Abstract
Objective
Junctional proteins are the most important component of the blood-testis barrier and maintaining the integrity of this barrier is essential for spermatogenesis and male fertility. The present study elucidated the effect of SARS-CoV-2 infection on the blood–testis barrier (BTB) in patients who died from severe acute respiratory syndrome coronavirus 2 (COVID-19) complications.
Methods
In this study, lung and testis tissue was collected from autopsies of COVID-19 positive (n = 10) and negative men (n = 10) and was taken for stereology, immunocytochemistry, and RNA extraction.
Results
Evaluation of the lung tissue showed that the SARS-CoV-2 infection caused extensive damage to the lung tissue and also increases inflammation in testicular tissue and destruction of the testicular blood barrier. Autopsied testicular specimens of COVID-19 showed that COVID-19 infection significantly changes the spatial arrangement of testicular cells and notably decreased the number of Sertoli cells. Moreover, the immunohistochemistry results showed a significant reduction in the protein expression of occluding, claudin-11, and connexin-43 in the COVID-19 group. In addition, we also observed a remarkable enhancement in protein expression of CD68 in the testes of the COVID-19 group in comparison with the control group. Furthermore, the result showed that the expression of TNF-α, IL1β, and IL6 was significantly increased in COVID-19 cases as well as the expression of occludin, claudin-11, and connexin-43 was decreased in COVID-19 cases.
Conclusions
Overall, the present study demonstrated that SARS-CoV-2 could induce the up-regulation of the pro-inflammatory cytokine and down-regulation of junctional proteins of the BTB, which can disrupt BTB and ultimately impair spermatogenesis.
Keywords: Blood–testis barrier, Inflammatory cytokines, COVID-19
Introduction
The worldwide outbreak of the new disease called coronavirus disease 2019 (COVID-19) arises from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has reached a pandemic condition in March 2020 [1, 2]. Existing data have indicated that SARS-CoV-2 is not usually limited to the respiratory system alone but might also attack other tissues in the body [3]. Reportedly, SARS-CoV-2 can also affect the male reproductive system, so it is observed in the semen and leads to damage in the spermatogenesis process [4, 5]. In the seminiferous tubules of the mammalian testis, Sertoli cells by providing nourishment and mechanical support, play a pivotal role in post-meiotic germ cells’ development during spermatogenesis. These cells also construct the blood–testis barrier (BTB) in the seminiferous epithelium that divides the seminiferous epithelium into the adluminal and the basal compartments [6], so there are spermatogonia and preleptotene spermatocytes in the basal compartment and other primary and secondary spermatocytes, round spermatids, and elongated spermatids in the adluminal compartment [7]. Ultrastructurally, the BTB consists of co-existing tight junctions (TJs), desmosome-like junctions, ectoplasmic specializations (ESs) and gap junctions which collectively play a crucial role in the maintenance of BTB integrity that is essential for spermatogenesis and male fertility [8]. In the testis, TJs are the most important component of the blood–testis barrier [7]. Claudins are transmembrane proteins which along with occludin, are essential for TJ formation [9]. Gap junctions and their constitutive proteins, connexins are another type of junction present in BTB. The most predominant connexins which are mainly found at the BTB are connexins-43, which probably leads to the synchronization of Sertoli cells [10].
BTB works not only as a physical barrier but rather allows the spermatogenesis process to take place in an immune-privileged environment and helps to inhibit testicular inflammation and immune response. But some viruses, such as the Mumps virus, Zika virus and HIV, can still penetrate the barriers and be seen in semen and induce inflammation, apoptosis and oxidative damage to the testis. Available data suggest that SARS-CoV-2 causes the release of several pro-inflammatory cytokines, such as interleukin 6 (IL-6), IL-1 and tumor necrosis factor-α (TNF-α) [11]. As reported in previous studies, inflammation is associated with disruption of spermatogenesis and testicular function [12].
All available studies indicate that COVID-19 could influence male reproductive health and it is suggested that the effect of SARS-CoV-2 in the male reproductive system should be considered [13]. Accordingly, this study was designed to elucidate the impacts of SARS-CoV-2 on the male reproductive system by investigating damage of BTB and histological change in human testis.
Materials and methods
Patient selection and patient criteria
Ten patient samples and ten healthy samples were included in the study (Table 1). For the control group, people in the age range (45–58 years) and reproductive health status who died due to accident, electric shock, or carbon monoxide poisoning and had no disease were used. The control group consisted of male adults without any exposure to SARS-CoV-2 infection. To prevent post-mortem delay, all control and COVID-19 groups were analyzed within a minimum period of time after death. None of the control group had a history of infertility or reproductive disorders. In addition, all of them had children.
Table 1.
