Abstract
Background
Dysregulated lipid metabolism is associated with more aggressive pathology and poorer prognosis in prostate cancer (PC). The primary aim of the study is to assess the relationship between the plasma lipidome and clinical outcomes in localised and metastatic PC. The secondary aim is to validate a prognostic circulating 3-lipid signature specific to metastatic castration-resistant PC (mCRPC).
Patients and methods
Comprehensive lipidomic analysis was performed on pre-treatment plasma samples from men with localised PC (N=389), metastatic hormone-sensitive PC (mHSPC)(N=44), or mCRPC (validation cohort, N=137). Clinical outcomes from our previously published mCRPC cohort (N=159) that was used to derive the prognostic circulating 3-lipid signature, were updated. Associations between circulating lipids and clinical outcomes were examined by Cox regression and latent class analysis.
Results
Circulating lipid profiles featuring elevated levels of ceramide species were associated with metastatic relapse in localised PC (HR 5.80, 95% CI 3.04–11.1, P=1×10−6), earlier testosterone suppression failure in mHSPC (HR 3.70, 95% CI 1.37–10.0, P=0.01), and shorter overall survival in mCRPC (HR 2.54, 95% CI 1.73–3.72, P=1×10−6). The prognostic significance of circulating lipid profiles in localised PC was independent of standard clinicopathological and metabolic factors (P<0.0002). The 3-lipid signature was verified in the mCRPC validation cohort (HR 2.39, 95%CI 1.63–3.51, P=1×10−5).
Conclusions
Elevated circulating ceramide species are associated with poorer clinical outcomes across the natural history of PC. These clinically actionable lipid profiles could be therapeutically targeted in prospective clinical trials to potentially improve PC outcomes.
INTRODUCTION
Lethal prostate cancer (PC) remains a global challenge with 359,000 associated deaths (2018)1. Therapies such as novel anti-androgens, taxanes, PARP inhibitors and targeted radioisotopes have significantly increased survival in metastatic disease2. However, long term control and cure of lethal PC will require therapeutic approaches that address multiple hallmarks of cancer such as the neoplastic epithelium, the tumor microenvironment and systemic metabolic factors including lipid metabolism, all of which contribute to cancer progression and treatment resistance3.
Epidemiological and molecular studies strongly indicate that perturbations in lipid metabolism contribute to the development of aggressive PC. For example, obesity is associated with higher rates of relapse after local therapy and PC-specific mortality4. Enhanced de novo lipogenesis in PC is well-documented and has underpinned recent efforts to target lipogenesis clinically5,6. Elevated circulating triglycerides are associated with increased risk of recurrence after radical prostatectomy, but elevated circulating cholesterol was only associated with recurrence in men with dyslipidemia7 despite the association of statin usage and improved PC outcomes8–10, suggesting that the beneficial effects of statin in PC is not due to its cholesterol-lowering effects. The association of other circulating lipids with PC outcomes is under-studied, despite the presence of over 500 unique lipid species in blood11 and the emerging role of other lipids in cancer pathogenesis.
Recently, our group undertook comprehensive plasma lipid profiling in men with metastatic castration-resistant prostate cancer (mCRPC), demonstrating that higher levels of sphingolipids such as ceramide and sphingomyelin species were associated with shorter overall survival (OS)12. However, the study raised further questions such as whether the prognostic lipidomic profile is only present in the mCRPC stage of PC. Ceramides are well-known for their inflammatory and metabolic dysfunction in diabetes and cardiovascular disease13, where elevated plasma ceramides is associated with increased risk of cardiovascular death independent of other commonly used lipid markers14 . There is now evidence suggesting that exogenous sphingolipids can alter PC cell metabolism and promote PC growth15.
We hypothesise that circulating lipid profiles that include ceramides are associated with poor prognosis and therapeutic resistance, and these profiles are metabolically actionable through drug and lifestyle interventions. The main aim of this study is to comprehensively profile the circulating lipidome across the natural history of PC spanning localised PC, metastatic hormone-sensitive PC (mHSPC) and mCRPC. The secondary aim is to validate our previously published prognostic mCRPC lipid signature12 in an independent mCRPC cohort.
PATIENTS AND METHODS
Study population
Plasma samples of localised PC were collected from fasted patients prior to radical prostatectomy at the Royal Adelaide Hospital (Adelaide, South Australia) from 2005 to 2016, by the Australian Prostate Cancer BioResource. Plasma samples from mHSPC patients (prior to testosterone suppression) and mCRPC patients (prior to first line chemotherapy) were sourced from an advanced PC biomarker registry at the Mayo Clinic (Rochester, MN, United States) from 2009 to 201416. This mCRPC cohort is referred to as the ‘mCRPC validation cohort’. Plasma collection methods are described in Supplementary S1.1.
Clinical outcomes of the Phase 1 and 2 cohorts of mCRPC patients from Australian hospitals in our previous lipidomic profiling study12 were updated after longer follow-up, and combined for increased statistical power in the analysis of progression-free-survival. This combined cohort is referred to as the ‘mCRPC discovery cohort’.
All participants provided written informed consent for blood collection and research (Royal Adelaide Hospital Human Ethics Committee 041011f; Mayo Clinic Institutional Review Board 09–1889; Royal Prince Alfred Hospital Human Research Ethics X19-0320; Australian-New Zealand Clinical Trials Registry ACTRN12607000077460).
Plasma lipidomic analysis
Lipidomic analysis of plasma samples was performed by liquid chromatography-mass spectrometry (LC-MS)17 (Supplementary Information S1). The mHSPC and mCRPC samples were analysed together, approximately 2 years after the localised PC samples, using a different LC-MS instrument with a larger coverage of lipids (Figure 1).
FIGURE 1.
