Abstract
Inadequate and poor quality of diet and malnutrition are common and associated with adverse health outcomes, including morbidity and mortality, among older persons. This review aimed to establish the latest evidence from studies investigating the association between oral function and nutrition among older adults.
An electronic search of MEDLINE using PubMed for literature published in English between March 2018 and March 2021 was conducted, and 27 papers were identified. The selected studies comprised 23 observational studies (17 cross-sectional and 6 longitudinal studies) and 4 interventional studies. Most of the observational studies demonstrated the following associations in older adults: older adults with poor oral function are likely to have poorer dietary intake and poorer nutritional status, and malnourished older adults are likely to have poorer oral function. The results of the intervention studies demonstrated that the combination of prosthodontic treatment and dietary counseling is more effective for improving dietary intake and nutritional status in older persons with tooth loss than the prosthodontic treatment alone.
Our review confirmed that a relationship exists between oral function and nutrition and revealed the need for additional high-quality studies investigating comprehensive oral function, rather than a single aspect of oral function, with regard to nutritional status.
Keywords: Oral health, Nutrition disorders, Aged, Review
1. Introduction
Inadequate and poor quality of diet [[1], [2], [3]] and malnutrition (undernutrition) [4] are common among older people. An inadequate and poor-quality diet and malnutrition are associated with adverse events, including longer length of hospital stay, high medical care costs, morbidity, and death [[5], [6], [7], [8], [9]]. Considering its impact on individuals and society, poor nutrition is a serious health problem among older persons.
Poor oral functions such as decreased ability to chew and swallow lead to unfavorable changes in food choices and a poor quantity and quality of food intake among older adults [[10], [11], [12]]. Inadequate dietary intake is a risk factor for malnutrition [13]. Therefore, a scenario where oral disease leads to reduced oral function, which subsequently leads to lack of dietary intake and thus malnutrition, can be expected [14]. To date, several review articles have been published on the association between oral health and nutritional status [[15], [16], [17], [18], [19], [20], [21]]. However, most of the included papers investigated the dentition status or the presence of dental prostheses alone, and oral function has not been studied in detail in the context of nutritional status.
Recently, the concept of oral frailty has been introduced in Japan. According to the Japan Dental Association, oral frailty presents as a series of phenomena and processes characterized by vulnerable oral health status due to age-related changes in different oral health conditions (number of teeth, oral hygiene, oral functions, etc.), which is accompanied by a decreased interest in oral health and physical and mental reserve capacity, which can lead to deterioration in eating function, potentially resulting in physical and mental disorders [22]. Oral frailty is used to educate the public on the importance of oral function.
The importance of oral function is receiving more attention from clinicians, researchers, and citizens than ever before. There is a need to establish the latest evidence from studies investigating the association between oral function and nutrition among older adults. In this review, we examined papers that describe associations between oral functions and nutrition that were published in the past 3 years (between March 2018 and March 2021).
2. Methods
The MEDLINE database was searched using PubMed (http://www.ncbi.nlm.nih.gov/pubmed) for papers published in English between March 2018 and March 2021. The terms used for the PubMed search query were as follows:
("Oral Health"[MH] OR "Mouth Diseases"[MH] OR " Mastication"[MH] OR " Deglutition Disorders"[MH] OR "Tooth Diseases"[MH] OR "Saliva"[MH] OR "oral frailty"[All Fields] OR "oral pain"[All Fields] OR "mouth pain"[All Fields] OR "tongue pressure"[All Fields] OR "occlusal force"[All Fields] OR "tongue-lip motor function"[All Fields] OR "functional dentition"[All Fields]) AND ("Malnutrition"[MH] OR "Nutritional Status"[MH] OR "Food"[MH] OR "Diet"[MH] OR "Thinness"[MH] OR "Body Weight"[MH] OR "Body Mass Index"[MH]) AND "Aged"[MH] AND "English"[Language] AND 2018/03/15[PDAT]: 2021/03/15[PDAT].
The studies identified by the electronic search were screened by examining the titles and abstracts. Papers were determined to be eligible if they explored the association between both subjectively and objectively assessed oral function and nutritional status. Full texts of these eligible studies were further screened based on the following exclusion criteria: (1) case reports, in vitro studies, animal experiments, letters to the editor, systematic or narrative reviews, guidelines, or comments; (2) observational or interventional studies that did not include any of the following oral variables: tooth loss, use of dental prosthesis, any index of oral dryness, occlusal force, tongue-lip motor function, tongue pressure, masticatory performance, and swallowing function; (3) observational or interventional studies that did not use any objective index for nutritional status or quantitative data for dietary intake; and (4) observational or interventional studies that did not include individuals aged ≥65 years old.
Finally, the methodological and reporting quality was evaluated using the National Heart Lung and Blood Institute (NHLBI) quality assessment tool [23]. Studies were excluded if they met <50% of the NHLBI criteria. The NHLBI tool for observational studies comprises 14 items; of these, 10 are applicable to cross-sectional studies, and all 14 are applicable to longitudinal studies. The NHLBI tool for interventional studies comprises 14 items. The NHLBI tool is a widely used assessment tool for evaluating qualities of epidemiological studies. The NHLBI tool allowed for assessment of methodological flaws, such as sampling, adjustment for confounders, study power and other relevant factors for the study [23,24].
3. Results
3.1. Selected articles
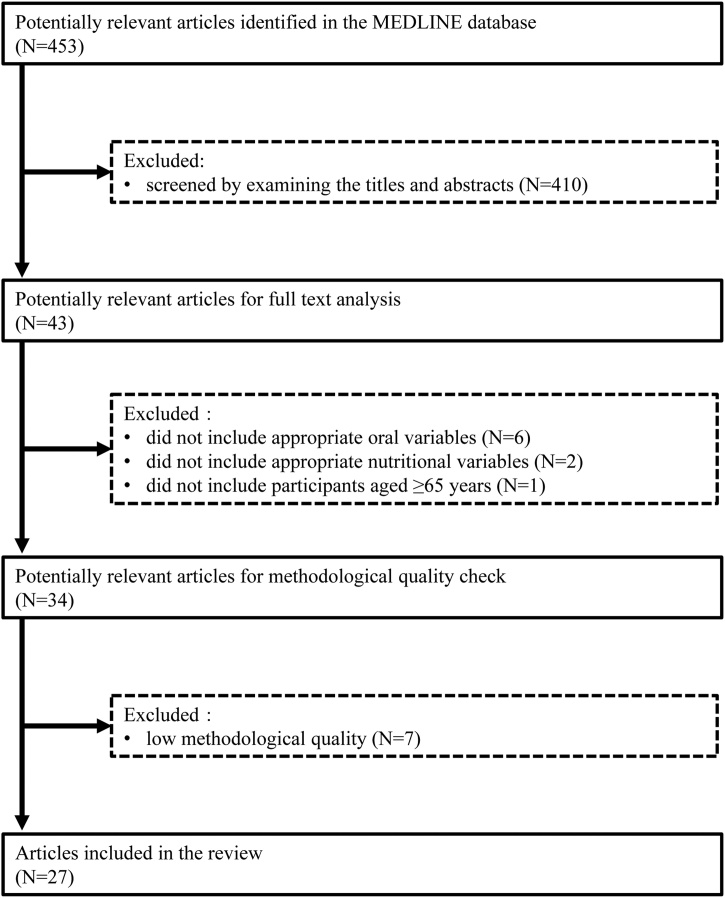
A total of 453 articles were identified. Of these, 410 articles were excluded because the contents of the article, based on the title and abstract, did not meet our criteria. The full texts of 43 articles were reviewed in detail, and 9 articles (6 did not include appropriate oral variables, 2 did not include appropriate nutritional variables, and 1 did not include participants aged ≥65 years) were excluded based on the criteria listed in the Methods section. The final group of 34 articles underwent a methodological quality assessment. Seven studies with low methodological quality were excluded. Ultimately, 27 articles were included in this review (Fig. 1).