Clinical characteristics of COVID-19 patients
| Case No. | Age (yr) | Disease duration (d) | Reproductive system disorders | Fertile and have children | Corticosteroid therapy | Comorbidity | Cause of death |
|---|---|---|---|---|---|---|---|
| C1 | 58 | 23 | Non | Yes | Yes | Hypertension, type 2 diabetes | COVID-19, acute respiratory distress syndrome, septic shock |
| C2 | 48 | 28 | Non | Yes | Yes | Non | COVID-19, pneumonia, acute respiratory distress syndrome, myocardial infarction |
| C3 | 49 | 30 | Non | Yes | Yes | Non | COVID-19, respiratory failure, myocardial infarction |
| C4 | 54 | 32 | Non | Yes | Yes | Hypertension | COVID-19, respiratory failure, septic shock |
| C5 | 57 | 27 | Non | Yes | Yes | Hypertension, type 2 diabetes | COVID-19, respiratory failure, septic shock |
| C6 | 55 | 20 | Non | Yes | Yes | Hypertension | COVID-19, respiratory failure, septic shock, renal failure |
| C7 | 60 | 25 | Non | Yes | Yes | Hypertension, type 2 diabetes | COVID-19, acute respiratory distress syndrome, septic shock, myocardial infarction |
| C8 | 58 | 31 | Non | Yes | Yes | Hypertension | COVID-19, pneumonia, acute respiratory distress syndrome, renal failure |
| C9 | 57 | 29 | Non | Yes | Yes | Non | COVID-19, respiratory failure, myocardial infarction |
| C10 | 62 | 30 | Non | Yes | Yes | Hypertension | COVID-19, respiratory failure, septic shock, myocardial infarction |
COVID-19 coronavirus disease 2019, yr year, d day
Postmortem examination and sampling of the testes
Postmortem human testes were collected from the Iranian Legal Medicine Organization. This study was conducted by the principles of the Declaration of Helsinki and the guidelines of the Chinese National Health Commission and was approved by the Ethics Committee at Shahid Beheshti University of Medical Sciences, (IR.SBMU.MSP.REC 1399.779). All cases were checked for their medical history of death, death certificate, autopsy report. Cases with a history of reproductive system disorders were excluded from the present study. According to the standard protocol, COVID-19 diagnosis was confirmed by positive nucleic acid testing of nasopharyngeal swabs, radiography (chest X-ray), computer tomography (CT) scan features of viral pneumonia, and clinical symptomatology. The deceased COVID-19 and control cases were transported by refrigerator from the hospital to forensic medicine. Postmortem examinations were carried out after informed consent from family members and were performed approximately 8 to 10 h after death. For ten deceased COVID-19 and ten control, a tissue sample of 3 × 3 cm2 was obtained via incisional autopsy for histological, cellular and molecular study.
Tissue preparation
Lung specimens were fixed in 10% formalin for 72 h and submitted for standard tissue processing, sectioning and hematoxylin and eosin (H&E) staining for histopathology evaluations. Evaluation of tissue sections was done by an expert histologist. The testes samples were kept in Bouin's for 48 h and then transfer to formalin 10% before placing them in paraffin blocks. We then made serial Sections 5 µm and 20 µm using a microtome (Leica RM2125 RTS, Germany) consistent with the stereological techniques. Systematic Uniform Random Sampling (SURS) was used to choose 10 sections in each sample by picking a random figure in the range of 1 to 10. They were then stained using H&E staining (Sigma, USA). It is worth noting that the testis cells were distinct in terms of morphology.
Analysis of TNF-α, IL1β, IL6, occludin, claudin-11 and connexin-43 expression using real-time PCR
After extracting the total RNA samples, they were treated with DNase I (Roche, Basel, Switzerland) to eliminate genomic DNA contamination. We used a commercial kit (Fermentas, Lithuania) to synthesize cDNA at 42 °C for 60 min in compliance with the protocols described in the manufacturer’s instructions. We used real-time PCR (TaqMan) based on QuantiTect SYBR Green RT-PCR kit (Takara Bio Inc, Japan) to quantify relative gene expression. All pairs of forward and reverse primers were designed by Primer 3 Plus software in an exon–exon junction manner to separate cDNA from genomic DNA. Before that, we tested PCR primers using the Primer-Blast tool available at the website, www.ncbi.nlm.nih.gov/tools/primer-blast (Table 2).
Table 2.
Primers design
| Genes | Primer sequences |
|---|---|
| GAPDH |
F: AACTTTGGTATCGTGGAAGG R: CAGTAGAGGCAGGGATGATGT |
| TNF-α |
F: CCTCACACTCACAAACCACCA R: ACAAGGTACAACCCATCGGC |
| IL1β |
F: CCACAGACCTTCCAGGAGAATG R: GTGCAGTTCAGTGATCGTACAGG |
| IL6 |
F: GCCTTCTTGGGACTGATGCT R: TGCCATTGCACAACTCTTTTCT |
| Occludin |
F: AACGATAACCTAGAGACACCT R: AATTATCACACATCAAGAGGA |
| Claudin-11 |
F: AAACCGTTTCTATTACTCTTC R: GACTGCGTCATGGCCACTGG |
| Connexin-43 |
F: GAACTCAAGGTTGCCCAAAC R: TTAGAGATGGTGCTTCCCG |
Number of testis cells
The testis cells’ number was estimated using the optical dissector method [14]. We computed the numerical density (Nv) of testis cells using the equation below:
where (ΣQ) is the number of cells and (ΣP) is the number of counting frame grid in all fields; (a/f) is the area of frame; (h) is the dissector height; (t) is the real thickness of the section and (BA) is the microtome section thickness. The total number of testis cells was obtained using the equation below:
Coefficient of error (CE)
CE (V) was computed according to the following equation:
where (B) and (A) are section boundary length and the section area, respectively. We calculated CE for the volume of testis and the number of testis cells using the equation below [14]:
CEs are depicted in Table 3.
Table 3.
Coefficients of error (CE) for total number of sertoli cells
| Groups | Number of sertoli cell |
|---|---|
| Control | 0.03 |
| COVID-19 | 0.04 |
Covariance function
The covariance function was measured using the following equation:
The both end points of dipoles (DP) of class size r = 1 (equivalent to 4.3 µm). To estimate “Vv”, “C(r)”, and “g(r)”, the distance between the points (DP) ranged from r = 0 (equivalent to 0 µm) to r = 49; so, the total distance was 127.4 µm (49 × 4.3 = 210.7 µm).
