Abstract
Background
Undegraded glycosaminoglycans (GAGs) induced by deficiency of enzymes are the primary cause of mucopolyscchardoses. Mucopolysacchardoses (MPS) are a group of rare lysosomal storage diseases (LSD). The quantification of a specific enzymatic activity is needed for accurate diagnosis. The objectives of this work were: first, to continue the study of mucopolysacchardoses disease in Egypt after the start of using the enzyme replacement therapy (ERT). Second, to define the commonest types among our population after 18 years experience with the disease. Third, to compare the different MPS types’ distribution, diagnosed after the start of the ERT, to identify the impact of using ERT on the number and type of diagnosed patients.
Method
Urinary GAGs were measured for all referred cases followed by two-dimensional electrophoretic separation for cases with high levels of GAGs; the specific enzyme activity was assayed for each type depending on the abnormal electrophoretic pattern obtained. Clinically suspected cases of Morquio syndrome were directly subjected to measuring the specific enzyme.
Results
Out of 1448 suspected cases, 622 (42.9%) MPS patients were diagnosed revealing the following distribution: MPS I (172, 27.7%), MPS II (57, 9.1%), MPS III [(177, 28.5%: 134 type B and 43 types A, C or D)], MPS IVA (124, 19.9%), MPS VI (90, 14.5%) and MPS VII (2, 0.3%). MPS III was the most commonly diagnosed type followed by MPS I and MPS IVA. MPS IVA represented the most common type receiving treatment, followed by MPS I, MPS II and MPS VI.
Conclusion
The presence of treatment encouraged the affected families and physicians to seek diagnosis. MPS III was the commonest type among our studied group after 7 years of diagnosis, while MPS IVA was the commonest type receiving treatment.
Keywords: Mucopolysacchardoses, Glycosaminoglycans, Diagnosis, Enzyme replacement therapy
Mucopolysacchardoses, Glycosaminoglycans, Diagnosis, Enzyme replacement therapy.
1. Introduction
The degradation of the glycosaminoglycans (GAGs) takes place in the lysosomes. Under physiological conditions, the main GAG chains — dermatan sulphate (DS), heparan sulphate (HS), keratan sulphate (KS) and chondroitin sulphate (CS) — are degraded by 11 lysosomal hydrolases enzymes [1, 2].
The deficit of any one of the 11 acid hydrolase activities gives rise to the progressive accumulation of GAGs in most tissues and organ systems, as well as in urine, resulting in 7 distinct types of mucopolysaccharidoses disorders (MPS). MPS I, MPS II, MPS III has 4 biochemical subtypes (A, B, C and D), MPS IV has 2 biochemical subtypes (A and B) and MPS IX is the rarest type which is recorded only in 4 patients worldwide [3, 4].
All MPS disorders follow an autosomal recessive inheritance pattern, with the exception of MPS II, which is X-linked [5].
Patients with MPS progressively develop growth impairment and deficiencies in respiratory, cardiovascular, musculoskeletal, nervous, gastrointestinal, auditory and visual systems [2].
All MPS disorders are heterogeneous with respect to genotype, clinical presentation and progression rate [4]. All MPS types may have severe or attenuated presentations depending on the residual enzymatic activity of the patient. Patients with severe forms experience symptoms in a wide range of organ systems and clinical recognition is therefore early; patients with attenuated forms and less obvious clinical manifestations may not be diagnosed until they reach adolescence or adulthood [4].
The assessment of GAG level (quantitative and qualitative), as well as the deficient enzyme activity, is crucial for the biochemical diagnosis of MPS [5].
The diagnosis of MPS starts with the measurement of GAG in the urine in suspected patients. A positive result is very suggestive of an MPS, but false-negative/positive results are sometimes obtained due to a lack of sufficient sensitivity and specificity. Further separation methods, as qualitative assessment of GAGs, are required. A confirmative diagnosis requires enzyme assay in cultured fibroblasts, leucocytes, plasma and dried blood spot, which is considered the gold standard for diagnosis [6, 7].
In addition, tandem mass spectrometry (MS/MS) have been established to measure GAGs level in different matrices, it gives a sensitive, specific and reproducible GAG analysis making it potentially useful for the screening, prognosis and monitoring of any therapeutic effect in MPS patients [6].
However, uGAGs measurement is widely used due to its simplicity, the availability of commercial kits and adaptation to an inexpensive qualitative visual test [8, 9] but, there are many previous studies interested in finding new biomarkers [9, 10, 11].
Alongside biochemical analysis, the molecular tests play a critical role in the identification of the genotype of MPS and potentially help in the genetic counseling. The estimated prevalence of MPS has been influenced by the fact that there is a significant variation among nations and ethnic backgrounds, depending on which type of MPS dominates a geographic area [5].
Early start of treatment helps reduce the accumulation of intracellular GAGs and the deceleration of progressive multiorgan worsening [12]. The Choices of treatment include bone marrow transplantation (HSCT) in MPS I, II, III, IV and VI and enzyme replacement therapy (ERT), which is developed and licensed from the beginning of the 2000s in MPSs I, VI, II, IVA and VII [13].
The aim of this report is to complete the mucopolysacchardoses survey in Egypt after the start of ERT at the National Research Centre by six years to compare the differences between the study done before in 2014 and the findings after six years; in an attempt to find out the effect of the presence of a treatment on the numbers of referred and diagnosed patients. Also, to define the commonest types among our population after a total period of 18 years. Also, to compare the commonest types diagnosed in the ERT era and the types receiving treatment verses the diagnosed one during the same period of time.
2. Patients and methods
2.1. Patients
This study included 1448 clinically suspected Egyptian MPS patients. They were referred from all over Egypt to the Biochemical Genetics Department, Human Genetics and Genome Research Division, National Research Centre during the last seven years (2014 till end of 2020) for the diagnosis or exclusion of MPS.
All patients were subjected to thorough medical history taking that included family history, pedigree construction for parental consanguinity and any other similarly affected family member.
Four to five ml urine samples were collected from all referred cases, 100 μl were used to measure the total levels of GAGs and 3 ml were used for the extraction of GAGs; followed by electrophoretic separation to detect the type of GAGs excreted. According to this type, we assayed the activity of the corresponding enzyme in 5 ml of whole blood on EDTA. Plasma and the leukocytes were separated to assay the different enzymes.
This study was approved by the Research Ethics Committee of the NRC according to the World Association Declaration of Helsinki and written informed consent was obtained from all patients’ legal guardians (National Research Centre, Medical Research Ethics Committee (MREC) # 1441022021).
2.2. Method
2.2.1. Quantitation of urinary GAGs
Quantitative determination of total GAGs in urine samples was carried out by the method of De Dong et al. (1989) [14]. This method is based on the reaction of urinary GAGs with the dye dimethylmethylene blue yielding a colored complex that can be measured spectrophotometrically at 520 nm and using dermatan sulfate as a standard. GAGs concentration (mg/dl) was subsequently normalized to urinary creatinine concentrations, which were determined by the method of Hortin and Gools by (1997) [15], to yield final reported values of GAGs concentrations in units of mg/mmol creatinine [16]. Total amounts of GAGs in the urine samples were compared with the age-dependent reference values of De Dong et al. 1992 [17].
