Abstract
There is a critical need for the establishment of an engineered model of the vocal fold epithelium that can be used to gain understanding of its role in vocal fold health and disease and facilitate the development of new treatment options. Towards this goal, primary vocal fold epithelial cells (VFECs) were isolated from healthy porcine larynxes and used within passage 3. Culture expanded VFECs expressed the suprabasal epithelial marker, cytokeratin 13 and intercellular junctional proteins, occludin, E-cadherin and zonula occludens-1. To establish the engineered model, VFECs were cultured on hyaluronic acid-derived synthetic basement membrane displaying fibronectin-derived integrin-binding peptide (RGDSP) and/or laminin 111-derived syndecan-binding peptide AG73 (RKRLQVQLSIRT). Our results show that matrix stiffness and composition cooperatively regulate the adhesion, proliferation and stratification of VFECs. Cells cultured on hydrogels with physiological stiffness (elastic shear modulus, G’ = 1828 Pa) adopted a cobblestone morphology with close cell-cell contacts, while those on softer matrices (G’ = 41 Pa) were spindle-shaped with extensive intracellular stress fibers. The development of stratified epithelium with proliferating basal cells and additional (1–2) suprabasal layers requires the presence of both RGDSP and AG73 peptide signals. Supplementation of cytokines produced by vimentin positive primary porcine vocal fold fibroblasts in the VFEC culture led to the establishment of 4–5 distinct cell layers. The engineered vocal fold epithelium resembled native tissue morphologically, expressed cytokeratin 13, mucin 1 and tight/adherens junction markers and secreted basement membrane proteins collagen IV and laminin 5. Collectively, our results demonstrate that stiffness matching, cell-matrix engagement and paracrine signaling cooperatively contribute to the stratification of VFECs. The engineered epithelium can be used as a versatile tool for investigations of genetic and molecular mechanisms in vocal fold health and disease.
Keywords: Vocal Fold Epithelial Cells, Stratification, Hydrogel, Hyaluronic Acid, Peptide
Graphical Abstract
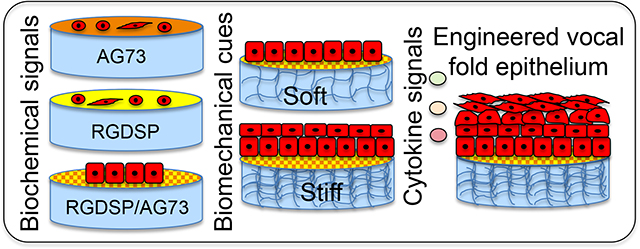
1. INTRODUCTION
When driven into a wave-like motion by the air from the lung, vocal folds produce a variety of sound that is essential to communication. The human vocal fold is composed of a lamina propria sandwiched between a stratified squamous epithelium and the vocalis muscle.1 The lamina propria is a loose connective tissue sparsely populated by fibroblasts (VFFs). 2–3 Anchored on a basement membrane, the epithelium consists of stacked epithelial cells (VFECs) held together by adherens junctions, desmosomes and tight junctions, with a cytokeratin 14 (KRT14) positive basal layer and up to 6 cytokeratin 13 (KRT13) positive suprabasal layers. During tissue turnover, VFECs divide in the basal layer and move superiorly and medially into the suprabasal layers. The outermost luminal cells are eventually replaced with new cells while old cells are sloughed off into the laryngeal lumen. The epithelium maintains tissue hydration, interfaces with the external environment, provides the structural stability to the vocal fold, and protects the underlying lamina propria from chemical/immunological irritation and biomechanical trauma. The epithelium, together with the superficial lamina propria, contributes to the propagation of the mucosa wave over the vocal fold during phonation. 4–7
Vocal folds can be damaged by exposure to chemicals, such as pollutant particles and refluxed stomach acids. Mechanical factors, such as trauma introduced during intubation or stress induced in voice abuse and overuse, can also result in vocal fold scarring.8 Accumulating knowledge suggests that VFECs play important roles in triggering and sustaining tissue fibrosis.9–10 Animal studies have shown that, following surgical striping of the epithelium, rapid re-epithelialization is achieved in 3–5 days, yet epithelial barrier function is not fully recovered.11 Excessive phonation can lead to destruction of tight/adherens junctions, shedding of surface VFECs, dilation of paracellular spaces and denudement of the basement membrane. The surviving or newly grown epithelial cells express different cytokeratins, are predisposed to further injury, secrete profibrotic mediators and promote abnormal repair.12
Despite the importance of VFECs in vocal fold homeostasis and diseases, few studies investigate the development of an artificial epithelium that resembles the structure and function of the native tissue. Mizuta et al. cultured rabbit VFECs on collagen IV coated tissue culture plates as a monolayer. To establish an epithelial cell multilayer expressing the epithelial cell markers KRT13, KRT14 and the tight junction proteins occludin and zonula occludens-1 (ZO-1), mouse 3T3 feeder cells were required.13 Separately, Erickson-DiRenzo et al. cultured porcine VFECs on tissue culture plates as a monolayer. Cells exhibited cobblestone appearance and were stained positive for pan-cytokeratin and expressed mucins (MUC) 1 and 4 at the transcript level.14 Although these studies laid the foundation for the development of physiologically relevant vocal fold tissue model, culturing VFECs on rigid tissue culture plates may inadvertently alter cell phenotype. Finally, Ling et al. cultured human VFECs on VFF-populated collagen gels under organotypic conditions.15 The bioengineered mucosae showed morphologic features of native tissue, proteome-level evidence of mucosal morphogenesis and emerging extracellular matrix complexity but did not exhibit mature barrier function.
The goal of this work is to gain fundamental understanding on how different biochemical and biomechanical signals regulate the attachment, proliferation and stratification of VFECs. Herein, primary VFECs were isolated from porcine larynxes and the contaminating VFFs were depleted by selective attachment. The epithelial cultures were expanded and characterized by immunostaining for stratified epithelial cell markers and tight/anchoring junction markers, and compared to the native porcine vocal fold tissue. Hyaluronic acid (HA)-based hydrogels of varying HA concentration and presenting fibronectin derived cell adhesive motif and laminin 111-derived syndecan binding peptide (AG73)16 were evaluated for initial cell attachment and proliferation. HA is naturally abundant in the lamina propria, contributing to the maintenance of optimal tissue mechanics.17–19 In addition, HA binds specific cell surface receptors and directs cell adhesion, wound healing and tissue morphogenesis.20–21 Thus, HA-based hydrogels presenting basement membrane protein signals recapitulate the epithelium-mesenchymal tissue boundary, allowing mechanistic interrogation of cell-matrix interactions necessary for VFEC stratification. Further enhancement in stratification was achieved by supplementing the epithelial culture with VFF-conditioned media. The engineered model can be used to investigate cellular events and molecular mechanisms contributing to the development of a disease phenotype.
2. MATERIALS AND METHODS
2.1. Hydrogel Synthesis and Characterization.
2.1.1. Synthesis of hydrogel building blocks.
Acrylate functionalized HA (HA-AES) was synthesized as previously reported.22 Briefly, sodium hyaluronate (5 kDa, Lifecore Biomedical, Chaska, MN) was converted to tetrabutylammonium salt, and reacted with mono-2-(acryloyloxy)ethyl succinate (AES) in DMSO. Purified product was collected after precipitation, ion-exchange and dialysis. An acrylate degree of modification of 50% was determined using 1H-NMR.22 Separately, sodium hyaluronate (430 kDa, Sanofi Genzyme Corporation, Cambridge, MA) was reacted with 3,3’dithiobispropanoic dihydrazide in deionized water in the presence of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride at pH 4.75 under rigorous mechanical stirring for 1 h. After treatment with 1,4-dithiothreitol, the product was purified by dialysis and lyophilized to produce HA-SH. Characterization by 1H-NMR indicated 60% thiol modification.23
Maleimide-functionalized peptides, MI-GGGRGDSPG23 and MI-GGGRKRLQVQLSIRT were synthesized on a PS-3 peptide synthesizer (Protein Technologies, Tucson, AZ). Fmoc solid phase synthesis was conducted at 0.25 mmol scale using Rink-Amide MBHA resin (EMD Millipore, Burlington, MA). Maleimide functionality was incorporated by reacting 4-maleimidobutyric acid (1.0 mmol) with the N-terminal glycines using N,N,N′,N′-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate (HBTU, 1.0 mmol) / N, N-diisopropylethylamine (DIPEA, 2.0 mmol) in DMF for 1 h to produce RGDSP-MI and AG73-MI. Peptides were cleaved with TFA/TIPS/H2O (95:2.5:2.5, v/v) and precipitated into diethyl ether. Peptides were purified with reverse phase high performance liquid chromatography (RP-HPLC). Peptide purity was analyzed at 220/280 nm (Figure S1) using a Shimadzu Prominence Series HPLC (Kyoto, Japan). A Waters UPLC LC-MS/MS system with an ESI source (Xevo G2-S QTof, Milford, MA) was used to confirm peptide mass (Figure S2).
2.1.2. Oscillatory rheology.
HA-SH and HA-AES were separately dissolved in PBS at pH 7.4 and mixed at 20/1 (v/v). Hydrogels prepared using 10 mg/mL HA-SH and 10 mg/mL HA-AES had a final HA concentration of 10 mg/mL (HA10) and those prepared using 20 mg/mL HA-SH and 100 mg/mL HA-AES had a final HA concentration of ∼24 mg/mL (HA24). The viscoelastic properties of HA gels were analyzed using a TA Instruments DHR-3 rheometer (TA, instruments, New Castle, DE) with a 12 mm stainless steel geometry and 500 μm gap size at 37 °C. Promptly after mixing, the liquid was loaded on a plate/plate geometry, and mineral oil was applied around the geometry. Time sweep was performed with 1.0% strain at 1 Hz. After 2 h, a frequency sweep from 0.1 to 10 Hz was performed at 1.0% strain. Measurements were conducted in triplicate, and the average storage (G′) and loss (G″) moduli are reported.
2.1.3. Characterization of peptide conjugation.
HA-SH and HA-ASE solutions were mixed and aliquoted into 12-mm cell culture inserts. Five minutes later, RGDSP-MI and AG73-MI (100 μL, 0.5 mM each) was added on top of the gel mixture and incubated for 30 min. The supernatant was aspirated and the hydrogel was washed with PBS (100 μL) three times. The supernatant and the wash solutions were pooled and lyophilized for the respective hydrogel. The dry product was dissolved in 100 μL deionized water and ran on the Shimadzu analytical HPLC by monitoring peptide elution at 220 nm. Pure peptides of known concentrations were analyzed similarly, and standard curves were constructed based on peak integration at each concentration. Peptide concentration in the combined wash solutions was determined using the standard curve.
2.2. Isolation and Characterization of VFECs.
2.2.1. Cell isolation and expansion.
Porcine larynxes were collected from healthy Yucatan mini-pigs, (male and female, 4–6 months old). All animal procedures were performed at the Philadelphia VA Medical Center with approval from the Institutional Animal Care and Use Committee and in accordance with policies set forth by the National Institutes of Health. The larynxes were transported in ice cold sterile Hank’s balanced salt solution (HBSS, with calcium and magnesium) supplemented with 100 IU/mL penicillin-streptomycin. The larynxes were then dissected along the mid-sagittal plane, and the true vocal folds were microdissected from its underlying thyroarytenoid muscle. The resected vocal folds were washed with calcium and magnesium free HBSS containing 100 IU/mL penicillin-streptomycin (HBSS+) and enzyme digested with 1 U/mL Dispase II (Sigma Aldrich, St. Louis, MO) in HBSS+ overnight at 4 °C.
After digestion, the epithelium was carefully peeled off the underlying lamina propria and placed in a tube containing HBSS+. Then the cells were isolated from the epithelium by incubating with 0.05% (wt/vol) trypsin-EDTA solution for 5 min and neutralized using a trypsin soybean inhibitor (Sigma Aldrich). The cell suspension was further neutralized and washed with flavonoid adenine dinucleotide (FAD) media containing Ham’s F-12/DMEM (3:1 ratio), 100 IU/mL penicillin−streptomycin, fetal bovine serum (2.5%), epidermal growth factor (10 ng/mL), insulin (5 μg/mL), hydrocortisone (0.4 μg/mL), cholera toxin (8.4 ng/mL) and adenine (24 μg/mL). 14
To prepare flaks for subculture, collagen IV (Sigma Aldrich) was diluted in sterile HBSS to a concentration of 50 μg/mL. Two milliliter of the solution was added to a T25 flask, and the flask was incubated at room temperature for 1 h. The flask was washed with sterile HBSS prior to cell seeding. The cell suspension was filtered through a 40-μm cell strainer and centrifuged. The supernatant was carefully removed, and the cell pellet was re-suspended in FAD media and seeded on collagen IV-coated cell culture flask. Isolated VFECs were cultured in a humidified incubator at 37 °C with 5% CO2 and 95% air. The growth and morphology of these VFECs were monitored daily and media was replaced every 4 days until they reach 50% confluency and from then every 2 days until they reach full confluency. Before passaging, VFECs were plated on uncoated cell culture flask to allow VFFs to attach. After 2 h of incubation, the floating cell population was collected and re-plated on collagen IV coated flask to obtain pure population of VFECs. The attached VFFs were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 100 IU/mL penicillin−streptomycin. Experiments in this work were conducted from passages 1–3 from at least 3 different animals.
