Summary
Antibody secreting cells (ASCs) are considered work horses of the humoral immune response for their tireless effort to produce large amounts of antibodies that fulfill an array of functions in host defense, inflammation, and maintenance of homeostasis. While traditionally considered largely senescent cells, surprising recent findings demonstrate that subsets of ASCs downmodulate ongoing immune responses independent of antibody formation. Such regulatory ASCs produce IL-10 or IL-35 and are implicated in maintaining tissue and immune homeostasis. They also serve to suppress pathogenic leukocytes in infection, allergy, and inflammatory diseases that affect tissues, such as the central nervous system and the respiratory tract. Additionally, regulatory ASCs infiltrate various cancer types and restrict effective anti-tumor T cell responses. While incompletely understood, there is significant overlap in factors that control ASC differentiation, IL-10 expression by B cells and the generation of ASCs that secrete both antibodies and IL-10. In this review, we will cover the biology, phenotype, generation, maintenance and function of regulatory ASCs in various tissues under pathological and steady states. An improved understanding of the development of regulatory ASCs and their biological roles will be critical for generating novel ASC-targeted therapies for the treatment of inflammatory diseases, infection, and cancer.
Keywords: antibody secreting cells, IL-10, immunosuppression, inflammation, cancer
1. Introduction
B cells and their terminally differentiated antibody-secreting stage are critical components of the immune response as they present antigens, secrete cytokines, and produce antibodies (reviewed in1). Elimination or control of pathogens and foreign material is essential for maintenance of long-term health; however, overly strong immune responses can lead to collateral tissue damage and disease. Therefore, mechanisms to suppress overt inflammation and maintain tissue homeostasis are necessary and are provided by various cell types. Regulatory B cells (Bregs) are subsets of B cells that are found among all main lineages of B cells and they are capable of suppressing immune responses through provision of anti-inflammatory cytokines, such as IL-10, their most well studied mechanism of action.2,3 B cells also regulate immune responses independent of IL-10 through expression of TGFβ, IL-35, IgM, PDL-1, FasL, and adenosine (reviewed in4,5). Recently, it has been recognized that many of the suppressive functions of regulatory B lineage cells are exerted by antibody secreting cells (ASCs), plasmablasts and plasma cells, that secrete both antibodies and the immunosuppressive cytokines IL-10 or IL-35. Plasmablasts are still dividing, produce high amounts of antibody and can further mature into terminally differentiated plasma cells. In this review, we will refer to plasmablasts and plasma cells collectively as ASCs. These regulatory ASCs are implicated in maintaining tissue and immune homeostasis, and they are beneficial or detrimental depending on the disease context. For example, the presence of IL-10 secreting ASCs in multiple sclerosis (MS) central nervous system (CNS) lesions is associated with limited neuroinflammation and a better prognosis in patients and is associated with milder disease course in mice with experimental autoimmune encephalomyelitis (EAE), the mouse model of MS.6,7 In contrast, the regulatory ASCs in prostate and liver cancer limit CD8+ T cell anti-tumor responses and correlate with poor survival.8,9
The signals and factors that control the generation of ASCs that secrete both antibodies and IL-10 are incompletely understood but there is significant overlap in factors that induce ASCs and IL-10 expression by B cells. Naïve B cells cannot secrete IL-10 without stimulation, but once activated by toll-like receptor (TLR) ligands, B cell receptor (BCR) antigens, CD40-ligand (CD40-L), co-stimulatory molecules (CD80, CD86) and inflammatory cytokines, B cells acquire the capacity to secrete IL-10 (reviewed in2,3). IL-10 producing ASCs either differentiate from IL-10+ B cells into ASCs10,11 or differentiated ASCs acquire the competence to secrete IL-10 upon antigenic stimulation.12 Such IL-10-inductive signals include IL-21,13–16 micro-RNAs (miRs) 21 and 155,17–19 and the transcription factors interferon regulatory factor 4 (IRF4) and B lymphocyte-induced maturation protein-1 (BLIMP-1).20–22 B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) mediate the survival of ASCs in traditional niches of the bone marrow and spleen,23–25 support the development of IL-10+ regulatory B cells and are found in non-traditional ASC sites, such as the skin in both homeostasis and inflammation and the inflamed CNS.26–29 This review focuses on the development and phenotype of IL-10+ ASCs, their role in maintaining homeostasis, as well as their functions in lymphoid and non-lymphoid organs in various disease settings.
2. Regulatory ASCs in different tissue niches
2.1. Regulatory ASCs in lymphoid tissues and maintenance of immune homeostasis
ASCs are formed in secondary lymphoid organs, and many long-lived ASCs reside permanently in the bone marrow where they are a major source of circulating antibodies.30 Long-lived ASCs also reside in the spleen, lung, and gut.31–35 In the spleen and bone marrow, ASCs have been demonstrated to be the most frequent IL-10-producing population based on the finding that approximately 60% or more IL-10+ cells have an ASC phenotype under homeostatic conditions.36,37 IL-10 production by ASCs has been observed among the main isotype producing ASCs (IgM, IgA, IgG).6,11,36–38 Short term culture without BCR or TLR antigen stimulation of IL-10+ ASCs isolated from the bone marrow leads to significantly more IL-10 secretion compared with B cells or non-B cells, indicating constitutive and plentiful secretion of IL-10 by bone marrow ASCs in vivo.36,37 Experimental data support the conclusion that secretion of IL-10 by B lineage cells promotes antibody production of and skewing towards or survival of IgG ASCs.36 Specifically, recombinant IL-10 supports, whereas blockade with anti-IL-10 significantly inhibits, ASC survival in vitro.36 In addition, in vivo, IL-10 production by adoptively transferred B lineage cells assists in the development of antigen-specific isotype switched ASCs upon antigen challenge.36 The authors also generated mixed bone marrow chimeras by reconstituting lethally irradiated recipient mice with bone marrow cells that were 90% derived from B-cell-deficient μMT mice, 10% from IL-10−/− mice or 10% from IL-10+/+ mice. This set-up resulted in chimeric mice, in which only the B lineage cells were IL-10-deficient. Following immunization, chimeric mice with IL-10−/− B cells have significantly augmented serum IgM and lower levels of serum IgG1 and IgG3 antibodies.36 However, further investigation is necessary to determine if IL-10 production by IgM+ ASCs supports the survival of class-switched ASCs and if there is an autocrine or paracrine mechanism by which IL-10 secretion supports IgM+ ASCs at steady state. (Figure 1A)
Figure 1. Regulatory ASCs in different tissue niches and disease settings.
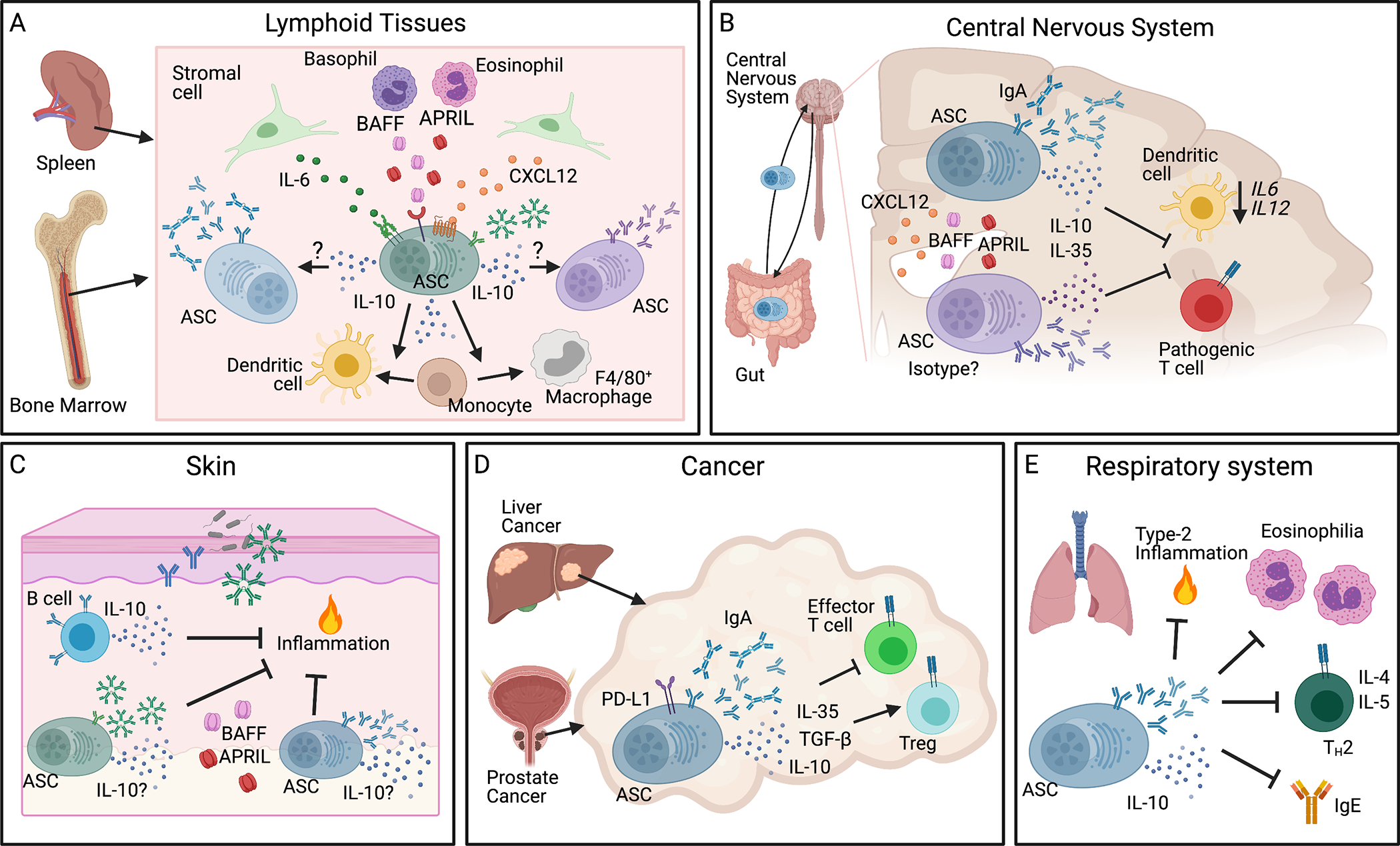
(A) ASCs reside in traditional lymphoid tissue niches, such as bone marrow and spleen, where stromal cells and nurturing hematopoietic cells, including eosinophils and basophils, provide an environment rich in CXCL12, IL-6, BAFF, and APRIL, necessary for the long-term survival of ASCs. A majority of ASCs produce IL-10 which enhances the survival of and immunoglobulin production by other resident ASCs. IL-10+ ASCs also support differentiation of CD11c+ dendritic cells and macrophages. (B) IL-10 and IL-35 producing ASCs localize to the CNS in neuroinflammation where CXCL12, BAFF, and APRIL are enriched. IgA+ ASCs traffic from the gut to the CNS in EAE and MS and produce IL-10 that inhibits dendritic cell transcription of IL6 and IL12 thereby suppressing pathogenic Th cell differentiation. (C) ASCs and IL-10+ B cells reside in unperturbed skin and increase during inflammation. Skin-resident ASCs are dependent on BAFF and/or APRIL and produce immunoglobulins to regulate inflammation and microbes in the microenvironment. We postulate that the ASCs are an additional source of IL-10 to mediate homeostasis. (D) Regulatory IgA+ ASCs localize to liver and prostate tumors. They express PD-L1, IL-10, IL-35, and TGF-β, all of which support Tregs and suppress effector T cells to dampen the immune response to cancer. (E) IL-10+ ASCs traffic to the respiratory system to restrict pulmonary inflammation, eosinophilia, Th2-cytokine production (IL-4 and IL-5), and IgE production. Image created with Biorender.com. APRIL, a proliferation-inducing ligand; ASCs, antibody secreting cells; BAFF, B-cell-activating factor; BLIMP-1, B lymphocyte-induced maturation protein-1; Bregs, regulatory B cells; EAE, experimental autoimmune encephalomyelitis; Ig, immunoglobulin; MS, multiple sclerosis; PD-L1, programmed death-ligand 1; Th, T-helper.
