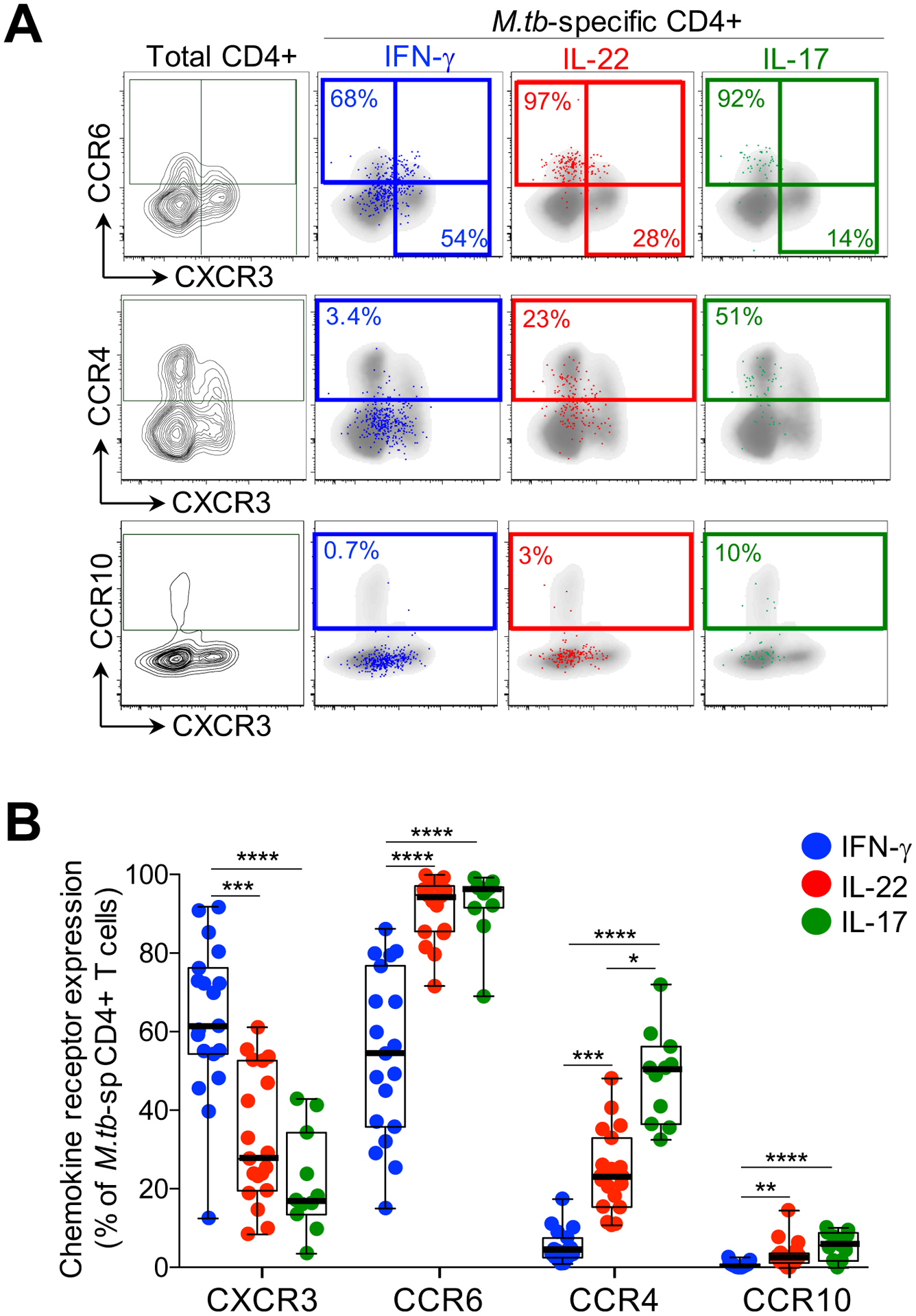
(A) Representative flow cytometry plots of the expression of CCR6, CCR4, CXCR3 and CCR10 on total CD4+ T cells in one individual. The overlays indicate the antigen specific CD4+ T cells producing IFN-γ (blue), IL-22 (red) or IL-17A (green). The frequencies of each subset are indicated. (B) The chemokine receptor distribution of cells producing IFN-γ (blue), IL-22 (red) or IL-17A (green) in response to M.tb lysate (n=19, 19 and 11, respectively). Only individuals with a positive cytokine response and more than 30 cytokine events were included in the phenotyping. Each dot represents one individual. Data are shown as box and whisker (interquartile range) plots and horizontal bars represent the median. Statistical comparisons were performed using Kruskal-Wallis and Dunn’s multiple comparison test. *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001
We also investigated the homing potential of M.tb-specific CD4+ Th subsets using KLRG1 and CD26. Killer cell lectin-like receptor G1 (KLRG1)-expressing cells appear to be retained within lung blood vasculature, while KLRG1− cells migrate to the lung parenchyma (40). Dipeptidyl peptidase IV (CD26) is involved in enzymatic chemokine modification that enhances T cell migration (41, 42). Expression of these markers was significantly different between Th subsets (Supplementary Figure S3A). Th22 cells were characterized by a near absence of KLRG1 expression compared to Th1 and Th17 cells. In contrast, 50% of Th22 cells expressed CD26, compared to a median of 34% of Th1 cells and 11% of Th17 cells (Supplemental Figure S3B). These data suggest that M.tb-specific Th22 are endowed with a distinct homing potential compare to Th1 and Th17 cells.
