Abstract
The treatment of patients with advanced acute heart failure is still challenging. Intra-aortic balloon pump (IABP) has widely been used in the management of patients with cardiogenic shock. However, according to international guidelines, its routinary use in patients with cardiogenic shock is not recommended. This recommendation is derived from the results of the IABP-SHOCK II trial, which demonstrated that IABP does not reduce all-cause mortality in patients with acute myocardial infarction and cardiogenic shock. The present position paper, released by the Italian Association of Hospital Cardiologists, reviews the available data derived from clinical studies. It also provides practical recommendations for the optimal use of IABP in the treatment of cardiogenic shock and advanced acute heart failure.
Keywords: Advanced heart failure, Cardiogenic shock, Intra-aortic balloon pump, Mechanical Circulatory Support (MCS)
State of the art and guideline recommendations
Historical background
The concept of ‘counterpulsation’ indicates the pumping of blood outside the canonical phases of the physiological heart cycle 1,2 This method was first applied in experimental animals by Adrian and Arthur Kantrowitz in 1952 .2 Six years later Harken proposed an extracorporeal pump able to remove the blood during the systole and re-infuse it quickly during the next diastole. However, only in 1961, he developed the first model of extracorporeal counterpulsation. The initial clinical results were poor due to several issues, such as complications related to arterial accesses (bilateral arteriotomy was required), massive haemolysis due to blood turbulence, and poor synchronization of the pump with the cardiac cycle.3 In the same year, Moulopoulos et al.4 developed an intra-aortic device which consisted of a catheter with a balloon placed in the aorta, inflating during left ventricular diastole and deflating in systole. The first clinical experience was described in 1968 by Kantrowitz et al.5 who reported the benefits observed in two patients with cardiogenic shock (CS), in terms of increased systemic blood pressure (BP) and urinary output, although only one patient survived till hospital discharge. At that time, device insertion required a surgical approach, which made the incidence of ischaemic vascular complications remarkably high. Notably, catheters had a diameter of 15 Fr. Due to these limitations, the indication was limited to end-stage heart failure. In 1980, Bregman et al.6 first described the insertion of intra-aortic balloon pump (IABP) catheter with a percutaneous approach in 25 patients, which led to a remarkable reduction in the rate of complications and a significant increase in its use. Since then, smaller balloon-catheter systems were developed (e.g. ‘sheathless’ technique or ‘low-profile” catheters), along with more efficient control systems able to adapt IABP to different haemodynamic and heart rhythm conditions. These developments allowed to further improve counterpulsation techniques and reduce complications.
The intra-aortic balloon pump
The IABP is the first and simplest mechanical circulatory support (MCS) device developed, which consists of an external machine connected to the balloon-catheter system. The external machine is composed of a console, a balloon inflation pump, and a helium cylinder. The console allows to control and adjust the haemodynamic parameters. The pump is capable to rapidly inflate and deflate the balloon synchronously with the cardiac cycle with a predetermined volume of gas (30–50 mL of helium). The sizing of the balloon should be carefully chosen before placement according to the anthropometric characteristics of the patient so that, when inflated, the balloon will fill 80–90% of the aortic diameter.2 The dedicated double-lumen (one lumen is for helium and the other for invasive pressure measurement) balloon catheter can have a diameter varying between 7 and 9.5 Fr.
The catheter can be easily inserted, either percutaneously or surgically, through the femoral artery (only in selected cases by the brachial artery) and is advanced until it reaches the correct position in the descending thoracic aorta. For proper positioning, the distal tip of the catheter should be placed about 2–3 cm below the origin of the left subclavian artery with the proximal extremity of the balloon above the origin of the renal arteries. The insertion manoeuvre requires about 20–30 min, it should be done under fluoroscopic guidance, and it may take place at the bedside.
Intra-aortic balloon pump remains the simplest, cheapest, most studied, and utilized MCS device and still represents the standard device in randomized clinical trials aiming to evaluate the safety and efficacy of new mechanical circulatory support systems.7 However, its use in recent years has seen a progressive reduction.8
Physiological principles of counterpulsation
The hydraulic model used for the description of the circulatory system is known as the Windkessel model or ‘fireman’s model’. The similarities between the two systems include the ability to transform a pulsating flow generated by a pulsating pump (the heart) into a continuous flow (in the vessels), considering the aorta as an elastic conduit. Thus, the circulatory system is conceived as an elastic central reservoir into which the heart pumps its content and from which the various tissues extract blood through non-elastic conduits. Therefore, ventricular-arterial coupling plays a key role in the normal function of the cardiopulmonary circulation. It is fundamental that the ‘heart system’ be adequately paired with the ‘vessels system’ in order to maintain a cardiac output able to ensure adequate tissue perfusion.
Coronary flow is directly proportional to the perfusion gradient and inversely proportional to the coronary resistance. It occurs mostly during diastole, and the driving pressure gradient is generated by the difference between the mean diastolic pressure in the aortic root and the mean right atrial pressure. For this reason, the diastolic arterial pressure determines the pressure at which the coronary arteries are filled and the coronary arteries perfusion pressure is usually around 50 mmHg.
The impact of counterpulsation is primarily due to an increase in the myocardial oxygen supply/demand ratio. This result is achieved through both a reduction in the afterload of the left ventricle (LV) and an increase in coronary perfusion in order to increase LV performance. Hence, the coupling between the left ventricle and the arterial system is promoted, that is of utmost importance in the setting of CS where a reduced ventricular elasticity (contractility) and an increase in arterial elasticity (after-load) are present.
The mechanism of counterpulsation is based on LV afterload modulation through the dislocation of a certain volume of blood in diastole with an increase in aortic pressure and its ‘restitution’ in systole with a decrease in aortic pressure. Of note, the displacement of blood due to balloon inflation is directed both towards the top (coronary arteries and supra-aortic trunks) and the bottom (renal arteries and peripheral circulation) of the balloon.
In order to allow proper functioning, the system requires that the balloon inflates during the cardiac diastole—immediately after the closing of the aortic valve—and deflates during the systole (i.e. the concept of ‘counterpulsation’). The volume shift induced by the balloon inflation increases the volume of blood present in the aortic arch and its pressure. Afterwards, the balloon must be rapidly deflated immediately preceding the systole (during the isovolumetric contraction) and must remain deflated during the entire duration of the systole.
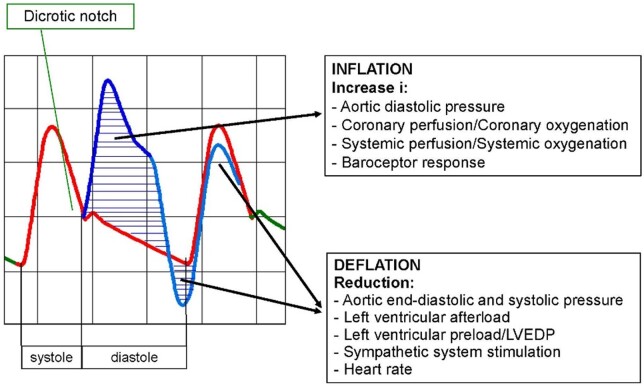
The overall haemodynamic effects of IABP therapy are summarized in Figure 1. Specifically, the systolic reduction in aortic pressure and volume generates the following consequences:
Figure 1.
Haemodynamic effects of intra-aortic balloon pump. LVEDP, left ventricular end-diastolic pressure.
a reduction in LV afterload with a resulting reduction in the myocardial consumption of oxygen
a more favourable balance between myocardial consumption and supply of oxygen and thus reduction of ischaemia
a reduction in peak systolic pressure due to LV workload reduction
an increase in cardiac output and ejection fraction
an improvement in the mechanical efficiency of the left ventricle in terms of contractility (due to the leftward shift of the pressure-volume curve).
