Objective
Pregnant women affected with a severe SARS-CoV-2 infection have worse clinical outcomes than nonpregnant women with SARS-CoV-2, which can include the greater risks for admission to the intensive care unit, the use of invasive mechanical ventilation, the need for extracorporeal membrane oxygenation, and death. In addition, SARS-CoV-2 infection is a risk factor for fetal death and preterm birth. Early during the COVID-19 pandemic, a preeclampsia-like syndrome was reported in pregnant women with SARS-CoV-2.1 This association has been confirmed by case series,2 systematic reviews, and meta-analyses.3 An important issue is whether COVID-19 causes preeclampsia. One of the Bradford Hill criteria to assess causality is the existence of a dose-response relationship between an exposure and the outcome of interest, which, in this case, is the severity of the SARS-CoV-2 infection and the likelihood of preeclampsia. This study was conducted to address this question.
Study Design
A retrospective observational study was conducted based on data from 14 National Health Service (NHS) maternity hospitals in the United Kingdom to assess the effects of SARS-CoV-2 infection in pregnancy. The institutions are listed as a footnote in the Supplemental Table. This study was considered exempt by the Institutional Review Board of the NHS Health Research Authority.
At each participating site, the electronic patient records were reviewed to identify cases with a diagnosis of SARS-CoV-2 in pregnant women based on a positive polymerase chain reaction test between February 1, 2020 and May 1, 2021. The maternal demographic characteristics, medical history, and pregnancy outcomes (ie, live birth or pregnancy loss, gestational age at delivery, birthweight, hypertensive disease in pregnancy, and dates of onset) were obtained from the hospital databases.
Individual patient records were reviewed for relevant information about SARS-CoV-2 infection and classified into 4 groups according to severity based on a modified spectrum used by the National Institutes of Health. The 4 groups are as follows: (1) asymptomatic—this group includes individuals who test positive for SARS-CoV-2 but who have no symptoms; (2) mild illness—includes individuals who have any of the various signs and symptoms of COVID-19 (such as fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, and loss of taste and smell) but who do not have shortness of breath, dyspnea, or abnormal chest imaging; (3) moderate illness—includes individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO2) ≥94% on room air; and (4) severe illness—includes individuals who require high dependency or intensive care secondary to respiratory impairment or failure or multiorgan dysfunction.
The primary outcome was the occurrence of preeclampsia in patients exposed to SARS-CoV-2. The other outcomes examined include preterm birth and gestational age at delivery. Preeclampsia was defined as hypertension (blood pressure ≥140 mm Hg/≥90 mm Hg) developing after 20 weeks of gestation in a previously normotensive woman or as chronic hypertension and development of new onset proteinuria (≥300 mg per 24 h or protein to creatinine ratio >30 mg/mmoL or >2 + on dipstick testing).
The effect of the severity of infection with SARS-CoV-2 (four group factor: asymptomatic, mild, moderate, or severe) on the rate of preeclampsia was assessed using robust Poisson regression models. Models were fit using the geepack package in the R statistical language and environment (www.r-project.org; R Foundation, Vienna, Austria). The asymptomatic group was used as a reference, and the model included adjustment for the prior risk of preeclampsia (log thereof), as defined, based on the maternal characteristics and medical history by using a competing risk model.4 We compared the risk of preeclampsia in the combined groups of moderate and severe COVID-19 patients against the risk in the groups of asymptomatic and mild disease patients. The effect of the severity of infection with SARS-CoV-2 on preterm birth (<37 weeks) was evaluated while adjusting for the maternal age, weight, height, race, method of conception, chronic hypertension, smoking, and diabetes. The selection of these variables was performed by backward elimination. A chi-square test for the trend was used to test the dose-response relationship between the severity of the SARS-CoV-2 infection and preeclampsia or preterm birth.
Results
The characteristics of patients included in this study (n=1223) are presented in the Supplemental Table. Of these, 51 (4.2%) had preeclampsia, 16 (1.3%) had a miscarriage, and 215 (17.6%) had preterm birth; there were 10 (0.81%) fetal deaths. Women with severe COVID-19 tended to be older and had a higher body mass index (P<.05 for both) (Supplemental Table). Of the 51 cases of preeclampsia, 21 were diagnosed before SARS-CoV-2 infection, 7 diagnosed at the same gestational age, and 23 diagnosed after SARS-CoV-2 infection. The 21 cases of preeclampsia diagnosed before SARS-CoV-2 infection were removed from further analysis. The median interval from SARS-CoV-2 infection to diagnosis among the 23 cases of preeclampsia diagnosed after the onset of a SARS-CoV-2 infection was 16 days (interquartile range, 7–61 days). Among the 30 cases included in the analysis, 13 had preterm preeclampsia (<37 weeks) and 17 had term preeclampsia.