While IL-10 acts on ASCs in the bone marrow, it can also affect other hematopoietic cells, by promoting differentiation of dendritic cells and F4/80+ macrophages. (Figure 1A) Over 50% of the IL-10 receptor (IL-10R) expressing cells in the bone marrow are CD115 (CSF-1R)+ myeloid lineage cells, with the highest transcript for Il10ra found in CD11c+ dendritic cells (DCs) and Ly6C+ monocytes.37 In vitro co-culture of IL-10+ bone marrow ASCs with purified monocytes promotes expansion of F4/80+ macrophages in an IL-10-signaling dependent manner.37 IL-10+ ASCs also support expansion of CD11c+ DCs; however, IL-10R blockade does not reverse this effect, indicating an IL-10-independent expansion of CD11c+ DCs by ASCs.37 Additionally, IL-10 production by ASCs suppresses the differentiation of osteoclast-like cells in vitro.37 IL-10 producing ASCs in the bone marrow may serve in part to regulate immune homeostasis, and the loss of IL-10+ ASCs is potentially a contributing factor to “inflammaging.” “Inflammaging” is a growing field of research concerned with the aging process contributing to low-grade chronic inflammation and the failures of the immune system that contribute to many age-associated diseases.39–41 In vivo studies support the findings that IL-10+ ASCs modulate the myeloid lineage cells in the bone marrow, and that this changes with age: 7-month-old B cell-specific IL-10 knockout mice have an increase in CD11c+ DCs and CD115+ myeloid cells with reduced F4/80 expression in the bone marrow, indicating a role for ASC-derived IL-10 in modulating local myeloid differentiation that is non-redundant. However, such effects by IL-10 from ASCs are not seen in 4-month-old mice.37 Through modulating myeloid cell differentiation in an age-dependent manner, IL-10 production by ASCs in the bone marrow promotes immune homeostasis. IL-10+ ASCs in the bone marrow as well as in other lymphoid tissues could be critical for maintaining the appropriate immune balance and response to aging and damaged tissues, and their loss may help to accelerate “inflammaging”. It would be intriguing to analyze aged mice (16–18 months) or elderly patient bone marrow samples to determine if there is further loss of IL-10+ ASCs and worsening defects in the myeloid and ASC compartments.
2.2. Regulatory ASCs and the gut-CNS Axis
Long-lived ASCs reside primarily in the bone marrow and gut but are also found in inflamed non-lymphoid tissues. Recent works by several groups have focused on the role of IL-10 secreting ASCs in the CNS in the control of neuroinflammation in EAE models.6,38,42 Matsumoto et al. investigated the role of IL-10 producing ASCs in EAE using IL-10 reporter mice and demonstrate that IL-10+ ASCs produce IL-10 in vivo.38 In this study, inflammation-limiting IL-10+ ASCs principally reside in the CNS-draining lymph nodes, but not in the spleen or spinal cords.38 These regulatory ASCs colocalize primarily with CD11c+ dendritic cells (DCs) in the extrafollicular region of the draining lymph nodes and secrete IL-10, which in turn binds to IL-10 receptor (IL-10R) on DCs, thereby decreasing transcription of Il6 and Il12 mRNA in the DCs. (Figure 1B) As a consequence, Th1 differentiation of MOG-specific encephalitogenic T cells is reduced by the addition of supernatants from wildtype ASC and DC co-cultures, indicating that IL-10+ ASCs inhibit DC functions to generate autoreactive T cells and thereby contribute to milder disease.38 (Figure 1B) Interestingly, Shen et al. generated mixed bone marrow chimeras by reconstituting lethally irradiated recipient mice with bone marrow cells that were 80% derived from B-cell-deficient JHT mice, 10% from IL-10−/− mice, and 10% from p35−/− mice. This set-up results in chimeric mice with 50% IL-10−/− B cells and 50% IL-35−/− B cells, in which the authors saw clinical symptoms in the EAE model that mirror chimeric mice reconstituted with wildtype bone marrow instead of JHT bone marrow cells.42 However, when the authors generated chimeric mice that were 100% IL-10−/− B cells or 100% p35−/− B cells (80% JHT bone marrow plus 20% IL-10−/− or 20% p35−/− bone marrow cells, respectively) those mice have a significantly exacerbated course of EAE, indicating that regulatory IL-10+ ASCs and IL-35+ ASCs act in parallel and are likely non-redundant.42 Future studies are required to dissect these intriguing results and reveal distinct tissue niches or cellular targets of IL-10 versus IL-35 expressing ASCs.
In neuroinflammation, both IgG+ and IgA+ ASCs localize to the brain and spinal cord of mice and humans.6,7,43 Rojas et al. demonstrated that at least a proportion of the IgA+ ASCs found in the CNS of mice with EAE originate from the gut and secrete IL-10 that ameliorates neuroinflammation.6 (Figure 1B) During the chronic phase of EAE, the authors have found a corresponding decrease in gut IgA+ ASCs, suggesting that the ASCs relocate from the gut into inflamed tissues.6 In line with this, during active relapse, MS patients have a significant reduction in IgA-bound gut bacteria from fecal samples, supporting the notion that IgA+ ASCs have relocated from the intestinal mucosa to the CNS.6,7 Expanding on the role of gut-microbiota specific IgA+ ASCs in regulating neuroinflammation, Pröbstel et al. recently published that clonally expanded IgA+ ASCs reside in the CNS of MS patients and accumulate adjacent to MS-associated demyelination.7 In mice, adoptive transfer of ASCs isolated from the small intestinal lamina propria reach the CNS of recipient mice and reduce symptoms of EAE.6 Trafficking of IgA+ ASCs from the gut is not restricted to the inflamed CNS. Using an influenza virus infection model, Rojas et al. demonstrated that rotavirus-specific IgA+ ASCs migrate to the inflamed lung that was challenged with influenza virus and also engage in low level homeostatic recirculation in the absence of inflammation.6 However, IgA+ ASCs do not traffic to the uninflamed CNS, indicating that ASCs are generally restricted from entering the CNS except during inflammation.6 Importantly, the ASCs reaching the CNS during EAE are commensal-reactive IgA+ IL-10+ ASCs and their secretion of IL-10 is required for reduction of EAE symptoms.6
Once IL-10 secreting ASCs reach the CNS, the appropriate signals are necessary to support their continued survival and function and, as discussed below in Maintenance and survival of regulatory ASCs, BAFF and/or APRIL present in inflamed CNS tissue could fulfill such a role. (Figure 1B) BAFF-Tg mice harbor an increased number of IL-10+ IgA+ ASCs in the gut and CNS during steady state, and these mice are resistant to EAE.6 In the same mice, IgG+ ASCs, which are absent in the CNS without local inflammation, increase in numbers in the CNS during EAE6, suggesting different requirements for CNS accumulation of IgG+ versus IgA+ ASCs. BAFF-Tg mice are also highly resistant to both MOG35–55 and rhMOG-induced models of EAE. Overexpression of BAFF affects the effector phase of EAE because adoptive transfer of pre-primed T cells into BAFF-Tg mice results in significantly attenuated disease compared with transfer into WT mice.6 Importantly, the authors demonstrate that IL-10 production by gut-derived ASCs is required for EAE resistance in BAFF-Tg mice.6 Contrasting the study by Rojas et al. showing increased resistance of BAFF-Tg mice to EAE, is a report by Zhou and colleagues that found that BAFF-Tg mice have exacerbated EAE symptoms and an increase in Th17 cells in the draining lymph nodes44. However the Zhou study did not analyze IL-10+ ASCs or B cells, and it is unclear if all animal facilities support the gut microbiome that induces regulatory IL-10+ ASCs. Thus, the discrepancies for the role of BAFF in EAE could be due to differences in animal facility microbiomes given that IgA+ ASCs are trafficking from the gut to the CNS during EAE and MS.6,7 Pröbstel and colleagues sequenced the IgA-bound microbial taxa and found differences in the abundance of specific taxa between MS patients and control donors in addition to absolute IgA levels in the gut between relapse and remission stages.7 The most prominent operational taxonal units (OTUs) bound by IgA in MS patients were Akkermansia muciniphila, Eggerthella lenta, Bifidobacterium adolescentis, and Ruminococcus with the first 3 OTUs previously reported to be increased in MS and to possess proinflammatory effects on T cells.7,45–47 In healthy controls, IgA preferentially bound to Bacteroides OTUs.7 These studies collectively indicate that the relative abundance of specific taxa in the gut microbiome of MS patients may contribute to MS development or exacerbation through the IL-10+ IgA+ ASCs that traffic from the gut to the CNS. Future research to harness this axis either through treatment that affects the gut microbiome or the ability of these cells to traffic to the brain will be critical for developing novel MS therapies.
Furthermore, mice deficient in TACI, a key receptor for BAFF and APRIL, develop exacerbated EAE symptoms.6 This result is in line with data from a clinical trial in which treatment of MS patients with atacicept (TACI-Ig), which blocks BAFF and APRIL, led to significant disease exacerbation that was accompanied by a significant reduction in serum IgM, IgA, and IgG levels suggestive of global ASC depletion.48 Interestingly, MS patients treated with anti-CD20 antibodies, which deplete B cells and spares ASCs, have an improvement in their clinical symptoms,49,50 demonstrating the unique role of ASCs in regulating symptoms of MS.
Cerebral spinal fluid levels of IgA increase significantly during active MS flares, whereas IgG levels, which are also higher in cerebrospinal fluid of MS patients than in that of healthy subjects,51 do not increase during relapse, indicating an activation of IgA+ ASCs during disease exacerbation.7 Oligoclonal bands or immunoglobulins are found in the cerebrospinal fluid of the majority of MS patients as well as in other chronic inflammatory CNS conditions.52,53 Some of these spinal fluid immunoglobulins of the IgG1 and IgG3 subclasses are suspected to contribute to pathogenesis of CNS inflammation; however, their contribution to disease pathogenesis is still controversial.53 It is possible that IgA+ ASCs that infiltrate the CNS are protective through provision of IL-10 and potentially IgA, while some IgG+ ASCs are potentially pathogenic via the various IgG effector mechanisms, including antibody-dependent cellular cytotoxicity, complement dependent cytotoxicity, and antibody-dependent cellular phagocytosis (reviewed in54). Based on the discovery that IL-10 production by IgA+ ASCs in mice with EAE is protective and the emerging presence of CNS IgA+ ASCs also in human MS, there is very likely a similar regulatory role for IL-10+ IgA+ ASCs in humans during MS relapse. However, future studies will need to investigate this in greater detail. Additional studies are also necessary to dissect which subclasses of CNS ASCs are protective vs. pathogenic, including the question of whether IgG+ ASCs may provide IL-10 to suppress inflammation in the context of MS or other neuroinflammatory conditions.
2.3. Regulatory ASCs of the respiratory tract.
Regulatory ASCs of the lung are discussed in detail below under Regulatory ASCs in specific disease settings.