Advanced heart failure: the dimensions of the problem
Definition and grading of cardiogenic shock
Cardiogenic shock is a clinical condition characterized by hypotension and hypoperfusion due to the inability of the heart to provide adequate cardiac output in presence of normal volemic status.9 Definitions of CS utilized in clinical trials and international guidelines are similar despite not completely uniform. Several clinical elements are constantly present across definitions: persistent hypotension (systolic blood pressure <90 mmHg) unresponsive to volume load and signs of end-organ hypoperfusion such as altered mental status, cold extremities, and oliguria (urinary output < 30 mL/h). Another essential parameter is hyperlactacidaemia (lactate > 2.0 mmol/L), a specific biochemical marker of tissue hypoperfusion. Low cardiac index (<2.2 L/min/m2) and high values of wedge pressure (>15 mmHg) are haemodynamic parameters that can contribute to define and characterize CS but are not essential for diagnosis.10 In the setting of CS, clinical and haemodynamic features have a variable spectrum of presentation, from mild hypoperfusion to refractory CS, and the outcome is directly related to the severity of clinical presentation.
Impending shock is a condition characterized by the presence of systolic blood pressure <100 mmHg, cardiac rate at the upper range, normal lactate values, cardiac index 2.0–2.2 L/min/m2 and need for one low dose inotrope/vasoactive drug. In overt CS these pathological alterations become more evident while in refractory CS they become severe with systolic blood pressure <90 mmHg, cardiac rate > 120 beat/min, obtunded mental status, lactate values > 4 mmol/L, cardiac index < 1.5 L/min/m2, and need for two or more vasoactive drugs.11 A clinical consensus statement on CS was published by the Society for Cardiovascular Angiography and Interventions (SCAI) in 2019, proposing an intuitive and innovative classification of CS in five stages from A (‘at risk’) to E (‘extremis’) and providing an accurate description of clinical signs, biomarkers, and haemodynamic parameters for each stage12 (Table 1).
Table 1.
Definition of advanced heart failure
| Stages of cardiogenic shock (SCAI CONSENSUS DOCUMENT) | ||
|---|---|---|
| Stage A | At risk | A patient who is not currently experiencing signs or symptoms of CS, but is at risk for its development. These patients may include those with large acute myocardial infarction or prior infarction acute and/or acute on chronic heart failure symptoms. |
| Stage B | Beginning cardiogenic shock | A patient who has clinical evidence of relative hypotension or tachycardia without hypoperfusion. |
| Stage C | Classic cardiogenic shock | A patient that manifests with hypoperfusion that requires intervention (inotrope, pressure or mechanical support, including ECMO) beyond volume resuscitation to restore perfusion. These patients typically present with relative hypotension. |
| Stage D | Deteriorating or Doom | A patient that is similar to category C but are getting worse. They have failure to respond to initial interventions |
| Stages E | Extremis | A patient that is experiencing cardiac arrest with ongoing CPR and/or ECMO, being supported by multiple interventions |
| ABP-shock II | ESC | SCAI |
|---|---|---|
|
|
|
Definition of advanced heart failure
Advanced heart failure [Stage D in the American College of Cardiology/American Heart Association classification (ACC/AHA)] is characterized by persistent signs and symptoms of heart failure despite the optimization of medical, surgical, and device therapy. Some coincident parameters can be found in both ACC and European society of Cardiology (ESC) definitions of advanced heart failure such as symptoms, number of heart failure hospitalization before index hospitalization, signs of end-organ dysfunction. Conversely, other parameters are reported only in one of the one definitions, such as intolerance to beta-blockers, Implantable Cardioverter Defibrillator (ICD) shocks, EF < 30%.13,14 The INTERMACS society (Interagency Registry for Mechanically Assisted Circulatory Support) proposed a classification made by seven stages characterized by progressively (from 7 to 1) more severe clinical and haemodynamic profiles. INTERMACS classification is used worldwide in both for clinical and scientific purposes15 (Table 2).
Table 2.
Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) classification for patients with advanced heart failure
| INTERMACS stages for classifying patients with advanced heart failure | ||
|---|---|---|
| INTERMACS 1 |
|
Haemodynamic instability in spite of increasing doses of catecholamines and/or mechanical circulatory support with critical hypoperfusion of target organs (severe cardiogenic shock). |
| INTERMACS 2 | Progressive decline despite inotropic support ‘Sliding on inotropes’ | Intravenous inotropic support with acceptable blood pressure but rapid deterioration of renal function, nutritional state, or signs of congestion. |
| INTERMACS 3 |
|
Haemodynamic stability with low or intermediate doses of inotropics, but necessary due to hypotension, worsening of symptoms, or progressive renal failure. |
| INTERMACS 4 |
|
Temporary cessation of inotropic treatment is possible, but patient presents with frequent symptoms recurrences and typically with fluid overload |
| INTERMACS 5 |
|
Complete cessation of physical activity, stable at rest, but frequently with moderate fluid retention and some level of renal dysfunction |
| INTERMACS 6 |
|
Minor limitation on physical activity and absence of congestion while at rest. Easily fatigued by light activity |
| INTERMACS 7 | ‘Placeholder’ | Patient in NYHA Class III with no current or recent unstable fluid balance. |
Table 3.
Clinical studies regarding the use of IABP in cardiogenic shock complicating acute myocardial infarction
| Study | Design | Patients (n) | STEMI | Shock definition | Treatment | Control | Primary endpoint | Result | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Ohman et al.31 | RCT | 57 | 100% | Hypotension | TL | No IABP | Total mortality | NS | 6 months |
| Sjauw et al.57 | Meta-Analysis | 1009 | 100% | NA | PCI | No IABP | Total mortality | NS | 30 days |
| Sjauw et al.57 | Meta-Analysis | 10529 | 100% | NA | TL | No IABP | Total mortality | Significant reduction | 30 days |
| Prondzinsky et al.56 | RCT | 40 | 65% | Hypotension | PCI | No IABP | APACHE II score | NS | 4 days |
| Abdel-Wahab et al.60 | Retrospective | 48 | 65% | Hypotension | PCI | IABP post PCI | Total mortality | Significant reduction | In-hospital |
| Romeo et al.63 | Meta-Analysis | 14186 | NA | NA | No reperfusion/TL/PCI | No IABP | Total mortality | NS | In-hospital |
| IABP-SHOCK II16 | RCT | 600 | 69% | Hypotension | PCI/CABG | No IABP | Total mortality | NS | 30 days |
| Unverzagt et al.35 | Meta-Analysis | 790 | NA | NA | PCI/TL/CABG | No IABP/LVAD | Total mortality | NS | 30 days |
| Hawranek et al.41 | Prospective registry | 991 | 81% | Hypotension | Failed PCI | No IABP | Total mortality | Significant reduction | 30 days |
CABG, coronary artery by-pass grafting; IABP, intra-aortic balloon pump; LVAD, left ventricular assist devices; NA, not available; NS, non-significant; PCI, percutaneous coronary intervention; RCT, randomized control trial; STEMI, ST elevation myocardial infarction; TL, thrombolysis.
