Summary
Rapid progress has recently been made regarding how the niche controls stem cell function, but little is yet known about how stem cells in the same niche interact with one another. In this study, we show that differentiation-defective Drosophila ovarian germline stem cells (GSCs) can outcompete normal ones for niche occupancy in a cadherin-dependent manner. The differentiation-defective bam or bgcn mutant GSCs invade the niche space of neighboring wild-type GSCs and gradually push them out of the niche by upregulating E-cadherin expression. Furthermore, the bam/bgcn-mediated GSC competition requires E-cadherin and normal GSC division but not the self-renewal-promoting BMP niche signal, while different E-cadherin levels can sufficiently stimulate GSC competition. Therefore, we propose that GSCs have a competitive relationship for niche occupancy, which may serve as a quality control mechanism to ensure that accidentally differentiated stem cells are rapidly removed from the niche and replaced by functional ones.
Introduction
Stem cells can self-renew to maintain a stable population and generate differentiated cells to replenish lost cells in adult animal tissues, and their self-renewal and proliferation are tightly controlled by signals from their niche (Li and Xie, 2005; Ohlstein et al., 2004). Normally, stem cells reside in the same niche or tissue site and are capable of repopulating the empty niche space left by a lost stem cell (Xie and Spradling, 2000). However, it remains unclear how the stem cells in the same or nearby niche quickly respond to the loss of stem cells and carry out subsequent repopulation of vacant niches. In this study, we provide experimental evidence that the stem cells in the same niche have a competitive relationship, which may provide a quality control mechanism for removing differentiated stem cells from the niche.
Drosophila germline stem cells (GSCs) have become an attractive system for studying stem cell biology, including the niche structure and function and self-renewal mechanisms. In a structure known as the germarium, at the tip of the Drosophila ovary, two or three GSCs can be reliably recognized by their size (the largest germ cells), location (in direct contact with cap cells) and anteriorly anchored spherical spectrosome (SS), and they can be effectively studied at the molecular and cellular level (Lin, 2002; Xie et al., 2005) (Figure 1A and 1B). At the tip of the germarium, somatic cap cells and possibly escort stem cells (ESCs), form the GSC niche (Figure 1A), where GSCs are anchored to the cap cells through E-cadherin-mediated cell adhesion (Song et al., 2002; Xie and Spradling, 2000). Such anchorage is essential for keeping GSCs in their niche for long-term self-renewal since the GSCs defective in E-cadherin-mediated cell adhesion are lost rapidly from the niche (Song et al., 2002). Cystoblasts, the immediate progeny of GSCs, divide synchronously four times with incomplete cytokinesis to form interconnected 16-cell cysts sharing a branched fusome (de Cuevas et al., 1997)(Figure 1B). Spectrosomes and fusomes are germ cell-specific organelles rich in membrane skeleton proteins such as adducin-like Hu li-tai shao (Hts) (Lin et al., 1994). In this study, we used this system to investigate how GSCs in the same niche interact with one another.
Figure 1.
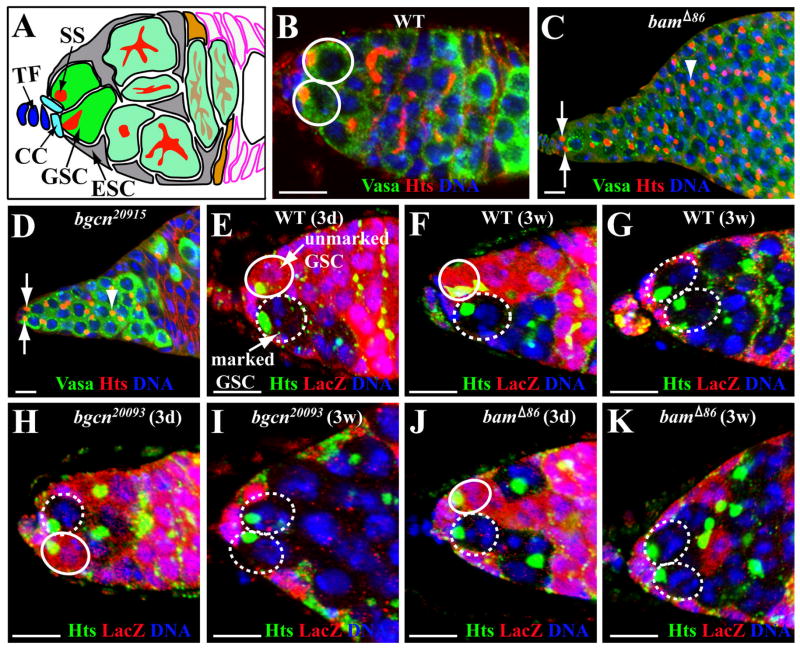
bgcn and bam mutant GSCs are more competitive than wild-type GSCs in occupying the niche. (A) A schematic diagram showing the GSC niche at the tip of the germarium. Two GSCs can be identified based on their location [contacting cap cells (CC) anteriorly and escort stem cells (ESC) laterally and in close to the terminal filament (TF) cells] and anteriorly localized spherical spectrosome (SS). The LacZ-negative marked GSCs and the LacZ-positive unmarked GSCs in E–K are highlighted by broken and solid circles, respectively. (B) A wild-type germarial tip showing two wild-type GSCs (circles). (C, D) Tumorous germarial tips showing two GSCs mutant for bam (C, arrows) or bgcn (D, arrows), and their undifferentiated progeny containing a spectrosome (arrowheads). (E–G) Germarial tips harboring a three-day-old wild-type partial GSC clone (E), a three-week-old wild-type partial GSC clone (F) and a three-week-old wild-type full GSC clone (G). (H, I) Germarial tips carrying a three-day-old partial bgcn20093 mutant clone (H) and a three-week-old bgcn20093 mutant full clone (I). (J, K) Germarial tips carrying a three-day-old partial bamΔ86 mutant clone (J) and a three-week-old bamΔ86 mutant full clone (K). The bars represent 10μm.
GSC self-renewal is primarily controlled by BMPs and piwi-mediated signals from the niche cells (Cox et al., 1998; Cox et al., 2000; Song et al., 2004; Xie and Spradling, 1998), and these signals maintain GSCs by repressing the expression of a differentiation-promoting gene, bag of marbles (bam) (Chen and McKearin, 2003a, 2005; Song et al., 2004; Szakmary et al., 2005). On the other hand, the differentiation of cystoblasts requires bam and bgcn (benign gonial cell neoplasm), since mutations in either bam or bgcn result in the accumulation of spectrosome-containing undifferentiated single germ cells (Lavoie et al., 1999; McKearin and Spradling, 1990; Ohlstein and al., 2000) (Figure 1C and 1D). These differentiation-defective single germ cells resemble GSCs based on their gene expression profiles (Kai et al., 2005). In this study, we show that bam and bgcn also have important functions in GSCs in controlling their relative competitiveness.
In Drosophila imaginal discs, where cell competition is extensively studied, its Drosophila homolog of human proto-oncogene myc (dmyc) regulates the process (de la Cova et al., 2004; Moreno and Basler, 2004). Namely, dmyc mutant cells grow poorly and are outcompeted by their more vigorous wild-type neighboring cells (Johnston et al., 1999), while local dmyc overexpression causes cell competition by inducing the apoptosis of their neighboring wild-type cells (de la Cova et al., 2004). In the Drosophila ovary, dMyc protein is abundantly expressed in the nucleus of both somatic and germ cells throughout oogenesis (Maines et al., 2004). dmyc mutant follicle cells and germ cells exhibit defects in growth and endoreplication (Maines et al., 2004). Although dmyc is highly expressed in the nuclei of GSCs, its role in stem cells has not been established. In this study, we have developed an effective system for studying stem cell competition, and then have shown that stem cells in the same niche have a competitive relationship, which is regulated by bam and bgcn but not dmyc.
Results
bam and bgcn Mutant GSCs Outcompete Wild-Type GSCs for Niche Occupancy
To investigate whether bgcn or bam mutant stem cells can have any competitive advantage over normal stem cells in occupying the niche, we chose bamΔ86, a deletion mutant of bam (McKearin and Spradling, 1990), and two bgcn mutants, bgcn20093 and bgcn20915, which bear a premature stop codon after residues 12 and 857, respectively (this study) to generate niches carrying a marked mutant bam or bgcn GSC and an unmarked wild-type GSC using the FLP-mediated FRT mitotic recombination (Xie and Spradling, 1998). Among one week-old mutant bamΔ86, bgcn20093 or bgcn20915 germaria labeled for Vasa (germ cells) and Hts (spectrosomes/fusomes), the bgcn20093 or bgcn20915 mutants contained only GSC-like single germ cells just like other previously characterized bgcn mutants (Ohlstein and al., 2000), and the bamΔ86 mutant germaria consistently had more GSC-like cells than those bgcn mutant germaria (Figure 1C and 1D), suggesting that the bam mutant GSC-like cells may proliferate faster than the bgcn mutants (Figure 1C and 1D).
