Abstract
Diabetic kidney disease (DKD) accounts for about half of individuals entering end-stage renal disease programs. Patients with DKD frequently have associated microvascular complications and are at very high risk for developing macrovascular complications. Comprehensive treatment involves slowing or preventing the decline in glomerular filtration rate (GFR) and preventing macrovascular and further microvascular complications. Maintaining an A1C <6.5% represents primary prevention; in established DKD, tight blood pressure control is essential. ACE inhibitors/angiotensin receptor blockers (ARBs) and sodium–glucose cotransporter 2 (SGLT2) inhibitors can be used in combination to slow the rate of decline in GFR. This article reviews the general approach to DKD treatment and summarizes renal outcomes in four cardiovascular outcomes trials of SGLT2 inhibitors. Together, these trials provide conclusive evidence that SGLT2 inhibitors, added to an ACE inhibitor or ARB, slow the progression of DKD.
Diabetic kidney disease (DKD) is rising worldwide; from 1997 to 2013, the years of life lost to chronic kidney disease (CKD) increased by 90%, compared with a 67% increase for years of life lost to diabetes (1). DKD is the leading cause of end-stage renal disease (ESRD) in the United States and worldwide, accounting for ∼50% of patients entering renal replacement programs (2,3). Unlike diabetic retinopathy, the prevalence of DKD has remained unchanged for the past 30 years (4). The cost of treating CKD and ESRD in the United States is $84 billion, or ∼20% of the Medicare budget (5). In patients with or without diabetes, the incidence of atherosclerotic cardiovascular disease (ASCVD) rises progressively with increasing albuminuria (6) and declining glomerular filtration rate (GFR) (7), adding to the burden of care for patients with DKD. Approximately 30% of patients with type 1 diabetes and ∼40% of those with type 2 diabetes develop CKD (8), but there is significant ethnic variability, with higher rates in Blacks, Hispanics, and American Indians.
Natural History of DKD
The natural history of DKD is shown in Figure 1 (9,10). Hyperfiltration (increased GFR), increased intraglomerular pressure, and glomerular hypertrophy (9–11) are present in the early stages of DKD in many, but not all, patients with diabetes and in some, but not all, studies have been found to predict the progression to late-stage renal disease in both type 1 diabetes (12,13) and type 2 diabetes (14). In a large, prospective study involving 1,388 kidney donors, a high single-nephron GFR (resulting from intraglomerular hypertension) was shown to be associated with biopsy-proven large glomeruli and glomerulosclerosis (15), providing support for glomerular hypertension and hypertrophy in the pathogenesis of DKD.
FIGURE 1.
Natural history of the development of DKD.
Despite the development of glomerulosclerosis, basement membrane thickening, increased mesangial matrix material, and tubule-interstitial disease, there are no clinical or laboratory clues that predict who is at risk for the development of DKD. In most individuals destined to develop DKD, the first laboratory evidence of renal disease is the development of microalbuminuria (30–300 mg/dL), followed ∼5 years later by the onset of overt albuminuria (>300 mg/day) (Figure 1). Within 5 years of the onset of macroalbuminuria, serum creatinine will have doubled, and within another 4–5 years, individuals will have progressed to ESRD and require dialysis or transplantation.
Two caveats need to be emphasized about albuminuria. First, in type 2 diabetes, microalbuminuria is a much stronger predictor of ASCVD than it is of DKD (16,17). Second, ∼20% of patients with diabetes progress to ESRD without albuminuria (18). Thus, urine albumin excretion lacks both the sensitivity and the specificity to detect early DKD, and novel markers to identify progressors are needed.
Pathogenesis of DKD
Multiple factors have been implicated in the development and progression of DKD (Table 1) (19). Hyperglycemia is the most important factor, and, if the A1C remains within the normal range (≤5.7%), DKD does not occur. Microalbuminuria is present in ∼15–20% of patients with prediabetes (A1C 5.8–6.4%) (20), but it is unknown how many of these individuals progress to advanced DKD as long as their A1C remains in the prediabetic range. When A1C rises above 7.0%, there is a gradual but increasingly steep rise in the incidence of microalbuminuria (21). The importance of good glycemic control in preventing diabetic microvascular complications has been underscored by the landmark Diabetes Control and Complications Trial (22) in type 1 diabetes, the UK Prospective Diabetes Study (23) in type 2 diabetes, and many subsequent studies.
TABLE 1.
Pathogenic Factors in the Development of DKD and Potential Renal Protective Mechanisms by Which SGLT2 Inhibitors Can Slow the Progressive Decline in GFR in Patients With Established DKD
| Etiological Factor | Potential SGLT2 Inhibitor Benefit? |
|---|---|
| Hyperglycemia | Yes* |
| Hypertension | Yes |
| Deranged tubuloglomerular feedback | Yes |
| Tubular hypertrophy/growth factors | Unknown |
| Renal hypoxia | Possible† |
| Podocyte injury/albumin toxicity | Possible† |
| Lipotoxicity | Possible† |
| Inflammation/reactive oxygen species | Possible† |
| Mitochondrial dysfunction | Unknown |
| Genetics | No |
Strict glycemic control (A1C <6.5%) represents primary prevention of DKD. However, the prevention of worsening of established DKD by SGLT2 inhibition is unrelated to the reduction in plasma glucose concentration and can be best appreciated in patients with diabetes with a GFR <45 mL/min/1.73 m2, in whom the decline in A1C is quite small.