Epidemiology
Cardiogenic shock is mainly due to acute myocardial infarction (AMI) complicated by left ventricle dysfunction (80%) followed by mechanical complications of myocardial infarction (13%). Myocarditis, cardiomyopathies, and electrical storm account for the remaining 7% of cases.16 CS complicates AMI in 5–8% of cases, with an incidence of 40 000–50 000 patient/year in the United States and 60 000–70 000 patient/year in Europe17. Recent data from a network of North American intensive care units showed a substantial modification in the epidemiology of CS due to an increase of non-ischaemic aetiology (28%) and ischaemic aetiology nonrelated to AMI (18%) and a decrease of CS complicating myocardial infarction (30%).18 Notably, the number of patients at risk of CS is constantly increasing due to progressive aging of the population and growing incidence of coronary artery disease and heart failure, as highlighted by a large Swedish register of 3,654 patients with CS due to AMI hospitalized in the period 1995–2013.19 The early mortality of CS is still elevated despite the progresses made in medical therapy, coronary revascularization techniques, and MCS devices. Thus, CS remains an unsolved clinical problem with a high rate of in-hospital mortality which has not significantly decreased over the last three decades. The lack of progress in terms of the outcome can be explained considering the increasing complexity and risk profile of CS patients in the last years. Indeed, these patients frequently show an advanced age, previous coronary events, and often a severe LV systolic dysfunction. In the late 90s, the SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) trial highlighted the positive impact of early revascularization on long-term outcomes in patients with AMI complicated by CS.20 As a consequence, more patients now survive to AMI increasing the number of patients with residual advanced heart failure, at risk for developing CS.
Prompt diagnosis
A prompt identification of signs and symptoms of hypoperfusion is crucial in patients with advanced heart failure, without overt CS, in order to prevent multi-organ failure refractory to any treatment. For this reason, the search for the aetiology of acute advanced heart failure and CS should proceed in parallel with its treatment. The main objective of CS treatment is the maintenance of adequate tissue perfusion and, when feasible, unloading of the LV and improving of coronary perfusion. In all cases of CS complicating AMI, an adequate pharmacological (inotropes and vasopressors), ventilatory and, if needed, mechanical support should be provided in addition to myocardial revascularization, in order to maintain an adequate perfusion10 (Figure 2). The presence of a ‘shock team’ is fundamental to manage these complex patients. The shock team should not be intended as a 24/7 available team, but rather as a model of management, a sort of diagnostic-therapeutic protocol applicable also in spoke hospitals. (Figures 3 and 4).
Figure 2.
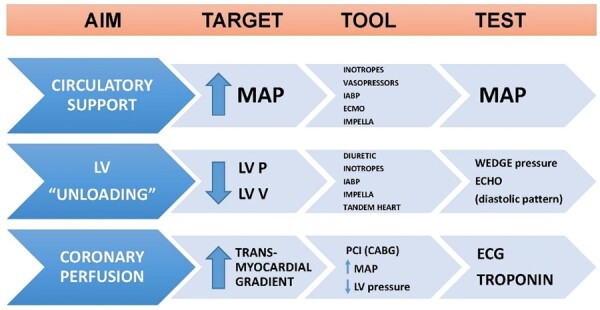
Targets in the treatment of cardiogenic shock. CABG, coronary artery bypass graft; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LV, left ventricle; P, pressure; MAP, mean arterial pressure; PCI, percutaneous coronary intervention; V, volume.
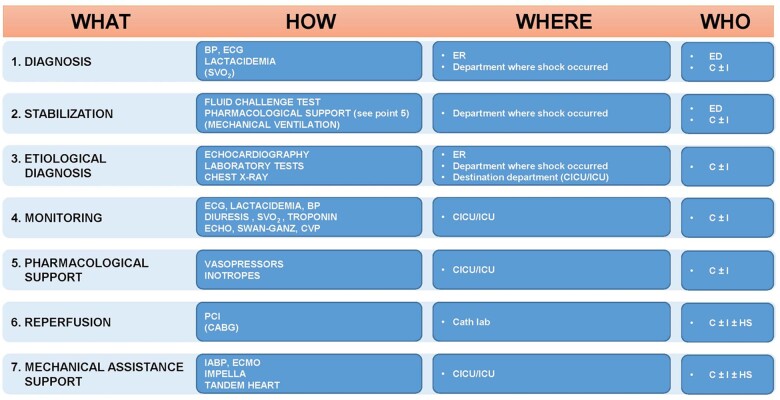
Figure 3.
The ≪Shock Team≫. BP, blood pressure; C, cardiologist; CABG, coronary artery bypass graft; HS, heart surgeon; ECMO, membrane extracorporeal oxygenation; IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention; ER, emergency room; CVP, central venous pressure; I, intensivist; SVO2, venous oxygen saturation; ICU, intensive care unit; ED, emergency medicine doctor; CICU, cardiological intensive care unit.
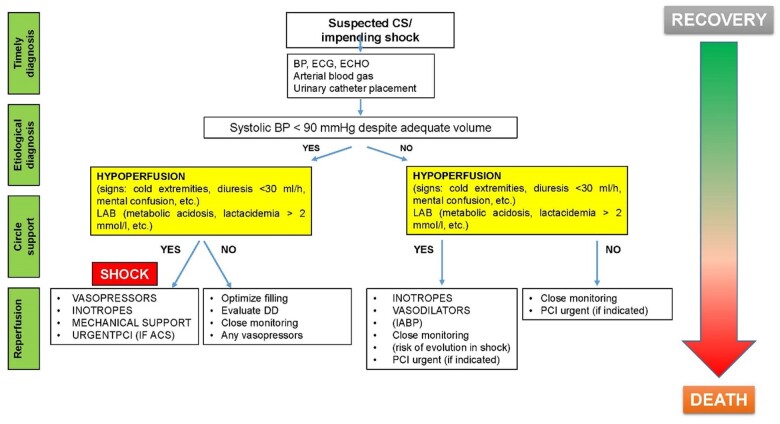
Figure 4.
Cardiogenic shock management protocol. CS, cardiogenic shock; BP, blood pressure; DO, differential diagnosis; IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome.
Guideline recommendations
ACC/AHA guidelines for ST-elevation myocardial infarction (STEMI) of 2004 assigned to IABP use in CS due to AMI a Class I, level of evidence B recommendation.21 In the following update of the same guidelines the recommendation was weaker (Class IIa, level of evidence B).22 Similarly, IABP use in patients with haemodynamic instability or CS was recommended in Class I (level of evidence C) in the 2008 ESC STEMI guidelines23 and 2010 ESC guidelines on myocardial revascularization.24 Afterwards, in 2012 ESC STEMI guidelines IABP received a class IIb recommendation.25 After the publication of IABP-SHOCK II trial,16 routine use of IABP in CS was downgraded to a Class III recommendation both in 2014 and 2018 guidelines on myocardial revascularization26 and in 2017 STEMI guidelines.27,28 Nonetheless, a Class IIa recommendation was left in case of mechanical complications after AMI. The progressive downgrading of routine IABP use in CS may have numerous consequences. First, a decrease in IABP use in clinical practice. Second, the need for cardiologists who still use this device for CS to motivate their choice from a legal perspective. Lastly, IABP could disappear as a standard therapy (control arm) in randomized clinical trials aiming to evaluate other MCS devices in the setting of CS.
Intra-aortic balloon pump contexts of use beyond cardiogenic shock
Since IABP was introduced in clinical practice, it has been used in several contexts in addition to CS (Figure 5).
Figure 5.