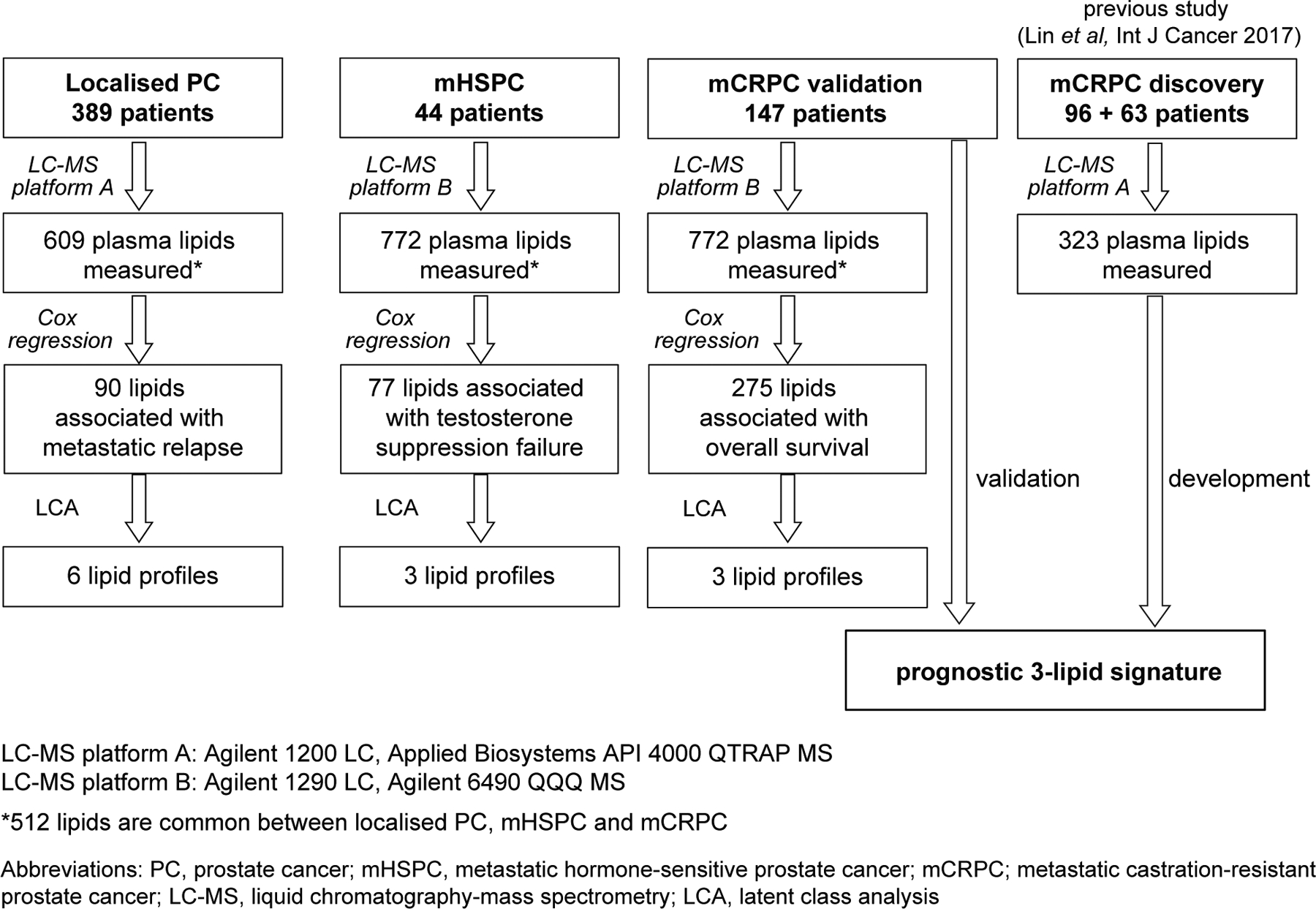
Prostate cancer study cohorts and analysis strategy.
Statistical analyses
The association of lipid levels (log2 of pmol/ml) with clinical outcome was determined by univariable Cox regression (R package survival, v2.41-3). Unique lipid profiles were identified by latent class analysis (LCA) of the levels of prognostic lipids categorised into quartiles (R package poLCA v1.4.1)18 (Supplementary Information S1.7). T-tests assessing lipid levels between the different lipid profiles were performed with R package multtest v2.24.0. Cox regression and t-test P-values<0.05 were considered as statistically significant.
RESULTS
Localised PC
The localised PC cohort consisted of 389 men, of whom 10% developed metastatic relapse at a median follow-up of 7.5 years (first quartile[Q1] 6.1, third quartile[Q3] 9.0) (Table 1, Figure 1).
TABLE 1.
Clinical characteristics of prostate cancer cohorts (n.d, no data; n.a, not applicable).