Fig. 1.
Flowchart of the study selection.
3.2. Study characteristics
The selected studies included 23 observational studies [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] (17 cross-sectional [[25], [26], [27], [28], [29], [30], [31],[33], [34], [35],37,38,40,42,43,45,46] and 6 longitudinal designs [28,30,32,36,39,44]) and 4 interventional studies [[47], [48], [49], [50]]. The number of study participants ranged between 32 and 28,738.
Various nutritional variables were used in the studies. These variables can be categorized as nutritional assessment or screening tools (listed in Table 1), measures of anthropometry including body composition (e.g., body mass index [BMI], muscle mass, and body fat), biochemical markers (e.g., serum albumin level), and diet quality and quantity. Different studies also had different definitions of oral function, which included dentition status, objectively and subjectively assessed oral functions such as masticatory and swallowing abilities, dysphagia assessment or screening tools (MD Anderson Dysphagia Inventory [MDADI], 10-item Eating Assessment Tool [EAT-10], and Functional Oral Intake Scale), and oral frailty defined as a multicomponent phenotype of poor oral function. Dentition status is a morphological aspect of oral health; however, this index is included in the component of oral frailty [51]. Therefore, we decided to include this index in our review.
Table 1.
Nutritional assessment or screening tools used in the studies included in our review.
| Nutritional assessment or screening tools | Score interpretation | [#Ref.] |
|---|---|---|
| Mini Nutritional Assessment | The lower the score, the greater the risk for malnutrition | [25,29,31,35,37,38,43,44] |
| Geriatric nutritional risk index | The lower the score, the greater the risk for malnutrition | [34] |
| Patient-Generated Subjective Global Assessment | The higher the score, the greater the risk for malnutrition | [36] |
| Prognostic nutritional index | The lower the score, the greater the risk for malnutrition | [52] |
| Minimal Eating Observation and Nutrition Form version II | The higher the score, the greater the risk for malnutrition | [27] |
| Malnutrition Universal Screening Tool | The higher the score, the greater the risk for malnutrition | [41] |
[#Ref.] indicates the reference number for the studies using the tools.
3.3. Observational cross-sectional studies investigating the association of oral functions with nutrition
Among the 17 observational cross-sectional studies [[25], [26], [27],29,31,[33], [34], [35],37,38,[40], [41], [42], [43],45,46,52] included in this review, 16 studies [[26], [27], [28], [29],[31], [32], [33], [34], [35], [36], [37], [38],[40], [41], [42], [43], [44], [45], [46],52] revealed an association between oral function and nutrition. One study [25] found no association. The author, publication year, setting, participants, measures (exposure and outcome), and results are summarized in Table 2. We presented key results of each study in a numerical form that includes estimates of associations and appropriate measures of variability and uncertainty (e.g., regression coefficients or odds ratios with confidence intervals).
Table 2.
Overview of included observational cross-sectional studies investigating the association of oral function with dietary intake and nutritional status.
| Author [#Ref.] | Year | Setting | Participants | Measures |
Results | ||
|---|---|---|---|---|---|---|---|
| n | Age | Exposure | Outcome | ||||
| Abe et al. [52] | 2019 | Hospital | 93 | Mean (s.d.) = 69.0 (9.2), 72.9 (8.8), and 73.5 (9.7) for the group with Eichner index A, B, and C | Eichner index | PNI | Compared with gastric cancer patients with Eichner index A, those with Eichner index C had a lower PNI (coefficient = −3.93, p < 0.01). |
| Bassim et al. [42] | 2020 | Community | 28,738 | Range = 45–85 | Number of self-reported oral health problems (e.g., dry mouth, chewing inadequacy) | Poor diet scale | Poorer oral health was associated with increased poor diet scale scores (linear regression model, standardized beta [95% CI] = 0.40 [0.20–0.61] for those with 1 oral health problem and 0.76 [0.57–0.95] for those with ≥2 oral health problems). |
| Blanař et al. [41] | 2019 | Hospital/nursing home | 17,580 | Mean (s.d.) = 68.5 (18.8) for the group with MUST of ≥1 and 67.1 (17.5) for the group with MUST of 0 | Self-reported swallowing problems | Malnutrition (defined as MUST of ≥1) | Patients with self-reported swallowing problems had higher odds of malnutrition (logistic regression model, adjusted OR [95% CI] = 2.2 [1.9–2.5]). |
| Chatindiara et al. [43] | 2018 | Hospital | 234 | Mean (s.d.) = 83.6 (7.6) | EAT-10 | MNA-SF | High EAT-10 score was associated with low MNA-SF score (Poisson regression model, adjusted PR [95% CI] = 0.98 [0.97–0.99]). |
| de Medeiros et al. [25] | 2020 | Nursing home | 344 | Mean (s.d.) = 77.7 (9.1) | Masticatory performance (variance of hue) | MNA-SF score, BMI, muscle mass (kg), and body fat (%) | The masticatory performance and swallowing threshold were not correlated with MNA-SF total score (r = −0.058, p = 0.30 for masticatory performance and r = 0.073, p = 0.20 for swallowing threshold), BMI (r = −0.015, p = 0.83 for masticatory performance and r = 0.053, p = 0.45 for swallowing threshold), muscle mass (r = 0.003, p = 0.96 for masticatory performance and r = −0.012, p = 0.86 for swallowing threshold) or body fat (r = −0.078, p = 0.26 for masticatory performance and r = 0.115, p = 0.10 for swallowing threshold). |
| Swallowing threshold (number of chewing cycles) | |||||||
| Fukutake et al. [26] | 2018 | Community | 164 | ≥69 | OSA | Dietary intake assessed using BDHQ | OSA score was associated with intake of green and yellow vegetables (linear regression model, β = 0.16, p = 0.03) and α-tocopherol (β = 0.16, p = 0.03). |
| Gaewkhiew et al. [40] | 2019 | Community | 788 | ≥60 | Presence of FD and dentures | Underweight (BMI < 18.5 kg/m2), overweight or obese (BMI > 25 kg/m2), and nutrient intake estimated using FFQ | Study participants with FD were less likely to be underweight than those with neither FD nor dentures (Poisson regression model, adjusted PR [95% CI] = 0.39 [0.16–0.95]). |
| Hägglund et al. [27] | 2019 | Institutionalized | 391 | Median (IQR) = 84 (78–89) | Swallowing dysfunction defined by a timed water swallow test | Malnutrition (MEONF-II ≥3) | Study participants with swallowing dysfunction had higher odds of malnutrition (logistic regression model, adjusted OR [95% CI] = 1.74 [1.04–2.92]). |