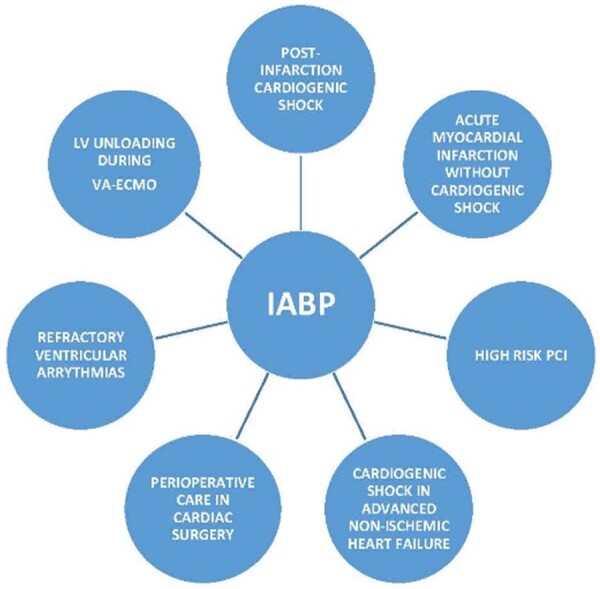
Contexts intra-aortic balloon pump of use. IABP, intra-aortic balloon pump; LV, left ventricle; VA-ECMO, veno-arterial extra-corporeal membrane oxygenation; PCI, percutaneous coronary intervention.
Cardiogenic shock complicating myocardial infarction: in the thrombolytic era, IABP was mainly implanted in patients with haemodynamic instability or CS with overall favourable results in registries or small randomized trials.29,30 In the 1990s, IABP use was so widespread that in the SHOCK trial20 86% of patients with CS complicating myocardial infarction were implanted with this device. In the following years, the era of primary percutaneous coronary intervention (pPCI), registries and trials showed no clear advantages in patients supported with IABP.31,32 In 2012 the IABP SHOCK II trial, the larger trial ever conducted on this topic, showed no improvement in outcome in patients who received IABP with a deep impact on following meta-analyses and international guidelines.33,34
Myocardial infarction without CS: despite the undisputed advantages of PCI, a small percentage of patients affected by myocardial infarction and treated with PCI still experience ‘no-reflow’, a phenomenon caused by a multifactorial mechanism.35 In this context, the prophylactic use of IABP has shown advantages both in experimental studies 36 and in a large registry of 1500 high-risk patients undergoing primary PCI.37 The randomized trial CRISP-AMI (Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction), was designed to assess whether IABP implantation before PCI could reduce infarct size evaluated by cardiac magnetic resonance in patients with anterior STEMI without CS. In this trial, the primary end-point was not reached,38 thus discouraging the use of IABP in this context. Nevertheless, a recent small randomized trial showed a non-significant survival benefit and a significant improvement in ST-segment resolution in the IABP group.39 In a recent prospective registry, Hawranek et al.40 assessed the impact of IABP use in patients with myocardial infarction complicated by CS according to the success of revascularization evaluated with final TIMI flow. Among patients with unsuccessful PCI (TIMI flow 0/1), those supported with IABP showed a significant lower 30-day mortality and 1-year mortality.
High-risk PCI: IABP has been used to prevent complications in patient undergoing high-risk PCI (defined according to clinical, haemodynamic, and anatomical criteria). The first clinical experiences in this context showed positive results , whereas the randomized trial Balloon Pump Assisted-Coronary Intervention Study (BCIS) showed controversial results. In this study, 150 patients received IABP before high-risk PCI and showed no benefit in terms of in-hospital and 6-month mortality and ischaemic cardiac or cerebral events compared with control group.0,41 Conversely, 2-years follow-up data showed a relative 34% decrease of all-cause mortality in the IABP group.42 In the last years, other percutaneous MCS devices have been used in high risk-PCI and practical algorithms for MCS device choice have been proposed.43
Non ischaemic CS and advanced heart failure: IABP can be implanted in these patients as ‘bridge to decision’ or ‘bridge to bridge’ while waiting for cardiac transplantation or left ventricular assist device (LVAD) implantation.44 Several studies highlighted predictors of successful IABP use in this context.45 The first randomized trial in this clinical scenario was recently published, comparing IABP with inotropes. The results showed a significant improvement in the primary endpoint (trend in venous oxygen saturation [SvO2] values at 3 h) and in other instrumental and laboratory parameters (cardiac power output, N-terminal fragment of the type-B natriuretic peptide) and a positive, albeit non-significant, trend in the rate of major adverse cardiovascular events at 30 and 90 days in patients implanted with IABP.46
Peri-operative care in cardiac surgery: the implantation of IABP before cardiac surgery in selected high-risk patients with LV dysfunction can reduce low flow syndrome/perioperative complications and intensive care length of stay.47,48
Refractory ventricular arrhythmias: the use of IABP as a mechanical support system in patients with LV dysfunction and sustained ventricular arrhythmias refractory to medical therapy has limited evidence. Goyal et al.49 described the efficacy of IABP in the treatment of refractory ventricular arrhythmias in a patient with dilated cardiomyopathy and normal coronary arteries. Fotopoulos et al.50 suggested in these patients an indirect beneficial mechanism mediated by the reduction of the adrenergic tone and therefore myocardial vulnerability to arrhythmias.
Left ventricle unloading during veno-arterial extracorporeal membrane oxygenation (VA ECMO) support: IABP reduces the afterload, thus it can be used as an unloading system of the LV during percutaneous VA-ECMO.51
Intra-aortic balloon pump in cardiogenic shock: a critical appraisal of the literature
Intra-aortic balloon pump is available since 1968, and it has been the most used MCS device in the last 40 years. Its wide use has been in part related to the Class I recommendation set in the previous European and American guidelines,52 despite a level of evidence of C and B respectively due to the small sample size of the supporting studies (mostly observational). In two small, randomized clinical studies in patients with AMI but without CS, IABP did not improve clinical outcomes and LV ejection fraction (EF) compared with medical therapy.53,54 However, in patients with anterior AMI without CS undergoing successful PCI, the use of IABP reduced the rate of re-occlusion of the infarct-related artery with a non-significant improvement of LVEF.55 In a randomized study including 57 patients with ST-elevation myocardial infarction (STEMI) and arterial hypotension, the use of IABP in addition to thrombolytic therapy reduced mortality with a borderline statistical significance only in patients in Killip Classes III and IV. Furthermore, in 45 patients with STEMI complicated by CS undergoing primary PCI, the addition of IABP was associated with only modest effects on the reduction of APACHE II score compared with medical therapy alone.56 In a meta-analysis of nine studies (only three of which including patients treated with primary PCI), IABP use did not improve 30-day survival or LVEF, and its use was associated with a significant increase in the rate of stroke and bleeding complications.57 However, all the aforementioned studies were not adequately powered either to investigate an association between IABP and mortality as a single Endpoint or to draw definite conclusions. Moreover, the wider use of primary PCI in patients with STEMI58 either complicated by CS or not, warranted a randomized clinical trial focused on the use of this device.