2.2.2. Growth kinetics.
VFECs were plated on collagen IV-coated tissue culture plates at 4 × 104 cells/cm2 and VFFs were seeded and maintained on uncoated plates at 5 × 103 cells/cm2. After 7 days of culture, cells were trypsinized, stained with trypan blue and counted using a hemocytometer. The doubling time was calculated as 2N = Cf/Ci, where N is the doubling time, Ci refers the initial cell count at the time of seeding and Cf is the final cell count on day 7.
2.2.3. Flow cytometry.
Cells were washed with PBS and stained with FITC-labeled anti-CD90 antibody (BD Biosciences, San Jose, CA) for 15 min at room temperature for cell surface staining. Next, cells were washed with cold PBS and fixed using 0.5% paraformaldehyde (PFA) / 0.1% Triton solution at a concentration of 2×106 cells/mL. Subsequently, cells were incubated for 10 min and washed with Perm/Wash buffer (BD Biosciences) consisting of 3% bovine serum albumin (BSA) and 0.1% Triton in PBS. Finally, cells were treated with Alexa Fluor® 647 monoclonal antibody that recognizes intracellular keratins 14, 15, 16 and 19 (KRT, BD Biosciences) for 30 min and washed using Perm/Wash buffer. Samples were ran using BD FACSAria II instrument (BD Biosciences) and analyzed using FlowJo software. Results were compared to their respective IgG controls. Antibody information can be found in Table S1.
2.3. Development of Hydrogel-Supported Epithelium.
2.3.1. Culture of VFECs on HA gels.
HA hydrogels were prepared as described above in 2.1.3. using sterile-filtered HA-SH, HA-AES, RGDSP-MI and AG73-MI. VFECs were seeded on the hydrogels at a concentration of 1 × 103 cells/μL. Cultures were submerged in FAD media until a cell monolayer was developed. Thereafter, cultures were maintained at the air-liquid interface, with media present only in the well surrounding the insert, until day 42. Alternatively, the FAD media was replaced with fibroblast-conditioned media produced by culturing VFFs isolated from the same animal in DMEM media at passage 3 until 80% confluency. Media was changed every other day.
2.3.2. Viability assay.
For LIVE/DEAD® cell staining, calcein AM (Life Technologies, Carlsbad, CA) and ethidium homodimer-1 (EthD-1, Life Technologies) was diluted at 1:1000 (v/v) and 1:2000 (v/v), respectively with sterile PBS. After aspirating media and washing with 300 μL of warm PBS, samples were incubated with 300 μL of the dye solution at 37 °C for 20 min. The constructs were assayed after 7, 14, 21 and 42 days of culture using a Zeiss LSM 880 confocal microscope (Oberkochen, Germany). Viability was quantified based on percentage of calcein positive area using ImageJ software.
2.3.3. Immunofluorescence.
Cells were fixed with 4% (v/v) PFA in PBS for 20 min. Following a 20-min permeabilization using 0.1 % Triton X-100 in a 3% BSA/PBS solution. The Triton solution was rinsed, and non-specific binding was blocked with 3% BSA for 1 h at room temperature. Samples were incubated with primary antibodies (Table S1) diluted in 3% BSA (1:100) at room temperature for 2 h. After rinsing, samples were incubated with secondary antibodies, Alexa Fluor 488 goat anti-mouse (Thermo Fisher Scientific, Waltham, MA) and Alexa Fluor 647 goat anti-rabbit (Thermo Fisher Scientific), diluted in 3% BSA (1:250) at room temperature for 1 h. Filamentous actin was labelled with Alexa Fluor 568 phalloidin (Thermo Fisher Scientific) using a 1:250 dilution in 3% BSA. Nuclei were visualized with DAPI (Thermo Fisher Scientific) at 1:1000 in 3% BSA. Fluorescent imaging was performed using a Zeiss (LSM 880.
2.3.4. Histology and immunohistochemistry.
Vocal folds dissected from porcine larynxes were fixed in 4% PFA and paraffin-embedded for tissue sectioning at Histochemistry and Tissue Processing Core, Nemours. Samples (5-μm sections) were stained with Hematoxylin and Eosin (H&E) following standard protocols, 24 visualized on a Nikon (Tokyo, Japan) TS100-F phase contrast microscope, and imaged with a Nikon Coolpix camera. H&E stained cell nuclei purple and the surrounding matrix pink. Five micron thick sections were collected, deparaffinized at 55 °C, and antigen retrieved using a citrate buffer. Sections were permeabilized using PBST (0.025% tween) and blocked using 10% BSA for 45 min at room temperature. Samples were then incubated in solutions of primary antibodies (1:100 dilution in 3% BSA) overnight at 4 °C, followed by 1-h incubation in solutions of secondary antibodies (1:250 dilution in 3% BSA) at room temperature. Sections were washed with PBS and counter stained for nuclei with DAPI (1:1000). Finally, mounting media (50% glycerol) was added with a coverslip and sections were images using a Zeiss LSM 880.
2.4. Statistical Analysis.
All data are shown as mean value with standard error of the mean from 3 technical repeats and 3–4 independent biological experiments. Based on the data set of each experiment, one-way analysis of variance (one-way ANOVA) or two-way analysis of variance (two-way ANOVA) was used for assessing significance levels for comparison of multiple groups. Unpaired two-tailed t-test was used from comparison between two groups. A value of p<0.05 was considered as statistically significant.
3. RESULTS.
3.1. Characterization of vocal fold tissues and cells.
Fresh porcine vocal fold tissues were obtained from animals subjected to experimental procedures that do not affect the larynx. We first characterized the porcine tissue by H&E staining to identify the distinct regions of vocal fold: the stratified squamous epithelium, the lamina propria and the muscle (Figure 1A). The thickness of the stratified epithelium was ∼50 μm. Immunostaining (Figure 1B) shows the confinement of occludin to cell-cell junctions, indicating a tight epithelial barrier. The tissue exhibits a multilayered structure consisting of a basal layer and 4–5 suprabasal layers. As key components of intermediate filaments, KRT13 was detected throughout the epithelial strata (Figure 1C), whereas KRT14 expression was confined to the basal layer (Figure 1D). Similar observations with spatial localization of KRT13 and 14 have been observed in human vocal fold epithelium.25
Figure 1.
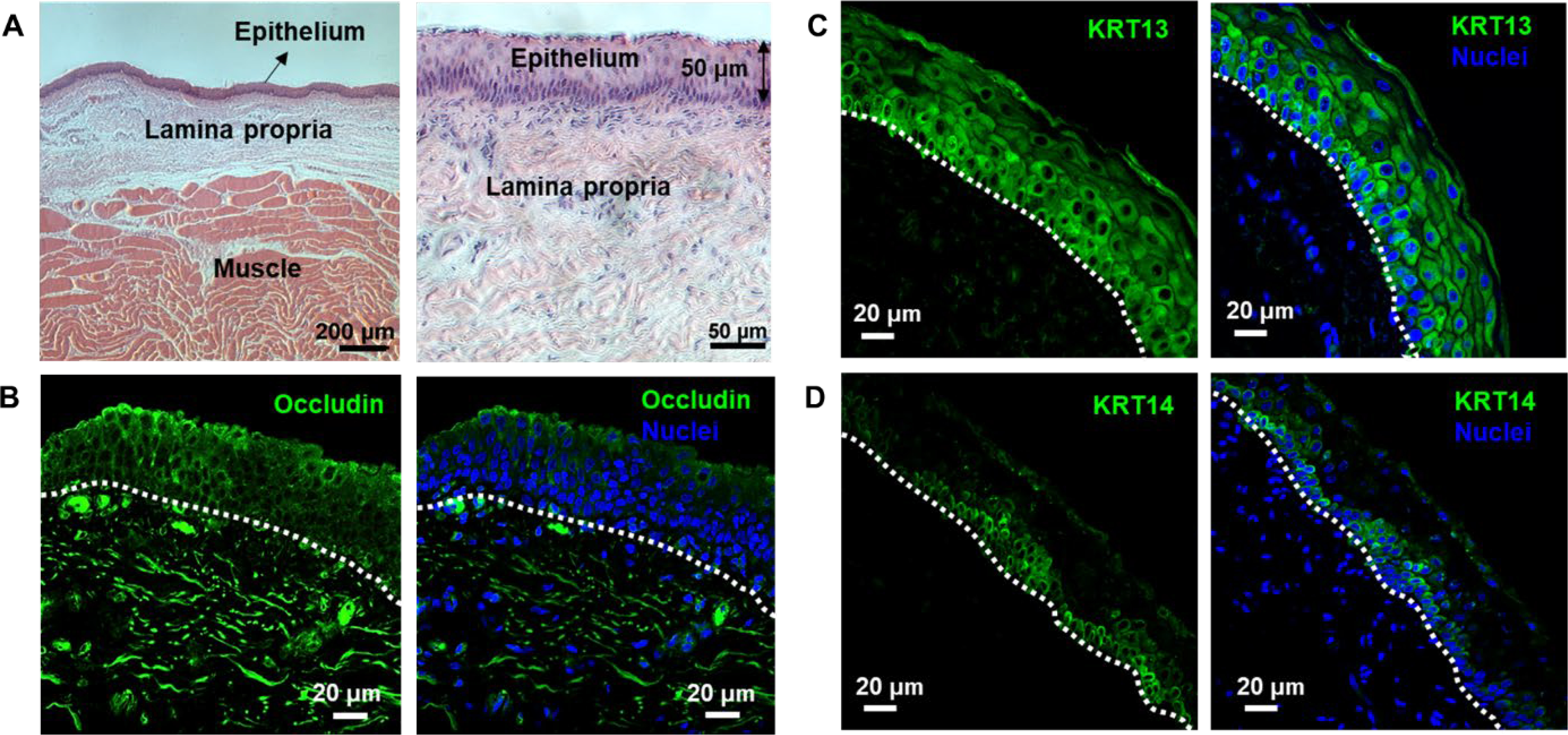
Characterization of porcine vocal folds by H&E (A) and immunohistochemistry (B-D). Tissues were collected from a 4-month old male pig. The stratified squamous epithelium contained five distinct cell layers with an estimated thickness of 50 μm (A). Tissues were stained positive for occludin, a tight junction marker (B). KRT13 expression extended throughout the entire stratified epithelium, sparing some basal cells that were directly attached to the basement membrane (C). KRT14 labeled the bottom three to four layers of epithelial cells (D). The right panels in (B-D) show markers overlaid with nuclei stained by DAPI. White dotted line indicates the basement membrane.
Primary VFECs and VFFs were released from the tissue via enzymatic digestion. We modified the established protocol14 to obtain pure populations of VFECs and VFFs. Our method relies on differential adhesion of VFFs and VFECs to coated and uncoated plates.15, 26–28 Cells isolated from the epithelium tissue were allowed to grow into confluence, and trypsinized. When re-plated on a flask without a collagen IV coating, VFFs readily attached, allowing collection of the non-adherent, floating population of cells that were primarily VFECs. These cells were re-plated on collagen IV coated flasks for expansion of a pure population of epithelial cells. The attached VFFs were cultured in fibroblast growth media to obtain a pure population of fibroblasts. This step is essential, as VFECs are sensitive to culture conditions; slight changes in isolation protocol, seeding density and subculture method led to compromised viability. Moreover, adventitious VFFs proliferated rapidly, suppressed VFEC proliferation and dominated the culture.
VFECs adopted a cobblestone morphology with close cell-cell contacts whereas VFFs maintained a spindle shaped morphology with elongated processes (Figure 2A). At passage 1, VFECs exhibited a doubling time of 37 h (Figure 2B). As the number of passages increased, the growth rate decreased, reaching a doubling time of 60 h by passage 6. Contrarily, VFFs continued to divide every 25 h even at higher passages. Overall, VFECs proliferated slowly compared to VFFs, in agreement with previous report.14 Thus, VFECs used in subsequent experiments were up to passage 3, while VFFs were used between passages 1 and 6.
