Abstract
Chylopericardium is a rare and benign condition. Apart from common causes like non-surgical trauma, tuberculosis, malignancy, radiation, and postoperative, mediastinal cystic hygroma presenting as chylopericardium is an extremely rare entity. Primary or idiopathic chylopericardium is diagnosed when the precise cause is not known. It is a diagnosis of exclusion. We report a 27-year-old lady with mediastinal cystic hygroma, presenting as spontaneous chylopericardium, who was managed surgically with no recurrence on 18 months follow-up. She was evaluated for complaints of discomfort in the upper abdomen region and breathing difficulty in left lateral position for 4 days, and was found to have a large pericardial effusion with impending tamponade. She underwent pericardiocentesis, and on fluid analysis, it was confirmed as chylous pericardial effusion. She was evaluated thoroughly and was taken up for right video-assisted thoracoscopy. The thoracic duct was clipped and a window was created in the pericardium, the cystic hygroma was excised, and pleurodesis was done. The postoperative period was uneventful. Histopathology of the pericardial window showed chronic inflammatory pathology and cystic lesion was confirmed as a chylous cyst.
Keywords: Mediastinal cystic hygroma, Chylopericardium, Pericardiocentesis, Thoracic duct ligation, Pericardial window
Introduction
Chylopericardium is a rare and benign condition. Usual causes are non-surgical trauma, tuberculosis, malignancy, radiation, and postoperatively. Less common causes are orthotopic heart transplantation, thrombosis of the subclavian vein, Behcet’s syndrome, Gorham syndrome, and endovascular treatment of superior vena cava syndrome. Primary or idiopathic chylopericardium is diagnosed when the precise cause is not known. Most of the time, it is a diagnosis of exclusion. Spontaneous chylopericardium in an adult due to mediastinal cystic hygroma is extremely rare [1]. We report a 27-year-old lady with mediastinal cystic hygroma presenting as spontaneous chylopericardium which was successfully managed surgically and doing well on 18 months of follow-up.
Case report
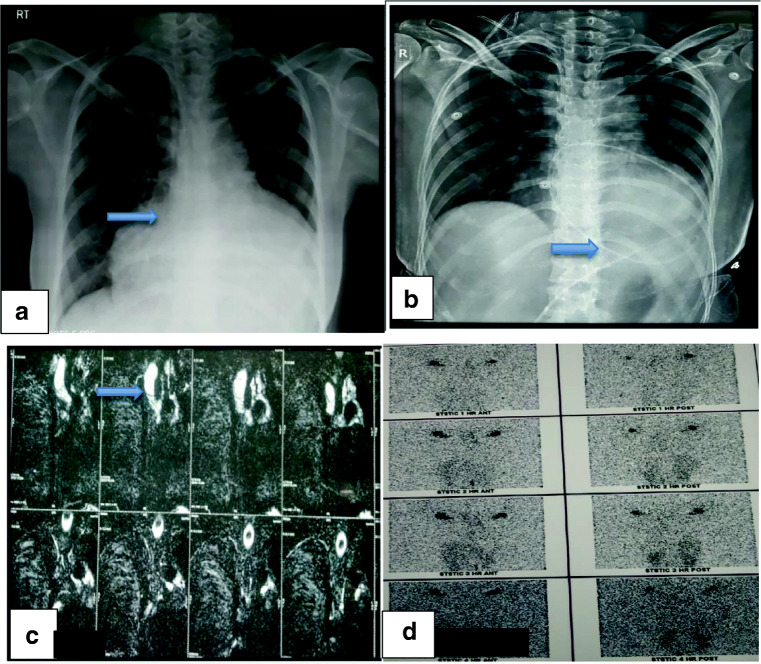
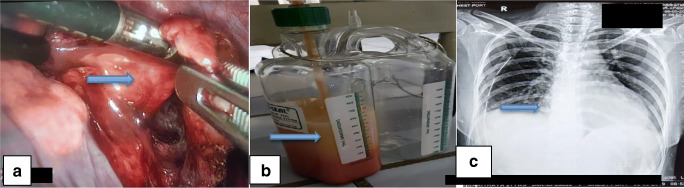
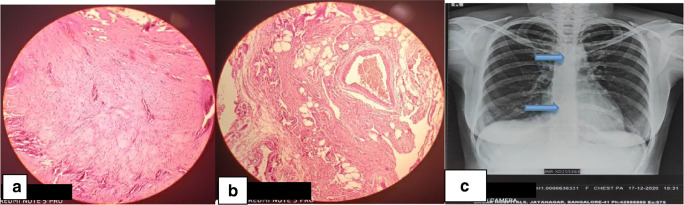
A 27-year-old female had complaints of discomfort in the upper abdomen region and breathing difficulty in the left lateral position for 4 days. On evaluation, she was found to have a large pericardial effusion—chest X-ray showed enlarged cardiac silhouette on a frontal view (Fig. 1a), electrocardiogram (ECG) showed sinus rhythm with low voltage complexes, and echocardiogram confirmed large pericardial effusion. In view of impending tamponade, pericardiocentesis was done and approximately 1.5 liters (l) of milky fluid was aspirated. On fluid analysis, it was confirmed as chylous pericardial effusion (fluid analysis—glucose, 99 milligrams per deciliter (mg/dl); total cholesterol, 50 mg/dl; triglyceride level, 902 mg/dl; total protein, 5.3 grams per deciliter (g/dl); lactate dehydrogenase (LDH), 158 units/l; ADA, 5.0). There were no microorganisms seen on Gram’s stain examination, culture and sensitivity showed no growth, and cytology for malignant cells was negative. In view of the recurrent accumulation of pericardial