2.4. The skin and other extralymphoid tissues: a potential residence for regulatory ASCs
The skin is a key barrier organ that provides protection from a variety of external insults including infections and allergens, in addition to mechanical, UV, thermal, and chemical damages. Our laboratory showed previously that B cells and ASCs constitutively recirculate through the unperturbed skin and that conventional B cells and IL-10+ innate like B cells reside in normal and inflamed skin of mice and humans.55,56 (Figure 1C) Supporting our findings, others reported the presence of B cells including IgG+ B cells in healthy and melanoma-associated human skin.57 In more recent work, we were able to show that IgM+ ASCs reside in healthy skin of both mice and humans, independently of T cells and microbiota, and provide natural antibodies to support homeostasis in the skin.27 Moreover, these ASCs increase significantly with chronic inflammation in mice and are found in human lesional skin from patients with the chronic inflammatory skin condition acne keloidalis.27 Cutaneous IgM ASCs are completely dependent upon the survival factors BAFF and APRIL, which are constitutively expressed in skin and upregulated in chronic inflammation.27 (Figure 1C) Treatment with anti-BAFF antibody during the course of inflammation also significantly reduces the accumulation of IgM ASCs in the skin, further demonstrating the continuous support that BAFF provides to cutaneous ASCs.27 We found that IL-10+ B cells migrate into the inflamed skin of mice and are critical for suppression of inflammation within the cutaneous environment.55,58 Positioning of natural antibody producing ASCs and IL-10+ B cells within the skin allows for appropriate defense and repair from insults while suppressing overt inflammation (Figure 1C) and it is likely that some proportion of the resident and infiltrating ASCs also produce IL-10 locally in the skin, which will be an interesting question to address in future work. Do the resident IgM+ ASCs in the skin constitutively produce IL-10? IgA+ ASCs are the second most common ASC found in mouse skin27 and are well established residents of human skin.59 Therefore, the questions arise whether skin ASCs produce IL-10 and what their relative contributions are in homeostasis and inflammation? Additional questions are whether inflammation induces IL-10 production by ASCs in distinct entities of skin inflammation, such as psoriasis or atopic dermatitis, and whether IL-10+ B cells that traffic into the skin during inflammation mature into IL-10+ ASCs that stay in the skin. If such IL-10+ ASCs were retained in the skin, would they be protective against future inflammatory insults from both external insults and new flare ups of an underlying inflammatory disease?
IL-10+ regulatory B cells (Bregs) play important roles in limiting local inflammation in extralymphoid tissue sites besides the skin, including joints and the colon; however, the role of IL-10+ ASCs in these sites is unknown. In joints, IL-10+ B cells suppress T-cell mediated inflammation in both collagen-induced arthritis60 and antigen-induced arthritis,61 mouse models in which the IL-10+ Bregs from spleen or lymph nodes limit the production of inflammatory cytokines by T cells. In patients with osteoarthritis and rheumatoid arthritis, IL-10+IgM+CD27+ B cells are found directly in the synovial fluid of affected joints and their relative frequency is decreased with more severe osteoarthritis.62 In the colonic lamina propria, resident enteric bacteria induce the development of IL-10+ B cells, which regulate T cell activation and maintain colonic homeostasis.63 IL-10+ Bregs from the peritoneal cavity also regulate colitis in both induced and spontaneous colitis mouse models by downregulating T cell activation.64 These sites are infiltrated with IL-10+ Bregs but the potential role of IL-10+ ASCs has not been explored and regulatory ASCs may play an important role in mediating immune suppression and homeostasis in a number of extra-lymphoid tissues.
3. Regulatory ASCs in specific disease settings
3.1. Multiple Sclerosis and EAE
The role of regulatory ASCs in MS and EAE is discussed in detail above under Regulatory ASCs in different tissue niches.
3.2. Regulatory ASCs in cancer
Robust anti-cancer responses require surveillance and tumoricidal activity by the host immune system, in particular by effector T cells. When infiltrating effector T cells fail to control malignant cell growth or are prevented from entering tumors, tumor growth is allowed to proceed. The role of tumor-infiltrating B lineage cells is less well studied but the presence of B cells and ASCs within tumors can have positive or negative effects on tumor growth depending on the individual type of cancer and the specific B lineage subset involved (reviewed in detail in65,66). Even at low numbers, tumor-infiltrating ASCs produce significant amounts of antibody.67,68 If the antibody produced recognizes tumor antigens, this will have a myriad of effects on tumor growth, including promotion of anti-tumor activity through antibody-dependent cellular cytotoxicity and phagocytosis, activation of complement, and enhanced antigen presentation by dendritic cells (reviewed in66). Tumor infiltrating B cells and ASCs can also suppress effective anti-tumor immune responses through secretion of cytokines, such as IL-10, IL-35, and TGFβ, and promote immunosuppressive myeloid cell differentiation, Treg development, and suppress effector T cell responses.8,9,66,69–74 Additionally the expression of PD-L1 on ASCs can strengthen the immunosuppression of effector T cell responses.8,9 The antibody isotype also affects the type of immune response generated, and formation of immune complexes between antibodies and tumor or non-tumor antigens within the tumor has been postulated to sustain chronic inflammation that weakens the anti-tumor response (reviewed in66). The Karin group convincingly showed that immunosuppressive IL-10-producing ASCs that also express both IgA and PD-L1 localize to prostate and liver cancers.8,9 (Figure 1D) The same authors go on to demonstrate that in a mouse model of prostate cancer, IL-10 producing PD-L1+ IgA+ ASCs impede T-cell dependent anti-tumor responses induced by oxaliplatin.8 Oxaliplatin is a type of immunogenic chemotherapy that causes a form of cell death that elicits both an innate and adaptive immune response.75 The appearance of the immunosuppressive ASCs within the tumors was dependent on TGFβ receptor signaling in the B lineage cells.8 Importantly, the tumor microenvironment frequently contains high levels of TGFβ, a cytokine known to promote B-cell isotype switching to IgA.76,77 and thus TGFβ-induced induction of immunosuppressive IgA ASCs appears as one of the many mechanisms by which tumors evade control by the host immune system. Loss of IgA+ ASCs in prostate tumors either through TGFβR2 knockout in B cells or ablation of IgA potentiates tumor control through enhanced CTL responses in response to oxaliplatin.8 Reconstitution of tumor-bearing B-cell-deficient Jh−/− recipient mice with B cells from either PD-L1−/− or IL-10−/− donors does not inhibit the efficacy of oxaliplatin treatment; however, reconstitution with wildtype B cells does ablate the efficacy of oxaliplatin-induced tumor control, indicating that PD-L1 and IL-10 co-expression are key mechanisms of immunosuppression by IgA+ ASCs.8 (Figure 1D) Human prostate cancer also contains IL-10+ IgA+ ASCs with a portion located in close proximity to CD8+ T cells.8 Approximately a quarter of IgA+ ASCs produced IL-10 in fresh surgical specimens, and IL-10+ IgA+ ASCs were enriched in malignant prostate tissue samples.8
Akin to IgA+ ASCs in prostate cancer, their counterparts in murine hepatocellular carcinoma (HCC) also express high levels of PD-L1 and secrete IL-10, which in turn suppress cytotoxic CD8+ T cell responses.9 (Figure 1D) PD-L1 neutralization leads to clonal expansion of HCC CD8+ T cell diversity.9 Ablation of IgA in mice reduces HCC burden significantly as IgA+ ASCs directly suppress CTL activation in vitro and in vivo through induction of CD8+ T cell exhaustion.9 PD-L1 blockade induced tumor regression and significantly reduced the accumulation of IgA+ IL10+ ASCs in the liver,9 potentially due to disruption of PD-L1:PD-1-dependent interactions between B cells and Tfh cells that are known to induce ASC maturation.78 Shalapour et al. additionally showed that PD-L1 neutralization does not decrease tumor burden in either IgA or CD8α deficient animals, demonstrating that immunosuppression requires both IgA+ ASCs and CD8+ T cells.9 It is possible that multiple tissues that turn malignant are promoted by IL-10+ IgA+ ASCs trafficking to the tissue and suppression of anti-tumor cytotoxic T cell responses. It would be interesting to study if mucosal or glandular tissues that have a high propensity to attract homing of IgA ASCs into the site79 still do so when they become malignant.
Infiltration of IgA+ ASCs into tumors does not always correlate with poor prognosis. Analysis of high-grade serous ovarian cancers demonstrate that infiltration with T cells predicts improved survival when co-infiltrated with B cells and ASCs, and intracellular and surface staining of the ASCs revealed IgA is the dominant isotype infiltrating these tumors.80 In these serous ovarian cancers, robust anti-tumor responses are mediated by tumor-antigen-specific and -nonspecific IgA secretion within the tumor.80 Additionally, transcytosis of IgA by the polymeric Ig receptor (PIGR) on ovarian cancer cells leads to transcriptional changes in inflammatory pathways and sensitization of the tumor cells to T cell-mediated cytotoxicity.80 However, the study did not assess IL-10, PD-L1, or LAG3 expression by the high-grade serous ovarian cancers-infiltrating IgA+ ASCs to determine whether these beneficial IgA+ ASCs express markers associated with regulatory or suppressive behaviors. Recent reviews on B cells and ASCs in cancer discuss in great detail the diverse roles B lineage cells play within tumor microenvironments.65,66 It will be necessary to investigate the functions of tumor-infiltrating ASCs, as well as specific ASC properties, within each cancer type to understand the outcomes associated with different types of infiltrating ASCs to design better therapies to enhance or restrict their activities.