Figure 2.
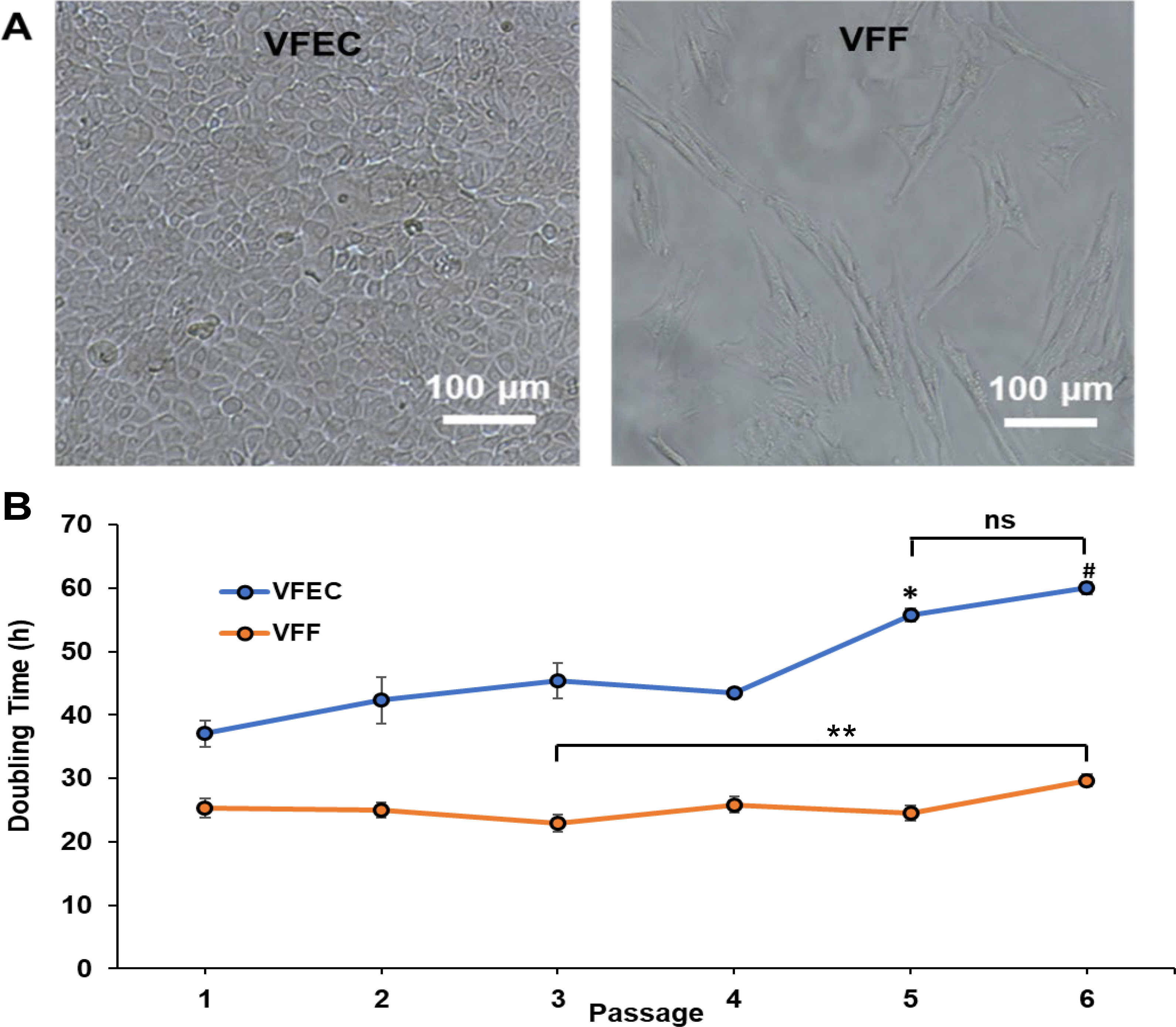
Morphology and growth kinetics of primary porcine VFECs and VFFs. (A) Phase contrast images of freshly isolated VFECs and VFFs grown on tissue culture plates. (B) Growth kinetics for VFECs and VFFs, in terms of doubling time as a function of passage number. */ # : Significantly different compared to passages 1 to 4 for VFECs. ns: Not significantly different between passages 5 and 6 for VFECs. **: Significantly different between passages 3 and 6 for VFFs (p<0.05, ANOVA, post hoc Tukey).
The purity of each cell type was confirmed by flow cytometry using CD90 as a fibroblast marker and cytokeratins 14, 15, 16 and 19 (KRT) as the epithelial marker (Figure 3A). Here, the relative expression level of CD90 and KRT is calculated based on low/high gating on a single-marker analysis. In agreement with prior literature, although both cell types expressed KRT and CD90,15 a significantly (p<0.05) higher levels of KRT was detected in culture expanded VFECs whereas a significantly (p<0.05) higher level of CD90 was detected in VFFs. The distribution of each cell population was further analyzed by double staining, and our results show that VFEC isolation was 70 % KRT+ and VFFs were 78% CD90+ (Figure 3B). Of note, approximately 30% of VFECs and 22% of VFFs were positive for both markers.
Figure 3.
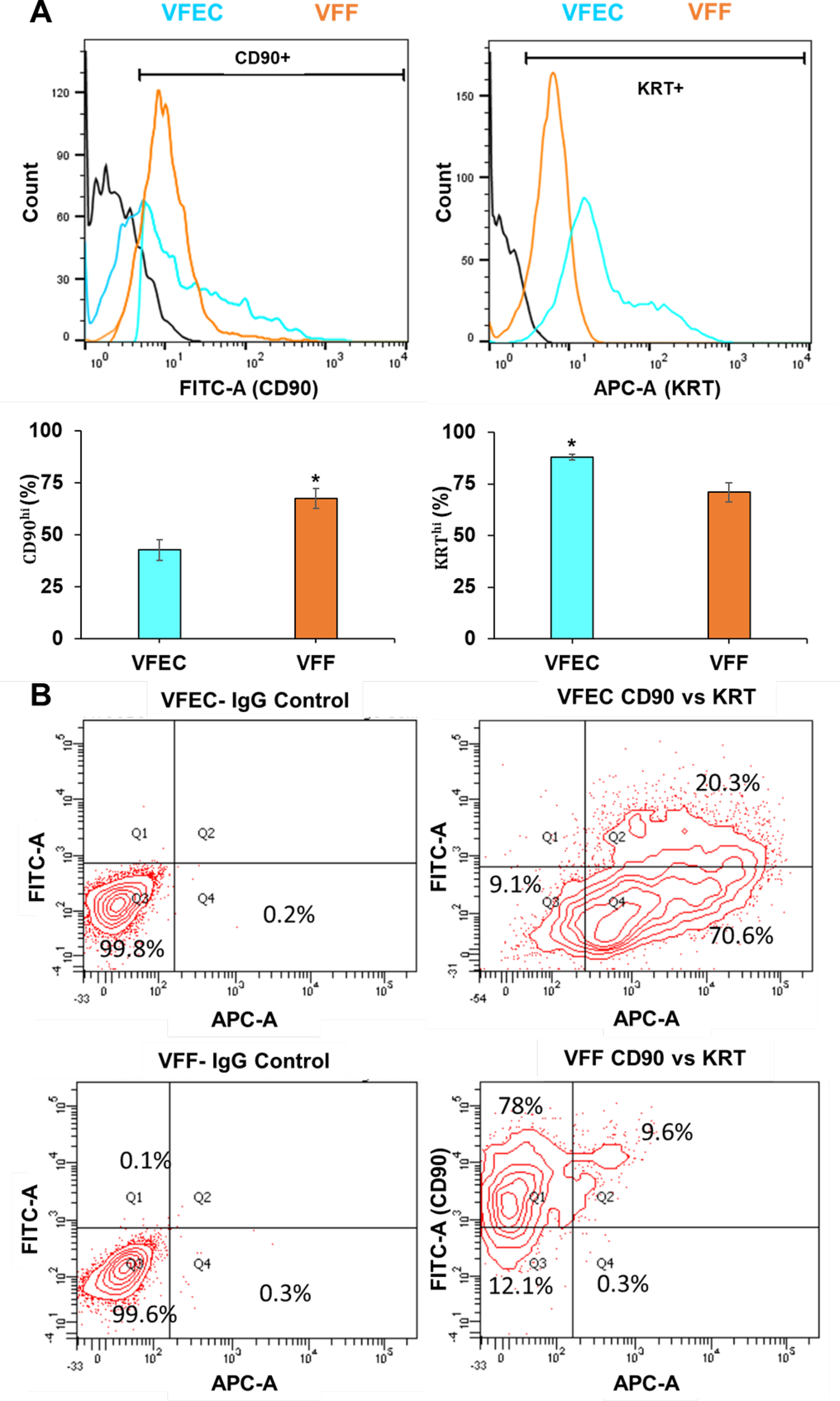
Flow cytometry analysis of primary porcine VFECs and VFFs. (A) Expression of CD90 and KRT in VFF and VFEC at P3. Fluorescence expression of FITC-CD90 and Alexa 647 KRT represented with low/high gates are shown in black. Percent positive cells was calculated based on the distribution of expression on the histogram. Data are means ± SEM (n = 4). *: Significantly different (p = 0.023 for CD90 expression in VFFs and 0.011 for KRT expression in VFECs, Student t test). (B) Representative CD90/KRT double staining contour plots for VFEC and VFF, along with their respective IgG controls.
Culture expanded cells were further characterized by immunofluorescence (Figure 4). Confluent VFEC cultures were composed entirely of polygonally shaped cells free of contamination from fibroblasts. Confluent VFF cultures were also morphologically pure, and cells were entirely spindle shaped with elongated processes. Isolated VFECs were stained positive for KRT13 and showed distinct paracellular localization of both anchoring and tight junction markers, E-cadherin, occludin and ZO-1. On the other hand, VFFs were stained negative for KRT13 or tight junction markers. Expression of classical mesenchymal marker, vimentin, confirmed the mesenchymal phenotype. Cells developed aligned F-actin stress fibers traversing the entire cell body. In summary, our method of isolation yielded a pure population of VFECs expressing epithelial markers, tight junction and anchoring junction markers similar to the native tissue.
Figure 4:
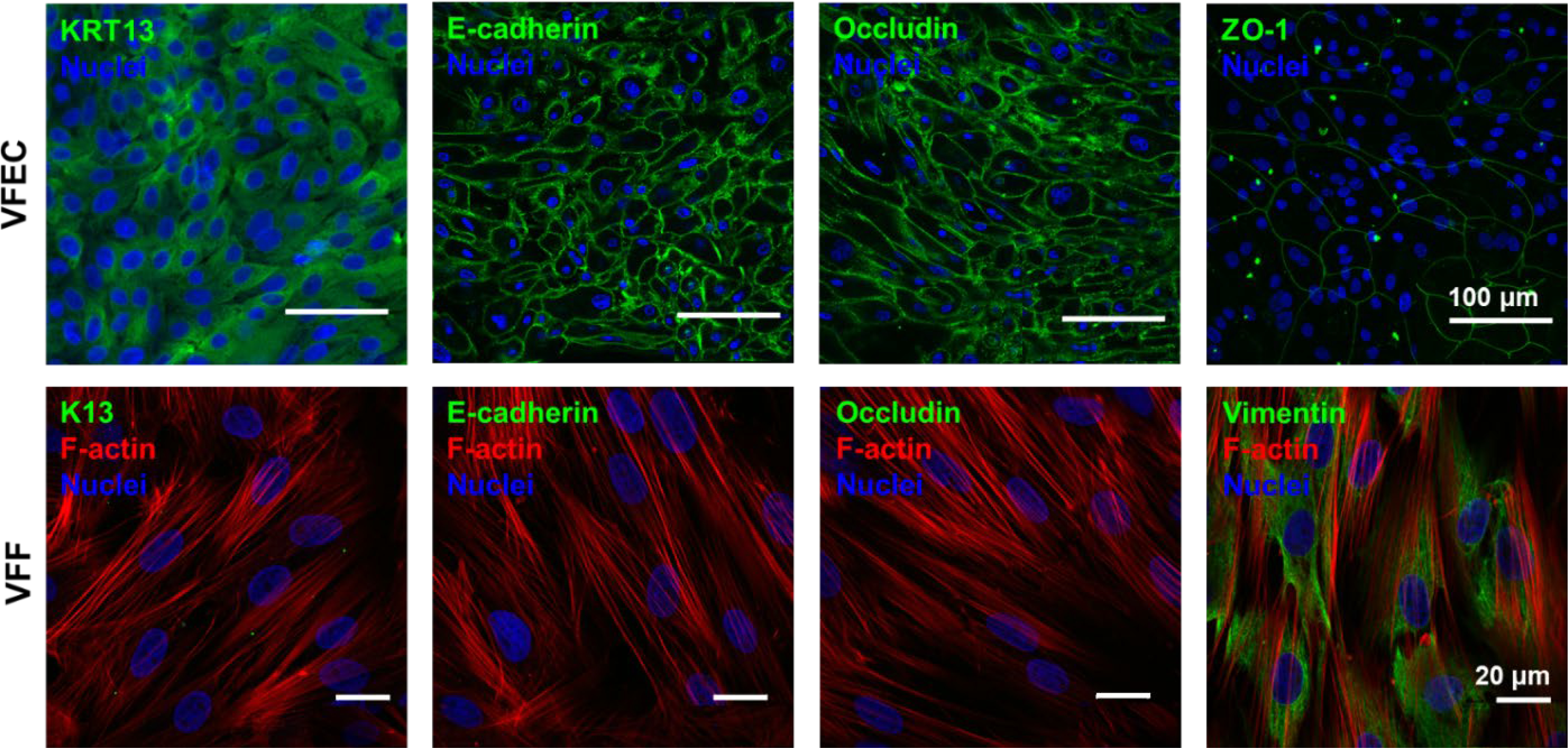
Phenotypic characterization of primary porcine VFECs and VFFs by immunofluorescence. VFECs were stained positive for stratified epithelial marker, KRT13, anchoring and tight junction markers, E-cadherin, occludin and ZO-1. VFFs were stained negative for KRT13, E-cadherin, occludin, but positive for vimentin, a classical mesenchymal marker.