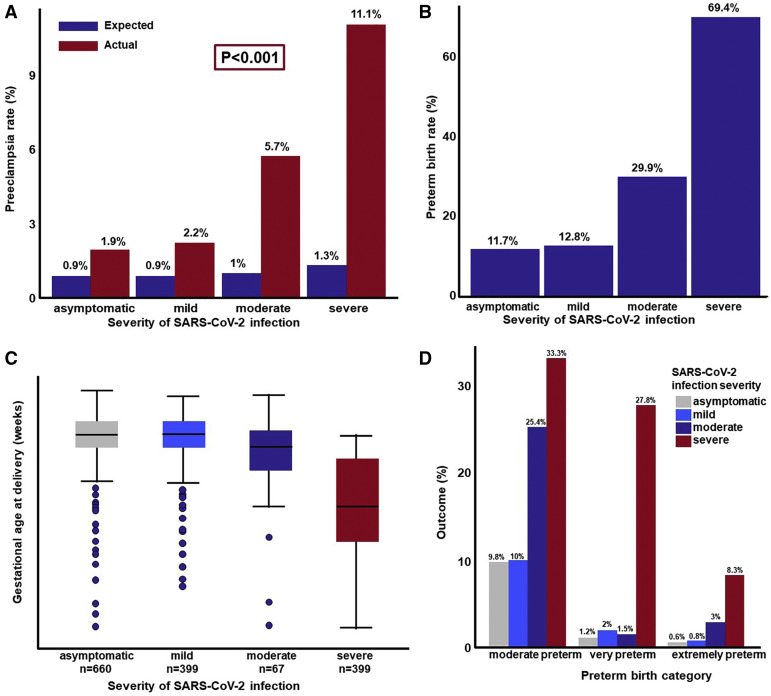
The prior risk of preeclampsia in a cohort of patients with comparable risk factors as those of the study population was approximately 1% (Figure , A). The observed rate of preeclampsia, after excluding the cases diagnosed before SARS-CoV-2 infection, was higher than expected: 1.9% in asymptomatic patients, 2.2% in patients with mild COVID-19, 5.7% with moderate disease, and 11.1% among patients with severe disease (Figure, A). This monotonic relationship between the severity of COVID-19 and the risk of developing preeclampsia was statistically significant (chi-square test for trend; P=.0017). We then compared the risk of preeclampsia between asymptomatic patients (reference group) and patients with COVID-19 symptoms while adjusting for differences in the prior risk of preeclampsia, as determined by the competing risk model. Severe COVID-19 disease was associated with a higher risk of preeclampsia (adjusted risk ratio [RR], 4.9; 1.56–15.38). There was a higher risk for patients with moderate or severe COVID-19 diagnosis as compared to those with asymptomatic or mild disease (adjusted RR, 3.3; 1.48–7.38).
Figure.
Association between SARS-CoV-2 infection severity and pregnancy outcomes
A, Expected and observed rates of preeclampsia in women with SARS-CoV-2 infection. B, Observed rates of preterm birth in women with SARS-CoV-2 infection who had a live neonate. C, Gestational age at delivery in women with SARS-CoV-2 infection who had a live neonate. D, Rate of moderate, very, and extreme preterm birth as a function of the severity of SARS-CoV-2 infection.
Lai. SARS-CoV-2, preeclampsia, and preterm birth. Am J Obstet Gynecol 2021.
Because others have proposed that preeclampsia predisposes a patient to COVID-19, we assessed this hypothesis within our dataset. We included in this analysis all women who developed preeclampsia before SARS-Cov2 and those who did not develop preeclampsia. We found a trend toward an increased risk of developing moderate or severe COVID-19 after a diagnosis of preeclampsia (unadjusted RR, 2.28; 0.92–5.61) (P=.07), (adjusted RR, 1.96; 0.8–4.84) (P=.14).