3.3. Regulatory ASCs in infection
Studies in IL-10+ reporter mice revealed that under physiological conditions and after various immune challenges (i.e. lipopolysaccharide (LPS), CpG oligodeoxynucleotide, goat anti-IgD, and murine cytomegalovirus) the majority of IL-10+ lymphocytes in secondary lymphoid tissues of mice are B lineage cells.81 The role of IL-10+ B cells in suppressing immune responses to a multitude of infections is well established (reviewed in3,82); however, the role of IL-10+ ASCs is less well studied. IL-10+ B cells are induced after various infections including Listeria monocytogenes, enteric bacteria, Mycobacterium tuberculosis, and Leishmania spp. and IL-10+ B cells are associated with exacerbation of infection and poor immune response to the invading pathogen.63,83–85 While induction of IL-10+ B cells can be seen as an escape mechanism by the microbe to evade protective immune responses, in some instances the development of IL-10+ B cells can be protective as it limits overzealous responses and immunopathology. For example, within 1–3 days after S. typhimurium infection, IL-10+ CD138+ B cells accumulate in the spleen.86 CD138 expression alone does not define ASCs,87,88 therefore it is unclear if the described IL-10+ B lineage cells represent B cells or ASCs. These IL-10+ CD138+ B cells are dependent on TLR (MyD88) signaling, and suppress the immune response required to clear the infection.86 Global deficiency in MyD88, an adaptor protein for TLRs, causes mice to succumb to S. typhimurium more rapidly relative to wildtype mice. In contrast, B-cell-specific lack of MyD88 or of TLR2 and TLR4 results in prolonged survival of mice infected with S. typhimurium86 Furthermore, mice with a B cell-specific deficiency in either IL-10 or Myd88 infected with S. typhimurium mount a more robust immune response in the infected livers relative to control mice, indicating that microbial sensing by B cells through TLR and subsequent IL-10 production leads to suppression of an effective immune response in this infection.86 The improved host survival in mice with B cell-specific MyD88 or IL-10 deficiency is characterized by increased neutrophil and NK responses early and a boost in IFNγ- and TNFα-producing CD4+ T cells 21 days post infection in the livers following S. typhimurium infection.86 Additional studies by Shen and colleagues in the S. typhimurium infection model show that distinct populations of immunosuppressive IL-10 or IL-35 producing ASCs are generated during the infection.42 Il10 mRNA and the subunits of IL-35, Ebi3 and p35, are only expressed by mature ASCs (CD138hiCD22–) that co-express Blimp1, while the less mature ASCs (CD138intCD22+) express little Il10 or Ebi3 mRNA following S. typhimurium infection.42 The findings indicate that the ability to produce IL-10 or IL-35 is acquired during ASC maturation.42. Interestingly, the ASCs generated during S. typhimurium infection were all Blimp1+IgM+CD138hiTACI+CXCR4+CD1dintTim1int, and a single-cell PCR analysis revealed that Il10 mRNA or Ebi3 and p35 mRNA were each expressed by subsets of 6–10% of these ASCs, but rarely did an individual cell express all three transcripts.42 It is an open question whether different signaling events upon B cell activation and differentiation into ASC or distinct B cell subsets give rise to the differentiation of IL-10 versus IL-35 producing ASCs. Shen et al. showed that, upon ex vivo stimulation, the regulatory ASCs generate significantly higher levels of IL-10 than B cells from the same mice.42 In addition, the regulatory ASCs are unable to produce IL-6, a pro-inflammatory cytokine that is expressed by B cells during Salmonella infection,89 indicating that IL-10+ or IL-35+ ASCs are the key regulatory B lineage subset that controls inflammation during Salmonella infection.42
3.4. Regulatory ASCs in allergic responses and allergen tolerance
As a key barrier organ, the lung is continuously exposed to microbes and allergens that the respiratory immune system must contain and clear without inducing significant airway inflammation. In a mouse model of ovalbumin(OVA)-driven allergic airway disease, IL-10-secreting CD138+ ASCs restrict pulmonary inflammation, eosinophilia, Th2-cytokine production (IL-4 and IL-5), and serum OVA-specific IgE levels.90 (Figure 1E) The development of these IL-10+ ASCs is dependent on CD19+CD138+ cells expression of semaphorin 4C, an axonal guidance molecule with potential roles in B cell development.90,91 Loss of semaphorin 4C in B cells inhibits IL-10+ CD138+ ASC development, increases the presence of pro-inflammatory IL-4+ CD138+ ASCs in the spleen, and leads to augmented type 2-airway inflammation following OVA exposure.90 (Figure 1E) The authors demonstrate a requirement for semaphorin 4C in IL-10+ ASC development and suppression of inflammation using mouse model with a B-cell specific deletion in Sema4 as well as adoptive transfer experiments.90 It would be critical to explore the antibody isotype of the IL-10+ ASCs that restrict allergic airway inflammation as well as their origin. Are they IgM+ and potentially come from the natural IgM B cell pool? Are they derived from IgA+ B cells educated within the respiratory tract, or do they represent IgA+ ASCs that relocated from the gut similar to rotavirus-specific IgA+ ASCs that were shown to traffic to the lung during influenza.6 Are they IgG+ ASCs specialized in restricting inflammation in the airways through both IL-10 and IgG-mediated antigen scavenging? Given the negative effects of IgE in allergic inflammation, it appears unlikely that the immunoregulatory IL-10+ ASCs are IgE+, however, this has not been formally ruled out and could represent a mechanism of immunological “self-restraint” akin to IL-10+IFNγ+ cytotoxic T lymphocytes that limit immunopathology in influenza.92
IgG4-related disease is a multi-organ immune-mediated condition characterized by lymphoplasmacytic infiltration, fibrosis and phlebitis, and one manifestation involves allergic diseases (reviewed by93). However, IgG4 is generally not believed to be a driver of pathogenesis but rather a non-inflammatory antigen sink.93 IgG4 is a noninflammatory antibody with low affinity for Fcγ receptors, engages in Fab arm exchange to form bi-specific antibodies, rendering it essentially monovalent and unable to crosslink antigens preventing activation of the complement cascade (reviewed in93,94). Peripheral allergen tolerance in individuals exposed to high-dose allergens is generated through multiple mechanisms, including allergen-specific IgG4 B cell induction distinct from IgG4-related disease.95–97 In a study on allergen-tolerant beekeepers that harbor B cells specific for the bee venom allergen phospholipase A2 (PLA), antigen-specific IgG4 production is significantly enhanced in the IL-10-producing B-cell subset.98 Patients with bee venom allergies were treated with honeybee venom immunotherapy, which leads to significantly increased frequency of IL-10+ PLA specific B cells.98 There is also a significant (100-fold) decrease in the anti-PLA IgE:IgG4 ratio in the serum due to significantly increased PLA-specific IgG4 levels compared to non-treated allergic controls.98 IL-10 indirectly downregulates IgE production via monocytes and also drives IgG4 synthesis by memory B cells.99–101 IL-10 and IgG4 co-expression by regulatory ASCs may be acting in concert to maintain immune tolerance in individuals exposed to high dose antigens, and this property may be utilized to provide relief to individuals with a variety of allergic diseases affecting the respiratory system.
Given the vast number of individuals with asthma or other airway inflammation – approximately 1 in 12 people or 25 million of the US population suffers from asthma alone,102 the role of IL-10+ ASCs in mediating suppression of allergen-specific T cell responses in the lung deserves further study as many open questions remain. For example, are individuals with asthma or chronic respiratory inflammation deficient in the generation of IL-10+ ASCs in the lung microenvironment?
4. The phenotype of regulatory ASCs
4.1. Surface markers
Thus far, little is known about the specific markers that distinguish immunomodulatory IL-10+ plasma cells and plasma blasts from their ‘regular’ non-IL-10-producing counterparts. In mice, ASCs are CD19lo/negCD138+B220lo/negIgD–CD93+CD44+VLA-4+IL6-R+BCMA+and CXCR4+; in humans they are CD27hiCD38hiCD138+IL6-R+BCMA+ and CXCR4+ (reviewed in23,25,103). Regulatory IL-10+ ASCs can be IgM or class-switched to IgA or IgG.6–9,36–38,42,66,81,86,90,98 The Fillatreau group recently described that expression of the inhibitory receptor LAG3 marks immunosuppressive natural regulatory ASCs that are poised to rapidly secrete IL-10 upon stimulation and subsequently suppress innate stimuli.104 In addition to LAG3, these cells express PD-L1, PD-L2, and CD200.104 LAG3, PD-L1, PD-L2, and CD200 are inhibitory receptors frequently associated with T cell exhaustion in the literature that are upregulated upon activation, and recent novel therapies, including anti-PD-1, -PD-L1, and -LAG3, have been designed around blockade of these “checkpoints” to enhance anti-tumor T cell responses (reviewed in105). Regulatory ASCs may express these inhibitory receptors as additional mechanisms to suppress an immune response in addition to secretion of IL-10 or IL-35.
5. Signals involved in the development of regulatory ASCs
Terminal differentiation of B cells into ASCs and the induction and maintenance of IL-10 expression by B lineage cells partially overlap. The transcription factors IRF4 and Blimp-1 as well as the cytokines IL-21 and IL-10 are associated with both induction of ASCs and IL-10 production in B cells and appear to support the development of IL-10 secreting ASCs. (Figure 2A) This section will cover the role of these factors in the differentiation of ASCs, IL-10+ B cells and IL-10+ ASCs.
Figure 2. Regulatory ASCs: phenotype, developmental and survival signals.
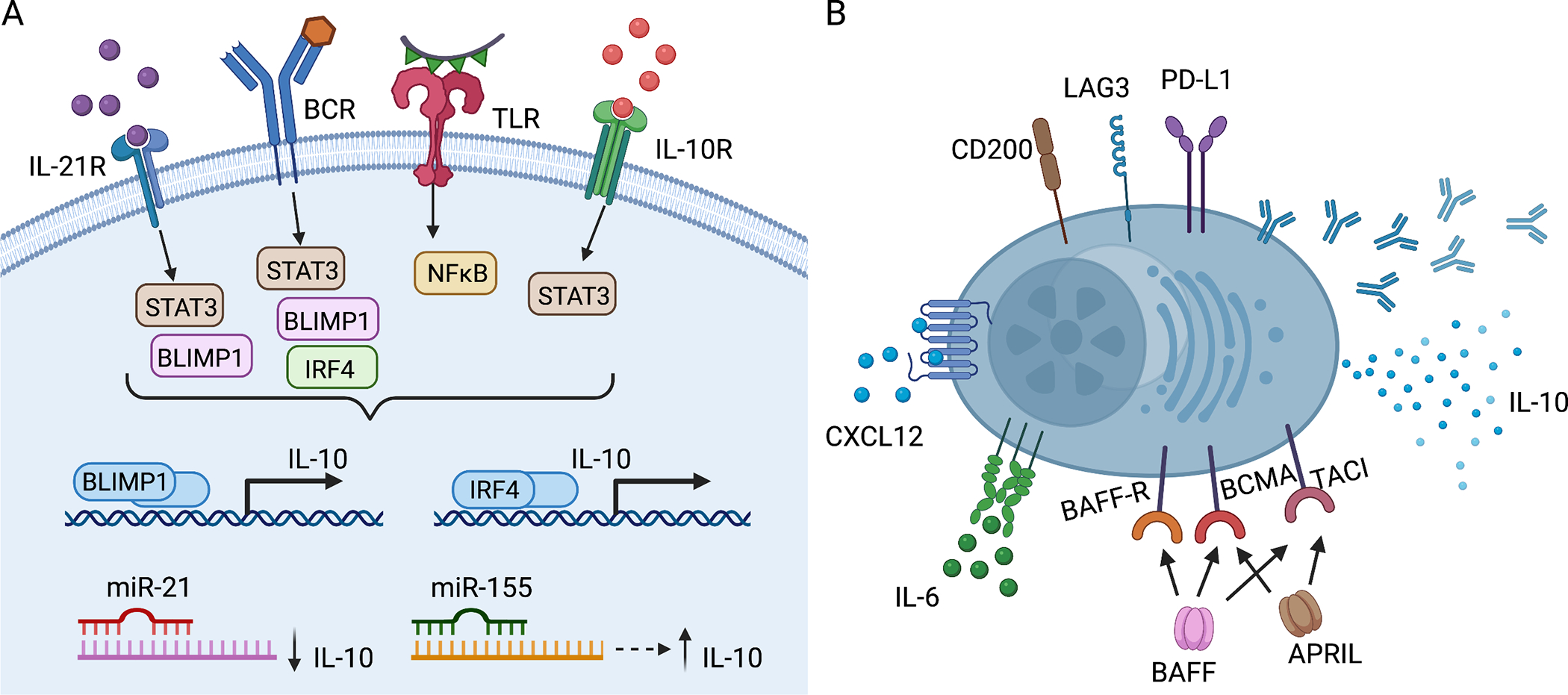
(A) Signals that support the development of IL-10+ B cells and ASCs overlap. Signaling through IL-21-IL-21R, IL-10-IL-10R and engagement of BCR and TLR lead to the upregulation of BLIMP1 and IRF4, which bind to regulatory regions of the IL10 locus turning on expression of IL-10. MiR-21 directly binds to IL-10 mRNA transcripts decreasing IL-10 expression. miR-155 acts indirectly through Jarid-2, which upregulates IL-10 expression. (B) Many IL-10+ ASCs express the inhibitory receptors, PD-L1, LAG3, and CD200. They express the IL-6 receptor and CXCR4, the receptor for CXCL12. They also express BAFF-R, BCMA, and/or TACI, surface receptors for the cytokines BAFF and APRIL, which mediate ASC survival. Image created with Biorender.com. APRIL, a proliferation-inducing ligand; ASCs, antibody secreting cells; BCR, B cell receptor; BAFF, B-cell-activating factor; BCMA, B-cell maturation antigen; BLIMP-1, B lymphocyte-induced maturation protein-1; IRF4, interferon regulatory factor 4; MiR, microRNA; LAG3, lymphocyte-activation gene 3; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PD-L1, programmed death-ligand 1; STAT3, Signal transducer and activator of transcription 3; TACI, transmembrane activator and calcium-modulating cyclophilin ligand interactor; TLR, toll-like receptor.