| Iwasaki et al. [29] | 2020 | Community | 1054 | Mean (s.d.) = 77.0 (4.8) | Oral frailty | Malnutrition (MNA-SF categorization and serum albumin) | Study participants with oral frailty had higher odds of malnutrition (logistic regression model, adjusted OR [95% CI] = 2.17 [1.58–2.98] for MNA-SF categorization and 1.59 [1.10–2.31] for serum albumin). |
| Izumi et al. [37] | 2020 | Hospital | 52 | ≥65 | Tongue pressure | Malnutrition (MNA-SF <12) | Greater tongue pressure was associated with lower odds of malnutrition (logistic regression model, adjusted OR [95% CI] = 0.88 [0.88–0.99]. |
| Lindroos et al. [31] | 2019 | Nursing home | 3123 | Mean (s.d.) = 84 (8), 85 (7), 85 (8), and 84 (8) in group with 0, 1, 2, and 3 oral health symptoms. | MNA | Chewing problems, swallowing difficulties, and dry mouth assessed by trained nurse (no specific tests were used) | Malnutrition evaluated using MNA classification was associated with several oral symptoms (logistic regression model, adjusted OR [95% CI] = 0.50 [0.41–0.59] for being at risk of malnutrition and 0.27 [0.20–0.36] for being well-nourished compared with the malnourished population). |
| Motokawa et al. [45] | 2021 | Community | 509 | ≥65 | Chewing ability assessed using color-changeable chewing gum | Malnutrition (serum albumin <4.0 g/dL) | Study participants with low chewing ability had higher odds of malnutrition (logistic regression model, adjusted OR [95% CI] = 1.5 [1.004–2.0]). |
| Natapov et al. [46] | 2018 | Community | 1776 | ≥65 | Self-reported chewing problems | Dietary intake estimated by 24 h dietary recall method | Dietary intakes of energy (1355 kcal [with chewing problems] vs. 1480 kcal [without chewing problems]), dietary fiber (15.3 g vs. 17.6 g), protein (3.2 servings vs. 3.5 servings), and vegetables (2.0 servings vs. 2.4 servings) were lower in study participants with chewing problems than in those without (linear regression model). |
| Nishida et al. [38] | 2021 | Community | 320 | ≥65 | Dysphagia defined as EAT-10 of ≥3 | Malnutrition (MNA-SF<12) and prefrailty or frailty assessed using CHS criteria | Dysphagia was associated with malnutrition (logistic regression model, adjusted OR [95% CI] = 4.0 [1.9–8.2]) and frailty status (logistic regression model, adjusted OR [95% CI] = 2.3 [1.0–5.2]). |
| Okamoto et al. [33] | 2019 | Community | 3134 | Median (IQR) = 71 (68–75) | Number of teeth and occlusal force | Low serum albumin (<4.4 g/dL) and low BMI (<21.0 kg/m2) | Compared with the women with an occlusal force of ≥500 N, those with an occlusal force of <300 N had higher odds of low serum albumin (logistic regression model, adjusted OR [95% CI] = 1.65 [1.06–2.55] for the group with 100–300 N and 1.95 [1.15–3.31] for the group with <100 N). |
| Saito et al. [34] | 2018 | Hospital | 165 | Median = 76 | Malnutrition defined as GNRI of <91.2 | Dysphagia defined as PAS of 4–8 | Compared with the patients without malnutrition, those with malnutrition had higher odds of dysphagia (logistic regression model, adjusted OR [95% CI] = 3.1 [1.1–9.1]). |
| Tagliaferri et al. [35] | 2019 | Community (outpatients) | 773 | Mean (s.d.) = 82.0 (7.1) | MNA-SF | Swallowing difficulties defined as EAT-10 of ≥3. | Higher MNA-SF score was associated with lower odds of swallowing difficulties (logistic regression model, adjusted OR [95% CI] = 0.91 [0.83–0.99]). |
β = standardized coefficient, BMI = body mass index, CHS = Cardiovascular Health Study, CI = confidence interval, EAT-10 = 10-item Eating Assessment Tool, FD = functional dentition, FFQ = food frequency questionnaire, GNRI = geriatric nutritional risk index, IQR = interquartile range, MEONF-II = Minimal Eating Observation and Nutrition Form version II, MUST = Malnutrition Universal Screening Tool, OR = odds ratio, OSA = oral stereognostic ability, PAS = penetration–aspiration scale, PNI = prognostic nutritional index, PR = prevalence ratio, r = correlation coefficient, s.d. = standard deviation.
Nine studies [26,29,33,35,38,40,42,45,46] included community-dwelling older adults. Bassim et al. [42] explored the cross-sectional associations among poor oral health, poor diet, and frailty in Canadian adults aged 45–85 years. This is the largest study included in this review. Oral functions were assessed using a self-administered questionnaire that included questions about oral health problems (dry mouth, chewing inadequacy, etc.). Based on the responses to these questions, a cumulative oral health deficit score (ranging from 0 to 24) was calculated and used to operationalize poor oral health. Dietary intake of seven food groups that are considered healthy—fruits, vegetables, nuts, legumes, fish, dairy, and meats (chicken and red meats)—was estimated by using a questionnaire. Based on the consumption level of each food group category, the overall diet score, ranging from 0 to 28, with a higher score indicating lower consumption of healthy food groups or a worse diet, was generated. Frailty was operationalized by calculating a frailty index that was created based on Rockwood’s frailty index [53]. Interrelationships among oral health deficit score, overall diet score, and frailty index were studied using path analysis. As a result, poorer oral health (higher oral health deficit score) was associated with a higher score on the poor diet scale. Moreover, path analysis revealed that poorer oral health indirectly affects frailty status through a poor score on the diet scale. This study is important because the study findings support long-discussed pathways linking poor oral health and frailty through diet. It should be noted, however, that the indirect effect of poor oral health through a poor diet was modest (i.e., representing only approximately 1% of the direct effect of poor oral health on frailty). Similar results were found among the National Health and Nutrition Examination Survey (NHANES) participants aged ≥60 years. Hakeem et al. [54] found that the number of teeth was negatively associated with frailty and that nutritional intake had a modest effect on this relationship among NHANES participants. The findings of Bassim’s study also indicate that oral functions were associated with diet and frailty in a dose-response manner. The cumulative effects of poor oral functions were operationalized as oral frailty [51]. We also obtained results consistent with Bassim’s study. We observed that oral frailty was associated with the presence and severity of malnutrition among community-dwelling older Japanese individuals [29]. The strength of our study was that unlike Bassim’s study, we used clinical data to assess oral function and defined nutritional status by using the Mini Nutritional Assessment-short form (MNA-SF), a validated and widely used nutrition screening tool, and serum albumin level.
