Summary
Pituitary hormone deficiency occurs in ∼1:4,000 live births. Approximately 3% of the cases are due to mutations in the alpha isoform of POU1F1, a pituitary-specific transcriptional activator. We found four separate heterozygous missense variants in unrelated individuals with hypopituitarism that were predicted to affect a minor isoform, POU1F1 beta, which can act as a transcriptional repressor. These variants retain repressor activity, but they shift splicing to favor the expression of the beta isoform, resulting in dominant-negative loss of function. Using a high-throughput splicing reporter assay, we tested 1,070 single-nucleotide variants in POU1F1. We identified 96 splice-disruptive variants, including 14 synonymous variants. In separate cohorts, we found two additional synonymous variants nominated by this screen that co-segregate with hypopituitarism. This study underlines the importance of evaluating the impact of variants on splicing and provides a catalog for interpretation of variants of unknown significance in POU1F1.
Keywords: alternative splicing, growth hormone deficiency, multiplexed assays of variant effects, transcriptional regulation, massively parallel splicing assay, variants of uncertain significance, hypopituitarism, PIT-1
Introduction
POU1F1 (formerly PIT-1 [MIM: 173110]) is a signature pituitary transcription factor that directly regulates the transcription of growth hormone (GH [MIM: 139250]), prolactin (PRL [MIM: 176760]), and both the alpha (CGA [MIM: 118850]) and beta (TSHB [MIM: 188540]) subunits of thyroid-stimulating hormone (TSH).1,2 In mice, Pou1f1 is expressed after the peak expression of Prop1 (MIM: 601538) at E14.5 and remains expressed into adulthood.3,4 A well-characterized mutant of Pou1f1 (Pou1f1dw/dw) carries a spontaneous missense mutation (p.Trp251Cys) in the homeodomain that disrupts DNA binding.4,5 The homozygous mutant mice have no somatotrophs, lactotrophs, or thyrotrophs except for the Pou1f1-independent rostral tip thyrotrophs.4,6, 7, 8 In humans, loss of POU1F1 function typically results in GH, TSH, and PRL deficiency.9
POU1F1 undergoes an evolutionarily conserved program of alternative splicing,10,11 resulting in a predominant isoform, alpha, that acts as a transcriptional activator and a minor isoform, beta, that acts as a transcriptional repressor.12, 13, 14 In the human pituitary gland, the beta isoform comprises approximately 1%–3% of POU1F1 transcripts.10,15 The POU1F1 beta isoform transcript is created by utilization of an alternative splice acceptor sequence for exon 2, located 78 bp upstream of the alpha acceptor, resulting in a 26 amino acid insertion that encodes an interaction domain for the transcription factor ETS1 (MIM: 164720). This insertion, which is absent in the alpha isoform, disrupts the POU1F1 transactivation domain at amino acid 48. The POU1F1 alpha and beta isoforms have different activities depending on the context of the target gene.12 For example, the POU1F1 alpha isoform activates its own expression, but the beta isoform does not, and the beta isoform interferes with alpha-isoform-mediated auto-activation.14 Although alternative splicing of POU1F1 is evolutionarily conserved among vertebrates, the functional significance of the minor, beta isoform remains unclear.10
The first case of a recessive POU1F1 loss of function was described in a child with combined pituitary hormone deficiency (CPHD [MIM: 613038, 262600, 221750, 262700, 601538, 173110, 615849, 600577, 182230, 612079, and 602146]) born to consanguineous parents;16 since then, many unique variants in POU1F1 have been reported in people with CPHD or isolated growth hormone deficiency (IGHD [MIM: 307200, 262400, 173100, 612781, 139250, 618157, 139191, 262500, 615925, and 618160)17, 18, 19, 20, 21, 22, 23(reviewed in Jadhav et al.24). A few dominant-negative mutations have been reported that most likely act by interfering with the function of POU1F1 dimers. The variant c.227C>T (p.Pro76Leu) alters the transactivation domain and causes completely penetrant IGHD;25 c.646A>G (p.Lys216Glu) interferes with the ability of POU1F1 to interact with retinoic acid receptors and CREBBP (p300 [MIM: 600140]);26 and c.811C>T (p.Arg271Trp) interferes with the ability of POU1F1 to be tethered to the nuclear matrix through MATR3 (MIM: 164015), SATB1 (MIM: 602075), and CTNNB1 (MIM: 116806).27 All of the reported mutations are located in domains shared by the alpha and beta isoforms of POU1F1 and were functionally tested with the alpha isoform only.
We found four missense variants, in four independent families, that shift splicing to favor the POU1F1 beta isoform almost exclusively while retaining its transcriptional repressor activity on the POU1F1 enhancer. We used a high-throughput assay to identify variants in and around exon 2 that cause exon skipping, isoform switching, or cryptic isoform use. With this splicing effect catalog, we evaluated additional families with hypopituitarism and identified two unrelated individuals carrying synonymous POU1F1 variants that affect its splicing without changing the amino acid sequence. This study underscores the importance of evaluating splicing defects as a disease mechanism.
Material and methods
Informed consent
The studies were approved by the following ethical committees: the local Comite de Eticae Pesquisa da Faculdade de Medicina da Universidade de São Paulo (CEP-FMUSP) and the National Comite Nacional de Etica em Pesquisa (CONEP) CAAE, 06425812.4.0000.0068; the Ethics Committee of the Faculty of Medicine, University of Leipzig (UL), Karl-Sudhoff-Institute for Medical History and Natural Sciences, Käthe-Kollwitz-Straße 82, 04109 Leipzig, Germany; and the Comité de Ética en Investigación (Research Ethics Committee) of the Hospital de Niños Ricardo Gutierrez (HNG), Gallo 1330, Ciudad Autónoma de Buenos Aires, Argentina (CEI no. 16.06). The GENHYPOPIT network collected anonymized information in a database declared to health authorities in accordance with local regulations at Aix-Marseille Université (AMU)-Conception Hospital (Assistance Publique-Hôpitaux de Marseille, AP-HM), and a declaration was made to the National Commission for Data Protection and Liberties (CNIL-France): 1991429 v.0. Adult individuals or the parents of children signed a written informed consent to participate. Families 1, 3, and 6 are historical cases that were referred to the GENHYPOPIT network for genetic testing. Limited information is available for families 1 and 3, and they were lost for follow-up. The University of Michigan (UM) Institutional Review Board found the study exempt because DNA samples were anonymized before exome sequencing at UM.
Genomic DNA sequencing
Individuals from families 1, 2, 4, and 5 underwent whole-exome sequencing (WES). Representative POU1F1 variants in families 3 and 6 were discovered in a traditional CPHD candidate gene screening via Sanger sequencing (PROP1, POU1F1, LHX3, and LHX4) (LHX3 [MIM: 600577], LHX4 [MIM: 602146]). WES of families 1 and 5 was carried out at UM as previously described.17 WES of family 2 was performed at the Broad Institute as previously described.28 WES of family 4 was performed at the Institute of Human Genetics at UL.
Expression vectors and cell culture
The open reading frame of either POU1F1 isoform alpha (GenBank: NM_000306.3) or beta (Genbank: NM_001122757.2) was cloned into pcDNA3.1+/C-(K)-DYK. We used site-directed mutagenesis to obtain each of the variant POU1F1 beta isoforms: p.Ser50Ala, p.Ile51Ser, p.Leu52Trp, and p.Ser53Ala in the beta isoform (Genscript). A firefly luciferase reporter gene was constructed in pNBm81-luc with 14 kb of the mouse Pou1f1 5′ flanking sequences that includes early and late enhancers and the promoter and 13 bp of the 5′ UTR. Cloning was performed with Infusion HD (Clontech) or NEBuilder HiFi DNA Assembly (New England Biolabs). Plasmid sequences were confirmed by Sanger sequencing. We used the pRL-TK Renilla (Promega) as a normalization control and pcDNA3.1(−) (Thermo Fisher) to keep the total DNA constant. COS-7 and GH3 cells were purchased from the American Type Culture Collection. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO, Grand Island, NY, USA) containing 10% fetal bovine serum and pen-strep (GIBCO). Plasmids were transiently transfected with ViaFect Transfection Reagent (Promega, Madison, WI, USA). Luciferase activities were measured as suggested by the manufacturer (dual-luciferase assay system; Promega).
Exon trapping assay
We cloned human POU1F1 exon 2, flanked by partial intron 1 (85 bp upstream) and intron 2 (178 bp downstream), into the BamHI cloning site of the pSPL3 plasmid (Invitrogen) to create an exon-trapping plasmid with a total insert size of 413 bp. Similarly, a minigene exon-trapping plasmid was constructed that included the last 85 bp of intron 1 and the first 85 bp of intron 5 for a total insert size of 3,442 bp including exons 2, 3, 4, and 5. We used site-directed mutagenesis to create the desired variants. Plasmids were transiently transfected into COS-7 cells. Total RNA was purified with RNeasy mini (QIAGEN). After reverse transcription, we analyzed exon trapping by using RT-PCR with the following primers: primers SD6 forward (5′-TCTGAGTCACCTGGACAACC-3′) and SA2 reverse (5′-ATCTCAGTGGTATTTGTGAGC-3′).29
POU1F1 saturation mutagenesis
The cloned POU1F1 fragment in pSPL3 was divided into four overlapping tiles of 150 bp each, spanning exon 2 plus flanking introns (79 bp upstream to 131 bp downstream). Mutant tile libraries containing every possible single-nucleotide variant were synthesized as a single 150-mer oligonucleotide pool by Twist Bio. We used HiFi Assembly to replace each wild-type (WT) tile with the respective mutant tile library amplified from the oligo pool. The resulting mutant minigene library pools were transformed in 10B E. coli (New England Biolabs) and had a minimum coverage of 90 clones per mutation.