The IABP-SHOCK II16 trial was a multicentre, open-label study, that enrolled 600 patients with STEMI complicated by CS undergoing planned early revascularization. Patients were randomly assigned to receive IABP in addition to optimal medical therapy. At 30 days, mortality was not different between IABP and control group [39.7% vs. 41.3%, respectively; relative risk 0.96; 95% confidence interval (CI) 0.79–1.17; P = 0.69]. No differences were found between the two groups with respect to the rates of stroke, bleeding, peripheral ischaemic complications, recurrent AMI, and stent thrombosis. IABP-SHOCK II is currently the largest available randomized clinical trial investigating the role of IABP in patients with AMI and CS, and the authors should be commended for their efforts. However, several study limitations are evident. First, only about 70% of the enrolled patients presented with STEMI and among these, more than a half with a non-anterior MI. Second, the timing of CS development has not been clearly reported, thus some CS cases may have experienced subacute presentation. Third, IABP was implanted after PCI in 87% of the cases, which is not coherent with a prompt treatment of CS and/or advanced acute heart failure. Forth, about 45% of enrolled patients experienced a resuscitated cardiac arrest (36% of those were treated with therapeutic hypothermia). Fifth, the median duration of counterpulsation was 3 days (interquartile range 2–4), with more than half of deaths occurring afterwards. Finally, 4.3% of patients enrolled in the IABP arm died before implantation and a cross-over from control group to IABP group occurred in 30 cases. Of note, the rate of LVAD implantation was higher in the control group (22 vs. 11). In the intention-to-treat analysis, the mortality rate in the control group was 41.3%, far from the 56% hypothesized by the authors for sample size calculation. Thus, the 8.8% absolute risk reduction obtained (lower than the expected 12%) decreased statistical power from 0.82 to 0.59. The results of IABP-SHOCK II trial have been confirmed at 6 years follow-up. However, it should be underlined that according to these data about 60% of patients with CS have died despite contemporary treatment with revascularization therapy.59
An important issue when considering the efficacy of IABP is the timing of insertion in relation to coronary angiography and PCI. It has been already reported that the insertion of IABP before PCI was associated with a significant reduction in mortality and adverse cardiovascular events.60 Recently, a study including patients with CS due to different aetiologies, confirmed that an early placement of IABP was an independent predictor of 30 days survival.61
In a subgroup analysis of the CRISP-AMI trial in patients with large anterior STEMI and persisting ischaemia after PCI, the use of IABP was associated with a significant mortality reduction at 6 months.62 Conversely, an updated meta-analysis of seven randomized studies (four comparing IABP vs. medical therapy and three comparing IABP with other MCS devices) including patients with STEMI complicated by CS, did not find significant differences in 30-days survival between the study groups.34 Subgroups analysis showed a beneficial impact of IABP use on prognosis in patients with young age, no prior MI, arterial hypertension, and in case of anterior MI.
In a recent prospective registry, Hawranek et al.40 investigated the efficacy of IABP in patients with AMI complicated by CS according to the success of revascularization evaluated with final TIMI flow. Since 2003 to 2014, more than 7200 patients were included in the study. Patients treated with IABP presented lower systolic arterial pressure and LVEF, higher heart rate, rate of multivessel coronary artery disease, and involvement of left main and left anterior descending artery. The use of IABP was associated with higher 30-day and 1-year mortality, recurrent MI, stroke, recurrent PCI, major bleeding, and cardiac arrest, due to the higher risk profile of patients treated with the device. However, in patients with final TIMI flow 0/1, IABP use was an independent predictor of lower 30-days mortality (HR 0.72, 95% CI 0.59–0.89; P = 0.002) despite a higher rate of bleeding, recurrent MI and lower LVEF. Conversely, in patients with final TIMI 2-3, IABP was an independent predictor of higher 30-day mortality (HR 1.18, 95% CI 1.08–1.30; P = 0.0004). Therefore, these hypothesis generating results might suggest a beneficial impact of IABP use in patients with AMI complicated by CS undergoing PCI with a final suboptimal angiographic result (TIMI 0–1 or no-reflow). Table 316,30,34,40,56,57,60,63 summarizes the results of the main studies on the use IABP in patients with MI complicated by CS.
Intra-aortic balloon pump vs. other percutaneous mechanical circulatory support devices in patients with ST-elevation myocardial infarction complicated by cardiogenic shock
Beyond IABP, the following percutaneous MCS (pMCS) devices are currently available with different circuit configurations:
Left ventricle → Aorta. Impella® 2.5 and CP (Abiomed, Danvers, MA, USA) is approved for short-term (7–14 days) support of the LV in patients with CS due to isolated LV dysfunction refractory to optimal medical therapy.
Left atrium → Aorta. TandemHeart, LivaNova London, UK.
Right atrium → Aorta. VA-ECMO.
Inferior vena cava → Pulmonary artery. Impella® RP is approved for CS due to right ventricle failure.
In the 65 patients with AMI complicated by CS enrolled in the ISAR-SHOCK (Efficacy Study of LV Assist Device to Treat Patients With Cardiogenic Shock)64 trial, the use of Impella® 2.5 appeared safe, feasible, and associated with an greater circulatory support compared to IABP. Nevertheless, overall 30 days mortality rate was elevated (46%) and did not differ between the two groups. The IMPRESS in Severe Shock (IMPella vs. IABP Reduces mortality in STEMI patients treated with primary PCI in Severe cardiogenic Shock) trial included 48 patients with STEMI complicated by severe CS (all treated with mechanical ventilation, 92% with cardiac arrest and refractory shock at return of spontaneous circulation) and reported no difference in mortality at 30 days and at 6 months between patients who received either Impella or IABP.65 The rate of major bleeding was higher in patients treated with Impella® (33% vs. 8%, P = 0.06). No difference on survival and an increased risk of bleeding was confirmed in the following registries comparing Impella® with the IABP in patients with CS surviving a cardiac arrest.66 A collaborative meta-analysis of four randomized trials aiming at investigating efficacy and safety of other pMCS devices (TandemHeart™ or Impella®) vs. IABP in CS reported no difference in 30-day mortality. However, other pMCS devices significantly increased median arterial pressure and decreased arterial lactate levels. Furthermore, although no significant difference was observed in the incidence of leg ischaemia, the rate of bleeding complications was significantly increased in patients treated with other pMCS devices compared with IABP.67 Schrage et al.68 performed a retrospective propensity-matched analysis comparing patients with MI complicated by CS managed with Impella® at several tertiary care European hospitals with patients enrolled in the IABP-SHOCK II trial. The authors found no difference in 30-days mortality (48.5% vs. 46.4%, P = 0.64). Notably, the use of Impella® was associated with a significant increase in severe or life-threatening bleeding (8.5% vs. 3.0%, P < 0.01) and peripheral vascular complications (9.8% vs. 3.8%, P = 0.01). Data from the National Cardiovascular Data Registry reported a significant increase over time in the use of Impella® in patients with AMI complicated by CS undergoing PCI: from 3.5% in 2015 to 8.7% in 2017. In the propensity-matched analysis performed within this cohort, total mortality was 45% in patients treated with Impella® and 34% in patients treated with IABP, while major bleedings were more frequent in the first group (31.3% vs. 16%).69Table 4 summarizes the main studies comparing Impella/TandemHeart with IABP.64–68,70
Table 4.
Studies comparing IABP vs. Impella/TandemHeart in patients with cardiogenic shock
| Study | Design | Patients (n) | Control | Primary Endpoint | Result | Follow-up |
|---|---|---|---|---|---|---|
| Seyfarth et al.64 | Randomized | 26 | Impella | Cardiac Index | Significant increase with Impella | 30 min |
| IMPRESS65 | Randomized | 48 | Impella | Mortality | NS | 30 days |
| Manzo-Silberman et al.66 | Retrospective | 78 | Impella | Mortality | NS | 30 days |
| Thiele et al.67 | Mata-Analysis | 148 | Impella/ TandemHeart | Mortality | NS | 30 days |
| Schrage et al.68 | Retrospective propensity matched | 372 | Impella | Mortality | NS | 30 days |
| Amin et al.70 | Retrospective propensity matched | 48306 | Impella | Mortality | Significant increase with Impella | In-hospital |
NS, not significant.
It should be noted that previous results have been obtained from observational studies. Thus residual confounders cannot be excluded despite statistical adjustment. Furthermore, several other study limitations should be considered. First, outcomes analysis has not been stratified for CS severity in the different studies. Second, it is likely that more severe patients with a higher risk of mortality have been treated with more complex pMCS devices. Third, most patients were treated with Impella® 2.5, thus the results of the studies may not be applied to Impella® 5.0 or CP. Finally, patients at higher risk who were initially treated with IABP and subsequently required an escalation to a more potent circulatory support have usually been excluded. Therefore, data from randomized clinical trials comparing Impella® 5.0 or CP with IABP are urgently needed.