Other studies of community-dwelling older adults revealed associations between oral stereognostic ability and dietary intake [26], between masticatory function assessed using color-changeable chewing gum and serum albumin level [45], between self-rated masticatory function and dietary intake [46], and between occlusal force and serum albumin level [33]. Unlike other studies conducted in a community setting that used oral function as an exposure, the study of Tagliaferri et al. [35] used low swallowing ability as an outcome. They reported that a higher MNA-SF score was associated with lower odds of swallowing difficulties, defined as an EAT-10 score ≥3.
The associations of oral aspects and function with nutritional status were also studied in older adults at a hospital, nursing home, and other health care facilities. Six studies [25,27,37,41,43,52] used oral function as an exposure. Among them, 3 studies found that low swallowing ability was associated with malnutrition assessed using the Malnutrition Universal Screening Tool [41], MNA-SF [43], or Minimal Eating Observation and Nutrition Form version II [27]. Abe et al. [52] found that study participants without functional dentition were likely to have poor nutritional status. Izumi et al. [37] found that greater tongue pressure was associated with a lower prevalence of malnutrition as defined by MNA-SF. One study [25] reported that masticatory performance that was assessed based on the degree of color mixing of a two-color chewing gum was not associated with MNA-SF or body composition.
On the other hand, 2 studies [31,34] used low swallowing ability as an outcome. These studies found that study participants with poor nutritional status tended to have poor oral functions.
3.4. Observational longitudinal studies investigating the association of oral functions with nutrition
Among the 6 longitudinal studies [28,30,32,36,39,44] included in this review, 4 studies [28,32,36,44] found an association between oral function and nutrition. Two studies [30,39] found no association. The author, publication year, setting, participants, measures (exposure and outcome), and results are summarized in Table 3.
Table 3.
Overview of included observational longitudinal studies investigating the association of oral function with dietary intake and nutritional status.
| Author [#Ref.] | Year | Setting | Participants |
Measures |
Results | ||
|---|---|---|---|---|---|---|---|
| n | Age | Exposure | Outcome | ||||
| Gaewkhiew et al. [39] | 2020 | Community | 651 | ≥60 | Presence of FD and dentures | BMI, WC, TSF, and nutrient intake estimated using FFQ | Presence of FD and dentures at baseline was not associated with 12-month changes in BMI, WC, TSF, and nutrient intake. |
| Hiratsuka et al. [28] | 2020 | Community | 891 | Mean (s.d.) = 75.5 (4.7) | Number of teeth | All-cause mortality | Having 1−9 teeth was associated with increased mortality mediated by malnutrition compared with having ≥20 teeth (mediation analysis, mediation proportion [95% CI] = 10.0 [3.0–28.7]). |
| Mediator = malnutrition (serum albumin <3.8 g/dL) | |||||||
| Kiesswettera et al. [30] | 2019 | Community | 893 | Mean (s.d.) = 67.6 (6.1) | Oral health characteristics based on self-report | Self-reported involuntary weight loss of ≥5% or low BMI (<20 kg/m2 and <22 kg/m2 in participants <70 and ≥70 years, respectively) | Toothache while chewing was associated with incidence of malnutrition (Cox-proportional hazard regression model, adjusted HR [95% CI] = 2.14 [1.10–4.19]). Self-rated oral health status (2.10 [0.88–4.98]) and xerostomia with edentulous (1.99 [0.93–4.28]) were close to the level of significance. |
| Logan et al. [32] | 2020 | Community | 1096 | Mean (s.d.) = 67.6 (6.1) | Dentition status: 21–28 teeth with and without dentures, 1–20 teeth with and without dentures, and edentate with dentures | Dietary intake estimated using FFQ | After an average time period of 13 years, having 21 or more natural teeth remaining was positively associated with dietary intake of fruit, vegetables, and nuts and resulted in higher diet quality scores (MDS and DDS) compared with those with severe tooth loss (i.e., 1–20 teeth or edentate) (linear regression model). |
| Maeda et al. [44] | 2019 | Hospital | 8768 | Mean (s.d.) = 76.1 (6.9) | BMI, MNA-SF, and amount of food intake at admission | Decline in swallowing ability indicated by FOIS of ≤5 at discharge | Malnutrition evaluated using MNA-SF (logistic regression model, adjusted OR [95% CI] = 0.92 [0.87–0.97]) and insufficient nutritional intake (OR [95% CI] = 2.33 [1.60–3.40]) were associated with swallowing disorder development. |
| Wang et al. [36] | 2020 | Hospital (patients with head and neck cancer undergoing RT) | 122 | Mean (s.d.) = 51.3 (15.2) | MDADI | Weight ratio (present weight/baseline weight × 100%) and PG-SGA | Poor swallowing functional outcomes related to a lower weight ratio (GEE, regression weight = 0.032, p = 0.01) and worsened nutritional status (regression weight = −0.115, p < 0.01). |
β = standardized coefficient, BMI = body mass index, CI = confidence interval, DDS = dietary diversity score, FD = functional dentition, FFQ = food frequency questionnaire, FOIS = functional oral intake scale, GEE = generalized estimating equation, HR = hazard ratio, IQR = interquartile range, MDADI = MD Anderson Dysphagia Inventory, MDS = Mediterranean diet score, OR = odds ratio, PG-SGA = Patient-Generated Subjective Global Assessment, RT = radiotherapy, s.d. = standard deviation, TSF = triceps skinfold thickness, WC = waist circumference.
Hiratsuka et al. [28] found that a lower number of teeth was associated with increased mortality mediated by low serum albumin levels. Logan et al. [32] found that having 21 or more natural teeth remaining was positively associated with dietary intake of fruit, vegetables, and nuts and resulted in higher diet quality scores compared with those of individuals with severe tooth loss. Wang et al. [36] collected clinical data from 122 patients with head and neck cancer undergoing radiotherapy (RT) at three time points: baseline, the third week of RT, and upon completion of RT. They found that the swallowing functional outcomes assessed using the MDADI worsened over the course of RT and were associated with a lower weight ratio (present weight/baseline weight × 100%) and worsened nutritional status assessed using the Patient-Generated Subjective Global Assessment. Maeda et al. [44] found that malnutrition at the time of admission was associated with a higher risk of swallowing disorder development at discharge among older adults. The link between malnutrition and swallowing disorders is thought to be that sarcopenic changes in eating-related muscles occur due to poor nutritional status [55]. It should be noted, however, that no definitive conclusion can be drawn because this interpretation is based on a single cross-sectional observational study [44]. It may be more biologically plausible that older people with swallowing disorders have problems getting an adequate diet and so end up poorly nourished.