Some supporting evidence is present in humans or in experimental models of DKD.
Hypertension is the second most important factor that accelerates the progression of DKD (24–26). The American Diabetes Association and the Eighth Joint National Committee recommend a blood pressure <140/90 mmHg in most people with diabetes and <130/80 mmHg in those at high risk for ASCVD and renal disease, whereas the American Heart Association/American College of Cardiology recommend a blood pressure <130/80 mmHg in all individuals with diabetes, with or without kidney disease (27).
Deranged tubuloglomerular feedback plays a central role in the pathogenesis of DKD (28–31). In people with either type 1 or type 2 diabetes, the filtered load of glucose is increased, leading to enhanced glucose along with sodium reabsorption by the sodium–glucose cotransporter 2 (SGLT2) protein in the proximal tubule. This process leads to a reduction in delivery to and absorption of sodium chloride by the macula densa cells. This reduction is perceived by the kidney as a decrease in circulating vascular volume and leads to 1) activation of the local renin-angiotensin system, resulting in efferent arteriolar vasoconstriction, glomerular hypertension, and renal hyperfiltration; and 2) decreased production of adenosine (a potent vasoconstrictor), leading to afferent arteriolar vasodilation, enhanced renal plasma flow, increased intraglomerular pressure, and hyperfiltration (Figure 2). On a long-term basis, the increase in intraglomerular pressure promotes glomerular sclerosis, albuminuria, and decline in GFR.
FIGURE 2.
Hemodynamic basis of diabetic nephropathy based on deranged tubuloglomerular feedback. JGA, juxtaglomerular apparatus; SGLT2i, SGLT2 inhibitor.
In animal models of diabetic nephropathy, tubular hypertrophy precedes the development of glomerular hypertrophy, and inhibition of tubular hypertrophy with ornithine decarboxylase prevents the development of glomerular hypertrophy, glomerular hypertension, glomerulosclerosis, and DKD (32). This observation suggests that tubular hypertrophy, stimulated by increased proximal tubular glucose-sodium reabsorption and/or one of the multiple growth factors (e.g., angiotensin, transforming growth factor-β, insulin-like growth factor 1, platelet-derived growth factor, and vascular endothelial growth factor) that are released by the diabetic kidney, precedes and contributes to the glomerular hypertrophy, which, once established, increases the intraglomerular pressure according to Laplace’s law.
Renal hypoxia has been demonstrated in both human and animal models of diabetes and has been implicated in the development and progression of DKD (33–36). In the diabetic kidney, the increase in renal plasma flow/GFR results in an increased filtered load of sodium and enhanced tubular sodium reabsorption, which requires adenosine triphosphate (ATP) production via the mitochondrial electron transport chain, a process that requires oxygen. Because renal blood flow, and therefore oxygen delivery, to the kidney is limited, a mismatch between supply and demand occurs, leading to renal hypoxia, which promotes a fibrotic response and diabetic nephropathy. The presence of renal hypoxia has been demonstrated using blood oxygenation level–dependent magnetic resonance imaging in humans and oxygen microelectrodes in diabetic animal models as well as by increased renal expression of hypoxia-inducible factor 1α (37).
The podocytes are an integral component of the glomerular filtration barrier. In DKD, podocyte number and attachment to the glomerular basement membrane are reduced (38), allowing albumin to escape into the glomerular filtrate. Albumin is toxic to the kidney and promotes glomerulosclerosis (39). Consistent with this, each 30% decrease in albuminuria reduces the risk of ESRD by ∼27% (40).
“Fatty kidney disease,” or obesity-related glomerulopathy, is a well-established cause of renal injury (41,42). Fatty kidney disease is diagnosed when no other primary nephropathy is evident and is characterized by glomerulomegaly with glomerulosclerosis, mesangial cell proliferation, podocyte loss, tubular hypertrophy, and glomerular hyperfiltration. Lipid deposition is demonstrable within podocytes, mesangial cells, and tubular cells (41–45), and many genes involved in lipid metabolism are differentially expressed (46). Thus, fatty kidney disease closely resembles the histologic changes observed in DKD. Of note, most patients with type 2 diabetes are obese, making it difficult to distinguish between the contribution of lipotoxicity and that of the diabetic state per se to the development of DKD.
Inflammation, increased reactive oxygen species, and mitochondrial dysfunction (47,48) also have been shown to play a role in the development of DKD, but these are likely to be late events triggered by the disturbances described above.
Finally, genetics plays an important role in the development of DKD, as demonstrated by the familial and ethnic clustering of the disease (49). Genome-wide association studies have identified >100 genetic variants associated with DKD in both type 1 and type 2 diabetes (50), but causality has yet to be established.
Treatment of DKD: General Approach
DKD does not occur in the absence of hyperglycemia; therefore, maintaining an A1C <6.5% is the most important treatment for primary prevention of DKD. In patients with established DKD, comprehensive treatment involves prevention of progressive renal disease, macrovascular complications, and further microvascular complications.