Following the FLP/FRT mediated mitotic recombination, the marked GSCs were identified by the absence of arm-lacZ expression, direct contact with cap cells and an anteriorly anchored spectrosome, whereas the unmarked GSCs were identified by the presence of arm-lacZ expression, and direct contact with cap cells and an anteriorly anchored spectrosome (Xie and Spradling, 1998)(Figure 1E–1K). In this study, a “partial clone” is defined as a germarium carrying a mixture of marked and unmarked GSCs (Figure 1E and 1F), whereas a marked “full clone” is a germarium containing only two or three marked GSCs (Figure 1G). In the control, most of the marked GSCs detected 3 days after clone induction (ACI) were still maintained in the germaria 3 weeks ACI (Figure 1E and 1F); 25.2% of them were lost due to natural turnover, which was consistent with previous results (Song et al., 2002; Xie and Spradling, 1998)(Table 1). Since a lost GSC can be efficiently repopulated by the progeny of a neighboring GSC in the same niche (Xie and Spradling, 2000), a partial clone in the germarium carrying only two GSCs will become a full clone after losing the unmarked GSC. Since marked wild-type GSCs should behave like unmarked wild-type GSCs, and a wild-type control germarium usually carries 2.5 GSCs (n=550), we would expect that the increased percentage of the germaria carrying a full clone is equal to about 40% of the percentage of the germaria losing a marked GSC. This agrees well with what we observed for wild-type control clones. From 3 days ACI to 3 weeks ACI, the percentage of the germaria carrying a partial clone decreased from 39.8% to 21.7% (18.1% net decrease), whereas the percentage of the germaria carrying a full clone increased from 1.8% to 9.4% (total 7.6% increase) (Table 1). Since natural GSC turnover is expected to be random, the lost marked wild-type GSCs should be roughly equal to the marked GSCs gained from replacement of the lost unmarked GSCs. Indeed, the percentage of marked GSCs versus total GSCs remained almost constant (without a net increase of the marked wild-type GSCs): 22.3% at 3 days ACI to 20.2% at 3 weeks ACI (Table 1). Together, this result shows that GSCs are an effective system to quantitatively study stem cell competition dynamics in the niche.
Table 1.
Competition between bgcn or bam mutant GSCs and their neighboring wild-type GSCs in the same niche requires E-cadherin-mediated cell adhesion.
| Genotypes | Ages | Percentagea of the germaria carrying a marked full GSC clone | Percentageb of the germaria carrying a marked partial GSC clone | Percentagec of the germaria carrying a marked GSC clone | Percentaged of the GSCs marked | Number of the germaria examined |
|---|---|---|---|---|---|---|
| Control (FRT42D) | 3 d | 1.8% | 39.8% | 41.6% | 22.3% | 113 |
| 1w | 7.6% | 33.8% | 41.4% | 23.6% | 225 | |
| 2w | 5.0% | 29.0% | 34.0% | 19.5% | 221 | |
| 3w | 9.4% | 21.7% | 31.1% | 20.2% | 212 | |
| bgcn20093 | 3 d | 6.6% | 38.3% | 44.9% | 25.1% | 183 |
| 1w | 13.1% | 24.9% | 38.0% | 26.3% | 221 | |
| 2w | 27.6% | 20.8% | 48.4% | 37.6% | 221 | |
| 3w | 32.4% | 10.3% | 42.7% | 37.5% | 213 | |
| bgcn20915 | 3 d | 6.9% | 38.9% | 45.8% | 26.0% | 175 |
| 1w | 12.4% | 37.6% | 50.0% | 33.6% | 226 | |
| 2w | 19.8% | 25.6% | 45.4% | 33.3% | 227 | |
| 3w | 27.1% | 13.8% | 40.9% | 34.9% | 225 | |
| bam Δ 86 | 3 d | 2.2% | 41.9% | 44.1% | 20.8% | 143 |
| 1w | 10.0% | 58.1% | 68.1% | 36.4% | 171 | |
| 2w | 32.4% | 53.7% | 86.1% | 61.6% | 205 | |
| 3w | 58.5% | 26.8% | 85.3% | 72.3% | 231 | |
| bgcn20093shgR69 | 3 d | 6.5% | 20.4% | 26.9% | 15.5% | 186 |
| 1w | 2.3% | 12.3% | 14.6% | 9.4% | 130 | |
| 2w | 9.8% | 5.8% | 15.6% | 12.6% | 173 | |
| 3w | 11.1% | 4.3% | 15.5% | 13.7% | 207 | |
| bgcn20915shg10469 | 3 d | 1.9% | 36.7% | 38.6% | 20.0% | 215 |
| 1w | 12.1% | 35.9% | 48.0% | 31.1% | 231 | |
| 2w | 19.8% | 19.4% | 39.2% | 29.7% | 217 | |
| 3w | 16.1% | 16.1% | 32.2% | 24.5% | 218 | |
| Control (FRT19A nos-gal4) | 1w | 2.9% | 13.3% | 16.3% | 8.4% | 173 |
| 2w | 4.3% | 9.8% | 14.1% | 7.6% | 163 | |
| 3w | 4.1% | 7.1% | 11.2% | 7.4% | 169 | |
| FRT19Anos-gal4; UAS-shg | 1w | 6.1% | 13.9% | 20.0% | 12.3% | 165 |
| 2w | 10.4% | 10.4% | 20.8% | 15.2% | 182 | |
| 3w | 11.8% | 5.6% | 17.4% | 14.2% | 195 |
The percentage of the germaria carrying a marked full GSC clone at a given time point = the number of germaria in which all GSCs are marked/total germaria examined.
The percentage of germaria carrying a partial marked GSC clone at a given time point = the number of germaria in which at least one GSC is not marked and also at least one GSC is marked/total germaria examined.
The percentage of germaria carrying a marked GSC clone at a given time point = the number of germaria carrying a marked full or partial GSC clone/total germaria examined.
The percentage of the GSCs marked = the number of marked GSCs/total GSCs examined.
Interestingly, the percentage of germaria carrying one or more marked bgcn20093 and bgcn20915 GSCs exhibited only a 4.9% and 10.7% reduction 3 weeks ACI, respectively, which is in contrast to a 25.2% reduction of marked wild-type GSCs, indicating that marked bgcn mutant GSCs are lost at a slower rate than marked wild-types (Table 1). The observation that blocking differentiation by bgcn mutations can prolong GSC lifespan also suggests that natural GSC turnover might be due to random differentiation. Moreover, we observed a dramatic increase in the percentage of germaria carrying a marked bgcn20093 or bgcn20915 full clone: from 6.6% at 3 days ACI to 32.4% at 3 weeks ACI for bgcn20093, and from 6.9% at 3 days ACI to 27.1% at 3 weeks ACI for bgcn20915, indicating that bgcn mutant GSCs can somehow replace their neighboring wild-type stem cells and occupy their niches (Figure 1H and 1I; Table 1). Similarly, we also observed a net increase in marked bgcn mutant GSCs: from 25.1% (3 days ACI) to 37.5% (3 weeks ACI) for bgcn20093 mutant GSCs and from 26.0% (3 days ACI) to 34.9% (3 weeks ACI) for bgcn20915 mutant GSCs (Table 1), which is in contrast to no obvious net increase of total marked wild-type GSCs. These results provide direct evidence that bgcn mutant GSCs can outcompete normal GSCs for their niches.
Since bgcn and bam have been proposed to function in the same genetic pathway to control GSC differentiation (Lavoie et al., 1999; Ohlstein and al., 2000), we would expect that bam mutant GSCs behave in the same way as bgcn mutant GSCs in stem cell competition. Surprisingly, the percentage of the germaria carrying a partial or full clone increased beyond the normal range of clone induction rate, from 44.1% at 3 days ACI to 86.1% at 2 weeks ACI (Table 1; Figure 1J and 1K), representing a three-fold net increase in total marked GSCs. The newly gained marked bam mutant GSCs must come from the mutant bam cystoblasts that occupied the niches, which were normally occupied by unmarked wild-type GSCs, indicating that bam mutant cystoblasts can successfully invade the niche occupied by wild-type GSCs and assume GSC identity. Interestingly, during the period from 2 weeks ACI to 3 weeks ACI, the percentage of the germaria carrying a partial marked GSC clone decreased, while the percentage of germaria carrying a full clone showed a dramatic increase, from 32.4% to 58.5%, indicating that bam mutant GSCs are able to outcompete wild-type GSCs for their niches like bgcn mutant GSCs (Figure 1J and 1K; Table 1). As a consequence, there was a dramatic net increase of the marked GSCs (from 61.6% at 14 days ACI to 72.3% at 21 days ACI) (Table 1). All these data indicate that bam mutant GSCs are much more superior to wild-type GSCs in niche competition. However, it remains unclear why mutant bgcn cystoblasts cannot invade the GSC niche as efficiently as the bam mutant cystoblasts.