Mutant library barcoding and sequencing
To tag each mutant minigene clone with a unique barcode, we inserted a random barcode sequence (N20) by HiFi Assembly into the MscI site within the common 3′ UTR. We used subassembly sequencing30 to pair each 3′ UTR barcode with its linked variant(s) in cis. Briefly, a fragment starting with the POU1F1 insert and ending at the N20 barcode (2.2 kb downstream) was amplified from the plasmid library DNA by PCR with 5′-phosphorylated primers. We re-circularized the resulting linear fragment by intramolecular ligation with T4 DNA ligase (NEB) to bring each barcode in close proximity to the mutagenized region. From this re-circularized product, paired-end amplicon sequencing libraries were generated such that each reverse read contained a plasmid barcode and the paired forward read contained a sequence from the associated POU1F1 insert. We clustered barcode reads with starcode31 (arguments “-d 1 -r 3”) to generate a catalog of known barcodes. We called variants within each barcode group by using freebayes32 and filtered them to require majority support and read depth ≥ 4 along the entire region targeted for mutagenesis. Barcode-variant pairing was confirmed by Sanger sequencing of 15 clones selected at random from the POU1F1 library; of those, 13/15 were found in the final catalog of reconstructed sequences and associated barcodes, and all 13 perfectly matched the Sanger-sequenced clones.
Pooled exon-trap transfection and RNA-seq
COS-7 cells were plated at 5 × 106 cells/60 mm plate. Each was transfected with 4 μg of the barcoded mutant exon-trap library with ViaFect reagent (3:1 ratio to DNA). After 24 h, we purified RNA as above, and we used 5 μg of total RNA to prepare first-strand cDNA by using the SuperScript III First-Strand Synthesis kit (Invitrogen) with oligo dT primers. Spliced transcript was amplified via nested PCR, initially for six cycles with the SD6F/SA2R primers, followed by 20 cycles with primers SD2F/jklab0046 (TGTAGTCAGTGCCATCTTGGATCT). Paired-end Illumina sequencing libraries were generated by tailing PCR (six cycles) with a forward primer within the constant upstream exon (GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTAGGGCATAGTGCCATCTTGGATCT) and a reverse primer immediately downstream of the N20 barcode (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGAGTGAACTGCACTGTGACAAGCTGC). Unique dual i5/i7 indices were added by a second round of tailing PCR (six cycles), and the resulting products were purified by SPRI bead cleanup and submitted for Illumina sequencing on a HiSeq 4000 and/or NovaSeq instrument.
RNA-seq processing pipeline
Reverse reads containing the plasmid barcode were searched for exact match to a known barcode from the plasmid library. Forward reads containing the spliced sequence were mapped to a variant-specific reference consisting of the POU1F1 exon trap reference sequence with the respective mutation introduced in silico via GMAP33 (arguments “-t 8 -f samse–microexon-spliceprob=1.0–allow-close-indels=2”). From the spliced reads, an isoform catalog was tallied requiring each isoform to be represented by at least three distinct barcodes and nine reads. We tallied spliced reads associated with each barcode to produce per-barcode isoform usage counts and percent spliced in (PSI) fractions. We then aggregated barcodes corresponding to the same POU1F1 variant (weighted by the number of reads obtained) to generate for each variant a mean PSI score for all known isoforms. Isoforms not matching a known isoform (beta, skip, or alpha) were placed in a catch-all category called “other.” Barcodes represented by fewer than three reads were discarded from further analyses.
Fold-change and significance testing
PSI distributions under the null hypothesis (no splicing difference) were approximated by bootstrap sampling. For each tested variant, the equivalent number of barcodes was drawn (with replacement) from intronic background region variants (defined as intronic variants > 20 bp from exon boundaries), repeated 1,000 times, and used to derive a null distribution against which each per-variant observed PSI values was converted to a Z score. For each variant, the Z scores were combined across replicates via Stouffer’s test. This process was repeated separately for each isoform. For each of the three tested alternative isoforms (beta, skip, or other), a fold-change over background was calculated for each variant. This was taken as the PSI value for that variant and isoform, divided by the sampling mean PSI for that isoform derived from the intron background region barcodes; the median of these values was then computed across replicates. Variants with a Z score > 4.16 (Bonferroni-corrected threshold for p = 0.05) and at least a 3-fold change from the average null distribution PSI for the beta, skip, or other isoform in at least half the replicates were nominated as splice disruptive variants (SDVs). Variants that met the Z score threshold but had a fold-change between 2 and 3, or that met the SDV criteria overall but failed to meet it individually in ≥7 replicates, were labeled as intermediate.
Comparison of bioinformatic predictors
HAL delta_psi scores,34 SPANR zdelta_psi scores,35 SpliceAI ds_max scores,35 and MMSplice delta_logit_psi scores36 were obtained from their original publications without modification. To compute per-variant ESRseq scores,37 we took the difference between the mean ESRseq Z scores of hexamers overlapping a variant position from that of hexamers overlapping the corresponding WT position. We obtained precision-recall curves to summarize each algorithm’s ability to predict the experimental determination of splice disruptiveness. For algorithms that output signed scores, area under the curve (prAUC) was separately computed with signed and absolute scores as input and the higher prAUC was taken.
Selection of candidate RNA-binding proteins
RNACompete Z scores38 were obtained from the cisBP-RNA database. At each position, WT and variant-containing Z scores were taken as the maximum among the overlapping k-mers, and the difference taken between the WT and variant scores. Motifs with high scoring matches (WT Z ≥ 3) to the WT sequence in the beta variant cluster (c.143 to c.167) were then pursued further.
Results
Mutations in the POU1F1 beta coding region cause hypopituitarism
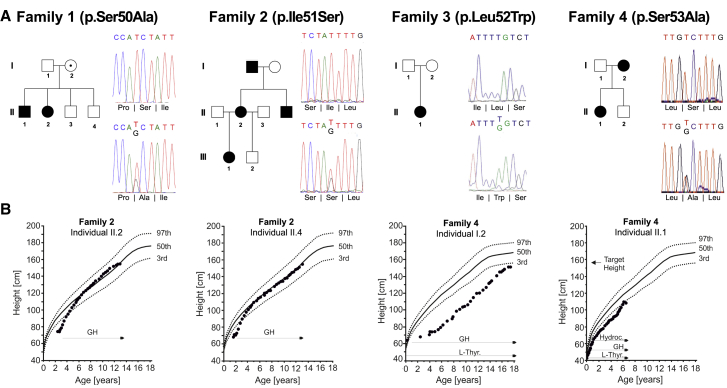
We initially focused on four cases of hypopituitarism from different cohorts in Europe and South America (Figure 1A). Affected individuals’ presentation was variable, ranging from multiple hormone deficiency with pituitary stalk interruption (family 1) to isolated GH deficiency (family 2) (Table 1, Table 2, Figure S1). The affected individuals had severe short stature and responded well to GH therapy (Figure 1B). To identify causal variants, we performed whole-exome sequencing (WES) for individuals in three families. Combined with conventional Sanger sequencing in another family, this revealed four missense variants in exon 2 of the POU1F1 beta isoform, each in an unrelated family (Figures 1A and 2A). The four individual POU1F1 missense variants are absent from Genome Aggregation Database (gnomAD) and in-house population-matched exome databases,39,40 and they are predicted to be damaging by several bioinformatic algorithms: CADD (18.65–25.8), SIFT (damaging), and MutationTaster (disease causing). Remarkably, these variants clustered in four consecutive codons within the beta isoform: c.148T>G (p.Ser50Ala), c.152T>G (p.Ile51Ser), c.155T>G (p.Leu52Trp), and c.157T>G (p.Ser53Ala) (Table 1). Only one of these (c.155T>G, family 3) appears to be de novo; the others were dominantly inherited and co-segregate with hypopituitarism phenotypes, except for c.148T>G, which was inherited from the apparently unaffected parent in family 1, indicating that if causal, this variant is incompletely penetrant. The other parent in family 1, the two affected children, and one unaffected relative also carried a variant of uncertain significance, SIX3 c.221 C>G (p.Pro74Arg) (MIM: 603714). No other variants in known hypopituitarism genes were detected.
Figure 1.
Clinical characteristics of the variants of POU1F1 beta coding region
(A) Pedigrees and the sequence of POU1F1 variants. Families 1–4 have variants in the POU1F1 beta coding region: c.148T>G (p.Ser50Ala), c.152T>G (p.Ile51Ser), c.155T>G (p.Leu52Trp), and c.157T>G (p.Ser53Ala).