3.2. Characterization of synthetic matrices
Hydrogels with varying HA concentrations (HA10 and HA24) were fabricated using HA-SH and HA-AES (Figure 5A). Because sulfhydryl groups were stoichiometrically in excess relative to the acrylate groups (18 and 4 molar excess for HA10 and HA24, respectively), both thiol-acrylate and thiol-thiol reactions contribute to the overall bulk mechanics.22 By oscillatory shear rheometry (Figure 5B), HA10 and HA24 exhibit an elastic modulus (G’) of 41.0 ± 7.0 and 1828.4 ± 180.3 Pa, respectively. Both gels were viscoelastic, having a loss modulus (G”) of 0.5 ± 0.2 and 5.4 ± 0.6 Pa, respectively.
Figure 5.
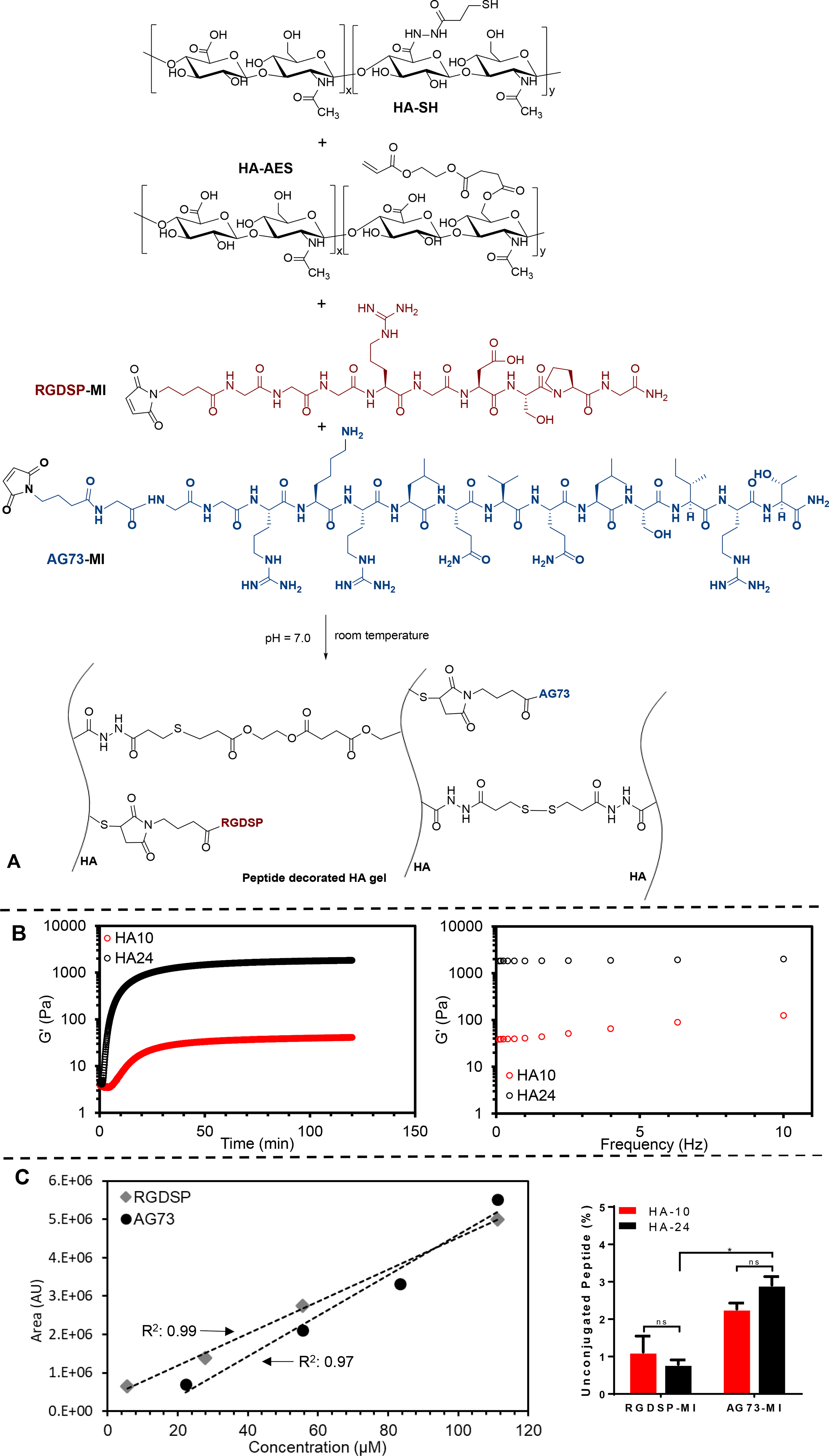
Synthesis and characterization of bioactive HA hydrogels. (A) Hydrogel was derived from HA-SH, HA-AES, RGDSP-MI and AG73-MI. (B) Rheological characterization of HA hydrogels. Left: Time sweep; Right: Frequency sweep. (C) HPLC characterization of peptide conjugation. Left: standard curves constructed from integrated peak areas for RGDSP-MI and AG73-MI, Solid symbol: raw data, Dotted line: linear fit; Right: percent unconjugated peptide as a function of peptide identity and hydrogel composition. *: Significant difference between the indicated groups, (p<0.05, two-way ANOVA, post hoc Tukey).
To promote cell attachment and proliferation, fibronectin-derived, integrin binding peptide (RGDSP)29–30 and laminin-derived, syndecan-1-binding peptide (AG73)31–32 were immobilized on the hydrogel surface via rapid and efficient thiol-maleimide reaction. In our procedure, equal volume of the peptide solution containing the same concentration of each peptide was introduced 5 min after HA-SH and HA-AES had been mixed to promote surface immobilization of peptide epitopes, although peptide conjugation in the bulk cannot be ruled out. Standard HPLC methods33 were employed to quantify the peptide concentration in the recovered supernatant and wash solutions after 30-min crosslinking/conjugation reaction. Our results (Figure 5C) show greater than 97% peptide conjugation under all conditions. For HA24, there was significantly (p<0.05) more unconjugated AG73-MI (2.91 ± 0.23%) than RGDSP-MI (0.79 ± 0.12%). For HA10, similar amounts of AG73-MI (2.27 ± 0.16%) and RGDSP-MI (1.12 ± 0.43%) were detected from the combined supernatant and wash solutions. Importantly, comparison of the same peptide conjugated to different substrates did not reveal any statistical significance (p>0.05). Collectively, these results indicate that the peptides were quantitatively incorporated to HA gels at similar levels between HA24 and HA10.
3.3. Development of hydrogel-supported epithelium.
Using HA-derived hydrogels, we established an in vitro cultivation procedure (Figure 6) for the establishment of engineered vocal fold epithelium. First, we evaluated the effects of peptide signals and substrate stiffness on VFEC attachment and proliferation (Figure 7). Cell-matrix interactions were analyzed based on the percentage of calcein positive area from Live/Dead confocal images. Minimal cell attachment was observed on HA gels without any peptide signals by day 7. When presented alone, RGDSP or AG73 enhanced cell attachment, but cells remain individually anchored. No significant difference was observed between soft and stiff hydrogels. Combination of RGDSP and AG73 signals led to significant enhancement of cell attachment with the formation of cell colonies only on HA24, but not HA10. When cultures were extended to day 14, no significant cell proliferation was observed on HA10 or HA24 with AG73 alone. Small cell colonies were detected on HA10 with RGDSP or RGDSP/AG73, but the surface coverage remained low (<20%). Significantly larger colonies were developed on HA24 with RGD or RGDSP/AG73. Compared to gels with RGDSP alone, HA24 with both RGDSP and AG73 signals led to the formation of large cell sheet with >90% surface coverage. By day 21, only HA24 presenting both AG73/RGDSP promoted the development of a confluent monolayer (Figure S3).
Figure 6.
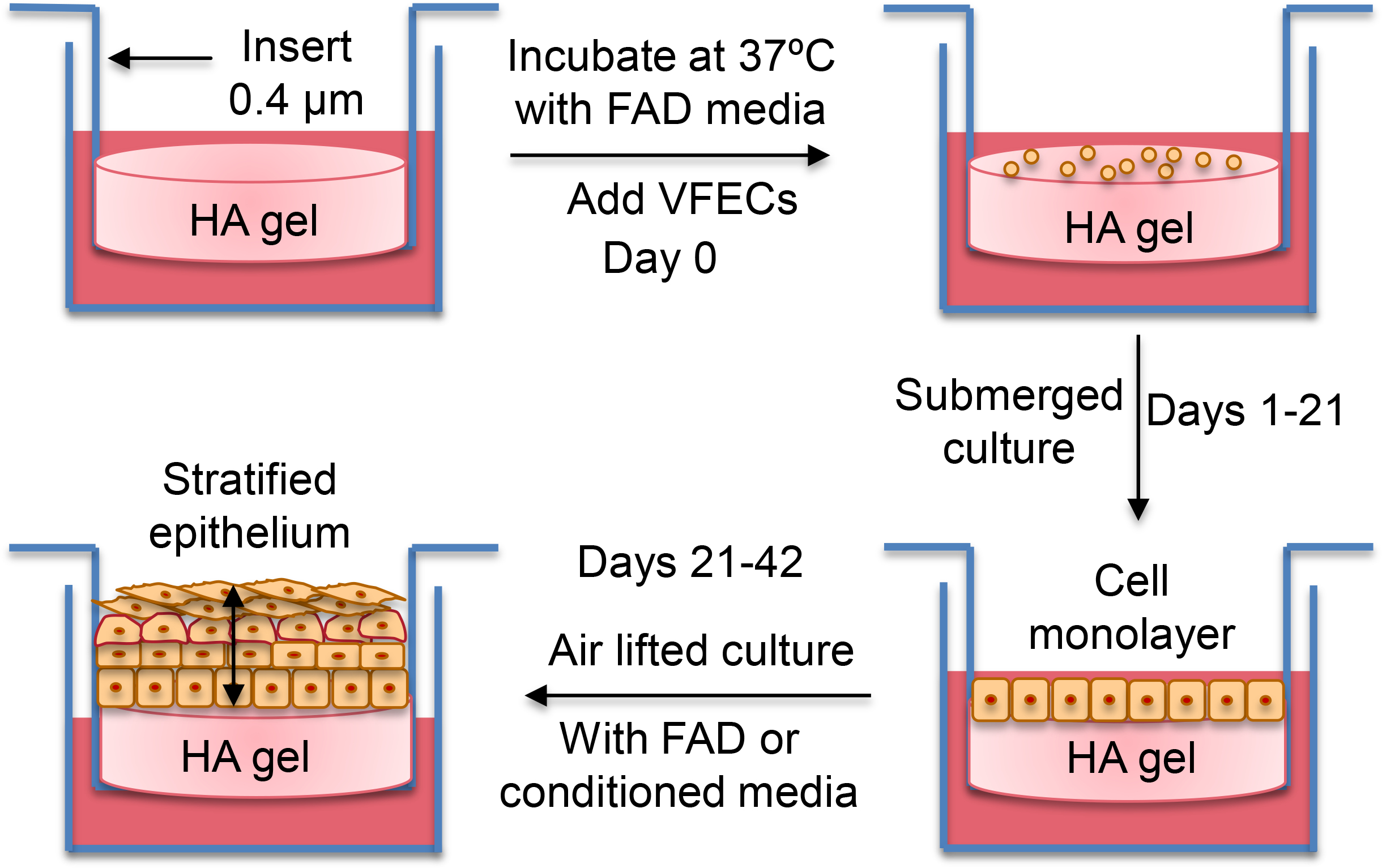
Experimental procedure for the establishment of stratified epithelium.