5.1. IRF4 and Blimp-1
IRF4 is expressed by B cells at all developmental stages except during the germinal center reaction and is highly upregulated in ASCs (reviewed in106). Blimp-1, encoded by prdm1, is expressed by ASCs and is required for ASC differentiation (reviewed in23). IRF4 is a transcriptional activator that cooperates with multiple other transcription factors throughout B cell development in a dose-dependent manner to control B cell fates: low amounts of IRF4 promote germinal center fate and class switch recombination, whereas high amounts repress Bcl6 and activate Blimp-1 and Zbtb20, thereby inducing ASC differentiation.107,108 Blimp-1 is a transcriptional repressor that drives terminal differentiation of B cells into ASCs by an overall shift from B cell programming to immunoglobulin secreting status. Blimp-1 represses expression of molecules critical for B cell programming including SPIB, ID3, AID, Bcl6, and Pax5.23,109,110 Each of these transcription factors is also known for its role in promoting IL-10 production in lymphocytes. Specifically, IRF4 and Blimp-1 play a role in activating IL-10 expression in both T cells111–113 and B cells.20–22 Using IL-10 reporter mice, Maseda et al. demonstrated that IL-10+ B cells from LPS stimulated mice express higher levels of transcripts for ASC-associated transcription factors, Prdm1, Xbp1, and Irf4.114 This finding is supported by work demonstrating that IL-10+ human B cells also have transcriptomic changes associated with ASC differentiation, class switching, and immunoglobulin production.11 IRF4 is essential for B-cell expression of IL-10 as Irf4−/− B cells are impaired in their ability to secrete IL-10 after TLR and BCR ligation.38 IRF4 binds to regulatory regions upstream of the IL10 transcription start site and controls NFAT-dependent IL-10 production.38,111 (Figure 2A) IRF4, in addition to IRF8 and/or BATF, is also recruited to regulatory motifs of the Il10, Ebi3, and Il12a promoter elements in activated B cells. Importantly, Ebi3 and Il12a are the genes encoding the subunits of IL-35, IL-12p35α and IL-27β.115 Blimp-1 also binds to regulatory regions of the IL-10 transcription start site.22 (Figure 2A) Blimp-1 expression is enriched in IL-10+ B cells but, surprisingly, Prdm1-deficient mice have an increase in IL-10+ B cells at steady state and with LPS stimulation; however, the IL-10+ B cells that arise are incapable of efficiently suppressing T cell proliferation.22 Additionally, Blimp-1-deficient IL-10+ B cells when stimulated with LPS fail to differentiate into IL-10+ ASCs.22 Wang et al. demonstrated that Blimp-1 represses Il10 in wildtype IL10+ B cells but after stimulation Blimp-1 acts together with STAT3 downstream of IL-21 signaling to enhance IL-10 transcription in B cells.22 Loss of either IRF4 or Blimp-1 specifically in B cells leads to a loss in IL-10+ ASCs, and localization of these cells to the draining lymph node is critical for control of EAE.38 TLR-mediated IL-10 production in B cells requires IκBNS, an inducible nuclear IκB protein, and absence of IκBNS results in a reduction of Irf4 and Prdm1 expression and a corresponding decrease in IL-10+ plasmablasts.116
5.2. IL-21
IL-21, a member of the common γ chain receptor family of cytokines, is one of the most important B cell-tropic cytokines and has roles in apoptosis, class switching, and ASC development.117,118 IL-21 signaling induces expression of plasma-cell related cell-surface markers and transcription factors in mice and humans including upregulation of CD138 surface expression, Prdm1, and Bcl6 mRNA transcripts and repression of Pax5 mRNA transcript119,120 and also leads to increases in IgM+ and IgG+ ASCs.119,121 Secretion of IL-21 by CXCR5+ follicular helper T cells during T-B cell interactions directly contributes to ASC differentiation and is superior to differentiation induced by other known ASC-driving cytokines including IL-2, IL-4, and IL-10.122,123 IL-21 acts through pSTAT3 activation to induce IL-10 expression by B cells.13–16 (Figure 2A) IL-21 and CD40 cognate interactions between B cells and T cells are required for the immunosuppressive functions of IL-10+ Bregs in mice induced with EAE.13
Activation with IL-21 is important for the development of IL-10+ ASCs. Rheumatoid arthritis patients have fewer IL-10+ B cells than healthy donors do, but IL-21 drastically increases the differentiation of both IL-10+ B cells and IL-10+ ASCs in RA and healthy donor B cells.16 IL-21 signaling also plays a role in the generation of autoreactive ASCs in systemic lupus erythematosus, in which patient CD11chiT-bet+ B cells express high levels of IL-21R and are poised to differentiate into autoreactive ASCs upon IL-21 stimulation.124 In contrast, ASCs differentiated from other B cell subsets in the same individuals are rarely autoreactive.124 These CD11chi B cells express high density of BAFF receptor and intermediate levels of TACI and are similar in phenotype to reported age-associated B cells (ABCs).124,125 The authors compared the transcriptome of CD11chi B cells to naïve and memory B cells and determined that they expressed high levels of ASC-related factors in addition to high levels of Il10 and low levels of Il6 mRNA transcripts.124 These cells correlate with specific disease subtype manifestations in systemic lupus erythematosus (e.g. malar rash and active nephritis) and localize to inflamed tissue lesions, indicating their direct pathogenic potential.124 There is some debate about the prevalence of IL-10-producing B lineage cells in autoimmune diseases with some studies demonstrating elevated IL-10+ B lineage cells and others demonstrating a paucity in these cells. These discrepancies could reflect attempts to autoregulate self-reactive lymphocytes or failure to suppress, respectively (reviewed in82,126) and could be dependent on the appropriate environmental signals such as IL-21 reaching the correct B cells to generate protection. Alternatively, the production of IL-10 could be a mechanism to autoregulate pathogenic ASCs.
5.3. IL-10-promoted differentiation of B cells into ASCs
In vitro and in vivo studies suggest a tight relationship between IL-10 and the differentiation into ASCs and vice versa. IL-10 acts in both a paracrine and autocrine manner to promote ASC differentiation (Figure 2A): in vitro addition of exogenous IL-10 induces differentiation of human B cells into ASCs of various isotypes, IgM, IgG, IgA and IgE.10,127,128 In addition to paracrine actions, activated B cells that secrete IL-10 signal in an autocrine fashion to enhance differentiation into ASCs in vitro, because blockade of IL-10R in cultures of purified IL-10+ B cells significantly reduces their differentiation to ASCs.11 Furthermore, IL-10 addition to human B cell cultures arrests memory B cell proliferation and pushes B cell differentiation to CD20–CD38+ primarily IgG-secreting ASCs.129 After treatment of IL-10 reporter mice with LPS, IL-10+ B cells transiently express IL-10 in vivo prior to differentiation to ASCs.114 IL-10+ B cells isolated from these reporter mice and stimulated in vitro with LPS, phorbol myristate acetate (PMA), and ionomycin then subjected to ELISPOT analyses revealed that many IL-10+ B cells differentiate into polyreactive or natural IgM, and antigen-specific IgG and IgM ASCs.114 Furthermore, adoptive transfer of IL-10+ B cells from naïve IL-10 reporter mice into Rag2−/− hosts have a greater capacity to contribute to serum IgM and IgG levels than do IL-10– B cells.114
5.4. ASCs acquire the ability to produce IL-10
While a subset of IL-10+ B cells differentiates into antibody-secreting cells upon stimulation, an unknown fraction of already existing ASCs acquires the ability to produce IL-10. It was previously accepted knowledge that ASCs lacked expression of surface immunoglobulin receptors. However, several independent studies demonstrated recently in both mice and humans that the vast majority of IgM and IgA ASCs express functional surface immunoglobulin receptors, whereas IgG ASCs do not.12,130,131 There is evidence that antigenic stimulation through surface BCRs on IgM and IgA ASCs leads to upregulation of activation markers and cytokine induction.12,130 Interestingly, upon antigenic stimulation of IgM+ ASCs, the top two gene ontology biological process terms defined are related to cytokine production, and the most highly differentially upregulated gene is for the chemokine Ccl5.12 Investigating the mRNA transcripts related to cytokine production, Blanc and colleagues found that in addition to Ccl5, transcripts for Il10 and Lag3 (lymphocyte-activation gene 3) are significantly upregulated in ASCs upon antigenic stimulation.12 The authors demonstrated that at steady state, there is constitutive transcription of IL-10 within polyclonal bone marrow ASCs,12 consistent with work from other groups.37,81 IL-10 expression is largely restricted to the IgM+ ASCs and is upregulated approximately 2-fold upon BCR ligation, but the authors did not confirm the upregulation of Lag3 or Ccl5 mRNA transcripts in ASCs by either qPCR or protein expression.12 CCL5 is a ligand for CCR1, CCR3, and CCR5 and attracts both innate immune cells, including NK cells and myeloid cells as well as activated T cells.132 While originally recognized as a T cell-secreted chemokine, other leukocytes, including B cells, also produce CCL5.132 B cells secrete CCL5 upon BCR activation and the chemokine acts in an autocrine fashion to support further B cell proliferation and antibody secretion133,134. LAG3, an inhibitory receptor often co-expressed with other inhibitory receptors, such as PD-L1 and PD-L2, is highly expressed on Tregs and is also expressed on activated B cells, a subset of malignant B cells, and on regulatory ASCs.104,135,136 Continued expression of functional BCR on ASCs may help their survival as well as mediate control of infections or inflammation in several ways through reprogramming ASCs towards a cytokine-producing population. We postulate that both IL-10 and CCL5 support ASC survival in limited niches in which secretion of CCL5 alerts other leukocytes to their location to control infection, but the co-secretion of IL-10 restricts overt inflammation induced by the infiltrating leukocytes. LAG3 may be co-induced to self-limit activation and could act in concert with IL-10 to restrict ASC activities. It will be interesting to further work out the mechanisms behind this axis of self-limiting antigen-specific immune responses by IL-10+ ASCs.