fluid, a pigtail catheter was inserted for drainage (Fig. 1b). During 6 weeks of conservative management (regular periodic pericardiocentesis, a medium-chain triglyceride and low-fat diet, and total parenteral nutrition), her serum albumin levels were reduced to 3.1 g/dl from 4.5 g/dl, and there was a loss of 8 kilograms (kg) of weight. She was investigated thoroughly to find out the cause for chylopericardium; magnetic resonance (MR) lymphangiography (Fig. 1c) showed suspicious disruption of the thoracic duct with periductal intensities at the T3 level and hyperintense cystic lesion, communicating with the thoracic duct, in the right subclavian region. Technetium-99m (Tc99m) sulfur colloid lymphoscintigraphy (Fig. 1d) showed abnormal tracer accumulation in the right subclavian region, with tracking along the thorax—concern for a chylous leak at this level. All these investigations and conservative management for 6 weeks were done elsewhere. In the intervening period of 6 weeks, pericardiocentesis was done once in every 3–4 days, aspirating 500 to 800 milliliters (ml) of milky fluid every time, and hence the patient was referred to us for further management. After reviewing all investigations, in view being non-responsive to conservative management and to prevent further deterioration of her metabolic, nutritional, and immunological profile, the patient was planned for surgical management, i.e., clipping the thoracic duct, pericardial window creation, and excision of cyst by a video-assisted thoracoscopic (VATS) approach. During surgery, first, the thoracic duct was clipped between the thoracic aorta and azygos vein, just above the diaphragm, using 3 clips placed at about 1 centimeter (cm) gap. Secondly, grossly distended, thickened pericardium was noted and a 2 × 2 cm pericardial window was created, draining about 700 ml of chylous fluid. Initially, we could not find the cystic lesion in the right subclavian region. Then, we searched on the left side, where we found a thin-walled, 5 × 6 cm-sized cystic lesion, hidden under the thymic remnant, on the medial aspect of the superior vena cava (Fig. 2a). This was excised as much as possible and clipping was done to remnants of the cystic lesion. Fibrin glue (TISSEL Lyo – Baxter) 2 ml was applied to remnants of the cystic lesion for sealing off any ill-defined lymphatic channels. The thymus was left in situ purposefully for the application of pressure after applying fibrin glue and for the tamponade effect. Finally, pleurodesis was performed using Steritalc powder (consists of talcum which is mined in France and is specifically processed for medical use). The patient withstood the procedure well. Postoperatively on day 1, intercostal chest drain (ICD) output was 700 ml chyle (Fig. 2b), which reduced to 200 ml on day 2, and sequentially thereafter ICD was removed on day 4. Two-dimensional (2D) echocardiography showed minimal pericardial effusion and chest X-ray showed good expansion of the lung (Fig. 2c). The patient was discharged on the fifth day postoperatively. Histopathology of the pericardial window showed chronic inflammatory pathology (Fig. 3a) and cystic lesion was confirmed as a chylous cyst (Fig. 3b). At 18 months of follow-up, the patient was doing well and no recurrence was observed (Fig. 3c).