Figure 7.
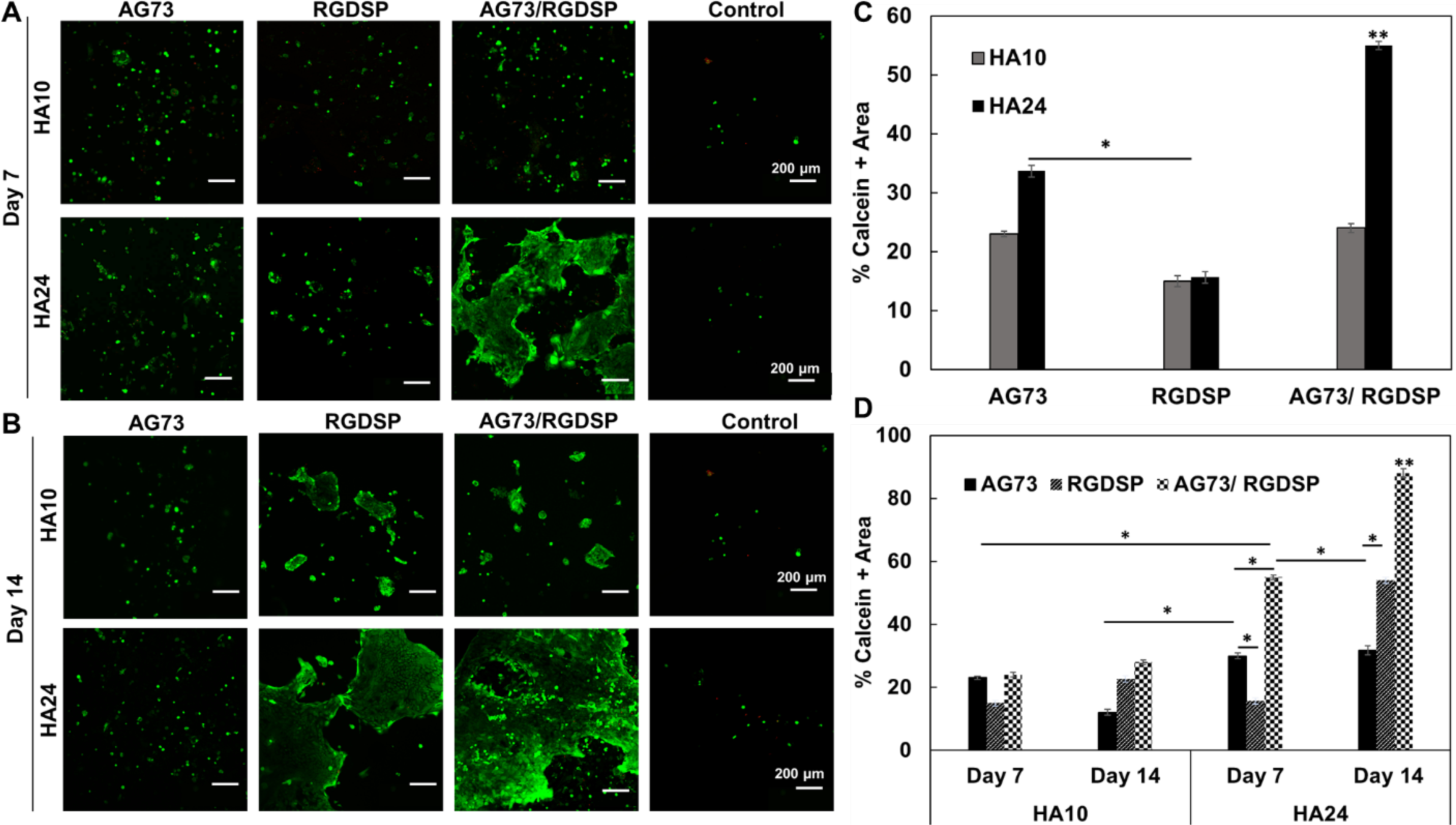
Effect of matrix composition and stiffness on VFEC attachment and proliferation under submerged culture conditions. (A, B) Confocal images of VFEC cultures after Live/Dead staining on day 7 (A) and 14 (B). Green: live cells; Red: dead cells. (C) Initial cell attachment on day 7, as percent calcein positive region, as a function of matrix composition. (D) Cell proliferation, as percent calcein positive regions on day 14 relative to day 7, as a function of matrix composition. Quantification was carried out using ImageJ software based on three separate 1024 × 1024 μm2 confocal images. Error represents standard error of the mean of three repeats. **: Significant compared to all other groups. *: Significant difference between the indicated groups, (p<0.05, ANOVA, post hoc Tukey).
Next, VFEC cultures on HA hydrogels were continued at the air-liquid interface to promote stratification. Live/dead staining (Figure 8A) shows that, by day 42, a few isolated cells were detected on AG73 decorated HA10 gels. Significantly more cells were attached to AG73 decorated HA24 gels, but cells lost epithelial morphology and became elongated. Small colonies of cells on RGDSP-containing HA10 coalesced into larger islands, and those on RGDSP-presenting HA24 gels formed a large sheet on top of a closely packed cell layer. Cells on HA24 gels with both AG73/RGDSP signals were completely covered by layers of cells. Although cell morphology varied from top to bottom, cell-cell proximity was still maintained. When presented on HA10 gels, the combined peptide signals led to formation of patchy cell sheets without stratification.
Figure 8.
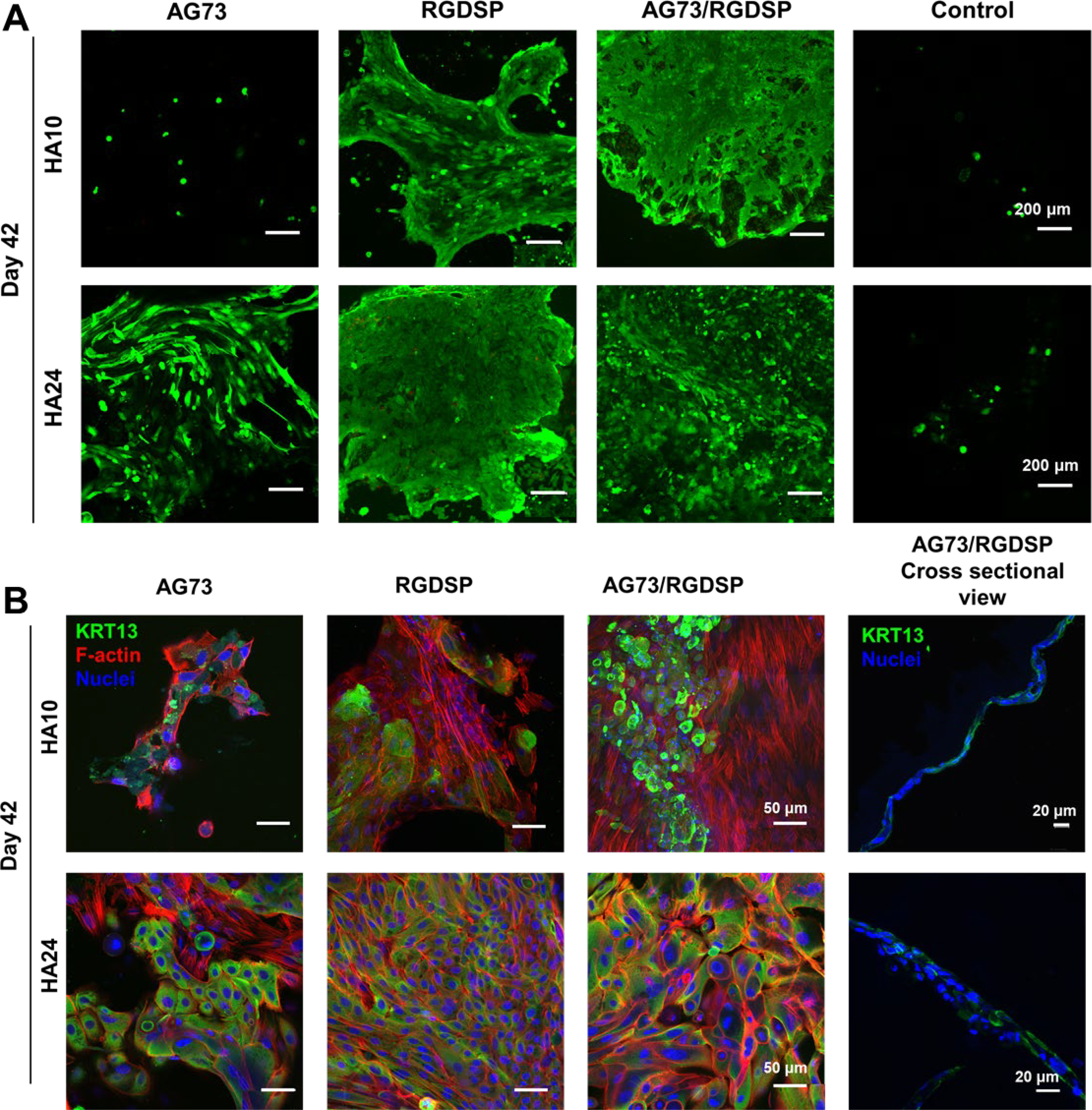
Effect of matrix composition and stiffness on VFEC stratification under air lifted culture conditions. (A) Confocal images of VFEC cultures on day 42 after Live/Dead staining. Green: live cells; Red: dead cells. (B) Representative confocal images of VFEC cultures on day 42 stained for KRT13 (green), F-actin (red) and nuclei (blue). HA10 down-regulated the expression of KRT13, suppressed the formation of cell-cell junctions and promoted the development F-actin stress fibers. HA24 with RGDSP and AG73/RGDSP maintained KRT13 expression and promoted the establishment of cell-cell junctions. In the presence of AG73/RGDSP, VFECs formed a monolayer on HA10, but stratified (2–3 layer of cells) on HA24.
Cultures were further stained for F-actin to assess cell morphology. As shown in Figure 8B, cells on HA24 under RGDSP or RGD/AG73 conditions were cuboidal shaped with cortical F-actin organized at cell-cell junctions. On HA24 with AG73, VFECs had partially differentiated to nonepithelial cells with F-actin stress fibers traversing the entire cell body. On HA10, irrespective of peptide identity, VFECs completely lost their cobblestone morphology and adopted fibroblast-like phenotype as evidenced by the presence of cytoskeletal stress fibers. Cells cultured on HA10, irrespective of the peptides, do not display robust staining of KRT13. Cells on HA24 with RGDSP or RGDSP/AG73 were stained consistently and uniformly for KRT13 throughout the cell layer. On AG73 decorated HA24, KRT13+ cells co-existed with fibroblast-like cells. 3D rendering of the z-stacks of structures developed on gels presenting both AG73/RGDSP signals shows a uniform monolayer on HA24 compared to HA10, where incomplete surface coverage was observed (Figure S4). The cross-sectional view of these hydrogels showed that there was an incomplete cellular monolayer on HA10 whereas HA24 had two disorganized cell layers (Figure 8B).
