Abstract
Complex Regional Pain Syndrome (CRPS) is a musculoskeletal pain condition that often develops after limb injury and/or immobilization. Although the exact mechanisms underlying CRPS are unknown, the syndrome is associated with central and autonomic nervous system dysregulation and peripheral hyperalgesia symptoms. These symptoms also manifest in alcoholic neuropathy, suggesting that the two conditions may be pathophysiologically accretive. Interestingly, people assigned female at birth (AFAB) appear to be more sensitive to both CRPS and alcoholic neuropathy. To better understand the biobehavioral mechanisms underlying these conditions, we investigated a model of combined CRPS and alcoholic neuropathy in female rats. Animals were pair-fed either a Lieber-DeCarli alcohol liquid diet or a control diet for ten weeks. CRPS was modeled via unilateral hind limb cast immobilization for seven days, allowing for the other limb to serve as a within-subject control for hyperalgesia measures. To investigate the role of circulating ovarian hormones on pain-related behaviors, half of the animals underwent ovariectomy (OVX). Using the von Frey procedure to record mechanical paw withdrawal thresholds, we found that cast immobilization and chronic alcohol drinking separately and additively produced mechanical hyperalgesia observed 3 days after cast removal. We then examined neuroadaptations in AMPA GluR1 and NMDA NR1 glutamate channel subunits, extracellular signal-regulated kinase (ERK), and cAMP response element-binding protein (CREB) in bilateral motor and cingulate cortex across all groups. Consistent with increased pain-related behavior, chronic alcohol drinking increased GluR1, NR1, ERK, and CREB phosphorylation in the cingulate cortex. OVX did not alter any of the observed effects. Our results suggest accretive relationships between CRPS and alcoholic neuropathy symptoms and point to novel therapeutic targets for these conditions.
Keywords: Alcohol, Cingulate Cortex, Complex Regional Pain Syndrome, Extracellular Signal-Regulated Kinase, Glutamate, Pain
1. Introduction
Chronic pain impacts over 20% of the population [1] and exacts a heavy toll on both physical and mental health [2]. This relationship appears to be particularly true for people assigned female sex at birth (AFAB; [3]). Complex regional pain syndrome (CRPS) describes a post-traumatic neuropathic pain condition of the limb [4, 5], and according to the McGill Pain Index is one of the most subjectively painful conditions known. CRPS disproportionally affects AFABs and often develops following limb injuries that require casting or limb immobilization. Extending beyond a simple somatic pain disorder, CRPS patients often suffer from a range of affective disorders, suggesting a dysregulation of higher emotional brain centers [6]. A recent examination found that 60% of CRPS patients suffered from depression while 18% misused alcohol or other substances [7]. Moreover, pain catastrophizing and other elements of pain-related negative affect contribute significantly to CRPS-related disability [8, 9].
Many chronic pain patients report higher levels of alcohol drinking and suffer from alcohol use disorder (AUD) at rates higher than the general population [10, 11]. Chronic pain and AUD are characterized by similar behavioral attributes, including cognitive dysfunction and enhanced negative affect [12, 13]. The profound negative emotional state generated by chronic pain is proposed to increase the risk of development AUD [14]. Heavy alcohol drinking itself often exacerbates nociceptive hypersensitivity in both humans and animal models [15, 16]. Self-reports of alcohol use with intention of pain management are common [17, 18]. Moreover, problem drinkers not only report more severe pain symptoms compared to non-drinkers, but also report a higher incidence of using alcohol to manage their pain [19]. Such data also suggest that frequent drinking in the context of AUD may be motivated in part by a desire to alleviate enhanced nociceptive sensitivity, or hyperalgesia [20].
Strong evidence demonstrates that the neurobiological substrates associated with alcohol reward overlap considerably with the supra-spinal substrates of the emotional aspects of pain processing [21]. Specifically, the affective component of pain appears to be strongly mediated by the cingulate cortex [22]. A recent role for the cingulate in the social transfer of pain has also been elucidated [23]. Various types of chronic pain are associated with specific neuronal plasticity in the cingulate cortex that closely associates with the affective or emotional dimension of pain, including increases in glutamatergic signaling [24], extracellular signal-regulated kinase (ERK) activity [25, 26], and closely associated phosphorylation and transcriptional activation of nuclear cAMP response-element binding protein (CREB; [27]). Much less is known about how chronic alcohol drinking dysregulates the cingulate cortex, although increases in glutamatergic activity and ERK phosphorylation are known to manifest throughout the brain in animal models of AUD [28, 29], indicating that this pathway may serve as a useful biomarker to interrogate how pain and alcohol interact to dysregulate nociceptive brain regions such as the cingulate.
To better understand the biobehavioral outcomes resulting from chronic alcohol drinking and limb immobilization, the objectives of the current study were to first examine mechanical nociceptive thresholds in a cast immobilization animal model of CRPS [30] in female rats exposed to an alcohol-containing diet. Half of the animals received ovariectomies (OVX) to determine the contributions of circulating ovarian hormones to development of CRPS. We also investigated resultant neuroadaptations in glutamatergic subunit (GluR1 and NR1) and intracellular signaling protein (ERK and CREB) phosphorylation levels within the motor cortex (ipsilateral and contralateral to the casted limb) and the cingulate cortex, representing potential points of intersection between peripheral injury, central brain nociceptive processing, and alcohol-mediated plasticity.
2. Material and Methods
2.1. Animals
Adult female Fischer 344 rats (n=4–5/group), 3 months old upon arrival, were purchased from Charles River and single-housed in a temperature and humidity controlled vivarium. Rats were maintained on a 12-hour light/dark cycle throughout the duration of the study. Rats were given one week to acclimate to the colony room prior to the start of experimental procedures. All animal care, use, and procedures in this study were approved by Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center (LSUSHC) in New Orleans, LA, and were in accordance with the National Institute of Health guidelines. For experimental timeline, see Figure 1. We utilized rats of the age typically employed in similar studies (e.g., [31]). It should also be pointed out that CRPS can affect individuals of any age, with age of onset ranging from as low as 18 to as high as 90 years of age [32].
Figure 1:
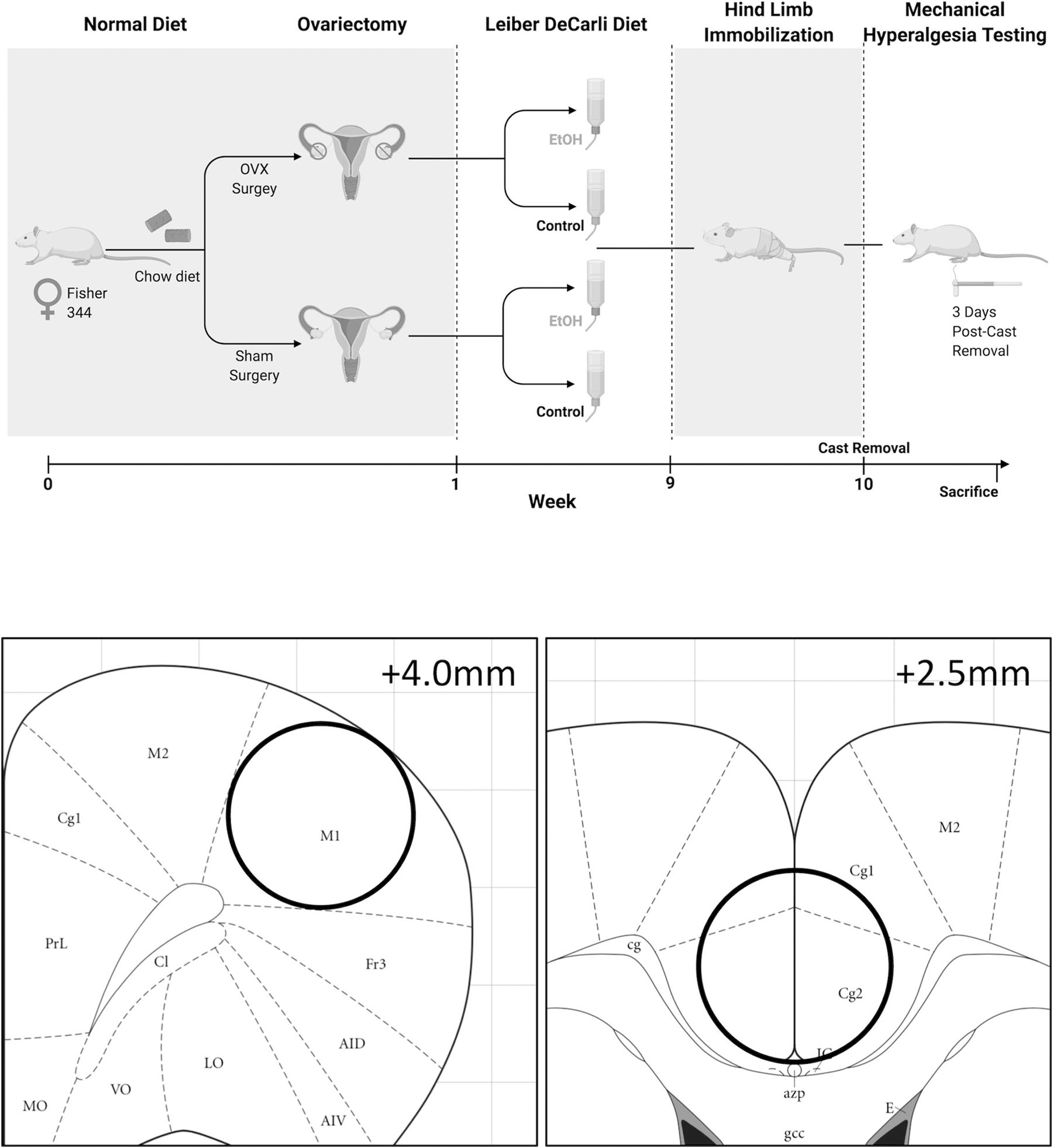
Experimental timeline and regional brain dissections for Western analyses.