(B) Growth curve of the affected individuals from families 2 and 4. GH replacement therapy was effective in reaching ideal height.
Table 1.
Clinical and molecular features of affected individuals
| Feature | Family 1 | Family 2 | Family 3 | Family 4 | Family 5 | Family 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individuals | II.1 | II.2 | I.1 | II.2 (i) | II.4 | III.1 | II.1 | I.2 | II.1 | I.1 | II.1 (i) | II.1 (i) | II.2 |
| Gender | male | female | male | female | male | female | female | female | female | male | male | male | male |
| Age at diagnosis | <5 years | <5 years | forties | <5 years | <5 years | <5 years | <5 years | <5 years | <5 years | preteen | <5 years | preteen | preteen |
| Hormone deficiency at diagnosis | GH, TSH | GH, TSH | GH | GH | GH | GH | GH, TSH, PRL | TSH | GH, TSH | GH | GH | GH | GH |
| Height at diagnosis (SDS) | N/A | N/A | −3.7 | −5 | −5.3 | N/A | −4 | −5.42 | −3.45 | −4.2 | −4.15 | N/A | N/A |
| rhGH treatment | N/A | N/A | no | yes | yes | yes | N/A | yes | yes | yes | yes | yes | N/A |
| Final height (cm/SDS) | N/A | N/A | 150/−3.7 | 156.5/−0.9 | 165/−1.5 | N/A | N/A | 150.9/−2.67 | still growing | 147.9/−3.66 | still growing | N/A | N/A |
| Pituitary hormone deficiencies | GH, TSH | GH, TSH | GH | GH | GH | GH | GH, TSH, PRL | GH, TSH, PRL, (ACTHa) | GH, TSH, PRL, (ACTHa) | GH | GH | GH | GH |
| Pituitary MRI | disrupted stalk | disrupted stalk | normal | normal | normal | N/A | normal | normal | normal | N/A | APH | normal | N/A |
| Brain MRI | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1 cm left frontal and parietal lobe abnormality | normal | normal | normal | N/A | N/A |
| Dysmorphic features | none noted | none noted | none noted | large forehead | none noted | N/A | N/A | intellectual disability, strabismus, astigmatism, nystagmus, dysplastic thyroid gland | macroglossia, bilateral hearing impairment, developmental delay, dysplastic thyroid gland | none noted | short stature, frontal bossing, high pitched voice | N/A | N/A |
| Molecular findings (heterozygous) | c.148T>G | c.148T>G | c.152T>G | c.152T>G | c.152T>G | c.152T>G | c.155T>G | c.157T>G | c.157T>G | c.150T>G | c.150T>G | c.153T>A | c.153T>A |
| p.Ser50Ala | p.Ser50Ala | p.Ile51Ser | p.Ile51Ser | p.Ile51Ser | p.Ile51Ser | p.Leu52Trp | p.Ser53Ala | p.Ser53Ala | p.Ser50= | p.Ser50= | p.Ile51= | p.Ile51= | |
Abbreviations are as follows: i, index case; N/A, not available; MRI, magnetic resonance imaging; rhGH, recombinant human growth hormone; APH, anterior pituitary hypoplasia; SDS, standard deviation score; ACTH, adrenocorticotropin.
Diagnosed in late twenties for I.2 and newborn for II.1.
Table 2.
Biochemical tests for hormone deficiencies in affected individuals
| Feature | Family 1 | Family 2 | Family 3 | Family 4 | Family 5 | Family 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individuals | II.1 | II.2 | I.1 | II.2 (i) | II.4 | III.1 | II.1 | I.2 | II.1 | I.1 | II.1 (i) | II.1 (i) | II.2 |
| GHSTs | N/A | N/A | clonidine, ITT | clonidine, ITT | clonidine | N/A | N/A | glucagon, insulin, clonidine | basal neonatal | insulin, l-dopa | arginine, clonidine | ITT | ITT |
| GH peak (ng/mL) | N/A | N/A | 7.6 | 0.9 | 0.5 | N/A | N/A | ND | ND | 2.9a | 2.7b | 3.7 mU/L | 3.6 mU/L |
| TSH (U/L) | N/A | N/A | 0.6 | 0.7 | 1.7 | N/A | N/A | N/A | 0.28 | N/A | 3.4 | normal | normal |
| Total T4 (ug/dL) | N/A | N/A | 6.4 | 6 | 5.6–7.3 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Free T4 (ng/dL) | N/A | N/A | 0.7 | 0.6–1.1 | 0.6–0.9 | N/A | N/A | N/A | 0.4 | 0.9 | 1.2 | N/A | N/A |
| Prolactin (ng/mL) | N/A | N/A | N/A | 3.8–12 | 3.2–6.6 | N/A | N/A | 2.0 mU/L | 9 mU/L | 8.3 | 4.4 | N/A | N/A |
| Cortisol (μg/dL) | N/A | N/A | N/A | peak ITT 42 | normal | N/A | N/A | treated in twenties | 36.2 nmol/L | 13 | 13.2 | N/A | N/A |
| LH/FSH | N/A | N/A | N/A | early puberty | normal puberty | N/A | N/A | delayed puberty, spontaneous pregnancy | normal | spontaneous puberty | 0.1/0.75 | spontaneous puberty | N/A |
Abbreviations are as follows: i, index case; N/A, not available; GHSTs, growth hormone stimulation tests; ND, non-detectable; ITT, insulin-tolerance test; LH, luteinizing hormone; FSH, follicle stimulating hormone.
Cut-off 4.8 ng/mL.
Cut-off 10 ng/mL.
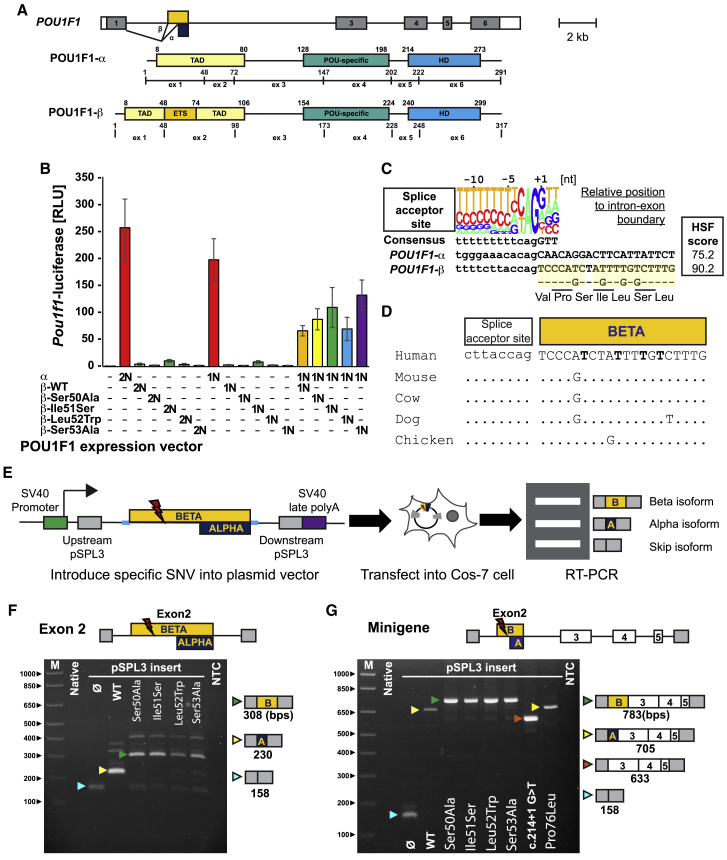
Figure 2.
Variants in the POU1F1 beta coding region suppress the function of the alpha isoform and lead to splicing abnormality
(A) Schematic of the human POU1F1 gene and protein isoforms produced by use of alternate splice acceptors at exon 2. The Pou1f1 beta isoform has an insertion of 26 amino acids located at amino acid 48 in the transactivation domain.
(B) COS-7 cells were transfected with a Pou1f1-luciferase reporter gene and expression vectors for POU1F1 alpha or beta isoforms either singly or together in the ratios indicated (2N and 1N). WT POU1F1 alpha has strong activation at 2N and 1N dosages. WT and variants of the POU1F1 beta isoform have no significant activation over background. A 50:50 mix of alpha and WT beta isoforms exhibited reduced activation. The variant beta isoforms suppress alpha-isoform-mediated activation to a degree similar to WT. The error bars represent standard deviation.
(C) Diagram of the splice acceptor site consensus and the genomic DNA sequence at the boundary between intron 1 and splice sites utilized in exon 2 of POU1F1.41
(D) Evolutionary conservation of the genomic sequence encoding the POU1F1 beta isoform in mammals and chickens.
(E) Exon trapping assay with pSPL3 exon trap vector containing exon 2 of POU1F1 and portions of the flanking introns.
(F) Ethidium bromide-stained gel of exon trap products from cells transfected with the indicated plasmid. Arrowheads indicate the expected products for exon skipping (blue), the alpha isoform (yellow), and the beta isoform (green).