5.5. Epigenetic and miRNA regulation of IL-10 expression in B cells and ASCs
Epigenetic changes related to IL-10 expression include alterations in methylation status at regulatory regions of the IL10 locus and changes in microRNAs (miRs) that modulate the translation of protein from Il10 mRNA transcripts. Murine IL-10 expressing B cells have lower levels of CpG methylation at the conserved non-coding sequence-9, conserved non-coding sequence-4.5, and conserved non-coding sequence+1.65 regions of the Il10 gene locus.137 Demethylation at conserved non-coding sequence-4.5 and partial demethylation at conserved non-coding sequence-9 is related the ability of B cells to secrete IL-10 upon activation.137 In humans, demethylation at the human conserved non-coding sequence-12.5 region (evolutionarily conserved with murine conserved non-coding sequence-9) is associated with IL-10 competence.137 This methylation signature is maintained in the ASCs of tonsil and bone marrow from humans and, interestingly, also in the plasma cell malignancy multiple myeloma.137 Fillatreau’s group demonstrated that IL-10+LAG3+CD138hi ASCs in mice are hypomethylated at regions around the IL10 gene in a similar manner as seen in B1a cells, which are enriched in IL-10+ expression relative to other B cells.104,138 The hypomethylated sites of the mouse IL10 locus overlap with B cell-specific DNase hypersensitive sites, which is consistent with an open-chromatin state for the IL10 locus and suggests that the cells are epigenetically poised or pre-programmed for IL-10 secretion.104
MicroRNAs (miRNA) are small, ~22-nt non-coding RNAs that modulate expression of other messenger RNAs139 and several miRNAs that regulate IL-10 expression19 also regulate ASC development and survival,140 including miR-21 and miR-155. (Figure 2A) Thus, miRNAs affect both IL-10 expression in B lineage cells and ASC development and maintenance. miR-21 directly targets the 3’ untranslated region of Il10 mRNA, thereby inhibiting its expression.17 Direct antagonism of miR-21 by antisense oligonucelotides is associated with an increase in IL-10+ B cells that protect from severe disease in an EAE model.17 (Figure 2A) miR-21 is not essential for ASC development; however, miR-21 is repressed by BLIMP-1 during IL-21 mediated ASC differentiation.141 On the other hand, miR-155 indirectly affects IL-10 expression by B cells through Jarid-2, because increased miR-155 inhibits Jarid-2 expression, which normally represses H3K27me3 binding to the IL10 promoter and expression of IL-10.18 (Figure 2A) miR-155 is also important in ASC differentiation by inhibiting PU.1 expression, which leads to Pax5 downregulation and initiation of ASC differentiation.142 In summary, decreased levels of miR-21 and increased levels of miR-155 each contribute to ASC development and also program IL-10 production in B lineage cells, emphasizing their likely central role in the development and maintenance of IL-10+ ASCs. Future research should formally test the requirement for miR-21 and miR-155 in the development of IL-10+ ASCs and the maintenance of IL-10 secretory ability in ASCs.
6. Maintenance and survival of regulatory ASCs
The majority of long-lived ASCs localize to the bone marrow of healthy individuals, where CXCL12-expressing stromal cells and accessory cells that secrete the survival factors IL-6, BAFF, and APRIL support their long-term residence (reviewed in23–25). (Figure 1A) It was recently demonstrated that over 60% of the IL-10+ cells residing in the bone marrow under steady state conditions are ASCs, indicating that the bone marrow niche is also supportive of IL-10+ ASC survival.37 In general, ASCs also take up residence in the spleen and other secondary lymphoid tissues, such as lymph nodes (reviewed in 143). Beyond these traditional ASC niches non-lymphoid tissues provide a residence for and support survival of ASCs, including skin,27 CNS,6,7 lung,32 and the lamina propria of mucosal tissue in the gut.33–35 These extralymphoid tissues facilitate accumulation of ASCs particularly well during inflammation (reviewed in 25). Among the many extrinsic and intrinsic factors that enhance ASC survival, the major cytokines and chemokines include IL-6, CXCL12, BAFF, and APRIL24–26, and those that support IL-10+ B cells include IL-21, IL-6, IL-1β, IFNα, IL-33, IL-35, BAFF, and APRIL (reviewed in detail in 144). (Figure 2B) There is some current debate about the niche requirements for ASC survival, and micro-niches potentially exist that support ASCs outside of the bone marrow and spleen (discussed in 26). The “physical niche model” proposes that specialized locations within the bone marrow contain accessory cells that provide the essential survival factors and appropriate cellular interactions for immature ASCs to mature and remain in the limited ASC niche long-term. However, it is possible that long-lived ASCs re-enter the circulation particularly during inflammation when bone marrow homeostasis is disrupted.26 Of note, IgA+ ASCs migrate also into the mammary gland as it becomes ready for lactation, a process guided by the CCR10-CCL28, receptor-ligand axis,145 and these ASC may also be recruited from existing survival niches rather than represent fresh ASCs generated from memory B cells. The “free access model” described by Wilmore and Allman proposes that ASCs are not limited to specialized physical niches and, instead, traffic freely to any CXCL12-rich tissues that support their survival through survival factors BAFF, APRIL, and IL-6 that are upregulated in inflamed sites.26 (Figure 2B) CXCL12 is found in various inflamed and non-inflamed tissues132 and presumably attracts CXCR4+ regulatory ASCs to these tissues. IL-6 originally defined by its role as a B-cell stimulating factor for immunoglobulin production,146 is a cytokine upregulated early in inflammation with critical roles in chronic inflammation, autoimmunity, and cancer (reviewed in147) and binds to IL-6R on ASCs in the inflamed tissues, supporting their survival.
It is becoming evident that the CNS is not just a site at which IL-10+ ASCs limit neuroinflammation (see section Regulatory ASCs and the gut-CNS Axis above), it is also transformed into an ASC survival niche by inflammatory events. Specifically, in humans, the CXCR4 ligand CXCL12 is upregulated in blood vessels lining the blood-brain barrier in the inflamed CNS of MS patients.148,149 In inflammation, polarized CXCL12 expression on the abluminal side of the blood vessels is lost and CXCL12 redistributes to the luminal side of vessels, allowing CXCR4+ leukocytes to migrate into the CNS.148,149 ASCs retain surface expression of CXCR4 and presumably use the CXCL12-CXCR4 axis to cross the blood-brain barrier in inflammation akin to their CXCR4-dependent trafficking into the bone marrow and secondary lymphoid organs.150 In EAE, CXCL12 is also upregulated in inflamed mouse CNS at the peak of disease.28 As discussed above, it was demonstrated that, IL-10+ IgA+ regulatory ASCs traffic from the gut into the inflamed CNS during EAE6 and human MS.7 In inflamed CNS tissue from EAE mice there is an increase in both APRIL and BAFF in the CNS at the peak of disease and ASCs are found to localize in the respective CNS areas.28 In humans, BAFF is produced by astrocytes and upregulated in MS brain lesions and primary CNS lymphomas29 and APRIL is secreted by a subset of macrophages infiltrating MS lesions.151 Thus, APRIL and BAFF expression in the CNS are well positioned to mediate the survival of regulatory ASCs in situ.
Overexpression of BAFF in transgenic mice using the liver-specific alpha-1 antitrypsin promoter with the ApoE enhancer152 generates mice with detectable BAFF in their serum153 and leads to an increase in IgA+ ASCs in the small intestinal lamina propria during steady state.154 These BAFF-Tg mice have an attenuated course of EAE that depends on IL-10 expression by ASCs and is correlated with increased infiltration of IgA+ ASCs into the CNS.6 The authors conclude that BAFF assists IL-10+ IgA+ ASCs during the effector phase of EAE to ameliorate EAE symptoms. In further support for BAFF in enhancing IL-10 expression in ASCs, are in vitro studies with recombinant BAFF, which augments both IL-10 and IgA2 production in B cells.155 Additional in vitro studies by Saulep-Easton and colleagues demonstrated that inhibition of signaling through the TACI receptor abrogates BAFF-induced IL-10 production.156 Furthermore TACI−/− mice have severely decreased IL-10 serum levels compared with wildtype mice, and BAFF-R−/− mice also have significantly reduced serum IL-10.156 Together, these results support the notion that BAFF signaling through its receptors, particularly TACI, is required for IL-10 production in these B lineage cells.156 However, a study by Matsushita and colleagues recently showed that BAFF neutralization in a scleroderma model in mice, abrogates IL-6 expressing pathogenic B cells while preserving IL-10+ Bregs that attenuate skin inflammation.157 It is an open question whether BAFF supports IL-10+ ASCs but not IL-10+ B cells or whether the organ site (intestine and CNS versus skin), or the type of inflammation determines these differences in BAFF regulation of IL-10 expression in B lineage cells.
APRIL also supports the development of IL-10+ B cells,158 and Fehres et al. demonstrated that APRIL induces a population of IgA+ PD-L1+ IL-10+ regulatory B cells, which suppress T cells and macrophage responses in vitro via IL-10 and PD-L1.159 The authors correlate the induction of IL-10+ regulatory B cells to APRIL signaling as APRIL-Tg mice have increased IL-10+ B cells and ameliorated EAE and contact hypersensitivity responses.159 Unfortunately, the authors did not assess antibody production, so it is unclear if the immunoregulatory role is exerted by IL-10+ ASCs or B cells. TACI is a receptor for both BAFF and APRIL and therefore either cytokine may support IL-10+ ASCs during CNS inflammation. Importantly, both BAFF and APRIL have known roles in the acquisition of IL-10 expression by B cells,144 and it will be important to tease apart the exact mechanisms and models in which diseases are controlled or exacerbated by IL-10+ B cells as compared to IL-10+ ASCs.
7. Conclusions and future directions
Recent findings have demonstrated that regulatory ASCs suppress immune responses in a number of tissue niches and disease states as described above. These studies expand the regulatory role of B lineage cells in infection, inflammation, and cancer, and it is likely that regulatory ASCs will be found to play critical immunosuppressive roles in additional diseases beyond what is discussed in this review. The particular microenvironmental signals that encourage the development and maintenance of regulatory ASCs as discussed above will demand additional study. Understanding the signals required for regulatory ASC development and function will allow us to harness their ability to provide antibodies, their capacity to secrete immunosuppressive cytokines, IL-10 and IL-35, as well as their provision of inhibitory checkpoint signals in various disease states and tissues. Co-secretion of anti-inflammatory antibodies, e.g., as is the case with natural IgM, and IL-10 or IL-35 may be two parallel mechanisms to suppress inflammation. Alternatively, these cytokines could serve a critical role in restricting inflammation generated by infiltrating immune cells as well as auto-regulating a pro-inflammatory ASC response. While evidence suggests that there is constitutive expression of IL-10 by ASCs in the bone marrow that maintains immune homeostasis, it is unclear if regulatory ASCs found in peripheral tissues constitutively express IL-10 or IL-35, or if these cytokines are only produced during the course of inflammation or infection. To enhance their study, future work is required to define cell surface markers in addition to LAG3 to easily identify regulatory ASCs. Additionally, are these cells long-lived residents within these tissues after resolution of disease, do they die, or do they return to the primary ASC niches? We predict that regulatory ASCs have continuous functions in barrier sites such as the skin, lung, and gut where antibodies are necessary to engage microbes and for the clearance of apoptotic cells, but overt inflammation needs to be restricted. It would be intriguing if any diseases mediated by pathogenic antibodies such as systemic lupus erythematosus or myasthenia gravis also contain a subset of IL-10 or IL-35 secreting ASCs that are antigen-specific, indicating an attempt at auto-restriction of an inflammatory response. Exploring the source of regulatory ASCs, whether they are generated within the target tissue or traffic into the tissues will provide avenues for targeting these cells therapeutically. In summary, regulatory ASCs is a growing research field that has widespread disease application therefore addressing many of the big and small open questions will provide multiple therapeutic avenues for harnessing the power of ASCs to regulate immune responses.
Acknowledgements
The authors were supported in part by NIH grants R01AR067751 and R01AI127389 (to GFD) and an Ethel Brown Foerderer Foundation Fellowship from Thomas Jefferson University and T32AI134646 (to SEM).
Abbreviations:
- APRIL
a proliferation-inducing ligand
- ASCs
antibody secreting cells
- BCR
B cell receptor
- BAFF
B-cell-activating factor
- BCMA
B-cell maturation antigen
- BLIMP-1
B lymphocyte-induced maturation protein-1
- Bregs
regulatory B cells
- CNS
central nervous system
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- ELISPOT
enzyme-linked immune absorbent spot
- Ig
immunoglobulin
- IRF4
interferon regulatory factor 4
- LAG3
lymphocyte-activation gene 3
- LPS
lipopolysaccharide
- MiR
microRNA
- MS
multiple sclerosis
- OTU
operational taxonal unit
- PD-L1
programmed death-ligand 1
- PMA
phorbol myristate acetate
- TACI
transmembrane activator and calcium-modulating cyclophilin ligand interactor
- TLR
, toll-like receptor
Footnotes
Conflict of Interest Statement
The authors state no conflict of interest.