Pair correlation function
The pair-correlation function is the normalized covariance function obtained by dividing the covariance by the reference value (squared volume fraction):
Voronoi tessellation for distribution of the testicular cells
Area of space with closely spaced cells. Thus, the polygon region represents the space occupied by a cell. Testicular cells were mapped using the Image J Voronoi Plugin, which involves drawing a polygonal area around each cell. The area and number of closest Voronoi polygons were obtained from different parts of the testis using the microscopic images of testicular tissue, an objective lens of 40 × was obtained. The data were then analyzed using "ImageJ". The variability of polygonal regions was easily analyzed by their variance. The coefficient of variation or CV (standard deviation of polygonal regions/mean × 100) provides an indicator for the spatial distribution of Sertoli cells: CV 33–64% is associated with a random distribution of testicular cells. CVs less than 33% have a regular pattern and those with more than 64% are considered a cluster distribution. This CV is a classification and not a statistical comparison.
Immunohistochemical staining
After deparaffinization and rehydration, the sections were retrieval with 10 mM citrate buffer and then incubated with primary antibodies (Sigma-Aldrich Corporation, St. Louis, Missouri), (diluted 1 in 100). Then, the appropriate secondary IgG antibody (diluted 1 in 200), the sections were incubated with 3'-diaminobenzidine (DAB) (Dako, Glostrup, Denmark). After the immunohistochemical reaction, sections were counter-stained with hematoxylin (Merck, White house, NJ) mounted, and observed under a light microscope.
Statistical analyses
In this study, we used the Pfaffl method and t tests for statistical analysis. A significant level of p ≤ 0.05 was assumed for all data.
Results
Histopathological feature of lung tissue
Histopathological examination of the pulmonary system showed a spectrum of diffuse alveolar damage in patients, which was evidenced by the presence of intra-alveolar fibrin, hyaline membranes in the alveolar septal walls. Airways and alveolar spaces contained large, reactive mononuclear inflammatory cells. Microscopic hemorrhage was identified with diffuse alveolar damage in patients. Most patients showed variable degrees of chronic interstitial inflammation, with some having more prominent perivascular lymphocytic inflammation (Fig. 1A and B).
Fig. 1.
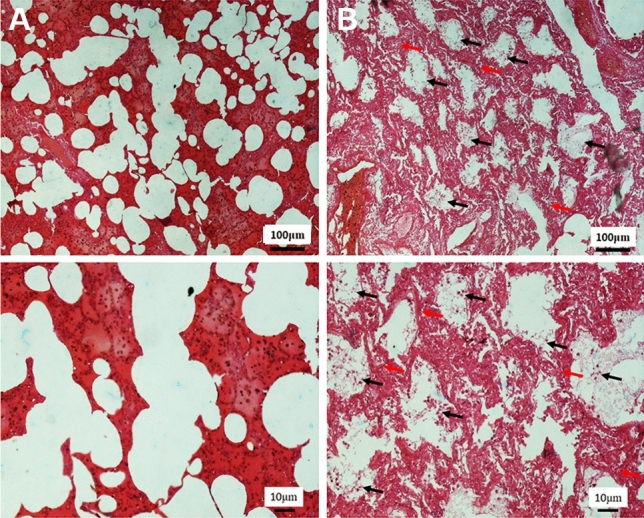
The effect of SARS-CoV-2 on the lung tissue in the human post-mortem. A In the control group, the lung parenchyma, including the alveolar sac and alveolar walls, has a normal appearance. B In the COVID-19 group, infiltration of lung tissue by mononuclear inflammatory cells (black arrow), along with desquamation of alveolar epithelium and formation of hyaline membrane together alveolar wall thickening was observed (red arrow)
SARS-CoV-2 increased the expression levels of genes involved in inflammation
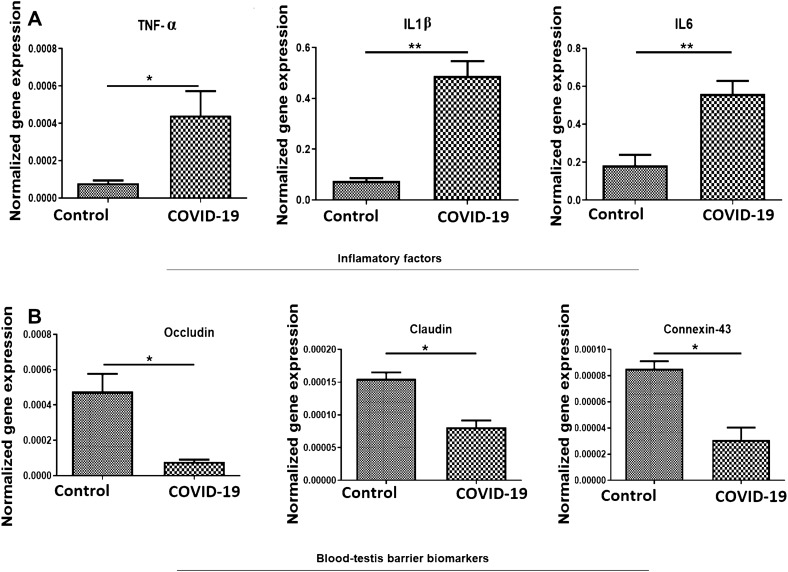
We normalized and quantified the relative mRNA expression levels of TNF-α, IL1β and IL6, in study groups. In comparison to the control group, the transcripts for TNF-α, IL1β and IL6 in the COVID-19 group were significantly upregulated (p < 0.05, p < 0.01 and p < 0.01, respectively) (Fig. 2A).
Fig. 2.
The effect of SARS-CoV-2 on the inflammatory cytokines and junctional proteins in the human post-mortem testicular tissue. A and B Real-time PCR analyses of testes. mRNA expression levels of TNF-α, IL1β, IL6, claudin-11, occludin and connexin-43 from control and COVID-19 groups. Mean ± SD of the mRNA expression levels of TNF-α, IL1β, IL6, claudin-11, occludin and connexin-43 of testis in the study groups (*p < 0.05 and **p < 0.01)
SARS-CoV-2 decreased the expression levels of genes involved in BTB
To investigate the impact of SARS-CoV-2 on the impairment of BTB at the molecular level, the number of transcripts for the three genes contributing to the structure of BTB (occluding, claudin and connexin-43) was analyzed. Furthermore, gene expression for occluding, claudin and connexin-43 was significantly (p < 0.05) lower in the COVID-19 group compared to that of the control group (Fig. 2B).