3.5. Intervention studies investigating the effect of prosthetic intervention on nutritional status
Four interventional studies (3 randomized controlled trials [RCTs] [[47], [48], [49]] and one non-RCT [50]) were identified (Table 4). These studies investigated the combined effect of dental prosthesis treatments, including complete or partial denture fabrication, and dietary advice or counseling on nutritional status among older adults. Of note, none of the included studies investigated the effect of dental prosthesis treatment alone. There is evidence that dietary advice or counseling may improve nutritional status in older people affected by undernutrition [[56], [57], [58]]. The three RCTs investigated complete denture (CD) fabrication and simple dietary advice provided by dentists using a pamphlet prepared with reference to the geriatric version of the Japanese Food Guide Spinning Top published by the Japanese Ministry of Agriculture, Forestry and Fisheries [59]. Compared to the control group (i.e., without dietary advice), the intervention group (i.e., with dietary advice) showed higher dietary intakes of several nutrients, including protein [48], at the 3-month posttreatment examinations and showed higher nutrient intake [47] and a higher MNA-SF score [49] at the 6-month posttreatment examinations. On the other hand, Nabeshima et al. [50] examined the effect of dietary counseling by nutritionists on dietary intake among older patients who received partial denture (PD) placements (before-after design with no control group). They observed that dietary intake of vegetables, α- and β-carotenes, and dietary fiber was higher at a follow-up evaluation 3 months after PD placement.
Table 4.
Overview of included intervention studies investigating the effect of prosthetic intervention on dietary intake and nutritional status.
| Author [#Ref.] | Year | Participants |
Design RCT/non-RCT | Study period | Measures |
Results | ||
|---|---|---|---|---|---|---|---|---|
| n | Age | Intervention | Outcome | |||||
| Kanazawa et al. [47] | 2019 | 70 (intervention: n = 35, control: n = 35) | Mean (s.d.) = 74.8 (8.0) in the intervention group and 78.6 (6.8) in the control group | RCT | 6 months | Intervention group: maxillary and mandibular CD fabrication and dietary advice | Dietary intake estimated by BDHQ | Higher dietary intakes of several nutrients including protein (ANCOVA, mean = 100.2 g in intervention group vs. 93.3 g in control group, p < 0.01) were seen in the intervention group at 3 months post-intervention, but not at 6 months post-intervention. |
| Control group: maxillary and mandibular CD fabrication | ||||||||
| Nabeshima et al. [50] | 2018 | 32 | Mean (s.d.) = 73.2 (7.7) | Non-RCT | 3 months | PD placement and dietary counseling | Dietary intake estimated by BDHQ | Dietary intake of vegetables (linear mixed model, effect size between post-intervention and baseline = 74 g), α- (70 μg) and β-carotenes (1036 μg), and dietary fiber (2.5 g) increased at follow-up evaluation 3 months after the PD placement. Serum concentration of carotenoids and vitamin C remained consistent. |
| Serum concentration of carotenoids and vitamin C | ||||||||
| Suzuki et al. [48] | 2018 | 62 (intervention: n = 31, control: n = 35) | Mean (s.d.) = 75.3 (8.2) in intervention group and 78.6 (6.6) in control group | RCT | 3 months | Intervention group: maxillary and mandibular CD fabrication and dietary advice | Dietary intake estimated by BDHQ | Higher dietary intakes of several nutrients, including protein (ANCOVA, mean = 101.6 g in intervention group vs. 93.5 g in control group, p < 0.01), were seen in the intervention group at 3 months postintervention. |
| Control group: maxillary and mandibular CD fabrication | ||||||||
| Suzuki et al. [49] | 2019 | 59 (intervention: n = 30, control: n = 29) | Mean (s.d.) = 74.8 (8.0) in intervention group and 78.6 (6.8) in control group | RCT | 6 months | Intervention group: CD fabrication and dietary advice | MNA-SF | Higher MNA-SF score (Mann–Whitney U test, median [IQR] = 13 [13,14] in intervention group vs. 13 [[11], [12], [13]] in control group, p < 0.01) was seen in the intervention group at 6 months postintervention. |
| Control group: CD fabrication | ||||||||
ANCOVA = one-way analysis of covariance, BDHQ = brief-type self-administered diet history questionnaire, CD = complete denture, PD = partial denture.
4. Discussion
Multiple studies published in the past 3 years have demonstrated the association between oral function and nutrition in older adults. Of note, two longitudinal studies [28,32] indicated that poor oral function increased the risk of malnutrition in community-dwelling older adults. One longitudinal study [36] also demonstrated temporal associations of poor oral function with malnutrition in hospitalized older adults. Furthermore, in the hospital setting, another longitudinal study [44] demonstrated an association between oral function and nutritional status in the opposite direction; that is, malnutrition led to poor oral function.
The prevalence of oral diseases increases with advancing age [60]. Oral diseases can cause poor oral function, including masticatory and swallowing hypofunction, which may result in a deterioration of nutritional intake, ultimately leading to malnutrition. On the other hand, malnutrition adversely affects muscle mass and strength [61]. This notion can also apply to eating-related muscles [44] and ultimately leads to a decline in oral function. Because more studies investigating the effects of malnutrition on oral function were conducted in inpatient study populations [31,34,44] than in outpatient populations [35], the adverse effects of malnutrition on muscle functions may be pronounced in frail persons. A recent intervention study revealed that a high-calorie diet provided energy for patients with dysphagia and improved their swallowing ability [62].
There is another potential pathway through which malnutrition might be connected to poor oral function. Malnutrition has adverse effects on the host defense system [63] and thus can increase the risk for oral diseases. In addition, several nutrients, such as carotenoids and vitamins C and E, have anti-inflammatory and antioxidant properties [64]. Inadequate intake of these nutrients due to poor oral function may increase the risk of periodontitis [65,66]. Overall, a negative cycle where poor oral function leads to malnutrition, which leads to oral diseases, which lead to poor oral function can be expected. Maintaining good oral function at an advanced age is thus important for preventing individuals from falling into such a negative cycle.
Swallowing function was the most used indicator for oral function across the studies included in this review. In a previous systematic review on the association between oral health and malnutrition in older adults in a long-term care facility, masticatory function was the most used indicator [15]. Another systematic review examined mastication function [19]. Recently, swallowing function has attracted increasing attention with regard to nutritional status.
Because individual oral health problems are interrelated and their prevalence increases with age, older adults frequently have coexisting oral health problems [29,67]. Oral frailty has been introduced as a state of deterioration of multiple oral functions [51]. Further studies investigating the association between comprehensive oral function and nutrition are warranted.