2.2.2. Characterization of urinary GAGs
Two-dimensional electrophoresis separation of GAGs was done to samples with high levels of total GAGs. Extraction of GAGs from urine for electrophoretic separation was carried out according to Whiteman (1973) [16]. One μl of extracted GAGs or reference standard GAGs solutions (1 mg/ml) was applied on cellulose acetate sheets. The separation proceeded into two phases; a first phase (pyridine, glacial acetic acid, water) for 1 h and a second phase (barium acetate buffer) for 3 h. Staining was done with 0.05% Alcian blue solution containing 50 mmol/l MgCl2 in 50 mmol/l sodium acetate buffer (pH 5.8) and washed with 5% acetic acid solution. Clear blue spots on a white background were obtained [16, 18]. According to the pattern of separation, each type of MPS was confirmed by assaying the activity of its specific deficient enzyme.
2.2.3. Analysis of enzyme activity in leukocytes or plasma
Before initiating enzymatic analysis, protein content was estimated according to Lowry et al. (1951) [19]. Activity of α-iduronidase for MPS I was assayed by Hopwood et al. (1979) [20]. Incubation of 4-methylumbelliferyl α-L-iduronide with peripheral blood leukocytes at 37 °C was done for 1 h, the reaction was stopped by sodium glycinate buffer (pH 10.3). The liberated 4-methylumbelliferone was measured fluorometrically at an excitation wavelength of 358 nm and an emission wavelength of 438 nm.
MPS II was diagnosed by assaying the activity of Iduronate-2-Sulfatase according to Voznyi et al., 2001 [21]. Leukocyte homogenate and 4-methylumbelliferyl-α-iduronate-2-sulfate were incubated at 37 °C for 4 h afterwards; McIlvain's buffer and iduronidase solution were added. Second incubation was carried out at 37 °C for 24 h. The reaction was stopped by sodium bicarbonate/sodium hydrogen carbonate buffer and read with an excitation wavelength of 365 nm and an emission wavelength of 450 nm.
MPS III B was confirmed by assaying the activity of N-acetyl- α-D-glucosaminidase [22]. Plasma and 4-methylumbelliferyl-N-acetyl-α-d-glucosaminide as buffer substrate were incubated at 37 °C for 2 h. The reaction was stopped with glycine-NaOH (pH 10.3) as stop solution and the fluorescence was read.
Before initiating enzymatic analysis, protein content was estimated according to Lowry et al. (1951) [19]. The activity of N-acetylgalactosamine-6-sulfatase for MPS IVA was assayed according to Van Diggelen et al., 1990 [23]. Leukocyte homogenates were sonicated in demineralized water. The tubes are centrifuged and the supernatant was dialyzed against the dialysis buffer. Dialyzed supernatant and 4-methylumbelliferyl-β-d-6-sulfo-N-acetylglucosaminide were incubated at 37 °C for 17 h. The reaction was stopped by glycine-carbonate buffer (pH 9.6) and read with an excitation wavelength of 365 nm and an emission wavelength of 450 nm.
Protein content was measured according to Lowry et al. (1951) [19] before starting the enzyme assay. The activity of N-acetylgalactosamine-4-sulfatase (arylsulfatase B) in patients with MPS VI was assayed according to Baum et al. (1959) [24]. Leukocyte homogenate was incubated with 4-nitrocatechol sulfate dipotassium salt as substrate buffer with sodium acetate buffer containing barium acetate at 37 °C for 90 min. The reaction was stopped with sodium hydroxide as stop solution and the absorbance was read at 490 nm.
Before initiating enzymatic analysis, protein content was estimated according to Lowry et al. (1951) [19]. Activity of beta-glucuronidase for MPS VII was measured according to Sly et al., 1973 [25]. 4-methylumbelliferyl-β-d-glucuronide was incubated with leukocyte homogenate at 37 °C for 45 min. The reaction was stopped with glycine-carbonate buffer (pH 9.6) and the fluorescence was read with an excitation wavelength of 365 nm and an emission wavelength of 450 nm.
3. Results
Throughout the time of the study (2014–2020), 1448 clinically MPS suspected cases were referred to the Biochemical Genetics Department. Positive parental consanguinity among the study group was recorded in 979 (67.6%) and was negative in 469 (32.4%). Patient's ages ranged from 10 days to 29 year. They were 911 males and 537 females with a ratio of 1.7:1 (Table 1).
Table 1.
Demographic data among 1448 suspected cases.
| Total number of suspected cases | Age range | Consanguinity [n (%)] |
Gender [n (%)] |
||
|---|---|---|---|---|---|
| Consanguineous | Non-consanguineous | Male | Female | ||
| 1448 | (10 days - 29 years) | 979 (67.6%) | 469 (32.4%) | 911 (63%) | 537 (37%) |
Quantitative determination of total GAGs in urine was done to all referred cases. It was found that 586 (40.5%) patients out of 1448 had high levels of urinary GAG (Table 3) as according to the age-dependent reference values of De Dong et al., 1992 ([17], Table 2). Good Confidence Intervals of age was calculated for each group Table 3.
Table 3.
Age group of 586 patients with confidence intervals (CI) of age and mean levels of total urinary glycosaminoglycans (GAGs) for each group.
| Age group | 95% CI | Number of patients (%) | Mean age | Mean (SD) Urinary GAGs (mg/mmol creatinine) |
|---|---|---|---|---|
| Group I (0–12 months) | [6.95,7.05] | 87 (14.9%) | 7 months | 45.8 (±28.3) |
| Group II (1–5 years) | [2.86, 3.14] | 288 (49.1 %) | 3 years. | 34.5 (±25) |
| Group III (5–10 years) | [6.78, 7.22] | 141 (24.1%) | 7 years | 30 (±22) |
| Group IV (>10 years) | [11.23, 12.77] | 70 (12%) | 12 years | 31 (±21) |
Table 2.
The age-dependent mean values of urinary glycosaminoglycans according to De Dong et al., 1992 [1,7].
| Age | Mean (SD) |
|---|---|
| (0–5 months). | 33.9 (9.2) |
| (6–12 months). | 23.3 (4.1) |
| 1 year. | 19.5 (5.2) |
| 2–3 years. | 14.5 (3.4) |
| 4–5 years. | 11 (1.7) |
| 6–7 years. | 9.3 (1.8) |
| 8–9 years. | 8.4 (1.6) |
| 10–14 years. | 7 (1.8) |
| 15–19 years. | 4.1 (1.3) |
| >20 years. | 3.3 (0.9) |
Two-dimensional electrophoretic separation was done to 586 cases. Eighty eight cases (15 %) out of 586 showed normal electrophoresis pattern while 498 cases (85%) showed pathologic pattern. Each type of abnormal electrophoretic pattern was confirmed by assaying the specific enzyme activity. Measurement of the enzyme activity was done to 498 patients. In addition, one hundred and twenty four patients (8.6%) out of 1448 were clinically suspected Morquio syndrome patients. The activity of Acetylgalactosamine-6-sulfatase was measured to confirm this clinical suspicion. The enzyme assay results proved that they were Morquio A, without measuring their GAGs levels or electrophoretic separation. The total number of MPS patients diagnosed in this study over 7 years was 622 (42.9%) out of 1448 referred cases Table 4.
Table 4.