SARS-CoV-2 decreased in the total number of Sertoli cells
At the cellular level, the total number of Sertoli cells using optical dissector was estimated. The assessment of testis tissue indicated a significant decrease in the number of Sertoli cells in the COVID-19 group compared to the normal control group (p < 0.001) (Fig. 3A–C).
Fig. 3.
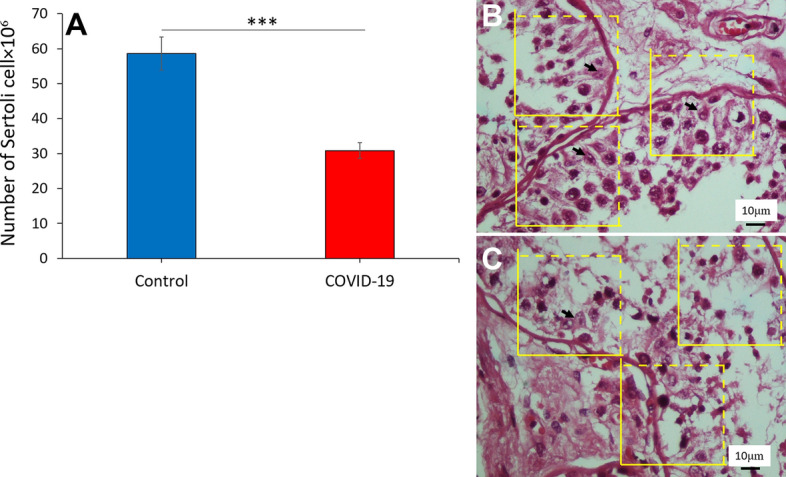
The effect of SARS-CoV-2 on the Sertoli cells number in the human post-mortem. A The total number of Sertoli cells in the COVID-19 group decreased in comparison with the control groups. (***p < 0 .001). Photomicrograph of the testis in control (B) and COVID-19 (C) groups stained with H&E, × 40. SC (Sertoli cell)
SARS-CoV-2 alters the spatial arrangement of Sertoli cells
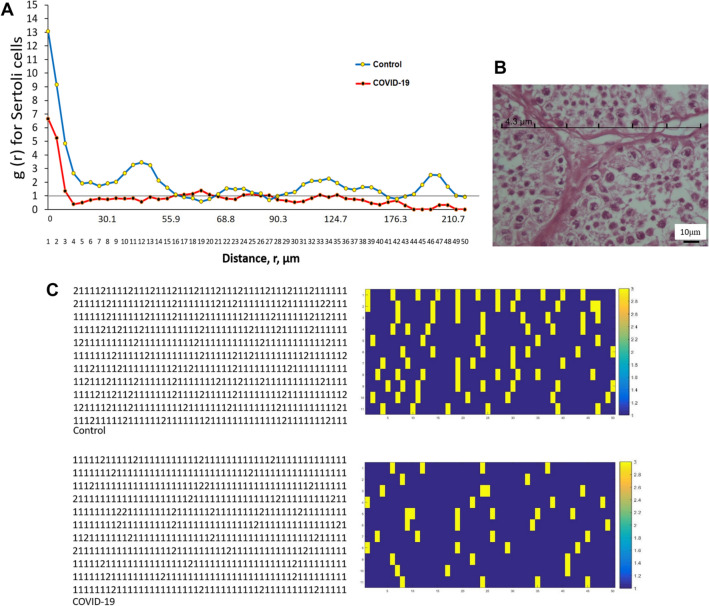
Assessments of g(r) for Sertoli cells and the dipole distances, were plotted versus each other (Fig. 4A and B). The estimated values from the start to the end of the curve (from r = 0 to 64.5 µm), (from r = 94.6 to 103.2 µm), (from r = 124.7 to 172 µm), (from r = 184.9 to 215 µm), revealed a significant difference among the two groups. After the gap, there are the data points for both the normal control and COVID-19 groups that were arranged randomly in longer distances (p < 0.05) (Fig. 4A and B).
Fig. 4.
The effect of SARS-CoV-2 on the spatial arrangement of Sertoli cells in the human post-mortem using the second-order stereological method. A Communication between pair-correlation function and dipole distance for Sertoli cells in control and COVID-19 groups. The mean points are g(r) in the control and COVID-19 groups. The horizontal reference axis relates to values expected for a random spatial arrangement [g(r) = 1]. B A micrograph that encodes cells to produce a matrix of a transparent lattice of points to serve as a set of dipole probes. Each row consists of 50 points and 49 equal distance intervals. For each testis, 11 tests were performed (a total of p = 550 test points). For each experiment, the nature of the tissue constituent at each test point was determined and all data were recorded in 11 × 50 matrices. The point interval (r) relates to a distance of 4.3 μm, which has a chance of being in the same Sertoli profile. If the dot was placed on Sertoli cells and connective tissue, 1 and 2 were encoded, respectively. C Convert data matrix to image type and display Sertoli cells (1) yellow and connective tissue (2) dark blue. A control group; Group B, COVID-19
Convert a data matrix into the image using MATLAB software
DM changed the testis cells’ spatial distribution, leading to the dissociation of these cells in some places. To give a clear picture of this event, we converted the data matrices of both COVID-19 and normal control groups into images by MATLAB software (Fig. 4C). Photomicrograph of the testis stained with H&E and cartoon images showed the changes of tissue structure and spatial pattern of testicular cells in the seminiferous tubules in the control and COVID-19 groups (Fig. 5A and B).