It should be noted that most of the studies included in this review investigated a single aspect of oral function with regard to nutrition. The results of intervention studies included in this review demonstrate the importance of dietary intervention during prosthetic treatment. These results are in agreement with those of earlier studies [68,69]. Moreover, previous RCTs did not produce consistent results supporting the effects of prosthetic treatment alone on older patients’ nutritional status [70,71]. Dietary advice or counseling is effective by itself to improve nutritional status in older people [[56], [57], [58]]. Overall, available evidence indicates the importance of multidisciplinary cooperation involving professionals in dentistry and nutrition to improve the nutritional status of older persons with tooth loss.
This review has some limitations. We searched a single electronic database. In addition, we did not search non-English language publications or unpublished studies. This limited search strategy may have led to some relevant studies being missed.
5. Conclusions
The current review revealed that research findings support a potential relationship of poor oral function with an inadequate and poor-quality diet and malnutrition. Older adults with poor oral function are likely to have poorer dietary intake and poorer nutritional status, and malnourished older adults are likely to have poorer oral function. Maintaining good oral function may be a key for longevity. However, currently, there is limited evidence from longitudinal studies, and comprehensive oral function is not studied in detail, indicating the need for additional high-quality studies.
Furthermore, the combined effects of dental and dietary interventions should be examined in more detail by conducting interventional studies. These studies should explore (1) the durations for which the intervention effects last and (2) whether the degree of the effects of dietary interventions differ based on the provider (i.e., professionals in nutrition or nonprofessionals) and the frequency and timing of the intervention. Moreover, well-designed studies that compare the nutritional outcomes between two groups with the same nutritional interventions but delayed or immediate dental interventions are necessary to better evaluate the combined effects of dental and dietary interventions. Such studies are important for gaining insight into a more effective interprofessional approach for nutritional management in older adults in dental clinical settings.
Conflict of interest
None.
Role of the funding source
This work was supported by JSPS KAKENHI (Grant Number 18K09919) and the MHLW GA Program (Grant Number JPMH20GA1003).
Data availability
The data supporting this review were obtained from previously reported studies and datasets, which have been cited.
Ethics approval
No ethical approval was needed because data from previously published studies in which informed consent was obtained by primary investigators were retrieved and analyzed.
References
- 1.Nieuwenhuizen W.F., Weenen H., Rigby P., Hetherington M.M. Older adults and patients in need of nutritional support: review of current treatment options and factors influencing nutritional intake. Clin Nutr. 2010;29:160–169. doi: 10.1016/j.clnu.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Ervin R.B. Healthy Eating Index scores among adults, 60 years of age and over, by sociodemographic and health characteristics: United States, 1999-2002. Adv Data. 2008:1–16. [PubMed] [Google Scholar]
- 3.Tangney C.C., Evans D.A., Bienias J.L., Clare Morris M. Healthy eating index of black and white older adults. Nutr Res. 2001;21:1411–1423. [Google Scholar]
- 4.Kaiser M.J., Bauer J.M., Ramsch C., Uter W., Guigoz Y., Cederholm T. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58:1734–1738. doi: 10.1111/j.1532-5415.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 5.Correia M.I., Waitzberg D.L. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235–239. doi: 10.1016/s0261-5614(02)00215-7. [DOI] [PubMed] [Google Scholar]
- 6.Houston D.K., Nicklas B.J., Ding J., Harris T.B., Tylavsky F.A., Newman A.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 7.Micha R., Peñalvo J.L., Cudhea F., Imamura F., Rehm C.D., Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317:912–924. doi: 10.1001/jama.2017.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George S.M., Ballard-Barbash R., Manson J.E., Reedy J., Shikany J.M., Subar A.F. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol. 2014;180:616–625. doi: 10.1093/aje/kwu173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford D.W., Hartman T.J., Still C., Wood C., Mitchell D.C., Erickson P. Body mass index, poor diet quality, and health-related quality of life are associated with mortality in rural older adults. J Nutr Gerontol Geriatr. 2014;33:23–34. doi: 10.1080/21551197.2014.875819. [DOI] [PubMed] [Google Scholar]
- 10.Savoca M.R., Arcury T.A., Leng X., Chen H., Bell R.A., Anderson A.M. Association between dietary quality of rural older adults and self-reported food avoidance and food modification due to oral health problems. J Am Geriatr Soc. 2010;58:1225–1232. doi: 10.1111/j.1532-5415.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshihara A., Watanabe R., Nishimuta M., Hanada N., Miyazaki H. The relationship between dietary intake and the number of teeth in elderly Japanese subjects. Gerodontology. 2005;22:211–218. doi: 10.1111/j.1741-2358.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 12.Ritchie C.S., Joshipura K., Hung H.C., Douglass C.W. Nutrition as a mediator in the relation between oral and systemic disease: associations between specific measures of adult oral health and nutrition outcomes. Crit Rev Oral Biol Med. 2002;13:291–300. doi: 10.1177/154411130201300306. [DOI] [PubMed] [Google Scholar]
- 13.Chen C.C., Schilling L.S., Lyder C.H. A concept analysis of malnutrition in the elderly. J Adv Nurs. 2001;36:131–142. doi: 10.1046/j.1365-2648.2001.01950.x. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki M., Taylor G.W., Manz M.C., Yoshihara A., Sato M., Muramatsu K. Oral health status: relationship to nutrient and food intake among 80-year-old Japanese adults. Community Dent Oral Epidemiol. 2014;42:441–450. doi: 10.1111/cdoe.12100. [DOI] [PubMed] [Google Scholar]