bam and bgcn Mutant GSCs Outcompete their Neighboring Wild-Type GSCs, but not through Inducing Differentiation and Apoptosis
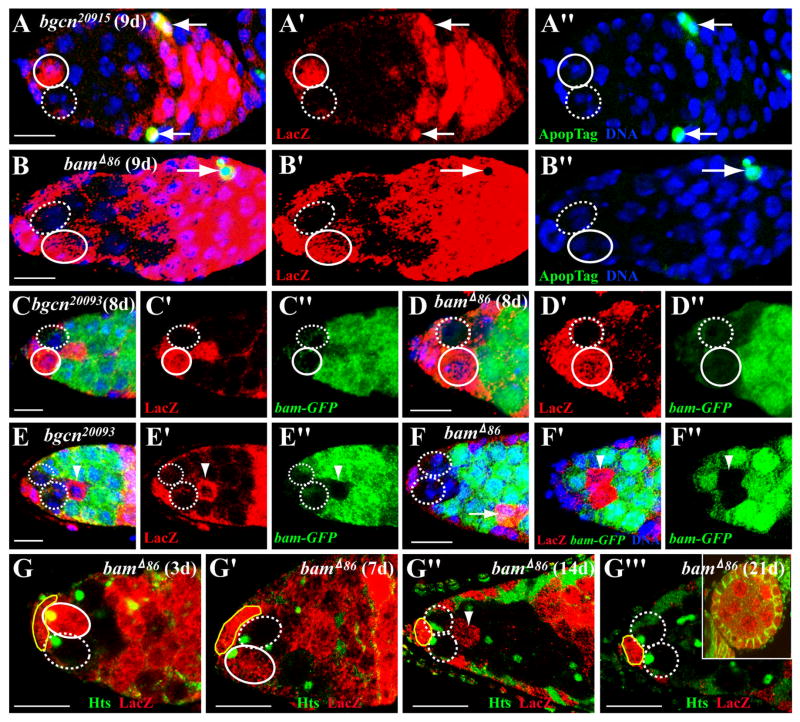
In the Drosophila wing imaginal disc, fast growing cells induced by higher dmyc expression can outcompete and eliminate slow growing neighbors by promoting their apoptosis (de la Cova et al., 2004). To investigate whether bgcn and bam mutant GSCs outcompete wild-type GSCs by the same mechanism, we performed the TUNEL assay to detect if there were apoptotic GSCs in the germaria carrying both marked bam or bgcn mutant and unmarked wild-type GSCs. Interestingly, in the germaria with both one wild-type GSC and one marked mutant bgcn20093 or bgcn20915 GSC (total 163 germaria examined), neither the wild-type (LacZ-positive) GSCs nor the marked bgcn (LacZ-negative) mutant GSCs were apoptotic (Figure 2A). The same is also true for those germaria harboring both wild-type and bam mutant GSCs (total 120 germaria examined) (Figure 2B). In addition, differentiating lacZ-positive wild-type cysts were seen within the germaria, intermingling with the bgcn and bam mutant cell mass. These observations indicate that it is unlikely that the loss of the wild-type GSCs neighboring the marked mutant bam or bgcn GSCs is due to cell death.
Figure 2.
bgcn or bam mutant GSCs push their neighboring wild-type GSC out of its niche space. The LacZ-negative marked GSCs and the LacZ-positive unmarked GSCs are highlighted by broken and solid circles, respectively. (A, B) Germarial tips showing no apoptosis for the 9-day-old marked bgcn20915 (A–A″) or bamΔ86 (B–B″) GSC and its neighboring unmarked GSC and the dying somatic cells in the midway of the germarium (arrows). (C, D) Germarial tips labeled showing no bam-GFP upregulation in the 8-day-old marked bgcn20093 (C–C″) or bamΔ86 (D–D″) GSC and its neighboring unmarked GSC. (E–E″) A germarial tip showing that a recently displaced wild-type GSC (arrowhead) lying posteriorly to the two marked bgcn20093 GSCs still does not upregulate bam-GFP. (F–F″) A germarial tip labeled for LacZ (red, F′), bam-GFP (green, F″) and DNA (blue) showing that the recently evacuated wild-type still connected GSC daughters (arrowhead; F′ and F″, on a different confocal section) lying posteriorly to the two marked bamΔ86 GSCs still do not upregulate bam-GFP. A LacZ-positive differentiated germ cell cyst (arrow, F) is positive for bam-GFP. (G–G‴) Different germarial tips showing a time course of forcing out a wild-type unmarked GSC by its marked bam mutant neighbor GSC. The three-day-old bamΔ86 mutant GSC and its neighboring wild-type GSC in (G) have similar contact areas with cap cells (yellow lines), whereas the one-week-old bamΔ86 mutant GSC has a larger contact area with cap cells than its neighboring wild-type GSC as shown in (G′). In the germarium carrying a two-week-old GSC clone (G″), a wild type GSC (arrowhead) has just been displaced from the niche, resulting in a full clone with two mutant GSCs. In the germarium carrying a three-week-old GSC full clone (G‴), the displaced wild-type GSC has differentiated into a normal egg chamber (inset). The bars represent 10μm.
To test whether the loss of unmarked wild-type GSCs is due to differentiation, we investigated the expression of bam-GFP in the unmarked wild-type GSCs sharing their niches with a marked bam or bgcn mutant GSC. As a GSC starts to differentiate, it immediately upregulates expression of bam-GFP (Chen and McKearin, 2003b). After examining 48 germaria carrying an 8-day-old bgcn20093 or bgcn20915 mutant GSC clone, we failed to observe bam-GFP expression in any of the unmarked wild-type GSCs (Figure 2C). Likewise, we did not observe any bam expression in any unmarked wild-type GSCs in the 45 germaria carrying an 8-day-old marked bam mutant GSC clone (Fig. 2D). We also noticed that the unmarked wild-type GSCs that had already moved out of their niches did not express bam-GFP, suggesting that the unmarked wild-type GSCs are pushed out of their niches before they differentiate (Figure 2E and 2F). Consistent with this idea, we observed a trend of gradual loss of the wild-type GSC sharing the niche with a marked mutant bam or bgcn GSC over time (Figure 2G–G‴). Therefore, our results indicate that bam or bgcn mutant GSCs outcompete their wild-type neighboring GSCs simply by forcing them out.
E-Cadherin is Required for bam/bgcn-Mediated GSC Competition
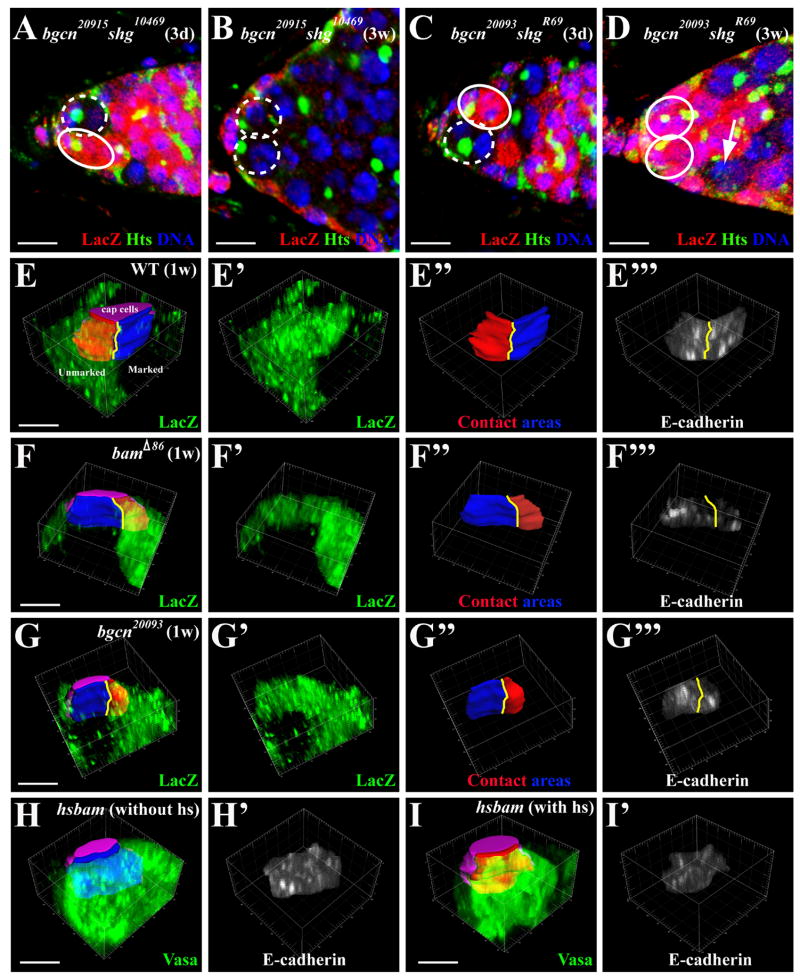
shotgun (shg) encodes E-cadherin, which is essential for anchoring GSCs in the niche (Song et al., 2002). To investigate whether E-cadherin participates in bgcn-mediated GSC competition, we generated marked bgcn and shg double mutant GSCs using two different shg alleles, shg10469 and shgR69, which represent a hypomorphic allele and a null allele, respectively. The observed frequency of the germaria carrying a bgcn20915 shg10469 mutant full GSC clone was similar to that of the germaria carrying a bgcn20915 mutant full GSC clone, indicating that the weak shg10469 mutation only had a slight effect on bgcn-mediated GSC competition (Figure 3A and 3B; Table 1). In contrast, the frequency of the germaria carrying a bgcn20093 shgR69 mutant full GSC clone was significantly lower than that of the germaria carrying a bgcn20093 mutant full GSC clone, but was comparable to that of the germaria carrying a wild-type full GSC clone, indicating that the null shg mutation almost completely abolishes the competitive advantage of the bgcn mutant GSCs over the wild-type ones (Figure 3C and 3D; Table 1). These results demonstrate that E-cadherin is involved in the bgcn-mediated GSC competition. As reported previously, over 95% of the marked shgR69 mutant GSCs detected at one week ACI are lost at three weeks ACI (Song et al., 2002). It is worth noting that over 50% of the marked bgcn20093 shgR69 double mutant GSCs detected at three days ACI still remained in the niche at three weeks ACI, indicating that preventing shgR69 mutant GSCs from differentiation stabilizes their interactions with cap cells. Due to their location on different chromosomes, we could not investigate whether E-cadherin also takes part in bam-mediated GSC competition.