In the recent IMPELLA-STIC,71 a small sample of patients with AMI complicated by CS stabilized by initial treatment with inotropes was randomized to receive Impella® LP 5.0. The use of the device was not associated with an improvement in LVEF, whereas it was associated with an increase in the rate of major bleeding at 1 month. The ongoing DanGer Shock72 trial randomizes patients with AMI complicated by CS on a 1:1 basis to Impella® CP or current guideline-driven therapy. The planned enrolment of 360 patients will provide an adequate statistical power to investigate the presence of an association between study treatment and survival in this clinical setting.
Veno-arterial extra-corporeal membrane oxygenation has been mainly studied in the setting of STEMI, myocarditis, post-cardiotomy shock, and refractory cardiac arrest. However, data on VA-ECMO mainly derive from observational data. Therefore, guidelines recommendation for its use are based only on experts opinion (Class IIb). Retrospective data by Sheu et al.73 and Baek et al.74 showed that an early use of VA-ECMO in patients with STEMI undergoing primary PCI complicated by refractory CS (defined as persistence of systolic blood pressure < 75 mmHg despite the use of vasopressors and IABP) improved outcome at 30 days, with an overall mortality of 43%. The use of the ENCOURAGE score based on 7 parameters [age > 60 years, female sex, body mass index > 25 kg/m2, Glasgow scale < 4, creatinine > 150 μmol/L, arterial lactates (<2, 2–8, or >8 mmol/L) and prothrombin activity <50%] before ECMO implantation might be a useful discriminatory tool to predict mortality in patients with AMI complicated by CS evaluated for VA-ECMO.75 Lastly, a recent retrospective study failed to show a significant difference in 30 days mortality in patients treated with VA-ECMO or Impella® CP/5.0.36
Practical recommendations on the use of intra-aortic balloon pump
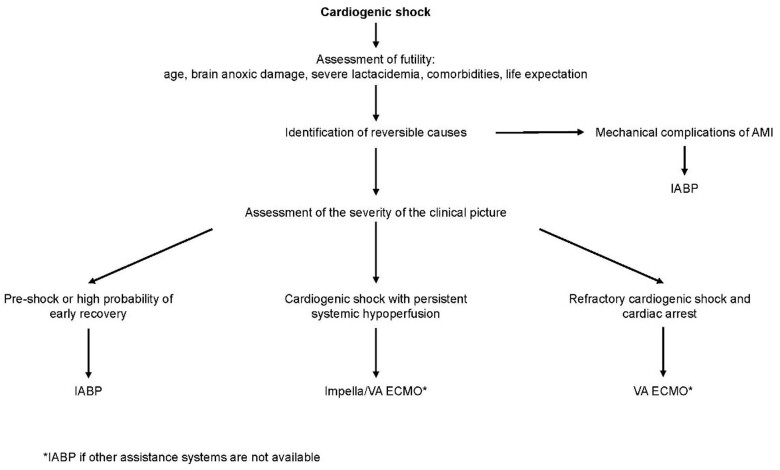
ANMCO aimed at focusing the proper setting for which IABP use is adequate, thus bridging the gap between Class III recommendation28 and its wide use in clinical practice. It is of utmost importance when selecting the proper percutaneous MCS device, a thorough evaluation of both the patient and the degree of ongoing acute heart failure/CS (Figure 6). The use of IABP should be considered in the very early phases of CS and in patients with impending shock, especially when other MCS are not available. Therefore, it is crucial to timely identify patients who are at risk of developing CS (or in CS initial phase) searching for early signs of CS such as initial increase in lactate levels in a setting of organ hypoperfusion.
Figure 6.
Choice of percutaneous mechanical assistance system in cardiogenic shock. IABP, intra-aortic balloon pump; VA ECMO, veno-arterial extracorporeal oxygenation to the arteria invenosus membrane; AMI acute myocardial infarction.
An adequate set up of IABP functions is warranted, with particular attention to balloon inflation and deflation timing (Figure 7).
Figure 7.
Correct intra-aortic balloon pump setting with appropriate balloon inflation and deflation timing according to cardiac cycle.
On the basis of previous data, safety, and ease of use of IABP, together with lack of prompt availability of new pMCS devices, we suggest the following practical recommendations for non-routinary use of IABP:
-
AMI with initial/impending CS:
AMI in ‘Pre-shock’ state (MAP 65–70 mmHg and/or SvO2/central venous saturation [ScvO2] < 65–70% and/or lactate increase and/or cardiac index 2–2.2 L/min/m2 with only one vasopressor/inotrope at low dosage) OR judged at high risk of developing CS [signs of pulmonary congestion, no response to pharmacological therapy (especially diuretics), oliguria, elevated HR, (SCAI Classification Class A and B)]
AMI showing persistent ischaemia/no-reflow after PCI, on top of standard therapy
-
AMI complicated by overt CS
AMI complicated by CS due to mechanical complications (bridge to surgery)
AMI with partially successful/unsuccessful PCI as initial device as a bridge to escalation to more potent pMCS devices placement (bridge to bridge) or LVAD placement/transplantation (bridge to decision)
AMI complicated by CS when other pMCS devices are not available
AMI complicated by CS when other pMCS severe aortic valvulopathy, severe peripheral artery disease, …).
-
CS due to non-ischaemic aetiology:
heart failure with non-ischaemic aetiology at high risk of developing CS (SCAI Classification Class A); ‘pre-shock’ (MAP 65–70 mmHg and/or SvO2/ScvO2 < 65–70%; normal lactates; cardiac index 2–2.2 L/min/m2 with only one vasopressor/inotrope at low dosage) especially if reversible cause are detected (bridge to recovery)
patients with CS in the presence of contra-indications to other pMCS devices placement
CS with non-ischaemic aetiology as initial device before other pMCS devices placement (bridge to bridge) or LVAD placement/transplantation (bridge to decision)
Back-up system (sheath insertion in femoral artery for rapid bail-out placement) in the context of high-risk PCI (Tables 5 and 6) based on clinical, anatomical, and procedural criteria, especially in the presence of contraindication for or unavailability of other MCS devices.
Perioperative setting use in cardiac surgery for high-risk patients to reduce peri-procedural complications and facilitate weaning from extra-corporeal circulation.
Ventricular arrhythmias refractory to pharmacological treatment as ‘bridge to recovery’ or ‘bridge to treatment’ (ablation, LVAD, transplantation).
LV unloading in patients undergoing VA-ECMO.
Table 5.
Factors contributing to define high-risk PCI
| Coronary artery disease | Clinical features | Haemodynamic aspects |
|---|---|---|
|
Comorbidities and cardiological conditions reducing tolerance to myocardial ischaemia:
|
|
Table 6.
Main elements in the choice of the type of percutaneous mechanical assistance for high -risk PCI in the absence of significant peripheral vascular disease
| IABP | IMPELLA | ECMO |
|---|---|---|
| High risk of haemodynamic instability | Very high risk of haemodynamic instability | Very high risk of haemodynamic instability with biventricular dysfunction |
| Echocardiographic aspect not relevant | No ventricular thrombosis, no severe aortic valve disease | Ventricular thrombosis, Severe aortic valve disease |
Nursing care in the patients with an intra-aortic balloon pump
Nursing care in the patients with an IABP lasts for the duration of IABP placement and consists of four steps:
Step 1: preparation of the patient for IABP placement
Step 2: assistance to the physician during IABP insertion
Step 3: monitoring the patient with IABP
Step 4: weaning phase and IABP removal
Step 1: preparation of the patient for intra-aortic balloon pump placement.