After hyperglycemia, hypertension is the single most important factor that accelerates the rate of progression of DKD (24–26). Therefore, maintaining tight blood pressure control—<130/80 mmHg—is essential for slowing the development and progression of DKD (27). A high-protein diet has been shown to accelerate the progression of kidney disease in people with or without diabetes (51), and amino acid infusion increases renal plasma flow and GFR (52) because of an increase in intraglomerular pressure. Therefore, ingestion of a diet high in protein should be avoided. In a large, prospective study, a low-protein diet in patients treated with an ACE inhibitor failed to slow the rate of progression of CKD (53). Therefore, a normal protein intake is recommended in patients with diabetes and renal impairment. Because obesity is associated with lipid deposition within the kidney and glomerulosclerosis (41–44), weight loss should be encouraged (54). Of course, these lifestyle interventions should be instituted in all patients with diabetes, along with strict glycemic control, to prevent the development of DKD.
Until recently, ACE inhibitors and angiotensin receptor blockers (ARBs) have been the pharmacologic mainstay for slowing the progression of DKD (55–57). As discussed previously, activation of the renin-angiotensin system plays a pivotal role in the development and progression of DKD by 1) causing vasoconstriction of the efferent arteriole, with a resultant increase in intraglomerular pressure (Figure 1); 2) its growth-promoting properties, leading to glomerular hypertrophy; and 3) activating proinflammatory and profibrotic pathways. Although inhibition of the renin-angiotensin-aldosterone system (RAAS) reduces hyperfiltration, it does not completely normalize it (58) and does not completely prevent kidney injury (59). Therefore, additional therapeutic interventions are required to stop the progression of DKD. As detailed in Table 1, multiple factors contribute to the development and progression of DKD. It follows, then, that multiple pharmacologic therapies will be required to slow the progression of DKD.
Mineralocorticoid receptor antagonists (MRAs), including spironolactone and eplerenone, reduce albuminuria when conjointly administered with an RAAS blocker in patients with diabetes (60,61), but hyperkalemia has tempered enthusiasm for this combination therapy. In the recent FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) trial (62), the nonsteroidal MRA finerenone was shown to reduce the combined primary end point of CKD progression, kidney failure, or kidney death when added to standard care in patients with chronic DKD. Although not yet approved by the U.S. Food and Drug Administration, this agent, added to ACE inhibitor or ARB therapy, may be an effective combination therapy for retarding the progression of DKD. However, monitoring for hyperkalemia still would be prudent. Although uric acid has been suggested to play a role in the progression of CKD, the recent PERL (Preventing Early Renal Loss in Diabetes) study (63) failed to demonstrate any benefit of uric acid lowering in slowing the rate of decline in GFR.
SLGT2 Inhibitors: Non-Renal Benefits
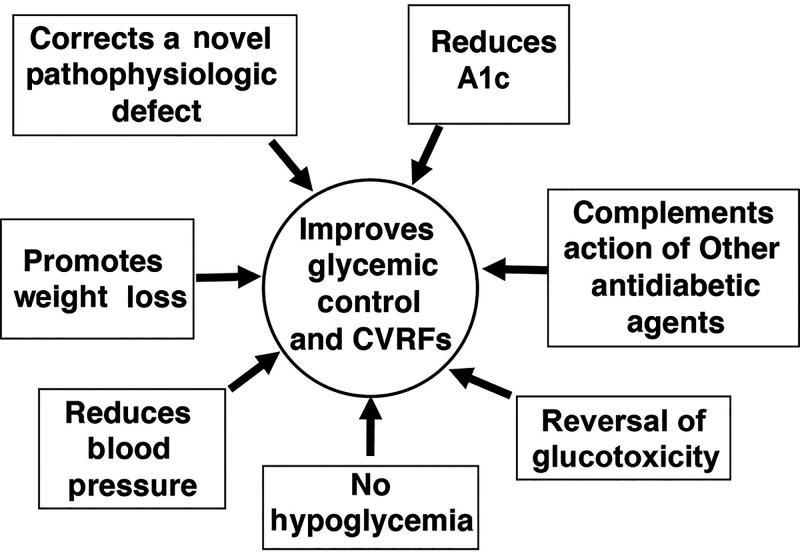
SGLT2 inhibitors have multiple nonrenal benefits that have been reviewed in detail and will be briefly covered here (Figure 3). Currently, there are four approved SGLT2 inhibitors in the United States: empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin.
FIGURE 3.
Benefits (noncardiac and nonrenal) of SGLT2 inhibitors in the treatment of type 2 diabetes. CVRF, cardiovascular risk factor.