The effect of HIV infection on the Th22 response to mycobacteria
Th1 responses to M.tb are impaired or reduced during HIV infection (3). However, little is known about the effect of HIV co-infection on the Th22 response to M.tb. Hence, we examined IFN-γ, IL-22 and IL-17A responses to BCG and PPD in 25 HIV-infected individuals with a median CD4 count of 619 cells/mm3 (IQR: 532.5–782) and a median plasma viral load of 6.38 ×103 copies/ml (IQR: 3.55–16.45 ×103; Table 1). Consistent with previous reports, the frequency of M.tb-specific CD4+ T cells producing IFN-γ was significantly lower in HIV-infected participants compared to uninfected participants in response to BCG (p=0.0004, medians 0.12% and 0.55%, respectively; Figure 6A). Notably, the IL-22 response to BCG was also lower in HIV-infected individuals, to a similar degree as the IFN-γ response (p=0.0005; medians 0.28% and 0.91%, respectively). Additionally, IL-17A responses were also significantly lower in HIV-infected individuals in response to BCG compared to the HIV-uninfected group (p<0.0001, medians 0% and 0.11%, respectively). After adjusting for CD4 count, these differences became even more evident (Figure 6B), despite the relatively well-preserved CD4+ T cell numbers in our HIV-infected cohort. HIV-infected participants had 8-fold (p<0.0001) and 3-fold (p=0.0003) fewer CD4+ T cells producing IFN-γ or IL-22, respectively, compared to uninfected participants. There were also fewer cells producing IL-17A in HIV-infected individuals (median 0; p<0.0001). Similar results were obtained for IFN-γ and IL-22 in response to PPD (Supplemental Figure S4A and B). Overall, HIV-infected participants had lower M.tb-specific IFN-γ, IL-22 and IL-17A responses. Whilst the decrease in M.tb-specific IFN-γ and IL-17A responses during HIV infection has been reported, we report here a striking loss of M.tb-specific CD4+ T cells producing IL-22.
Figure 6: CD4+ T cell responses to BCG in HIV-infected and uninfected individuals and their relationship with CD4 count and HIV viral load.
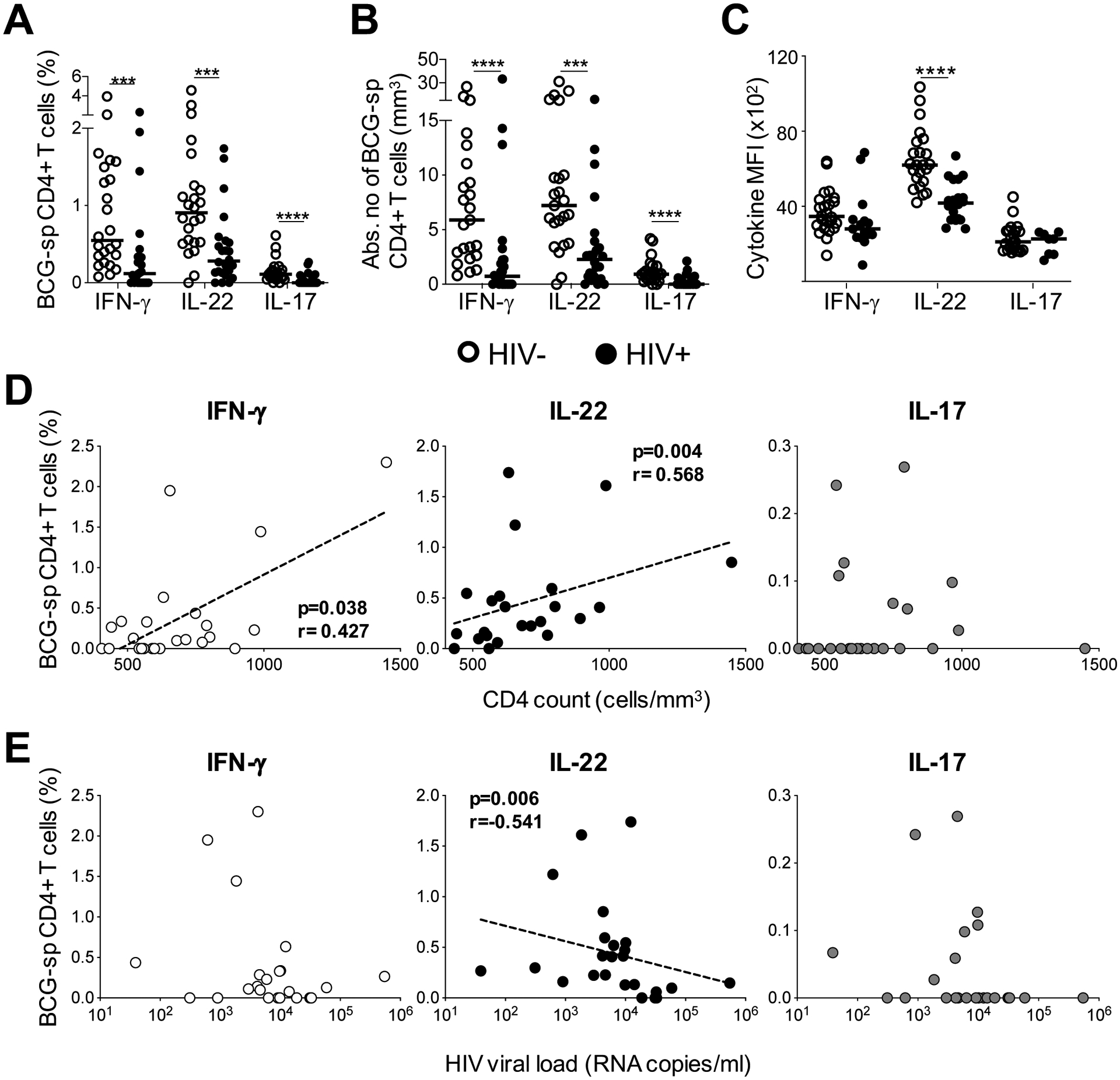
(A) The individual IFN-γ, IL-22 or IL-17A responses in HIV uninfected or infected individuals in response to BCG (n=24 in each group). (B) The cytokine frequency adjusted for CD4 count in HIV-infected and HIV-uninfected individuals in response to BCG. (C) The median fluorescent intensity (MFI) of IFN-γ, (n=24 and n=15 for HIV-uninfected and infected, respectively), IL-22 (n=23 and n=20 for HIV-uninfected and infected, respectively) and IL-17A (n=22 and n=8 for HIV-uninfected and infected, respectively) in response to BCG. For each cytokine, MFI was only graphed for individuals with positive cytokine responses. HIV-uninfected participants are shown with open circles and HIV-uninfected individuals with closed circles. Each dot represents one individual. Horizontal bars represent the median. (D) The association between IFN-γ (white), IL-22 (black) or IL-17A (gray) responses to BCG and CD4 count or (E) viral load (n=24). The dotted line indicates linear regression for statistically significant correlations. Statistical comparisons were performed using a non-parametric Mann Whitney test, and a non-parametric Spearman rank test for the correlations. *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
To further investigate the impact of HIV on BCG-specific Th22 responses, we measured the amount of IL-22 produced per cell, using median fluorescent intensity (MFI). The MFI of IL-22 was significantly lower in HIV-infected individuals compared to uninfected individuals (p<0.0001; medians 4169 and 6215, respectively; Figure 6C), whereas no differences in the MFI of IFN-γ and IL-17A was observed. This suggests that HIV may have a unique effect on Th22 cells in response to BCG. However, we found no differences in the MFI of any cytokines produced in response to PPD (Supplemental Figure S4C).