2.2. Ovariectomy
At the start of the experiment, animals were randomly assigned to receive an ovariectomy (OVX) or sham surgery, as previously described [33]. Animals were anesthetized using ketamine/xylazine and were given a dose of slow-release buprenorphine prior to surgery. To perform the OVX, a bilateral skin and muscle incision were made and ovaries located in the fat pad were drawn out using blunt forceps. Ovarian blood vessels were ligated with 4–0 silk, the ovaries were excised, and skin and muscle wall incisions were sutured separately. Animals receiving sham surgery underwent a similar procedure but did not receive clamping or excision of the ovaries. All animals were allowed to recover for three days. Ovarian hormone loss was confirmed via uterine weight at the time of sacrifice.
2.3. Lieber-DeCarli Liquid Diet
One week following surgery, animals were randomly assigned to receive either an alcohol-containing (ethanol diet F1258SP; Bio-Serve, Frenchtown, NJ, USA) or a pair-fed control (control diet F1259SP; Bio-Serve) Lieber-DeCarli liquid diet for the duration of the experiment. Animals were transitioned onto the Lieber-DeCarli liquid diet over a five-day period of decreasing solid food and increasing the liquid diet. In a recent cohort of animals run in parallel with the current study (Levitt et al., 2020), administration of a Lieber-DeCarli diet produced average blood alcohol levels of 103.9 mg/dL (n=7, SEM = 15.5), consistent with other studies utilizing this model to examine alcohol-related neuropathy [34].
2.4. Hind Limb Immobilization
Previous work has demonstrated that hind limb immobilization produces mechanical hypersensitivity, even in the absence of bone fracturing [30], similar to that reported in clinical studies [35]. All animals underwent unilateral hind limb immobilization 9 weeks following OVX surgery. Animals were anesthetized using ketamine/xylazine and hind limbs were casted using plaster of Paris as previously described [33]. The casted leg was randomized across animals such that there was an equal number of right and left casted legs. Animals were casted with slight hip flexion and knee extension, with dorsal aspects of the ankle and the feet left un-casted to allow for movement and unobstructed blood flow. The animals were immobilized for seven days and allowed to recover for three days prior to mechanical hyperalgesia testing.
2.5. von Frey Filament Mechanical Hyperalgesia Testing
One day prior to sacrifice, mechanical sensitivity was determined by obtaining hind paw withdrawal thresholds, similar to what is described in [15]. Animals were first acclimated for 10 minutes to individual Plexiglas compartments set on top of a mesh stand. A series of nylon von Frey filaments were applied perpendicularly to the plantar surface of the hind paw until they buckled for 2 seconds, and a sharp withdrawal of the stimulated hind paw before or within the 2 seconds indicated a positive response. Testing was initiated with the filament corresponding to 15 grams of force and continued in accordance with the up- and-down method [36]. The 50% paw withdrawal threshold was determined by the formula Xf+kδ, where Xf = last von Frey filament used, k = Dixon value corresponding to response pattern, and δ = mean difference between stimuli.
2.6. Western Blot Analysis
Western analyses of brain tissue were performed as previously described [37]. Briefly, all animals were sacrificed by decapitation under light isofluorane anesthesia procedures that permit detection of brain protein phosphorylation levels. Brains were rapidly dissected, snap-frozen in −30°C isopentane, and stored at −80°C until microdissection, during which time three 0.5mm thick regional punches were taken from coronal brain sections using a 13 gauge needle (motor cortex dissections ranged from 4.0mm to 2.5mm from Bregma and cingulate cortex dissections ranged from 2.5 to 1.0mm from Bregma, Figure 1). A homogenization buffer consisting of 320 mm sucrose, 5 mm HEPES, 1 mm EGTA, 1 mm EDTA, 1% SDS, protease inhibitor cocktail (diluted 1:100), and phosphatase inhibitor cocktails II and III (diluted 1:100) (Sigma, St. Louis, MO, USA) was added to brain punches prior to sonication to homogenize the tissue. Following sonication, tissue homogenates were heated to 90°C for 5 minutes, and total protein concentration was measured using a detergent-compatible Lowry protein assay (Bio-Rad, Hercules, CA, USA). Aliquots of tissue homogenates were loaded into 10% acrylamide gels and samples of protein were separated by SDS-polyacrylamide gel electrophoresis using a Tris/Glycine/SDS buffer system (Bio-Rad). Gels were electrophorectically transferred onto polyvinylidene difluoride membranes (GE Healthcare, Piscataway, NJ, USA), and following transfer, membranes were blocked for 1 hour at room temperature in 5% non-fat milk. Primary antibody incubation for the following antibodies occurred overnight at 4°C in 2.5% non-fat milk: phospho-ERK (1:5,000–1:10,000; Cell Signaling, Cat #9106), total ERK (1:5,000–1:10,000; Cell Signaling, Cat #9102), phospho-CREB (1:20,000; Millipore, Cat #06–519), total CREB (1:20,000; Millipore, Cat #06–863), phospho-NR1 (Ser897) (1:5,000; Millipore, Cat #ABN99), total NR1 (1:5,000; Cell Signaling, Cat #5704), phospho-GluR1 (Ser845) (1:2,000–1:5,000; Cell Signaling, Cat #8084S), total GluR1 (1:2,000–1:5,000; Cell Signaling, Cat #13185S), and Tubulin (1:1,000,000; Santa Cruz Biotechnology, Inc., Cat #sc-53140). Membranes were then washed, incubated with species-specific peroxidase-conjugated secondary antibody (1:10,000; Bio-Rad) for 1 hour at room temperature, washed again, incubated in Immobilon® Crescendo Western HRP substrate chemiluminescent reagent (MilliporeSigma, Cat #WBLUR0500) and exposed to film. After films were developed, membranes were stripped for 20 minutes at room temperature (Restore; Thermo Scientific) and re-probed for either total protein levels or Tubulin. Band immunoreactivity was detected using densitometry (Image J 1.45S, Bethesda, MD, USA). Densitized phosphoprotein values were normalized to either total protein densitometry values to generate phosphorylated:total protein ratio values or to loading control Tubulin if significant changes were observed in total protein between experimental groups. Densitized values are expressed as a percentage of the mean of control (control diet-sham surgery) values for each gel to normalize data across blots.