In this phase, the nurse prepares the patient for IABP insertion, and:
Cleans the groin area and, if necessary, perform trichotomy from the groin until the knee
Talks to the patient (previously informed by the physician) and explains further details, if necessary
Step 2: assistance to the physician during intra-aortic balloon pump placement.
The assistance for IABP placement includes both preparation of materials and direct assistance to the physician during insertion manoeuvre:
Gathering the material:
sterile sheets, gauzes and gloves, face masks, protective glasses;
dressing and treatment trolley: disinfectant, sutures, local anaesthetic, various syringes;
pressure bag with saline solution (in some centres saline solution is heparinized).
Preparing the kit and the device:
check the completeness of the kit
predisposition of IABP device (check cables, helium tank filling level, correct tank position, and opening)
preparation of invasive blood pressure monitoring kit
Tasks during IABP placement:
plug in the device
connect pressure and heart rate monitoring cables to the patient’s monitor (where available)
monitoring patient’s vital parameters, level of consciousness, cognition, and agitation
co-operate with the physician for insertion and positioning of the catheter
co-operate with the physician in IABP connection and setting
remove all the utilized material once the catheter is placed, with special attention to the sharps
prepare a dressing at the insertion site
adjust the bed and the patient in a comfortable position (always keep an inclination <30°)
Step 3: Monitoring the patient with intra-aortic balloon pump
In this phase, a prompt identification of early and late complications of IABP is warranted.
Early complications
It is important to monitor:
vital parameters (HR, BP, diuresis, peripheral saturation, fever) ensuring that the target values are reached and maintained. Reduction in urinary output refractory to diuretic therapy could be due to balloon displacement, thus correct position should be checked.
insertion site (percutaneous or surgical) and its dressing, in order to promptly identify bleeding complications.
proper IABP device functioning
circuit integrity. In case blood is detected in the connecting pipe between IABP and the catheter, IABP should be immediately stopped and the physician informed.
level of the battery. IABP is usually plugged. Nevertheless, the patient may need to be moved to undergo diagnostic test. Thus, batteries should be kept fully charged and must be able to provide adequate power supply.
helium tank residual capacity. Check the helium tank light when starting to use the device and subsequently perform daily check.
daily coagulation tests, especially if patient is treated with anticoagulant therapy (such as unfractioned heparin).
peripheral pulses, colour, and temperature of the limb where the catheter is placed.
patient’s psychological state.
Nursing manoeuvre during counterpulsation
The nurse should:
pay attention to bedsores during daily patient hygiene, as the patient must constantly keep a supine position without the possibility to move the lower limb in which IABP is inserted.
put IABP in ‘standby’ mode (if feasible) during any manoeuvre in which the catheter could be moved. Once the manoeuvre is ceased, the device can be re-activated pressing ‘START’ button on the console.
keep the patient in supine position with an inclination always < 30° to avoid kinking of the catheter.
Phase 4: from weaning phase to intra-aortic balloon pump removal
Weaning phase. Either set IABP in 2:1 ratio (i.e. 1 cycle inflation/deflation every two cardiac cycles) or reduce balloon inflation. Monitor haemodynamic stability for a few hours and check coagulation tests.
-
IABP removal phase. In this phase, the catheter is removed in close collaboration with the physician. The nurse should:
inform the patient of the forthcoming procedure
check the coagulation tests
monitor vital parameters during weaning from IABP
-
prepare the material for IABP removal:
sterile gloves and gauzes, masks, protective glasses, non-sterile single-use sheet, blades
hazardous waste containers
Ensure the IABP is switched off and disconnected from the catheter during removal (be sure the balloon is not accidentally inflated)
catheter disposal
prepare a compression dressing to be left in place for at least 12 h once the physician has terminated to compress the insertion site
monitor patient’s limb and insertion site to exclude bleeding
monitor vital parameters at close intervals (every hour for the first 12 h)
Removal phase is a very delicate stage as the deflated balloon cannot pass through the sheath and, thus, must be removed together with the sheath requiring careful attention to vessel haemostasis.
Conclusion
Prognosis of patients with acute advanced heart failure and CS is still poor in spite of coronary reperfusion. A prompt diagnosis of multi-organ hypoperfusion and therapeutic intervention aimed at restoring an adequate arterial pressure is crucial. The neutral results of the IABP-CHOCK II trial might be related to a late IABP implantation, which occurred in the vast majority of cases after PCI. It seems reasonable to proceed with IABP implantation in patients with impending shock/CS, provided it is implanted in the very early phases of heart failure/CS, especially in Centres that do not have more potent pMCS systems.
Conflict of interest: none declared.
Disclaimers
This Position Paper was originally published in the Italian language in ‘Position paper ANMCO: Ruolo del contropulsatore aortico nel paziente con insufficienza cardiaca acuta avanzata’, official journal of Italian Federation of Cardiology (IFC), published by Il Pensiero Scientifico Editore. Translated by a representative of the Italian Association of Hospital Cardiologists (ANMCO) and reprinted by permission of IFC and Il Pensiero Scientifico Editore.
Reviewers: Vincenzo Amodeo, Nadia Aspromonte, Andrea Di Lenarda, Stefano Domenicucci, Giuseppina Maura Francese, Adriano Murrone, Stefano Urbinati
References
- 1.Trost JC, Hillis D.. Intra-aortic balloon counterpulsation. Am J Cardiol 2006;97:1391–1398. [DOI] [PubMed] [Google Scholar]
- 2.Kantrowitz A.Experimental augmentation of coronary flow by retardation of the arterial pressure pulse. Surgery 1952;14:678–687. [PubMed] [Google Scholar]
- 3.Clauss RH, Birtwell WC, Albertal G, Lunzer S, Taylor WJ, Fosberg AM, Harken DE.. Assisted circulation. I. The arterial counterpulsator. J Thorac Cardiovasc Surg 1961;41:447–458. [PubMed] [Google Scholar]
- 4.Moulopoulos SD, Topaz S, Kolff WJ.. Diastolic balloon pumping (with carbon dioxide) in the aorta – a mechanical assistance to the failing circulation. Am Heart J 1962;63:669–675. [DOI] [PubMed] [Google Scholar]
- 5.Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL. Jr., Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA 1968;203:113–118. [PubMed] [Google Scholar]
- 6.Bregman D, Nichols AB, Weiss MB, Powers ER, Martin EC, Casarell WJ.. Percutaneous intraaortic balloon insertion. Am J Cardiol 1980;46:261–264. [DOI] [PubMed] [Google Scholar]
- 7.Pappalardo F, Ajello S, Greco M, Celińska-Spodar M, De Bonis M, Zangrillo A, Montisci A.. Contemporary applications of intra-aortic balloon counterpulsation for cardiogenic shock: a ‘real world’ experience. J Thorac Dis 2018;10:2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah M, Patnaik S, Patel B, Ram P, Garg L, Agarwal M, Agrawal S, Arora S, Patel N, Wald J, Jorde UP.. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol 2018;107:287–303. [DOI] [PubMed] [Google Scholar]
- 9.Valente S, Marini M, Battistoni I, et al. Lo shock cardiogeno e una malattia rara che necessita di una rete dedicata. G Ital Cardiol 2017;18:719–726. [DOI] [PubMed] [Google Scholar]