Our results show that HA24 with both RGDSP and AG73 is most conducive to the development of stratified epithelium. Compared to the native tissue, however, the engineered tissue in thinner and less organized. Thus, biochemical and biomechanical signals from the matrix alone are not sufficient for stratification of VFECs. Studies have highlighted the importance of cytokine signals from the underlying stromal tissue in promoting stratification of epithelial cells.34–36 Here, we used VFF-conditioned media to regulate the stratification of VFECs on HA24 gel presenting both AG73/RGDSP peptide signals. Replacing the FAD media with VFF-conditioned DMEM growth media on day 14 resulted in further stratification of VFECs. As shown in Figure 9, the engineered epithelium contained 4–5 cell layers. Cells expressed KRT13, E-cadherin, MUC1 and occludin throughout the strata. Involucrin was expressed in the superficial and middle layers, but not the basal layer, consistent with what is observed in human tissues. 25 Integrin β1 was more concentrated in the basal layer indicating stronger cell-substrate interactions. Collagen IV and laminin 5 were detected beneath the basal cells. The engineered epithelium is morphologically similar to the native tissue (Figure 10), although the latter contained one additional cell layer. Both tissues exhibit homogenous distribution of KRT13 throughout the strata and proliferative basal cells stained positive for Ki67. The positive staining for tight junction and anchoring junction markers (E-cadherin and occludin) and basement membrane proteins (collagen IV and laminin 5) indicates an intact epithelium. These observations confirmed that cytokine signals from the fibroblasts are necessary for stratification of VFECs.
Figure 9.
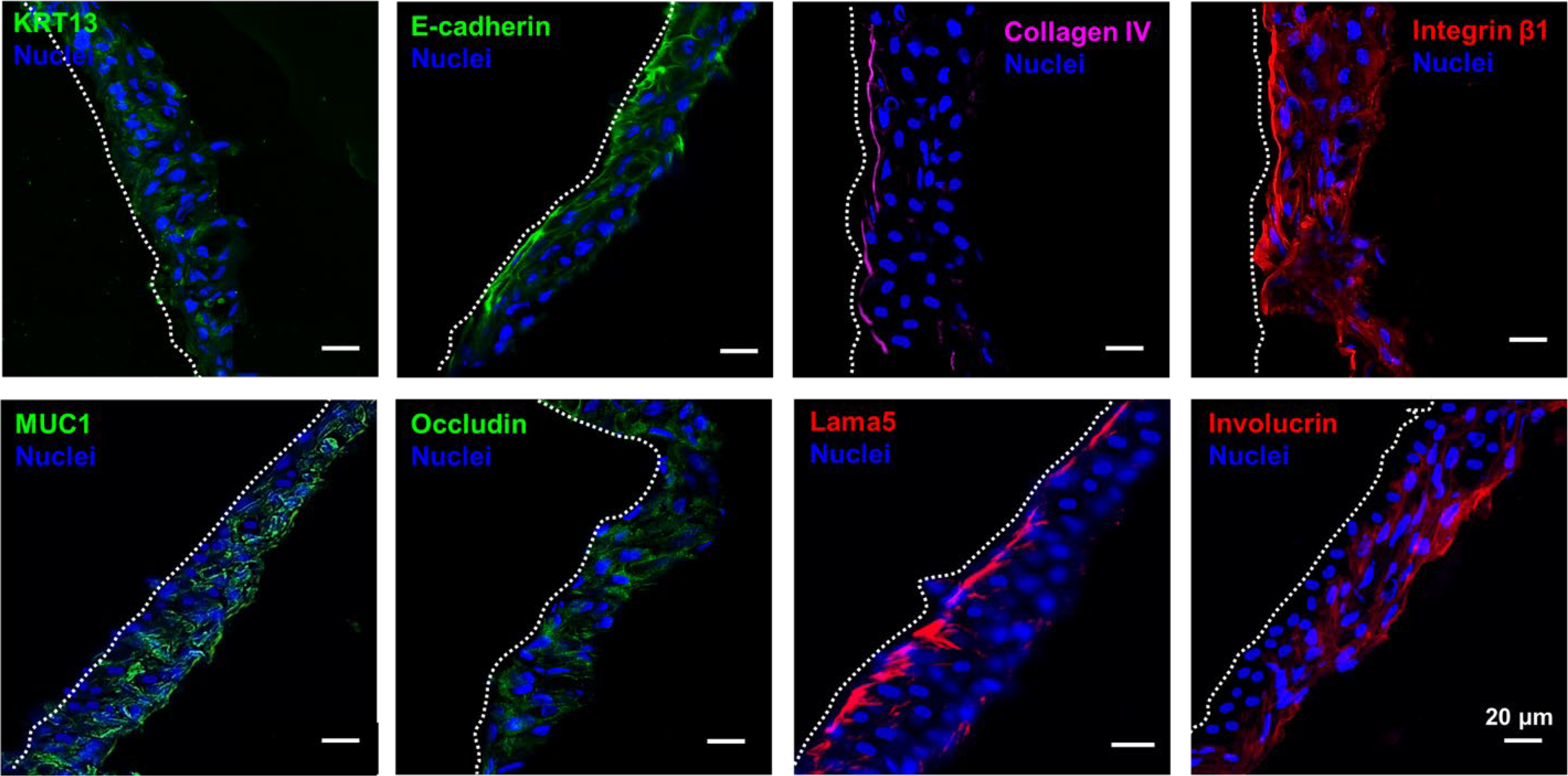
Characterization of stratified epithelium grown on HA24 with AG73 and RGDSP in the presence of VFF conditioned media. Cells expressed KRT13, E-cadherin, integrin β1, MUC1 and occludin throughout the strata. Collagen IV and Lama 5 were expressed by the basal cells, while involucrin+ cells were found in the suprabasal layers.
Figure 10.
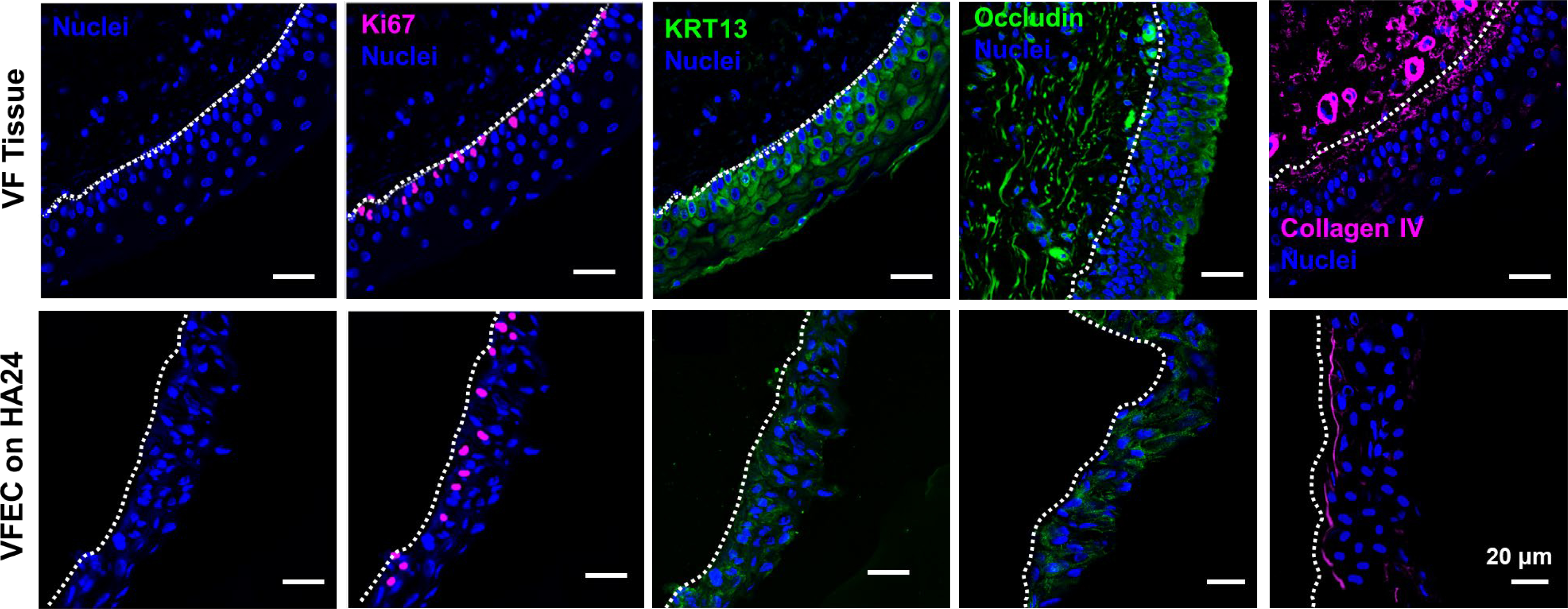
Comparison of engineered epithelium with the native tissue. The native tissue has 5–6 layers of stratified squamous epithelial cells (nuclei, blue), while the engineered tissue has 4–5 cell layers. Both native and engineered tissues exhibited a basal, proliferative cell layer, stained positive for Ki67 (pink). Both native and engineered tissues were stained positive for KRT13, occludin and collagen IV, with similar staining patterns. White dotted line delineates tissue boundary (basement membrane).
4. DISCUSSION.
Vocal fold epithelium is a multilayered tissue with distinct cellular organizations25, 37 similar to that covering the cornea,35, 38 vagina,39 oral cavity, and esophagus.40 Held together by tight and adherent junctions,41 the epithelium serves as a protective barrier, controls solute diffusion and maintains tissue homeostasis.42–43 Our strategy for the establishment of a vocal fold epithelium model relies on the availability of pure primary cells. Literature report on in vitro culture of VFECs is sparse; these cells were isolated from humans,15, 44 rabbits13 and pigs14. Primary human VFEC cultures are not feasible owing to the inaccessibility of the tissue, its susceptibility to surgical damage, and the scarcity of healthy human tissues. A porcine model was chosen for the current study because of the morphological and functional similarity of porcine vocal fold epithelium to that of humans, and the availability and abundance of healthy porcine tissues. One of the main challenges associated with the expansion and stratification of primary epithelial culture is the requirement of feeder (e.g. murine 3T3 fibroblasts13) or supporting cells (e.g. VFFs15). A recent study has shown successful culture of porcine VFEC without feeder cells in FAD media. However, under the experimental conditions employed, only cell monolayer was developed.14
Our cell isolation protocol yielded relatively pure epithelial and fibroblast cell populations. Previous work on cells isolated from human vocal fold tissues shows that, by single marker analysis, percent KRT+ cells in VFFs and VFECs was comparable and low, while percent CD90+ cells in VFFs is significantly higher than in VFECs. Our isolation resulted in VFEC population with higher percentage of KRT+ cells compared to CD90+ cells and VFF population with high percent of CD90+ cells compared to KRT+ cells. In both cases, the difference is significant. The double marker contour plot confirmed successful separation into CD90hi/KRTlo VFFs and CD90lo/KRThi VFECs.
Here, we report the first example of a hydrogel-supported stratified vocal fold epithelium without the co-cultured fibroblast. HA-based hydrogels with immobilized bioactive peptides were developed to mimics the native tissue mechanically and compositionally. It is well accepted that substrate stiffness has a profound influence on adhesion, proliferation and differentiation of cells of both mesenchymal and epithelial origins. In general, cells spread and proliferate more readily on stiff substrates, forming larger, stable, elongated focal adhesions and phosphorylate ERK in response to growth factor stimulation.45–46 For stratified epithelial cells, physiologically relevant soft and compliant substrates promote higher proliferation and stratification, colony formation and the maintenance of stem/progenitor cell markers, whereas stiffer substrates promote differentiation.47–48 Note, in the literature, soft substrates are physiologically relevant and stiff substrates often refer to non-physiologic or pathological tissues. Here, both HA10 and HA24 are relatively soft.
The vocal fold epithelium is attached to a basement membrane that is anchored on the underlying connective tissue, lamina propria. Mechanically, the basement membrane couples the epithelium with the superfacial lamina propria to form a cohesive mucosa.49 The pig vocal fold had a two-layer lamina propria, with the superfacial layer being mostly ground substance (HA), and the deep layer consisted mostly of collagen and elastin.50–51 By torsional wave experiment, we have previously shown that adult tissues (the lamina propria and the epithelium combined) had an average storage modulus of 2,309 ± 1,394 Pa at frequencies of 36 – 200 Hz. The vocal folds of young pigs were significantly more compliant, with a storage modulus of 394 ± 142 Pa between 14 and 30 Hz. When analyzed by commercial rheometer at 0–10 Hz, tissues are significantly softer, from 220–1,036 Pa at 0.016 Hz to 5,976–8,214 Pa at 10 Hz.52 Thus, in terms of stiffness, HA24 matches the native tissue more closely than HA10.