2.7. Statistical Analysis
All data were analyzed using Prism 8 (GraphPad Software, Inc.; La Jolla, CA, USA). Behavioral data was analyzed using a three-way ANOVA (alcohol x OVX x limb). Two-way ANOVAs (alcohol x OVX) were used to measure the effects of each treatment and their interaction on phosphoprotein and total protein levels. Pearson’s r correlations and linear regressions were used to analyze the relationships between individual phosphoprotein levels and paw withdrawal thresholds. All data are presented as mean ± SEM. Statistical significance was established at p < 0.05.
3. Results
3.1. Hind limb cast immobilization and chronic alcohol separately and additively produce mechanical hyperalgesia.
Both hind limb cast immobilization and chronic alcohol induced mechanical hyperalgesia as measured by the von Frey test, indicating that we have successfully modeled both CRPS and alcoholic neuropathy (Figure 2). Three-way ANOVA indicated a significant main effect of alcohol (F1,30 = 5.721, p = 0.0232) and a significant main effect of casting (F1,30 = 8.318, p = 0.0072) to decrease mean paw withdrawal threshold. However, there was no effect of OVX (F1,30 = 0.1548, p = 0.6968) or alcohol x casting (F1,30 = 0.9746, p = 0.3314), alcohol x OVX (F1,30 = 0.1836, p = 0.5595), or alcohol x OVX x casting (F1,30 = 0.2457, p = 0.6237) interaction on mechanical hyperalgesia.
Figure 2:
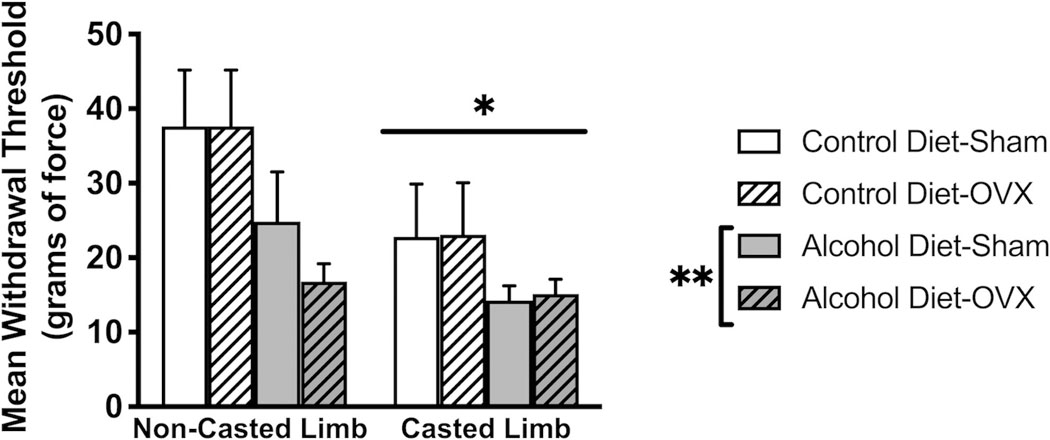
Hind limb cast immobilization and chronic alcohol separately and additively produce mechanical hypersensitivity. There was a significant main effect of alcohol (p=0.0072) and a significant main effect of casting (p=0.0232) to decrease paw withdrawal thresholds. There was no significant main effect of ovariectomy (OVX) or significant OVX interactions. Data were analyzed using 3-way ANOVA. Data are represented as mean ± SEM. Control diet + sham surgery, solid navy (n = 5); control diet + OVX, navy dotted (n = 5); alcohol diet + sham surgery, solid orange (n = 5); alcohol diet + OVX, orange dotted (n = 4).
3.2. Hind limb cast immobilization-induced mechanical hyperalgesia correlates with ERK phosphorylation in the contralateral motor cortex
We have previously examined within-subjects correlations between protein phosphorylation status and pain behavior as a marker of neurobiological signaling mechanisms underlying pain states [38]. Utilizing this approach, we discovered a significant positive correlation (r = 0.6211, p = 0.0045) between ERK phosphorylation in the motor cortex contralateral to the casted limb and mean paw withdrawal thresholds of the casted limb (Figure 3A). Conversely, there was no correlation (r = −0.1020, p = 0.6777) between ERK phosphorylation in the motor cortex contralateral to the non-casted limb and mean paw withdrawal thresholds of the non-casted limb (Figure 3B). Because this correlation is only observed in the motor cortex corresponding to the immobilized limb, these data suggest reduced capacity for plasticity in association with immobilization-induced hyperalgesia.
Figure 3:
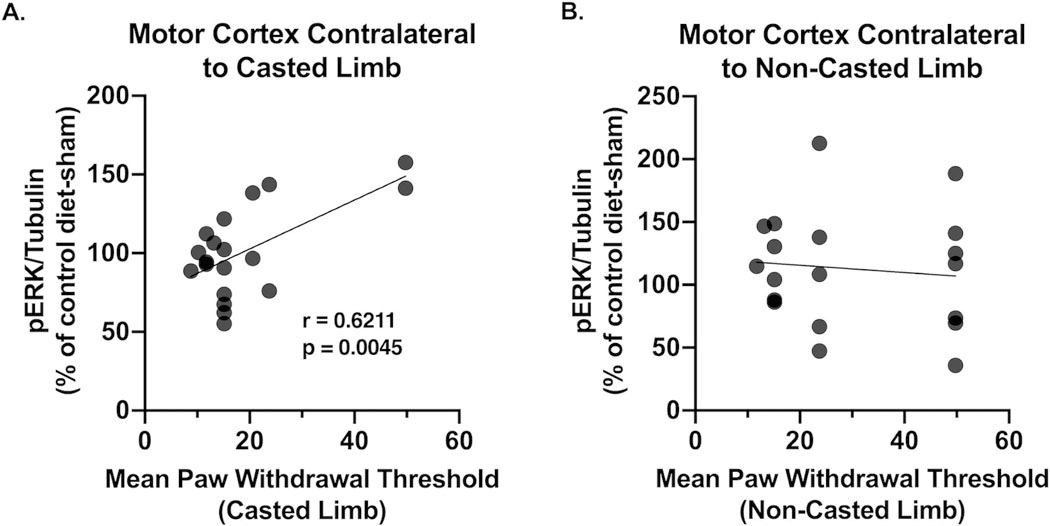
(A) CRPS-induced mechanical hyperalgesia correlates with ERK phosphorylation in the contralateral motor cortex (r = 0.6221; p = 0.0045). (B) There is no correlation between ERK phosphorylation in the motor cortex contralateral to the non-casted limb and paw withdrawal thresholds in the non-casted limb. Data were analyzed using Pearson’s linear regression. n = 4–5/group.
3.3. Chronic alcohol increases total and PKA-phosphorylated levels of glutamate receptor channel subunits in the cingulate cortex
To determine if chronic alcohol in the context of CRPS altered excitatory signaling within the cingulate cortex, we first examined the phosphorylation status of glutamate receptors AMPA GluR1 and NMDAR1 at the serine 845 and 897 phosphorylation sites, respectively. Phosphorylation at these residues is specific to protein kinase A (PKA) and corresponds to increased AMPA and NMDA receptor currents and membrane trafficking. We observed a significant effect of alcohol to increase both phosphorylated and total levels of GluR1 (F1,16 = 13.96, p = 0.0018; F1,16 = 5.157, p = 0.0395) and NMDAR1 (F1,16 = 7.502, p = 0.0146; F1,16 = 5.277, p = 0.0354; Figure 4). The observed increase in total GluR1 (Figure 4B) and NMDAR1 (Figure 4D) levels suggests that chronic alcohol produces an increase in GluR1- and NR1- containing AMPA and NMDA receptors in the cingulate cortex. However, when normalized to tubulin, we still observed an increase in receptor phosphorylation (Figure 4A,C), suggesting an increase in receptor activity and glutamatergic signaling. There was no effect of OVX (F1,16 = 0.06364, p = 0.8041; F1,16 = 0.3746, p = 0.5491) or alcohol x OVX interaction (F1,16 = 0.9300, p = 0.3492; F1,16 = 0.009589, p = 0.9232) on phosphorylated or total levels of GluR1 or NMDAR1. Increases in phosphorylation of both AMPA GluR1 and NMDAR1 suggest an increase in excitatory glutamatergic signaling in the cingulate cortex following chronic alcohol use. Representative images are shown in Figure 6A.