Biochemical results and demographic data of 622 diagnosed patients with MPS.
| MPS TYPES (N, %) | Mean GAGs levels (mg/mmol creatinine) | Electrophoretic Pattern |
Mean activity of deficient enzyme |
Reference range of enzyme activity | Age in years(y) mean (range) | Consanguinity |
Male N (%)/Female N (%) | |
|---|---|---|---|---|---|---|---|---|
| Consanguineous N (%) | Non-consanguineous N (%) | |||||||
| MPS I (172, 27.7%) | 40.5 | Dermatan sulfate, Heparan sulfate. | α-L-Iduronidase = 0.54 μmol/gp/h. | 10–40 μmol/gp/h. | 0.17 y (0.17 y-0.33 y) | 142 (82.5%) | 30 (17.5%) | 102 (59.3%)/70 (40.7%) |
| 4 y (0.5 y-15 y) | ||||||||
| 26 y (25 y-27 y) | ||||||||
| MPS II (57, 9%) | 35.8 | Dermatan sulfate, Heparan sulfate. | Iduronate-2-sulfate sulfatase = 0.34 nmol/4 h/ml. | 167–475 nmol/4 h/ml. | 0.17 y (0.17 y-0.17 y) | 19 (33%) | 38 (67%) | 57 (100%)/0 |
| 4.5 y (1.2 y-14 y) | ||||||||
| MPS III (134 type B and 43 type A, C or D) (177, 28.5%) | 30.8 | Heparan sulfate. | N-Acetyl- α-D-glucosaminidas type B = 0.2 mmol/l/h | 10–45 μmol/l/h | 0.5 y (0.17 y-0.75 y) | 131 (74%) | 46 (26%) | 100 (56.5%)/77 (43.5%) |
| 3.8 y (1.8 y-7 y) | ||||||||
| 10 y (8 y-16 y) | ||||||||
| MPS IVA (124, 20%) | 18 | Normal pattern | N-Acetylgalactosamine-6- sulfatase = 90.8 pmol/gp/h. | 400–2000 pmol/gp/h. | 0.25 y (0.17 y-0.42 y) | 90 (72.5%) | 34 (27.5%) | 68 (54.8%)/56 (45.2%) |
| 6 y (1.5 y-16 y) | ||||||||
| 29 y (29 y) | ||||||||
| MPS VI (90, 14.5%) | 36.6 | Dermatan sulfate | Arylsulfatase B = 0.17 mmol/gp/h | 5–70 μmol/gp/h. | 0.55 y (0.25 y-0.66 y) | 65 (72.2%) | 25 (27.7%) | 51(56.6%)/39 (43.4%) |
| 3.4 y (1 y-5.6 y) | ||||||||
| 9.8 y (6 y-17 y) | ||||||||
| MPS VII (2, 0.3%) | 34 | Chondroitin-4 and -6 sulphate, Dermatan sulfate Heparan sulfate. | beta-glucuronidase = 1.6 μmol/gp/h | 100–800 μmol/gp/h | 3.3 y (2.5 y-4 y) | 2 (100%) | - | 0/2 (100%) |
MPS I (Hurler, Scheie, Hurler/Scheie syndrome), MPS II (Hunter syndrome), MPS III (Sanfilippo syndrome), MPS IVA (Morquio syndrome), MPS VI (Maroteaux-Lamy syndrome) and MPS VII (Sly syndrome).
The mean age of diagnosis was around 4–6 years as follows for each disorder.
Within the age range of MPS I (0.17 y- 27 y), there were 29 patients with higher age from 6 to 15 years. In addition, there were 5 patients with an age of (2–4 months) and 2 patients with 25 and 27 years, Table 4.
For MPS II, there were 9 patients with relatively high age (8-14 y), beside 2 patients with an age of 0.17 year, Table 4.
In the case of MPS III, 23 patients had high age range (8-16 y). Twelve patients had the age range of 0.17–0.75 year, Table 4.
In MPS IVA, 36 patients had a relatively high age range (6-16 y), three patients with an age range of 0.17–0.42 year and one patient was 29 years old, Table 4.
In MPS VI, there were 14 patients with an age range of 6 to 17 y, beside 7 patients with an age range of 0.25–0.66 year, Table 4.
The highest consanguinity was found among MPS I patients (82.5%). In the case of MPS II, although an x-linked disorder, still the consanguinity was 33%. Totally, the consanguinity was reported in 449 patients (72.2%) out of 622 patients, if we exclude MPS II which is X-linked, consanguinity will rise to (76.1%), Table 4.
Excluding MPS II which is an X-linked disorder, the number of male patients were 321 out of 565 (56.8%) while female patients were 244 out of 565 (43.2%), Table 4.
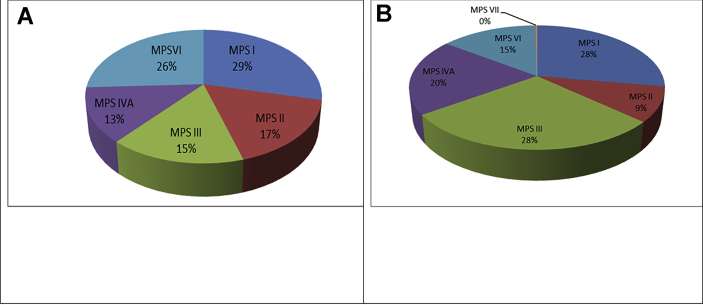
N-acetylglucosaminidase activity was measured in the plasma of 177 patients, with big heparan and heparan sulfate spots in electrophoretic pattern specific for MPSIII. Forty three patients (24.3%) had either MPS III A, C or D while 134 (75.7%) had MPS III B, Table 4. Patients of MPS III (28.5%) and MPS II (27.7%) were the most frequently diagnosed types in this study, followed by MPS IVA (20%), MPS VI (14.5%) then MPS II (9%), and at the end was MPS VII (0.3%) (as seen in Table 4, Figure 4B).
Figure 4.
A: Types of mucopolysaccharidoses among 278 diagnosed patients in eleven years (2002–2013) [27]. B: Types of mucopolysaccharidoses among 622 diagnosed patients in six years (2014–2020).
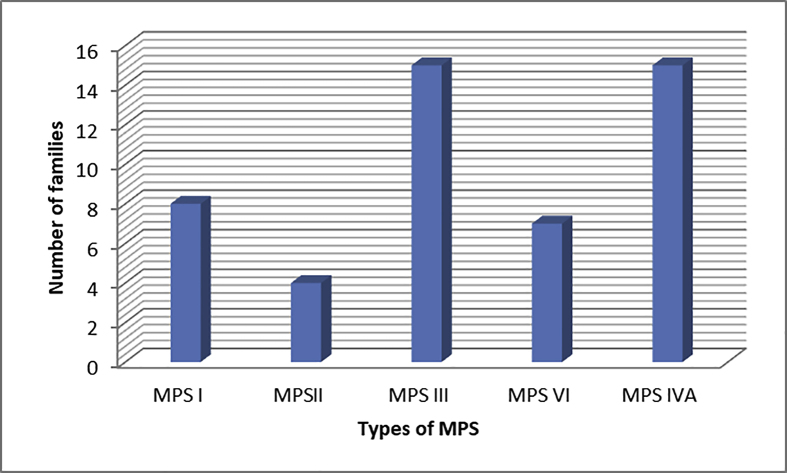
Out of 622 patients, there were 80 samples from siblings (13%), the highest number of families with previously affected patients was found in MPS III and MPS IVA followed by MPSs VI, I and II, Figure 1.
Figure 1.
Families with more than one affected siblings.
The following figures summarize the comparison between the present study and our previous one (2002–2013), which was published in 2014 [22].