| Median [first quartile, third quartile] or number [% of cohort] | ||||
|---|---|---|---|---|
| Localised | mHSPC | CRPC discovery | CRPC validation | |
| Number of men | 389 | 44 | 159 | 137 |
| Median age, years | 63 [59, 68] | 67 [63, 78] | 70 [64,75] | 72 [67, 77] |
| Median baseline PSA, μg/l | 7.0 [5.2, 9.2] | 25 [7,43], 9% n.d | 111 [40,407], 2% n.d | 16 [4, 66], 5% n.d |
| Median alkaline phosphatase, U/l | n/a | 98 [72,131], 23% n.d | 135 [93,299], 4% n.d | 92 [68, 131], 4% n.d |
| Median haemoglobin, g/l | n/a | 139 [136,147] 93% n.d | 125 [111,135], 2% n.d | 127 [121,137], 69% n.d |
| Median lactase dehydrogenase | n/a | 172 [166,196], 82% n.d | n.d | 188 [159,223], 53% n.d |
| Median body mass index | 27 [25, 30], 55% n.d | 29 [26, 32] 11% n.d | 27 [25, 31] 23% n.d | 30 [27, 34], 3% n.d |
| Metastatic relapse | 40 [10%] | n/a | n/a | n/a |
| Biochemical relapse | 157 [40%] | n/a | n/a | n/a |
| Testosterone suppression failure | n/a | 28 [64%] | n/a | n/a |
| Dead | 0 | 24 [55%] | 124 [78%] | 122 [89%] |
| Gleason grade | ||||
| 7 | 274 [70%] | 10 [23%] | 34 [21%] | 49 [36%] |
| ≥9 | 22 [5.6%] | 16 [36%] | 52 [33%] | 41 [30%] |
| Pathological stage | n/a | n.a | n/a | |
| PT2 | 205 [53%] | |||
| Extraprostatic extension | 177 [46%] | n/a | n/a | n/a |
| Positive surgical margin | 143 [37%] | n/a | n/a | n/a |
| Seminal vesicle invasion | 45 [12%] | n/a | n/a | n/a |
| Metastasis | n/a | |||
| Visceral | 0 | 59[37%](soft tissue) | 10 [7%] | |
| mHSPC therapy | n/a | n/a | n/a | |
| With docetaxel | 2 [5%] | |||
| CRPC 1st line therapy | n/a | n/a | ||
| Docetaxel +/− atrasentan | 0 | 3 [2%] | ||
| Cisplatin + etoposide | 0 | 1 [0.7%] | ||
| Abiraterone | 0 | 3 [2%] | ||
| Sipuleucel-T | 0 | 1 [0.7%] | ||
| CRPC 2nd line therapy | n/a | n/a | ||
| Enzalutamide | 3 [2%] | 14 [10%] | ||
| Mitoxantrone | 13 [8%] | 1 [0.7%] | ||
| Sipuleucel-T | 0 | 2 [1%] | ||
| Diabetes | 12 [3.1%], 36% n.d | n.d | 23 [14%], 4% n,d | n.d |
| Statin medication | 81 [21%], 36% n.d | n.d | 49 [31%], 4% n.d | n.d |
| Hypertension medication | 119 [31%], 36% n.d | n.d | n.d | n.d |
Metastasis-free survival is an established surrogate for OS in localised PC unlike biochemical relapse19, thus we focused on metastatic relapse as the endpoint. The levels of 90 lipids were significantly associated with metastatic relapse (Figures 1 & 2A, Supplementary List A). The top 20 significant lipid species mainly consisted of ceramide, acylcarnitine, alkenylphosphatidylethanolamine and alkylphosphatidylethanolamine (Figure 2A, Supplementary List A). Elevated levels of ceramide, acylcarnitine and triacylglycerol species; and lower levels of alkenylphosphatidylethanolamine and alkylphosphatidylethanolamine species were associated with a shorter time to metastatic relapse (Figure 2A).
FIGURE 2. Association of plasma lipids with clinical outcomes in localised PC, mHSPC and mCRPC.
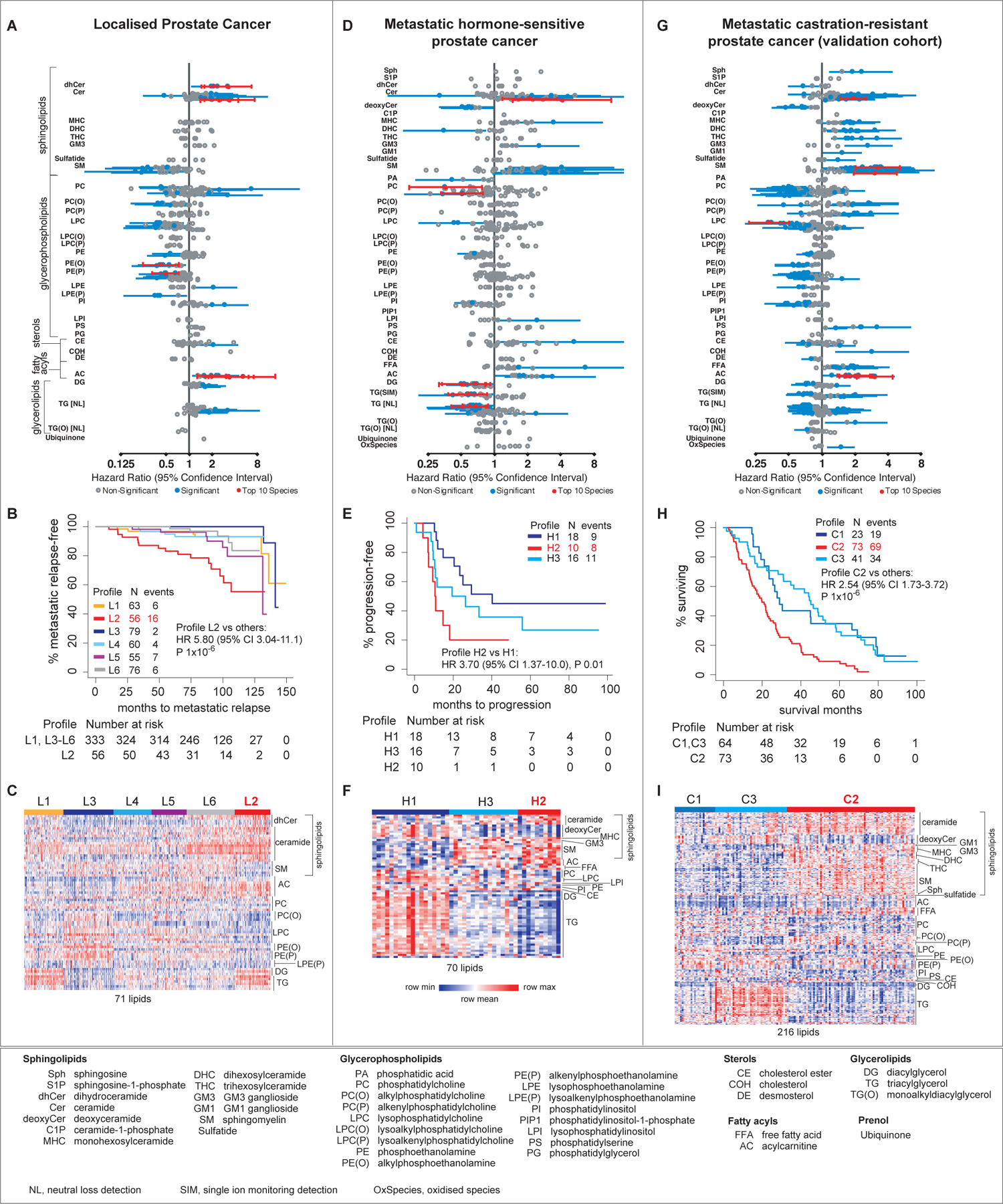
Forest plots of the hazard ratios of levels of plasma lipids (A,D,G); Kaplan-Meir curves (B,E,H) and heatmaps (C,F,I) of lipid profiles associated with clinical outcomes. The heatmaps show the levels of prognostic lipids that were significantly different between the profile with the worse outcome compared to the other profiles, except for mHSPC where the comparison is between Profile H2 and H1.
