Abstract
Arterial switch operation for transposition of great arteries (TGA) is the choice of surgical treatment for this condition. Conventional “open” coronary transfer technique has been commonly employed with good results in experienced hands. A modified “closed” technique of coronary transfer, with a more accurate coronary artery placement taking into account a distended aorta, along with anterior interrupted sutures to reduce purse stringing and other advantages is described.
Keywords: Arterial switch technique, Closed coronary transfer, ASO modified technique
Introduction
The arterial switch operation (ASO) is the procedure of choice for the surgical treatment of transposition of great arteries today [1]. While most of the technique of ASO has been standardized, the method of coronary artery (CA) transfer is still variable, considering the varied anatomy of the coronary arteries, including sinus of origin, proximal course, looping, branching pattern, subtle changes in the anatomy of the great vessels, and the individual experience of the surgeon. However, as in the many circumstances encountered in congenital heart surgery, the “wise” surgeon should be aware of all the various techniques so as to execute a successful CA transfer in a given situation. An accurate CA transfer is the most important determinant to ensure a successful outcome of the ASO. The “open” technique of transfer, before the restoration of neo-aortic continuity, was the conventional technique, which was reproducible, and has yielded excellent outcomes in many hands. The single most important drawback of this technique is the predictability of the final lie of the CA in a full aorta, as the CA translocation is done in a collapsed state of the aorta, before restoration of the aortic continuity.
The second important issue of concern in the “open technique” is the definite need to manage the proximal and distal neo-aorta mismatch which is augmented by at least 1.5 times after addition of both the coronary buttons to the already existing larger proximal pulmonary artery (PA).
This may not be a factor of concern for the experienced surgeon, but assumes significance for a young inexperienced surgeon doing ASO, and in the presence of CA anomalies. In the latter situation, oftentimes on termination of cardiopulmonary bypass (CPB) and if coronary insufficiency is encountered, the whole neo-aortic and CA anastomosis ensemble needs to be taken down to address the problem. If we could separate the aortic continuity sutures from the CA implantation suture line, maybe this could be avoided, the aortic clamp times could be reduced, and the CA anastomosis revised more expeditiously.
Technique
A modified closed technique of CA transfer after arterial switch has been followed in our unit since more than 10 years, which is detailed below. The modified closed technique is based on the earlier description by Bove [2]. We will describe our modified technique for arterial switch operation. Standard median sternotomy is done. After total thymectomy, the pericardium is harvested and kept in normal saline. Anatomy is inspected. Marking suture is placed on the anterior surface of the aorta, which acts as a center point during neo-aortic reconstruction. CPB is instituted using aorto-right atrial appendage cannulation. Patient is cooled to 24 °C. Patent ductus arteriosus is then dissected and divided between 5/0 polypropylene sutures. PA end is reinforced with another suture. Branch PAs are dissected up to the hilum and aorta is cross clamped. Cold Del-nido cardioplegia is given. Aorta is transected, the coronaries are explanted and mobilized in their proximal course so as to reach the neo-aorta without tension. Once the PA prior to its branching has been transected, three marking sutures of 7/0 polypropylene corresponding to the commissures of the pulmonary (neo-aortic) valve (in a tri-leaflet valve) are taken on the proximal PA externally on its wall (Fig. 1). The neo-aorta is reconstructed end-to-end with posterior continuous, and anterior multiple evenly spaced interrupted sutures, after the Le-Compte maneuver using 7/0 polypropylene suture. Plication of a pulmonary sinus is done, if there is a great size mismatch, to match the diameter of the distal aorta. The proximal PA (which becomes the neo-aorta) is plicated using multiple interrupted 7/0 polypropylene sutures, to match the size of the distal aorta. The aortic clamp is thereafter temporarily released to distend the reconstructed neo-aorta and the site for precise placement of coronary buttons is marked by 7/0 polypropylene marking sutures. This shows the exact lie of the CA in a fully distended aorta. The previously taken marking sutures help in knowing the exact position of the neo-aortic valve commissures and help to safeguard them during making incisions for CA implantation.
Fig. 1.
Posterior aortic continuous suture line
Distending the aorta at this stage also has two other advantages:
It helps to see the bleeding sites at the neo-aortic suture line and secure them.
It closes the neo-aortic valve leaflets, so that they move away from the wall and are safeguarded when making the incision for CA implantation.
Incision on the neo-aortic sinus for reimplantation of CA could be a medial/lateral based trapdoor [1] or a plain simple incision, as dictated by the anatomy and probable best lie of the CAs. This could be even abreast the neo-aortic suture line, or even into the distal aorta. The other modification added to the technique in the course of our experience is the placement of posterior continuous and anterior interrupted 7/0 polypropylene sutures for neo-aortic reconstruction (Fig. 2). We initially adopted this technique for the neo-PA reconstruction, and seeing its better lie and advantages of no purse stringing, extrapolated it to the neo-aortic reconstruction. We have found this to be particularly helpful, especially in low birth weight neonates with small diameter aortas, and those with extreme size mismatch, where even a small amount of purse stringing gives rise to gradients.