Fig. 1.
a Chest X-ray showed enlarged cardiac silhouette on frontal view. b In view of the patient’s persistent pericardial collection (chylopericardium), a pigtail catheter was inserted for drainage. c MR lymphangiography showed suspicious disruption of the thoracic duct with periductal intensities at the T3 level and hyperintense cystic lesion communicating with the thoracic duct in the right subclavian region. d Tc99m sulfur colloid lymphoscintigraphy showed abnormal tracer accumulation in the right subclavian region with tracking along the thorax—concern for a chylous leak at this level
Fig. 2.
a 5 × 6 cm chylous cyst below the thymus and above the medial aspect of the superior vena cava. b Postoperatively on day 1 ICD bottle filled with 700 ml chyle. c Postoperatively on day 5 chest X-ray showed good expansion of the lung
Fig. 3.
a Histopathology of the pericardial window showed chronic inflammatory pathology. b Histopathology of cystic lesion confirmed as a chylous cyst. c Follow-up chest X-ray showed metal clips applied after partial excision of chylous cyst and clips applied on the thoracic duct with normal cardiac silhouette
Discussion
The accumulation of chyle in the pericardial cavity is a rare phenomenon. When there is no obvious cause found after thorough evaluation, then idiopathic chylopericardium should be considered. First reported in 1886 by Hasebrock, it was Groves and Effler who proposed primary chylopericardium nomenclature for idiopathic chylopericardium [2]. Possible explanations for primary idiopathic chylopericardium are the presence of damaged lymphatics with abnormal communications between the thoracic duct and pericardial lymphatics, chylous reflux, and elevated pressure in the thoracic duct secondary to lymphatic abnormalities like lymphangiectasis, lymphangioma/cystic hygroma, and valvular incompetence in the main thoracic duct and its branches, which results in elevated pressure in the thoracic duct and its branches leading to reflux of chyle into the pericardium and chylopericardium. It can occur in all age groups, but most commonly in young adults and children. Cystic hygroma is usually a disease of childhood, with most of the cases occurring below the age of 7 years. Mediastinal cystic hygroma presenting as spontaneous chylopericardium is extremely rare. To the best of our knowledge, to date, only very few cases have been reported [1–4]. Majority of patients are asymptomatic and very few patients may have symptoms because of cardiac tamponade (4%). Dyspnea and orthopnea are the commonest symptoms reported. Chest X-ray usually demonstrates an enlargement of the cardiac silhouette [5]. 2D-echo demonstrates pericardial effusion. Pericardiocentesis is diagnostic and sometimes therapeutic. To diagnose chylopericardium, the characteristic features are milky appearance of fluid, triglyceride level > 500 mg/dl, cholesterol/triglyceride ratio < 1, fluid culture sterile, negative cytology for malignant cells, and fluid cell count showing lymphocytic predominance. Each characteristic feature carries 1 point. For diagnosis of chylopericardium, a score of 2 is required (sensitivity and specificity 100%) [2]. In our patient, all of these features were present. Sometimes chyle mixed with blood may look orange and usually indicates an underlying malignant cause. Pathophysiology of chylopericardium, firstly proposed by Dunn, includes thoracic duct obstruction, abnormal reflux of chyle with elevated pressure within the duct, lymphatic valvular incompetence, and lymphangiectasia. A recent theory suggests that accumulation of chyle may be due to slow transudation of fluid from small, pathologic lymphatic channels, rather than through direct communication of the thoracic duct [3, 5]. According to a report, cystic hygroma of the neck may be associated with generalized abnormalities in the lymphatic system resulting in raised pressure in lymphatic channels and reflux of chyle into the pericardial cavity. In our case, cystic hygroma of the mediastinum was present and this may be the probable mechanism [3]. Various imaging modalities may be helpful for diagnostic purposes and to identify underlying secondary causes. Lymphangiogram and lymphoscintigraphy (like Tc99m red blood cell (RBC) scan, oral 131I–triolein lymphangiography, pedal Tc99m sulfur colloid scan) demonstrate the communicating channels between the main thoracic duct and lymphatic channels of the pericardium. However, it is still not possible to demonstrate these communicating channels in 50% of the cases. Computed tomography (CT) scan of the chest and abdomen, MR lymphangiogram, and bone scan are helpful investigations to rule out secondary causes for chylopericardium [3]. Once the diagnosis of chylopericardium is confirmed, it is important to identify the cause. The treatment of secondary chylopericardium is to treat the underlying cause. Treatment of primary chylopericardium is initially conservative, provided the patient is hemodynamically stable, which includes repeated pericardiocentesis, pericardiotomy by the subxiphoid approach, pigtail catheter insertion for the recurrent accumulation of chyle, medium-chain triglyceride diet, total parenteral nutrition, and low-fat diet for 2 to 3 weeks. Except for subxiphoid pericardiotomy, the rest of all modalities were tried in our patient, but without success. Review of the literature reveals the success rate of conservative management in primary chylopericardium to be up to 51%. Conservative management can be tried up to 3 weeks maximum. In our case, it was tried elsewhere for 6 weeks without success. There are reports where conservative management was tried between 2 and 146 days [4]. Surgical management is indicated in cases of chylopericardium with failed conservative management; patients with hemodynamic instability; the cause is well defined like mediastinal tumors; trauma; and an obstructive lesion in the mediastinum, impairing chyle flow, to prevent nutritional deterioration, immunological impairment, and metabolic complications. The surgical approach can be open thoracotomy (right/left, right thoracotomy preferable because of easy access to the thoracic duct) and VATS [5] based on available expertise. Surgical options include combined thoracic duct ligation immediately above the diaphragm, as above this level, congenital anomalies are common, and pericardial window creation over the anterior surface, to prevent complications like constrictive pericarditis. Pericardial window creation alone is associated with a recurrence in 50% of cases. Combined thoracic duct ligation and pericardial window creation are associated with nearly 100% success [6, 7].