Figure 4:
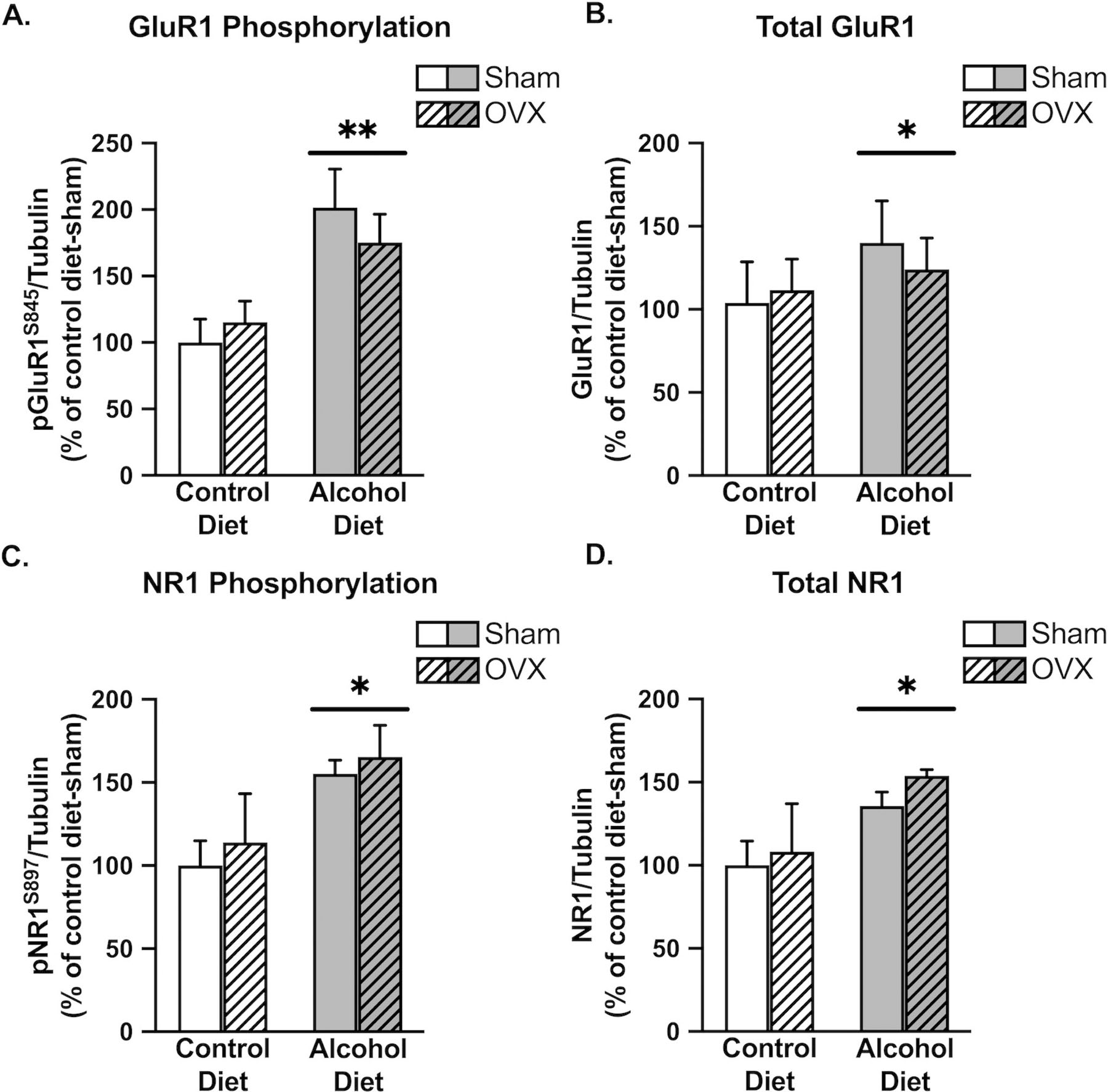
Chronic alcohol increases both phosphorylated and total levels of glutamate receptor channel subunits in the cingulate cortex. There was a significant main effect of alcohol to increase GluR1 phosphorylation (A; p=0.0018), total levels of GluR1 (B; p=0.0395), NR1 phosphorylation (C; p=0.0146), and total levels of NR1 (D; p=0.0354). Control diet + sham surgery, solid navy (n = 5); control diet + OVX, navy dotted (n = 5); alcohol diet + sham surgery, solid orange (n = 5); alcohol diet + OVX, orange dotted (n = 4).
Figure 6:
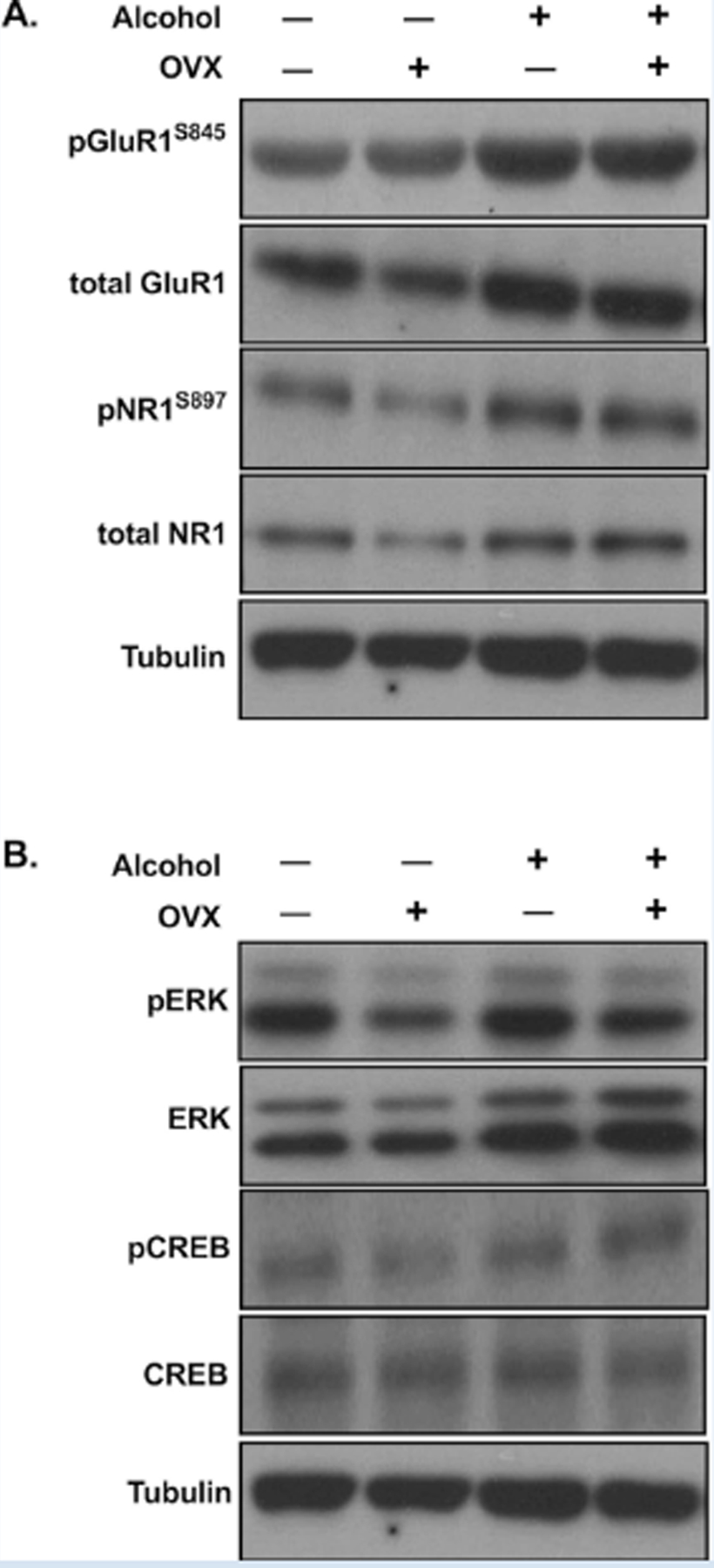
Representative Western blots corresponding to Figure 4 (A) and Figure 5 (B).