Fig. 5.
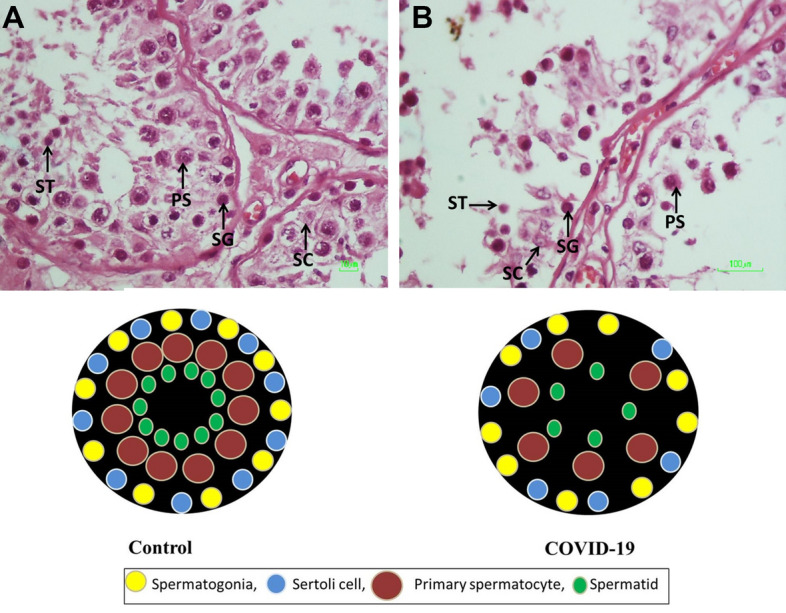
The effect of SARS-CoV-2 on the testicular cells morphology in the human post-mortem. A Photomicrograph of the testis stained with H&E, ×40. SG (spermatogonia), PS (primary spermatocyte), ST (round spermatid), SC (Sertoli cell). A and B are cartoons that illustrate the differing morphologies of testicular tubules in control (A) and COVID-19 (B) groups
The spatial distribution of the testicular cells
Voronoi tessellation of the testicular cells in the control and COVID-19 groups is shown in Fig. 6A–E. The data showed that 8% of the areas of the polygons of the testicular cells in the testis in the COVID-19 samples were located in the range of ˃ 120 μm2, while in the control samples, just 17% of the areas of the polygons were located in this range. The results also showed that 50% of the areas of the polygons of the testicular cells in the testis in the COVID-19 samples were located in the range of 120–130 μm2, while in the control samples, just 60% of the areas of the polygons were located in this range.
Fig. 6.
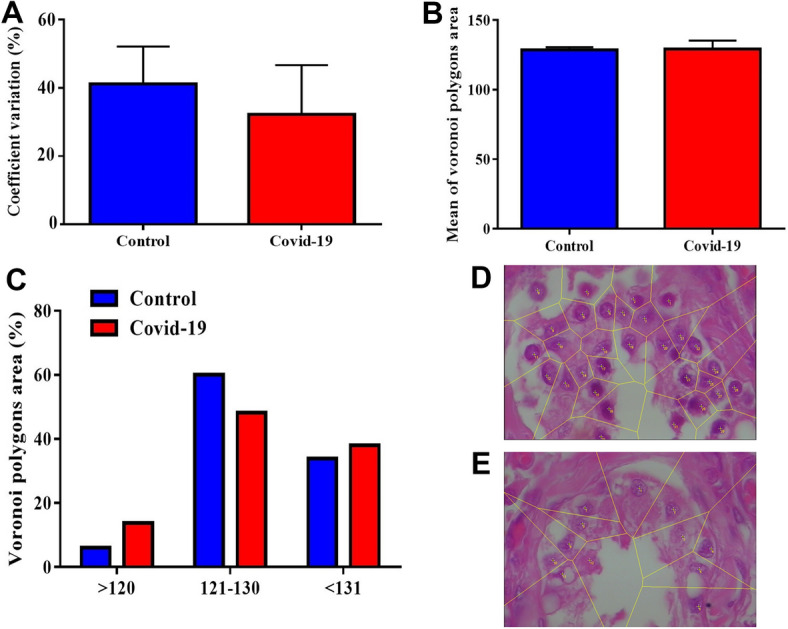
The effect of SARS-CoV-2 on the spatial pattern of testicular cells in the human post-mortem using Voronoi tesselation. A–C Mean ± SD of Voronoi polygon area (%) and Coefficient of Variation (CV) within the testis in study groups. D and E Representative photograph, polygon area, and schematic of Voronoi tessellation of the testicular cells in the control (D) and COVID-19 (E) groups
Additionally, our results also showed that 40% of the areas of the polygons of the testicular cells in the testis in the COVID-19 samples were located in the range of ˂ 130 μm2, while in the control samples, just 37% of the areas of the polygons were located in this range. Furthermore, the COVID-19 samples showed fewer testicular cells in the range of 120–130 μm2 than control samples (Fig. 6A–E). Based on the coefficient of variation (CV) classification, the mean CV of polygon areas in both groups is located in a random range (33–64%) (Fig. 6A–E).
Expression of CD68 in testis based on immunocytochemistry
Immunostaining showed that CD68 was and strongly expressed in the interstitial tissue of testis in the COVID-19 group when compared with the control group (p < 0.001) (Fig. 7C). In the interstitium of testicular tissue, there was mild inflammatory infiltrate composed predominantly of macrophage (CD68-positive cells) (Fig. 7A and B) as confirmed immunohistochemistry.
Fig. 7.