(G) POU1F1 minigenes spanning from intron 1 to 5, with all of the intervening exons, were engineered with the indicated variants and assayed for splicing. WT and p.Pro76Leu POU1F1 splice to produce the alpha isoform, the G>T change in the splice acceptor causes exon skipping (red arrow), and the other variants all splice to produce the POU1F1 beta isoform.
Sequence variants retain POU1F1 beta isoform repressor function
We used a transient transfection assay to determine whether these variants disrupt the ability of POU1F1 to transactivate its own highly conserved distal enhancer element42, 43, 44 (Figure 2B). As expected, a Pou1f1 promoter reporter was strongly activated when co-transfected with cDNA of POU1F1 alpha isoform, which does not include the variant sites. Neither WT POU1F1 beta isoform nor any of the four missense variants found in affected individuals showed significant activation of the Pou1f1-luc reporter. Consistent with a repressive role for POU1F1 beta, co-transfection with alpha at a 1:1 ratio significantly suppressed activation compared to the equivalent amount of the alpha isoform alone. The four POU1F1 beta variants and WT beta repressed POU1F1 alpha activity to a similar degree.
Missense variants disrupt normal POU1F1 splicing to favor the beta isoform
Alpha is normally the predominant POU1F1 isoform, comprising 97%–99% of the POU1F1 transcripts in human pituitary gland,10 but its splice acceptor is predicted to be much weaker than the beta isoform acceptor 78 bp upstream (MaxEntScan;45 scores, alpha: −3.63, beta: 6.96) (Figure 2C). The beta isoform splice acceptor sequence and coding region are evolutionarily conserved in mammals and birds (Figure 2D). We reasoned that splice repressor and/or enhancer sequences in POU1F1 may dictate the normal balance of alpha over beta isoforms, and these may be disrupted by the four T>G transversions detected in individuals with hypopituitarism. To test the effect of these variants directly, we cloned POU1F1 exon 2 beta and portions of the flanking introns into the exon trap splice reporter pSPL3 and introduced each variant by site directed mutagenesis (Figure 2E). These small minigenes were transfected into COS-7 cells, and RNA was analyzed by RT-PCR. As expected, the WT minigene produced almost exclusively alpha isoform, while variants carried by affected individuals predominantly produced the beta isoform (Figure 2F). We tested these small minigenes in GH3 cells, a rat pituitary tumor cell line that secretes growth hormone. The results were the same, suggesting that the splicing of rat exon 2 is the same in pituitary and non-pituitary cell lines (Figure S2). This is consistent with previous studies of Pou1f1 splicing.46 Finally, we tested splicing with larger minigenes, which contain portions of intron 1 and intron 5 with intact exons 2, 3, 4, and 5 as well as introns 2, 3, and 4, and obtained similar results, indicating the additional sequence context does not strongly influence the observed splicing pattern (Figure 2G). We also tested two previously reported POU1F1 variants in the longer minigene context. The c.214+1G>T caused skipping of exon 2, as expected, resulting in an in-frame POU1F1 protein that lacks 80% of the transactivation domain.47 This variant is associated with mild hypopituitarism. The p.Pro76Leu variant is located in the transactivation domain, enhances POU1F1 interaction with other proteins, and is associated with severe, dominant IGHD.25 The effect of this variant on splicing had not been assessed previously, and we found that it produced predominantly alpha isoform expression, indistinguishable from WT.
Saturation mutagenesis screen for splice disruptive effects
We set out to systematically identify SDVs in POU1F1 exon 2 by using a massively parallel splice reporter assay. We designed oligonucleotide pools containing every possible single-nucleotide variant across exon 2 beta (150 bp) and 210 bp of the flanking introns (N = 1,080 variants) and generated libraries of this allelic series placed into the pSPL3 reporter. To track the splicing outcomes associated with each mutation, we placed a degenerate 20-mer barcode in the downstream 3′ UTR. We subjected the mutant plasmid library to subassembly sequencing30 to establish the pairing between each unique barcode and its associated POU1F1 mutation. In total, the mutant library contained 255,023 distinct barcoded clones, among which 188,772 (74.0%) had exactly one programmed mutation. Nearly every targeted mutation appeared in this library (1,070/1,080, 99.1%) with a high degree of redundancy (median 75.0 distinct barcodes/mutation, Figure S3).
The splice reporter library was transfected as a pool into COS-7 cells and processed similarly to the single mutation constructs. Spliced reporter transcripts were read out en masse via paired-end RNA-seq (Figure 3A), and each forward read measured an individual splicing outcome and the paired reverse read contained the 3′ UTR barcode that identifies the mutation(s) present in the primary transcript. We performed 14 biological replicates, across which 94.2% (81.8%–93.4%, mean 87.4%) of barcodes associated with single-nucleotide variants in the clone library were detected. As expected, alpha was the predominant POU1F1 isoform (69.2% of reads overall), followed by exon 2 skipping (25.6%) and beta (1.6%). We created a catch-all category (“other”) for the remaining reads (3.6%) derived from the 262 other isoforms detected. Most of those noncanonical isoforms were only scarcely used; among them, the top 20 accounted for >80% of the reads from that category. For each POU1F1 variant, a percent spliced in (PSI) value was computed for each isoform (alpha, skip, beta, other), averaged over the associated barcodes. PSI values were highly reproducible across replicates (median pairwise Pearson’s r: 0.92; Figure S4), and the effects measured in the pooled screen were corroborated by individual assays of 17 variants selected for validation (Figure S5).
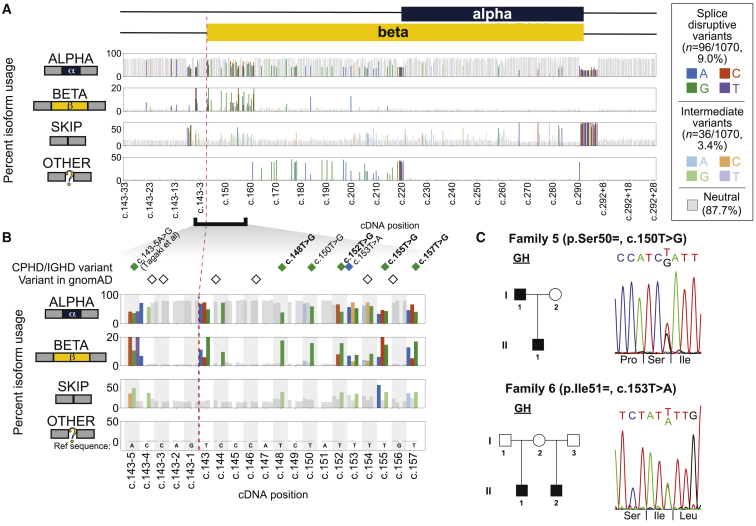
Figure 3.
Splicing effect map in POU1F1 exon 2 and flanking introns and identification of IGHD-affected families with synonymous changes
(A) Percent usage of POU1F1 exon 2 alpha (top panel), beta (second panel), skip (third panel), and other isoforms (bottom panel) by variant position, as measured by massively parallel minigene assay. Gray bars denote splicing-neutral variants, while shaded bars indicate the base pair change of each SDV (dark colors) and intermediate variant (light colors). Cropped intronic regions are shown in Figure S6.
(B) A cluster of SDVs near the beta isoform splice acceptor leads to increased usage of the beta isoform and, in some cases, intermediately increased exon skipping. Diamonds colored by the alternate allele indicate variants in individuals with hypopituitarism, and empty diamonds indicate variants reported in gnomAD. Missense variants’ labels are in bold text.
(C) Families 5 and 6 each had two individuals affected with IGHD and synonymous variants that were splice disruptive. Pedigrees and Sanger sequence confirmation of variants are shown.
SDVs across POU1F1 exon 2
We measured the impacts upon splicing of 1,070 single-nucleotide variants (Figure 3A, Figure S6). Of these, 96 (9.0%) were SDVs, which we defined as those that increased usage of beta, skip, or other isoforms by at least 3-fold (Bonferroni-corrected p < 0.05; mean observed fold-change 8.10). SDVs using other isoforms or increasing beta usage were the most frequent (n = 35/96 variants associated with each outcome) followed by those increasing exon skipping (n = 30/96); some variants (n = 4/96) impacted usage of multiple isoforms (Figure S7). Variants leading to each outcome tended to cluster in distinct regions; notably, the beta-increasing SDVs were located near the 5′ end of the beta isoform. Intronic SDVs tended to lead to skipping. A few variants that increased skipping were scattered across exon 2, and there was some enrichment in the 5′ end of the beta isoform coding region, but most were enriched near splice donor and acceptor sites: 25 of 26 intronic SDVs were within ± 20 bp of exon 2. We identified an additional 36 intermediate variants that had weaker but still significant effects (2- to 3-fold increase in beta, skip, or other isoforms usage; Bonferroni p < 0.05). The majority of these intermediate variants increased exon skipping (n = 22/36; 61.1%) and they clustered similarly to the SDVs associated with each isoform.