Fig. 2.
Anterior interrupted sutures
Other advantages of the anterior interrupted sutures that we have noted are:
Allows growth
Even distribution of tension, hence more hemostatic with far less bleeding
Allows “even cheating” when there is a size mismatch with no purse stringing
Gives us the flexibility to site the CA implantation across the neo-aortic suture line, if the anatomy dictates it for a favorable lie, without having to undo the whole aortic suture line, unlike when a continuous suture is used. Trapdoor or a box flap could also be created if found necessary (Fig. 3).
Fig. 3.
Right coronary anastomosis across the aortic suture line made easier especially circumflex from sinus 2 arrangement
Very frequently, the two ends of the posterior continuous suture line of the neo-aorta are kept taut and left untied, till the coronary sites are marked on a distended aorta, after the completion of the anterior interrupted suture line. This helps in sitting the CAs more accurately, without having to take down the whole posterior continuous suture line, if the ends had been tied down earlier.
-
5.
Does not add tissue to increase the proximal neo-aortic diameter, particularly if there is a size mismatch
The perceived disadvantages of this technique are:
A little increase in cross clamp time
Increased usage of polypropylene sutures
Possible damage to the neo-aortic leaflets/commissures, if care is not taken
A note of caution: Allowance has to be made for a full ventricle in determining the site of coronary transfer, which has to be a bit higher when seen on a full aorta, to avoid kinking, if it is translocated in a lower sinus position
We feel these drawbacks are minor and are compensated by a perfect CA lie, less bleeding, less use of blood products, less gradients, more primary closures of chest, and possibly good great artery growth on follow-up. Almost all varieties of CA have been translocated successfully by this technique (Table 1), with no revisions found necessary. The other advantage noticed is a bit more lateral implantation of CA, that is, permitted in most anatomies. This helps to avoid CA compression by the anteriorly translocated neo-PA.
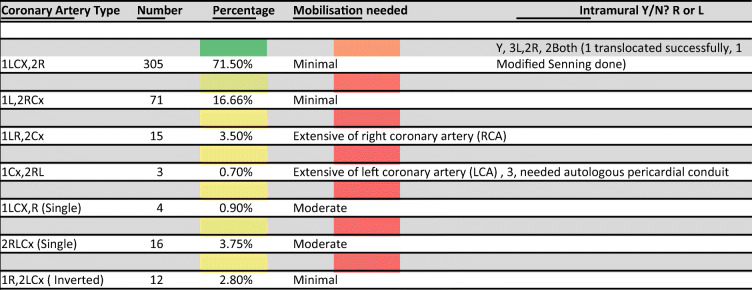
Table 1.
Coronary artery anatomy encountered, N = 426
Cx, left circumflex artery; R, right coronary artery; L, left anterior descending artery; Y, yes; N, no
Results
In the period between July 2009 and June 2020, 426 neonates underwent arterial switch operation at our center. Of these, 305 children (71%) had the CA anatomy “normal for transposition” (1LCx,2R (L—left anterior descending, Cx—left circumflex; R—right coronary artery)), 71 (16.7%) had 1L-2RCx pattern, and smaller percentages of cases had more complex and rarer forms of coronary anatomy as shown in Table 1. The Leiden Convention [3, 4] has been used to describe the CA anatomy). Majority of the cases having the anatomy 1LCx-2R had transposition with intact ventricular septum, while the other patterns were seen mostly in transposition with ventricular septal defect (VSD) and Taussig Bing anomalies. The rarer forms were encountered more frequently in Taussig Bing anomalies particularly those associated with side-by-side great artery relationships. Nearly all of these types of CA anomalies could be successfully translocated with the modified closed technique. Three needed addition of a small piece of the pericardium to correct the angulation in the early part of our experience, and three others with the anatomy 1Cx-2RL needed an autologous pericardial tube for a successful translocation. Intramural origin and course of CAs were encountered in 7 cases (3LCA, 2RCA, and 2 both CAs). We could manage a successful transfer in six of them, all with the closed technique, except one patient. Of the two patients with intramural origin of both CA, one patient had origin of both CAs juxta-commissurally from separate sinuses, which could be successfully transferred after unroofing the respective orifices. The other patient had a successful modified Senning operation, as the intramural segments were very long and the orifices small. It was felt that the CA transfer carried a very high risk of failure, and hence, an atrial switch was decided upon, before explanting the CA from the aortic sinuses. There were no coronary issues encountered with this technique. No revisions were required. The overall operative mortality for the entire series has been 8 patients (8/426, 1.8%). None of these mortalities was related to coronary issues. Open technique for coronary transfer also has comparable results in experienced hands. The real advantages of either technique will only be realized in the long-term follow-up.