To investigate whether the lower cytokine responses to mycobacterial antigens in HIV-infected individuals related to clinical parameters, the association between IFN-γ, IL-22 and IL-17A responses and CD4 count or plasma viral load was examined. We observed a significant positive correlation between both the IFN-γ and IL-22 response to BCG and CD4 count (p=0.04, r=0.43; and p=0.004, r=0.57, respectively; Figure 6D). Likewise, in response to PPD, IFN-γ (p=0.03, r=0.45) and IL-22 (p=0.004, r=0.57) correlated directly with CD4 count (data not shown). This suggests that the decrease in these responses could be a consequence of overall CD4+ T cell depletion, despite the relatively narrow CD4 count range and modest CD4 decreases in our study (84% of participants had CD4 counts >500 cells/mm3). No association between the frequency of IL-17A and CD4 count was observed for either BCG (Figure 6D) or PPD (data not shown). Finally, there were no significant associations between plasma viral load and IFN-γ or Th17 responses to BCG; Figure 6E) or any cytokine in response to PPD (data not shown). However, the frequency of Th22 cells responding to BCG was significantly inversely correlated with plasma viral load (p=0.006, r=−0.54, Figure 6E, middle panel).
Overall, we demonstrate the detrimental effect of HIV infection on CD4+ T helper subsets in response to mycobacteria. In particular, the Th22 subset exhibited both a decrease in the magnitude of the response to mycobacteria, and a defect in IL-22 production on a per cell basis. Furthermore, unlike the other cytokine-producing subsets examined (Th1 and Th17), the frequency of Th22 cells correlated inversely with HIV viral load, suggesting a direct relationship between HIV infection and the loss of Th22 cells specific for mycobacteria.
DISCUSSION
Th1/IFN-γ responses are needed for an effective response to TB (8), however a range of immune mechanisms beyond Th1 immunity may also contribute to protection from TB (6). Since HIV-infected individuals are considerably more susceptible to TB disease (3), key components required for effective immune control of M.tb are likely to be defective in these individuals, and we sought to identify these. In addition to IFN-γ/Th1 immunity, this study examined IL-17A and IL-22 responses to mycobacteria in M.tb-sensitized, HIV-infected and uninfected individuals. Consistent with previous studies, we identified distinct populations of CD4+ T cells expressing IFN-γ, IL-17A or IL-22 in response to mycobacterial antigens (43, 44). The IL-22 response was unexpectedly abundant, contributing up to 50% of the mycobacterial response measured using these three cytokines, and the source was a distinct subset of CD4+ T cells producing IL-22 alone. Importantly, IL-22 response was impaired in HIV-infected individuals in both magnitude and function, suggesting that depletion of this subset may contribute to TB risk.
IL-22 has classically been characterized as a Th17-related cytokine, since in mice it is co-secreted with IL-17A and has overlapping functions with IL-17A (27). However, IL-22 is a member of the IL-10 family (45), and in humans, IL-22 is not co-expressed with IL-17A (28–30). Consequently, ‘Th22’ cells were proposed as a novel CD4+ T helper cell lineage in humans, with shared but distinct features and functions compared to Th17 cells.
The role for IL-17 and Th17 responses in TB is well appreciated in mouse models (21, 24–26, 46, 47), with accumulating evidence from non-human primates (48, 49). In humans, genetic mutations and polymorphisms that suppress Th17 function were associated with the development of TB disease in humans (50, 51). Consequently, lower Th17 responses were found in individuals with active TB and in those who went on to develop active disease (19, 43). Importantly, IL-17 producing tissue-resident cells in the human lung enhanced immune control of M.tb (52). However, other studies report associations between IL-17 responses and TB pathogenesis (18, 53–55). More studies are clearly required to determine the conditions in which Th17 cells confer protection. Here, we found that IL-17A responses made only a modest contribution to the total mycobacterial response in M.tb-exposed individuals, consistent with previous reports (20, 43). In contrast, we detected ample mycobacteria-specific IL-22 production from CD4+ T cells in the absence of IL-17A (and IFN-γ), consistent with a distinct Th22 subset and in agreement with earlier observations in LTBI and TB disease (43, 56). Of the CD4+ T cells producing IL-22, we found only a small proportion (10%) also expressed IL-17. Thus, our data, along with previous reports, emphasize that Th22 cells should be considered a distinct contributor to the human mycobacterial response in their own right.
Phenotypic profiling demonstrated that whilst the memory differentiation phenotype of Th22 cells was similar to that of Th1 and Th17 cells, the bulk of Th22 cells expressed CCR6, with expression frequencies of CXCR3, CCR4 and CCR10 intermediate between Th1 and Th17 cells, somewhat consistent with published reports (28, 30). M.tb-specific Th22 cells were also characterized by higher CD26 and absent KLRG1 expression compared to both Th1 and Th17 cells. Altogether, these characteristics emphasize the shared and unique features of mycobacteria-specific Th22 cells relative to Th1 and Th17 cells, which may relate to distinct homing capabilities.