To summarize, the management of chylopericardium starts with investigations to find out the cause, using various non-invasive and invasive modalities, and moves towards treatment. The latter includes dietary intervention (low-fat diet, medium-chain triglyceride-rich diet, and total parenteral nutrition), pharmacological intervention (injection of octreotide infusion), radiological intervention (drain insertion, pigtail catheter insertion, image-guided pericardiocentesis), and surgical intervention as mentioned above. In our case, apart from the above, we excised the cystic hygroma partially and applied clips and fibrin glue to seal off any invisible leaks, followed by pleurodesis. It is well proven that the VATS approach has less morbidity compared to the open method [6, 7]. We are reporting this case because mediastinal cystic hygroma presenting as spontaneous chylopericardium in adults is extremely rare and to the best of our knowledge, this is the first case of its kind in its management. In 18 months of follow-up, the patient is doing well and no recurrence has been observed.
Conclusion
Mediastinal cystic hygroma presenting as spontaneous chylopericardium is an extremely rare condition with very few reports in literature and surgery is the treatment of choice.
Acknowledgements
The authors would like to thank Dr. Vineela Malae, Dr. Nishanth Lakshmikantha, and Dr. Ramakrishna H K for proofreading the manuscript and for their suggestions.
Author contribution
Both authors have contributed equally.
Funding
None.
Data availability
We have the patient data available.
Declarations
Informed consent
We have taken the patient written and informed consent for reporting the case.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chemuru Munisekhar Reddy, Email: chemuru.munisekharreddy@gmail.com.
H. V. Rajashekara Reddy, Email: reddyvats@gmail.com.
References
- 1.Dib C, Tajik AJ, Park S, Kheir MEL, Khandieria B, Mookadam F. Chylopericardium in adults: a literature review over the past decade (1996–2006) J Thorac Cardiovasc Surg. 2008;136:650–656. doi: 10.1016/j.jtcvs.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 2.Han Z, Li S, Jing H, Liu H. Primary idiopathic chylopericardium: a retrospective case series. BMC Surg. 2015;15:61. doi: 10.1186/s12893-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hattori H, Dakeshita E, Nakazato J, Takahashi T, Wake M, Hirata K, Yasumoto H, Tengan T, Mototake H. Primary chylopericardium treated by surgery: report of two cases. J Cardiol Cases. 2011;3:e106–e110. doi: 10.1016/j.jccase.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho BC, Kang SM, Lee SC, Moon JG, Lee DH, Lim SH. Primary idiopathic chylopericardium associated with cervicomediastinal cystic hygroma. Yonsei Med J. 2005;46:439–444. doi: 10.3349/ymj.2005.46.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsui K, Namiki K, Matsumoto H, Konno F, Yoshida R, Miura S. Thoracoscopic treatment for primary chylopericardium: report of a case. Surg Today. 2005;35:76–79. doi: 10.1007/s00595-004-2869-3. [DOI] [PubMed] [Google Scholar]
- 6.Vinayakumar D, Arunkumar G, Sajeev CG, Rajesh G, Muneer K, Haridasan V, Babu K, Krishnan MN. Cystic lymphangioma of pericardium presenting as isolated chylopericardium–a case report. Indian Heart J. 2014;66:119–121. doi: 10.1016/j.ihj.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, Jia N, Ye S, Zhou M, Liu D. Primary chylopericardium: a case report and literature review. Exp Ther Med. 2018;15:419–425. doi: 10.3892/etm.2017.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have the patient data available.