Peptide free HA substrates, irrespective of the stiffness, do not support the attachment and growth of VFECs. Although mesenchymal cells or epithelial cells that have undergone mesenchymal transition can bind HA via the cell surface receptor, CD44 or RHAMM, healthy epithelial cells do not express CD44, thus unable to interact with HA directly. Moreover, CD44-mediated cell adhesion is weak and dynamic, not sufficient to permanently anchor cells to a substrate.53 Compositionally, the basement membrane is composed primarily of laminin, fibronectin and collagen IV. Epithelial cells are known to bind fibronectin derived RGDSP peptide through specific integrin receptors, such as α6β1 and α8β1.54 Integrin mediated cell-ECM interactions play a key role in establishing and maintaining association of cytoskeleton in neighboring cells through cadherins.55–56
As reported previously, syndecan-binding AG73 has been shown to promote the development of acini-like structures from salivary gland epithelial cells.32 When conjugated to chitosan, AG73 promoted the attachment and proliferation of keratinocytes in vivo.57 AG73 alone does not promote cell adhesion,58 but when combined with integrin binding peptides, it synergistically accelerates cell adhesion and neurite outgrowth.16, 59
Our work shows that the initial attachment and stratification of VFECs requires substrates with an optimal stiffness (1828 Pa) displaying both integrin and syndecan binding peptides. Our results further show that cells cultured on soft substrates (41 Pa) with RGDSP, alone or together with AG73, lost the cuboidal epithelial cell morphology and adopted mesenchymal-like morphology with elongated, F-actin rich cell processes. Interestingly, a fraction of cells attached to gels with HA24/AG73 had also undergone morphological changes. VFF contamination is unlikely as our isolation protocol resulted in relatively pure populations of VFFs and VFECs. Moreover, if VFFs were present in our epithelial culture, they would appear under all culture conditions.
Epithelial cells exhibit cellular plasticity and dedifferentiation is often associated with tissue repair. For example, differentiated airway epithelial cells can revert into cells that are morphologically indistinguishable and functionally comparable to basal stem cells in repairing epithelial injury.60 In kidney during recovery from ischemia/reperfusion injury, surviving tubular epithelial cells dedifferentiate into cells with apparent stem cell markers; it is these dedifferentiated cells that are responsible for restoring tubular integrity.61 Retinal pigment epithelium cells are also known to dedifferentiate in culture. When cultured on relatively soft substrates, these cells dedifferentiate into fibroblast-like cells and the mechanical environment was the dominating factor and Activin A was unable to rescue these cells.62
In our case, all HA10-based substrates, as well as HA24 with AG73, do not promote the establishment of epithelial cell colonies with cell-cell contacts. These substrates failed to establish strong cellar adhesion through integrin. The surviving epithelial cells that do attach adapt to the loss of adjacent cells by dedifferentiating, possibly by degradation and/or relocalization of the junction proteins. On the other hand, VFECs cultured on HA24 with RGDSP/AG73 can generated large local substrate deformations that appeared to recruit adjacent epithelial cells into joining an evolving colony. Our study therefore highlights the importance of culturing VFECs on substrates with tissue-like stiffness and appropriate adhesive ligands to promote robust cell-matrix, and subsequently cell-cell adhesion. These cells are primed to be responsive to exogenous factors from VFF-conditioned media to improve epithelium function.
Further stratification is achieved through paracrine signaling without the need for direct epithelial/fibroblast contacts using VFF-conditioned media. With 4–5 cell layers, the engineered tissue express tight junction marker occludin and adherens junction marker E-cadherin, confirming the stability of the tissue. The expression of MUC1 by the engineered tissue confirms the maintenance of VFECs function and the establishment of mucus barrier. Basal expression of Ki67 confirmed the regenerative capacity of VFECs. Cellular deposition of basement membrane proteins, laminin 5 and collagen IV suggests the establishment of barrier function. The positive staining in the luminal cell layers for interlucrin, a protein to be expressed at the surface of stratified squamous epithelium, including the vocal fold and the epidermis, 25 further confirm successful stratification.
Earlier work on epithelial stem cells of the thymus and epidermis, cytokine signals from the underlying stromal tissue and expression of p63 are important for stratification of epithelial cells.34–36 A number of studies have identified cytokine signals contributing to the stratification of epithelial cells. For example, it has been reported that fibroblast growth factor-7 (FGF-7) is essential for bladder urothelial stratification63, FGF-10 is required for stratification of vaginal epithelium.64 Transforming growth factor beta (TGF-β1) regulates the esophageal epithelial barrier65 and bone morphogenetic protein (BMP7) signaling is required for stratification of mouse esophageal epithelium cells.66 Identification of specific cytokines conductive to VFEC stratification will allow for the establishment of engineered vocal fold epithelium under defined conditions using appropriate factors at appropriate concentrations. This is the subject of our current investigation.
4. Conclusion
Towards the goal of establishing an in vitro model of vocal fold cover, primary porcine VFECs were isolated, purified and expanded in culture. HA-based hydrogels recapitulating the biochemical composition of the basement membrane and the biomechanical properties of the underlying lamina propria were prepared using thiolated HA, acrylated HA and maleimide-functionalized integrin and syndecan binding peptides. VFECs attached and proliferated on HA gels that exhibited an elastic shear modulus of 1828 Pa and contained both RGDSP and AG73, but not on gels with a G’ of 40 Pa or with only RGDSP or AG73 peptide. Following a 21-day submerged culture and 21-day air-lifted culture, a triayered epithelium with proliferating cells was developed. Culture of VFECs in VFF-conditioned media led to more robust stratification; the epithelium consisted of 4–5 distinct cell layers, expressing Ki67, collagen IV and laminin 5 at the basal layer and KRT13, E-cadherin and occludin throughout the epithelial strata. This work highlights the importance of matrix properties, cytokine signaling and cell-cell communication in VFEC function, in terms of attachment, proliferation and stratification.
Supplementary Material
Acknowledgements.
This work was supported in part by National Institutes of Health (NIDCD, R01DC014461), National Science Foundation (NSF, DMR 1809612) and Delaware Bioscience Center. Instrumentation was supported by NIH grants P30GM110758, P20GM104316, S10RR026962, S10OD016267 and NSF grants CHE-0840401, CHE-1229234, and CHE-1048367. We thank Dr. Robert Mauck for providing porcine tissues, Dr. Jeffrey Caplan for his advice on confocal imaging, Chen-Yuan Kao for his guidance on flow cytometry, and Terry Kokas for assistance with tissue embedding and sectioning. We acknowledge Sanofi/Genzyme for providing HA.
Footnotes
Supporting Information: Antibody information and characterization of peptides by ESI-MS and HPLC, confocal images of hydrogel-supported epithelium. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gray SD, Cellular Physiology of the Vocal Folds. Otolaryngol Clin North Am. 2000, 33 (4), 679–698. DOI: 10.1016/s0030-6665(05)70237-1. [DOI] [PubMed] [Google Scholar]
- 2.Chen X; Thibeault SL, Characteristics of age-related changes in cultured human vocal fold fibroblasts. The Laryngoscope 2008, 118 (9), 1700–4. DOI: 10.1097/MLG.0b013e31817aec6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jetté ME; Hayer SD; Thibeault SL, Characterization of human vocal fold fibroblasts derived from chronic scar. The Laryngoscope 2013, 123 (3), 738–45. DOI: 10.1002/lary.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leydon C; Imaizumi M; Yang D; Thibeault SL; Fried MP, Structural and functional vocal fold epithelial integrity following injury. The Laryngoscope 2014, 124 (12), 2764–9. DOI: 10.1002/lary.24818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levendoski EE; Leydon C; Thibeault SL, Vocal fold epithelial barrier in health and injury: a research review. Journal of speech, language, and hearing research : JSLHR 2014, 57 (5), 1679–91. DOI: 10.1044/2014_jslhr-s-13-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima T; Van Deusen M; Jerome WG; Garrett CG; Sivasankar MP; Novaleski CK; Rousseau B, Quantification of acute vocal fold epithelial surface damage with increasing time and magnitude doses of vibration exposure. PloS one 2014, 9 (3), e91615. DOI: 10.1371/journal.pone.0091615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima T; Valenzuela CV; Novaleski CK; Van Deusen M; Mitchell JR; Garrett CG; Sivasankar MP; Rousseau B, Effects of phonation time and magnitude dose on vocal fold epithelial genes, barrier integrity, and function. The Laryngoscope 2014, 124 (12), 2770–8. DOI: 10.1002/lary.24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano S, Current Treatment of Vocal Fold Scarring. Curr Opin Otolaryngol Head Neck Surg. 2005, 13 (3), 143–147. [DOI] [PubMed] [Google Scholar]
- 9.Leydon C; Imaizumi M; Bartlett RS; Wang SF; Thibeault SL, Epithelial Cells are Active Participants in Vocal Fold Wound Healing: An in vivo Animal Model of Injury. PloS one 2014, 9 (12), e115389. DOI: 10.1371/journal.pone.0115389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novaleski CK; Carter BD; Sivasankar MP; Ridner SH; Dietrich MS; Rousseau B, Apoptosis and Vocal Fold Disease: Clinically Relevant Implications of Epithelial Cell Death. J Speech Lang Hear Res. 2017, 60 (5), 1264–1272. DOI: 10.1044/2016_jslhr-s-16-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leydon C; Imaizumi M; Yang D; Thibeault SL; Fried MP, Structural and Functional Vocal Fold Epithelial Integrity Following Injury. Laryngoscope. 2014, 124 (12), 2764–2769. DOI: 10.1002/lary.24818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai N; Tager AM, Fibrosis of Two: Epithelial Cell-Fibroblast Interactions in Pulmonary Fibrosis. Biochim. Biophys. Acta 2013, 1832 (7), 911–921. DOI: 10.1016/j.bbadis.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizuta M; Kurita T; Kimball EE; Rousseau B, Structurally and Functionally Characterized in vitro Model of Rabbit Vocal Fold Epithelium. Tissue Cell 2017, 49 (3), 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson-DiRenzo E; Leydon C; Thibeault SL, Methodology for the Establishment of Primary Porcine Vocal Fold Epithelial Cell Cultures. Laryngoscope. 2019. DOI: 10.1002/lary.27909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling C; Li Q; Brown ME; Kishimoto Y; Toya Y; Devine EE; Choi KO; Nishimoto K; Norman IG; Tsegyal T; Jiang JJ; Burlingham WJ; Gunasekaran S; Smith LM; Frey BL; Welham NV, Bioengineered Vocal Fold Mucosa for Voice Restoration. Sci Transl Med. 2015, 7 (314), 314ra187. DOI: 10.1126/scitranslmed.aab4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weeks BS; Nomizu M; Ramchandran RS; Yamada Y; Kleinman HK, Laminin-1 and the RKRLQVQLSIRT Laminin-1 α1 Globular Domain Peptide Stimulate Matrix Metalloproteinase Secretion by PC12 Cells. Exp. Cell Res. 1998, 243 (2), 375–382. [DOI] [PubMed] [Google Scholar]
- 17.Chan RW; Gray SD; Titze IR, The Importance of Hyaluronic Acid in Vocal Fold Biomechanics. Otolaryngol Head Neck Surg. 2001, 124 (6), 607–614. [DOI] [PubMed] [Google Scholar]