The previously unappreciated, sizeable contribution Th22 cells make to the mycobacterial response prompts the question of whether Th22 responses play a role in protective immunity against M.tb. Previous studies demonstrated that deficiency or neutralization of IL-22 in mice did not affect control of laboratory strains of M.tb (H37Rv and Erdman) (32–35). However, renewed interest in IL-22 has been garnered since the observation that IL-22 deficient mice infected with a hypervirulent clinical strain of M.tb (HN878) have an impaired ability to control the infection, resulting in both increased bacterial burden and greater dissemination of infection (36). Additional evidence from a range of models suggest that IL-22 may indeed participate in TB immunity. IL-22 has been found at sites of TB disease; soluble IL-22 and IL-22 transcripts were elevated in the airways, lung tissue, granuloma, and in pleural and pericardial effusions during TB disease (43, 56–60). Along with IFN-γ, IL-22 was one of the strongest genes upregulated in bovine TB (61), and gene expression signatures revealed that IFN-γ and IL-22 were the dominant correlates of protection from bovine TB in blood in BCG-vaccinated cattle (62). Human genetic studies demonstrated the association between increased susceptibility to TB and a single nucleotide polymorphism in the IL-22 promoter that decreased IL-22 expression (60).
If IL-22 is involved in TB immunity, how might it mediate a protective function? IL-22 functions as a key regulator of tissue-specific antimicrobial immunity (31). The receptor for IL-22 is a heterodimer consisting of the IL-10R2 and the IL-22R, and expression is primarily restricted to non-hematopoietic cells, particularly epithelial cells in the skin, digestive tract and respiratory tract (31). IL-22 has been shown to be essential for mediating protective immunity to a range of extracellular and intracellular bacteria, such as Klebsiella and Chlamydia in the lung and Citrobacter in the intestine (63–66). Neutralization of IL-22 led to bacterial dissemination, exacerbated pathology, and lower Th1 and Th17 responses in the lung (65). The protective role at barrier sites appears to be mediated by three distinct functions, namely; maintenance of barrier integrity by promotion of epithelial homeostasis, stimulating epithelial proliferation and preventing apoptosis, as well as enhancing mucin production and tight junction formation; inducing antimicrobial peptides such as β-defensins; and regulating chemokine secretion from epithelial cells to co-ordinate recruitment of immune cells, such as neutrophils, to inflamed tissue (27, 29, 65, 67). Indeed, Treerat and colleagues demonstrated that the TB-protective role of IL-22 resulted from the secretion of S100 and Reg3γ from epithelial cells, and induction of CCL2 that mediated macrophage recruitment to the infected lung (36). It is worth noting that several studies have independently documented IL-22R expression on M.tb-infected monocyte-derived macrophages (MDMs), as well as macrophages in TB granulomas in humans and non-human primates (36, 68, 69). Consistent with these findings, IL-22 from CD4+ T cells and innate cells, as well as recombinant IL-22, reduced mycobacterial replication in MDMs by improving phagolysosome fusion (36, 68–70). These data suggest that direct effector function for IL-22 in limiting mycobacterial growth cannot be ruled out.
HIV-infected individuals remain one of the most vulnerable populations at risk of TB (3). The early depletion of M.tb-specific Th1 responses, considered fundamental to TB immunity, has been reported during HIV infection (9, 10). Here, we investigated the relative effect of HIV on Th22 and Th17 responses to mycobacteria compared to Th1 responses. An important and novel finding from our study was that the mycobacteria-specific Th22 response was depleted during HIV infection, to a similar extent as Th1/IFN-γ responses. Mycobacterial Th17 responses, albeit low in magnitude, were also significantly lower during HIV infection. Several studies have described a global and preferential loss of Th22 and Th17 cells during HIV/SIV infection, leading to mucosal gut damage and systemic immune activation, driving HIV disease progression (37, 71–74). The CCR6+CD4+ T cell subset (within which all Th22 and Th17 cells reside) is more permissive to HIV infection and replication, and is enriched for HIV DNA (75–77). Elevated expression of HIV co-receptors CCR5 and CXCR4 has been reported on CCR6+CD4+ T cells, which could facilitate HIV entry (78). In addition, post-entry mechanisms appear to create a more permissive cellular environment for HIV replication in CCR6-expressing cells, demonstrated by specific transcriptional signatures favoring HIV replication (79–81). We report here that higher HIV plasma viral load correlates with lower frequencies of Th22 cells specific for mycobacteria, consistent with a mechanism of direct, preferential infection of Th22 cells by HIV. Overall, multiple mechanisms may contribute to the loss of Th22, Th17 and Th1 subsets specific for M.tb (82, 83), and their combined depletion may contribute to TB risk during HIV infection. Although ART leads to expansion of the CD4+ T cell compartment, mycobacteria-specific T cells may not recover proportionally (84–86). Indeed, the incomplete reconstitution of M.tb-specific Th1 cells in individuals on ART has been described by our group, with the extent of reconstitution dependent on the memory differentiation phenotype (87). Thus, given that the memory phenotype of Th1, Th17 and Th22 cells were similar in the current study, we may speculate that ART would lead to a similar, partial recovery of Th17 and Th22 subsets. Studies examining recovery of these subsets in individuals on ART are ongoing.
Another important observation from our study is that while IL-22 is readily detectable from stimulated whole blood, it is not easily detected in a traditional PBMC assay, which may partly account for its unappreciated contribution to the mycobacterial T cell response. Interestingly, the IL-22 response in PBMC could be recovered when these cells were cultured with whole blood followed by stimulation with M.tb, suggesting that CD4+ T cells capable of producing IL-22 are not lost in PBMC, but that there are factors in whole blood that are required for IL-22 production. These could be cell types responsible for antigen presentation and co-stimulation, such as granulocytes, or soluble factors in plasma. A range of innate cytokines have been shown to enhance IL-22 expression (27, 28, 83). Experiments to understand the conditions necessary for IL-22 secretion are underway.