We next examined the splicing isoforms in the “other” category. The associated 35 SDVs were nearly all located within the coding region unique to the beta isoform and at the alpha isoform acceptor site (n = 34/35; 97.1%). Of these, most (28/34) create a cryptic acceptor AG dinucleotide that outcompetes the more distal, native alpha acceptor (Figure S8). Most of these (20/28) result in a frameshifted transcript with a premature truncation codon predicted to result in non-sense mediated decay. In contrast, every one of the six possible variants in the native alpha acceptor “AG” dinucleotide activate a cryptic acceptor six bases downstream, leading to in-frame deletion of two codons (Figure S9). By contrast to the cryptic acceptors, of 99 SNVs creating a GT dinucleotide, only one was used as a novel splice donor: c.290C>T, located 4 bp upstream of the native exon 2 donor.
We next checked how the splicing disruption map scored the four POU1F1 missense variants found in families 1–4. All four showed strongly increased beta isoform usage (beta PSI increased 9.63- to 11.01-fold over background), as seen in individual minigene assays (Figure 3B). Our results also recapitulate previously described effects of two variants found in CPHD individuals: first, an upstream intronic variant c.143−5A>G,48 which led to increased beta usage and intermediately elevated skipping (Figure 3C), and an essential splice donor variant c.292+1G>T, which led to near-complete skipping (Figure S9).47
We also examined the incidence of splice-disruptive POU1F1 variants in the general population. gnomAD contains 93 of the variants measured here; among those, six (6.5%) are splice disruptive and four (4.3%) are intermediate and all are individually rare (minor allele frequency ≤ 1.6 × 10−5; Figure S10). Overall, variants found in gnomAD were not significantly depleted for splice-disruptive/intermediate effects relative to randomly selected subsets of the tested single-nucleotide variants (p = 0.74, Fisher’s exact test). Thus, POU1F1 SDVs are tolerated to a similar extent as other predicted-loss-of-function variants (stop gain, frameshift, splice site), which are observed throughout POU1F1 at low frequencies in gnomAD.
Additional SDVs, including silent variants, in individuals with hypopituitarism
We next examined the splicing impacts of synonymous variants, which would typically be given low priority during genetic screening because of their expected lack of coding impact. Of the 108 synonymous variants tested, 14 were splice disruptive and an additional 12 were intermediate (13.0% SDV; 11.1% intermediate; Figure S7). We identified unrelated individuals with IGHD carrying two of these synonymous SDVs in the beta isoform coding region near the 5′ end of exon 2 (Figure 3C), both of which were absent in gnomAD and population-matched control databases. The first, c.150T>G (p.Ser50=), was found among an Argentinian cohort (n = 171) in a family with two individuals with severe short stature and IGHD (Table 1) for whom WES did not reveal any likely pathogenic variants in known CPHD or IGHD genes. The index individual had pituitary hypoplasia, and the individual responded well to recombinant GH treatment. The second, c.153T>A (p.Ile51=), was found in a French family in relatives with severe IGHD. The parent’s DNA was not available for testing, and the parent could be an unaffected carrier or an example of gonadal mosaicism. Each of these two silent variants increased beta isoform usage to a degree similar to that of the four missense variants (beta fold change = 10.7 and 4.15 for c.150T>G and c.153T>A, respectively).
Comparison to bioinformatic splicing effect predictions
We examined how scores from splicing effect prediction algorithms compared with these experimental measurements. We scored each single-nucleotide variant in the targeted region of POU1F1 by using SpliceAI,36 MMSplice,36 SPANR,49 HAL,34 and ESRseq scores.37 Among these, only SpliceAI predicted a high density of SDVs specific to the exon 2 beta region surrounding the disease-causing variants (Figure S11). To benchmark each bioinformatic prediction, we took our SDV calls as a truth set and computed for each algorithm the area under the precision recall curve (Figure S12). SpliceAI was the most highly concordant with our results for both exonic variants (prAUC = 0.843 versus other tools’ range: 0.251–0.351) and intronic variants (prAUC = 0.663 versus other tools’ range: 0.549–0.585). Nevertheless, SpliceAI disagreed with our measurements for numerous variants: at the minimum threshold needed to capture all six variants seen in individuals with hypopituitarism as disruptive (SpliceAI deltaMax score ≥ 0.18), it achieved 80.2% sensitivity (n = 19 SDVs according to the assay but not predicted by SpliceAI) and 97.3% specificity (n = 26 variants predicted by SpliceAI but not identified by our assay) for predicting the SDVs we identified. The degree of concordance with SpliceAI was largely insensitive to the fold change threshold used to call variants as splice disruptive (Figure S12). Additional studies will be required to resolve the discordant predictions for variants observed during clinical screening.
Discussion
We found six unrelated individuals with CPHD or IGHD that can be explained by variants that shift splicing to favor the repressive beta isoform of POU1F1. The missense variants, p.Ser50Ala, p.Ile51Ser, p.Leu52Trp, and p.Ser53Ala, retain repressive function. They act in a dominant-negative manner by suppressing the ability of the POU1F1 alpha isoform, expressed from the WT allele, to transactivate expression of POU1F1 and other downstream target genes. Suzuki et al. recently reported a splice-disruptive p.Ile51Ser variant in a family with hypopituitarism.50 Using saturation mutagenesis coupled to a high-throughput RNA-seq splicing readout, we systematically tested nearly every possible single-nucleotide variant in or near POU1F1 exon 2 for splice-disruptive potential (Table S1). We identified 96 SDVs and an additional 36 intermediate SDVs, which similarly activate usage of the beta isoform or cause other aberrant splicing outcomes, such as exon skipping.
In addition to the four missense variants we identified initially, this screen also nominated 26 synonymous variants that were SDVs or intermediately disruptive, together accounting for nearly a quarter of the possible synonymous variants in POU1F1 exon 2. We identified two of these in unrelated families with IGHD, c.150T>G (p.Ser50=) and c.153T>A (p.Ile51=), each of which increased beta isoform usage similarly to the four missense variants that initially drew our attention. These findings underscore the need to closely examine variants for splice-disruptive effects, particularly synonymous variants that could be overlooked by traditional exome sequencing filtering pipelines.
The clinical features varied among the six families, although they were consistent within a family. Families 1, 3, and 4 presented with CPHD, while families 2, 5, and 6 had IGHD. Moreover, family 4 developed cortisol deficiency. The reason for this variability in presentation is unknown. However, there are precedents for variable clinical features and incomplete penetrance with other cases of hypopituitarism.18 Approximately 50% of IGHD progresses to CPHD, and this can even occur when the mutated gene is only expressed in GH-producing cells, i.e., GH1.51 Even individuals with the same POU1F1 mutation (i.e., p.Glu230Lys) can present with either IGHD or CPHD,52 indicating a contributing role for genetic background, epigenetic, and/or environmental factors. Both affected relatives in family 1 had stalk disruption, a phenotype not currently associated with any other POU1F1 variants. This feature may be due to the presence of an additional variant in SIX3, p.Pro74Arg, that was carried by two unaffected relatives. Heterozygous loss of function of SIX3 is associated with incompletely penetrant and highly variable craniofacial abnormalities, including CPHD and holoprosencephaly, and there is precedent in mice for Six3 loss of function to exacerbate the phenotype caused by mutations in other CPHD genes such as Hesx1.53, 54, 55
Autosomal-dominant inheritance is clear in family 2, in which there were four affected individuals over three generations, as well as families 4, 5, and 6. POU1F1 acts as a heterodimer.56 Some other dominant mutations in POU1F1 act as negative effectors because of the ability of the mutant protein to interfere with the action of the WT protein produced from the other allele.26,57,58 The negative effect of POU1F1 beta on the transactivation properties of POU1F1 alpha are context dependent and have differential effects on Gh, Prl, and Pou1f1 reporter genes.46 The strongest effect was reported for autoregulation of POU1F1 expression via the distal, late enhancer, dampening the auto-activation of POU1F1 expression and adversely affecting differentiation of the entire POU1F1 lineage, resulting in anterior lobe hypoplasia.
The lack of significant depletion for POU1F1 SDVs among ostensibly healthy adult populations underscores the possibility of variable expressivity and/or penetrance for POU1F1 SDVs. This is consistent with the apparently unaffected parents in families 1 and 6. A subset of these variants, like the variant c.219A>G, which disrupts the alpha isoform acceptor and causes a frame-preserving two-codon deletion, may retain partial or complete function. Still others may cause loss of function without dominant-negative effects and would not be expected to be strongly depleted.
In human genes, canonical splice site motifs contain less than half of the information content needed for proper splicing.59 Additional specificity is provided by short (6–10 nt) motifs termed exonic or intronic silencers and enhancers, which are bound by RNA-binding proteins that promote or antagonize splicing.60 Although transcriptome-wide atlases have been developed to map these sites37,61 and derive motif models,62 it often remains unclear how genetic variants impact their binding and, in turn, the eventual splicing output. Our splicing effect map identifies a cluster of SDVs at the 5′ end of the POU1F1 exon 2 beta, each of which increases the usage of that normally repressed isoform. These results suggest the presence of an exonic splice silencer (ESS), which may normally suppress utilization of the beta isoform acceptor. We do not expect any cell-type-specific factors to be involved because WT minigene assays in pituitary cell lines and heterologous cell lines mimic the ratios of alpha:beta isoform transcripts found in normal pituitary glands.46 We mined the cisBP-RNA database38 and identified eight candidate motifs with strong matches to the U-rich WT sequence in this region (c.143 to c.167) corresponding to known splicing factors, including ELAVL1 (HuR), RALY, TIA1, and U2AF2 (Figure S13). All six hypopituitarism-associated variants replaced a U with another base (G in five of six bases), which may disrupt these motifs at high information content positions (Figure S14). Other variants predicted to disrupt these motifs tended to be beta-promoting more often than intermediate/neutral in our map (p < 0.05, Fisher’s exact test). These trends suggest that U-rich ESS serves to inhibit production of POU1F1 beta and this inhibition is disrupted by CPHD-associated variants, although conclusively identifying the specific cognate-binding factor will require further study.