The primary mechanism of action of all SGLT2 inhibitors is inhibition of glucose/sodium reabsorption by the SGLT2 transporter in the proximal tubule (64). In type 2 diabetes, the normal renal threshold (180 mg/dL) for glucose spillage into the urine is increased and rises progressively with increasing A1C. Treatment of patients with type 2 diabetes, as well as those with normal glucose tolerance, with an SGLT2 inhibitor decreases the renal threshold for glucose spillage into urine to ∼40 mg/dL, leading to the excretion of ∼70–80 g glucose per day (65,66). Because the renal threshold is reduced to below the fasting plasma glucose concentration (∼80–90 mg/dL) in normal glucose-tolerant subjects, even patients without diabetes respond with a glucosuric response of ∼50–60 g/day. However, hypoglycemia does not occur because the liver reads the amount of glucose lost in the urine and augments its production of glucose to quantitatively match the amount excreted in the urine. Consequently, in patients with type 2 diabetes who are treated with an SGLT2 inhibitor, hypoglycemia does not occur (64,65). Of note, the weight loss and blood pressure–lowering effects of SGLT2 inhibitors are maintained even in individuals with diabetes who have markedly reduced GFR. Furthermore, because of the marked reduction in the renal threshold, there is no such thing as a nonresponder, as long as renal function remains relatively intact (GFR >60 mL/min/1.73 m2). Additionally, because the primary mechanism of action of the SGLT2 inhibitors is on the kidney, they can be used in combination with all other antidiabetic agents to further reduce A1C (63,64).
SGLT2 inhibition reduces both fasting and postprandial plasma glucose concentrations, leading to the reversal of glucotoxicity. This process results in a marked increase in insulin secretion (67,68) and a modest improvement in insulin sensitivity (69). Because calories are lost in the urine, weight loss in the amount of 2–3 kg occurs within the initial 3–6 months of SGLT2 inhibitor therapy (63,64). Because SGLT2 inhibitors block sodium along with glucose absorption, a negative sodium balance of ∼100 mEq and a negative fluid balance of ∼750 cc is observed within the first 48 hours (66), leading to a decrease in systolic/diastolic blood pressure of 4–5/1–2 mmHg (63,64).
SGLT2 Inhibitors: Cardiovascular Benefits
There have been four major cardiovascular outcomes trials (CVOTs) of SGLT2 inhibitors: EMPA-REG OUTCOME (BI 10773 [Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) (70), the CANVAS (Canagliflozin Cardiovascular Assessment Study) Program (71), DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events) (72), and VERTIS CV (Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Assess Cardiovascular Outcomes Following Treatment With Ertugliflozin [MK-8835/PF-04971729] in Subjects With Type 2 Diabetes Mellitus and Established Vascular Disease) (73). In all four trials (70–73), a composite of major adverse cardiovascular events (MACE) was the primary end point. Their results have been summarized in two recent meta-analyses (74,75).
In two CKD trials, CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) (76) and DAPA-CKD (A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease) (77), MACE was a secondary outcome.
In the combined analysis (71,75), MACE was reduced by 12%, cardiovascular death by 17%, and myocardial infarction by 12%. There was no significant reduction in stroke risk in any of the four trials (hazard ratio [HR] 0.96, 95% CI 0.86–1.09). Hospitalization for heart failure (HHF) was reduced by 32%, and the composite end point of cardiovascular death and HHF was reduced by 24%. Heterogeneity for cardiovascular mortality was noted, primarily resulting from the large 38% reduction in cardiovascular death in the EMPA-REG OUTCOME trial. However, it should be noted that 99% of the patients with type 2 diabetes in that trial (70) had a prior cardiovascular event, compared with ∼66% in the CANVAS Program (71) and ∼40% in the DECLARE-TIMI 58 trial (72). Thus, the heterogeneity for cardiovascular mortality most likely was secondary to differences in the patient population (i.e., the percentage of patients with a previous cardiovascular event versus the percentage of patients with cardiovascular risk factors but without a prior event) than to intrinsic differences between the three SGLT2 inhibitors.
In the VERTIS CV trial (73), the MACE end point was not significantly different from placebo (HR 0.97). In the recently published DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trial (78), patients with and without diabetes who had heart failure and reduced ejection fraction experienced a 35% decrease in the primary end point of worsening heart failure or cardiovascular death. Similar findings have been reported with empagliflozin in the EMPEROR-Reduced (Cardiovascular and Renal Outcomes With Empagliflozin in Heart Failure) trial (79).
Collectively, these studies provide conclusive evidence that SGLT2 inhibitors provide protection against cardiovascular death, myocardial infarction, and HHF in patients with type 2 diabetes who have had a prior cardiovascular event (70–73), as well as in those with type 2 diabetes who have risk factors for ASCVD (72). The results of these CVOTs and potential mechanisms for the cardioprotective effects of SGLT2 inhibitors are discussed in more detail in other articles in this Diabetes Spectrum From Research to Practice section (pp. 214–256) and elsewhere (78,80).
SGLT2 Inhibitors: Renal Benefits
The effect of SGLT2 inhibitors on renal function was a secondary outcome in the EMPA-REG OUTCOME trial (70), the CANVAS Program (71), and the DECLARE-TIMI 58 trial (72). A composite renal outcome including doubling of the serum creatinine or >40% sustained reduction in GFR, renal replacement therapy (dialysis or transplantation), and renal death was examined in all three studies and found to be significantly reduced. Furthermore, benefit was demonstrated for each component of the composite outcome individually. In a meta-analysis (75) including all three CVOTs, the renal composite outcome was reduced by 38% (HR 0.62, 95% CI 0.56–0.70, P <0.001) with no heterogeneity among the studies. Although these results are impressive, these studies mainly included subjects with normal to mildly impaired renal function; only a minority (10–25%) had a baseline estimated GFR (eGFR) <60 mL/min/1.73 m2.