In conclusion, our new findings add to a growing body of evidence in support of a role for IL-22 in protective immunity to TB. However, a number of questions remain unanswered. Does IL-22 contribute to protective immunity to TB, or only during infection with specific clinical strains, or during HIV infection, when multiple immunological defects manifest? Does IL-22 assume a direct effector or indirect regulatory role in immunity to TB, or both? Does the inflammatory context dictate whether IL-22 might be beneficial to the host or pathological (88)? Ultimately, will it be necessary to induce Th22 responses for a TB vaccine to be effective? Notwithstanding these gaps in our knowledge, our study highlights the substantial contribution that Th22 cells make to mycobacterial immunity, and the importance of further elucidating the role of IL-22 in the control of M.tb infection and disease.
Supplementary Material
KEY POINTS.
IL-22 responses to mycobacterial antigens contributed ~50% of the total response.
Mycobacterial Th22 responses were significantly lower in HIV-infected individuals.
ACKNOWLEDGEMENTS
We thank the study participants for providing samples and for their time and commitment to the study, and to the clinical staff at the Ubuntu HIV-TB clinic. We thank Dr Shaun Barnabas and Rene Goliath for phlebotomy. We thank Dr Mario Roederer for excellent discussions. We are grateful to BEI Resources, NIAID, NIH, for the following reagent: Mycobacterium tuberculosis, Strain H37Rv, Whole Cell Lysate, NR-14822.
Funding:
This project is part of the EDCTP2 programme supported by the European Union (EU)’s Horizon 2020 programme (Training and Mobility Action TMA2016SF-1535-CaTCH-22, to WAB, TMA2017SF-1951-TB-SPEC to CR and TMA2020CDF-3187 to RB). The work was conducted at CIDRI-Africa, which is supported by core funding from the Wellcome Trust [203135/Z/16/Z]. Additionally, WAB was funded by the SAMRC, NRF SA (92755) and NHLS Trust (2016-2DEV04). RB was a Carnegie Corporation Fellow and received PhD funding from the University of Cape Town and the Canada Africa Prevention Trials (CAPT) Network. RJW is supported by the Wellcome Trust (203135 and 104803), NIH (U01 AI115940), the Francis Crick Institute (Cancer Research UK, MRC UK and Wellcome FC0010218), NRF SA (96841) and SAMRC (SHIP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed are those of the authors, and the funders are not responsible for any use that may be made of the information contained herein.
Footnotes
Potential conflicts of interest: The authors of this manuscript do not have commercial or other associations that pose a conflict of interest.
REFERENCES
- 1.World Health Organization. 2020. Global Tuberculosis Report.
- 2.El-Sadr WM, Perlman DC, Denning E, Matts JP, and Cohn DL. 2001. A review of efficacy studies of 6-month short-course therapy for tuberculosis among patients infected with Human Immunodeficiency Virus: differences in study outcomes. Clin. Infect. Dis 32: 623–632. [DOI] [PubMed] [Google Scholar]
- 3.Esmail H, Riou C, du Bruyn E, Lai RP-J, Harley YXR, Meintjes G, Wilkinson KA, and Wilkinson RJ. 2018. The Immune Response to Mycobacterium tuberculosis in HIV-1-Coinfected Persons. Annu. Rev. Immunol 36: 1–36. [DOI] [PubMed] [Google Scholar]
- 4.GBD. 2018. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect. Dis 18: 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD, Myer L, Edwards D, Bekker L-GG, and Wood R. 2009. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 23: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai S, Mayer-Barber KD, and Barber DL. 2014. Defining features of protective CD4 T cell responses to Mycobacterium tuberculosis. Curr. Opin. Immunol 29: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E, Ayles HM, Henostroza G, Thienemann F, Scriba TJ, Diacon A, Blatner GL, Demoitié M-A, Tameris M, Malahleha M, Innes JC, Hellström E, Martinson N, Singh T, Akite EJ, Khatoon Azam A, Bollaerts A, Ginsberg AM, Evans TG, Gillard P, and Tait DR. 2018. Phase 2b Controlled Trial of M72/AS01 E Vaccine to Prevent Tuberculosis. N. Engl. J. Med 379: 1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, and Berry MPR. 2013. The immune response in tuberculosis. Annu. Rev. Immunol 31: 475–527. [DOI] [PubMed] [Google Scholar]
- 9.Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, Saathoff E, Boehme C, Geis S, Maboko L, Singh M, Minja F, Meyerhans A, a Koup R, and Hoelscher M. 2008. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J. Infect. Dis 198: 1590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, Casazza JP, Ambrozak DR, Louder M, Ampofo W, Pollakis G, Hill B, Sanga E, Saathoff E, Maboko L, Roederer M, Paxton WA, Hoelscher M, and Koup RA. 2010. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J. Exp. Med 207: 2869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day CL, Abrahams DA, Harris LD, van Rooyen M, Stone L, de Kock M, and Hanekom WA. 2017. HIV-1 Infection is associated with depletion and functional impairment of Mycobacterium tuberculosis–specific CD4 T cells in individuals with latent tuberculosis infection. J. Immunol 199: 2069–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strickland N, Müller TL, Berkowitz N, Goliath R, Carrington MN, Wilkinson RJ, Burgers WA, and Riou C. 2017. Characterization of Mycobacterium tuberculosis–specific cells using MHC Class II tetramers reveals phenotypic differences related to HIV infection and tuberculosis disease. J. Immunol 199: 2440–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalsdorf B, Scriba T, Wood K, Day CL, Dheda K, Dawson R, Hanekom WA, Lange C, and Wilkinson RJ. 2009. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am. J. Respir. Crit. Care Med 180: 1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jambo KC, Sepako E, Fullerton DG, Mzinza D, Glennie S, Wright AK, Heyderman RS, and Gordon SB. 2011. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax 66: 375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunjun R, Riou C, Soares AP, Thawer N, Müller TL, Kiravu A, Ginbot Z, Oni T, Goliath R, Kalsdorf B, Von Groote-Bidlingmaier F, Hanekom W, Walzl G, Wilkinson RJ, and Burgers WA. 2017. Effect of HIV on the frequency and number of Mycobacterium tuberculosis-specific CD4 + T Cells in blood and airways during latent M. tuberculosis Infection. J. Infect. Dis 216: 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallegos AM, van Heijst JWJ, Samstein M, Su X, Pamer EG, and Glickman MS. 2011. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 7: e1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riou C, Strickland N, Soares AP, Corleis B, Kwon DS, Wherry EJ, Wilkinson RJ, and Burgers WA. 2016. HIV Skews the Lineage-Defining Transcriptional Profile of Mycobacterium tuberculosis –specific CD4 + T Cells. J. Immunol 196: 3006–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen H, and Chen ZW. 2018. The crucial roles of Th17-related cytokines/signal pathways in M.Tuberculosis infection. Cell. Mol. Immunol 15: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scriba TJ, Penn-Nicholson A, Shankar S, Hraha T, Thompson EG, Sterling D, Nemes E, Darboe F, Suliman S, Amon LM, Mahomed H, Erasmus M, Whatney W, Johnson JL, Boom WH, Hatherill M, Valvo J, De Groote MA, Ochsner UA, Aderem A, Hanekom WA, Zak DE, and other members of the ACS cohort study team. 2017. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog. 13: e1006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray LW, Satti I, Meyerowitz J, Jones M, Willberg CB, Ussher JE, Goedhals D, Hurst J, Phillips RE, McShane H, van Vuuren C, Frater J, van Vuuren C, and Frater J. 2018. Human Immunodeficiency Virus infection impairs Th1 and Th17 Mycobacterium tuberculosis–specific T cell responses. J. Infect. Dis 217: 1782–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopal R, Monin L, Slight S, Uche U, Blanchard E, a Fallert Junecko B, Ramos-Payan R, Stallings CL, a Reinhart T, Kolls JK, Kaushal D, Nagarajan U, Rangel-Moreno J, and Khader SA. 2014. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 10: e1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, Kalinski P, and Khader SA. 2012. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immunol 42: 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, and Khader SA. 2013. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 6: 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khader S. a, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, and Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol 8: 369–377. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto Yoshida Y, Umemura M, Yahagi A, O’Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, and Matsuzaki G. 2010. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol 184: 4414–22. [DOI] [PubMed] [Google Scholar]
- 26.Domingo-Gonzalez R, Das S, Griffiths KL, Ahmed M, Bambouskova M, Gopal R, Gondi S, Muñoz-Torrico M, Salazar-Lezama MA, Cruz-Lagunas A, Jiménez-Álvarez L, Ramirez-Martinez G, Espinosa-Soto R, Sultana T, Lyons-Weiler J, Reinhart TA, Arcos J, de la Luz Garcia-Hernandez M, Mastrangelo MA, Al-Hammadi N, Townsend R, Balada-Llasat J-M, Torrelles JB, Kaplan G, Horne W, Kolls JK, Artyomov MN, Rangel-Moreno J, Zúñiga J, and Khader SA. 2017. Interleukin-17 limits hypoxia-inducible factor 1α and development of hypoxic granulomas during tuberculosis. JCI insight 2: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang SC, Tan X-Y, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, and Fouser LA. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med 203: 2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, and Sallusto F. 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol 10: 857–63. [DOI] [PubMed] [Google Scholar]
- 29.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, and Cavani A. 2009. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest 119: 3573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trifari S, Kaplan CD, Tran EH, Crellin NK, and Spits H. 2009. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol 10: 864–71. [DOI] [PubMed] [Google Scholar]
- 31.Sonnenberg GF, Fouser LA, and Artis D. 2011. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol 12: 383–90. [DOI] [PubMed] [Google Scholar]
- 32.Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, Grigg M, Collins M, Fouser L, and Wynn TA. 2010. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J. Immunol 184: 4378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khader SA, Guglani L, Rangel-moreno J, Gopal R, F. Junecko a Beth, Fountain JJ, Martino C, Pearl JE, Tighe MM, Lin Y, Slight S, Kolls JK, Reinhart TA, Randall TD, Cooper AM, and Junecko BAF. 2011. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J. Immunol 187: 5402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behrends J, Renauld J, Ehlers S, and Hölscher C. 2013. IL-22 is mainly produced by IFNγ-secreting cells but is dispensable for host protection against Mycobacterium tuberculosis infection. PLoS One 8: e57379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segueni N, Tritto E, Bourigault ML, Rose S, Erard F, Le Bert M, Jacobs M, Di Padova F, Stiehl DP, Moulin P, Brees D, Chibout SD, Ryffel B, Kammüller M, and Quesniaux VF. 2016. Controlled Mycobacterium tuberculosis infection in mice under treatment with anti-IL-17A or IL-17F antibodies, in contrast to TNFα neutralization. Sci. Rep 6: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treerat P, Prince O, Cruz-Lagunas A, Muñoz-Torrico M, Salazar-Lezama MA, Selman M, Fallert-Junecko B, Reinhardt TA, Alcorn JF, Kaushal D, Zuñiga J, Rangel-Moreno J, Kolls JK, and Khader SA. 2017. Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection. Mucosal Immunol. 10: 1069–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim CJ, Nazli a, Rojas OL, Chege D, Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LYY, Grin A, Kandel G, Loutfy M, Ostrowski M, Gommerman JL, Kaushic C, and Kaul R. 2012. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 5: 670–80. [DOI] [PubMed] [Google Scholar]
- 38.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, Murray RA, Hawkridge A, Haslett PAJ, Ress S, Hussey GD, and Kaplan G. 2004. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J. Immunol. Methods 291: 185–95. [DOI] [PubMed] [Google Scholar]
- 39.Roederer M, Nozzi JL, and Nason MC. 2011. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry. A 79: 167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, and Barber DL. 2014. Cutting Edge: Control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T Cells. J. Immunol 192: 2965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwata S, Yamaguchi N, Munakata Y, Ikushima H, Lee JF, Hosono O, Schlossman SF, and Morimoto C. 1999. CD26/dipeptidyl peptidase IV differentially regulates the chemotaxis of T cells and monocytes toward RANTES: Possible mechanism for the switch from innate to acquired immune response. Int. Immunol 11: 417–426. [DOI] [PubMed] [Google Scholar]