3.4. Chronic alcohol increases intracellular activity markers ERK and CREB in the cingulate cortex
We next investigated the phosphorylation status of intracellular and nuclear activity markers in the cingulate cortex. We first examined ERK phosphorylation as a general marker of activity and observed a significant effect of alcohol (F1,16 = 5.649, p = 0.0303) to increase ERK phosphorylation in the cingulate cortex (Figure 5A). We observed a similar alcohol effect (F1,16 = 5.602, p = 0.0309) on phosphorylation of the transcription factor CREB at the serine 133 site, which initiates target gene transcription (Figure 5C). Alcohol also significantly increased total levels of ERK (F1,16 = 5.694, p = 0.0297, Figure 5B), but not CREB (F1,16 = 2.899, p = 0.1080, Figure 5D). Neither ERK phosphorylation or CREB phosphorylation were affected by OVX (F1,16 = 0.00136, p = 0.9710; F1,16 = 0.5084, p = 0.4861), and no alcohol x OVX interactions were observed (F1,16 = 0.03742, p = 0.8491; F1,16 = 8.011e-005, p = 0.9930). These findings indicate that chronic alcohol in the context of CRPS increases intracellular and nuclear activity within the cingulate cortex, further suggesting a widespread increase in cingulate cortex activity following chronic alcohol drinking. Representative western blot images are shown in Figure 6.
Figure 5:
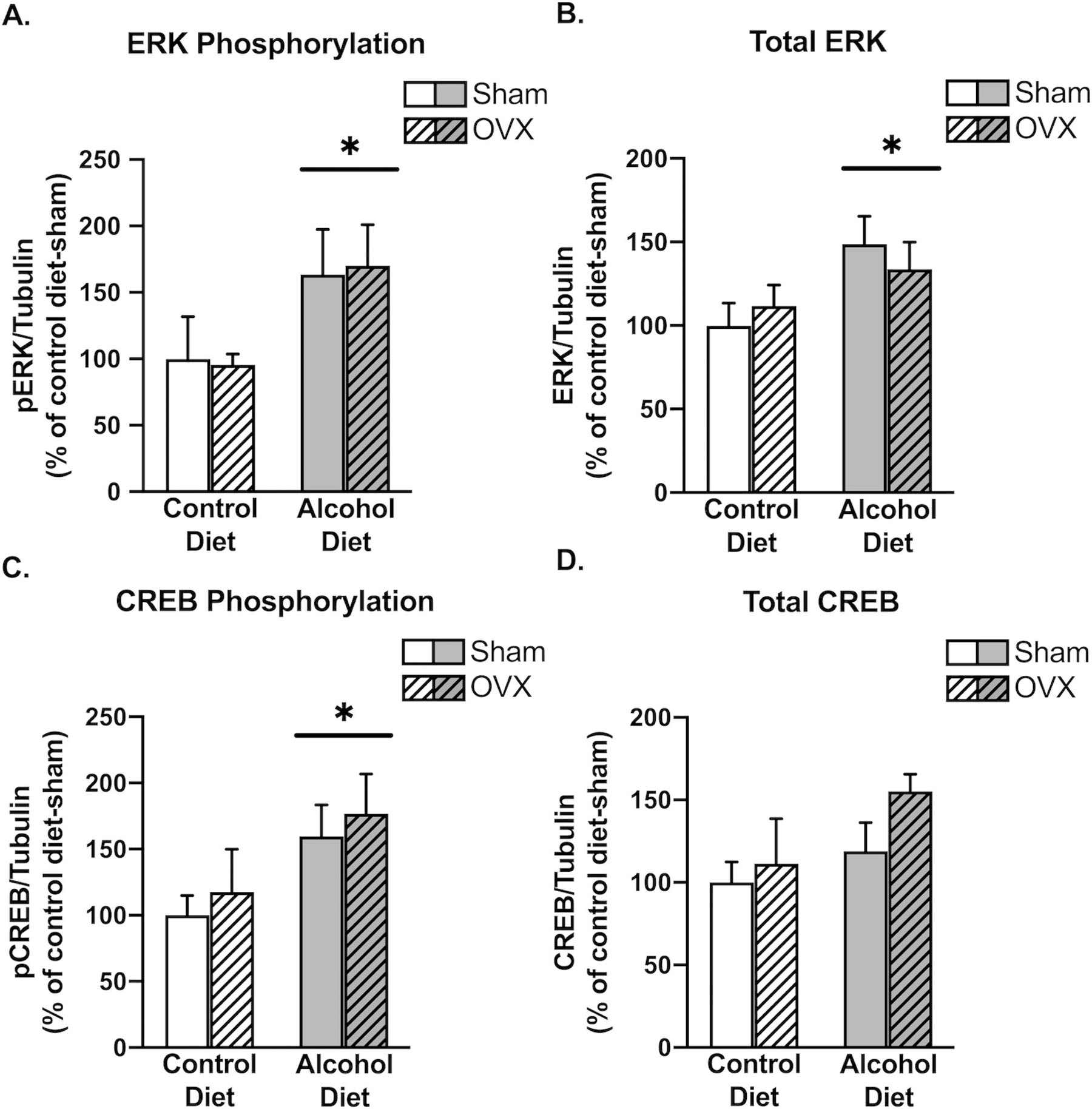
Chronic alcohol increases intracellular activity markers in the cingulate cortex. There was a significant main effect of alcohol to increase ERK phosphorylation (A; p=0.0303), total levels of ERK (B; p=0.0297), and CREB phosphorylation (C; p=0.0048). Alcohol did not increase total levels of CREB (D; p=0.1080). Control diet + sham surgery, solid navy (n = 5); control diet + OVX, navy dotted (n = 5); alcohol diet + sham surgery, solid orange (n = 5); alcohol diet + OVX, orange dotted (n = 4).
4. Discussion
Heavy alcohol use and chronic pain are highly comorbid [39, 40]. Moreover, chronic alcohol use is associated with increased risk of injury [41], and regular use of alcohol is frequently reported as an analgesic in both chronic pain and alcohol use disorder (AUD) patients [17, 42]. The current study sought to develop and validate an animal model of combined CRPS and alcoholic neuropathy and identify associated neuroadaptations in a key central pain-related brain area (the cingulate cortex). Of particular interest is the role of the cingulate cortex in organizing behavioral goals (such as avoiding pain) in the context of injury-associated nociception [43]. Evidence for this intersection in frontocortical regions, including the cingulate cortex, has been demonstrated in preclinical pain models [25, 27]. Thus, we hypothesized that significant neurobiological interactions would exist between chronic alcohol drinking and pain within the cingulate as reflected by altered neuronal activity (indexed via both synaptic and intracellular protein phosphorylation measures). Dysregulated cingulate activity has also been described in relation to pain processing by CRPS patients [44–46]. Based on its regulation of sympathetic outflow [47–49], over-activation of the cingulate cortex may also underlie the characteristic autonomic symptoms of CRPS [50]. Indeed, increased cingulate cortex activity has been observed in several animal models of neuropathic pain [51, 52]. Further, glutamate-mediated long-term potentiation (LTP) in the cingulate cortex and glutamate receptor neuroadaptations have also been observed in animal models of neuropathic pain and following injury [53–55]. Interestingly, increased activity in the cingulate cortex also appears to serve as a risk factor for alcohol dependence [56]. Additional studies using this model are recommended to parse out neuroadaptations in excitatory (pronociceptive) versus inhibitory (anti-nociceptive) neurons within the cingulate cortex (e.g., by utilizing a c-Fos mapping strategy).