LCA of the 90 prognostic lipids classified the cohort into six groups with distinct lipid profiles, referred to as Profile L1 to L6 (Figure 2B). These groups had different clinical outcomes – men with Profile L2 had the highest rate of metastatic relapse (Hazard Ratio [HR] 5.80, 95% CI 3.04–11.1, P=1×10−6), whereas men with Profile L3 had the lowest rate of metastatic relapse (Figure 2B). Men with Profile L2 had significantly higher plasma levels of the prognostic species of ceramide, dihydroceramide, acylcarnitine and triacylglycerol, and lower levels of alkenylphosphatidylethanolamine and alkylphosphatidylethanolamine species than other men (Figure 2C, Supplementary List A). Interestingly, the lipid profile of men with Profile L2 was the inverse of men with Profile L3, who had the lowest rate of metastatic relapse (Figure 2C).
The association of lipid profiles with metastatic relapse was independent of standard clinicopathological factors (P=6×10−6, Model 1 in Table 2) and key metabolic indicators (P=2×10−4 when modelled with diabetes, Model 2 in Table 2; P≤3×10−5 when modelled with other indicators in Supplementary Information S2). The addition of the lipid profile to a Cox regression model of Gleason Score and pathological stage (Concordance Index [C-index] 0.83, P=2×10−22) improved the model’s predictive ability (C-index 0.86, P=4×10−25). The lipid profile also improved the discriminatory value of the American Joint Committee on Cancer’s TNM staging, where men with Stage 3 cancer (highest TNM stage in the cohort) had a higher metastatic relapse rate if they had Profile L2 (HR 6.30, 95% CI 3.12–12.7, P=2×10−6)(Supplementary Figure S3.1).
TABLE 2.
Cox regression analyses of the association of metastatic relapse with lipid profiles, metabolic factors and clinicopathological factors in the localised prostate cancer cohort.
| Clinicopathological factor | case | event | Univariable cox regression | Multivariable cox regression | ||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Lipid profile Profile L2 vs others | 389 | 40 | 5.80 (3.04–11.1) | 1×10−7 | 4.79 (2.43–9.44) | 6×10−6 | 4.06 (1.94–8.56) | 2×10−4 |
| Gleason score ≤7 vs >7 | 389 | 40 | 10.5 (5.38–20.3) | 4×10−12 | 6.29 (3.00–13.2) | 1×10−6 | 6.25 (2.87–13.6) | 4×10−6 |
| P-stage PT2 vs PT3 | 389 | 40 | 14.3 (5.02–40.7) | 6×10−7 | 11.4 (3.91–33.3) | 8×10−6 | 5.63 (1.89–16.8) | 0.002 |
| Pre-operative PSA* | 389 | 40 | 1.05 (1.02–1.07) | 4×10−4 | 1.02 (0.99–1.05) | 0.2 | - | - |
| Surgical margin Positive vs negative | 389 | 40 | 1.72 (0.92–3.20) | 0.09 | - | - | - | - |
| Age* | 389 | 40 | 1.07 (1.01–1.13) | 0.01 | - | - | - | - |
| Diabetes Yes vs no | 251 | 33 | 5.25 (1.81–15.2) | 2×10−3 | - | - | 2.13 (0.67–6.73) | 0.2 |
| Statin usage Yes vs no | 251 | 33 | 1.70 (0.85–3.37) | 0.1 | - | - | - | - |
| Hypertension Yes vs no | 249 | 32 | 1.49 (0.74–3.00) | 0.3 | - | - | - | - |
continuous variable
mHSPC
The mHSPC cohort consisted of 44 men, of whom 64% developed resistance to testosterone suppression at a median follow-up of 4.4 years (Q1=3.1, Q3=6.8)(Table 1, Figure 1).
The levels of 77 lipids were significantly associated with testosterone suppression failure (Figures 1 & 2D, Supplementary List B). The top significant lipid species mainly consisted of ceramide, diacylglycerol and triacylglycerol. Higher levels of the ceramide species and lower levels of diacylglycerol and triacylglycerol species were associated with early testosterone suppression failure (Figure 2D, Supplementary List B).