Moreover, we examined the relationship between the severity of COVID-19 and the rate of preterm birth; we excluded those who did not have a live birth (n=1162) from the data set. The rate of preterm birth was 11.7% in asymptomatic patients, 12.8% in patients with mild COVID-19, 29.9% in patients with moderate COVID-19, and 69.4% in patients with severe COVID-19 (Figure, B). Similarly, the risk of preterm birth increased as a function of the severity of SARS-CoV-2 (chi-square for trend, P<.0001). Compared to asymptomatic patients, women with moderate or severe disease had a higher risk of preterm birth (moderate: adjusted RR, 2.47, 1.61–3.78; and severe: adjusted RR, 5.64, 4.09–7.79). Moreover, there was a dose-response relationship between the gestational age at delivery and the severity of SARS-CoV-2 infection. (Figure, C). The mean gestational age at delivery was significantly earlier in women with moderate or severe SARS-CoV-2 infection than in those who were asymptomatic (asymptomatic: 38.7, moderate 37.5, severe 33 weeks, P<.001 for both comparisons). The risk of moderate (32 to <37 weeks), very preterm (28 to <32 weeks), and extreme (<28 weeks) preterm birth increased as a function of the severity of the SARS-CoV-2 infection (chi-square for trend, P<.0001 for each) (Figure, D).
Conclusion
The principal finding here is that there is a dose-response relationship between the severity of SARS-CoV-2 infection and the risk of subsequent development of preeclampsia and preterm birth. This conclusion is based on a large number of pregnant patients who tested positive for SARS-CoV-2 and a calculation of the individualized risk of preeclampsia and preterm birth for each patient based on maternal characteristics and obstetrical history. Patients with severe COVID-19 have a 5-fold greater risk of preeclampsia than asymptomatic patients. Moreover, the relative risk of developing preeclampsia in women with moderate or severe COVID-19 was 3.3-fold higher than in those with asymptomatic or mild infection. Notably, this estimate of relative risk was higher than the 1.96 estimate obtained when testing the reverse hypothesis that preeclampsia causes moderate or severe COVID-19 reported by other authors.5 Our findings are consistent with those reported by Metz et al2 in a cohort of 1219 patients and with those of a systematic review and meta-analysis, which found that patients with symptomatic illness (odds ratio [OR], 2.11; 95% confidence interval [CI], 1.59–2.81) were more likely to be diagnosed with preeclampsia than those who were asymptomatic (OR, 1.59; 95% CI, 1.21–2.10).3
There was a dose-response relationship between the severity of SARS-CoV-2 infection and the risk of spontaneous preterm birth (P<.0001). This is consistent with other reports. We have no information about the relative contribution of medically indicated preterm birth vs spontaneous preterm birth. The fact that 43% (13/30) of the cases of preeclampsia diagnosed after SARS-CoV-2 infection were preterm preeclampsia (<37 weeks) suggests that COVID-19 may be a cause of medically indicated preterm birth, which contributes to the excess preterm birth delivery rate reported previously.6
This study was designed to examine whether the relationship between SARS-CoV-2 and preeclampsia or preterm birth is causal. Nonetheless, we observed the cases in which preeclampsia preceded infection with SARS-CoV-2. Whether preeclampsia can predispose a patient to COVID-19 in some cases, or that the 2 conditions may co-occur because they share similar risk factors, requires further investigation.5
In conclusion, we present evidence that the more severe the infection with SARS-CoV-2, the greater the risk of preeclampsia and preterm birth. SARS-CoV-2 infection can lead to endothelial dysfunction, intravascular inflammation, proteinuria, activation of thrombin, and hypertension, which are all features of preeclampsia. Therefore, a causal relationship must be considered.
Footnotes
The authors report no conflict of interest.
This research was supported by a grant from the Fetal Medicine Foundation (charity no: 1037116). This research was supported, in part by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and in part with Federal funds from NICHD/NIH/DHHS under contract no. HHSN275201300006C.
R.R. has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Supplementary Material
Supplemental Table.
Demographic and clinical characteristics of the study population
| Demographics | Severity of SARS-CoV-2 infection |
|||
|---|---|---|---|---|
| Asymptomatic (n=696) | Mild (n=417) | Moderate (n=72) | Severe (n=38) | |
| Age (y) | 30.7 (26.4–34.9) | 31.6 (27.5–35.4)a | 32.6 (27.1–35.9)a | 32.8 (30.3–35.1)a |
| Weight (kg) | 69 (60–79) | 70 (61–81)a | 72 (62.5–83.8) | 80.5 (71.2–92.8)b |
| Height (cm) | 162 (157–166) | 163 (159–168)b | 162.5 (157–168) | 162 (160–165) |
| BMI (kg/m2) | 26.5 (23.4–30.4) | 26.3 (23.2–30.4) | 26.8 (24.5–30.9) | 30.1 (27.4–35.5)b |
| Race | ||||
| White | 342 (49.1) | 208 (49.9) | 22 (30.6)b | 14 (36.8) |
| Black | 99 (14.2) | 90 (21.6)b | 22 (30.6)b | 9 (23.7) |
| South Asian | 238 (34.2) | 97 (23.3)b | 25 (34.7) | 12 (31.6) |
| East Asian | 7 (1) | 6 (1.4) | 1 (1.4) | 0 (0) |
| Mixed | 10 (1.4) | 16 (3.8)a | 2 (2.8) | 3 (7.9)a |
| Diabetes | ||||
| None | 680 (97.7) | 412 (98.8) | 71 (98.6) | 36 (94.7) |
| Type I | 6 (0.9) | 2 (0.5) | 0 (0) | 0 (0) |
| Type II | 10 (1.4) | 3 (0.7) | 1 (1.4) | 2 (5.3) |
| History of preeclampsia | ||||
| Multiparous no PE | 413 (59.3) | 245 (58.8) | 41 (56.9) | 23 (60.5) |
| Multiparous PE | 11 (1.6) | 10 (2.4) | 2 (2.8) | 1 (2.6) |
| Nulliparous | 272 (39.1) | 162 (38.8) | 29 (40.3) | 14 (36.8) |
| Twins | 6 (0.9) | 6 (1.4) | 0 (0) | 1 (2.6) |
| Smoker | 51 (7.3) | 16 (3.8)a | 2 (2.8) | 1 (2.6) |
| Chronic hypertension | 13 (1.9) | 11 (2.6) | 1 (1.4) | 1 (2.6) |
| Conception method | ||||
| Spontaneous | 684 (98.3) | 406 (97.4) | 69 (95.8) | 38 (100) |
| In vitro fertilization | 12 (1.7) | 11 (2.6) | 3 (4.2) | 0 (0) |
| Birthweight (g) | 3210 (2825–3545) | 3268 (2900–3580) | 3090 (2520–3490) | 2320 (1657.5–2850)b |
| Preterm birth | 100 (14.4) | 66 (15.8) | 23 (31.9)b | 26 (68.4)b |
| Preeclampsia | 25 (3.6) | 14 (3.4) | 6 (8.3) | 6 (15.8)b |
| Fetal death | 7 (1) | 2 (0.48) | 1 (1.4) | 0 (0) |
| Miscarriage | 8 (1.2) | 7 (1.7) | 1 (1.4) | 0 (0) |
Continuous data are presented as median (interquartile range) whereas categorical data as number (percentage).
Differences between symptomatic groups and asymptomatic patients were assessed using t tests for continuous variables and Fisher exact test for categorical variables.
There were 11 hospitals in London (Kings College Hospital, Princess Royal Hospital, St Thomas’ Hospital, St Mary’s Hospital, Northwick Park Hospital, Homerton Hospital, The Royal London Hospital, Whipps Cross Hospital, Newham Hospital, Queen Elizabeth Hospital, Lewisham Hospital) and 3 in Birmingham (Birmingham Heartlands Hospital, Queen Elizabeth Hospital Birmingham and Good Hope Hospital).
BMI, body mass index; PE, preeclampsia.
Lai. SARS-CoV-2, preeclampsia, and preterm birth. Am J Obstet Gynecol 2021.
Significance P<.05 is denoted with respect to the asymptomatic group
Significance P<.01 is denoted with respect to the asymptomatic group.
References
- 1.Mendoza M., Garcia-Ruiz I., Maiz N., et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127:1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metz T.D., Clifton R.G., Hughes B.L., et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conde-Agudelo A., Romero R. SARS-COV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.07.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright D., Syngelaki A., Akolekar R., Poon L.C., Nicolaides K.H. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am J Obstet Gynecol. 2015;213:62.e1–62.e10. doi: 10.1016/j.ajog.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Papageorghiou A.T., Deruelle P., Gunier R.B., et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289.e1–289.e17. doi: 10.1016/j.ajog.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Perez O., Prats Rodriguez P., Muner Hernandez M., et al. The association between SARS-CoV-2 infection and preterm delivery: a prospective study with a multivariable analysis. BMC Pregnancy Childbirth. 2021;21:273. doi: 10.1186/s12884-021-03742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]