In addition to the observed change in the cingulate cortex, our results show a significant correlation between paw withdrawal thresholds and ERK phosphorylation levels in the motor cortex contralateral (but not ipsilateral) to the immobilized limb across all animals, indicating that hyperalgesia symptoms are linked to compromised ERK activity in the motor cortex. Although this may appear to conflict with clinical data suggesting decreased inhibition [57, 58] and increased activation in the CRPS-corresponding motor cortex [59], a limitation of the current level of analysis (Western blot) is the inability of discriminating ERK phosphorylation changes in excitatory pyramidal neurons vs. inhibitory interneurons (or other cell types) within the motor cortex. Our previous study confirmed a reduction in corresponding quadriceps muscle mass with hind limb immobilization [33]. The current findings extend these functional deficits into associated motor centers in the brain. Interestingly, CRPS patients exhibit altered functional connectivity between motor and cingulate cortices that associate with neuropathic pain intensity [60].
CRPS and alcoholic neuropathy present with similar symptoms, including neurogenic inflammation, central and autonomic nervous system dysregulation, and peripheral hyperalgesia. While the bidirectional relationship between alcohol use and pain has been an intense area of recent research [18, 61], alcohol use in the context of CRPS remains under-investigated. Our current findings are consistent with other studies describing the development of mechanical and thermal hyperalgesia in animal models of AUD [15, 34, 62]. Importantly, we observed hyperalgesia symptoms in animals continuously exposed to the Liber-DeCarli diet (even in the absence of alcohol withdrawal), consistent with previous studies [34]. Future studies should examine additional analgesic substances that may be frequently used by CRPS patients, including opioids. Interestingly, combined morphine and NMDA glutamate receptor antagonist treatment attenuates cingulate activation during movement of the affected limb in CRPS patients [63]. However, opioid therapy should be approached with caution as, similar to alcohol, chronic opioid use also gradually produces paradoxical hyperalgesia symptoms [15, 38]. Collectively, our findings suggest that alcohol drinking worsens CRPS-induced pain, in line with clinician recommendations for CRPS patients to avoid alcohol consumption.
Future studies should also examine how alcohol-drinking levels are altered in the casting model of CRPS. A recent study of C57BL/6J male and female mice that were in a state of chronic inflammatory pain and given continuous access to alcohol reported that male (but not female) mice consumed significantly more alcohol in the context of pain [64]. Another study in male rats suggested that relationships between alcohol drinking and hyperalgesia symptoms are altered over the course of time in an inflammatory pain state [65]. In humans, a confluence of studies appear to indicate that pain may be more likely to increase drinking and relapse in those who have begun the transition to AUD [19, 66–68]. Another transitory relationship exists between the analgesic efficacy of alcohol in humans relative to AUD status. While regular alcohol consumption is associated with reduced pain symptoms in most chronic pain sufferers [66, 69], alcohol appears to increase pain and pain-related disability in problem drinkers and those with an AUD diagnosis [19, 68, 70]. Our findings warrant additional longitudinal studies examining how CRPS-related pain affects alcohol physiology in subjects transitioning from non-dependent to alcohol-dependent states to better understand how these relationships change over time.
A recent meta-analysis of alcohol-mediated analgesia in human subjects discovered a linear relationship between alcohol dose and analgesia, with blood alcohol levels that corresponded to binge-like alcohol exposure (0.08 g/dL) producing a clinically relevant reduction in pain intensity [71]. Administration of a Liber-DiCarli alcohol diet approximates this level of drinking over an extended period, producing an average blood alcohol concentration of 0.10 g/dL [33]. While alcohol produces reliable analgesia in rodent models [72], additional studies are necessary to determine the efficacy of alcohol to manage CRPS-related pain in either animal models or humans. However, binge alcohol exposure is also considered a primary risk factor for the eventual development of AUD based on its engagement of brain reward areas, including the cingulate cortex [73].
In addition to shared overlapping symptomology, CRPS and alcoholic neuropathy also disproportionately affect people assigned female at birth (AFAB). CRPS is two to four times more prevalent in AFAB people [74], and AFAB individuals display both higher rates and more severe symptoms of alcoholic neuropathy [75]. Further, AFAB individuals report higher incidence of chronic pain, greater sensitivity to painful stimuli, and more frequent pain compared to their male counterparts [76, 77]. Additionally, AFAB individuals display more rapid progression from onset of drinking to dependence [78, 79], greater rates of alcohol-related health consequences [80], and are the fastest-growing population of alcohol users in the United States [81]. Based on these sex differences, the present study investigated only female animals and utilized ovariectomy (OVX) to determine the effects of circulating ovarian hormones on mechanical pain sensitivity and underlying neurobiology. We originally hypothesized that OVX would facilitate hyperalgesia symptoms, since AFAB individuals suffering from CRPS exhibit reduced estradiol levels [82]. It is possible that hyperalgesia produced by limb immobilization and chronic alcohol drinking precluded the expected OVX effects on pain sensitivity. The absence of OVX-related factors in the current study may also suggest that non-sex hormonal factors may disproportionately predispose individuals to CRPS, including psychosocial determinants of pain sensitivity, brain organizational factors, and/or sex differences in inflammation and oxidative stress status following immobilization-related injury [83]. Indeed, various genetic and psychosocial factors have been shown to influence the development of pain in AFAB individuals [84–87]. The first and only study to date investigating the role gender identity on chronic pain found that transgender and cisgender women report similar pain summation and chronic pain severity, both of which were greater than their cisgender male counterparts [88]. This supports anecdotal evidence that gender identity plays a more significant role in pain than genetic sex. While the present study focused on an animal model of CRPS, these findings may also contribute to our understanding of other under-investigated pain syndromes that disproportionally affect AFAB individuals, as well as sex-dependent medication strategies for treating alcohol-induced hyperalgesia symptoms [89].
Some limitations of the current study should be pointed out. First, our measure of hyperalgesia was limited to von Frey analysis of mechanical hypersensitivity, and no morphological or electrophysiological measures were conducted to verify a neuropathic state. Future studies should examine additional pain modalities (e.g., thermal) as well as measures of unprovoked or spontaneous pain, such as pain-avoidance assays [90]. While the current design incorporated a within-subject control for the casted leg, future studies including a non-casted control could shed additional light on how alcohol drinking impacts CRPS-related symptoms. We did not track estrous cycle in the current study due to potentially confounding influences on the measured behavior. However, in relation to this, a case-control study of AFAB CRPS patients found no association between current or cumulative endogenous estrogen exposure and CRPS [91]. Finally, our study employed a relatively low number of animals. While we were able to detect clear main effects of some of the factors under investigation, future studies incorporating additional variables may require considerably more subjects to uncover valuable interactions.
5. Conclusions
To our knowledge, this is the first study investigating pain behavior and neuroadaptations in a combined model of CRPS and alcoholic neuropathy. We found that 1) CRPS and alcohol drinking separately and additively produced mechanical hyperalgesia, 2) immobilization-induced hyperalgesia is associated with a potentially altered capacity for motor cortex plasticity, 3) chronic alcohol drinking in the context of CRPS appears to facilitate cingulate cortex hyperexcitability, and 4) circulating ovarian hormones do not affect mechanical hyperalgesia or associated neuroadaptations in our model of combined CRPS and alcoholic neuropathy. These findings suggest that the cingulate cortex may serve as a novel target for understanding the pathophysiology and treatment of these disorders.