Figure 3.
The bgcn/bam-mediated GSC competition requires E-cadherin. In the germarial tips (A–D), the marked GSCs and the unmarked GSCs are highlighted by broken and solid circles, respectively. (A, B) Germarial tips harboring a three-day-old partial bgcn20915 shg10469 mutant GSC clone (A), and a three-week-old full mutant bgcn20915 shg10469 clone (B). (C, D) The germarial tips showing a three-day-old partial bgcn20093 shgR69 mutant GSC clone (C), and a recently lost three-week-old partial bgcn20093 shgR69 clone (arrow, D). (E–G) 3-D projections of germarial tips (pointing into the page) carrying control (E), bamΔ86 (F) and bgcn20093 (G) partial GSC clones, showing that the lacZ-negative (black) marked GSC (blue-contact area with cap cells colored purple) and the lacZ-positive (green) unmarked GSC (red-contact area) are separated by yellow lines. E′–G′ and E″–G″ show LacZ staining and the contact areas with cap cells for the marked GSC (red) and the unmarked GSC (blue), respectively. E‴–G‴ represent E-cadherin staining intensity in stem cell-niche junction. (H, I) The 3-D projections of a hs-bam germarial tip without (H) or with (I) heatshock treatments showing the stem cell-niche junction (H, blue; I, red) and E-cadherin accumulation in the junction (H′ and I′) between cap cells (purple) and GSCs (green, Vasa). The bars in A–D and in E–I represent 10μm and 5 μm, respectively.
The bam/bgcn Pathway Negatively Regulates E-Cadherin Accumulation in the GSC-Niche Junction
It is possible that bam or bgcn mutant GSCs express higher levels of E-cadherin in the stem cell-niche junction and thus have a higher affinity for cap cells (niche cells) and a more competitive advantage for niche occupancy. We used the three-dimensional reconstruction of confocal sections (see Materials and Methods) to quantify E-cadherin in the stem cell-niche junction and the contact area with cap cells in the germaria carrying a wild-type GSC and a marked bam or bgcn mutant GSC. In the control germaria, the marked and unmarked wild-type GSCs in the same niche had similar contact areas in the stem cell-niche junction (38.2±9.9 μm2 for the unmarked GSC and 37.4±9.0 μm2 for the marked one; p=0.70; n=19), and also had similar amounts of E-cadherin accumulation in the junction with cap cells (the ratio of the unmarked GSC to the marked one=1.09; p=0.38; n=19) (Figure 3E–E‴). In contrast, the marked bamΔ86 mutant GSC had a significantly larger contact area with cap cells than the unmarked wild-type (35.1±18.5 μm2 for the bam mutant GSC and 23.1±12.5 μm2 for the wild-type one; p=0.001; n=20) and also had significantly more E-cadherin accumulation in the stem cell-niche junction than the unmarked wild-type in the same niche (the ratio of the bam mutant GSC to the wild-type =2.31; p=0.002; n=20) (Figure 3F–F‴). Similarly, the marked bgcn20093 mutant GSC had a significantly larger contact area with cap cells than the unmarked wild-type (44.2±12.9 μm2 for the bgcn mutant GSC and 32.3±11.6 μm2 for the wild-type; p=0.0009; n=15) and also had significantly more E-cadherin accumulation in the stem cell-niche junction than the unmarked marked wild-type in the same niche (the ratio of the bgcn mutant GSC to the wild-type=1.73; p=0.01; n=15) (Figure 3G–G‴). The observation that the bam and bgcn mutant GSCs have larger contact areas with cap cells than their wild-type counterparts in the same niches further supports the notion that the wild-type GSCs are gradually dislodged from the niche as a result of the invasion of the mutant GSCs. Therefore, our results strongly suggest that the increase of E-cadherin in the junction between mutant GSCs and cap cells is likely one of the primary determining factors for their stronger competitiveness.
The aforementioned findings also raise an interesting possibility that bam/bgcn might negatively regulate E-cadherin accumulation in the GSC. To test the possibility, we performed three-dimensional reconstruction to determine E-cadherin expression in the stem cell-niche junction 9 hours after bam overexpression using the hs-bam transgene, when the GSCs still remained in the niches and contained an anteriorly anchored spectrosome. Since the germaria carrying three stem cells could have a larger contact area and possibly more E-cadherin between the stem cells and cap cells (the niche) than the ones containing two stem cells, we focused our analyses only on the germaria carrying two stem cells. In the control experiments, the germaria from the control (yw) females (not carrying hs-bam) with heatshock treatments and the ones without heatshock treatments had similar E-cadherin accumulation in the stem cell-niche junction (the ratio=1.00; p=0.49; n=14). In contrast, in the germaria of females carrying hs-bam, there was significantly less E-cadherin accumulation in the stem cell-niche junction after heatshock treatments, compared with those without heatshock treatments (the ratio=0.68; p=0.001; n=15) (Figure 3H–3I′). This result demonstrates that bam upregulation in the GSC can sufficiently downregulate E-cadherin expression, which sheds light on why differentiated stem cells are detached from the niche. In conjunction with the fact that E-cadherin expression is increased in bam and bgcn mutant GSCs, this result leads us to conclude that bam/bgcn normally negatively regulate E-cadherin accumulation in the stem cell-niche junction.
Different Expression Levels of E-Cadherin can Induce GSC Competition
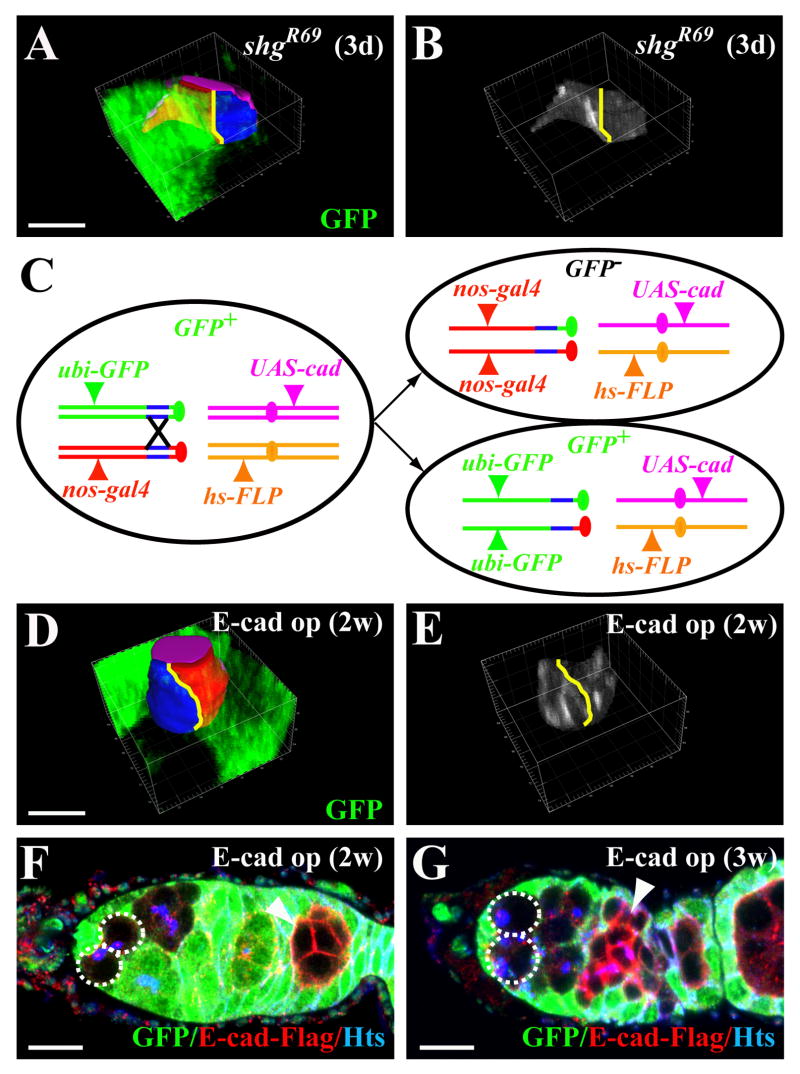
Our earlier results also imply that the mutant GSCs defective in adhesion would have reduced their contact area with cap cells, and thus are gradually competed out by their wild-type neighbors. To directly test this, we quantified the contact areas of the marked mutant shg GSC and the unmarked wild-type GSC in the mosaic germaria, which only host a shg mutant GSC marked by loss of arm-lacZ expression and an unmarked wild-type one with the expression of arm-lacZ. As shown in our previous study, most marked shg mutant GSCs are lost two weeks after clone induction (Song et al., 2002). Thus, we only examined the one-week-old germaria carrying a mutant shg GSC and a wild-type GSC. As predicted, the unmarked wild-type GSCs indeed had a larger contact area with cap cells (52.2±12.9 μm2) than their companion mutant shg GSCs (26.1±7.7 μm2) in the same niche (n=11; p=0.0004; Figure 4A and 4B), indicating that the mutant shg GSCs are indeed gradually competed out by their neighboring wild-type GSCs.