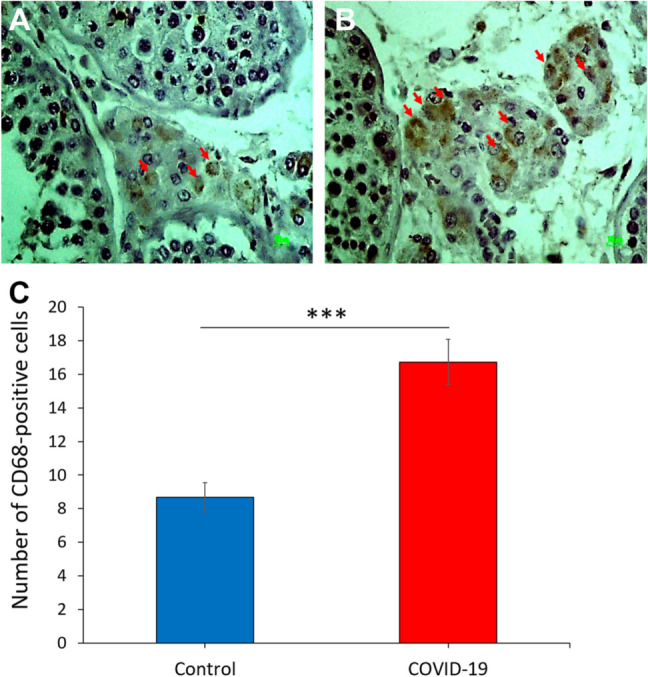
The effect of SARS-CoV-2 on the macrophage biomarker in the human post-mortem testcular tissue. Photomicrograph of the testis immunohistochemistry staining for CD68, ×40. A Control group and B COVID-19 group. C Total number of CD68-positive cells in study groups. (***p < 0.001). Arrows are CD68-positive cells
Immunohistochemistry of occluding, claudin-11 and connexin-43
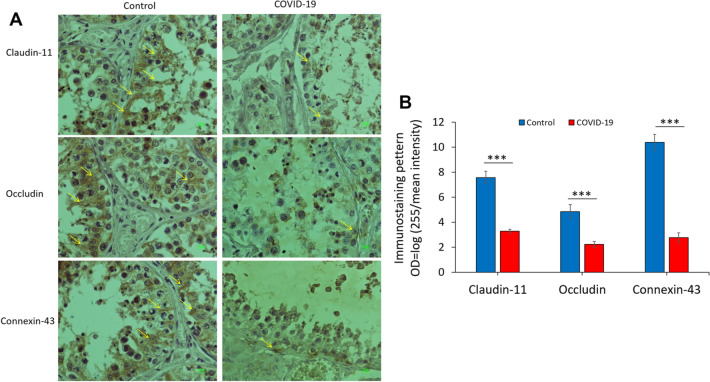
Figure 8A shows the expression of claudin-11, occludin and connexin-43 in testicular tissues in COVID-19 groups. A significant difference was observed in the expression of target proteins that are involved in the establishment of BTB. The expression of claudin-11, occludin and connexin-43 in testis showed a significant decrease in the COVID-19 group when compared with the normal control group (p < 0.001, p < 0.001, p < 0.001, respectively) (Fig. 8B). Immunohistochemical staining showed that SARS-CoV-2 induced disrupts BTB by decreasing BTB junction proteins, such as claudin, occludin and connexin-43. The brownish color was considered to be evidence of a positive expression of claudin-11, occludin and connexin-43 in the testis. To convert the intensity and quantification of immunoreactivity of protein expression, we used the optical density (OD), following this formula: OD = log (max intensity/Mean intensity), where max intensity = 255 for 8-bit images.
Fig. 8.
The effect of SARS-CoV-2 on the junctional proteins of BTB in the human post-mortem testicular tissue. A The patterns of immunostaining of claudin-11, occluding, and connexin-43 in the control and COVID-19 groups. Immunoreactivity pattern (OD = log(max intensity/Mean intensity) of claudin-11, occluding, and connexin-43 in testis in the study groups. B The significant difference between COVID-19 with the control groups is indicated. (***p < 0.001). Arrows indicate junctional proteins
Discussion
Some researchers have confirmed that COVID-19 could induce male reproductive dysfunction, but the exact mechanisms of COVID-19 on the male reproductive system are still not clear. The analysis within this report provides evidence that the COVID-19 could induce structural changes of the seminiferous tubules and alterations of the blood–testis barrier which gives insight into the damage to the seminiferous tubules associated with COVID-19.
Then, we first proved that COVID-19 infection would disrupt BTB integrity through producing higher levels of pro-inflammatory cytokines, including TNF-α, IL-1b and IL-6 as well as decreasing expressions of genes and proteins related to BTB, including occludin, claudin and connexin-43.