Data Availability Statement
Data sharing is not applicable to this article as no new data were generated or analyzed in this review.
References
- 1.Cyster JG, Allen CDC. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell. 2019;177(3):524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–612. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Fu Y, Chu Y. Regulatory B Cells. Adv Exp Med Biol. 2020;1254:87–103. [DOI] [PubMed] [Google Scholar]
- 4.Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells, A to Z. Allergy. 2021. [Google Scholar]
- 5.Ray A, Wang L, Dittel BN. IL-10-independent regulatory B-cell subsets and mechanisms of action. Int Immunol. 2015;27(10):531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas OL, Probstel AK, Porfilio EA, et al. Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Cell. 2019;176(3):610–624 e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Probstel AK, Zhou X, Baumann R, et al. Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. 2020;5(53). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalapour S, Font-Burgada J, Di Caro G, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521(7550):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalapour S, Lin XJ, Bastian IN, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551(7680):340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdin N, Van Kooten C, Galibert L, et al. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J Immunol. 1995;154(6):2533–2544. [PubMed] [Google Scholar]
- 11.Heine G, Drozdenko G, Grun JR, Chang HD, Radbruch A, Worm M. Autocrine IL-10 promotes human B-cell differentiation into IgM- or IgG-secreting plasmablasts. Eur J Immunol. 2014;44(6):1615–1621. [DOI] [PubMed] [Google Scholar]
- 12.Blanc P, Moro-Sibilot L, Barthly L, et al. Mature IgM-expressing plasma cells sense antigen and develop competence for cytokine production upon antigenic challenge. Nat Commun. 2016;7:13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshizaki A, Miyagaki T, DiLillo DJ, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Yang J, Chu Y, et al. T follicular helper cells mediate expansion of regulatory B cells via IL-21 in Lupus-prone MRL/lpr mice. PLoS One. 2013;8(4):e62855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yang J, Chu Y, et al. T follicular helper cells and regulatory B cells dynamics in systemic lupus erythematosus. PLoS One. 2014;9(2):e88441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banko Z, Pozsgay J, Szili D, et al. Induction and Differentiation of IL-10-Producing Regulatory B Cells from Healthy Blood Donors and Rheumatoid Arthritis Patients. J Immunol. 2017;198(4):1512–1520. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Xu W, Shao Q, Ding Q. miR-21 silencing ameliorates experimental autoimmune encephalomyelitis by promoting the differentiation of IL-10-producing B cells. Oncotarget. 2017;8(55):94069–94079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y, Ge W, Ma Y, et al. miR-155 Regulates IL-10-Producing CD24(hi)CD27(+) B Cells and Impairs Their Function in Patients with Crohn’s Disease. Front Immunol. 2017;8:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerqueira C, Manfroi B, Fillatreau S. IL-10-producing regulatory B cells and plasmocytes: Molecular mechanisms and disease relevance. Semin Immunol. 2019;44:101323. [DOI] [PubMed] [Google Scholar]
- 20.Rangaswamy US, Speck SH. Murine gammaherpesvirus M2 protein induction of IRF4 via the NFAT pathway leads to IL-10 expression in B cells. PLoS Pathog. 2014;10(1):e1003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matar CG, Rangaswamy US, Wakeman BS, Iwakoshi N, Speck SH. Murine gammaherpesvirus 68 reactivation from B cells requires IRF4 but not XBP-1. J Virol. 2014;88(19):11600–11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YH, Tsai DY, Ko YA, et al. Blimp-1 Contributes to the Development and Function of Regulatory B Cells. Front Immunol. 2019;10:1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15(3):160–171. [DOI] [PubMed] [Google Scholar]
- 24.Chu VT, Berek C. The establishment of the plasma cell survival niche in the bone marrow. Immunol Rev. 2013;251(1):177–188. [DOI] [PubMed] [Google Scholar]
- 25.Khodadadi L, Cheng Q, Radbruch A, Hiepe F. The Maintenance of Memory Plasma Cells. Front Immunol. 2019;10:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilmore JR, Allman D. Here, There, and Anywhere? Arguments for and against the Physical Plasma Cell Survival Niche. J Immunol. 2017;199(3):839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson RP, McGettigan SE, Dang VD, et al. IgM Plasma Cells Reside in Healthy Skin and Accumulate with Chronic Inflammation. J Invest Dermatol. 2019;139(12):2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollok K, Mothes R, Ulbricht C, et al. The chronically inflamed central nervous system provides niches for long-lived plasma cells. Acta Neuropathol Commun. 2017;5(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krumbholz M, Theil D, Derfuss T, et al. BAFF is produced by astrocytes and upregulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 2005;201(2):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69(3):1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363–372. [DOI] [PubMed] [Google Scholar]
- 32.Wolf AI, Mozdzanowska K, Quinn WJ 3rd, et al. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. J Clin Invest. 2011;121(10):3954–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemke A, Kraft M, Roth K, Riedel R, Lammerding D, Hauser AE. Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice. Mucosal Immunol. 2016;9(1):83–97. [DOI] [PubMed] [Google Scholar]
- 34.Kunisawa J, Gohda M, Hashimoto E, et al. Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice. Nat Commun. 2013;4:1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahnsen FL, Baekkevold ES, Hov JR, Landsverk OJ. Do Long-Lived Plasma Cells Maintain a Healthy Microbiota in the Gut? Trends Immunol. 2018;39(3):196–208. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki-Yamazaki N, Yanobu-Takanashi R, Okamura T, Takaki S. IL-10 production in murine IgM(+) CD138(hi) cells is driven by Blimp-1 and downregulated in class-switched cells. Eur J Immunol. 2017;47(3):493–503. [DOI] [PubMed] [Google Scholar]
- 37.Meng L, Almeida LN, Clauder AK, et al. Bone Marrow Plasma Cells Modulate Local Myeloid-Lineage Differentiation via IL-10. Front Immunol. 2019;10:1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumoto M, Baba A, Yokota T, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41(6):1040–1051. [DOI] [PubMed] [Google Scholar]
- 39.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 40.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagen M, Derudder E. Inflammation and the Alteration of B-Cell Physiology in Aging. Gerontology. 2020;66(2):105–113. [DOI] [PubMed] [Google Scholar]
- 42.Shen P, Roch T, Lampropoulou V, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507(7492):366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabatino JJ Jr., Probstel AK, Zamvil SS B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20(12):728–745. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Xia Z, Lan Q, et al. BAFF promotes Th17 cells and aggravates experimental autoimmune encephalomyelitis. PLoS One. 2011;6(8):e23629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berer K, Gerdes LA, Cekanaviciute E, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017;114(40):10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114(40):10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan TG, Sefik E, Geva-Zatorsky N, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci U S A. 2016;113(50):E8141–E8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kappos L, Hartung HP, Freedman MS, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol. 2014;13(4):353–363. [DOI] [PubMed] [Google Scholar]
- 49.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688. [DOI] [PubMed] [Google Scholar]
- 50.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. 2017;376(3):221–234. [DOI] [PubMed] [Google Scholar]
- 51.Felgenhauer K, Reiber H. The diagnostic significance of antibody specificity indices in multiple sclerosis and herpes virus induced diseases of the nervous system. Clin Investig. 1992;70(1):28–37. [DOI] [PubMed] [Google Scholar]
- 52.Deisenhammer F, Zetterberg H, Fitzner B, Zettl UK. The Cerebrospinal Fluid in Multiple Sclerosis. Front Immunol. 2019;10:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu X, Graner M, Kennedy PGE, Liu Y. The Role of Antibodies in the Pathogenesis of Multiple Sclerosis. Front Neurol. 2020;11:533388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Taeye SW, Rispens T, Vidarsson G. The Ligands for Human IgG and Their Effector Functions. Antibodies (Basel). 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geherin SA, Gomez D, Glabman RA, Ruthel G, Hamann A, Debes GF. IL-10+ Innate-like B Cells Are Part of the Skin Immune System and Require alpha4beta1 Integrin To Migrate between the Peritoneum and Inflamed Skin. J Immunol. 2016;196(6):2514–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geherin SA, Fintushel SR, Lee MH, et al. The skin, a novel niche for recirculating B cells. J Immunol. 2012;188(12):6027–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saul L, Ilieva KM, Bax HJ, et al. IgG subclass switching and clonal expansion in cutaneous melanoma and normal skin. Sci Rep. 2016;6:29736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aira LE, Debes GF. Skin-homing regulatory B cells required for suppression of cutaneous inflammation. J Invest Dermatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Metze D, Jurecka W, Gebhart W, Schmidt J, Mainitz M, Niebauer G. Immunohistochemical demonstration of immunoglobulin A in human sebaceous and sweat glands. J Invest Dermatol. 1989;92(1):13–17. [DOI] [PubMed] [Google Scholar]
- 60.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosser EC, Oleinika K, Tonon S, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat Med. 2014;20(11):1334–1339. [DOI] [PubMed] [Google Scholar]
- 62.Sun H, Zhang Y, Song W, et al. IgM(+)CD27(+) B cells possessed regulatory function and represented the main source of B cell-derived IL-10 in the synovial fluid of osteoarthritis patients. Hum Immunol. 2019;80(4):263–269. [DOI] [PubMed] [Google Scholar]
- 63.Mishima Y, Oka A, Liu B, et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J Clin Invest. 2019;129(9):3702–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maseda D, Candando KM, Smith SH, et al. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-gamma+CD4+ T cell numbers during colitis development in mice. J Immunol. 2013;191(5):2780–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wouters MCA, Nelson BH. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin Cancer Res. 2018;24(24):6125–6135. [DOI] [PubMed] [Google Scholar]
- 66.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20(5):294–307. [DOI] [PubMed] [Google Scholar]
- 67.Coronella JA, Spier C, Welch M, et al. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol. 2002;169(4):1829–1836. [DOI] [PubMed] [Google Scholar]
- 68.Montfort A, Pearce O, Maniati E, et al. A Strong B-cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin Cancer Res. 2017;23(1):250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66(15):7741–7747. [DOI] [PubMed] [Google Scholar]
- 70.Pylayeva-Gupta Y, Das S, Handler JS, et al. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016;6(3):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Morgan R, Chen C, et al. Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses. Int Immunol. 2016;28(9):423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olkhanud PB, Damdinsuren B, Bodogai M, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71(10):3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. 2016;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bodogai M, Moritoh K, Lee-Chang C, et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res. 2015;75(17):3456–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Locher C, Conforti R, Aymeric L, et al. Desirable cell death during anticancer chemotherapy. Ann N Y Acad Sci. 2010;1209:99–108. [DOI] [PubMed] [Google Scholar]
- 76.van Vlasselaer P, Punnonen J, de Vries JE. Transforming growth factor-beta directs IgA switching in human B cells. J Immunol. 1992;148(7):2062–2067. [PubMed] [Google Scholar]
- 77.Stavnezer J, Kang J. The surprising discovery that TGF beta specifically induces the IgA class switch. J Immunol. 2009;182(1):5–7. [DOI] [PubMed] [Google Scholar]
- 78.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11(6):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3(10):822–829. [DOI] [PubMed] [Google Scholar]
- 80.Biswas S, Mandal G, Payne KK, et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature. 2021;591(7850):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madan R, Demircik F, Surianarayanan S, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183(4):2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dasgupta S, Dasgupta S, Bandyopadhyay M. Regulatory B cells in infection, inflammation, and autoimmunity. Cell Immunol. 2020;352:104076. [DOI] [PubMed] [Google Scholar]
- 83.Horikawa M, Weimer ET, DiLillo DJ, et al. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol. 2013;190(3):1158–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dai YC, Wang WD, Zhang JA, et al. MTB driven B cells producing IL-35 and secreting high level of IL-10 in the patients with active pulmonary tuberculosis. Mol Immunol. 2019;112:175–181. [DOI] [PubMed] [Google Scholar]
- 85.Schaut RG, Lamb IM, Toepp AJ, et al. Regulatory IgDhi B Cells Suppress T Cell Function via IL-10 and PD-L1 during Progressive Visceral Leishmaniasis. J Immunol. 2016;196(10):4100–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neves P, Lampropoulou V, Calderon-Gomez E, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33(5):777–790. [DOI] [PubMed] [Google Scholar]
- 87.Wilmore JR, Jones DD, Allman D. Protocol for improved resolution of plasma cell subpopulations by flow cytometry. Eur J Immunol. 2017;47(8):1386–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pracht K, Meinzinger J, Daum P, et al. A new staining protocol for detection of murine antibody-secreting plasma cell subsets by flow cytometry. Eur J Immunol. 2017;47(8):1389–1392. [DOI] [PubMed] [Google Scholar]
- 89.Barr TA, Brown S, Mastroeni P, Gray D. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol. 2010;185(5):2783–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xue D, Kaufman GN, Dembele M, et al. Semaphorin 4C Protects against Allergic Inflammation: Requirement of Regulatory CD138+ Plasma Cells. J Immunol. 2017;198(1):71–81. [DOI] [PubMed] [Google Scholar]
- 91.Xue D, Desjardins M, Kaufman GN, et al. Semaphorin 4C: A Novel Component of B-Cell Polarization in Th2-Driven Immune Responses. Front Immunol. 2016;7:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15(3):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385(9976):1460–1471. [DOI] [PubMed] [Google Scholar]
- 94.van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317(5844):1554–1557. [DOI] [PubMed] [Google Scholar]
- 95.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116(3):608–613. [DOI] [PubMed] [Google Scholar]
- 96.James LK, Shamji MH, Walker SM, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127(2):509–516 e501-505. [DOI] [PubMed] [Google Scholar]
- 97.Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129(5):1290–1296. [DOI] [PubMed] [Google Scholar]
- 98.van de Veen W, Stanic B, Yaman G, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131(4):1204–1212. [DOI] [PubMed] [Google Scholar]
- 99.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160(7):3555–3561. [PubMed] [Google Scholar]
- 100.Punnonen J, de Waal Malefyt R, van Vlasselaer P, Gauchat JF, de Vries JE. IL-10 and viral IL-10 prevent IL-4-induced IgE synthesis by inhibiting the accessory cell function of monocytes. J Immunol. 1993;151(3):1280–1289. [PubMed] [Google Scholar]
- 101.Lin AA, Freeman AF, Nutman TB. IL-10 Indirectly Downregulates IL-4-Induced IgE Production by Human B Cells. Immunohorizons. 2018;2(11):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Control CfD. Asthma in the US. 2011; https://www.cdc.gov/vitalsigns/asthma/index.html.Accessed April 19, 2021.