These results extend the breadth of endocrine disorders caused by disrupted splicing. For example, in a large cohort with IGHD from Itabaianinha, Brazil, affected individuals are homozygous for a mutation in the splice donor dinucleotide (c.57+1G>A) in the growth hormone-releasing hormone receptor gene (GHRHR).63 In addition, most mutations that cause dominant IGHD type II affect splicing of the growth hormone (GH1) gene.64 Mutations in splice sites or splice enhancer sequences result in skipping exon 3 and production of a dominant-negative 17.5 kD isoform of growth hormone that lacks amino acids 32–71.65 The severity of the disease is variable and correlates inversely with the ratio of 17.5 to 20 kD GH. Finally, severe short stature associated with Laron syndrome, or GH resistance, can be caused by generation of a cryptic splice site in the GH receptor gene. Individuals from El Oro and Loja in southern Ecuador are homozygous for a p.Glu180= codon variant (GAA to GAG) that does not change the amino acid encoded but creates a splice acceptor site 24 nt upstream of the normally utilized site.66 It is notable that antisense oligonucleotide therapies hold promise for treating diseases caused by abnormal splicing, including IGHD.67,68
Splicing disruption accounts for a significant minority of the genetic burden in endocrine disorders, as in human genetic disease more generally.69,70 Some estimates from large-scale screens indicate that 10% of SNVs within exons alter splicing, and a third of all disease-associated SNVs impact splicing efficiency.71 Variants at or near canonical spice sites are readily recognized as pathogenic,72 and these can be predicted with high accuracy by algorithms such as SpliceAI. However, for exonic variants, particularly those farther from exon junctions, splicing defects may be more challenging to identify bioinformatically.73, 74, 75 Efforts to interpret these variants will need to account for the functional impacts of changing the encoded protein sequence as well as its splicing. Finally, as our results illustrate, different variants in a single gene may lead to distinct splicing outcomes with diverse consequences ranging from the straightforward loss of function to dominant-negative effects.
Declaration of interests
J.O.K. is a scientific advisor to MyOme, Inc. The authors declare no other competing interests.
Acknowledgments
This work was supported by The National Institutes of Health (R01HD097096 to S.A.C. and R01GM129123 to J.O.K.), the Japan Society for Promotion of Science (H.B.), grant 2013/03236-5 from the São Paulo Research Foundation (FAPESP) (I.J.P.A.), a grant from Pfizer (R.P.), and the Argentinean National Agency of Scientific and Technical Promotion, PICT 2016-2913 and PICT 2017-0002 (M.I.P.M.).
Published: July 15, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2021.06.013.
Contributor Information
Jacob O. Kitzman, Email: kitzmanj@umich.edu.
Sally A. Camper, Email: scamper@med.umich.edu.
Data and code availability
Raw sequence reads and processed counts are available at the NCBI GEO (GEO: GSE172504).
Web resources
cisBP-RNA, http://cisbp-rna.ccbr.utoronto.ca
Code repository on GitHub, https://github.com/kitzmanlab/pou1f1_splicing
Supplemental information
References
- 1.Ingraham H.A., Flynn S.E., Voss J.W., Albert V.R., Kapiloff M.S., Wilson L., Rosenfeld M.G. The POU-specific domain of Pit-1 is essential for sequence-specific, high affinity DNA binding and DNA-dependent Pit-1-Pit-1 interactions. Cell. 1990;61:1021–1033. doi: 10.1016/0092-8674(90)90067-o. [DOI] [PubMed] [Google Scholar]
- 2.Gordon D.F., Haugen B.R., Sarapura V.D., Nelson A.R., Wood W.M., Ridgway E.C. Analysis of Pit-1 in regulating mouse TSH beta promoter activity in thyrotropes. Mol. Cell. Endocrinol. 1993;96:75–84. doi: 10.1016/0303-7207(93)90097-4. [DOI] [PubMed] [Google Scholar]
- 3.Davis S.W., Keisler J.L., Pérez-Millán M.I., Schade V., Camper S.A. All Hormone-Producing Cell Types of the Pituitary Intermediate and Anterior Lobes Derive From Prop1-Expressing Progenitors. Endocrinology. 2016;157:1385–1396. doi: 10.1210/en.2015-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S., Crenshaw E.B., 3rd, Rawson E.J., Simmons D.M., Swanson L.W., Rosenfeld M.G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 5.Camper S.A., Saunders T.L., Katz R.W., Reeves R.H. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics. 1990;8:586–590. doi: 10.1016/0888-7543(90)90050-5. [DOI] [PubMed] [Google Scholar]
- 6.Simmons D.M., Voss J.W., Ingraham H.A., Holloway J.M., Broide R.S., Rosenfeld M.G., Swanson L.W. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990;4:695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- 7.Slabaugh M.B., Lieberman M.E., Rutledge J.J., Gorski J. Growth hormone and prolactin synthesis in normal and homozygous Snell and Ames dwarf mice. Endocrinology. 1981;109:1040–1046. doi: 10.1210/endo-109-4-1040. [DOI] [PubMed] [Google Scholar]
- 8.Lin S.C., Li S., Drolet D.W., Rosenfeld M.G. Pituitary ontogeny of the Snell dwarf mouse reveals Pit-1-independent and Pit-1-dependent origins of the thyrotrope. Development. 1994;120:515–522. doi: 10.1242/dev.120.3.515. [DOI] [PubMed] [Google Scholar]
- 9.Pfäffle R., Klammt J. Pituitary transcription factors in the aetiology of combined pituitary hormone deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:43–60. doi: 10.1016/j.beem.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Wallis M. Evolution of the POU1F1 transcription factor in mammals: Rapid change of the alternatively-spliced β-domain. Gen. Comp. Endocrinol. 2018;260:100–106. doi: 10.1016/j.ygcen.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Schanke J.T., Conwell C.M., Durning M., Fisher J.M., Golos T.G. Pit-1/growth hormone factor 1 splice variant expression in the rhesus monkey pituitary gland and the rhesus and human placenta. J. Clin. Endocrinol. Metab. 1997;82:800–807. doi: 10.1210/jcem.82.3.3791. [DOI] [PubMed] [Google Scholar]
- 12.Konzak K.E., Moore D.D. Functional isoforms of Pit-1 generated by alternative messenger RNA splicing. Mol. Endocrinol. 1992;6:241–247. doi: 10.1210/mend.6.2.1569967. [DOI] [PubMed] [Google Scholar]
- 13.Haugen B.R., Wood W.M., Gordon D.F., Ridgway E.C. A thyrotrope-specific variant of Pit-1 transactivates the thyrotropin beta promoter. J. Biol. Chem. 1993;268:20818–20824. [PubMed] [Google Scholar]
- 14.Jonsen M.D., Duval D.L., Gutierrez-Hartmann A. The 26-amino acid beta-motif of the Pit-1beta transcription factor is a dominant and independent repressor domain. Mol. Endocrinol. 2009;23:1371–1384. doi: 10.1210/me.2008-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consortium G.T., GTEx Consortium The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–1330. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatsumi K., Miyai K., Notomi T., Kaibe K., Amino N., Mizuno Y., Kohno H. Cretinism with combined hormone deficiency caused by a mutation in the PIT1 gene. Nat. Genet. 1992;1:56–58. doi: 10.1038/ng0492-56. [DOI] [PubMed] [Google Scholar]
- 17.Fang Q., George A.S., Brinkmeier M.L., Mortensen A.H., Gergics P., Cheung L.Y., Daly A.Z., Ajmal A., Pérez Millán M.I., Ozel A.B. Genetics of Combined Pituitary Hormone Deficiency: Roadmap into the Genome Era. Endocr. Rev. 2016;37:636–675. doi: 10.1210/er.2016-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gergics P. Pituitary Transcription Factor Mutations Leading to Hypopituitarism. Exp Suppl. 2019;111(Suppl):263–298. doi: 10.1007/978-3-030-25905-1_13. [DOI] [PubMed] [Google Scholar]
- 19.Birla S., Vijayakumar P., Sehgal S., Bhatnagar S., Pallavi K., Sharma A. Characterization of a Novel POU1F1 Mutation Identified on Screening 160 Growth Hormone Deficiency Patients. Horm. Metab. Res. 2019;51:248–255. doi: 10.1055/a-0867-1026. [DOI] [PubMed] [Google Scholar]