It is right to point out that based on previous studies, the spikes of the SARS-CoV-2 virus interact with angiotensin-converting enzyme 2 (ACE2) to infect the cell. Except for the lung and some other organs, ACE2 expresses extensively on the surface of most testicular cells, interstitial cells, Sertoli cells, Leydig cells and the early stage of germ cells [15–17]. As reported in other studies, angiotensinogen is converted via renin to angiotensin I (Ang I). ACE2 regulates negatively the function of ACE by converting Ang I to Ang 1–9 and Ang II to Ang 1–7 [5, 18, 19]. In general, the activated ACE-Ang-II-AT1R axis leads to pro-inflammatory cytokines. On the other hand, the ACE-2-Ang- (1–7)-Mas axis has an anti-inflammatory effect [20–22]. It is also suggested that the attachment of SARS-CoV-2 to the ACE2 receptor may trigger an inflammatory response that could lead to Sertoli and Leydig cells dysfunction [23]. Our previous results unpublished showed that the expression of the ACE2 gene and protein was a reduction in testicular cells in the COVID-19 samples compared with the healthy control samples. Therefore, the possibilities of SARS-CoV-2 virus entry to the testicular cells are unlikely. Therefore, it can be said, following the down-regulation of ACE2 and consequently reduction of Ang 1–7, SARS-CoV-2 has been able to increase the level of TNF-α, IL-1b and IL-6 gene expressions in the COVID-19 group in comparison with the control group [24]. The TNF-α, mediated by activation of the ERK1/2 signaling pathway, leads to increase tight junction permeability [25]. Our present study showed COVID-19 increased the mRNA levels of TNF-α, IL-1β and IL-6, suggesting that COVID-19 induced inflammation in testis. And we speculated that COVID-19 reduced protein levels of occludin, claudin-11 and connexin-43 associated with inflammation in testis. Several inflammatory cytokines, such as TGF-b3, TNF-α, and IL-1, play an effective role in the regulation of blood–testis barrier (BTB) dynamics [26]. Furthermore, IL6 might play a role in the downregulation of occludin expression and the modulation of BTB permeability [27]. Additionally, TNF-α temporarily facilitates permeability of the BTB during spermatogenesis to germ cell migration [28] and it has been reported that TNF-α inhibits the expression of claudin-11 in Sertoli cells [29]. Therefore, based on these studies, it can be explained, how an increase in expression of inflammatory cytokines, including TNF-α, IL-1β and IL-6, has caused a decrease in BTB proteins expression including occludin, claudin-11 and connexin-43 in the COVID-19 group. Another study has reported that the levels of pro-inflammatory chemokines and cytokines, including IL-6, TNF-α, and MCP-1, play a noteworthy role in the immunopathology of SARS-CoV-2, in semen samples of COVID-19 patients increased [30]. However, one of the mechanisms underlying COVID-19-associated barrier disruption is increasing the susceptibility of testicular cells to interactions with other immune cells, leading to increased infiltration across the barrier 31). The rapid proliferation of the virus upon arrival may lead to the secretion of immune cells and apoptosis of endothelial cells in the testes with subsequent release of inflammatory biomarkers including cytokines, chemokines, and adhesion molecules [32]. Also, inflammatory cytokines including TNF-α, IL-1β may cause oxidative stress in Sertoli cells and alter BTB integrity [33].
On the other hand, based on previous studies, connexin-43, the predominant gap junction protein in the testis, is necessary for tight junction reassembly at the BTB [34] and is essential for the initiation and maintenance of spermatogenesis [35]. Furthermore, reduced expression of the gap junction protein connexin-43 can lead to damage to BTB. Tight junction proteins, such as JAM-A, claudin-11, occludin, ZO-1, and N-cadherin, play a key role in keeping the integrity of the structure and the stability of the cell's internal environment. The present study showed that COVID-19 infection disrupts BTB permeability and spatial arrangement by reducing the expression of junctional molecules, which is in agreement with the fact that tight junctions play a critical role in the junctions between Sertoli cells. Additionally, the current study is the first to do an investigation to evaluate the spatial arrangement of testicular cells using second-order stereology and Voronoi tessellation test by diminished populations of testicular cells in the testes after infection. [31–33], suggests that there is a correlation between the disruption of the spatial arrangement of the testicular cells and SARS-CoV-2. The results of this study indicate that Sertoli cells are damaged, in patients infected by SARS-CoV-2. The plots of the pair-correlation function of Sertoli cells indicated a wide range of gaps in the spatial distribution of Sertoli cells in the COVID-19 group. Our Voronoi tessellation findings also showed that changes in the spatial arrangement of testicular cells as well as we observed testicular cells were randomly distributed in the control and COVID-19 groups. The spatial arrangement of the cell is highly associated with cell-to-cell interactions. Thus, these events lead to the loss of a spermatogenic cell in the testes and will undoubtedly change the spatial pattern of the Sertoli and spermatogenic cells. The number of Sertoli cells determines efficient spermatogenesis and directs correlation of the number of germ cells with the number of functional Sertoli cells. Decreasing the number of Sertoli cells and subsequent changes in the spatial arrangement of the cells disrupt the structure and function of BTB and lead to impaired spermatogenesis. In this study, we observed that the coronavirus can disrupt the BTB. Given that coronavirus is an emerging virus that has recently negatively affected the lives of many people around the world, we still have to wait for more extensive research to better identify the chronic complications of this disease. In conclusion, the present study demonstrated that SARS-CoV-2 could induce the up-regulation of the pro-inflammatory cytokine and down-regulation of junctional proteins that cause a disruption of BTB and impairment of spermatogenesis. This implies the importance of applying further care in reproductive health in men infected by SARS-CoV-2. Screening of these patients is necessary to look over the reversibility of these impacts and improve standard therapeutic strategies to aid these patients.
Acknowledgements
This work has been performed at the School of Medicine, Shahid Beheshti University of Medical Science (Registration No: 1399. 26274). The present article is supported by “Research Department of the School of Medicine Shahid Beheshti University of Medical Sciences” (Registration No, 26274). We would like to express our special thanks to the forensic center of Tehran, Iran.