LCA of the 77 prognostic lipids classified the cohort into three groups with distinct lipid profiles referred to as Profile H1 to H3 (Figure 2E). Men with Profile H2 had the shortest time to testosterone suppression failure, whereas men with Profile H1 had the best outcome (Profile H2 versus H1: HR 3.70, 95% CI 1.37–10.0, P=0.01). Men with Profile H2 had significantly higher levels of the prognostic species of ceramide, sphingomyelin and acylcarnitine; and lower levels of prognostic species of deoxyceramide and triacylglycerol than men with Profile H1(Figure 2F).
mCRPC
The mCRPC validation cohort consisted of 137 men, of whom 51% subsequently received first-line chemotherapy and 20% were still alive at the end of the study period (Table 1). The median follow-up time was 26 months (Q1=14, Q3=46).
The levels of 275 lipids were significantly associated with OS (Figures 1 & 2G, Supplementary List C). The top 20 significant lipids mainly consisted of species of ceramide, sphingomyelin and acylcarnitine, where higher levels of these lipids were associated with shorter OS (Figure 2G, Supplementary List C).
LCA of these 275 prognostic lipids classified the cohort into three groups with distinct lipid profiles referred to as Profile C1 to C3. Men with Profile C2 had the shortest OS compared to the other groups (HR 2.54, 95% CI 1.73–3.72, P=1×10−6, Figure 2H). The levels of the ceramide, sphingomyelin, ganglioside, hexosylceramide and acylcarnitine species were significantly higher for men with Profile C2 compared to the other groups, whereas the levels of the species of deoxyceramide and triacylglycerol were lower in the poor prognostic group (Figure 2I). The mCRPC lipid profiles were independently associated with OS when modelled with age and BMI (P=1×10−5, Supplementary Table S4.1), or with PSA or alkaline phosphatase (P≤1×10−4, Supplementary Table S4.2).
External validation of a prognostic 3-lipid signature in mCRPC
The association of the lipid profiles of the mCRPC validation cohort with OS is consistent with that of our previously described mCRPC discovery cohort, where plasma levels of sphingolipid species of ceramide, sphingomyelin, ganglioside and hexosylceramide were associated with shorter OS12. The circulating 3-lipid signature of poor prognosis (ceramide(d18:1/24:1), sphingomyelin(d18:2/16:0), phosphatidylcholine(16:0/16:0))(Figure 3A) previously derived from our mCRPC discovery cohort12, was re-analysed with additional follow-up and retained prognostic ability (HR 2.80, 95% CI 1.89–4.13, P=1×10−6, Figure 3B). Furthermore, all patients had received docetaxel as first-line mCRPC therapy and those with the 3-lipid signature had a shorter time to PSA progression (HR 1.67, 95% CI 1.14–2.44, P=0.01, Figure 3C).
FIGURE 3. Prognostic mCRPC 3-lipid signature and ceramide species.
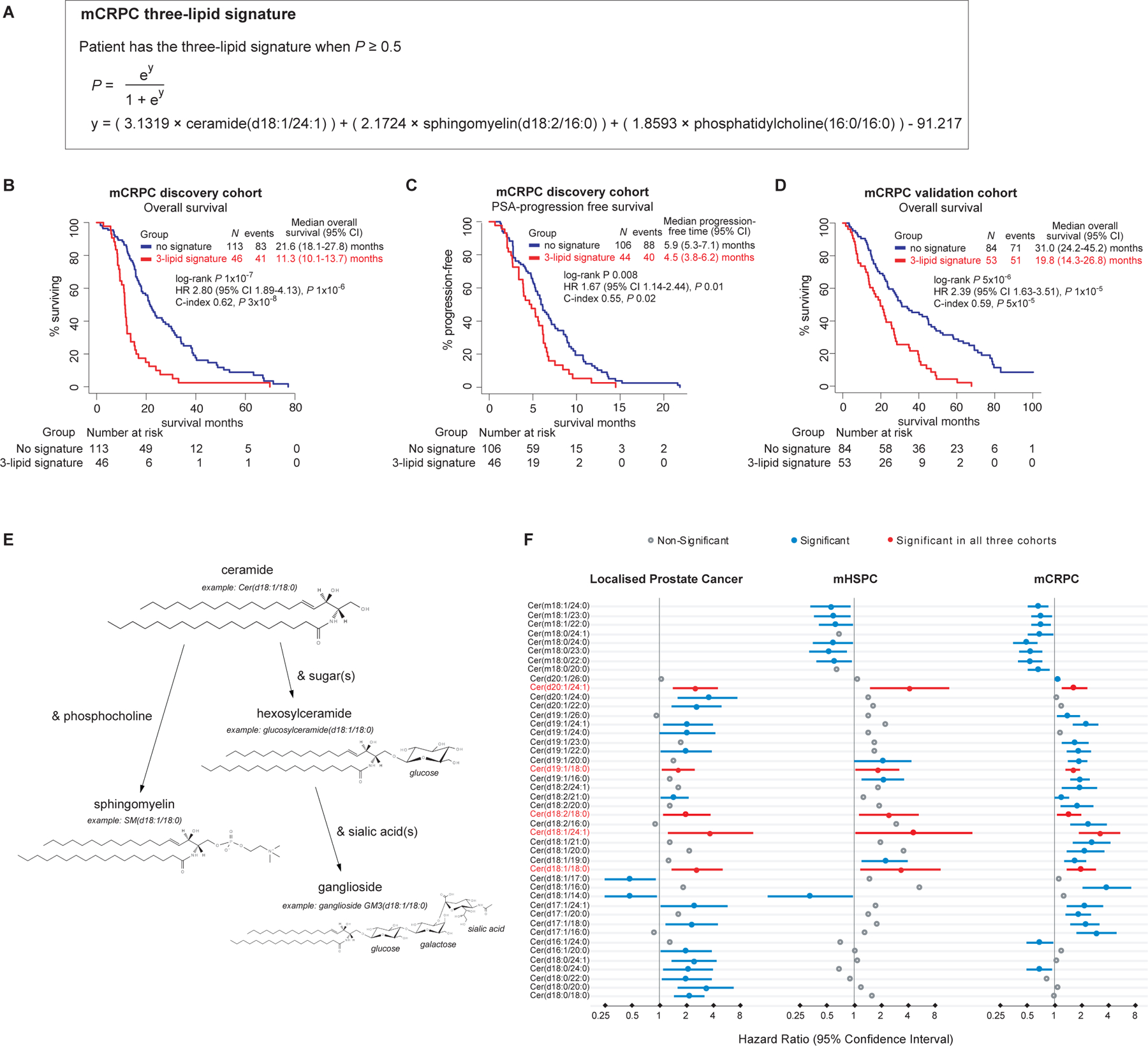
Formula of prognostic mCRPC 3-lipid signature derived in previous study of discovery cohort12 (A); overall survival (B) and PSA progression-free curves (C) of discovery cohort classified by the 3-lipid signature; overall survival curves of validation cohort classified by the 3-lipid signature (D); metabolism of ceramide and other sphingolipids (E); forest plots of the hazard ratios of ceramide species that are prognostic in localised PC, mHSPC or mCRPC validation cohorts (F).