- 15.Van Lancker A., Verhaeghe S., Van Hecke A., Vanderwee K., Goossens J., Beeckman D. The association between malnutrition and oral health status in elderly in long-term care facilities: a systematic review. Int J Nurs Stud. 2012;49:1568–1581. doi: 10.1016/j.ijnurstu.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Toniazzo M.P., Amorim P.S., Muniz F., Weidlich P. Relationship of nutritional status and oral health in elderly: systematic review with meta-analysis. Clin Nutr. 2018;37:824–830. doi: 10.1016/j.clnu.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Kossioni A.E. The association of poor oral health parameters with malnutrition in older adults: a review considering the potential implications for cognitive impairment. Nutrients. 2018;10:1709. doi: 10.3390/nu10111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiesswetter E., Poggiogalle E., Migliaccio S., Donini L.M., Sulmont-Rossé C., Feart C. Functional determinants of dietary intake in community-dwelling older adults: a DEDIPAC (DEterminants of DIet and Physical ACtivity) systematic literature review. Public Health Nutr. 2018;21:1886–1903. doi: 10.1017/S1368980017004244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tada A., Miura H. Systematic review of the association of mastication with food and nutrient intake in the independent elderly. Arch Gerontol Geriatr. 2014;59:497–505. doi: 10.1016/j.archger.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Gaewkhiew P., Sabbah W., Bernabé E. Does tooth loss affect dietary intake and nutritional status? A systematic review of longitudinal studies. J Dent. 2017;67:1–8. doi: 10.1016/j.jdent.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki M., Sato M., Yoshihara A., Miyazaki H. Malnutrition and oral disease in the elderly––is there any bidirectional relationship? Curr Oral Health Rep. 2017;4:70–78. [Google Scholar]
- 22.Japan Dental Association . 2019. Manual for oral frailty at dental clinics.https://www.jda.or.jp/dentist/oral_flail/pdf/manual_all.pdf [Accessed 16 April 2021] [Google Scholar]
- 23.National Heart L, and Blood Institute. Study quality assessment tools. [Accessed 16 April 2021] https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 24.Fawole H.O., Idowu O.A., Abaraogu U.O., Dell’Isola A., Riskowski J.L., Oke K.I. Factors associated with fatigue in hip and/or knee osteoarthritis: a systematic review and best evidence synthesis. Rheumatol Adv Pract. 2021;5:rkab013. doi: 10.1093/rap/rkab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Medeiros M.M.D., Pinheiro M.A., de Figueredo O.M.C., de Oliveira L.F.S., Wanderley R.L., Cavalcanti Y.W. Masticatory function in nursing home residents: correlation with the nutritional status and oral health–related quality of life. J Oral Rehabil. 2020;47:1511–1520. doi: 10.1111/joor.13096. [DOI] [PubMed] [Google Scholar]
- 26.Fukutake M., Ikebe K., Okubo H., Matsuda K.I., Enoki K., Inomata C. Relationship between oral stereognostic ability and dietary intake in older Japanese adults with complete dentures. J Prosthodont Res. 2019;63:105–109. doi: 10.1016/j.jpor.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Hägglund P., Fält A., Hägg M., Wester P., Levring Jäghagen E. Swallowing dysfunction as risk factor for undernutrition in older people admitted to Swedish short-term care: a cross-sectional study. Aging Clin Exp Res. 2019;31:85–94. doi: 10.1007/s40520-018-0944-7. [DOI] [PubMed] [Google Scholar]
- 28.Hiratsuka T., Komiyama T., Ohi T., Tanji F., Tomata Y., Tsuji I. Contribution of systemic inflammation and nutritional status to the relationship between tooth loss and mortality in a community-dwelling older Japanese population: a mediation analysis of data from the Tsurugaya project. Clin Oral Investig. 2020;24:2071–2077. doi: 10.1007/s00784-019-03072-y. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki M., Motokawa K., Watanabe Y., Shirobe M., Inagaki H., Edahiro A. Association between oral frailty and nutritional status among community-dwelling older adults: the Takashimadaira study. J Nutr Health Aging. 2020;24:1003–1010. doi: 10.1007/s12603-020-1433-1. [DOI] [PubMed] [Google Scholar]
- 30.Kiesswetter E., Hengeveld L.M., Keijser B.J., Volkert D., Visser M. Oral health determinants of incident malnutrition in community-dwelling older adults. J Dent. 2019;85:73–80. doi: 10.1016/j.jdent.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Lindroos E.K., Saarela R.K.T., Suominen M.H., Muurinen S., Soini H., Kautiainen H. Burden of oral symptoms and its associations with nutrition, well-being, and survival among nursing home residents. J Am Med Dir Assoc. 2019;20:537–543. doi: 10.1016/j.jamda.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Logan D., McEvoy C.T., McKenna G., Kee F., Linden G., Woodside J.V. Association between oral health status and future dietary intake and diet quality in older men: the PRIME study. J Dent. 2020;92 doi: 10.1016/j.jdent.2019.103265. 103265. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto N., Amano N., Nakamura T., Yanagi M. Relationship between tooth loss, low masticatory ability, and nutritional indices in the elderly: a cross-sectional study. BMC Oral Health. 2019;19:110. doi: 10.1186/s12903-019-0778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito T., Hayashi K., Nakazawa H., Yagihashi F., Oikawa L.O., Ota T. A significant association of malnutrition with dysphagia in acute patients. Dysphagia. 2018;33:258–265. doi: 10.1007/s00455-017-9855-6. [DOI] [PubMed] [Google Scholar]
- 35.Tagliaferri S., Lauretani F., Pelá G., Meschi T., Maggio M. The risk of dysphagia is associated with malnutrition and poor functional outcomes in a large population of outpatient older individuals. Clin Nutr. 2019;38:2684–2689. doi: 10.1016/j.clnu.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Zhang L., Jin S., Li H., Gong L., Wang Y. Swallowing functional outcomes and nutritional status in head and neck cancer radiotherapy: longitudinal study. BMJ Support Palliat Care. 2020;10:452–461. doi: 10.1136/bmjspcare-2020-002216. [DOI] [PubMed] [Google Scholar]
- 37.Izumi M., Sonoki K., Ohta Y., Fukuhara M., Nagata M., Akifusa S. Impact of tongue pressure and peak expiratory flow rate on nutritional status of older residents of nursing homes in Japan: a cross-sectional study. J Nutr Health Aging. 2020;24:512–517. doi: 10.1007/s12603-020-1347-y. [DOI] [PubMed] [Google Scholar]
- 38.Nishida T., Yamabe K., Honda S. The Influence of dysphagia on nutritional and frailty status among community-dwelling older adults. Nutrients. 2021;13:512. doi: 10.3390/nu13020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaewkhiew P., Sabbah W., Bernabé E. Functional dentition and 12-month changes in body measurements among Thai older adults. Int J Environ Res Public Health. 2020;17:4200. doi: 10.3390/ijerph17124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaewkhiew P., Sabbah W., Bernabé E. Functional dentition, dietary intake and nutritional status in Thai older adults. Gerodontology. 2019;36:276–284. doi: 10.1111/ger.12408. [DOI] [PubMed] [Google Scholar]
- 41.Blanař V., Hödl M., Lohrmann C., Amir Y., Eglseer D. Dysphagia and factors associated with malnutrition risk: a 5-year multicentre study. J Adv Nurs. 2019;75:3566–3576. doi: 10.1111/jan.14188. [DOI] [PubMed] [Google Scholar]
- 42.Bassim C., Mayhew A.J., Ma J., Kanters D., Verschoor C.P., Griffith L.E. Oral health, diet, and frailty at baseline of the Canadian longitudinal study on aging. J Am Geriatr Soc. 2020 doi: 10.1111/jgs.16377. [DOI] [PubMed] [Google Scholar]