Author contributions
MAA devised the present research, implemented a stereological investigation, and drafted the manuscript. TP presented the clinical information and was involved in the draft of the manuscript. AA, BEF, and SZ implemented the clinical information and conducted statistical analyses. In addition VE and AA accomplished the histological study. Molecular examination and immunohistochemistry study was also performed by NM, SA, AR, and MKG. Prepare tissue and molecular autopsy sample was also performed by MF, MG, and GRM. Each of the authors read and confirmed the resulting paper.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica: Atenei Parmensis. 2020;91(1):157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciotti M, Ciccozzi M, Terrinoni A, Jiang W-C, Wang C-B, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57(6):365–388. doi: 10.1080/10408363.2020.1783198. [DOI] [PubMed] [Google Scholar]
- 3.Olaniyan OT, Dare A, Okotie GE, Adetunji CO, Ibitoye BO, Bamidele OJ, et al. Testis and blood-testis barrier in Covid-19 infestation: role of angiotensin-converting enzyme 2 in male infertility. J Basic Clin Physiol Pharmacol. 2020;31(6). [DOI] [PubMed]
- 4.Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:100604. doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vishvkarma R, Rajender S. Could SARS-CoV-2 affect male fertility? Andrologia. 2020;52(9):e13712. doi: 10.1111/and.13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 7.Mruk DD, Cheng CY. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. 2015;36(5):564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopera IA, Bilinska B, Cheng CY, Mruk DD. Sertoli–germ cell junctions in the testis: a review of recent data. Philos Trans R Soc B: Biol Sci. 2010;365(1546):1593–1605. doi: 10.1098/rstb.2009.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mruk DD, Cheng C. Tight junctions in the testis: new perspectives. Philos Trans R Soc B: Biol Sci. 2010;365(1546):1621–1635. doi: 10.1098/rstb.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pointis G, Gilleron J, Carette D, Segretain D. Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos Trans R Soc B: Biol Sci. 2010;365(1546):1607–1620. doi: 10.1098/rstb.2009.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;43:203. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuppe H-C, Meinhardt A. Immunology of gametes and embryo implantation. Karger Publishers; 2005. Immune privilege and inflammation of the testis; pp. 1–14. [DOI] [PubMed] [Google Scholar]
- 13.Pascolo L, Zito G, Zupin L, Luppi S, Giolo E, Martinelli M, et al. Renin angiotensin system, COVID-19 and male fertility: any risk for conceiving? Microorganisms. 2020;8(10):1492. doi: 10.3390/microorganisms8101492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed M, Howard C, De Yanés GS. One-stop stereology: the estimation of 3D parameters using isotropic rulers. J Micros. 2010;239(1):54–65. doi: 10.1111/j.1365-2818.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Lou D. Rising concern on damaged testis of COVID-19 patients. Urology. 2020;142:42. doi: 10.1016/j.urology.2020.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abobaker A, Raba AA. Does COVID-19 affect male fertility? World J Urol. 2020;39:1–2. doi: 10.1007/s00345-020-03208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9(4):920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta PK, Liu F, Fischer T, Rappaport J, Qin X. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10(16):7448. doi: 10.7150/thno.48076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younis JS, Abassi Z, Skorecki K. Is there an impact of the COVID-19 pandemic on male fertility? The ACE2 connection. American Physiological Society Rockville; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalan R, Bornstein SR, El-Armouche A, Rodionov RN, Markov A, Wielockx B, et al. The ACE-2 in COVID-19: foe or friend? Horm Metab Res. 2020;52(5):257. doi: 10.1055/a-1155-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Carvalho SM, Dutra MF, Vago JP, Lima KM, Galvão I, de Souza-Neto FP, et al. Angiotensin-(1–7) and alamandine promote anti-inflammatory response in macrophages in vitro and in vivo. Mediat Inflamm. 2019;2019:1–14. doi: 10.1155/2019/8146257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee B, Thakur SS. ACE2 as a potential therapeutic target for pandemic COVID-19. RSC Adv. 2020;10(65):39808–39813. doi: 10.1039/D0RA08228G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Illiano E, Trama F, Costantini E. Could COVID-19 have an impact on male fertility? Andrologia. 2020;52:e13654. doi: 10.1111/and.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youssef K, Abdelhak K. Male genital damage in COVID-19 patients: are available data relevant? Asian J Urol 2020. [DOI] [PMC free article] [PubMed]
- 25.Al-Sadi R, Guo S, Ye D, Ma TY. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol. 2013;183(6):1871–1884. doi: 10.1016/j.ajpath.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Yin Y, Wang G, Liu Z, Liu L, Sun F. Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci Rep. 2014;4:4260. doi: 10.1038/srep04260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez CV, Sobarzo CM, Jacobo PV, Pellizzari EH, Cigorraga SB, Denduchis B, et al. Loss of occludin expression and impairment of blood-testis barrier permeability in rats with autoimmune orchitis: effect of interleukin 6 on Sertoli cell tight junctions. Biol Reprod. 2012;87(5):122. doi: 10.1095/biolreprod.112.101709. [DOI] [PubMed] [Google Scholar]
- 28.Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, et al. Tumor necrosis factor α reversibly disrupts the blood–testis barrier and impairs Sertoli–germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190(2):313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 29.Hellani A, Ji J, Mauduit C, Deschildre C, Tabone E, Benahmed M. Developmental and hormonal regulation of the expression of oligodendrocyte-specific protein/claudin 11 in mouse testis. Endocrinology. 2000;141(8):3012–3019. doi: 10.1210/endo.141.8.7625. [DOI] [PubMed] [Google Scholar]
- 30.Renu K, Subramaniam MD, Chakraborty R, Haritha M, Iyer M, Bharathi G, et al. The role of Interleukin-4 in COVID-19 associated male infertility—a hypothesis: running title: IL-4 and its role in COVID-19 associated male infertility. J Reprod Immunol. 2020;142:103213. doi: 10.1016/j.jri.2020.103213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Q, Wang B, Mao J. Cytokine storm in COVID-19 and treatment. J Infect 2020. [DOI] [PMC free article] [PubMed]
- 33.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 is critical to maintain the homeostasis of the blood–testis barrier via its effects on tight junction reassembly. Proc Natl Acad Sci. 2010;107(42):17998–18003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pointis G, Segretain D. Role of connexin-based gap junction channels in testis. Trends Endocrinol Metab. 2005;16(7):300–306. doi: 10.1016/j.tem.2005.07.001. [DOI] [PubMed] [Google Scholar]