Figure 4.
Different levels of E-cadherin can induce GSC competition. (A, B) 3-D projections of a germarial tip showing that the GFP-negative (black) marked shg mutant GSC (blue-contact area, A) has a smaller contact area with cap cells and much less E-cadherin (B) in the stem cell-niche junction than the GFP-positive (green) unmarked wild-type GSC (red-contact area, A). Solid yellow lines indicate the boundary between the marked GSC and the unmarked GSC. (C) A diagram explaining the genetic strategy for overexpressing different levels of E-cadherin in a marked GSC (carrying two copies of nos-gal4VP16; GFP−) and its neighboring unmarked GSC (carrying one copy of nos-gal4VP16; GFP+). (D, E) 3-D projections of a germarial tip showing that the GFP-negative (black) marked E-cadherin-overexpressing GSC (blue-contact area, D) has a larger contact area with cap cells and more E-cadherin in the stem cell-niche junction (E) than the GFP-positive (green) unmarked wild-type GSC (red-contact area, D). Solid yellow lines indicate the boundary between the marked GSC and the unmarked GSC. (F, G) Germaria carrying two-week-old (F) and three-week-old (G) marked full GSC clones, in which both the GSCs are indicated by broken lines. The bars in A and D and the ones in F and G represent 5 and 10 μm, respectively.
We also further tested whether different E-cadherin expression levels in the GSCs in the same niche are sufficient to determine their relative competitiveness for niche occupancy. To generate a marked GSC that expresses different levels of E-cadherin from its neighboring GSCs, we utilized a combination of the FLP-mediated FRT recombination and the binary UAS-Gal4 expression system. In these experiments, Drosophila females carry a nanos (nos)-gal4VP16 located distal to FRT19A on one X chromosome and the ubi-GFP (a ubiquitin promoter driven GFP) on the other X chromosome distal to FRT19A (Figure 4C). The nos-gal4VP16 was used to drive a UASp-E-cad-FLAG (a C-terminal FLAG-tagged E-cadherin controlled by a UASp promoter) to overexpress E-cadherin specifically in germ cells (Van Doren et al., 1998); (Pan et al., 2007), while ubi-GFP is ubiquitously expressed in all the cells of the germarium. After clone induction, the marked GFP-negative GSCs would carry two copies of nos-gal4VP16, while the GFP-positive GSCs carry only one copy of nos-gal4VP16. In order to test if the GSCs carrying two copies of nos-gal4 can indeed express more E-cadherin than the ones carrying only one copy of nos-gal4, we used the 3-D reconstruction to measure expression levels of E-cadherin in the GFP− marked GSCs and their neighboring GFP+ unmarked GSCs. Indeed, the GFP-negative marked GSCs carrying two copies of nos-gal4 had significantly higher levels of E-cadherin than the GFP-positive unmarked GSCs carrying only one copy of nos-gal4 (the ratio of the former to the latter: 2.15; p=0.0001; n=13) (Figure 4D). Consequently, the GSCs expressing more E-cadherin (54.8±17.8 μm2) had larger contact areas with cap cells than the GSCs expressing less E-cadherin (37.6±9.8 μm2) (p=0.001; n=13; Figure 4E).
We then determined if the E-cadherin-overexpressing GSCs can expel their neighboring stem cells out of their niche. In this set of experiments, the initial clone induction rates at one week ACI for the control and E-cadherin overexpression were 16.3% and 20.0%, respectively, both of which were lower than earlier experiments. At three weeks ACI, the percentage of the germaria carrying a marked full clone in the control was 4.1%, which was much lower than that of the germaria carrying a partial clone (7.1%) (Table 1). In contrast, the germaria carrying a GFP-negative marked full clone carrying two copies of nos-gal4VP16 continued to increase from one week to three weeks ACI, and they became more prevalent than the germaria carrying a partial clone three weeks ACI (11.8% of the germaria carrying a full clone versus 5.6% of the germaria carrying a partial clone) (Figure 4F and 4G; Table 1). This result indicates that the GSCs expressing more E-cadherin are consistently more competitive than their neighbors expressing less E-cadherin. Together, our loss-of-function and gain-of-function experiments demonstrate that different levels of E-cadherin expression in GSCs within the same niche can stimulate stem cell competition.
Slow Proliferation of GSCs Compromises their Competitiveness
As reported above, bam GSCs are more competitive than bgcn mutant GSCs and bam mutant tumors are larger than bgcn mutant tumors, suggesting that the stronger competitiveness of bam mutant stem cells may result from their faster proliferation rate. We therefore used BrdU labeling to determine if the bam mutant GSCs indeed divide faster than bgcn mutant GSCs. In the 320 germaria carrying a lacZ-negative marked bam mutant GSC and a lacZ-positive unmarked wild-type GSC, there were 23 of the lacZ-negative bam mutant GSCs that were BrdU positive, while only 11 of the lacZ-positive wild-type GSCs were labeled by BrdU (the χ2 test: p=0.04), indicating that bam mutant GSCs divide significantly faster than wild-type neighbors. In contrast, in the 258 germaria carrying a lacZ-negative marked bgcn mutant GSC and a lacZ-positive unmarked wild-type GSC, 11 of the lacZ-negative bgcn mutant GSCs and 15 of the lacZ-positive wild-type GSCs were positive for BrdU (the χ2 test: p=0.433), indicating that bgcn mutant GSCs and wild-type neighbors divide at similar rates. These results suggest that bam mutant GSCs divide faster than bgcn mutant GSCs.