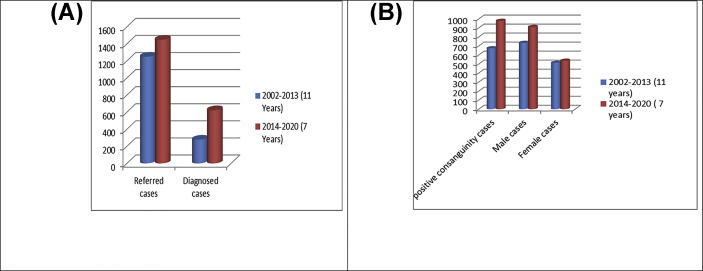
The duration of the previous study (11 years) was almost double the duration of the present study (6 years) equal to 1.8:1. A higher number of referred cases was reported in the current work (1448 vs 1249) with ratio 1.15:1, and a higher number of diagnosed cases was observed in the present work [622 (42.9%) vs 278 (22.3%)] with ratio 2.2:1 as well, Figure 2(A).
Figure 2.
(A): Comparison between the numbers of referred and diagnosed cases in the two studies. (B): Comparison in demographic data among referred cases in the two studies.
Also, the number of positive consanguinity and male cases were higher in the present study in the referred case than the previous one with ratios 1.5:1 and 1.4:1 respectively. The number of female cases was similar in both studies, as shown in Figure 2(B).
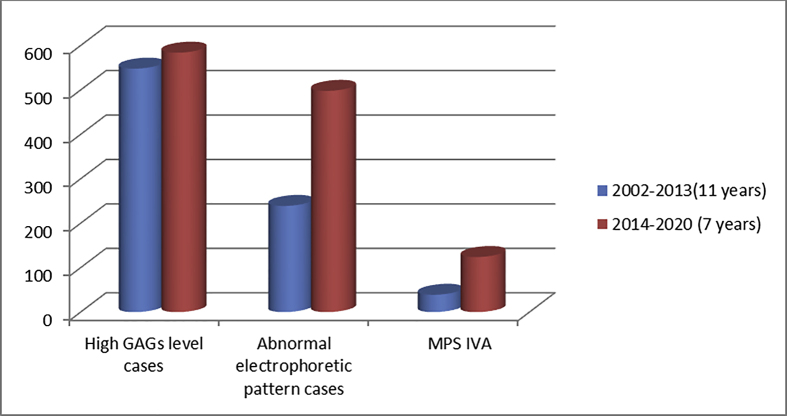
Although the number of cases with high GAGs levels was similar in both studies (548 vs 584). The numbers of abnormal electrophoretic patterns were higher in the current study (239 vs 498), almost the double (1:2.1), Figure 3.
Figure 3.
Comparsion between biochemical results among referred cases in the two studies.
To confirm the diagnosis, the enzyme activity was assayed in 239 patients in the previous study, while in the current study; enzyme activity was measured in 498 patients, Figure 3.
In addition to the above, the enzyme activity for the clinically suspected MPS IVA patients was measured in 104 cases in the previous study and in 124 cases in the present one. The results showed that, the numbers of MPS IVA patients were higher in the present study (124) than the previous one (39), with ratio 3.2:1, Figure 3.
Totally, the number of diagnosed patients in the current study was higher (622) than the previous study (278) (2.2:1).
In the previous study [26] MPSs I and VI were the most prevalent types, followed by MPSs II and III, while in the present study, MPSs III and I had high incidence, followed by MPSs IVA, VI and II, Figure 4.
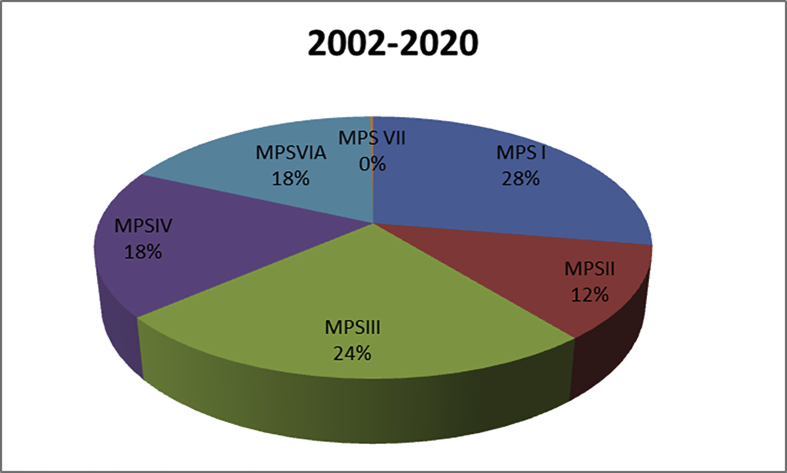
The total number of MPS diagnosed patients during the time period from 2002 to 2020 (eighteen years) is 900, distributed as follows: MPS I in the first order with ratio 28% (251 out of 900), then MPS III in the second order 24.4% (220 out of 900), then MPS IVA 18.1% (163 out of 900), then MPS VI 18% (161 out of 900), then MPSII 11.5% (103 out of 900) and MPS VII 0.2% (2 out of 900), Figure 5.
Figure 5.
Types of mucopolysaccharidoses among 900 diagnosed patients during the time of the study (2002–2020).
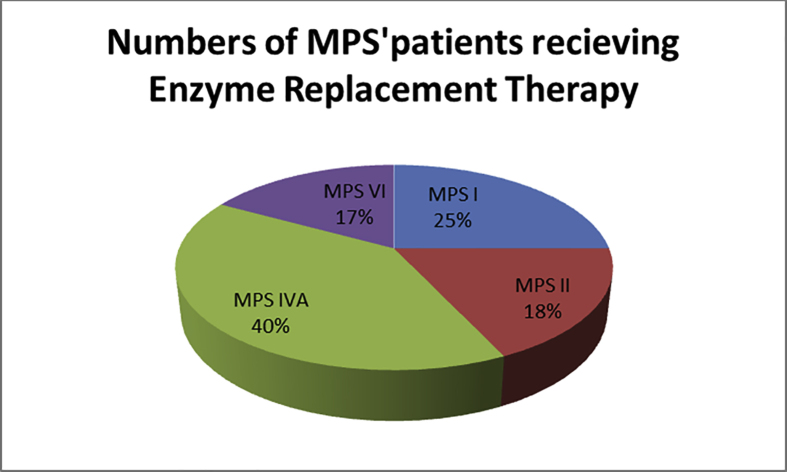
Enzyme replacement therapy has started in December 2013 at the National Research Centre. At the moment, 77 patients receive the treatment at the Enzyme Replacement Therapy Unit (ERTU), NRC.
Thirty-one MPS IVA patients receive the treatment (40.2%) followed by MPS I (19, 25%) then MPS II (14, 18%) and MPS VI (13, 17%), Figure 6.
Figure 6.
Types and percent of MPS′ patients receiving treatment at ERT unit in NRC.
4. Discussion
The term mucopolysaccharidosis defines a series of rare disorders (accounting for <0.1% of all genetic diseases). They are characterized by defects in the catabolism of glycosaminoglycans [1] which causes the progressive accumulation of these macromolecules in cellular lysosomes in different tissues [2].
This is the second retrospective study from the Biochemical Genetics Department (the reference laboratory for Inborn Errors of Metabolism at National Research Centre, Egypt) regarding the MPS types diagnosed.
Relative to our report in 2014 (Fateen et al., 2014) [26], in the present study, we analyzed more numbers of clinically suspected patients (1448 versus 1294) in a less time period (6 years vs 11 years). We diagnosed more than double the number of positive cases (622, 42.9% versus 278, 22.3%). This may be attributed to increased awareness among the population and clinicians especially with the availability of treatment, Figures 2(A) and 3.
Previous Egyptian studies matched with our results; a study was done over 15 years (1995–2010) by Fateen et al reported that lysosomal storage diseases (LSD) came in the first order among inborn errors of metabolism (69.4%). Of this percent, 49% had MPS [27]. Elmonem et al showed that 94 (44.5%) patients out of 211 were diagnosed with mucopolysaccharidoses in a study carried out between (2008–2014) [28], indicating that mucopolysaccharidoses were the most common group of lysosomal storage diseases diagnosed in Egypt.