The CREDENCE trial (74), which included people with type 2 diabetes and an eGFR of 30–90 mL/min/1.73 m2 or urine albumin-to-creatinine ratio (UACR) of 300–5,000 mg/g, has provided definitive evidence about the renal protective effect of SGLT2 inhibitors. Canagliflozin versus placebo reduced the renal composite outcome (doubling of serum creatinine; sustained decrease in GFR to <15 mL/min/1.73 m2 for ≥30 days; dialysis for ≥30 days or renal transplant; or renal death) by 34% (HR 0.66, 95% CI 0.53–0.81, P <0.001) over a follow-up period of 2.6 years (Figure 4). Furthermore, each component of the primary outcome was significantly reduced individually. When stratified by eGFR or UACR, subjects with an eGFR <60 mL/min/1.73 m2 or UACR >1,000 had the greatest renal benefit. Among 1,000 patients treated for 1.5 years, 22 would need to be treated with canagliflozin to prevent the renal-specific composite outcome.
FIGURE 4.
Effect of canagliflozin and dapagliflozin on the renal-specific composite outcome. Adapted from Refs. 74 and 75.
The results of the DAPA-CKD trial (75) were similar to those of the CREDENCE trial. The renal composite end point (sustained GFR decline >50%, ESRD, or renal death) was decreased by 44% (HR 0.56, 95% CI 0.45–0.68, P <0.001) over a follow-up period of 2.4 years (Figure 4). Each component of the renal composite end point was significantly reduced individually, and patients with or without diabetes benefited similarly.
Of great importance, all patients in the CREDENCE and DAPA-CKD trials were required to be on a stable dose of ACE inhibitor or ARB for at least 4 weeks before randomization. Thus, the renal benefit of canagliflozin and dapagliflozin was in addition to that provided by an ACE inhibitor or ARB. Few patients in these trials had an eGFR <25–30 mL/min/1.73 m2. However, there is no reason to believe that the benefit observed in patients with type 2 diabetes and an eGFR of 30–45 mL/min/1.73 m2 would be any different from that in individuals with an eGFR <30 mL/min/1.73 m2, nor would one expect the side effect profile to be any more adversely affected. Therefore, the authors believe that SGLT2 inhibitor therapy should be maintained until the time a patient is ready for renal replacement therapy. The similar results in the CREDENCE and DAPA-CKD trials support the concept that the renal-protective effect of the SGLT2 inhibitors is a class effect.
Now that two classes of drugs, SGLT2 inhibitors and ACE inhibitors/ARBs, are available for the treatment of DKD (note that these two classes are also cardioprotective), clinicians must consider whether both drugs should be started simultaneously at the time of DKD diagnosis or whether they should be added sequentially. Because of the ominous prognosis for patients with DKD, the authors favor starting both medications simultaneously or within a 2- to 3-week interval until a large prospective study can directly address this question. Another unanswered question is at what level of renal impairment (i.e., microalbuminuria versus macroalbuminuria or eGFR <60 versus >60 mL/min/1.73 m2) should an SGLT2 inhibitor be started to derive the maximum benefit? The authors favor the earlier start, recognizing that the predictive value of microalbuminuria for future development of ESRD is much less than that of macroalbuminuria and that ∼20% of patients with diabetes and DKD do not manifest macroalbuminuria.
SLGT2 Inhibition and Renal Protection: Potential Mechanisms
The mechanisms that contribute to the development of DKD were reviewed above and are the subject of several recent reviews (11,19,81). In patients with type 2 diabetes and an eGFR <45 mL/min/1.73 m2, the glucosuric effect and, therefore, the glucose-lowering effect of SGLT2 inhibitors is markedly attenuated, making improved glycemic control an unlikely explanation for their renal protective effect. Furthermore, it remains to be determined at what level of albuminuria intensive glycemic control fails to retard the progression of established DKD. When high levels of macroalbuminuria (>0.5–1.0 g/day) are present, improved glucose control is unlikely to halt the progression of DKD (82,83), although it may be able to slow progression down (84,85).
Strict glycemic control (A1C <6.5%) is of paramount importance in preventing the onset of DKD (i.e., primary prevention). To the extent that SGLT2 inhibitor therapy helps to normalize A1C in patients with newly diagnosed type 2 diabetes without evident renal disease, it can help prevent the development of DKD.
Hypertension is a key factor that accelerates the progression of DKD. SGLT2 inhibitors routinely reduce systolic/diastolic blood pressure by 4–5/1–2 mmHg (64) and thus could contribute to slowing the progression of DKD. However, the reduction in blood pressure is modest, and factors in addition to blood pressure reduction must play a more central role in the renal protective effect of SGLT2 inhibitors observed in the EMPA-REG OUTCOME, CANVAS Program, DECLARE-TIMI 58, CREDENCE, and DAPA-CKD trials (70–74).