Importantly, the 3-lipid signature was also associated with shorter OS in the independent, external mCRPC validation cohort (HR 2.39, 95% CI 1.63–3.51, P 1×10−5, Figure 3D). Patients with the 3-lipid signature had higher levels of sphingolipids including ceramide species, similar to the mCRPC discovery cohort (Supplementary Figure S4.1). Ceramide(d18:1/24:1) alone was comparable to the 3-lipid signature (HR 3.2 (95% CI 1.88–5.40, P 4×10−5) on univariable analysis (Supplementary Table S4.3), but the 3-lipid signature performed better in the prediction of 1 year survival (ROC analyses in Supplementary Figure S4.2). Post-treatment progression data was not available for the mCRPC validation cohort so PSA progression-free survival was not assessable.
Significant circulating lipids across all PC phases
Acylcarnitine and ceramide species were the only lipids associated with poorer outcomes in all phases of PC progression. Ceramide is of particular interest as dysregulation of ceramide metabolism has been implicated in cancer and other pathological conditions20. Interestingly, higher levels of several other circulating sphingolipids which can be linked to ceramide such as sphingomyelin, hexosylceramide, and ganglioside species (Figure 3E), were associated with worse outcomes in mHSPC and mCRPC but not localised PC (Figure 2).
The number of ceramide species identified as prognostic differed between localised PC, mHSPC and mCRPC although all ceramide species had the same direction of association – elevated ceramides were associated with poorer clinical outcomes (Figure 3F). Nevertheless, five prognostic ceramides were common between all three disease phases and included ceramide(d18:1/24:1), which is part of our previously published and now validated prognostic 3-lipid signature for mCRPC.
Overall, these findings suggest that aberrations in sphingolipid metabolism are associated with aggressive PC, beginning with ceramide metabolism in localised PC and progressively encompassing the metabolism of other sphingolipids over the natural history of the disease.
DISCUSSION
The key finding of this study is that elevated circulating ceramide levels are associated with poor outcomes across the key timepoints of PC progression from localised PC to mHSPC to mCRPC. Patients with elevated ceramide levels are more likely to have metastatic relapse, therapeutic failure (testosterone suppression/docetaxel chemotherapy) and shorter OS, while our previously published prognostic 3-lipid signature for mCRPC was successfully validated in an external independent cohort. The lipidomic assay used in this study has been extensively utilised and validated in cardiovascular disease and diabetes, identifying plasma lipidomic signatures that predict cardiovascular events in high-risk patients and the general population, and are now driving changes in the treatment of cardiac patients17,21,22. Importantly, the 3-lipid signature in mCRPC described in our study is potentially actionable using existing metabolic drugs that target ceramide metabolism.
Circulating sphingolipids are mainly derived from the liver, transported in lipoprotein pools23, and can be increased by systemic inflammation24. However, some circulating sphingolipids may originate from the tumour as PC cells express the relevant biosynthetic enzymes (The Cancer Genome Atlas) of which some are associated with poorer PC outcomes (Supplementary Information S5)25. Exosomes secreted by PC cells are also enriched in sphingolipids26.
One hypothesis is that ceramides may also be promoting aggressive PC via their metabolite, sphingosine-1-phosphate (S1P). S1P is produced by a series of enzymatic reactions involving acid ceramidase and sphingosine kinase (SPHK) which are reported to have high expression/activity in PC cancers27,28. Furthermore, elevated SPHK gene expression in localised PC is associated with disease progression (Supplementary Information S5.2). S1P can promote cancer cell proliferation, survival and metastasis; and regulate lymphocyte trafficking by acting on S1P-specific receptors present on immune cells and cancer cells29. Mice lacking SPNS2, the lymphoid tissue-specific transporter of S1P, have reduced metastases30. Drugs that inhibit S1P production have anti-cancer properties, such as SPHK inhibitors31, of which ABC294640 completed a Phase I trial for advanced solid tumours (NCT01488513) and is undergoing Phase IIA clinical trials for cholangiocarcinoma (NCT03377179).
Aberrant ceramide metabolism in PC could also be modulated by targeting the metabolic environment of the host. High-fat feeding increases circulating ceramides32, and promoted inflammation and metastasis through S1P signalling in a breast cancer mouse model33. Importantly, this metabolic state can be pharmacologically normalised; cardiovascular and obesity studies demonstrate that elevated circulating ceramides can be decreased using cholesterol-lowering drugs (statins and PCSK9 inhibitors)34,35 and exercise36. In summary, targeting ‘host’ or tumour sphingolipid metabolism are both clinically feasible approaches and may improve the outcome of PC patients.
The tumour-promoting effect of obesity is attributed to circulating metabolic and inflammatory mediators associated with the chronic inflammatory state of adipose tissue3. However, the type of circulating lipids appears to be an important mediator, as we found that lipid profiles were independently associated with clinical outcomes when modelled with BMI. This suggests that a subset of men with PC, irrespective of their obesity status, have a metabolic signature that affects their cancer outcome.