In Tunisia during the period 1970–2005, there were 96 (72.7%) confirmed MPS patients out of 132 referred cases [29]. In Libya over 12 years (2001–2012), lysosomal storage diseases (LSD) came in the third order among the inborn errors of metabolism pattern, where five patients (33.3%) out of 15 had MPS disease [30]. In Saudi Arabia, a study carried out from 2001-2014, MPS was diagnosed in 15/187 (8%) [31]. While in the Eastern Province of Saudi Arabia, a study covered 33 years (1983–2016), MPS came in the second place 37% (33 of the 89 patients) in the prevalence of LSDs [32]. But still the numbers of diagnosed patients are very small compared with our experience and publications. This is mostly due to the large Egyptian population number (100 million) and the availability of testing at our laboratory which make it easier for patients and physician to receive a diagnosis. This is not available in many other neighboring countries.
Arab populations have a deeply rooted tradition of consanguineous marriages mostly due to socio-cultural factors. The consanguinity rate among Egyptians in general ranged between 30% to 40% [33, 34]. In the present study, the parental consanguinity was found in 67.6% of the referred cases, Table 1. This is higher than what was reported by Fateen et al. 2014 where parental consanguinity was 54.1% among the referred cases [26]. This might be due to the larger number of the study group, but also it is an important marker of the increase in consanguineous marriages mostly due to socioeconomic factors as well.
A high consanguinity rate among the referred cases was also found in Fateen et al., 2014 [27], 74% (8998 out of 12,148) and in Fateen and Abdallah, 2019 [35], 76% (3897 out of 5128).
Parental consanguinity among the diagnosed MPS patients in this study was 76 % Table 4. This matched with the previous studies of Shawky et al (77.1%) [36], Shawky et al (80%) [37] and Ben Turkia et al., (83%) [29]. MPS II was an exception, although it is an X-linked disorder, 33% of the diagnosed MPS II patients were to consanguineous marriages.
The higher male to female ratio of referred (1.7:1) and diagnosed cases (1.4:1), Tables 1 and 4 is a tradition among the Arab population, who give more care to males than females especially in rural regions. We did report these findings in different studies and publications before such as: Shawky et al., 2008, the M:F ratio was 1.2:1 [37], Fateen et al., 2014, it was 3:2 [27], Elmonem et al, 2016 it was 1.3:1 [28], Selim et al, 2016 it was 2.1:1 [38], Ben Turkia et al., 2009 it was 1.2:1 [29] and Al Obaidy, 2013 it was 1.2:1 [30].
The severely affected patients showed signs and symptoms in the first year of life while the attenuated forms started in childhood or adolescence [2, 4] and are often under diagnosed and generally taken into account only when signs and symptoms become clearly evident.
Individuals with severe MPS I usually have symptoms apparent by the age 6 months, most of our MPS I patients are diagnosed at an age from 6 months to 3 years. Also, 5 patients were diagnosed at an age of 0.17–0.33 year. These were siblings to previously affected families. The attenuated form may not become apparent until after the age of 3 years. Many attenuated MPS I patients were not diagnosed until late childhood or even later age. That was seen in some of our patients with high age at diagnosis, around 6–15 years, and in two patients at the age of 25 and 27 years, Table 4 [39].
For severe cases of MPS II, a diagnosis is often made between the ages of 1.5 and 3 years of age. This agrees with our findings that 46 patients (80.7% out of 57) were diagnosed from 1.2 to 5 years. Two patients were diagnosed at the age 0.17 year; they were siblings to affected patients, whereas the attenuated form was diagnosed between the ages of 4 and 8 years. Some of our attenuated form patients were diagnosed between 6 and 14 years, Table 4 [40].
Symptoms of MPS III usually begin to appear between 2 and 6 years of age. It was found that 145 (82 %) patients with MPS III were diagnosed at the age from 1.8 to 7 years in the present study. Nine patients were diagnosed at the age from 0.17-0.75 year, which were siblings to affected families. There were 23 patients with higher age (8–16 years), they are relatives of previously affected patients, Table 4 [41].
Patients with MPS IVA appear healthy at birth like many other lysosomal disorders. The age of onset is usually between 1 and 3 years. Most of our patients were diagnosed around the age of 1.5–5 years. Three patients were diagnosed earlier between 0.17 to 0.42 year as they were siblings to previously affected patients. Thirty six patients were diagnosed later; between 6 to16 years and one patient was 29 years old. Many of them were siblings and the others showed the attenuated form, Table 4 [42].
Patients with the classical form of MPS VI generally present within the first 3 years of life. Seventy four patients were diagnosed between 0.5 to 5 years. Two patients were diagnosed around age 0.17 and 0.25 year, as they were relatives to affected patients. In contrast, patients with non-classical MPS VI present later in life with more subtle disease manifestations, 14 patients were diagnosed at the age of 6–17 years, Table 4 [43]. We can conclude that most of the MPS patients are diagnosed early except for the mild forms which present late.
According to our laboratory experience in the biochemical diagnosis of MPS [26], the diagnostic process started with a set of full GAG profile including both quantitative and qualitative analysis. Most MPS patients have higher GAG excretion in urine compared with age-matched normal subjects. Our result showed that, the mean value of GAG of (40.5%) patients was high in respect to the age of matched controls, Table 3.
Mahalingam et al. stated that MPS III and MPS IVA patients in general excrete fewer amounts of GAGs in their urine which is observed in our study, Table 4 [44]. Patients with MPS types are not only different in the total amount of GAG, but also in the relative proportion of various types of GAG. [2], so qualitative analysis of GAG using two-dimensional electrophoresis is a diagnostic step for MPS disorders. The electrophoretic results revealed that 88 cases (15 %) with high GAG level in urine had normal electrophoretic pattern. This is much lower than what we reported previously were 56.4% of the referred cases showed normal pattern, Figure 3 [26] this means that the diagnostic skills of the physicians rise with the increased flow of cases.
Mucopolysaccharidoses share many signs and symptoms with other disorders like mucolipidosis II, oligosaccharidosis and multiple sulfatase deficiency. High level of GAG could be detected in these conditions as well and in many dysplasia conditions [45, 46]. Consequently, the level of GAG is not diagnostic of MPS but it could be used as a screening test only [6].
Sheth et al. mentioned that overlapping clinical phenotypes are a diagnostic challenge to the clinician in the cases of mucolipidosis (ML) and mucopolysaccharide disorders [47]. Also, Khan and Tomatsu stated that these disorders share characteristic bone deformities. In addition, several lysosomal glycosidases and sulfatases enzymes are deficient in ML lysosomes and are involved in a step by step degradation of GAGs [48]. During the study period, 47 mucolipidosis cases were diagnosed.
Previous literature mentioned that, patients with MPS I excrete more dermatan sulfate (DS) than heparan sulfate (HS), and patients with MPS II excrete heparan sulfate more than dermatan sulfate, throughout our study in some patients we could not observe these differences. Quantitative measurements are needed to confirm such observations. Also, quantitative measurements of the different GAGs are needed for monitoring the response to treatment. We are currently working on establishing this technique by LC MS/MS. Measuring both the α-iduronidase and iduronate-2-sulfate sulfatase enzyme activities is required to confirm the diagnosis [49, 50].