- 103.Brynjolfsson SF, Persson Berg L, Olsen Ekerhult T, et al. Long-Lived Plasma Cells in Mice and Men. Front Immunol. 2018;9:2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lino AC, Dang VD, Lampropoulou V, et al. LAG-3 Inhibitory Receptor Expression Identifies Immunosuppressive Natural Regulatory Plasma Cells. Immunity. 2018;49(1):120–133 e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patel SA, Minn AJ. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity. 2018;48(3):417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Silva NS, Simonetti G, Heise N, Klein U. The diverse roles of IRF4 in late germinal center B-cell differentiation. Immunol Rev. 2012;247(1):73–92. [DOI] [PubMed] [Google Scholar]
- 107.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25(2):225–236. [DOI] [PubMed] [Google Scholar]
- 108.Ochiai K, Maienschein-Cline M, Simonetti G, et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity. 2013;38(5):918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shaffer AL, Lin KI, Kuo TC, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17(1):51–62. [DOI] [PubMed] [Google Scholar]
- 110.Lin KI, Angelin-Duclos C, Kuo TC, Calame K. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol Cell Biol. 2002;22(13):4771–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cretney E, Xin A, Shi W, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12(4):304–311. [DOI] [PubMed] [Google Scholar]
- 112.Ahyi AN, Chang HC, Dent AL, Nutt SL, Kaplan MH. IFN regulatory factor 4 regulates the expression of a subset of Th2 cytokines. J Immunol. 2009;183(3):1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martins GA, Cimmino L, Shapiro-Shelef M, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7(5):457–465. [DOI] [PubMed] [Google Scholar]
- 114.Maseda D, Smith SH, DiLillo DJ, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188(3):1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu CR, Choi JK, Uche AN, Egwuagu CE. Production of IL-35 by Bregs is mediated through binding of BATF-IRF-4-IRF-8 complex to il12a and ebi3 promoter elements. J Leukoc Biol. 2018;104(6):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miura M, Hasegawa N, Noguchi M, Sugimoto K, Touma M. The atypical IkappaB protein IkappaB(NS) is important for Toll-like receptor-induced interleukin-10 production in B cells. Immunology. 2016;147(4):453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karnell JL, Ettinger R. The Interplay of IL-21 and BAFF in the Formation and Maintenance of Human B Cell Memory. Front Immunol. 2012;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. [DOI] [PubMed] [Google Scholar]
- 119.Ozaki K, Spolski R, Ettinger R, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173(9):5361–5371. [DOI] [PubMed] [Google Scholar]
- 120.Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175(12):7867–7879. [DOI] [PubMed] [Google Scholar]
- 121.Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298(5598):1630–1634. [DOI] [PubMed] [Google Scholar]
- 122.Kuchen S, Robbins R, Sims GP, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179(9):5886–5896. [DOI] [PubMed] [Google Scholar]
- 123.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179(12):8180–8190. [DOI] [PubMed] [Google Scholar]
- 124.Wang S, Wang J, Kumar V, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun. 2018;9(1):1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118(5):1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miyagaki T, Fujimoto M, Sato S. Regulatory B cells in human inflammatory and autoimmune diseases: from mouse models to clinical research. Int Immunol. 2015;27(10):495–504. [DOI] [PubMed] [Google Scholar]
- 127.Defrance T, Vanbervliet B, Briere F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175(3):671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kobayashi N, Nagumo H, Agematsu K. IL-10 enhances B-cell IgE synthesis by promoting differentiation into plasma cells, a process that is inhibited by CD27/CD70 interaction. Clin Exp Immunol. 2002;129(3):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choe J, Choi YS. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur J Immunol. 1998;28(2):508–515. [DOI] [PubMed] [Google Scholar]
- 130.Pinto D, Montani E, Bolli M, et al. A functional BCR in human IgA and IgM plasma cells. Blood. 2013;121(20):4110–4114. [DOI] [PubMed] [Google Scholar]
- 131.Reynolds AE, Kuraoka M, Kelsoe G. Natural IgM is produced by CD5- plasma cells that occupy a distinct survival niche in bone marrow. J Immunol. 2015;194(1):231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bachelerie F, Ben-Baruch A, Burkhardt AM, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66(1):1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mueller CG, Boix C, Kwan WH, et al. Critical role of monocytes to support normal B cell and diffuse large B cell lymphoma survival and proliferation. J Leukoc Biol. 2007;82(3):567–575. [DOI] [PubMed] [Google Scholar]
- 134.Sullivan NL, Eickhoff CS, Zhang X, Giddings OK, Lane TE, Hoft DF. Importance of the CCR5-CCL5 axis for mucosal Trypanosoma cruzi protection and B cell activation. J Immunol. 2011;187(3):1358–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Keane C, Law SC, Gould C, et al. LAG3: a novel immune checkpoint expressed by multiple lymphocyte subsets in diffuse large B-cell lymphoma. Blood Adv. 2020;4(7):1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maruhashi T, Sugiura D, Okazaki IM, Okazaki T. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tonon S, Mion F, Dong J, et al. IL-10-producing B cells are characterized by a specific methylation signature. Eur J Immunol. 2019;49(8):1213–1225. [DOI] [PubMed] [Google Scholar]
- 138.Sun J, Wang J, Pefanis E, et al. Transcriptomics Identify CD9 as a Marker of Murine IL-10-Competent Regulatory B Cells. Cell Rep. 2015;13(6):1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ambros V The functions of animal microRNAs. Nature. 2004;431(7006):350–355. [DOI] [PubMed] [Google Scholar]
- 140.Meinzinger J, Jack HM, Pracht K. miRNA meets plasma cells “How tiny RNAs control antibody responses”. Clin Immunol. 2018;186:3–8. [DOI] [PubMed] [Google Scholar]
- 141.Barnes NA, Stephenson S, Cocco M, Tooze RM, Doody GM. BLIMP-1 and STAT3 counterregulate microRNA-21 during plasma cell differentiation. J Immunol. 2012;189(1):253–260. [DOI] [PubMed] [Google Scholar]
- 142.Lu D, Nakagawa R, Lazzaro S, et al. The miR-155-PU.1 axis acts on Pax5 to enable efficient terminal B cell differentiation. J Exp Med. 2014;211(11):2183–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tangye SG. Staying alive: regulation of plasma cell survival. Trends Immunol. 2011;32(12):595–602. [DOI] [PubMed] [Google Scholar]
- 144.Mohd Jaya FN, Garcia SG, Borras FE, Chan GCF, Franquesa M. Paradoxical role of Breg-inducing cytokines in autoimmune diseases. J Transl Autoimmun. 2019;2:100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. 2004;200(6):805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324(6092):73–76. [DOI] [PubMed] [Google Scholar]
- 147.Hirano T IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33(3):127–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129(Pt 1):200–211. [DOI] [PubMed] [Google Scholar]
- 149.McCandless EE, Piccio L, Woerner BM, et al. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008;172(3):799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194:48–60. [DOI] [PubMed] [Google Scholar]
- 151.Baert L, Benkhoucha M, Popa N, et al. A proliferation-inducing ligand-mediated anti-inflammatory response of astrocytes in multiple sclerosis. Ann Neurol. 2019;85(3):406–420. [DOI] [PubMed] [Google Scholar]
- 152.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Batten M, Fletcher C, Ng LG, et al. TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphoma. J Immunol. 2004;172(2):812–822. [DOI] [PubMed] [Google Scholar]
- 154.McCarthy DD, Kujawa J, Wilson C, et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121(10):3991–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.den Hartog G, van Osch TLJ, Vos M, et al. BAFF augments IgA2 and IL-10 production by TLR7/8 stimulated total peripheral blood B cells. Eur J Immunol. 2018;48(2):283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saulep-Easton D, Vincent FB, Quah PS, et al. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia. 2016;30(1):163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Matsushita T, Kobayashi T, Mizumaki K, et al. BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci Adv. 2018;4(7):eaas9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hua C, Audo R, Yeremenko N, et al. A proliferation inducing ligand (APRIL) promotes IL-10 production and regulatory functions of human B cells. J Autoimmun. 2016;73:64–72. [DOI] [PubMed] [Google Scholar]
- 159.Fehres CM, van Uden NO, Yeremenko NG, et al. APRIL Induces a Novel Subset of IgA(+) Regulatory B Cells That Suppress Inflammation via Expression of IL-10 and PD-L1. Front Immunol. 2019;10:1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were generated or analyzed in this review.