Limb immobilization and alcohol increase mechanical nociceptive sensitivity
Immobilization hyperalgesia correlates with motor cortex ERK phosphorylation
Alcohol in the context of immobilization increases cingulate protein phosphorylation
Acknowledgements
This work was generously supported by funds through the Department of Physiology, LSU Health-New Orleans, as well as research and training grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA): F31AA028445 (JACR), K01AA02449403 (LSP), R01AA025996 (SE), T35AA021097 (PEM), and T32AA007577 (PEM) and National Institute of General Medical Sciences (NIGMS): R25GM121189.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- 1.Dahlhamer J., et al. , Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep, 2018. 67(36): p. 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elman I, Borsook D, and Volkow ND, Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol, 2013. 109: p. 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chancay MG, Guendsechadze SN, and Blanco I., Types of pain and their psychosocial impact in women with rheumatoid arthritis. Womens Midlife Health, 2019. 5: p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harden NR, et al. , Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain, 2010. 150(2): p. 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birklein F and Dimova V., Complex regional pain syndrome-up-to-date. Pain Rep, 2017. 2(6): p. e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shim H., et al. , Complex regional pain syndrome: a narrative review for the practising clinician. Br J Anaesth, 2019. 123(2): p. e424–e433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bass C and Yates G., Complex regional pain syndrome type 1 in the medico-legal setting: High rates of somatoform disorders, opiate use and diagnostic uncertainty. Med Sci Law, 2018. 58(3): p. 147–155. [DOI] [PubMed] [Google Scholar]
- 8.Bean DJ, et al. , Factors Associated With Disability and Sick Leave in Early Complex Regional Pain Syndrome Type-1. Clin J Pain, 2016. 32(2): p. 130–8. [DOI] [PubMed] [Google Scholar]
- 9.Bean DJ, et al. , Do psychological factors influence recovery from complex regional pain syndrome type 1? A prospective study. Pain, 2015. 156(11): p. 2310–2318. [DOI] [PubMed] [Google Scholar]
- 10.Vowles KE, et al. , Alcohol and Opioid Use in Chronic Pain: A Cross-Sectional Examination of Differences in Functioning Based on Misuse Status. J Pain, 2018. 19(10): p. 1181–1188. [DOI] [PubMed] [Google Scholar]
- 11.Edwards S., et al. , Alcohol and Pain: A Translational Review of Preclinical and Clinical Findings to Inform Future Treatment Strategies. Alcohol Clin Exp Res, 2020. 44(2): p. 368–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards S and Koob GF, Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol, 2010. 5(3): p. 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elman I and Borsook D., Common Brain Mechanisms of Chronic Pain and Addiction. Neuron, 2016. 89(1): p. 11–36. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc DM, et al. , The affective dimension of pain as a risk factor for drug and alcohol addiction. Alcohol, 2015. 49(8): p. 803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards S., et al. , Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology, 2012. 62(2): p. 1142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang S., et al. , Downregulation of M-channels in lateral habenula mediates hyperalgesia during alcohol withdrawal in rats. Sci Rep, 2019. 9(1): p. 2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley JL 3rd and King C., Self-report of alcohol use for pain in a multi-ethnic community sample. J Pain, 2009. 10(9): p. 944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cucinello-Ragland JA and Edwards S., Neurobiological aspects of pain in the context of alcohol use disorder. Int Rev Neurobiol, 2021. 157: p. 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan PL, Schutte KK, and Moos RH, Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction, 2005. 100(6): p. 777–86. [DOI] [PubMed] [Google Scholar]
- 20.Zale EL, Maisto SA, and Ditre JW, Interrelations between pain and alcohol: An integrative review. Clin Psychol Rev, 2015. 37: p. 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egli M, Koob GF, and Edwards S., Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev, 2012. 36(10): p. 2179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George O and Koob GF, Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev, 2010. 35(2): p. 232–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith ML, et al. , Anterior Cingulate Cortex Contributes to Alcohol Withdrawal-Induced and Socially Transferred Hyperalgesia. eNeuro, 2017. 4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansen JP and Fields HL, Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci, 2004. 7(4): p. 398–403. [DOI] [PubMed] [Google Scholar]
- 25.Johansen JP, Fields HL, and Manning BH, The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A, 2001. 98(14): p. 8077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei F and Zhuo M., Activation of Erk in the anterior cingulate cortex during the induction and expression of chronic pain. Mol Pain, 2008. 4: p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao H., et al. , Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction of long-term potentiation in rats. Neurosci Bull, 2009. 25(5): p. 301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanna PP, et al. , ERK regulation in chronic ethanol exposure and withdrawal. Brain Res, 2002. 948(1–2): p. 186–91. [DOI] [PubMed] [Google Scholar]
- 29.Zamora-Martinez ER and Edwards S., Neuronal extracellular signal-regulated kinase (ERK) activity as marker and mediator of alcohol and opioid dependence. Front Integr Neurosci, 2014. 8: p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo TZ, et al. , Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture in rats. J Pain, 2014. 15(10): p. 1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamaue Y., et al. , Immobilization-induced hypersensitivity associated with spinal cord sensitization during cast immobilization and after cast removal in rats. J Physiol Sci, 2013. 63(6): p. 401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beerthuizen A., et al. , Demographic and medical parameters in the development of complex regional pain syndrome type 1 (CRPS1): prospective study on 596 patients with a fracture. Pain, 2012. 153(6): p. 1187–1192. [DOI] [PubMed] [Google Scholar]
- 33.Levitt DE, et al. , Chronic Alcohol Dysregulates Skeletal Muscle Myogenic Gene Expression after Hind Limb Immobilization in Female Rats. Biomolecules, 2020. 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dina OA, et al. , Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci, 2000. 20(22): p. 8614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terkelsen AJ, Bach FW, and Jensen TS, Experimental forearm immobilization in humans induces cold and mechanical hyperalgesia. Anesthesiology, 2008. 109(2): p. 297–307. [DOI] [PubMed] [Google Scholar]
- 36.Dixon WJ, Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol, 1980. 20: p. 441–62. [DOI] [PubMed] [Google Scholar]
- 37.McGinn MA, et al. , Withdrawal from Chronic Nicotine Exposure Produces Region-Specific Tolerance to Alcohol-Stimulated GluA1 Phosphorylation. Alcohol Clin Exp Res, 2016. 40(12): p. 2537–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pahng AR, et al. , Neurobiological Correlates of Pain Avoidance-Like Behavior in Morphine-Dependent and Non-Dependent Rats. Neuroscience, 2017. 366: p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boissoneault J, Lewis B, and Nixon SJ, Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol, 2019. 75: p. 47–54. [DOI] [PubMed] [Google Scholar]
- 40.Moskal D., et al. , Effects of experimental pain induction on alcohol urge, intention to consume alcohol, and alcohol demand. Exp Clin Psychopharmacol, 2018. 26(1): p. 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cremonte M and Cherpitel CJ, Alcohol intake and risk of injury. Medicina (B Aires), 2014. 74(4): p. 287–92. [PMC free article] [PubMed] [Google Scholar]
- 42.Alford DP, et al. , Primary Care Patients with Drug Use Report Chronic Pain and Self-Medicate with Alcohol and Other Drugs. J Gen Intern Med, 2016. 31(5): p. 486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogt BA, Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci, 2005. 6(7): p. 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freund W., et al. , The role of periaqueductal gray and cingulate cortex during suppression of pain in complex regional pain syndrome. Clin J Pain, 2011. 27(9): p. 796–804. [DOI] [PubMed] [Google Scholar]
- 45.Freund W., et al. , Different activation of opercular and posterior cingulate cortex (PCC) in patients with complex regional pain syndrome (CRPS I) compared with healthy controls during perception of electrically induced pain: a functional MRI study. Clin J Pain, 2010. 26(4): p. 339–47. [DOI] [PubMed] [Google Scholar]