In addition, patients with MPS VI have 100% concentration of DS, while MPS I has higher concentration of DS than HS [51]. We found an overlap between the patterns of these types; accordingly, the deficient enzyme must be measured for both types for accurate diagnosis.
MPS diagnosis should not be confirmed nor ruled out on the basis of a single GAG quantification test, it should be followed by qualitative analysis of GAG to identify the abnormal pattern, which highly reduces the cost of diagnosis by circumventing the assay of all 11 enzymes in all suspected cases. Measuring the activity of the accused MPS enzyme is universally acted as the key component of diagnosis according to the GAG results and clinical suspicion.
Regarding MPS IVA, we did not depend on the total GAGs levels, because their levels were mostly close to the normal range, this agrees with the finding of Tomatsu et al., 2004 [52], Tomatsu et al., 2014 [53] and Sawamoto et al., 2020 [54]. Besides, we could not detect the keratan spot by using two-dimensional electrophoresis, Table 4, as mentioned by Tomatsu et al., 2014 [53], we depend on the clinical and radiologic features of MPS IVA, that agrees with a previous statement, “this syndrome has distinct clinical and radiologic features that are characteristic and do not overlap with any other MPS types” [55]. Hence, the deficient enzyme activity was determined in clinically suspected cases [23].
Sometimes, there can be a delay in the diagnosis of MPS due to the late onset or less progressive phenotypes. Less awareness by physicians especially in the rural areas, can delay the diagnosis. They may detect few signs or symptoms, subsequently patients are sent to different medical departments before diagnosis [56]. This was obviously seen in the high age range of diagnosis in severe and attenuated forms between our patients in each type as mentioned previously, Table 4.
MPS is a genetic disease and the recurrence is 25%, possibility with each pregnancy. Counseling of the parents is important especially with the availability of prenatal diagnosis. Prenatal diagnosis is routinely done at our laboratory to all our MPS patients’ parents [57].
Differences in incidence rely on many factors, among which are consanguinity and cultural characteristics [58]. In the previous report, the distribution of MPS ʼtypes between the diagnosed patients was MPSs I (29%), VI (26%) followed by MPS II (17%), MPS III (15%) and MPS IVA (13%) [26], Figure (4A). In this study, MPSs III (28.5%) and I (27.7%) were the most prevailing types, followed by MPSs IVA (20%), VI (14.5%) and MPS II (9%), Figure (4B).
The sample sizes in both studies were 1249 and 1448 but the study periods were 11 years versus 6 years. Although this report studied retrospectively the last six years while our previous report (2014) [27] reported eleven years‘ experience, the number of diagnosed cases in this six years is almost double that diagnosed in the previous eleven years study, Figure (2A).
The availability of enzyme replacement therapy at ERTU in NRC since December 2013, Figure 6, encouraged the affected families to seek for diagnosis thereby increasing their numbers that attend clinics and in turn encouraging many physicians to refer their patients. This with time led to an increase in the awareness among affected families and an increase in the experience among physicians.
If we add the patients numbers in both studies to give the frequency of each MPS type diagnosed over 18 years, which will give a more accurate look on the distribution of each MPS type among the Egyptian population according to our studies, we will find out that MPS I is the most common type in both studies by 28%. In the second place comes MPS III by 24.5%, followed by MPS IVA by 18.1%, MPS VI by 18% and MPS II by 11.5%, Figure 5.
A study carried on 132 suspected cases in Tunisia over 35 years from 1970-2005 found that MPS I and III were the most frequent MPS types observed, which matches our finding but the rest of the MPS types showed a different distribution [29].
The current findings differ from results that have been reported in other Arab countries. In Saudi Arabia, MPS VI represented the most common type, followed by MPS I, IVA and MPS III in a study from 1983 to 2008 [59]. While in Emiratis, MPS IIIB, IVA, and VI were the most common types of mucopolysaccharidoses [60].
In Asia, the frequency of MPS types were MPS II, MPSI, MPS III then MPS IV and MPS VI according to the studies carried on China and Japan [61, 62].
In Germany and the Netherlands MPS III came first, followed by MPS I, MPS II, MPS IV and MPS VI [63]. While in Denmark and Norway, a study from the 1970s–2000s reported that MPS I was the most common prevailing type. This agrees with our findings as MPS I came first in distribution [64]. The same finding was observed in studies carried out in America and Canada while the rest of MPS types were different. In America MPS I came first then MPS II followed by MPS III, MPS IV and MPS VI, while in Canada the ranking was MPS IV, MPS VI, MPS III then MPS II [65]. This might be attributed to the large number of Asian population in the USA. In Brazil, a study carried out from 1994 to 2018 indicated that MPS II was the first condition, followed by MPS VI, MPS I, then MPS III, MPS IV and MPS VII [66].
This study's main limitation was that, enzymes activity for MPS III A, C and D were not diagnosed in our lab. They are rare in Mediterranean region. Measure of these enzymes was done abroad. At the moment we did start measuring the activity of MPS IIIA and may be in the future we will add the measuring activity of MPS IIIC and D.
4.1. In conclusion
The presence of treatment for four MPS types promoted the diagnosis exponentially as we diagnosed double the patients number in almost half the period of time.The presence of treatments encourages the parents to seek diagnosis and equally challenged the physicians.
Although MPS III is the most prevalence type during the present study, but MPS I is the most common type among our Egyptian population after 18 years of precise diagnosis. Counseling of the parents is an essential part of the diagnosis.
The follow up of the response to treatment needs accurate quantitative tools for the different GAGs types beside the clinical evaluation.
The level of inbreeding is high due to the lack of awareness and commitment of individuals to their traditional cultural values. These leads to an increase in the incidence of autosomal recessive diseases, including Mucopolysacchardoses, multidisciplinary preventive programs are needed to alert the population, for example: prenatal screening, carrier detection, genetic counseling and public health education.