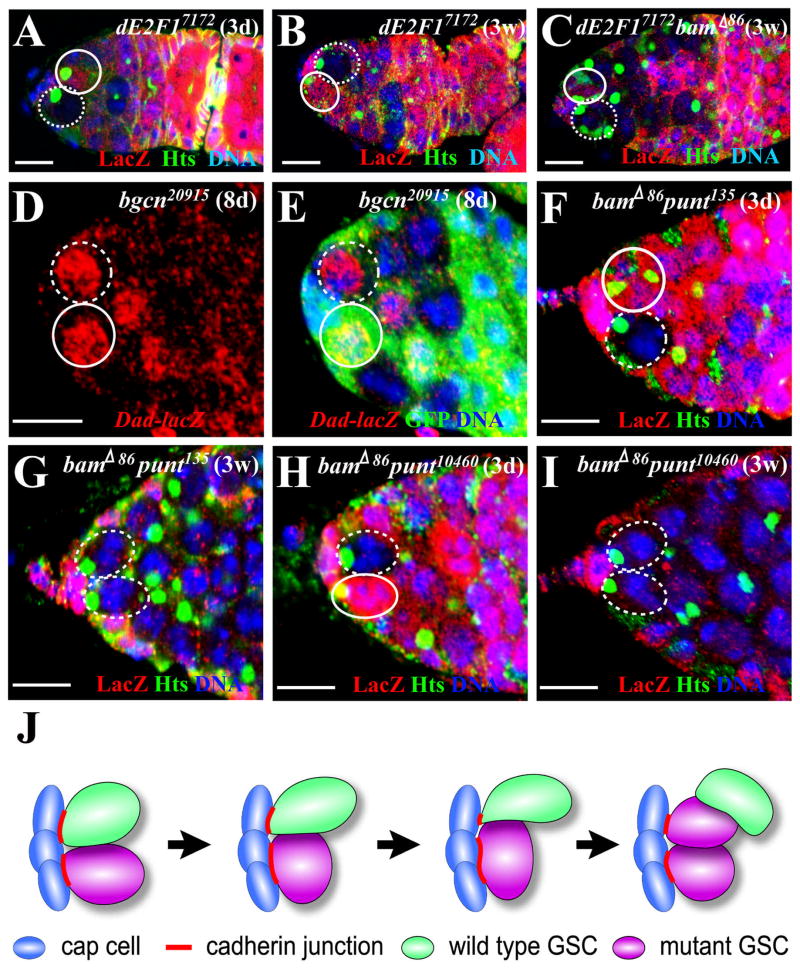
Based on the finding that bam mutant GSCs divide faster and are more competitive than bgcn mutant GSCs, we hypothesized that the rate of cell proliferation contributes to stem cell competitiveness. To test this hypothesis, we used the same strategy described earlier to generate marked GSCs mutant for both bam and dE2F1. dE2F1 is known to be important for controlling cell cycle progression (Korenjak and Brehm, 2005), and two moderate dE2F1 mutants, dE2F17172 and dE2F1rM729, were used to disrupt dE2F1 function (Asano et al., 1996). In comparison with the control, the marked GSCs mutant for dE2F17172 and dE2F1rM729 were maintained for three weeks just like or close to the wild-type GSCs, and the marked mutant dE2F1GSC clones detected at one week ACI could be frequently observed at three weeks ACI, indicating that these two dE2F1 mutations do not dramatically affect stem cell maintenance (Table 2; Figure 5A and 5B). The relative division rate for a marked GSC is determined by the number of cysts generated by a marked mutant GSC divided by the number of cysts generated by a marked wild-type GSC (Xi and Xie, 2005); and the marked mutant dE2F17172 and dE2F1rM729 GSCs had relative division rates of 0.45 (17 mutant GSCs examined) and 0.47 (16 mutant GSCs examined), respectively. These results indicate that different division rates are not sufficient for inducing stem cell loss, and that the two dE2F1 mutants are suitable for studying the effect of proliferation on stem cell competition. Interestingly, the marked GSC mutant for both bam and dE2F1 became much less competitive than the marked GSCs only mutant for bam. For example, at three weeks ACI, only 22.5% and 23.5% of the germaria carried bamΔ86 dE2F17172 and bamΔ86 dE2F1rM729 full clones in comparison with 58.5% of the germaria carrying marked bamΔ86 GSC full clones (Tables 1 and 2); the germaria carrying a partial bam dE2F1 mutant GSC clone were frequently observed (Figure 5C). These results indicate that different cell proliferation potentials contribute to, but do not sufficiently change, the relative competitiveness of stem cells for niche occupancy.
Table 2.
Compromised stem cell proliferation but not BMP signaling weakens its competition capacity.
| Genotypes | Ages | Percentagea of the germaria carrying a marked full GSC clone | Percentageb of the germaria carrying a marked partial GSC clone | Percentagec of the germaria carrying a marked GSC clone | Number of the germaria examined |
|---|---|---|---|---|---|
| Control (FRT82B) | 1w | 1.6% | 31.3% | 33.0% | 182 |
| 2w | 3.2% | 23.9% | 27.1% | 218 | |
| 3w | 4.3% | 22.2% | 26.5% | 185 | |
| dE2F17172 | 1w | 4.6% | 38.3% | 32.2% | 174 |
| 2w | 6.0% | 27.6% | 27.7% | 184 | |
| 3w | 10.0% | 21.7% | 24.2% | 190 | |
| dE2F17172bam Δ 86 | 1w | 11.4% | 38.9% | 50.3% | 167 |
| 2w | 23.2% | 31.9% | 55.1% | 185 | |
| 3w | 30.6% | 31.8% | 62.4% | 173 | |
| dE2F1rM729 | 1w | 4.6% | 27.6% | 33.3% | 171 |
| 2w | 4.8% | 20.2% | 25.0% | 168 | |
| 3w | 5.5% | 10.9% | 16.4% | 183 | |
| dE2F1rM729bam Δ 86 | 1w | 9.1% | 46.3% | 55.4% | 175 |
| 2w | 19.7% | 41.0% | 60.7% | 173 | |
| 3w | 28.7% | 38.9% | 67.7% | 167 | |
| bam Δ 86punt10460 | 3 d | 0.9% | 50.2% | 51.1% | 209 |
| 1w | 10.5% | 65.0% | 75.5% | 161 | |
| 2w | 32.2% | 50.7% | 83.9% | 217 | |
| 3w | 60.2% | 28.0% | 88.2% | 211 | |
| bam Δ 86punt135 | 3d | 0.6% | 37.6% | 38.2% | 157 |
| 1w | 6.3% | 58.0% | 64.3% | 207 | |
| 2w | 24.2% | 38.9% | 63.1% | 198 | |
| 3w | 54.6% | 28.2% | 82.8% | 227 |
The percentage of the germaria carrying a marked full GSC clone at a given time point = the number of germaria in which all GSCs are marked/total germaria examined.
The percentage of germaria carrying a partial marked GSC clone at a given time point = the number of germaria in which at least one GSC is not marked and also at least one GSC is marked/total germaria examined.
The percentage of germaria carrying a marked GSC clone at a given time point = the number of germaria carrying a marked full or partial GSC clone/total germaria examined.
Figure 5.
The bam/bgcn-mediated GSC competition is affected by the ability to proliferate but not by BMP signaling. Marked mutant GSCs and unmarked wild-type GSCs are highlighted by broken and solid circles, respectively. (A, B) Germaria carrying a three-day-old (A) or three-week-old (B) partial dE2F17172 mutant GSC clone. (C) A germarium carrying a three-week-old partial dE2F17172 bamΔ86 double mutant GSC clone. (D, E) A germarial tip showing that Dad expression does not change in an 8-day-old GFP-negative marked bgcn20915 GSC in comparison with its neighboring GFP-positive unmarked wild-type GSC. (F, G) Germarial tips showing a marked punt135 bamΔ86 partial clone (F) and a marked punt135 bamΔ86 full clone (G). (H, I) Germarial tips showing a marked punt10460 bamΔ86 partial clone (E) and a marked punt10460 bamΔ86 full clone (F). The bars represent 10μm. (J) A working model explaining bam/bgcn-mediated GSC competition. At the beginning, the newly generated marked bgcn or bam mutant GSC (purple) has a similar contact area (similar amount E-cadherin accumulation, red) with cap cells (blue) to that of the wild-type GSC (green). Over time, the mutant GSC expands its interface with cap cells, and consequently more E-cadherin accumulates in the interface between the mutant GSC and cap cells. Eventually, the mutant GSC divides to generate two daughters that both contact cap cells and push out the wild-type GSC.
The GSC Competition Does not Require BMP Signaling and dMyc Function
Since bam is proposed to negatively regulate BMP signaling (Casanueva and Ferguson, 2004), one explanation for the bam/bgcn-mediated GSC competition is that BMP signaling activities may be upregulated in the bam or bgcn mutant GSCs. The Dad-lacZ line has been extensively used to monitor BMP signaling activities in different tissue types, including the ovary (Casanueva and Ferguson, 2004; Kai and Spradling, 2003; Song et al., 2004). Interestingly, the marked bgcn mutant GSCs and their neighboring unmarked wild-type GSCs expressed Dad-lacZ at similar levels, indicating that BMP signaling activity does not change in bgcn mutant GSCs (Figure 5D and 5E). To directly test whether blocking BMP signaling could compromise bam-mediated stem cell competition, we used weak (punt10460) and strong (punt135) mutations of punt, which encodes the type II receptor essential for BMP signaling (Letsou et al., 1995; Ruberte et al., 1995), to block BMP signaling in bam mutant GSCs. The marked double punt10460 bamΔ86 or punt135 bamΔ86 GSCs still retained a similar ability to those marked bam mutant GSCs to outcompete wild-type GSCs in the same niches based on the percentages of germaria carrying a marked GSC full clone 3weeks ACI, indicating that BMP signaling is not required for bam-mediated GSC competition (Figure 5F–5I; Tables 1 and 2). Corroborating the Dad-lacZ expression result, these results demonstrate that BMP signaling is not involved in the bam/bgcn-mediated GSC competition.