- 42.Ikushima H, Munakata Y, Iwata S, Ohnuma K, Kobayashi S, Dang NH, and Morimoto C. 2002. Soluble CD26/dipeptidyl peptidase IV enhances transendothelial migration via its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cell. Immunol 215: 106–110. [DOI] [PubMed] [Google Scholar]
- 43.Scriba TJ, Kalsdorf B, Abrahams D-A, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, and Hanekom WA. 2008. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol 180: 1962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye ZJ, Zhou Q, Yuan ML, Du RH, Yang WB, Xiong XZ, Huang B, and Shi HZ. 2012. Differentiation and recruitment of IL-22-producing helper T cells stimulated by pleural mesothelial cells in tuberculous pleurisy. Am. J. Respir. Crit. Care Med 185: 660–669. [DOI] [PubMed] [Google Scholar]
- 45.Dumoutier L, Louahed J, and Renauld JC. 2000. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol 164: 1814–1819. [DOI] [PubMed] [Google Scholar]
- 46.Wozniak TM, Saunders BM, Ryan AA, and Britton WJ. 2010. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect. Immun 78: 4187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freches D, Korf H, Denis O, Havaux X, Huygen K, and Romano M. 2013. Mice genetically inactivated in interleukin-17a receptor are defective in long-term control of Mycobacterium tuberculosis infection. Immunology 140: 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gideon HP, Phuah J, Myers AJ, Bryson BD, a Rodgers M, Coleman MT, Maiello P, Rutledge T, Marino S, Fortune SM, Kirschner DE, Lin PL, and Flynn JL. 2015. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog. 11: e1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shanmugasundaram U, Bucsan AN, Ganatra SR, Ibegbu C, Quezada M, Blair RV, Alvarez X, Velu V, Kaushal D, and Rengarajan J. 2020. Pulmonary Mycobacterium tuberculosis control associates with CXCR3- And CCR6-expressing antigen-specific Th1 and Th17 cell recruitment. JCI Insight 5: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okada S, Henderson LA, Marzouqa H, Shamma J, and Gonzalez M. 2015. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. 349: 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang M, Xu G, Lü L, Xu K, Chen Y, Pan H, Burstrom B, Burstrom K, and Wang J. 2016. Genetic polymorphisms of IL-17A, IL-17F, TLR4 and miR-146a in association with the risk of pulmonary tuberculosis. Sci. Rep 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogongo P, Tezera LB, Ardain A, Nhamoyebonde S, Ramsuran D, Singh A, Ngoepe A, Karim F, Naidoo T, Khan K, Dullabh KJ, Fehlings M, Lee BH, Nardin A, Lindestam Arlehamn CS, Sette A, Behar SM, Steyn AJC, Madansein R, Kløverpris HN, Elkington PT, and Leslie A. 2021. Tissue resident-like CD4+ T cells secreting IL-17 control Mycobacterium tuberculosis in the human lung. J. Clin. Invest [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh S, Maniakis-Grivas G, Singh UK, Asher RM, Mauri F, Elkington PT, and Friedland JS. 2018. Interleukin-17 regulates matrix metalloproteinase activity in human pulmonary tuberculosis. J. Pathol 244: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper AM 2009. IL-17 and anti-bacterial immunity: Protection versus tissue damage. Eur. J. Immunol 39: 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basile JI, Geffner LJ, Romero MM, Balboa L, Sabio Y García C, Ritacco V, García A, Cuffré M, Abbate E, López B, Barrera L, Ambroggi M, Alemán M, Sasiain MC, and De La Barrera SS. 2011. Outbreaks of Mycobacterium tuberculosis MDR strains induce high IL-17 T-cell response in patients with MDR tuberculosis that is closely associated with high antigen load. J. Infect. Dis 204: 1054–1064. [DOI] [PubMed] [Google Scholar]
- 56.Qiu Y, Huang Y, Chen J, Qiao D, Zeng G, and Cai J. 2013. Depletion of IL-22 during culture enhanced antigen-driven IFN-γ production by CD4(+)T cells from patients with active TB. Immunol. Lett 150: 48–53. [DOI] [PubMed] [Google Scholar]
- 57.Matthews K, Wilkinson KA, Kalsdorf B, Roberts T, Diacon A, Walzl G, Wolske J, Ntsekhe M, Syed F, Russell J, Mayosi BM, Dawson R, Dheda K, Wilkinson RJ, a Hanekom W, and Scriba TJ. 2011. Predominance of interleukin-22 over interleukin-17 at the site of disease in human tuberculosis. Tuberculosis (Edinb). 91: 587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semple PL, Binder AB, Davids M, Maredza A, van Zyl-Smit RN, and Dheda K. 2013. Regulatory T cells attenuate mycobacterial stasis in alveolar and blood-derived macrophages from patients with tuberculosis. Am. J. Respir. Crit. Care Med 187: 1249–58. [DOI] [PubMed] [Google Scholar]
- 59.Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC, and Chen ZW. 2010. Differentiation, distribution and gammadelta T cell-driven regulation of IL-22-producing T cells in tuberculosis. PLoS Pathog. 6: e1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang G, Chen X, Chan L, Zhang M, Zhu B, Wang L, Zhu X, Zhang J, Zhou B, and Wang J. 2011. An SNP selection strategy identified IL-22 associating with susceptibility to tuberculosis in Chinese. Sci. Rep 1: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aranday-Cortes E, Hogarth PJ, Kaveh DA, Whelan AO, Villarreal-Ramos B, Lalvani A, and Vordermeier HM. 2012. Transcriptional profiling of disease-induced host responses in bovine tuberculosis and the identification of potential diagnostic biomarkers. PLoS One 7: e30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhuju S, Aranday-Cortes E, Villarreal-Ramos B, Xing Z, Singh M, and Vordermeier HM. 2012. Global gene transcriptome analysis in vaccinated cattle revealed a dominant role of IL-22 for protection against bovine tuberculosis. PLoS Pathog. 8: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, and Weaver CT. 2012. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37: 1061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, and Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med 14: 282–289. [DOI] [PubMed] [Google Scholar]