- 43.Chatindiara I., Allen J., Popman A., Patel D., Richter M., Kruger M. Dysphagia risk, low muscle strength and poor cognition predict malnutrition risk in older adults at hospital admission. BMC Geriatr. 2018;18:78. doi: 10.1186/s12877-018-0771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeda K., Ishida Y., Nonogaki T., Shimizu A., Yamanaka Y., Matsuyama R. Development and predictors of sarcopenic dysphagia during hospitalization of older adults. Nutrients. 2019;12 doi: 10.3390/nu12010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motokawa K., Mikami Y., Shirobe M., Edahiro A., Ohara Y., Iwasaki M. Relationship between chewing ability and nutritional status in Japanese older adults: a cross-sectional study. Int J Environ Res Public Health. 2021;18:1216. doi: 10.3390/ijerph18031216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Natapov L., Kushnir D., Goldsmith R., Dichtiar R., Zusman S.P. Dental status, visits, and functional ability and dietary intake of elderly in Israel. Isr J Health Policy Res. 2018;7:58. doi: 10.1186/s13584-018-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanazawa M., Suzuki H., Komagamine Y., Iwaki M., Amagai N., Minakuchi S. Combined effects of new complete denture fabrication and simplified dietary advice on nutrient intake in edentulous elderly patients for 6 months. Clin Oral Investig. 2019;23:2245–2252. doi: 10.1007/s00784-018-2669-6. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki H., Kanazawa M., Komagamine Y., Iwaki M., Jo A., Amagai N. The effect of new complete denture fabrication and simplified dietary advice on nutrient intake and masticatory function of edentulous elderly: a randomized-controlled trial. Clin Nutr. 2018;37:1441–1447. doi: 10.1016/j.clnu.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki H., Kanazawa M., Komagamine Y., Iwaki M., Amagai N., Minakuchi S. Changes in the nutritional statuses of edentulous elderly patients after new denture fabrication with and without providing simple dietary advice. J Prosthodont Res. 2019;63:288–292. doi: 10.1016/j.jpor.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Nabeshima G., Fueki K., Inamochi Y., Wakabayashi N. Effect of dietary counselling with prosthetic restoration on fruit and vegetable intake in partially dentate patients: a prospective study. J Oral Rehabil. 2018;45:618–626. doi: 10.1111/joor.12647. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T., Takahashi K., Hirano H., Kikutani T., Watanabe Y., Ohara Y. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 2018;73:1661–1667. doi: 10.1093/gerona/glx225. [DOI] [PubMed] [Google Scholar]
- 52.Abe A., Kurita K., Hayashi H., Ishihama T., Ueda A. Correlation between prognostic nutritional index and occlusal status in gastric cancer. Oral Dis. 2020;26:465–472. doi: 10.1111/odi.13242. [DOI] [PubMed] [Google Scholar]
- 53.Rockwood K., Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 54.Hakeem F.F., Bernabé E., Sabbah W. Association between oral health and frailty among American older adults. J Am Med Dir Assoc. 2021;22:559–563.e552. doi: 10.1016/j.jamda.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 55.Fujishima I., Fujiu-Kurachi M., Arai H., Hyodo M., Kagaya H., Maeda K. Sarcopenia and dysphagia: position paper by four professional organizations. Geriatr Gerontol Int. 2019;19:91–97. doi: 10.1111/ggi.13591. [DOI] [PubMed] [Google Scholar]
- 56.Munk T., Tolstrup U., Beck A.M., Holst M., Rasmussen H.H., Hovhannisyan K. Individualised dietary counselling for nutritionally at-risk older patients following discharge from acute hospital to home: a systematic review and meta-analysis. J Hum Nutr Diet. 2016;29:196–208. doi: 10.1111/jhn.12307. [DOI] [PubMed] [Google Scholar]
- 57.Baldwin C., Weekes C.E. Dietary advice with or without oral nutritional supplements for disease‐related malnutrition in adults. Cochrane Database Syst Rev. 2011;2011:CD002008. doi: 10.1002/14651858.CD002008.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milne A.C., Potter J., Vivanti A., Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009;2009:CD003288. doi: 10.1002/14651858.CD003288.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ministry of agriculture, forestry and fisheries. The geriatric version of the Japanese food guide spinning top. [Accessed 16 April 2021] https://www.maff.go.jp/j/balance_guide/b_sizai/pdf/korei_all.pdf.
- 60.Dye B.A., Tan S., Smith V., Lewis B.G., Barker L.K., Thornton-Evans G. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat. 2007;11:1–92. [PubMed] [Google Scholar]
- 61.Fujita S., Volpi E. Nutrition and sarcopenia of ageing. Nutr Res Rev. 2004;17:69–76. doi: 10.1079/NRR200481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimizu A., Fujishima I., Maeda K., Wakabayashi H., Nishioka S., Ohno T. Nutritional management enhances the recovery of swallowing ability in older patients with sarcopenic dysphagia. Nutrients. 2021;13:596. doi: 10.3390/nu13020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scrimshaw N.S., SanGiovanni J.P. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–477S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 64.Rock C.L., Jacob R.A., Bowen P.E. Update on the biological characteristics of the antioxidant micronutrients: vitamin C, vitamin E, and the carotenoids. J Am Diet Assoc. 1996;96:693–702. doi: 10.1016/S0002-8223(96)00190-3. quiz 703–694. [DOI] [PubMed] [Google Scholar]
- 65.Iwasaki M., Manz M.C., Taylor G.W., Yoshihara A., Miyazaki H. Relations of serum ascorbic acid and alpha-tocopherol to periodontal disease. J Dent Res. 2012;91:167–172. doi: 10.1177/0022034511431702. [DOI] [PubMed] [Google Scholar]
- 66.Iwasaki M., Moynihan P., Manz M.C., Taylor G.W., Yoshihara A., Muramatsu K. Dietary antioxidants and periodontal disease in community-based older Japanese: a 2-year follow-up study. Public Health Nutr. 2013;16:330–338. doi: 10.1017/S1368980012002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petersen P.E., Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2005;33:81–92. doi: 10.1111/j.1600-0528.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 68.Bartlett D.W., Maggio B., Targett D., Fenlon M.R., Thomas J. A preliminary investigation into the use of denture adhesives combined with dietary advice to improve diets in complete denture wearers. J Dent. 2013;41:143–147. doi: 10.1016/j.jdent.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Bradbury J., Thomason J.M., Jepson N.J., Walls A.W., Allen P.F., Moynihan P.J. Nutrition counseling increases fruit and vegetable intake in the edentulous. J Dent Res. 2006;85:463–468. doi: 10.1177/154405910608500513. [DOI] [PubMed] [Google Scholar]
- 70.Hamdan N.M., Gray-Donald K., Awad M.A., Johnson-Down L., Wollin S., Feine J.S. Do implant overdentures improve dietary intake? A randomized clinical trial. J Dent Res. 2013;92:146S–153S. doi: 10.1177/0022034513504948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muller F., Duvernay E., Loup A., Vazquez L., Herrmann F.R., Schimmel M. Implant-supported mandibular overdentures in very old adults: a randomized controlled trial. J Dent Res. 2013;92:154s–160s. doi: 10.1177/0022034513509630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this review were obtained from previously reported studies and datasets, which have been cited.