- 20.Baş F., Abalı Z.Y., Toksoy G., Poyrazoğlu Ş., Bundak R., Güleç Ç., Uyguner Z.O., Darendeliler F. Precocious or early puberty in patients with combined pituitary hormone deficiency due to POU1F1 gene mutation: case report and review of possible mechanisms. Hormones (Athens) 2018;17:581–588. doi: 10.1007/s42000-018-0079-4. [DOI] [PubMed] [Google Scholar]
- 21.Blum W.F., Klammt J., Amselem S., Pfäffle H.M., Legendre M., Sobrier M.L., Luton M.P., Child C.J., Jones C., Zimmermann A.G. Screening a large pediatric cohort with GH deficiency for mutations in genes regulating pituitary development and GH secretion: Frequencies, phenotypes and growth outcomes. EBioMedicine. 2018;36:390–400. doi: 10.1016/j.ebiom.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertko E., Klammt J., Dusatkova P., Bahceci M., Gonc N., Ten Have L., Kandemir N., Mansmann G., Obermannova B., Oostdijk W. Combined pituitary hormone deficiency due to gross deletions in the POU1F1 (PIT-1) and PROP1 genes. J. Hum. Genet. 2017;62:755–762. doi: 10.1038/jhg.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birla S., Khadgawat R., Jyotsna V.P., Jain V., Garg M.K., Bhalla A.S., Sharma A. Identification of Novel PROP1 and POU1F1 Mutations in Patients with Combined Pituitary Hormone Deficiency. Horm. Metab. Res. 2016;48:822–827. doi: 10.1055/s-0042-117112. [DOI] [PubMed] [Google Scholar]
- 24.Jadhav S., Diwaker C., Lila A.R., Gada J.V., Kale S., Sarathi V., Thadani P.M., Arya S., Patil V.A., Shah N.S., Bandgar T.R. POU1F1 mutations in combined pituitary hormone deficiency: differing spectrum of mutations in a Western-Indian cohort and systematic analysis of world literature. Pituitary. 2021 doi: 10.1007/s11102-021-01140-9. [DOI] [PubMed] [Google Scholar]
- 25.Sobrier M.L., Tsai Y.C., Pérez C., Leheup B., Bouceba T., Duquesnoy P., Copin B., Sizova D., Penzo A., Stanger B.Z. Functional characterization of a human POU1F1 mutation associated with isolated growth hormone deficiency: a novel etiology for IGHD. Hum. Mol. Genet. 2016;25:472–483. doi: 10.1093/hmg/ddv486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen L.E., Zanger K., Brue T., Wondisford F.E., Radovick S. Defective retinoic acid regulation of the Pit-1 gene enhancer: a novel mechanism of combined pituitary hormone deficiency. Mol. Endocrinol. 1999;13:476–484. doi: 10.1210/mend.13.3.0251. [DOI] [PubMed] [Google Scholar]
- 27.Skowronska-Krawczyk D., Ma Q., Schwartz M., Scully K., Li W., Liu Z., Taylor H., Tollkuhn J., Ohgi K.A., Notani D. Required enhancer-matrin-3 network interactions for a homeodomain transcription program. Nature. 2014;514:257–261. doi: 10.1038/nature13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo M.H., Shen Y., Walvoord E.C., Miller T.C., Moon J.E., Hirschhorn J.N., Dauber A. Whole exome sequencing to identify genetic causes of short stature. Horm. Res. Paediatr. 2014;82:44–52. doi: 10.1159/000360857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nisson P.E., Ally A., Watkins P.C. Protocols for trapping internal and 3′-terminal exons. PCR Methods Appl. 1994;4:S24–S39. doi: 10.1101/gr.4.1.s24. [DOI] [PubMed] [Google Scholar]
- 30.Hiatt J.B., Patwardhan R.P., Turner E.H., Lee C., Shendure J. Parallel, tag-directed assembly of locally derived short sequence reads. Nat. Methods. 2010;7:119–122. doi: 10.1038/nmeth.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zorita E., Cuscó P., Filion G.J. Starcode: sequence clustering based on all-pairs search. Bioinformatics. 2015;31:1913–1919. doi: 10.1093/bioinformatics/btv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrison E., Marth G. Haplotype-based variant detection from short-read sequencing. arXiv. 2012 https://arxiv.org/abs/1207.3907 1207.3907. [Google Scholar]
- 33.Wu T.D., Watanabe C.K. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics. 2005;21:1859–1875. doi: 10.1093/bioinformatics/bti310. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg A.B., Patwardhan R.P., Shendure J., Seelig G. Learning the sequence determinants of alternative splicing from millions of random sequences. Cell. 2015;163:698–711. doi: 10.1016/j.cell.2015.09.054. [DOI] [PubMed] [Google Scholar]
- 35.Jaganathan K., Kyriazopoulou Panagiotopoulou S., McRae J.F., Darbandi S.F., Knowles D., Li Y.I., Kosmicki J.A., Arbelaez J., Cui W., Schwartz G.B. Predicting Splicing from Primary Sequence with Deep Learning. Cell. 2019;176:535–548.e24. doi: 10.1016/j.cell.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Cheng J., Nguyen T.Y.D., Cygan K.J., Çelik M.H., Fairbrother W.G., Avsec Ž., Gagneur J. MMSplice: modular modeling improves the predictions of genetic variant effects on splicing. Genome Biol. 2019;20:48. doi: 10.1186/s13059-019-1653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ke S., Shang S., Kalachikov S.M., Morozova I., Yu L., Russo J.J., Ju J., Chasin L.A. Quantitative evaluation of all hexamers as exonic splicing elements. Genome Res. 2011;21:1360–1374. doi: 10.1101/gr.119628.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray D., Kazan H., Cook K.B., Weirauch M.T., Najafabadi H.S., Li X., Gueroussov S., Albu M., Zheng H., Yang A. A compendium of RNA-binding motifs for decoding gene regulation. Nature. 2013;499:172–177. doi: 10.1038/nature12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerario A.M., Mohan D.R., Montenegro L.R., Funari M.F.A., Nishi M.Y., Narcizo A.M., Benedetti A.F.F., Oba-Shinjo S.M., Vitorino A.J., Santos R.A.S.X.D. SELAdb: A database of exonic variants in a Brazilian population referred to a quaternary medical center in São Paulo. Clinics (São Paulo) 2020;75:e1913. doi: 10.6061/clinics/2020/e1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vishnopolska S.A., Turjanski A.G., Herrera Piñero M., Groisman B., Liascovich R., Chiesa A., Marti M.A. Genetics and genomic medicine in Argentina. Mol. Genet. Genomic Med. 2018 doi: 10.1002/mgg3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma S.L., Vega-Warner V., Gillies C., Sampson M.G., Kher V., Sethi S.K., Otto E.A. Whole Exome Sequencing Reveals Novel PHEX Splice Site Mutations in Patients with Hypophosphatemic Rickets. PLoS ONE. 2015;10:e0130729. doi: 10.1371/journal.pone.0130729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho Y., Cooke N.E., Liebhaber S.A. An autoregulatory pathway establishes the definitive chromatin conformation at the pit-1 locus. Mol. Cell. Biol. 2015;35:1523–1532. doi: 10.1128/MCB.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajas F., Delhase M., De La Hoya M., Verdood P., Castrillo J.L., Hooghe-Peters E.L. Nuclear factor 1 regulates the distal silencer of the human PIT1/GHF1 gene. Biochem. J. 1998;333:77–84. doi: 10.1042/bj3330077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiMattia G.E., Rhodes S.J., Krones A., Carrière C., O’Connell S., Kalla K., Arias C., Sawchenko P., Rosenfeld M.G. The Pit-1 gene is regulated by distinct early and late pituitary-specific enhancers. Dev. Biol. 1997;182:180–190. doi: 10.1006/dbio.1996.8472. [DOI] [PubMed] [Google Scholar]
- 45.Yeo G., Burge C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 2004;11:377–394. doi: 10.1089/1066527041410418. [DOI] [PubMed] [Google Scholar]
- 46.Theill L.E., Hattori K., Lazzaro D., Castrillo J.L., Karin M. Differential splicing of the GHF1 primary transcript gives rise to two functionally distinct homeodomain proteins. EMBO J. 1992;11:2261–2269. doi: 10.1002/j.1460-2075.1992.tb05285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue H., Mukai T., Sakamoto Y., Kimura C., Kangawa N., Itakura M., Ogata T., Ito Y., Fujieda K., Japan Growth Genome Consortium Identification of a novel mutation in the exon 2 splice donor site of the POU1F1/PIT-1 gene in Japanese identical twins with mild combined pituitary hormone deficiency. Clin. Endocrinol. (Oxf.) 2012;76:78–87. doi: 10.1111/j.1365-2265.2011.04165.x. [DOI] [PubMed] [Google Scholar]
- 48.Takagi M., Kamasaki H., Yagi H., Fukuzawa R., Narumi S., Hasegawa T. A novel heterozygous intronic mutation in POU1F1 is associated with combined pituitary hormone deficiency. Endocr. J. 2017;64:229–234. doi: 10.1507/endocrj.EJ16-0361. [DOI] [PubMed] [Google Scholar]