- 10.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017;136:e232-68–e268. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson TM, Ohman EM, O'Neill WW, Rab T, Cigarroa JE; Interventional Scientific Council of the American College of Cardiology. A practical approach to mechanical circulatory support in patients undergoing percutaneous coronary intervention: an interventional perspective. JACC Cardiovasc Interv 2016;9:871–883. [DOI] [PubMed] [Google Scholar]
- 12.Baran DA, Grines CL, Bailey S.. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29–37. [DOI] [PubMed] [Google Scholar]
- 13.Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin-Adams M, Dickinson MG, Levy P, Stough WG, Sweitzer NK, Teerlink JR, Whellan DJ, Albert NM, Krishnamani R, Rich MW, Walsh MN, Bonnell MR, Carson PE, Chan MC, Dries DL, Hernandez AF, Hershberger RE, Katz SD, Moore S, Rodgers JE, Rogers JG, Vest AR, Givertz MM; Heart Failure Society of America Guidelines Committee. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail 2015;21:519–534. [DOI] [PubMed] [Google Scholar]
- 14.Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge-Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska-Migaj E, McDonagh T, Seferovic P, Ruschitzka F.. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:1505–1535. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, Naftel DC, Ulisney K, Desvigne-Nickens P, Kirklin JK.. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant 2009;28:535–541. [DOI] [PubMed] [Google Scholar]
- 16.Thiele H, Zeymer U, Neumann F-J, Ferenc M, Olbrich H-G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K; IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–1296. [DOI] [PubMed] [Google Scholar]
- 17.Thiele H, Ohman EM, Desch S, Eitel I, De Waha S.. Management of cardiogenic shock. European Heart Journal 2015;36:1223–1230. 10.1093/eurheartj/ehv051 25732762 [DOI] [PubMed] [Google Scholar]
- 18.Berg DD, Bohula EA, van Diepen S, et al. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes 2019;12:e005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redfors B, Angerås O, Råmunddal T, Dworeck C, Haraldsson I, Ioanes D, Petursson P, Libungan B, Odenstedt J, Stewart J, Lodin E, Wahlin M, Albertsson P, Matejka G, Omerovic E.. 17-year trends in incidence and prognosis of cardiogenic shock in patients with acute myocardial infarction in western Sweden. Int J Cardiol 2015;185:256–262. [DOI] [PubMed] [Google Scholar]
- 20.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH.. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med 1999;341:625–634. [DOI] [PubMed] [Google Scholar]
- 21.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110:588–636. [DOI] [PubMed] [Google Scholar]
- 22.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso JE, Tracy CM, Woo YJ, Zhao DX; CF/AHA Task Force. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:529–555. [DOI] [PubMed] [Google Scholar]
- 23.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M; ESC Committee for Practice Guidelines (CPG). Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2008;29:2909–2945. [DOI] [PubMed] [Google Scholar]
- 24.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D; Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501–2555. [DOI] [PubMed] [Google Scholar]
- 25.Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D; Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 26.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 27.Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 28.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 29.Anderson RD, Ohman EM, Holmes DR Jr, Col I, Stebbins AL, Bates ER, Stomel RJ, Granger CB, Topol EJ, Califf RM.. Use of intraaortic balloon counterpulsation in patients presenting with cardiogenic shock: observations from the GUSTO-I Study. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol 1997;30:708–715. [DOI] [PubMed] [Google Scholar]
- 30.Ohman EM, Nanas J, Stomel RJ, Leesar MA, Nielsen DWT, O'Dea D, Rogers FJ, Harber D, Hudson MP, Fraulo E, Shaw LK, Lee KL; TACTICS Trial. Thrombolysis and counterpulsation to improve survival in myocardial infarctioncomplicated by hypotension and suspected cardiogenic shock of heart failure: results of the TACTICS trial. J Thromb Thrombolysis 2005;19:33–39. [DOI] [PubMed] [Google Scholar]
- 31.Zeymer U, Hochadel M, Hauptmann K-E, Wiegand K, Schuhmacher B, Brachmann J, Gitt A, Zahn R.. Intra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: results of the ALKK-PCI registry. Clin Res Cardiol 2013;102:223–227. [DOI] [PubMed] [Google Scholar]
- 32.Timoteo AT, Nogueira MA, Rosa SA, Belo A, Ferreira RC; ProACS Investigators. Role of intra-aortic balloon pump counterpulsation in the treatment of acute myocardial infarction complicated by cardiogenic shock: evidence from the Portuguese nationwide registry. Eur Heart J Acute Cardiovasc Care 2016;5:23–31. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad Y, Sen S, Shun-Shin MJ, Ouyang J, Finegold JA, Al-Lamee RK, Davies JER, Cole GD, Francis DP.. Intra-aortic balloon pump therapy for acute myocardial infarction: a meta-analysis. JAMA Intern Med 2015;175:931–939. [DOI] [PubMed] [Google Scholar]
- 34.Unverzagt S, Buerke M, de Waha A, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev 2015;CD007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niccoli G, Burzotta F, Galiuto L, Crea F.. Myocardial no-reflow in humans. J Am Coll Cardiol 2009;54:281–292. [DOI] [PubMed] [Google Scholar]
- 36.LeDoux JF, Tamareille S, Felli PR, Amirian J, Smalling RW.. Left ventricular unloading with intra-aortic counter pulsation prior to reperfusion reduces myocardial release of endothelin-1 and decreases infarction size in a porcine ischemia-reperfusion model. Catheter Cardiovasc Interv 2008;72:513–521. [DOI] [PubMed] [Google Scholar]
- 37.Brodie BR, Stuckey TD, Hansen C, Muncy D.. Intra-aortic balloon counterpulsation before primary percutaneous transluminal coronary angioplasty reduces catheterization laboratory events in highrisk patients with acute myocardial infarction. Am J Cardiol 1999;84:18–23. [DOI] [PubMed] [Google Scholar]
- 38.Patel MR, Smalling RW, Thiele H, Barnhart HX, Zhou Y, Chandra P, Chew D, Cohen M, French J, Perera D, Ohman EM.. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA 2011;306:1329–1337. [DOI] [PubMed] [Google Scholar]
- 39.Nunen LX, Veer M, Zimmermann FM, Wijnbergen I, Brueren GRG, Tonino PAL, Aarnoudse WA, Pijls NHJ.. Intra-aortic balloon pump counterpulsation in extensive myocardial infarction with persistent ischemia: the SEMPER FI pilot study. Catheter Cardiovasc Interv 2020;95:128–135. [DOI] [PubMed] [Google Scholar]
- 40.Hawranek M, Gierlotka M, Pres D, Zembala M, Gąsior M.. Nonroutine use of intra-aortic balloon pump in cardiogenic shock complicating myocardial infarction with successful and unsuccessful primary percutaneous coronary intervention. JACC Cardiovasc Interv 2018;11:1885–1893. [DOI] [PubMed] [Google Scholar]
- 41.Perera D, Stables R, Thomas M, BCIS-1 Investigators. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA 2010;304:867–874. [DOI] [PubMed] [Google Scholar]
- 42.Perera D, Stables R, Clayton T, De Silva K, Lumley M, Clack L, Thomas M, Redwood S; BCIS-1 Investigators. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation 2013;127:207–212. [DOI] [PubMed] [Google Scholar]
- 43.Burzotta F, Russo G, Basile E, et al. Come orientarsi tra contropulsatore, Impella e ossigenazione a membrana extracorporea. G Ital Cardiol 2018;19:5S–13S. [DOI] [PubMed] [Google Scholar]
- 44.Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200.;. [DOI] [PubMed] [Google Scholar]
- 45.Imamura T, Juricek C, Nguyen A, et al. Predictors of hemodynamic improvement and stabilization following intraaortic balloon pump implantation in patients with advanced heart failure. J Invasive Cardiol 2018;30:56–61. [PMC free article] [PubMed] [Google Scholar]
- 46.den Uil CA, Van Mieghem NM, Bastos MB, Jewbali LS, Lenzen MJ, Engstrom AE, Bunge JJH, Brugts JJ, Manintveld OC, Daemen J, Wilschut JM, Zijlstra F, Constantinescu AA.. