Abstract
Background:
Sensitive and specific biomarkers for use in progressive multiple sclerosis (MS) have not been established. We investigate neurofilament light (NfL) as a treatment response biomarker in progressive MS.
Objective:
To evaluate whether ibudilast 100 mg/d alters serum and CSF levels of NfL in progressive MS
Methods:
In a protocol-defined exploratory analysis from a 2-year, phase 2 clinical trial of ibudilast in progressive MS (NCT01982942), serum samples were collected from 239 subjects and a subset contributed CSF and assayed using SIMOA immunoassay. A mixed model for repeated measurements yielded log(NfL) as the response variable.
Results:
The geometric mean baseline serum NfL was 31.9 and 28.8 pg/ml in placebo and ibudilast groups, respectively. The geometric mean baseline CSF NfL was 1150.8 and 1290.3 pg/ml in placebo and ibudilast groups, respectively. Serum and CSF NfL correlations were r = 0.52 and 0.78 at week 48 and 96, respectively. Over 96 weeks, there was no between-group difference in NfL in either serum (P=0.76) or CSF (P=0.46). After controlling for factors that may affect NfL, no effect of ibudilast on NfL in either serum or CSF was observed.
Conclusions:
Ibudilast treatment was not associated with a change in either serum or CSF NfL.
Keywords: multiple sclerosis, biomarker, neurofilament
Introduction
Therapeutic biomarkers indicate a response to a therapeutic intervention. In relapsing remitting multiple sclerosis (RRMS), biomarkers for an anti-inflammatory therapeutic response include focal lesions seen on MRI. MRI treatment response in RRMS has a close association with clinical treatment response, and these measures have become standard readouts in clinical trials.1 In contrast, a sensitive and specific biomarker for use in progressive MS remains to be established.2
Neurofilaments are a family of cytoskeletal proteins expressed in neurons of the central and peripheral neuroaxonal compartments.3 Neurofilaments are released upon neuronal injury and can be measured in the CSF. Neurofilaments diffuse into the serum, where their levels correlate with CSF levels, albeit at a lower concentration. Neurofilaments are the major components of axonal cytoskeleton proteins, which are made up of three differently sized chains. Neurofilament light chain (NfL) is a 69 kDa neurofilament and is a commonly measured and reported neurofilament in neurologic diseases, as it relates closely to acute axonal damage.3 NfL concentrations are elevated in many diseases that affect the neuroaxonal compartment, including stroke, Alzheimer’s disease, and multiple sclerosis (MS).4 NfL levels increase with age in healthy individuals and increase with comorbidities such as small vessel disease.5 These findings support the application of NfL as a biomarker for neurodegenerative disease and emphasize the complexity in interpreting and using NfL data to indicate pathophysiology or intervention efficacy.6 Several studies have shown that serum NfL levels are reduced following treatment with anti-inflammatory therapies in relapsing-remitting MS.7, 8 NfL levels are also reduced in response to some MS anti-inflammatory therapies in progressive MS.9
Recently, we reported that ibudilast reduces MRI measures of progressive tissue injury in patients with progressive MS (NCT01982942).10 In the central nervous system (CNS), ibudilast reduces inflammatory activities of several non-neuronal cell types and also reduces oligodendrocyte cell toxicity, astrocyte apoptosis, and microglial activation in a dose-dependent manner.11 Ibudilast also reduces reactive oxygen species, the production and activity of inflammatory cytokines, and tumor necrosis factor (TNF)-release from the astrocytes and microglial cells.12 However, evidence suggests that, rather than acting as an anti-inflammatory, ibudilast may protect neurons from persistent damage after acute inflammation (17). In either case, treatment of progressive MS with ibudilast may reduce NfL levels in the CSF and serum. Here, we evaluated NfL as a therapeutic response biomarker in progressive MS.
Materials and Methods
The SPRINT-MS study was a Phase 2 trial of ibudilast (MN-166, Medinova, La Jolla, CA) in subjects with either primary progressive MS (PPMS) or secondary progressive MS (SPMS) and was conducted within the National Institutes of Neurological Diseases and Stroke NeuroNEXT clinical trial network. In short, 255 subjects were randomized 1:1 to either up to 100 mg daily oral ibudilast or matching placebo. Details of the study were described previously.13 Brain MRIs were obtained every 24 weeks using a standardized protocol and were read at a central reading center (NeuroRx, Montreal, Canada). Human subject protection was provided by the NeuroNEXT Central IRB at the Massachusetts General Hospital. All subjects provided informed consent for biomarker collection and analysis, including optional consent to contribute CSF. In all enrolled subjects, serum was collected at screening (n=239), 8 weeks (n=222), 48 weeks (n=209), and 96 weeks (n=178). Serum was separated and stored at −80C at the clinical site and then shipped on dry ice to a central laboratory for storage and then to a single laboratory for NfL analysis. All testing was performed in a single laboratory atthe University Hospital Basel, Switzerland. Subjects were offered the option to undergo voluntary lumbar puncture to collect CSF at screening (n=58), 48 weeks (n=56), and 96 weeks (n=37). Concentrations of NfL were measured using the ultrasensitive single-molecule assay (Simoa) immunoassay described previously, which can identify measurable NfL in essentially all human serum samples.14 Inter-assay coefficients of variation (CV) for 3 native serum samples were below 11%. The mean intra-assay CV of duplicate determinations for concentration was 6.8%. Measurements were performed on coded samples. All laboratory personnel remained blinded to treatment allocation and clinical data. See Fox et al, NEJM 2018 for original trial protocol and statistical analysis plan.
Statistical analysis
In a protocol-defined exploratory analysis, performed using R, data was analyzed according to the intention-to-treat (ITT) principle and included all available NfL values in the analysis (n = 609 samples). Analysis utilized mixed models for repeated measurements with log(NfL) as the response variable, adjusted for treatment (placebo vs ibudilast), age, and log(baseline NFL) for serum and adjusted for treatment and age for CSF NfL. (Adjustment for log(baseline CSF NfL) was not included in CSF modeling because of lack of model convergence.) Log transformation was utilized because both serum and CSF NfL values are not normally distributed. The model further included visit-by-treatment and visit-by-log(baseline NfL) interactions for serum NfL, and only visit-by-treatment interactions for CSF NfL, using an unstructured covariance matrix. In an exploratory sensitivity analysis, we included concurrent use of injectable disease modifying therapy as a potential confounder in the mixed model.
Because active inflammation and other brain tissue injury can cause elevations of NfL and, thus, may affect efforts to measure the potential neuroprotective effects of a therapy, we conducted several post-hoc analyses to control for these events. Censored Cohort 1 excluded time points:
at or after onset of neurologic serious adverse events (SAE; n=4 samples from a total of 3 subjects);
within 6 months after a clinical relapse (n=3 samples); and
when MRI showed any new or enlarging T2 lesions (n=103 samples)
The neurologic serious adverse events were cerebral hemorrhage, ataxia, and convulsion. All serum NfL data from week 8 were excluded in these two sensitivity analyses because no MRI was performed at week 8. Censored Cohort 2 was the same as censored Cohort 1 except exclusion c) was relaxed to only exclude time points when MRI showed 3 or more new or enlarging T2 lesions (n=38 samples). A total of 105 samples were excluded in censored Cohort 1 and 40 samples were excluded in censored Cohort 2 (the individual categories do not add up to the total because some samples were excluded for multiple reasons). Otherwise, statistical analyses were conducted in an identical manner. In a similar sensitivity analysis, we repeated the mixed model analysis and included the censored cohort definitions as a single composite confounding variable. Additionally, we performed a “per protocol” analysis after excluding subjects who stopped ibudilast study medication prior to sample collection (n=15 samples).
We examined the correlation between serum and CSF NfL levels through Pearson correlations after log transformation. Blood volume varies by body size,15 so as recently suggested,16 we explored controlling blood/CSF NfL correlations for body mass index. In some patients, blood and serum collection was on disparate dates, so we also explored limiting correlations to only collections on the same day.
Results
A total of 239 subjects were included in the NfL analysis, and 58 subjects participated in the CSF sub-study (Table 1). See Fox et al, NEJM 2018 for CONSORT flow diagram. Geometric mean serum NfL at baseline was 31.9 pg/ml in the placebo group and 28.8 pg/ml in the ibudilast group. Geometric mean CSF NfL at baseline was 1150.8 pg/ml in the placebo group and 1290.3 pg/ml in the ibudilast group. Serum and CSF NfL demonstrated modest correlations: r = 0.52 at week 48 (95%CI 0.29 – 0.69), and r = 0.78 at week 96 (Fig. 1). After removing one very high outlying value at week 96, the correlation at week 96 was r = 0.59 (95% CI 0.30 – 0.79). When controlling for baseline BMI (again excluding the single outlier), correlations at week 48 and 96 were 0.53 and 0.58, respectively. Finally, when only using samples collected on the same day (n = 42 at week 48 and n=19 at week 96), correlation was similar at r = 0.57 and r = 0.62, respectively.
Table 1:
Study Subject Baseline Characteristics
| Placebo (n=120) | Ibudilast (n=119) | |
|---|---|---|
| Age, average (SD) | 56.6 (6.6) | 54.6 (7.8) |
| Sex, females (%) | 65 (54%) | 65 (52%) |
| Disease Duration, median years (range) | 9.0 (0–36) | 11.0 (0–41) |
| Disability, median EDSS step (range) | 6.0 (3.0–7.0) | 6.0 (2.5, 6.5) |
| Concurrent injectable therapy, # (%) | 39 (32%) | 39 (31%) |
| Baseline serum NfL, geometric mean (95% CI) | 31.9 (28.8, 35.4) | 28.8 (26.3, 31.7) |
| Baseline CSF NfL, geometric mean (95% CI) (# subjects) | 1150.8 (947, 1398) (n=28) | 1290.3 (1029, 1617) (n=30) |
| Censored Cohort 1 | ||
| Baseline Serum NfL, mean | 29.7 (n=94) | 28.2 (n=91) |
| Baseline CSF NfL, mean | 1086.2 (n=26) | 1175.7 (n=23) |
| Censored Cohort 2 | ||
| Baseline Serum NfL, mean | 30.7 (n=105) | 27.7 (n=103) |
| Baseline CSF NfL, mean | 1095.4 (n=27) | 1275.2 (n=28) |
Figure 1.
Correlation plots of log (serum NfL) and log (CSF NfL) at Weeks 48 and 96 (after removing the outlier data point in the Week 96 plot, r = 0.58).
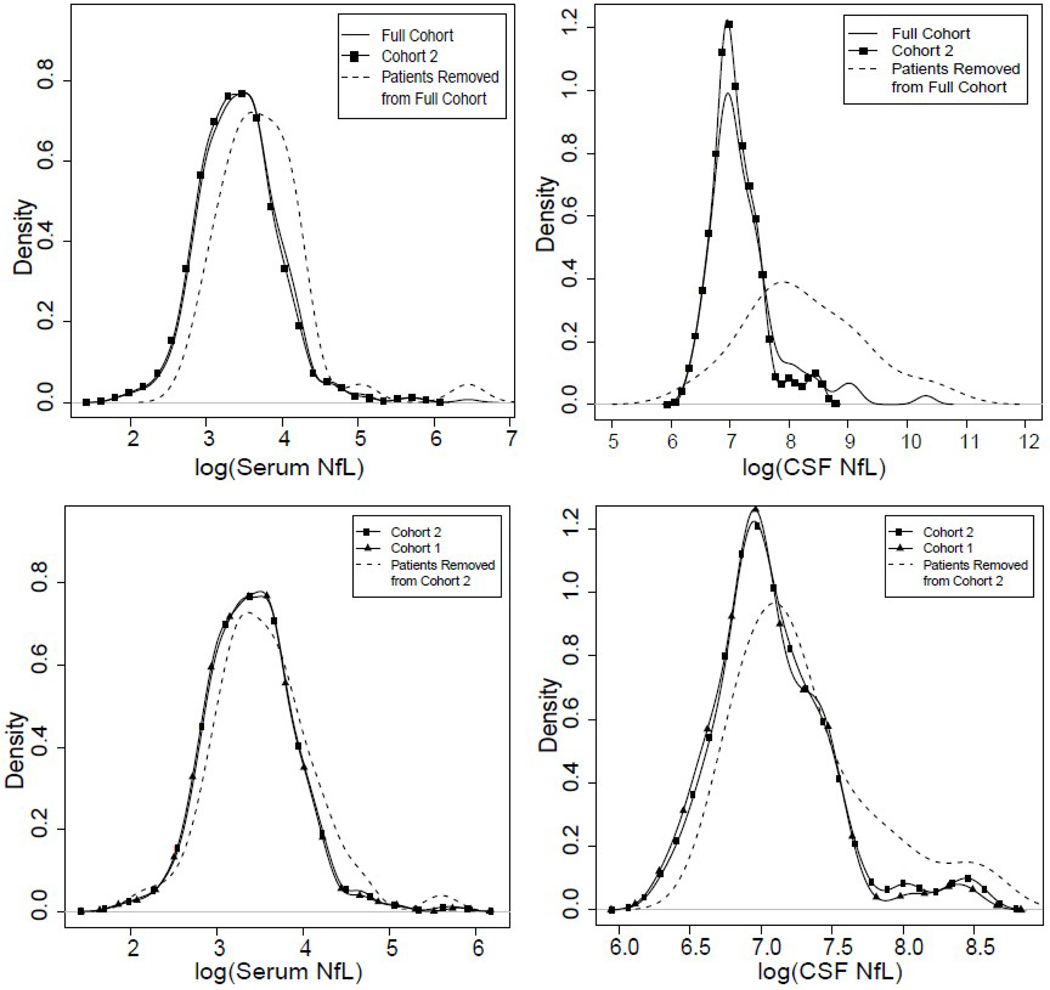
NfL at time points following either brain tissue injury from neurologic SAEs or inflammatory activity as measured by clinical relapse or ≥3 new lesions on MRI was higher in both serum (P=0.002, Fig 2A) and CSF (P<0.001, Fig 2B) compared to the full cohort. Comparison of the most restrictive censoring (any new/enlarging T2 lesions, Cohort 1) to a less restrictive (3 or more new/enlarging T2 lesions, Cohort 2) found essentially no change in either serum NfL (P=0.09, Fig 2C) or CSF NfL (P=0.1, Fig 2D), although 11% of subjects from both serum and CSF datasets were lost with the more restrictive censoring (Table 1).
Figure 2.
Density plots of NfL values in serum (A, C) and CSF (B, D) between full study cohort and two censored cohorts.
Over the course of the study, NfL in both serum and CSF increased in the overall study population (P<0.001) (Fig. 3 A, B). When using the full dataset, no between-group differences for study drug allocation in NfL were observed in either serum (P=0.76; Fig 3A) or CSF (P=0.46; Fig 3B). Similarly, after excluding NfL at time points following either brain tissue injury from neurologic SAEs or inflammatory activity (Cohorts 1 and 2), there was no between-group difference (ibudilast vs placebo) in either serum (cohort 1, P=0.93; cohort 2, P=0.89) or CSF (cohort 1 P=0.48; cohort 2, P=0.24) (Fig 3C-F).
Figure 3.
NfL (error bars represent 95% confidence intervals) in the full cohort for ibudilast-treated (squares) and placebo (triangles) in serum (A) and CSF(B); NfL in Cohort 1 in serum (C) and CSF (D); NfL in Cohort 2 in serum(E) and CSF (F).
For other exploratory sensitivity analyses, potential confounders (neurological serious adverse events, relapses, and new MRI lesions) were collapsed into a composite variable and included in a model as a potential source of heterogeneity. Time points accompanying neurologic SAEs, clinical relapse, or new MRI lesions were associated with higher serum NfL (P<0.001). After adjusting for that composite variable, there remained no difference in serum NfL between placebo and ibudilast groups over 96 weeks (P=0.3). In addition, the potential confounding of concurrent immune modulating therapy (interferon-beta or glatiramer acetate) was evaluated using a mixed model. When using concurrent immune modulating therapy as a potential confounder, there remained no effect of ibudilast on serum NfL (P=0.66). Finally, a per-protocol analysis that included only time points when subjects were still taking assigned study medication excluded 15 samples (<2% of total samples available) and was unchanged from the overall analysis (P=0.80).
Discussion
In a Phase 2 trial in progressive MS, ibudilast treatment was associated with a reduction in progression of whole brain atrophy, cortical atrophy, and decline in magnetization transfer ratio in normal-appearing brain tissue.18 However, we were surprised here to find that ibudilast was not associated with a change in either serum or CSF NfL compared with placebo. This result was surprising given benefits seen in the primary and secondary imaging outcomes from the same trial.10 One previous study of fingolimod and preliminary reports of several approved MS therapies, including alemtuzumab, natalizumab, pegilated interferon, and ocrelizumab, found that these therapies are associated with lower serum NfL levels.17–19 Although ibudilast has several anti-inflammatory effects in vitro (described above), it does not reduce the development of new inflammatory brain lesions in either relapsing MS20 or our trial of progressive MS21 and thus does not appear to have clinically-relevant anti-inflammatory activity in MS. Rather than acting as an anti-inflammatory in the brain of patients with progressive MS, ibudilast may protect neurons in lesions from continued damage and decline after previous inflammatory injury (17).
We examined whether active inflammation or other CNS injury in our study population affected NfL levels and altered our findings. In a post-hoc analysis, we excluded data points when a new or enlarging lesion developed in the previous six months, a clinical relapse was reported in the previous six months (which would help capture spinal cord inflammation), or if a subject experienced a CNS serious adverse event such as stroke. As expected, serum and CSF NfL levels were elevated in patients with active inflammation or CNS serious adverse events. However, after excluding patients with inflammation and brain injury, we did not observe a difference in either serum or CSF NfL between the treatment groups. Interestingly, we found no difference in NfL levels in either censored Cohort 1 (1 or more new or enlarging T2 lesions on brain MRI) or censored Cohort 2 (3 or more new or enlarging T2 lesions). This observation suggests that the elevation in NfL observed in the serum and CSF of progressive MS patients is not driven primarily by subjects with a large number of new lesions. Similarly, when including inflammation and brain injury as a composite variable, we did not observe a difference between treatment groups.
Despite having positive effects on MRI measures of progressive tissue injury, ibudilast did not have a beneficial effect on the development of new or enlarging T2 lesions.22 This dissociation between measures of neurodegeneration and neuroinflammation, along with our observations that NfL was unchanged by ibudilast, suggests that NfL may be more sensitive to track neuroaxonal injury that is associated with acute focal inflammatory disease activity in MS (i.e. new gadolinium-enhancing lesions and T2 lesions) than other chronic pathologies present in progressive MS (i.e. progressive atrophy), as the quantities of released NfL are likely far higher in the former. In fact, the outlier subject depicted in Figure 1B had developed 14 new/enlarging lesions between weeks 48 and 96, highlighting the large contribution of focal inflammation to NfL levels. Dissociation between inflammation and neurodegeneration has been observed in other MS studies, including preliminary reports from progressive MS trials, in which both fingolimod (primary progressive MS) and natalizumab (secondary progressive MS) have been shown to reduce NfL levels17, 19 despite no significant benefit on relapse independent progression of clinical disability.23, 24 In a meta-analysis, CSF NfL levels in patients with RRMS were five times higher during remission and nine times higher during a relapse than CSF NfL levels in controls.25. For patients with progressive MS, CSF NfL levels were twice that of controls but were, however, significantly less than those with RRMS during remission?.25 This association between CSF NfL and acute inflammation, suggests that NfL is measuring neuro-axonal injury by acute focal inflammation. The role in quantifying chronic neurodegeneration is less certain to date, hence it remains unknown whether serum NfL may be a useful and sensitive measure of neuroprotection in progressive MS in the absence of active inflammation and further study is needed.
Serum NfL has been proposed as an estimate of CSF levels of NfL.26 We paired serum and CSF samples from 54 subjects at week 48 and 30 subjects at week 96, and found that serum NfL accounted for only 27–35% of the variance in CSF NfL (r = 0.52–0.59, after excluding one extreme outlier at week 96). Previous serum/CSF correlation studies found somewhat higher correlations of r = 0.62 – 0.80.14, 16, 27 All three of these other studies included relapsing MS subjects who have a broader range of CNS inflammation than the progressive MS patients included in our study. In the Disanto study, almost half of subjects had gadolinium-enhancing lesions at the time of sampling.14 The Novakova study reported r = 0.62 in MS patients, but only r = 0.385 in healthy controls. These NfL levels in healthy controls are more similar to the levels observed in the progressive MS patients reported here, although they used a different NfL assay, which makes direct comparisons difficult.27 As described in a recent review, the NfL levels are higher in RRMS than SPMS in CSF but then are the highest in the serum of SPMS so that a possible contribution to the serum NfL levels from the periphery may play a role in reducing the CSF/serum correlation.[ref PMID 33146954] Moreover, our somewhat reduced correlation coefficient between CSF and blood NfL is likely to be secondary to the reduced observed range of NfL values. As with any correlation calculation, restricting the range of values will reduce the derived correlation coefficient.28Finally, although the timecourse of NfL in CSF and blood following MS disease activity are unknown and may not be linear, since progressive MS has less disease activity than relapsing MS, this is less likely in progressive MS to impact correlations between serum and CSF levels.
The results from these other studies, together with our results, suggest that serum NfL levels have limited correlation with those in concurrent CSF samples when the expected range of NfL levels is relatively low. This observation may have implications when monitoring other neurologic conditions where NfL levels are only modestly elevated relative to healthy controls, such as Alzheimer’s disease.29 Although all NfL in the peripheral blood is presumed to come from neurons in either the central or peripheral nervous system, the kinetics of NfL in the CSF and peripheral blood and its transit from CSF to the peripheral is not well understood. The lower association we observed compared to other reports may represent differential kinetics of NfL in the two body compartments. In contrast to a previous study,16 adjusting for body mass index had a negligible effect on the correlation, although this observation may be secondary to the relatively low levels of NfL values. Restricting correlations to just those serum and CSF samples collected on the same day led to little change in the correlation, suggesting that our observation was not due to asynchronous serum and CSF collections. Importantly, our observation regarding serum and CSF NfL correlations does not indicate that measures of serum NfL are not useful in progressive MS. Indeed, NfL secretion into the CSF may be more variable, leaving serum NfL as a better cumulative measure of NfL turnover. Further studies of serum and CSF NfL are needed to better understand both the kinetics of NfL turn-over in each body fluid compartment and the role of NfL as a treatment response biomarker in neurologic conditions such as MS.
Limitations of this study include the lack of data on either gadolinium-enhancing lesions or spinal cord lesions, both of which indicate inflammation and correlate with increased NfL levels.30 Blood sampling only once a year prevented more granular assessments of the treatment effect of NfL and potential confounding by disease activity and CNS injury. Many sensitivity analyses were post-hoc efforts to interpret potential confounders and should be interpreted as exploratory and hypothesis-generating.
In summary, we observed no effect of ibudilast on either serum or CSF levels of NfL in a Phase 2 trial of ibudilast in progressive MS. We observed NfL elevations in patients with either active inflammation or other CNS injury, which may have confounded our use of NfL to measure the potential neuroprotective effect of ibudilast, although removal of those patients didn’t affect results. In progressive MS, with its lower range of NfL concentration in the CSF and serum, the correlation between these compartments appears lower than that seen in other MS studies involving relapsing MS patients. Our results indicate that the optimal use of NfL as a biomarker in progressive MS remains to be defined. In addition, our data highlight the complexities in the interpretation of NfL as a biomarker in progressive MS trials.
Acknowledgements
The investigators are indebted to the Clinical Coordinating Center (Massachusetts General Hospital), the Data Coordinating Center (University of Iowa), all the staff at the clinical sites, the Protocol Steering Committee, NINDS, National MS Society, and NeuroRx for their support of the SPRINT-MS trial. A special thanks to all the people living with progressive MS who participated in the SPRINT-MS trial.
The authors acknowledge David Leppert, University of Basel, and John Fredieu, Cleveland Clinic, for critical review of this manuscript, and Aryn Giffi Scibona, Cleveland Clinic, for editorial support of this manuscript.
Funding:
Supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) (U01NS082329) and the National Multiple Sclerosis Society (RG 4778-A-6) and by MediciNova through a contract with the National Institutes of Health (NIH). The NeuroNEXT Network is supported by the NINDS (Central Coordinating Center, U01NS077179; Data Coordinating Center, U01NS077352; and individual grants to each trial site) and the Swiss National Science Foundation (320030_189140).
Footnotes
Disclosures:
R. Fox has received personal consulting fees from Actelion, Biogen, Celgene, EMD Serono, Genentech, Immunic, Novartis, and Teva, served on advisory committees for Actelion, Biogen, Immunic, and Novartis, and received clinical trial contract and research grant funding from Biogen and Novartis.
P. Raska reports no disclosures relevant to this manuscript
C. Barro reports no disclosures relevant to this manuscript
C. Goebel reports no disclosures relevant to this manuscript
M. Karafa reports no disclosures relevant to this manuscript
V. Konig reports no disclosures relevant to this manuscript
R. Bermel has served as a consultant for Biogen, Genzyme, Genentech, and Novartis, received research support from Biogen, Genentech, and Novartis, and shares rights to intellectual property underlying the Multiple Sclerosis Performance Test, currently licensed to Qr8 Health and Biogen.
M. Chase reports no disclosures relevant to this manuscript
C. Coffey reports no disclosures relevant to this manuscript
A. Goodman has received personal compensation for consulting from Adamas, EMD-Serono, MedDay, Greenwich Bioscience, Celgene, Teva, Sun Pharma, Novartis, Sanofi Genzyme, Genentech-Roche, Biogen, Atara, Acorda Therapeutics and received research support from Teva, Sun Pharma, Novartis, Sanofi Genzyme, Genentech-Roche, Biogen, Atara, Acorda Therapeutics.
E. Klawiter has received consulting fees from Alexion, Biogen, EMD Serono, Genentech and MedDay and received research funding from Abbvie, Biogen, EMD Serono, Genzyme, and Roche.
R. Naismith has received consulting fees for Alexion, Alkermes, Bayer AG, Biogen, Celgene, EMD Serono, Genentech, Genzyme, NervGen, Novartis, TG Therapeutics, Third Rock Ventures, Viela Bio.
J. Kuhle has received research funding from Bayer, Biogen, Celgene, Merck, Novartis, Roche, Sanofi, Swiss MS Society, Swiss National Research Foundation, University of Basel.
References
- 1.Sormani MP and Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol 2013; 12: 669–676. DOI: 10.1016/S1474-4422(13)70103-0. [DOI] [PubMed] [Google Scholar]
- 2.Fox RJ. Feast or famine in multiple sclerosis therapeutics. Lancet Neurol 2020 2020/01/26. DOI: 10.1016/S1474-4422(19)30487-9. [DOI] [PubMed] [Google Scholar]
- 3.Teunissen CE and Khalil M. Neurofilaments as biomarkers in multiple sclerosis. Mult Scler 2012; 18: 552–556. 2012/04/12. DOI: 10.1177/1352458512443092. [DOI] [PubMed] [Google Scholar]
- 4.Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. Jama Neurology 2019; 76: 1035–1048. DOI: 10.1001/jamaneurol.2019.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duering M, Konieczny MJ, Tiedt S, et al. Serum Neurofilament Light Chain Levels Are Related to Small Vessel Disease Burden. J Stroke 2018; 20: 228–238. 2018/06/12. DOI: 10.5853/jos.2017.02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor R, Smith K, Allegretta M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology 2020; 95: 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92: E1007–E1015. DOI: 10.1212/Wnl.0000000000007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141: 2382–2391. DOI: 10.1093/brain/awy154. [DOI] [PubMed] [Google Scholar]
- 9.Christensen JR, Komori M, von Essen MR, et al. CSF inflammatory biomarkers responsive to treatment in progressive multiple sclerosis capture residual inflammation associated with axonal damage. Multiple Sclerosis Journal 2019; 25: 937–946. DOI: 10.1177/1352458518774880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. New England Journal of Medicine 2018; 379: 846–855. DOI: 10.1056/NEJMoa1803583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolan P, Hutchinson M and Johnson K. Ibudilast: a review of its pharmacology, efficacy and 2009/11/26safety in respiratory and neurological disease. Expert Opin PharmacotherF 2009; 10: 2897–2904. DOI: 10.1517/14656560903426189 [DOI] [PubMed] [Google Scholar]
- 12.Suzumura A, Ito A, Yoshikawa M, et al. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res 1999; 837: 203–212. 1999/08/06. DOI: 10.1016/s0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- 13.Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials 2016; 50: 166–177. 2016/08/16. DOI: S1551-7144(16)30207-5 10.1016/j.cct.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disanto G, Barro C, Benkert P, et al. Serum Neurofilament Light: A Biomarker of Neuronal Damage in Multiple Sclerosis. Ann Neurol 2017; 81: 857–870. DOI: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadler SB, Hidalgo JH and Bloch T. Prediction of blood volume in normal human adults. Surgery 1962; 51: 224–232. 1962/02/01. [PubMed] [Google Scholar]
- 16.Manouchehrinia A, Piehl F, Hillert J, et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann Clin Transl Neurol 2020; 7: 139–143. 2020/01/02. DOI: 10.1002/acn3.50972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhle J, Kropshofer H, Haring DA, et al. Neurofilament light levels in the blood of patients with secondary progressive MS are higher than in primary progressive MS and may predict brain atrophy in both MS subtypes. Multiple Sclerosis Journal 2018; 24: 111. [Google Scholar]
- 18.Kuhle J, Kropshofer H, Barro C, et al. Siponimod Reduces Neurofilament Light Chain Blood Levels in Secondary Progressive Multiple Sclerosis Patients. Neurology 2018; 90. [Google Scholar]
- 19.Kapoor R, Sellebjerg F, Hartung HP, et al. Natalizumab Reduces Serum Concentrations of Neurofilament Light Chain in Secondary Progressive Multiple Sclerosis Patients From the Phase 3 ASCEND Study. Neurology 2019; 92. [Google Scholar]
- 20.Barkhof F, Hulst HE, Drulovic J, et al. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology 2010; 74: 1033–1040. 2010/03/05. DOI: WNL.0b013e3181d7d651 [pii] 10.1212/WNL.0b013e3181d7d651. [DOI] [PubMed] [Google Scholar]
- 21.Naismith RT, Bermel RA, Coffey CS, et al. Effects of Ibudilast on MRI measures in the Phase 2 SPRINT-MS study. Neurology in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naismith RTB, Coffey RA, Goodman CS, Fedler AD, Chase J, Klawiter M, Nakamura EC, Narayanan K, Goebel S, Yankey CV, Klingner J, Fox E, R.J. Effects of Ibudilast on MRI measures in the Phase 2 SPRINT-MS study. . Manuscript submitted for publication, 2020. [Google Scholar]
- 23.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016. 2016/02/02. DOI: S0140-6736(15)01314-8 [pii] 10.1016/S0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- 24.Kapoor R, Ho PR, Campbell N, Chang I, Deykin A, Forrestal F, Lucas N, Yu B, Arnold DL, Freedman MS, Goldman MD, Hartung HP, Havrdová EK, Jeffery D, Miller A, Sellebjerg F, Cadavid D, Mikol D, Steiner D. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. The Lancet Neurology 2018; 17: 405–415. DOI: 10.1016/S1474-4422(18)30069-3. [DOI] [PubMed] [Google Scholar]
- 25.Martin SJ, McGlasson S, Hunt D, et al. Cerebrospinal fluid neurofilament light chain in multiple sclerosis and its subtypes: a meta-analysis of case-control studies. J Neurol Neurosurg Psychiatry 2019; 90: 1059–1067. 2019/05/28. DOI: 10.1136/jnnp-2018-319190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nature Reviews Neurology 2018; 14: 577–589. DOI: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 27.Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89: 2230–2237. DOI: 10.1212/Wnl.0000000000004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Encyclopedia of Research Design. 2010. DOI: 10.4135/9781412961288. [DOI] [Google Scholar]
- 29.Zhou W, Zhang J, Ye F, et al. Plasma neurofilament light chain levels in Alzheimer’s disease. Neurosci Lett 2017; 650: 60–64. 2017/04/22. DOI: 10.1016/j.neulet.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Fox RJ CB, De Sèze J, Gold R, Hartung H- P, Jeffery D, Kappos L, Montalbán X, Weinstock-Guttman B, Singh C, Campbell N, Ho P- R, Su R, Engle B, Sangurdekar D, de Moor C, Fisher E, Kieseier BC,Rudick RA, Plavina T. Temporal Relationship of Serum Neurofilament Light (NfL) Levels and Radiological Disease Activity in MS Patients; ECTRIMS Online Library. 2018, October; 10; 228376; P532. [Google Scholar]