The association of circulating sphingolipids with poorer clinical outcomes have also been reported recently by two metabolomic studies on localised PC. Clendinen et al (2019) profiled the levels of 450 lipids in pre-radical prostatectomy serum samples from 40 patients with biochemical recurrence and 40 in remission, and found that ceramide levels were increased in those with biochemical recurrence37. Snider et al (2020) performed metabolomic analysis on plasma from 159 treatment-naïve men and found that circulating levels of glycosphingolipids, ceramides and sphingomyelins were increased in men with more aggressive cancer as defined by Gleason grade, PSA levels and tumour stage38. The total number of lipids profiled was not specified. Interestingly, one of the significant sphingolipids identified from both Clendinen et al and Snider et al was Cer(d18:1/24:1), which was prognostic in all our cohorts and part of the prognostic 3-lipid signature. Overall the findings of these studies are consistent with ours, which show that perturbations in ceramide metabolism is associated with aggressive prostate cancer. The strength of our study as opposed to these is that we had sufficient follow-up to define metastasis-free survival as the endpoint, which was demonstrated by the ICECaP working group as the only surrogate endpoint associated with prostate-cancer specific survival19. The limitations of our study are the small size of the mHSPC cohort, and the limited availability of clinicopathological and metabolic data for some of the metastatic PC cohorts. Furthermore, there were some minor differences in the panel of lipid species profiled across all three cohorts due to improvements in analytical methodology where a larger number of lipids were profiled in the metastatic cohorts compared to localised PC. Thus, direct comparisons of certain lipid species could not be made, such as the ceramide species with different isoforms of the sphingoid backbone. Our prognostic 3-lipid signature is only applicable to mCRPC and not prognostic in the localised and mHSPC cohorts (data not shown), which is not suprising as the signature was originally derived from a mCRPC cohort12. The biology of prostate cancer will change through treatment and progression, thus some changes in the lipidomic profiles over the course of the disease are expected. The development of specific and accurate prognostic lipid signatures for localised and mHSPC will require future validation cohorts.
CONCLUSION
Elevated circulating ceramide species are associated with poorer clinical outcomes across the natural history of PC, from localised PC to mHSPC to mCRPC. Furthermore, our previously published prognostic mCRPC 3-lipid signature was validated in an independent mCRPC cohort. Precision oncology is commonly used to describe genomic-driven treatment, however, based on our data there is a case for personalised metabolic therapy in conjunction with standard-of-care to facilitate the optimal therapeutic environment. Prospectively-designed clinical trials with ceramide-targeting therapy using the lipid signature to guide treatment decisions in metastatic PC are now warranted to demonstrate its clinical utility and potentially improve patient outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge Maurene Giles and Cassandra Gordon (Australian Prostate Cancer BioResource) for collection and annotation of clinical follow-up data for the localised PC cohort; Anne-Maree Haynes (Garvan Institute of Medical Research), Lisa Graham (Chris O’Brien Lifehouse), Jessica Savage and Kayla Bremert (South Australian node coordinators of the Australian Prostate Cancer BioResource), the PRIMe consortium, and research nurses for collection of blood specimens and clinical data; Alexander Smith and Gavriel Olshansky (Baker Heart and Diabetes Institute) for their useful discussion on statistical analysis; our consumer representative Tony Maxwell for reviewing the paper; and the patients for their participation.
Financial Support
National Health and Medical Research Council of Australia (GNT0614296), Cancer Institute New South Wales (10/TPG/1-04, 2018/TPG001), Australian Prostate Cancer Research Centre-New South Wales, Australian Department of Health and Aging, the Movember Foundation and the Prostate Cancer Foundation of Australia (Revolutionary Team Award MRTA3), Cancer Council New South Wales (PG 10-01), Cancer Council South Australia (Beat Cancer Project Principal Cancer Research Fellowship, PRF1117), The Victorian Government’s Operational Infrastructure Support Program, National Institutes of Health grant award to M.K (RO1-CA212097).