- 46.Maihofner C and Speck V., Graded motor imagery for complex regional pain syndrome: where are we now? Eur J Pain, 2012. 16(4): p. 461–2. [DOI] [PubMed] [Google Scholar]
- 47.Critchley HD, et al. , Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain, 2003. 126(Pt 10): p. 2139–52. [DOI] [PubMed] [Google Scholar]
- 48.Gentil AF, et al. , Physiological responses to brain stimulation during limbic surgery: further evidence of anterior cingulate modulation of autonomic arousal. Biol Psychiatry, 2009. 66(7): p. 695–701. [DOI] [PubMed] [Google Scholar]
- 49.Gillies MJ, et al. , Direct neurophysiological evidence for a role of the human anterior cingulate cortex in central command. Auton Neurosci, 2019. 216: p. 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knudsen LF, et al. , Complex regional pain syndrome: a focus on the autonomic nervous system. Clin Auton Res, 2019. 29(4): p. 457–467. [DOI] [PubMed] [Google Scholar]
- 51.Sellmeijer J., et al. , Hyperactivity of Anterior Cingulate Cortex Areas 24a/24b Drives Chronic Pain-Induced Anxiodepressive-like Consequences. J Neurosci, 2018. 38(12): p. 3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao R., et al. , Neuropathic Pain Causes Pyramidal Neuronal Hyperactivity in the Anterior Cingulate Cortex. Front Cell Neurosci, 2018. 12: p. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bliss TV, et al. , Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci, 2016. 17(8): p. 485–96. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L., et al. , NMDA and AMPA receptors in the anterior cingulate cortex mediates visceral pain in visceral hypersensitivity rats. Cell Immunol, 2014. 287(2): p. 86–90. [DOI] [PubMed] [Google Scholar]
- 55.Xu H., et al. , Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci, 2008. 28(29): p. 7445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vollstadt-Klein S., et al. , Increased activation of the ACC during a spatial working memory task in alcohol-dependence versus heavy social drinking. Alcohol Clin Exp Res, 2010. 34(5): p. 771–6. [DOI] [PubMed] [Google Scholar]
- 57.Eisenberg E., et al. , Evidence for cortical hyperexcitability of the affected limb representation area in CRPS: a psychophysical and transcranial magnetic stimulation study. Pain, 2005. 113(1–2): p. 99–105. [DOI] [PubMed] [Google Scholar]
- 58.Schwenkreis P., et al. , Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology, 2003. 61(4): p. 515–9. [DOI] [PubMed] [Google Scholar]
- 59.Maihofner C., et al. , The motor system shows adaptive changes in complex regional pain syndrome. Brain, 2007. 130(Pt 10): p. 2671–87. [DOI] [PubMed] [Google Scholar]
- 60.Bolwerk A, Seifert F, and Maihofner C., Altered resting-state functional connectivity in complex regional pain syndrome. J Pain, 2013. 14(10): p. 1107–1115e8. [DOI] [PubMed] [Google Scholar]
- 61.Robins MT, Heinricher MM, and Ryabinin AE, From Pleasure to Pain, and Back Again: The Intricate Relationship Between Alcohol and Nociception. Alcohol Alcohol, 2019. 54(6): p. 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roltsch Hellard EA, Impastato RA, and Gilpin NW, Intra-cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats. Addict Biol, 2017. 22(3): p. 692–701. [DOI] [PubMed] [Google Scholar]
- 63.Gustin SM, et al. , NMDA-receptor antagonist and morphine decrease CRPS-pain and cerebral pain representation. Pain, 2010. 151(1): p. 69–76. [DOI] [PubMed] [Google Scholar]
- 64.Yu W., et al. , Chronic inflammatory pain drives alcohol drinking in a sex-dependent manner for C57BL/6J mice. Alcohol, 2019. 77: p. 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adrienne McGinn M, Edwards KN, and Edwards S., Chronic inflammatory pain alters alcohol-regulated frontocortical signaling and associations between alcohol drinking and thermal sensitivity. Neurobiol Pain, 2020. 8: p. 100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ekholm O., et al. , Alcohol and smoking behavior in chronic pain patients: the role of opioids. Eur J Pain, 2009. 13(6): p. 606–12. [DOI] [PubMed] [Google Scholar]
- 67.Jakubczyk A., et al. , Reductions in physical pain predict lower risk of relapse following alcohol treatment. Drug Alcohol Depend, 2016. 158: p. 167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witkiewitz K., et al. , Pain as a predictor of heavy drinking and any drinking lapses in the COMBINE study and the UK Alcohol Treatment Trial. Addiction, 2015. 110(8): p. 1262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macfarlane GJ and Beasley M., Alcohol Consumption in Relation to Risk and Severity of Chronic Widespread Pain: Results From a UK Population-Based Study. Arthritis Care Res (Hoboken), 2015. 67(9): p. 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeung EW, et al. , The Association Between Alcohol Consumption and Pain Interference in a Nationally Representative Sample: The Moderating Roles of Gender and Alcohol Use Disorder Symptomatology. Alcohol Clin Exp Res, 2020. 44(3): p. 645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson T., et al. , Analgesic Effects of Alcohol: A Systematic Review and Meta-Analysis of Controlled Experimental Studies in Healthy Participants. J Pain, 2017. 18(5): p. 499–510. [DOI] [PubMed] [Google Scholar]
- 72.Neddenriep B., et al. , Pharmacological mechanisms of alcohol analgesic-like properties in mouse models of acute and chronic pain. Neuropharmacology, 2019. 160: p. 107793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pahng AR, et al. , The Prefrontal Cortex as a Critical Gate of Negative Affect and Motivation in Alcohol Use Disorder. Curr Opin Behav Sci, 2017. 13: p. 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Mos M., et al. , The incidence of complex regional pain syndrome: a population-based study. Pain, 2007. 129(1–2): p. 12–20. [DOI] [PubMed] [Google Scholar]
- 75.Ammendola A., et al. , Gender and peripheral neuropathy in chronic alcoholism: a clinical-electroneurographic study. Alcohol Alcohol, 2000. 35(4): p. 368–71. [DOI] [PubMed] [Google Scholar]
- 76.Bartley EJ and Fillingim RB, Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth, 2013. 111(1): p. 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mogil JS and Bailey AL, Sex and gender differences in pain and analgesia. Prog Brain Res, 2010. 186: p. 141–57. [DOI] [PubMed] [Google Scholar]
- 78.Diehl A., et al. , Alcoholism in women: is it different in onset and outcome compared to men? Eur Arch Psychiatry Clin Neurosci, 2007. 257(6): p. 344–51. [DOI] [PubMed] [Google Scholar]
- 79.Fama R, Le Berre AP, and Sullivan EV, Alcohol’s Unique Effects on Cognition in Women: A 2020 (Re)view to Envision Future Research and Treatment. Alcohol Res, 2020. 40(2): p. 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agabio R., et al. , Sex Differences in Alcohol Use Disorder. Curr Med Chem, 2017. 24(24): p. 2661–2670. [DOI] [PubMed] [Google Scholar]
- 81.Grant BF, et al. , Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 2017. 74(9): p. 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buryanov A, Kostrub A, and Kotiuk V., Endocrine disorders in women with complex regional pain syndrome type I. Eur J Pain, 2017. 21(2): p. 302–308. [DOI] [PubMed] [Google Scholar]
- 83.Tang C., et al. , Sex differences in complex regional pain syndrome type I (CRPS-I) in mice. J Pain Res, 2017. 10: p. 1811–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mogil JS, et al. , The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A, 2003. 100(8): p. 4867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fillingim RB, et al. , Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol, 2005. 69(1): p. 97–112. [DOI] [PubMed] [Google Scholar]
- 86.Olsen MB, et al. , Pain intensity the first year after lumbar disc herniation is associated with the A118G polymorphism in the opioid receptor mu 1 gene: evidence of a sex and genotype interaction. J Neurosci, 2012. 32(29): p. 9831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu S., et al. , Genes known to escape X chromosome inactivation predict comorbid chronic musculoskeletal pain and posttraumatic stress symptom development in women following trauma exposure. Am J Med Genet B Neuropsychiatr Genet, 2019. 180(6): p. 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strath LJ, et al. , Sex and Gender are Not the Same: Why Identity Is Important for People Living with HIV and Chronic Pain. J Pain Res, 2020. 13: p. 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bergeson SE, et al. , Binge Ethanol Consumption Increases Inflammatory Pain Responses and Mechanical and Cold Sensitivity: Tigecycline Treatment Efficacy Shows Sex Differences. Alcohol Clin Exp Res, 2016. 40(12): p. 2506–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pahng AR and Edwards S., Measuring Pain Avoidance-Like Behavior in Drug-Dependent Rats. Curr Protoc Neurosci, 2018. 85(1): p. e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Mos M., et al. , Estrogens and the risk of complex regional pain syndrome (CRPS). Pharmacoepidemiol Drug Saf, 2009. 18(1): p. 44–52. [DOI] [PubMed] [Google Scholar]