Declarations
Author contribution statement
Ekram Fateen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Zeinab Y Abdallah: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mona Mahmoud: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Walaa Nazim: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Amira Radwan: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.National Organization for Rare Disorders Rare Disease Database Mucopolysaccharidoses. https://rarediseases.org/rare-diseases/mucopolysaccharidoses/ Available online:
- 2.Muenzer J. Overview of the mucopolysaccharidoses. Rheumatol. 2011;50(5):v4–v12. doi: 10.1093/rheumatology/ker394. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell J., Berger K.I., Borgo A., Braunlin E.A., Burton B.K., Ghotme K.A., Kircher S.G. Unique medical issues in adult patients with mucopolysaccharidoses. Eur. J. Int. Med. 2016;34:2–10. doi: 10.1016/j.ejim.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Scarpa M., Buffone E., La Marca P. Difficulties in diagnosing slowly progressive mucopolysaccharidosis VI: a case series. J. Pediatr. Rehabil. Med. 2002;3:71–75. doi: 10.3233/PRM-2010-0104. [DOI] [PubMed] [Google Scholar]
- 5.Celik B., Tomatsu S.C., Tomatsu S., Khan S.A. Epidemiology of mucopolysaccharidoses update. Diagnostics. 2021;11(2):273. doi: 10.3390/diagnostics11020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filocamo M., Tomanin R., Bertola F., Morrone A. Biochemical and molecular analysis in mucopolysaccharidoses: what a paediatrician must know. Ital. J. Pediatr. 2018;44 doi: 10.1186/s13052-018-0553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nan H., Park C., Maeng S. Review Article Mucopolysaccharidoses I and II: brief review of therapeutic options and supportive/palliative therapies. Hindawi Bio. Med. Rese Int. 2020:1–18. doi: 10.1155/2020/2408402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrencea R., Brownb J.R., Loreyc F., Dicksond P.I., Crawfordb B.E., Eskoa J.D. Glycan-based biomarkers for mucopolysaccharidoses. Mol. Genet. Metabol. 2014 Feb;111(2):73–83. doi: 10.1016/j.ymgme.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubaski F., Poswar F.O., Michelin-Tirelli k, Burin M.G., Rojas-Málaga D., Brusius-Facchin A.C. Review diagnosis of mucopolysaccharidoses. Diagnostics. 2020;10(3) doi: 10.3390/diagnostics10030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saville J.T. Glycosaminoglycan fragments as a measure of disease burden in the mucopolysaccharidosis type I mouse. Mol. Genet. Metabol. 2018;123(2):112–117. doi: 10.1016/j.ymgme.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Fujitsuka H., Sawamoto K., Peracha H., Mason R.W., Mackenzie W., Kobayashi Biomarkers in patients with mucopolysaccharidosis type II and IV. Mol. Genet. Metab. Rep. 2019 Jun:19. doi: 10.1016/j.ymgmr.2019.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vairo F., Federhen A., Baldo G., Riegel M., Burin M., Leistner-Segal S., Giugliani R. Diagnostic and treatment strategies in mucopolysaccharidosis VI. Appl. Clin. Genet. 2015;8:245–255. doi: 10.2147/TACG.S68650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omara A., Jalila J.A., Shakrina N.M., Ngub L.H., Yunusa Z.M. Selective screening for detection of mucopolysaccharidoses in Malaysia; A two-year study (2014–2016) Mol. Genet. Metab. Rep. 2019;19:100469. doi: 10.1016/j.ymgmr.2019.100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Dong J.G., Wevers R.A., Laarakkers C., Poorthuis B.J. Dimethylene-blue based procedure for mucopolysaccharidoses. Clin. Chem. 1989;1989(35):1472–1477. [PubMed] [Google Scholar]
- 15.Hortin G.L., Goolsby K. Lipemia interference with a rate-blanked creatinine method. Clin. Chem. 1997;43:408–410. [PubMed] [Google Scholar]
- 16.Whiteman P. The quantitative determination of glycosaminoglycans in urine with Alcian blue 8GX. Biochem. J. 1973;131:351–357. doi: 10.1042/bj1310351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Dong J.G., Wevers R.A., Liebrand-van Sambeek R. Measuring urinary glycosaminoglycans in the presence of protein: an improved screening procedure for mucopolysaccharidosis based on dimethyleneblue. Clin. Chem. 1992;38:803–807. [PubMed] [Google Scholar]
- 18.Mossman J., Patrick A. Prenatal diagnosis of mucopolysaccharidoses by two-dimensional electrophoresis of amniotic fluid glycosaminoglycans. Prenat. Diagn. 2005;2:169–176. doi: 10.1002/pd.1970020305. [DOI] [PubMed] [Google Scholar]
- 19.Lowry O.H., Rosenbrough N.J., Farr A., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Hopwood J., Muller V., Simthson A., Baggett N. A fluorometric assay using 4- methylumbeliferyl a-L-iduronidate for the estimation of a-L-iduronidase activity and the detection of Hurler and Scheie syndromes. Clin. Chim. Acta. 1979;92:257–265. doi: 10.1016/0009-8981(79)90121-9. [DOI] [PubMed] [Google Scholar]
- 21.Voznyi Y.V., Keulemans J.L.M., van Diggelen O.P. A fluorimetric assay for the diagnosis of MPS II (Hunter’s disease) J. Inherit. Metab. Dis. 2001;24:675–680. doi: 10.1023/a:1012763026526. [DOI] [PubMed] [Google Scholar]
- 22.Chow P., Weissmann B. 4-Methylumbelliferyl 2-acetamldo-2-deoxy-x-d-glucopyranoside a fluorogenic substrate for n-acetyl-x-d-glucosaminidase. Carbohydr. Res. 1981;96:87–93. doi: 10.1016/s0008-6215(00)84698-4. [DOI] [PubMed] [Google Scholar]
- 23.Van Diggelen O.P., Zhao H., Kleijer W.J., Janse H.C., Poorthuis J.H.M., Van Pelt J.M. A fluorometric enzyme assay for the diagnosis of Morquio disease type A (MPS IV A) Clin. Chim. Acta. 1990;187:131–139. doi: 10.1016/0009-8981(90)90339-t. [DOI] [PubMed] [Google Scholar]
- 24.Baum H., Dodgson K.S., Spencer B. The assay of arylsulfatase A and B in human urine. Clin. Chim. Acta. 1959;4:453–455. doi: 10.1016/0009-8981(59)90119-6. [DOI] [PubMed] [Google Scholar]
- 25.Sly W.S., Quinton B.A., McAlister W.H., Rjmoin D.L. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J. Pediatr. 1973;82(2):249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 26.Fateen E.M., Ibrahim M.M., Gouda A.S., Youssef Z.A. Biochemical diagnosis of mucopolysaccharidoses over 11 years: the Egyptian experience. Middle East J. Med. Genet. 2014;3:16–23. [Google Scholar]
- 27.Fateen E.M., Gouda A.S., Ibrahim M.M., Abdallah Z.Y. Fifteen years experience: Egyptian metabolic lab. Egypt J. Med. Hum. Genet. 2014;15:379–385. [Google Scholar]
- 28.Elmonem M.A., Mahmoud I.G., Mehaney D.A., Sharaf S.A., Hassan S.A., Orabi A., Salem F. Lysosomal storage disorders in Egyptian children. Indian J. Pediatr. 2016;83:805–813. doi: 10.1007/s12098-015-2014-x. [DOI] [PubMed] [Google Scholar]
- 29.Ben Turkia H., Tebib N., Azzouz H., Abdelmoula M.S., Chehida A.B., Chemli J. Incidence of mucopolysaccharidoses in Tunisia. La tuni Med. 2009;87:782–785. [PubMed] [Google Scholar]
- 30.AlObaidy H. Patterns of inborn errors of metabolism: a 12 year single-center hospital-based study in Libya. QMJ. 2013;18 doi: 10.5339/qmj.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alfadhel M., Benmeakel M., Hossain M.A., Al Mutairi F., Al Othaim A., Alfares A.A., Al Balwi M., Alzaben A., Eyaid W. Thirteen year retrospective review of the spectrum of inborn errors of metabolism presenting in a tertiary center in Saudi Arabia. Orphanet J. Rare Dis. 2016;11:126. doi: 10.1186/s13023-016-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Sannaa N.A., Al-Abdulwahed H.Y., Al-Ghamdi M.S. Lysosomal storage disorders (LSDs): the prevalence in the eastern Province of Saudi Arabia. Int. J. Neurol. Dis. 2017;1(2):38–43. [Google Scholar]