Discussion
Coronary artery translocation plays an important role in ensuring the success of an ASO. Techniques of implantation have to be tailored to the anatomy. In an anterior-posterior relationship of great arteries (GA), as is usually seen in transposition of great arteries (TGA), the coronary translocation involves rotation of the coronary button by as much as 90°, or more sometimes. Usually, it is around 30 to 45°. To reduce this twist and torsion, Mee described the medially based trapdoor technique that has given excellent outcomes [1]. However, the precise place to translocate the CA by the conventional open technique in a collapsed neo-aorta is open to judgment. It increases the diameter of the proximal PA (neo-aorta) and may cause distortion of CA in a distended state of the neo-aorta. There could be kinks of CAs if the implantation site is lower and the CA length is more. Also, the complete neo-aortic suture line has to be undone, if CA implantation has to be revised, when CA insufficiency is encountered on termination of CPB. The closed technique, initially described by Bove [2] and reported by Dedemoğlu and colleagues recently [5], has been adopted by us since 2007, with no revisions found necessary in any given kind of coronary anatomy coming up for ASO. The interrupted anterior suture reconstruction of aorta modification helps growth, reduces bleeding due to even distribution of tension, and smoothens tailoring of a size mismatch. It also gives more flexibility in planning the perfect placement of the CA implantation site, even abreast the neo-aortic suture line, if found necessary. This is accomplished by just removing a few interrupted sutures and fashioning the implantation site as needed, by a trapdoor or a box incision, resulting in a generous coronary opening, sans kinks and twists. We agree with Brown et al. [6] that this technique permits a safe CA transfer in almost all complex CA anatomies, and all this, without having to take down the whole neo-aortic suture line to revise the CA translocation. We have not found any limitations to this technique, except for a marginally longer aortic cross clamp time and a few more sutures. However, this increased cost appears to be compensated by less bleeding and use of blood products with more instances of primary chest closure.
Conclusion
Modified closed coronary artery transfer during the arterial switch operation for transposition of the great arteries is a reliable and safe method that facilitates precise transfer of the coronary buttons into the neo-aortic root. This technique minimizes the risk of tension on the anastomosis, facilitates proper alignment, and can be done with minimal coronary artery mobilization. The use of external marking sutures to identify the location of the neo-aortic valve commissures has proved to be safe, avoiding injury to the valve, when the incision in the sinus of Valsalva is made. The use of multiple interrupted sutures on the neo-aortic suture line is one of the most important modification, as it makes it very useful even in side-by-side relationship, where the posterior coronary artery can be implanted across the suture line, by just removing a few sutures and not disturbing the whole suture line.
Acknowledgments
The authors would like to acknowledge Dr. Shraddha Harsh Seth for the art works.
Funding
None.
Declarations
Ethics approval
Retrospective study, hence approval was waived.
Statement of human and animal rights
Not applicable being review article.
Consent for manuscript publication
Yes.
Conflict of interest
The authors declare no competing interests.
Disclaimers
None.
Footnotes
Previous publication/presentations of the article
None
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mee. RBBB: The arterial switch operation, In Stark J, de Leval M. Editors. Surgery for Congenital Heart defects, 3rd Ed. John Wiley and Sons Limited; 2006: 471–487.
- 2.Bove EL. The arterial switch procedure: closed coronary artery transfer. Oper Tech Thorac Cardiovasc Surg. 2009;14:309–316. doi: 10.1053/j.optechstcvs.2009.11.004. [DOI] [Google Scholar]
- 3.Yacoub MH, Radley-Smith R. Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax. 1978;33:418–424. doi: 10.1136/thx.33.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gittenberger-de Groot AC, Sauer U, Oppenheimer-Dekker A, Quaegebeur J. Coronary arterial anatomy in transposition of the great arteries: a morphologic study. Pediatr Cardiol. 1983;4:15–24. [Google Scholar]
- 5.Dedemoğlu M, Coşkun G, Özdemir F, Yurdakök O, Korun O, Çiçek M, Biçer M, Coşkun Fİ, Aydemir NA, Şaşmazel A. Modified closed coronary transfer is a good alternative to the trap-door method during arterial switch operation: a retrospective propensity-matched comparison. Braz J Cardiovasc Surg. 2020;35:329–338. doi: 10.21470/1678-9741-2019-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JW, Park HJ, Turrentine MW. Arterial switch operation: factors impacting survival in the current era. Ann Thorac Surg. 2001;71:1978–1984. doi: 10.1016/S0003-4975(01)02529-2. [DOI] [PubMed] [Google Scholar]