CONFLICT OF INTEREST
A.Azad: Consultant - Astellas, Janssen, Novartis; Speakers Bureau - Astellas, Janssen, Novartis, Amgen; Honoraria - Astellas, Janssen, Novartis, Tolmar, Amgen, Pfizer, Telix; Scientific Advisory Board - Astellas, Novartis, Sanofi, AstraZeneca, Tolmar, Pfizer, Telix; Research Funding - Astellas, Merck Serono, Bristol Myers Squibb (institutional), Astra Zeneca (institutional), Aptevo Therapeutics (institutional), Glaxo Smith Kline (institutional), Pfizer (institutional), MedImmune (institutional), Astellas (institutional), SYNthorx (institutional), Bionomics (institutional), Sanofi Aventis (institutional), Novartis (institutional). L.Horvath: Research funding - Astellas; Travel sponsorship - Janssen, Pfizer; Honoraria - Imagion Biosystems. All remaining authors have declared no conflicts of interest.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018; 68(6): 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Sartor O, de Bono JS. Metastatic Prostate Cancer. N Engl J Med 2018; 378(7): 645–657. [DOI] [PubMed] [Google Scholar]
- 3.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol 2016; 34(35): 4270–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol 2013; 63(5): 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Daniels G, Lee P, Monaco ME. Lipid metabolism in prostate cancer. Am J Clin Exp Urol 2014; 2(2): 111–120. [PMC free article] [PubMed] [Google Scholar]
- 6.Zadra G, Ribeiro CF, Chetta P, Ho Y, Cacciatore S, Gao X et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2019; 116(2): 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK et al. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol Biomarkers Prev 2014; 23(11): 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S-Y, Fang S-C, Shih H-J, Wen Y-C, Shao Y-HJ. Mortality associated with statins in men with advanced prostate cancer treated with androgen deprivation therapy. Eur J Cancer 2019; 112: 109–117. [DOI] [PubMed] [Google Scholar]
- 9.Van Rompay MI, Solomon KR, Nickel JC, Ranganathan G, Kantoff PW, McKinlay JB. Prostate cancer incidence and mortality among men using statins and non-statin lipid-lowering medications. European Journal of Cancer 2019; 112: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raval AD, Thakker D, Negi H, Vyas A, Salkini MW. Association between statins and clinical outcomes among men with prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2016; 19(2): 151–162. [DOI] [PubMed] [Google Scholar]
- 11.Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med 2011; 365(19): 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin HM, Mahon KL, Weir JM, Mundra PA, Spielman C, Briscoe K et al. A distinct plasma lipid signature associated with poor prognosis in castration-resistant prostate cancer. Int J Cancer 2017; 141(10): 2112–2120. [DOI] [PubMed] [Google Scholar]
- 13.Summers SA, Chaurasia B, Holland WL. Metabolic Messengers: ceramides. Nat Metab 2019; 1(11): 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016; 37(25): 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vykoukal J, Fahrmann JF, Gregg JR, Tang Z, Basourakos S, Irajizad E et al. Caveolin-1-mediated sphingolipid oncometabolism underlies a metabolic vulnerability of prostate cancer. Nat Commun 2020; 11(1): 4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohli M, Tan W, Zheng T, Wang A, Montesinos C, Wong C et al. Clinical and genomic insights into circulating tumor DNA-based alterations across the spectrum of metastatic hormone-sensitive and castrate-resistant prostate cancer. EBioMedicine 2020; 54: 102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh K, Barlow CK, Jayawardana KS, Weir JM, Mellett NA, Cinel M et al. High-Throughput Plasma Lipidomics: Detailed Mapping of the Associations with Cardiometabolic Risk Factors. Cell Chem Biol 2019; 26(1): 71–84.e74. [DOI] [PubMed] [Google Scholar]
- 18.Linzer DA, Lewis JB. poLCA: An R Package for Polytomous Variable Latent Class Analysis. J Stat Softw 2011; 42(10): 1–29. [Google Scholar]
- 19.Xie W, Regan MM, Buyse M, Halabi S, Kantoff PW, Sartor O et al. Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J Clin Oncol 2017; 35(27): 3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008; 9(2): 139–150. [DOI] [PubMed] [Google Scholar]
- 21.Meikle PJ, Wong G, Tan R, Giral P, Robillard P, Orsoni A et al. Statin action favors normalization of the plasma lipidome in the atherogenic mixed dyslipidemia of MetS: potential relevance to statin-associated dysglycemia. J Lipid Res 2015; 56(12): 2381–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundra PA, Barlow CK, Nestel PJ, Barnes EH, Kirby A, Thompson P et al. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight 2018; 3(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res 2009; 50(3): 574–585. [DOI] [PubMed] [Google Scholar]
- 24.Lightle S, Tosheva R, Lee A, Queen-Baker J, Boyanovsky B, Shedlofsky S et al. Elevation of ceramide in serum lipoproteins during acute phase response in humans and mice: role of serine-palmitoyl transferase. Arch Biochem Biophys 2003; 419(2): 120–128. [DOI] [PubMed] [Google Scholar]
- 25.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2(5): 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski A et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta 2013; 1831(7): 1302–1309. [DOI] [PubMed] [Google Scholar]
- 27.Malavaud B, Pchejetski D, Mazerolles C, de Paiva GR, Calvet C, Doumerc N et al. Sphingosine kinase-1 activity and expression in human prostate cancer resection specimens. Eur J Cancer 2010; 46(18): 3417–3424. [DOI] [PubMed] [Google Scholar]
- 28.Seelan RS, Qian C, Yokomizo A, Bostwick DG, Smith DI, Liu W. Human acid ceramidase is overexpressed but not mutated in prostate cancer. Genes Chromosomes Cancer 2000; 29(2): 137–146. [DOI] [PubMed] [Google Scholar]
- 29.Ogretmen B Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 2018; 18(1): 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Weyden L, Arends MJ, Campbell AD, Bald T, Wardle-Jones H, Griggs N et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature 2017; 541(7636): 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos WL, Lynch KR. Drugging sphingosine kinases. ACS Chem Biol 2015; 10(1): 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem 2008; 283(20): 13538–13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagahashi M, Yamada A, Katsuta E, Aoyagi T, Huang WC, Terracina KP et al. Targeting the SphK1/S1P/S1PR1 Axis That Links Obesity, Chronic Inflammation, and Breast Cancer Metastasis. Cancer Res 2018; 78(7): 1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilvo M, Simolin H, Metso J, Ruuth M, Oorni K, Jauhiainen M et al. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis 2018; 269: 159–165. [DOI] [PubMed] [Google Scholar]
- 35.Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, Hurme R et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab 2014; 99(1): E45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasumov T, Solomon TP, Hwang C, Huang H, Haus JM, Zhang R et al. Improved insulin sensitivity after exercise training is linked to reduced plasma C14:0 ceramide in obesity and type 2 diabetes. Obesity (Silver Spring) 2015; 23(7): 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clendinen CS, Gaul DA, Monge ME, Arnold RS, Edison AS, Petros JA et al. Preoperative Metabolic Signatures of Prostate Cancer Recurrence Following Radical Prostatectomy. J Proteome Res 2019. [DOI] [PubMed] [Google Scholar]
- 38.Snider AJ, Seeds MC, Johnstone L, Snider JM, Hallmark B, Dutta R et al. Identification of Plasma Glycosphingolipids as Potential Biomarkers for Prostate Cancer (PCa) Status. Biomolecules 2020; 10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