Since dmyc is capable of inducing cell competition in the Drosophila wing imaginal disc (de la Cova et al., 2004; Moreno and Basler, 2004), we sought to determine if dmyc is also involved in GSC competition by generating the marked GSCs homozygous for strong dmyc mutations, dm2 and dm4 (Maines et al., 2004; Pierce et al., 2004). Surprisingly, 69.7% and 73.5% of the marked dm2 and dm4 mutant GSCs detected at one week ACI were maintained at three weeks ACI, respectively, indicating that dmyc mutant GSCs are as stable as the marked controls (Table S1 and Figure S1A–1D). Furthermore, the marked dm2 and dm4 mutant GSCs had normal division rates, which are 1.0 (n=16) and 0.91 (n=39), respectively. To further investigate if overexpressing dmyc in the marked GSCs can strengthen their competitiveness, we used the same strategy for overexpressing E-cadherin to overexpress dmyc in marked GSCs. Interestingly, the percentages of the germaria carrying a GFP-negative marked GSC full clone, which carried two copies of nos-gal4 and should express more dmyc, decreased over time indicating that dmyc overexpression does not make GSCs more competitive. Furthermore, the germaria carrying a marked GSC clone overexpressing dmyc appeared to be lost slightly faster than the controls (Figure S1E–1H). Together, our results show that dmyc is not essential for GSC competition.
Discussion
In this study, we have shown that the differentiation-defective bam or bgcn mutant GSCs can drive wild-type stem cells away from their niche in an E-cadherin-dependent manner in the Drosophila ovary (Figure 5J). Our genetic and cell biological analyses indicate that different levels of E-cadherin expression in GSCs determine their competency in occupying the niche and that the bam/bgcn pathway controls E-cadherin protein expression levels in the GSC. Furthermore, we demonstrate that cell proliferation, but not BMP signaling or dMyc, modulates bam/bgcn-mediated GSC competition. This study also offers new insight into how a differentiated GSC is forced out of its niche by its neighboring stem cells. Its departure from the niche is probably caused by the upregulation of bam and consequent downregulation of E-cadherin. Such competition may serve as a quality control mechanism to ensure that the niche is always occupied by functional stem cells. Finally, the knowledge gained from studying stem cell competition may make it possible to deliver stem cells to diseased tissues by replacing nonfunctional stem cells in the future.
bam/bgcn Controls GSC Competition for Niche Occupancy by a Novel Mechanism
bam, encoding a novel protein with no previously known functional domain, is primarily expressed in a fraction of cystoblasts and proliferating cysts (McKearin and Ohlstein, 1995; McKearin and Spradling, 1990), while bgcn, encoding a putative RNA binding protein, is expressed in GSCs, cystoblasts, and proliferating cysts (Ohlstein et al., 2000). Mutations in bam and bgcn lead to accumulation of cystoblast-like or GSC-like single germ cells, further supporting the view that their functions are restricted to cystoblasts and proliferating cysts. In this study, however, we have shown that both bam and bgcn are required in GSCs to control their competitiveness in niche occupancy. This study also suggests that natural GSC turnover might be caused by the fluctuation of bam/bgcn function in GSCs. The competition mechanism we have discovered in this study could account for stem cell quality control and efficient replacement of lost GSCs due to their upregulated bam expression. Although bam is transcriptionally repressed by BMP signaling, low levels of bam transcription in the GSC can still be detected using the bam-GFP transgenic line (Figure S2). It is, therefore, possible that low levels of bam transcription detected in GSCs may provide a function for controlling GSC competitiveness.
Although mutations in bam and bgcn generate similar germ cell tumor phenotypes, there are two obvious differences in their mutant phenotypes. One difference is that bam mutant ovaries have more single germ cells than bgcn mutant ovaries. Our results show that bam mutant GSCs are more mitotically active than bgcn mutants, suggesting that bam has a bgcn-independent function in controlling GSC and/or cystoblast proliferation rate. The second difference is that mutant bam GSCs are more competitive than mutant bgcn GSCs in niche occupancy. This could be due to their difference in mitotic activities since we have shown in this study that the competitiveness of GSCs is modulated by mitotic activity. Taken together, we conclude that bam can function in bgcn-dependent and bgcn-independent manners to control the competitiveness of GSCs.
In the Drosophila imaginal disc, dmyc mediates cell competition by inducing apoptosis in disadvantaged cells (expressing less dmyc). This competitive behavior correlates with, and can be corrected by, the activation of the BMP/Dpp survival signaling pathway (Moreno and Basler, 2004). As shown in this study, however, dmyc and BMP signaling are dispensable for GSC competition, suggesting that GSCs use a distinct mechanism to control their competition. Indeed, we have shown that bam or bgcn mutant stem cells do not promote apoptosis or differentiation of their wild-type counterparts in the same niche. Instead, bam/bgcn mutant GSCs push their wild-type counterparts out of the niche through their adhesive advantage, which is supported by our observation that the mutant GSCs gradually increase E-cadherin accumulation at the GSC/cap cell junction. In addition, unlike in the imaginal disc, slowly dividing E2F1 mutant GSCs do not exhibit any obvious competitive disadvantages over wild-type neighbors. However, slow GSC division does compromise bam/bgcn-mediated GSC competition since we observed that mutant bam dE2F1 GSCs are less competitive than bam mutant GSCs. The proliferation of the advantaged GSC may help produce a physical force to push the disadvantaged GSC out of its niche and occupy the space of the doomed GSC. Therefore, our study has revealed a novel function of bam/bgcn in stem cell competition and a novel mechanism for cell competition.
The bam/bgcn Pathway Controls GSC Competition by Regulating E-Cadherin Accumulation in the Stem Cell-Niche Junction
Our previous study showed that the mutant GSCs lacking E-cadherin are lost rapidly from their niche, but the underlying mechanism is not determined (Song et al., 2002). In this study, we provide an important insight into the mechanism by showing that the GSCs lacking E-cadherin lose their competition for the niche to their neighboring wild-type GSCs. This observation suggests that different levels of E-cadherin can induce competition among the GSCs in the same niche. Indeed, we have further shown that the GSCs expressing more E-cadherin become more competitive than the neighboring GSCs expressing less E-cadherin. Therefore, this study has demonstrated that different levels of E-cadherin in GSCs can sufficiently stimulate GSC competition for niche occupancy.
This study has also offered new mechanistic insights into why bam/bgcn mutant GSCs are more competitive than their neighboring wild-type GSCs. First, we show that bam and bgcn mutant GSCs accumulate more E-cadherin in the stem cell-niche junction than their neighboring wild-type counterparts. Second, bam overexpression is sufficient to downregulate E-cadherin accumulation in the junction. Together, these observations demonstrate that the bam/bgcn pathway is necessary and sufficient for controlling E-cadherin accumulation in the stem cell-niche junction. This bam/bgcn-mediated E-cadherin regulation may represent a quality control mechanism to ensure that a differentiated GSC triggered by a spontaneous mutation or abnormal upregulation of bam can be efficiently removed from the niche and then be replaced with a functional GSC generated by its neighboring GSC. Consistent with the notion that E-cadherin is involved in bam/bgcn-mediated cell competition, we showed that the removal of E-cadherin can abolish the competitive advantage of GSCs gained from bgcn mutations. However, it remains unclear why bam/bgcn mutant GSCs contain more E-cadherin in the stem cell-niche junction than their neighboring wild-type GSCs. Since bam/bgcn have been proposed to regulate translation (Ohlstein et al., 2000), it is possible that bam/bgcn normally control E-cadherin expression in GSCs by repressing its translation. Elucidating biochemical functions of Bam and Bgcn proteins will be essential for understanding how the bam/bgcn pathway controls GSC competitiveness.
Materials and Methods
Generation of marked GSC clones
To determine the roles of bgcn and bam in GSC competition, we used the FLP-mediated mitotic recombination technique to generate and analyze mutant GSC clones of the following genotypes: (1) hs-FLP; FRT42D/FRT42D arm-lacZ; (2) hs-FLP; FRT42D bgcn20093/FRT42D arm-lacZ; (3) hs-FLP; FRT42D bgcn20915/FRT42D arm-lacZ; (4) hs-FLP; FRT82B bamΔ86/FRT82B arm-lacZ. To determine the potential role of E-cadherin or BMP signaling in regulating the bgcn/bam-mediated GSC competition, we performed analysis on double mutant GSC clones of the following genotypes: (1) hs-FLP; FRT42D bgcn20093 shgR69/FRT42D arm-lacZ; (2) hs-FLP; FRT42D bgcn20915 shg10469/FRT42D arm-lacZ; (3) hs-FLP; FRT82B bamΔ86 punt135/FRT82B arm-lacZ; (4) hs-FLP; FRT82B bamΔ86 punt10460/FRT82B arm-lacZ.
To determine the role of GSC division in regulating the bgcn/bam-mediated GSC competition, we performed analysis on dE2F1 single or dE2F1 bam double mutant GSC clones of the following genotypes: (1) hs-FLP; FRT82B/FRT82B arm-lacZ; (2) hs-FLP; FRT82B dE2F17172/FRT82B arm-lacZ; (3) hs-FLP; FRT82B dE2F1rM729/FRT82B arm-lacZ; (4) hs-FLP; FRT82B bamΔ86 dE2F17172/FRT82B arm-lacZ; (5) hs-FLP; FRT82B bamΔ86 dE2F1rM729/FRT82B arm-lacZ. To determine if dmyc is involved in regulating GSC competition, we analyzed marked dmyc mutant GSC clones of the following genotypes: (1) FRT19A/Ubi-GFP FRT19A; hs-FLP; (2) dmycdm2 FRT19A/Ubi-GFP FRT19A; hs-FLP; (3) dmycdm4 FRT19A/Ubi-GFP FRT19A; hs-FLP.