- 49.Xiong H.Y., Alipanahi B., Lee L.J., Bretschneider H., Merico D., Yuen R.K., Hua Y., Gueroussov S., Najafabadi H.S., Hughes T.R. RNA splicing. The human splicing code reveals new insights into the genetic determinants of disease. Science. 2015;347:1254806. doi: 10.1126/science.1254806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki S., Matsuo K., Ito Y., Kobayashi A., Kokumai T., Furuya A., Ueda O., Mukai T., Yano K., Fujieda K. A mutation of the β-domain in POU1F1 causes pituitary deficiency due to dominant PIT-1β expression. Eur. J. Endocrinol. 2021;185:1–12. doi: 10.1530/EJE-20-1313. [DOI] [PubMed] [Google Scholar]
- 51.Cerbone M., Dattani M.T. Progression from isolated growth hormone deficiency to combined pituitary hormone deficiency. Growth Horm. IGF Res. 2017;37:19–25. doi: 10.1016/j.ghir.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Turton J.P., Reynaud R., Mehta A., Torpiano J., Saveanu A., Woods K.S., Tiulpakov A., Zdravkovic V., Hamilton J., Attard-Montalto S. Novel mutations within the POU1F1 gene associated with variable combined pituitary hormone deficiency. J. Clin. Endocrinol. Metab. 2005;90:4762–4770. doi: 10.1210/jc.2005-0570. [DOI] [PubMed] [Google Scholar]
- 53.Solomon B.D., Mercier S., Vélez J.I., Pineda-Alvarez D.E., Wyllie A., Zhou N., Dubourg C., David V., Odent S., Roessler E., Muenke M. Analysis of genotype-phenotype correlations in human holoprosencephaly. Am. J. Med. Genet. C. Semin. Med. Genet. 2010;154C:133–141. doi: 10.1002/ajmg.c.30240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Domené S., Roessler E., El-Jaick K.B., Snir M., Brown J.L., Vélez J.I., Bale S., Lacbawan F., Muenke M., Feldman B. Mutations in the human SIX3 gene in holoprosencephaly are loss of function. Hum. Mol. Genet. 2008;17:3919–3928. doi: 10.1093/hmg/ddn294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaston-Massuet C., Andoniadou C.L., Signore M., Sajedi E., Bird S., Turner J.M., Martinez-Barbera J.P. Genetic interaction between the homeobox transcription factors HESX1 and SIX3 is required for normal pituitary development. Dev. Biol. 2008;324:322–333. doi: 10.1016/j.ydbio.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holloway J.M., Szeto D.P., Scully K.M., Glass C.K., Rosenfeld M.G. Pit-1 binding to specific DNA sites as a monomer or dimer determines gene-specific use of a tyrosine-dependent synergy domain. Genes Dev. 1995;9:1992–2006. doi: 10.1101/gad.9.16.1992. [DOI] [PubMed] [Google Scholar]
- 57.Rhodes S.J., Chen R., DiMattia G.E., Scully K.M., Kalla K.A., Lin S.C., Yu V.C., Rosenfeld M.G. A tissue-specific enhancer confers Pit-1-dependent morphogen inducibility and autoregulation on the pit-1 gene. Genes Dev. 1993;7:913–932. doi: 10.1101/gad.7.6.913. [DOI] [PubMed] [Google Scholar]
- 58.Cohen R.N., Brue T., Naik K., Houlihan C.A., Wondisford F.E., Radovick S. The role of CBP/p300 interactions and Pit-1 dimerization in the pathophysiological mechanism of combined pituitary hormone deficiency. J. Clin. Endocrinol. Metab. 2006;91:239–247. doi: 10.1210/jc.2005-1211. [DOI] [PubMed] [Google Scholar]
- 59.Lim L.P., Burge C.B. A computational analysis of sequence features involved in recognition of short introns. Proc. Natl. Acad. Sci. USA. 2001;98:11193–11198. doi: 10.1073/pnas.201407298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cartegni L., Chew S.L., Krainer A.R. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat. Rev. Genet. 2002;3:285–298. doi: 10.1038/nrg775. [DOI] [PubMed] [Google Scholar]
- 61.Cheung R., Insigne K.D., Yao D., Burghard C.P., Wang J., Hsiao Y.E., Jones E.M., Goodman D.B., Xiao X., Kosuri S. A Multiplexed Assay for Exon Recognition Reveals that an Unappreciated Fraction of Rare Genetic Variants Cause Large-Effect Splicing Disruptions. Mol. Cell. 2019;73:183–194.e8. doi: 10.1016/j.molcel.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.König J., Zarnack K., Luscombe N.M., Ule J. Protein-RNA interactions: new genomic technologies and perspectives. Nat. Rev. Genet. 2012;13:77–83. doi: 10.1038/nrg3141. [DOI] [PubMed] [Google Scholar]
- 63.Salvatori R., Hayashida C.Y., Aguiar-Oliveira M.H., Phillips J.A., 3rd, Souza A.H., Gondo R.G., Toledo S.P., Conceicão M.M., Prince M., Maheshwari H.G. Familial dwarfism due to a novel mutation of the growth hormone-releasing hormone receptor gene. J. Clin. Endocrinol. Metab. 1999;84:917–923. doi: 10.1210/jcem.84.3.5599. [DOI] [PubMed] [Google Scholar]
- 64.Alatzoglou K.S., Dattani M.T. Phenotype-genotype correlations in congenital isolated growth hormone deficiency (IGHD) Indian J. Pediatr. 2012;79:99–106. doi: 10.1007/s12098-011-0614-7. [DOI] [PubMed] [Google Scholar]
- 65.Shariat N., Holladay C.D., Cleary R.K., Phillips J.A., 3rd, Patton J.G. Isolated growth hormone deficiency type II caused by a point mutation that alters both splice site strength and splicing enhancer function. Clin. Genet. 2008;74:539–545. doi: 10.1111/j.1399-0004.2008.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berg M.A., Guevara-Aguirre J., Rosenbloom A.L., Rosenfeld R.G., Francke U. Mutation creating a new splice site in the growth hormone receptor genes of 37 Ecuadorean patients with Laron syndrome. Hum. Mutat. 1992;1:24–32. doi: 10.1002/humu.1380010105. [DOI] [PubMed] [Google Scholar]
- 67.Miletta M.C., Lochmatter D., Pektovic V., Mullis P.E. Isolated growth hormone deficiency type 2: from gene to therapy. Endocr. Dev. 2012;23:109–120. doi: 10.1159/000341766. [DOI] [PubMed] [Google Scholar]
- 68.Kuijper E.C., Bergsma A.J., Pijnappel W.W.M.P., Aartsma-Rus A. Opportunities and challenges for antisense oligonucleotide therapies. J. Inherit. Metab. Dis. 2021;44:72–87. doi: 10.1002/jimd.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cummings B.B., Marshall J.L., Tukiainen T., Lek M., Donkervoort S., Foley A.R., Bolduc V., Waddell L.B., Sandaradura S.A., O’Grady G.L., Genotype-Tissue Expression Consortium Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci. Transl. Med. 2017;9:eaal5209. doi: 10.1126/scitranslmed.aal5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scotti M.M., Swanson M.S. RNA mis-splicing in disease. Nat. Rev. Genet. 2016;17:19–32. doi: 10.1038/nrg.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soemedi R., Cygan K.J., Rhine C.L., Wang J., Bulacan C., Yang J., Bayrak-Toydemir P., McDonald J., Fairbrother W.G. Pathogenic variants that alter protein code often disrupt splicing. Nat. Genet. 2017;49:848–855. doi: 10.1038/ng.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lord J., Gallone G., Short P.J., McRae J.F., Ironfield H., Wynn E.H., Gerety S.S., He L., Kerr B., Johnson D.S., Deciphering Developmental Disorders study Pathogenicity and selective constraint on variation near splice sites. Genome Res. 2019;29:159–170. doi: 10.1101/gr.238444.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan M., Cornelis S.S., Pozo-Valero M.D., Whelan L., Runhart E.H., Mishra K., Bults F., AlSwaiti Y., AlTalbishi A., De Baere E. Resolving the dark matter of ABCA4 for 1054 Stargardt disease probands through integrated genomics and transcriptomics. Genet. Med. 2020;22:1235–1246. doi: 10.1038/s41436-020-0787-4. [DOI] [PubMed] [Google Scholar]
- 74.Chen J.M., Lin J.H., Masson E., Liao Z., Férec C., Cooper D.N., Hayden M. The Experimentally Obtained Functional Impact Assessments of 5′ Splice Site GT’GC Variants Differ Markedly from Those Predicted. Curr. Genomics. 2020;21:56–66. doi: 10.2174/1389202921666200210141701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dionnet E., Defour A., Da Silva N., Salvi A., Lévy N., Krahn M., Bartoli M., Puppo F., Gorokhova S. Splicing impact of deep exonic missense variants in CAPN3 explored systematically by minigene functional assay. Hum. Mutat. 2020 doi: 10.1002/humu.24083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence reads and processed counts are available at the NCBI GEO (GEO: GSE172504).