Increased intraglomerular pressure is a well-established pathophysiologic factor in the development of diabetic nephropathy (28–31). SGLT2 inhibitors, by inhibiting sodium along with glucose transport in the proximal tubule, enhance the delivery of sodium to the juxtaglomerular apparatus, where increased sodium absorption by the macula densa cells leads to increased adenosine production, resulting in vasoconstriction of the afferent arteriole, decreased renal plasma flow, and reduced intraglomerular pressure. On a long-term basis, this process provides protection against DKD. This scenario is well established in both animal models of and humans with type 1 diabetes (28–31,86). However, a recent study suggests that, in human type 2 diabetes, the renal hemodynamic effects of SGLT2 inhibitors are caused by post-glomerular (efferent) vasodilation rather than pre-glomerular vasoconstriction (87).
Renal hypoxia is a characteristic feature of the diabetic kidney (31–37,88). Renal oxygen consumption is very high, second only to that of the heart. The hyperfiltering diabetic kidney, in combination with an elevated plasma glucose concentration, markedly increases the filtered load of glucose that is reabsorbed, along with sodium, primarily by the SGLT2 transporter in the proximal tubule by the sodium–potassium adenosine triphosphatase pump, and this requires energy in the form of ATP. Because the kidney has a limited ability to increase renal blood flow, oxygen demand exceeds supply, leading to hypoxia, which can promote DKD. The SGLT2 inhibitors, by blocking glucose–sodium cotransport in the proximal tubule, could possibly lead to a reduction in oxygen demand and prevention of DKD.
Podocytes are an integral component of the glomerular permselectivity barrier, and podocyte loss correlates strongly with albuminuria, which is toxic to the kidney, and with the decline in GFR in humans (89). Lipid accumulation in podocytes, as well as in mesangial and tubular cells, promotes glomerulosclerosis and tubulointerstitial renal disease (41–43). SGLT2 inhibitors cause a switch from glucose to fatty acid oxidation (64,67,68). By increasing fat oxidation in the kidney and reversing lipotoxicity, these drugs would be expected to improve podocyte function, decrease albuminuria, and slow the progression of DKD.
The end product of fatty acid oxidation is ketones, and, not surprisingly, SLGT2 inhibitors also increase the production of ketones (64,68,90). Ketones are freely taken up by the kidney in proportion to their plasma concentration. Furthermore, oxidation of ketones generates more ATP with less oxygen consumption than glucose. This process would reduce renal hypoxia, thereby contributing to the improvement in renal function. Because ketones are avidly taken up by the heart, provide a source of acetyl CoA for the tricarboxcillic acid cycle, and generate more ATP per molecule of oxygen consumed than glucose, a similar mechanism could explain the beneficial effects of the SGLT2 inhibitors on the heart (90).
Although diabetic ketoacidosis is rare in patients with type 2 diabetes who are treated with an SGLT2 inhibitor, it occurs in 3–4% of patients with type 1 diabetes who take an SGLT2 inhibitor (91). Although not approved for these patients in the United States, SGLT2 inhibitors are approved in some European countries for the treatment of patients with type 1 diabetes, and their use should be monitored closely in this group.
Summary
DKD accounts for ∼50% of patients entering ESRD programs. Hyperglycemia is the primary factor responsible for initiating the cascade of pathophysiologic events that result in ESRD. Therefore, tight glycemic control (A1C <6.5%) is essential to prevent the development of diabetic glomerulosclerosis and progressive decline in GFR. ACE inhibitors, ARBs, and MRAs all have been shown to slow the progression of DKD. Most recently, five large prospective studies (the EMPA-REG OUTCOME, CANVAS Program, DECLARE-TIMI 58, CREDENCE, and DAPA-CKD trials) have provided conclusive evidence that SGLT2 inhibitors, when added to ACE inhibitor/ARB therapy, reduce a renal composite outcome including progression to ESRD in patients with type 2 diabetes.
Article Information
Duality of Interest
R.A.D. serves on advisory boards for AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; has received research support from AstraZeneca, Boehringer Ingelheim, Janssen, and Merck; and is a speaker’s bureau member for AstraZeneca and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
Both authors researched data and wrote and edited the manuscript. R.A.D. is the guarantor of this work and, as such, had full access to all the data and takes responsibility for the integrity of this review.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015;385:1975–1982 [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Diabetes and Digestive and Kidney Diseases . U.S. Renal Disease System report. Available from https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/us-renal-data-system-report. Accessed 19 March 2021
- 4.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 2014;370:1514–1523 [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System . Home page. Available from https://www.usrds.org. Accessed 19 March 2021
- 6.Miettinen H, Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects. Stroke 1996;27:2033–2039 [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 8.Nelson RG, Tuttle KR, Bilous RW, et al.; National Kidney Foundation . KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012;60:850–886 [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA. Diabetic nephropathy: etiologic and therapeutic considerations. Diabetes Rev (Alex) 1995;3:510–564 [Google Scholar]
- 10.Tuttle KR, Stein JH, DeFronzo RA. The natural history of diabetic nephropathy. Semin Nephrol 1990;10:184–193 [PubMed] [Google Scholar]
- 11.Lytvyn Y, Bjornstad P, van Raalte DH, Heerspink HL, Cherney DZI. The new biology of diabetic kidney disease: mechanisms and therapeutic implications. Endocr Rev 2020;41:202–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogensen CE. Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand J Clin Lab Invest 1986;46:201–206 [DOI] [PubMed] [Google Scholar]
- 13.Bjornstad P, Cherney DZ, Snell-Bergeon JK, et al. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with type 1 diabetes. Nephrol Dial Transplant 2015;30:1706–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruggenenti P, Porrini EL, Gaspari F, et al.; GFR Study Investigators . Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 2012;35:2061–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denic A, Mathew J, Lerman LO, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med 2017;376:2349–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol 2006;17:2100–2105 [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC, Mann JF, Yi Q, et al.; HOPE Study Investigators . Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001;286:421–426 [DOI] [PubMed] [Google Scholar]
- 18.Klimontov VV, Korbut AI. Albuminuric and non-albuminuric patterns of chronic kidney disease in type 2 diabetes. Diabetes Metab Syndr 2019;13:474–479 [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Reeves B, Awad A. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibition. Nature Nephrol Rev 2021;17:319–334 [DOI] [PubMed] [Google Scholar]
- 20.Markus MRP, Ittermann T, Baumeister SE, et al. Prediabetes is associated with microalbuminuria, reduced kidney function and chronic kidney disease in the general population: the KORA (Cooperative Health Research in the Augsburg Region) F4-Study. Nutr Metab Cardiovasc Dis 2018;28:234–242 [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association . 6. Glycemic targets: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S55–S64 [DOI] [PubMed] [Google Scholar]
- 22.Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 23.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parving H-H, Andersen AR, Smidt UM, Svendsen PA. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet 1983;1:1175–1179 [DOI] [PubMed] [Google Scholar]
- 25.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387:435–443 [DOI] [PubMed] [Google Scholar]
- 26.Patney V, Whaley-Connell A, Bakris G. Hypertension management in diabetic kidney disease. Diabetes Spectr 2015;28:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252–289 [DOI] [PubMed] [Google Scholar]
- 28.Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 2012;74:351–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hostetter TH, Troy JL, Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int 1981;19:410–415 [DOI] [PubMed] [Google Scholar]
- 30.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597 [DOI] [PubMed] [Google Scholar]
- 31.Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron, Physiol 2009;111:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 2001;107:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol 2010;6:667–678 [DOI] [PubMed] [Google Scholar]
- 34.Blantz RC, Deng A, Miracle CM, Thomson SC. Regulation of kidney function and metabolism: a question of supply and demand. Trans Am Clin Climatol Assoc 2007;118:23–43 [PMC free article] [PubMed] [Google Scholar]
- 35.Friederich-Persson M, Thörn E, Hansell P, Nangaku M, Levin M, Palm F. Kidney hypoxia, attributable to increased oxygen consumption, induces nephropathy independently of hyperglycemia and oxidative stress. Hypertension 2013;62:914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol 2011;22:1429–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp Nephrol 2008;110:e1–e7 [DOI] [PubMed] [Google Scholar]
- 38.Tamsma JT, van den Born J, Bruijn JA, et al. Expression of glomerular extracellular matrix components in human diabetic nephropathy: decrease of heparan sulphate in the glomerular basement membrane. Diabetologia 1994;37:313–320 [DOI] [PubMed] [Google Scholar]
- 39.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006;17:2974–2984 [DOI] [PubMed] [Google Scholar]
- 40.Heerspink HJL, Greene T, Tighiouart H, et al.; Chronic Kidney Disease Epidemiology Collaboration . Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 2019;7:128–139 [DOI] [PubMed] [Google Scholar]
- 41.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2011;6:2364–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res 2014;55:561–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chertow GM, Hsu CY, Johansen KL. The enlarging body of evidence: obesity and chronic kidney disease. J Am Soc Nephrol 2006;17:1501–1502 [DOI] [PubMed] [Google Scholar]
- 44.Grove KJ, Voziyan PA, Spraggins JM, et al. Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. J Lipid Res 2014;55:1375–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper ME, Jandeleit-Dahm KA. Lipids and diabetic renal disease. Curr Diab Rep 2005;5:445–448 [DOI] [PubMed] [Google Scholar]
- 46.Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem 2002;277:18919–18927 [DOI] [PubMed] [Google Scholar]
- 47.Pérez-Morales RE, Del Pino MD, Valdivielso JM, Ortiz A, Mora-Fernández C, Navarro-González JF. Inflammation in diabetic kidney disease. Nephron 2019;143:12–16 [DOI] [PubMed] [Google Scholar]
- 48.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787–790 [DOI] [PubMed] [Google Scholar]
- 49.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease: evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 1989;320:1161–1165 [DOI] [PubMed] [Google Scholar]
- 50.Igo RP Jr, Iyengar SK, Nicholas SB, et al.; Family Investigation of Nephropathy and Diabetes Research Group . Genomewide linkage scan for diabetic renal failure and albuminuria: the FIND study. Am J Nephrol 2011;33:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evanoff G, Thompson C, Brown J, Weinman E. Prolonged dietary protein restriction in diabetic nephropathy. Arch Intern Med 1989;149:1129–1133 [PubMed] [Google Scholar]
- 52.Tuttle KR, Bruton JL, Perusek MC, Lancaster JL, Kopp DT, DeFronzo RA. Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N Engl J Med 1991;324:1626–1632 [DOI] [PubMed] [Google Scholar]
- 53.Zeller K, Whittaker E, Sullivan L, Raskin P, Jacobson HR. Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. N Engl J Med 1991;324:78–84 [DOI] [PubMed] [Google Scholar]
- 54.Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant 2013;28(Suppl. 4):iv82–iv98 [DOI] [PubMed] [Google Scholar]
- 55.Lewis EJ, Hunsicker LG, Bain RP; The Collaborative Study Group . The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 56.Barnett AH. Preventing renal complications in diabetic patients: the Diabetics Exposed to Telmisartan And enalaprIL (DETAIL) study. Acta Diabetol 2005;42(Suppl. 1):S42–S49 [DOI] [PubMed] [Google Scholar]
- 57.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 2006;17:1703–1709 [DOI] [PubMed] [Google Scholar]
- 58.de Azevedo MJ, Ramos OL, Gross JL. Lack of effect of captopril on glomerular hyperfiltration in normoalbuminuric normotensive insulin-dependent diabetic patients. Horm Metab Res 1997;29:516–519 [DOI] [PubMed] [Google Scholar]
- 59.Fried LF, Emanuele N, Zhang JH, et al.; VA NEPHRON-D Investigators . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–1903 [DOI] [PubMed] [Google Scholar]
- 60.Morales E, Millet VG, Rojas-Rivera J, et al. Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol Dial Transplant 2013;28:405–412 [DOI] [PubMed] [Google Scholar]
- 61.Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 2006;1:940–951 [DOI] [PubMed] [Google Scholar]
- 62.Bakris GL, Agarwal R, Anker SD, et al.; FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–2229 [DOI] [PubMed] [Google Scholar]
- 63.Afkarian M, Polsky S, Parsa A, et al.; PERL Study Group . Preventing Early Renal Loss in Diabetes (PERL) study: a randomized double-blinded trial of allopurinol: rationale, design, and baseline data. Diabetes Care 2019;42:1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017;13:11–26 [DOI] [PubMed] [Google Scholar]
- 65.DeFronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013;36:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Jobori H, Daniele G, Cersosimo E, et al. Empagliflozin and kinetics of renal glucose transport in healthy individuals and individuals with type 2 diabetes. Diabetes 2017;66:1999–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merovci A, Mari A, Solis-Herrera C, et al. Dapagliflozin lowers plasma glucose concentration and improves β-cell function. J Clin Endocrinol Metab 2015;100:1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 71.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 72.Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE-TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 73.Cannon CP, Pratley R, Dagogo-Jack S, et al.; VERTIS CV Investigators . Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425–1435 [DOI] [PubMed] [Google Scholar]
- 74.Arnott C, Li Q, Kang A, et al. Sodium–glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 77.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 78.McMurray JJV, Solomon SD, Inzucchi SE, et al.; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 79.Packer M, Anker SD, Butler J, et al.; EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424 [DOI] [PubMed] [Google Scholar]
- 80.Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care 2016;39:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muskiet MHA, Wheeler DC, Heerspink HJL. New pharmacological strategies for protecting kidney function in type 2 diabetes. Lancet Diabetes Endocrinol 2019;7:397–412 [DOI] [PubMed] [Google Scholar]
- 82.Tamborlane WV, Puklin JE, Bergman M, et al. Long-term improvement of metabolic control with the insulin pump does not reverse diabetic microangiopathy. Diabetes Care 1982;5(Suppl. 1):58–64 [PubMed] [Google Scholar]
- 83.Ciavarella A, Vannini P, Flammini M, Bacci L, Forlani G, Borgnino LC. Effect of long-term near-normoglycemia on the progression of diabetic nephropathy. Diabete Metab 1985;11:3–8 [PubMed] [Google Scholar]
- 84.Brocco E, Velussi M, Cernigoi AM, et al. Evidence of a threshold value of glycated hemoglobin to improve the course of renal function in type 2 diabetes with typical diabetic glomerulopathy. J Nephrol 2001;14:461–471 [PubMed] [Google Scholar]
- 85.Perkovic V, Heerspink HL, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 2013;83:517–523 [DOI] [PubMed] [Google Scholar]
- 86.Kidokoro K, Cherney DZI, Bozovic A, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 2019;140:303–315 [DOI] [PubMed] [Google Scholar]
- 87.van Bommel EJM, Muskiet MHA, van Baar MJB, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int 2020;97:202–212 [DOI] [PubMed] [Google Scholar]
- 88.Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl 1998;65:S74–S78 [PubMed] [Google Scholar]
- 89.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 1997;99:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016;39:1108–1114 [DOI] [PubMed] [Google Scholar]
- 91.Peters AL, McGuire DK, Danne T, et al. Diabetic ketoacidosis and related events with sotagliflozin added to insulin in adults with type 1 diabetes: a pooled analysis of the inTandem 1 and 2 studies. Diabetes Care 2020;43:2713–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]