To assay whether mutations in bgcn or bam affect BMP signaling in GSCs, flies of the following genotypes were used: (1) hs-FLP; FRT42D bgcn20093/FRT42D ubi-GFP; Dad-lacZ; (2) hs-FLP; FRT42D bgcn20915/FRT42D ubi-GFP; Dad -lacZ. bam-GFP, which is under the control of the endogenous bam promoter (Chen and McKearin, 2003b), was used in this study to monitor bam expression in both bam and bgcn mutant GSCs. To carry out this experiment, flies of the following genotypes were generated: (1) hs-FLP; FRT42D bgcn20093/FRT42D arm-lacZ; bam-GFP; (2) hs-FLP; FRT42D bgcn20915/FRT42D arm-lacZ; bam-GFP; (3) hs-FLP; bam-GFP; FRT82B bamΔ86/FRT82B arm-lacZ.
To determine if E-cadherin and dMyc overexpression can induce GSC competition, we used the following genotypes: (1) nos-gal4VP16 FRT19A/Ubi-GFP FRT19A; hs-FLP; (2) nos-gal4VP16 FRT19A/Ubi-GFP FRT19A; UAS-E-cadherin-Flag/hs-FLP; (3) nos-gal4VP16 FRT19A/Ubi-GFP FRT19A; UAS-dmyc/hs-FLP.
Adult females, 3- to 5-days old, were heat-shocked twice daily at 37°C for 1 hour with a 7- to 8-hour interval, for three consecutive days. Unless otherwise specified, fly ovaries were removed at 3 days, 1 week, 2 weeks and 3 weeks after the last heat-shock, and were then fixed for immunostaining as previously described (Xie and Spradling, 1998). For BrdU labeling of one-week-old marked bam and bgcn GSC clones, the ovaries from the females carrying appropriate genotypes for bam or bgcn were processed one week after heatshock treatments, and BrdU detection was performed as described previously (Zhu and Xie, 2003).
Immunostaining
The following antisera were used: monoclonal mouse anti-Hts antibody 1B1 (1:3, DSHB), monoclonal mouse anti-Arm N2 7A1 (1:3, DSHB), monoclonal rat anti-DE-Cadherin DCAD2 (1:3, DSHB), polyclonal rabbit anti-β-galactosidase antibody (1:300, Cappel), monoclonal mouse anti-β-galactosidase antibody (1:100, Promega), polyclonal rabbit anti-GFP antibody (1:200, Molecular Probes), rat anti-Vasa antibody (1:200, kindly provided by P. Lasko), Alexa 488 and Alexa 568-conjugated goat anti-mouse, anti-rabbit IgG and anti-rat (1:300, Molecular Probes), and Cy5-conjugated goat anti-mouse IgG (1:200, Jackson ImmunoResearch). The immunostaining protocol and the TUNEL assay using an ApopTag kit from Chemion have been described previously (Kawase et al., 2004). All micrographs were taken using a Leica TCS SP2 confocal microscope.
Measuring contact areas and E-cadherin between GSCs and cap cells
Using a Lecia SP2 confocal microscope, z-stacks were taken along the Z axis (0.5 μm each step) from those germaria that only bear one mutant and one control GSC or two GSCs in bam over-expression experiments. All images were acquired within the dynamic range of the detector at 12 bit depth. A contour plot for E-cadherin was generated with Imaris Bitplane (Saint Paul MN). The contour was drawn along the boundary of a single GSC (or two GSCs in bam over-expressing germaria) and cap cells on each focal plane. After 3-D reconstruction, a contour surface should represent the contact area between a single GSC (or two GSCs in bam over-expressing germaria) and cap cells. The intensity of E-cadherin staining on the contour surface was measured with Imaris Bitplane. All the p values were generated from the paired t-test using Microsoft Excel unless otherwise specified.
Supplementary Material
Acknowledgments
We would like to thank B. Edgar, N. Dyson, P. Lasko, D. Mckearin, T. Murphy, H. Oda, R. Padgett, D. Stein and U. Tapass for reagents, Developmental Studies Hybridoma Bank (DSHB) for antibodies, D. Zhu for advice on statistical analysis, R. Krumlauf, R. Li and N. Zhang for comments, the Xie laboratory members for stimulating discussions, the Stowers Institute facilities for services, and Dorothy Stanley for administrative assistance. This work is supported by a grant from NIH (1R01 GM64428-01) and the Stowers Institute for Medical Research.
References
- Asano M, Nevins JR, Wharton RP. Ectopic E2F expression induces S phase and apoptosis in Drosophila imaginal discs. Genes Dev. 1996;10:1422–1432. doi: 10.1101/gad.10.11.1422. [DOI] [PubMed] [Google Scholar]
- Casanueva MO, Ferguson EL. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development. 2004;131:1881–1890. doi: 10.1242/dev.01076. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003a;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin D. Gene circuitry controlling a stem cell niche. Curr Biol. 2005;15:179–184. doi: 10.1016/j.cub.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003b;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DN, Chao A, Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127:503–514. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Lilly MA, Spradling AC. Germline cyst formation in Drosophila. Annu Rev Genet. 1997;31:405–428. doi: 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–790. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Williams D, Spradling AC. The expression profile of purified Drosophila germline stem cells. Dev Biol. 2005;283:486–502. doi: 10.1016/j.ydbio.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- Korenjak M, Brehm A. E2F-Rb complexes regulating transcription of genes important for differentiation and development. Curr Opin Genet Dev. 2005;15:520–527. doi: 10.1016/j.gde.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Lavoie CA, Ohlstein B, McKearin DM. Localization and function of Bam protein require the benign gonial cell neoplasm gene product. Dev Biol. 1999;212:405–413. doi: 10.1006/dbio.1999.9346. [DOI] [PubMed] [Google Scholar]
- Letsou A, Arora K, Wrana JL, Simin K, Twombly V, Jamal J, Staehling-Hampton K, Hoffmann FM, Gelbart WM, Massague J, et al. Drosophila Dpp signaling is mediated by the punt gene product: a dual ligand-binding type II receptor of the TGF beta receptor family. Cell. 1995;80:899–908. doi: 10.1016/0092-8674(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3:931–940. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Maines JZ, Stevens LM, Tong X, Stein D. Drosophila dMyc is required for ovary cell growth and endoreplication. Development. 2004;131:775–786. doi: 10.1242/dev.00932. [DOI] [PubMed] [Google Scholar]
- McKearin D, Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- McKearin DM, Spradling AC. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, et al. The Drosophila cystoblast differentiation factor, benign gonial cell neoplasm, is related to DExH-box proteins and interacts genetically with bag-of-marbles. Genetics. 2000;155:1809–1819. doi: 10.1093/genetics/155.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Curr Opin Cell Biol. 2004;16:693–699. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Lavoie CA, Vef O, Gateff E, McKearin DM. The Drosophila cystoblast differentiation factor, benign gonial cell neoplasm, is related to DExH-box proteins and interacts genetically with bag-of-marbles. Genetics. 2000;155:1809–1819. doi: 10.1093/genetics/155.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call GB, Zhu D, Tang H, Zhang N, Xie T. Stem cell aging is controlled by both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:458–468. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Yost C, Britton JS, Loo LW, Flynn EM, Edgar BA, Eisenman RN. dMyc is required for larval growth and endoreplication in Drosophila. Development. 2004;131:2317–2327. doi: 10.1242/dev.01108. [DOI] [PubMed] [Google Scholar]
- Ruberte E, Marty T, Nellen D, Affolter M, Basler K. An absolute requirement for both the type II and type I receptors, punt and thick veins, for dpp signaling in vivo. Cell. 1995;80:889–897. doi: 10.1016/0092-8674(95)90292-9. [DOI] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- Szakmary A, Cox DN, Wang Z, Lin H. Regulatory Relationship among piwi, pumilio, and bag-of-marbles in Drosophila Germline Stem Cell Self-Renewal and Differentiation. Curr Biol. 2005;15:171–178. doi: 10.1016/j.cub.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol. 1998;8:243–246. doi: 10.1016/s0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- Xi R, Xie T. Stem cell self-renewal controlled by chromatin remodeling factors. Science. 2005;310:1487–1489. doi: 10.1126/science.1120140. [DOI] [PubMed] [Google Scholar]
- Xie T, Kawase E, Kirilly D, Wong MD. Intimate relationships with their neighbors: tales of stem cells in Drosophila reproductive systems. Dev Dyn. 2005;232:775–790. doi: 10.1002/dvdy.20317. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Zhu C, Xie T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development. 2003;130:2579–2588. doi: 10.1242/dev.00499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.