- 65.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, and Kolls JK. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med 14: 275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng Y, Gao X, Yang J, Shekhar S, Wang S, Fan Y, Zhao W, and Yang X. 2014. Interleukin-22 promotes T helper 1 (Th1)/Th17 immunity in chlamydial lung infection. Mol. Med 20: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aujla SJ, and Kolls JK. 2009. IL-22: a critical mediator in mucosal host defense. J. Mol. Med. (Berl) 87: 451–4. [DOI] [PubMed] [Google Scholar]
- 68.Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LVM, and Vankayalapati R. 2009. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J. Immunol 183: 6639–45. [DOI] [PubMed] [Google Scholar]
- 69.Zeng G, Chen CY, Huang D, Yao S, Wang RC, and Chen ZW. 2011. Membrane-bound IL-22 after de novo production in tuberculosis and anti-Mycobacterium tuberculosis effector function of IL-22+ CD4+ T cells. J. Immunol 187: 190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhiman R, Venkatasubramanian S, Paidipally P, Barnes PF, Tvinnereim A, and Vankayalapati R. 2014. Interleukin 22 inhibits intracellular growth of Mycobacterium tuberculosis by enhancing calgranulin A expression. J. Infect. Dis 209: 578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, and Douek DC. 2008. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112: 2826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klatt NR, and Brenchley JM. 2010. Th17 cell dynamics in HIV infection. Curr. Opin. HIV AIDS 5: 135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Page EE, Greathead L, Metcalf R, Clark S-A, Hart M, Fuchs D, Pantelidis P, Gotch F, Pozniak A, Nelson M, Boasso A, Gazzard B, and Kelleher P. 2014. Loss of Th22 cells is associated with increased immune activation and IDO-1 activity in HIV-1 infection. J. Acquir. Immune Defic. Syndr 67: 227–35. [DOI] [PubMed] [Google Scholar]
- 74.Ryan ES, Micci L, Fromentin R, Paganini S, McGary CS, Easley K, Chomont N, and Paiardini M. 2016. Loss of function of intestinal IL-17 and IL-22 producing cells contributes to inflammation and viral persistence in SIV-infected Rhesus Macaques. PLoS Pathog. 12: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gosselin A, Monteiro P, Chomont N, Diaz-Griffero F, Said EA, Fonseca S, Wacleche V, El-Far M, Boulassel M-R, Routy J-P, Sekaly R-P, and Ancuta P. 2010. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+ CD4+ T cells are highly permissive to HIV-1 infection. J. Immunol 184: 1604–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gosselin A, Wiche Salinas TR, Planas D, Wacleche VS, Zhang Y, Fromentin R, Chomont N, Cohen ÉA, Shacklett B, Mehraj V, Ghali MP, Routy J-P, and Ancuta P. 2017. HIV persists in CCR6+CD4+ T-cells from colon and blood during antiretroviral therapy. AIDS 31: 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monteiro P, Gosselin A, Wacleche VS, El-Far M, Said EA, Kared H, Grandvaux N, Boulassel M-R, Routy J-P, and Ancuta P. 2011. Memory CCR6+CD4+ T Cells Are preferential targets for productive HIV type 1 infection regardless of their expression of integrin 7. J. Immunol 186: 4618–4630. [DOI] [PubMed] [Google Scholar]
- 78.Alvarez Y, Tuen M, Shen G, Nawaz F, Arthos J, Wolff MJ, Poles MA, and Hioe CE. 2013. Preferential HIV infection of CCR6+ TH17 cells is associated with higher levels of virus receptor expression and lack of CCR5 ligands. J. Virol 87: 10843–10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernier A, Cleret-Buhot A, Zhang Y, Goulet JP, Monteiro P, Gosselin A, DaFonseca S, Wacleche VS, Jenabian MA, Routy JP, Tremblay C, and Ancuta P. 2013. Transcriptional profiling reveals molecular signatures associated with HIV permissiveness in Th1Th17 cells and identifies peroxisome proliferator-activated receptor gamma as an intrinsic negative regulator of viral replication. Retrovirology 10: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cleret-Buhot A, Zhang Y, Planas D, Goulet JP, Monteiro P, Gosselin A, Wacleche VS, Tremblay CL, Jenabian MA, Routy JP, El-Far M, Chomont N, Haddad EK, Sekaly RP, and Ancuta P. 2015. Identification of novel HIV-1 dependency factors in primary CCR4 + CCR6 + Th17 cells via a genome-wide transcriptional approach. Retrovirology 12: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Planas D, Zhang Y, Monteiro P, Goulet J, Gosselin A, Grandvaux N, Hope TJ, Fassati A, Routy J, and Ancuta P. 2017. HIV-1 selectively targets gut-homing CCR6+CD4+ T cells via mTOR-dependent mechanisms. JCI insight 2: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, Martin JN, Hecht FM, Deeks SG, and McCune JM. 2010. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med 2: 32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klatt NR, Estes JD, Sun X, Ortiz a M., Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, Tabb B, Canary LA, Dang Q, Hirsch VM, Alter G, Belkaid Y, Lifson JD, Silvestri G, Milner JD, Paiardini M, Haddad EK, and Brenchley JM. 2012. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 5: 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schluger NW, Perez D, and Liu YM. 2002. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest 122: 597–602. [DOI] [PubMed] [Google Scholar]
- 85.Jambo KC, Banda DH, Afran L, Kankwatira AM, Malamba RD, Allain TJ, Gordon SB, Heyderman RS, Russell DG, and Mwandumba HC. 2014. Asymptomatic HIV-infected individuals on antiretroviral therapy exhibit impaired lung CD4(+) T-cell responses to mycobacteria. Am. J. Respir. Crit. Care Med 190: 938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilkinson KA, Seldon R, Meintjes G, Rangaka MX, Hanekom WA, Maartens G, and Wilkinson RJ. 2009. Dissection of Regenerating T-Cell Responses against Tuberculosis in HIV-infected Adults Sensitized by Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med 180: 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riou C, Tanko RF, Soares AP, Masson L, Werner L, Garrett NJ, Samsunder N, Abdool Karim Q, Abdool Karim SS, and Burgers WA. 2015. Restoration of CD4+ responses to copathogens in HIV-infected individuals on antiretroviral therapy is dependent on t cell memory phenotype. J. Immunol 195: 2273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, and Artis D. 2010. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med 207: 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