- 33.Temtamy S.A., Loutife A. Surgical aspects of cleft lip-cleft palate problems in Egypt. CL Palate J. 1970:578–581. [PubMed] [Google Scholar]
- 34.Temtamy S.A., Aglan M.S., Meguid N.A. In: Genetic Disorders Among Arab Population. Teebi A.S., editor. Springer; 2010. Genetic disorders in the Egyptians. [Google Scholar]
- 35.Ekram F., Zeinab Y.A. Twenty- five years of biochemical diagnosis of Gaucher disease: the Egyptian experience. Helicon. 2019 doi: 10.1016/j.heliyon.2019.e02574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shawky R.M., Abd el Monim M.T., el Sebai A.A., el Sayed S.M. Cardiac and ocular manifestations in Egyptian patients with mucopolysaccharidoses. East. Mediterr. Health J. 2001;7(6):981–991. [PubMed] [Google Scholar]
- 37.Shawky R., Zaki E., Fateen E., Refaat M., Bahaa Eldin N. Profile of Egyptian patients with mucopolysaccharidoses. Egypt J. Med. Hum. Genet. 2008;9:11–21. [Google Scholar]
- 38.Selim L., Abdelhamid N., Salama E., Elbadawy A., Gamaleldin I., Abdelmoneim M., Selim A. Cardiovascular abnormalities in Egyptian children with mucopolysaccharidoses. J. Clin. Diagn. Res. 2016 Nov;10(11):SC05–SC08. doi: 10.7860/JCDR/2016/21135.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grosse S.D., Lam W.K.K., Wiggins L.D., Kemper A.R. Cognitive outcomes and age of detection of severe mucopolysaccharidosis type 1. Genet. Med. 2017;19:957–982. doi: 10.1038/gim.2016.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wraith J.E., Scarpa M., Beck M. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 2008;167(3):267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nijmeijer S.C.M., Van den Born L.I., Kievit A.J.A., Stepien K.M., Langendonk J., Marchal J.P. The attenuated end of the phenotypic spectrum in MPS III: from late-onset stable cognitive impairment to a non-neuronopathic phenotype. Orphanet J. Rare Dis. 2019;14:249. doi: 10.1186/s13023-019-1232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regier D.S., Oetgen M., Tanpaiboon P. Mucopolysaccharidosis type IVA. Gen. Rev. 2016 doi: 10.2147/TACG.S69080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vairo F., Federhen A., Baldo G., Riegel M., Burin M., Leistner-Segal S., Giugliani R. Diagnostic and treatment strategies in mucopolysaccharidosis VI. Appl. Clin. Genet. 2015;8:245–255. doi: 10.2147/TACG.S68650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahalingam N.K., Priya S.J.S., Elango E.M., Sundari R.M. Diagnosis of mucopolysaccharidoses : how to avoid false positives and false. Indian J. Pediatr. 2004;71(1):29–32. doi: 10.1007/BF02725652. [DOI] [PubMed] [Google Scholar]
- 45.Tomatsu S., Okamura K., Maeda H. Keratan sulfate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- 46.Muenzer J. The mucopolysaccharidoses: a heterogeneous group of disorders with variable pediatric presentations. J. Pediatr. 2004;144:27–34. doi: 10.1016/j.jpeds.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 47.Sheth J., Mistri M., Kamate M., Vaja S., Sheth F.J. Diagnostic strategy for mucolipidosis II/III. Indian Pediatr. 2012;10 doi: 10.1007/s13312-012-0247-6. [DOI] [PubMed] [Google Scholar]
- 48.Khan S.A., Tomatsu S.C. Mucolipidoses overview: past, present, and future. Int. J. Mol. Sci. 2020;21:6812. doi: 10.3390/ijms21186812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehman T.J.A., Miller N., Norquist B., Underhill L., Keutzer J. Diagnosis of the mucopolysaccharidoses. Rhumatologie. 2011;50:v41–v48. doi: 10.1093/rheumatology/ker390. [DOI] [PubMed] [Google Scholar]
- 50.Giugliani R., Harmatz P., Wraith J.E. Management guidelines for mucopolysaccharidosis VI. Pediatric. 2007;120:405–418. doi: 10.1542/peds.2006-2184. [DOI] [PubMed] [Google Scholar]
- 51.Burlingame R.W., Thomas G.H., Stevens R.L., Schmid K., Moser H.W. Direct quantitation of glycosaminoglycans in 2 ml of urine from patients with mucopolysaccharidoses. Clin. Chem. 1981;27:124–128. [PubMed] [Google Scholar]
- 52.Tomatsu S., Okamura K., Taketani T., Orii K.O., Nishioka T., Gutierrez M.A. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr. Res. 2004;55(4):592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- 53.Tomatsu S., Yasuda E., Patel P., Ruhnke K., Shimada T., Mackenzie W.G. Morquio A syndrome: diagnosis and current and future therapies. Pediatr. Endocrinol. Rev. 2014;12(1):141–151. [PMC free article] [PubMed] [Google Scholar]
- 54.Sawamoto K., González J.V.A., Piechnik M., Otero F.J., Couce M.L., Suzuki Y. Mucopolysaccharidosis IVA: diagnosis, treatment, and management. Int. J. Mol. Sci. 2020 Feb;21(4):1517. doi: 10.3390/ijms21041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wood T.C., Harvey K., Beck M., Burin M.G., Chien Y.H., Church H.J. Diagnosing mucopolysaccharidoses IVA. J. Inherit. Metab. Dis. 2013;36:293–307. doi: 10.1007/s10545-013-9587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donati M.A., Pasquini E., Spada M., Polo G., Burlina A. Newborn screening in mucopolysaccharidoses. Ital. J. Pediatr. 2018;44:126. doi: 10.1186/s13052-018-0552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aboul Nasr A., Fateen E. Prenatal diagnosis of mucopolysacchardosis (MPS): the first Egyptian experience. Bratisl. Lek. Listy. 2004;105(9):291–340. [PubMed] [Google Scholar]
- 58.Ferreira C.A., Gahl W.A. Lysosomal storage diseases. Transl. Sci. Rare Dis. 2017;25:1–71. doi: 10.3233/TRD-160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moammar H., Cheriyan G., Mathew R., Al-Sannaa N. Incidence and patterns of inborn errors of metabolism in the Eastern Province of Saudi Arabia, 1983-2008. Ann. Saudi Med. 2010;30(4):271–277. doi: 10.4103/0256-4947.65254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Jasmi F.A., Tawfig N., Berniah A., Ali B.R., Taleb M., Hertecant J.L. Prevalence and novel mutations of lysosomal storage disorders in United Arab Emirates. JIMD Rep. 2013:1–9. doi: 10.1007/8904_2012_182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X., Qiu W., Ye J., Han L., Gu X., Zhang H. Demographic characteristics and distribution of lysosomal storage disorder subtypes in Eastern China. J. Hum. Genet. 2016;61:345–349. doi: 10.1038/jhg.2015.155. [DOI] [PubMed] [Google Scholar]
- 62.Khan S.A., Peracha H., Ballhausen D., Wiesbauer A., Rohrbach M. Molecular genetics and metabolism. Mol. Genet. Metabol. 2017;121(3):227–240. doi: 10.1016/j.ymgme.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poorthuis B.J., Wevers R.A., Kleijer W.J., Groener J.E., de Jong J.G., van Weely S. The frequency of lysosomal storage diseases in The Netherlands. Hum. Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 64.Malm G., Lund A.M., Mansson J.E., Heiberg A. Mucopolysaccharidoses in the Scandinavian countries: incidence and prevalence. Acta paedi. 2008;97:1577–1581. doi: 10.1111/j.1651-2227.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 65.Lowry R.B., Applegarth D.A., Toone J.R., MacDonald E., Thunem N.Y. An update on the frequency of mucopolysaccharide syndromes in British Columbia. Hum. Genet. 1990;85:389–390. doi: 10.1007/BF00206770. [DOI] [PubMed] [Google Scholar]
- 66.Josahkian J.A., Trapp F.B., Burin M.G., Michelin-Tirelli K., De Magalhães A.P.P.S., Sebastião F.M. Updated birth prevalence and relative frequency of mucopolysaccharidoses across Brazilian regions. Genet. Molec. Biol. 2021;44(1) doi: 10.1590/1678-4685-GMB-2020-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.