- 18.Butler JE; Hammond TH; Gray SD, Gender‐related Differences of Hyaluronic Acid Distribution in the Human Vocal Fold. Laryngoscope. 2001, 111 (5), 907–911. [DOI] [PubMed] [Google Scholar]
- 19.Dicker KT; Gurski LA; Pradhan-Bhatt S; Witt RL; Farach-Carson MC; Jia X, Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater. 2014, 10 (4), 1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turley EA; Noble PW; Bourguignon LY, Signaling Properties of Hyaluronan Receptors. J Biol Chem. 2002, 277 (7), 4589–4592. DOI: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 21.Jiang D; Liang J; Noble PW, Hyaluronan in Tissue Injury and Repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 435–461. DOI: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemir T; Fowler EW; Liu S; Harrington DA; Witt RL; Farach-Carson MC; Pradhan-Bhatt S; Jia X, Tuning Hydrogel Properties to Promote the Assembly of Salivary Gland Spheroids in 3D. ACS Biomater. Sci. Eng. 2016, 2 (12), 2217–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerdoum AB; Fowler EW; Jia X, Induction of Fibrogenic Phenotype in Human Mesenchymal Stem Cells by Connective Tissue Growth Factor in a Hydrogel Model of Soft Connective Tissue. ACS Biomater. Sci. Eng. 2019, 5 (9), 4531–4541. DOI: 10.1021/acsbiomaterials.9b00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suvarna SK; Layton C; Bancroft JD, Theory and Practice of Histology Techniques. 8th ed.; Elsevier: 2019. [Google Scholar]
- 25.Dowdall JR; Sadow PM; Hartnick C; Vinarsky V; Mou H; Zhao R; Song PC; Franco RA; Rajagopal J, Identification of Distinct Layers within the Stratified Squamous Epithelium of the Adult Human True Vocal Fold. Laryngoscope. 2015, 125 (9), E313–E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouger K; Fornasari B; Armengol V; Jouvion G; Leroux I; Dubreil L; Feron M; Guevel L; Cherel Y, Progenitor Cell Isolation from Muscle-derived Cells based on Adhesion Properties. J Histochem Cytochem. 2007, 55 (6), 607–618. DOI: 10.1369/jhc.6A6954.2007. [DOI] [PubMed] [Google Scholar]
- 27.Riva F; Casasco A; Casasco M; Calligaro A; Cornaglia AI, Growth and Stratification of Epithelial Cells in Minimal Culture Conditions. In Epidermal Cells. Methods Mol Biol., Springer: 2010; Vol. 585, pp 25–43. DOI: 10.1007/978-1-60761-380-0_3. [DOI] [PubMed] [Google Scholar]
- 28.Ilmarinen T; Laine J; Juuti‐Uusitalo K; Numminen J; Seppänen‐Suuronen R; Uusitalo H; Skottman H, Towards a Defined, Serum‐and Feeder‐free Culture of Stratified Human Oral Mucosal Epithelium for Ocular Surface Reconstruction. Acta Ophthalmol. 2013, 91 (8), 744–750. [DOI] [PubMed] [Google Scholar]
- 29.Peppas N; Huang Y; Torres-Lugo M; Ward J; Zhang J, Physicochemical Foundations and Structural Design of Hydrogels in Medicine and Biology. Annu. Rev. Biomed. Eng. 2000, 2 (1), 9–29. [DOI] [PubMed] [Google Scholar]
- 30.Ruoslahti E, RGD and Other Recognition Sequences for Integrins. Annu. Rev. Cell Dev. Biol. 1996, 12 (1), 697–715. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman MP; Engbring JA; Nielsen PK; Vargas J; Steinberg Z; Karmand AJ; Nomizu M; Yamada Y; Kleinman HK, Cell type-specific Differences in Glycosaminoglycans Modulate the Biological Activity of a Heparin-binding Peptide (RKRLQVQLSIRT) from the G Domain of the Laminin α1 Chain. J Biol Chem. 2001, 276 (25), 22077–22085. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman MP; Nomizu M; Roque E; Lee S; Jung DW; Yamada Y; Kleinman HK, Laminin-1 and Laminin-2 G-Domain Synthetic Peptides Bind Syndecan-1 and are Involved in Acinar Formation of a Human Submandibular Gland Cell Line. J Biol Chem. 1998, 273 (44), 28633–28641. [DOI] [PubMed] [Google Scholar]
- 33.Lough WJ; Wainer IW, High Performance Liquid Chromatography: Fundamental Principles and Practice. Springer: 1995. [Google Scholar]
- 34.Senoo M; Pinto F; Crum CP; McKeon F, p63 is Essential for the Proliferative Potential of Stem Cells in Stratified Epithelia. Cell 2007, 129 (3), 523–536. DOI: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 35.Koizumi N; Inatomi T; Suzuki T; Sotozono C; Kinoshita S, Cultivated Corneal Epithelial Stem Cell Transplantation in Ocular Surface Disorders. Ophthalmology 2001, 108 (9), 1569–1574. [DOI] [PubMed] [Google Scholar]
- 36.Koster MI; Kim S; Mills AA; DeMayo FJ; Roop DR, p63 is the Molecular Switch for Initiation of an Epithelial Stratification Program. Genes Dev. 2004, 18 (2), 126–131. DOI: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levendoski EE; Leydon C; Thibeault SL, Vocal Fold Epithelial Barrier in Health and Injury: A Research Review. J Speech Lang Hear Res. 2014, 57 (5), 1679–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yánez-Soto B; Leonard BC; Raghunathan VK; Abbott NL; Murphy CJ, Effect of Stratification on Surface Properties of Corneal Epithelial Cells. Invest. Ophthalmol. Visual Sci. 2015, 56 (13), 8340–8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houghton O; McCluggage WG, The Expression and Diagnostic Utility of p63 in the Female Genital Tract. Adv. Anat. Pathol. 2009, 16 (5), 316–21. DOI: 10.1097/PAP.0b013e3181b507c6. [DOI] [PubMed] [Google Scholar]
- 40.Squier CA; Kremer MJ, Biology of Oral Mucosa and Esophagus. J Natl Cancer Inst Monogr. 2001, 2001 (29), 7–15. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, Regulation of Intestinal Epithelial Permeability by Tight Junctions. Cell. Mol. Life Sci. 2013, 70 (4), 631–659. DOI: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasegawa H; Fujita H; Katoh H; Aoki J; Nakamura K; Ichikawa A; Negishi M, Opposite Regulation of Transepithelial Electrical Resistance and Paracellular Permeability by Rho in Madin-Darby Canine Kidney Cells. J Biol Chem. 1999, 274 (30), 20982–8. DOI: 10.1074/jbc.274.30.20982. [DOI] [PubMed] [Google Scholar]
- 43.Sivasankar M; Erickson E; Rosenblatt M; Branski RC, Hypertonic Challenge to Porcine Vocal Folds: Effects on Epithelial Barrier Function. Otolaryngol.--Head Neck Surg. 2010, 142 (1), 79–84. DOI: 10.1016/j.otohns.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rees LE; Gunasekaran S; Sipaul F; Birchall MA; Bailey M, The Isolation and Characterisation of Primary Human Laryngeal Epithelial Cells. Mol. Immunol. 2006, 43 (6), 725–730. [DOI] [PubMed] [Google Scholar]
- 45.Paszek MJ; Zahir N; Johnson KR; Lakins JN; Rozenberg GI; Gefen A; Reinhart-King CA; Margulies SS; Dembo M; Boettiger D; Hammer DA; Weaver VM, Tensional Homeostasis and the Malignant Phenotype. Cancer Cell. 2005, 8 (3), 241–254. DOI: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Janmey PA; Winer JP; Murray ME; Wen Q, The Hard Life of Soft Cells. Cell Motil. Cytoskeleton 2009, 66 (8), 597–605. DOI: 10.1002/cm.20382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarkoob H; Bodduluri S; Ponnaluri SV; Selby JC; Sander EA, Substrate Stiffness Affects Human Keratinocyte Colony Formation. Cell. Mol. Bioeng. 2015, 8 (1), 32–50. DOI: 10.1007/s12195-015-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gouveia RM; Vajda F; Wibowo JA; Figueiredo F; Connon CJ, YAP, ΔNp63, and β-Catenin Signaling Pathways Are Involved in the Modulation of Corneal Epithelial Stem Cell Phenotype Induced by Substrate Stiffness. Cells 2019, 8 (4). DOI: 10.3390/cells8040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dion GR; Jeswani S; Roof S; Fritz M; Coelho PG; Sobieraj M; Amin MR; Branski RC, Functional Assessment of the ex vivo Vocal Folds through Biomechanical Testing: A Review. Mater. Sci. Eng., C 2016, 64, 444–453. DOI: 10.1016/j.msec.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garrett CG; Coleman JR; Reinisch L, Comparative Histology and Vibration of the Vocal Folds: Implications for Experimental Studies in Microlaryngeal Surgery. Laryngoscope. 2000, 110 (5 Pt 1), 814–24. DOI: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Tang SS; Mohad V; Gowda M; Thibeault SL, Insights Into the Role of Collagen in Vocal Fold Health and Disease. J Voice. 2017, 31 (5), 520–527. DOI: 10.1016/j.jvoice.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teller SS; Farran AJ; Xiao L; Jiao T; Duncan RL; Clifton RJ; Jia X, High-frequency Viscoelastic Shear Properties of Vocal Fold Tissues: Implications for Vocal Fold Tissue Engineering. Tissue Eng., Part A 2012, 18 (19–20), 2008–19. DOI: 10.1089/ten.TEA.2012.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dicker KT; Gurski LA; Pradhan-Bhatt S; Witt RL; Farach-Carson MC; Jia X, Hyaluronan: a simple polysaccharide with diverse biological functions. Acta biomaterialia 2014, 10 (4), 1558–70. DOI: 10.1016/j.actbio.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown AC; Rowe JA; Barker TH, Guiding Epithelial Cell Phenotypes with Engineered Integrin-specific Recombinant Fibronectin Fragments. Tissue Eng., Part A 2011, 17 (1–2), 139–50. DOI: 10.1089/ten.TEA.2010.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z; Symons JM; Goldstein SL; McDonald A; Miner JH; Kreidberg JA, (Alpha)3(beta)1 Integrin Regulates Epithelial Cytoskeletal Organization. J Cell Sci. 1999, 112 ( Pt 17), 2925–2935. [DOI] [PubMed] [Google Scholar]
- 56.Benoit YD; Groulx JF; Gagné D; Beaulieu JF, RGD-Dependent Epithelial Cell-Matrix Interactions in the Human Intestinal Crypt. J. Signal Transduction 2012, 2012, 248759. DOI: 10.1155/2012/248759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikemoto S; Mochizuki M; Yamada M; Takeda A; Uchinuma E; Yamashina S; Nomizu M; Kadoya Y, Laminin Peptide‐conjugated Chitosan Membrane: Application for Keratinocyte Delivery in Wounded Skin. J. Biomed. Mater. Res., Part A 2006, 79 (3), 716–722. [DOI] [PubMed] [Google Scholar]
- 58.Nomizu M; Kim WH; Yamamura K; Utani A; Song S-Y; Otaka A; Roller PP; Kleinman HK; Yamada Y, Identification of Cell Binding Sites in the Laminin α1 Chain Carboxyl-Terminal Globular Domain by Systematic Screening of Synthetic Peptides. J Biol Chem. 1995, 270 (35), 20583–20590. [DOI] [PubMed] [Google Scholar]
- 59.Yamada Y; Hozumi K; Katagiri F; Kikkawa Y; Nomizu M, Laminin-111-Derived Peptide-Hyaluronate Hydrogels as a Synthetic Basement Membrane. Biomaterials 2013, 34 (28), 6539–6547. [DOI] [PubMed] [Google Scholar]
- 60.Tata PR; Mou H; Pardo-Saganta A; Zhao R; Prabhu M; Law BM; Vinarsky V; Cho JL; Breton S; Sahay A; Medoff BD; Rajagopal J, Dedifferentiation of Committed Epithelial Cells into Stem Cells in vivo. Nature 2013, 503 (7475), 218–223. DOI: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duffield JS; Park KM; Hsiao LL; Kelley VR; Scadden DT; Ichimura T; Bonventre JV, Restoration of Tubular Epithelial Cells during Repair of the Postischemic Kidney Occurs Independently of Bone Marrow-derived Stem Cells. J Clin Invest. 2005, 115 (7), 1743–55. DOI: 10.1172/jci22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White CE; Kwok B; Olabisi RM, Activin A improves Retinal Pigment Epithelial Cell Survival on Stiff but not Soft Substrates. J. Biomed. Mater. Res., Part A 2018, 106 (11), 2871–2880. DOI: 10.1002/jbm.a.36476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tash JA; David SG; Vaughan EE; Herzlinger DA, Fibroblast Growth Factor-7 Regulates Stratification of the Bladder Urothelium. J. Urol. 2001, 166 (6), 2536–2541. [PubMed] [Google Scholar]
- 64.Nakajima T; Hayashi S; Iguchi T; Sato T, The Role of Fibroblast Growth Factors on the Differentiation of Vaginal Epithelium of Neonatal Mice. Differentiation 2011, 82 (1), 28–37. DOI: 10.1016/j.diff.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen N; Fernando SD; Biette KA; Hammer JA; Capocelli KE; Kitzenberg DA; Glover LE; Colgan SP; Furuta GT; Masterson JC, TGF-β1 Alters Esophageal Epithelial Barrier Function by Attenuation of Claudin-7 in Eosinophilic Esophagitis. Mucosal Immunol. 2018, 11 (2), 415–426. DOI: 10.1038/mi.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez P; Da Silva S; Oxburgh L; Wang F; Hogan BL; Que J, BMP Signaling in the Development of the Mouse Esophagus and Forestomach. Development. 2010, 137 (24), 4171–4176. DOI: 10.1242/dev.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


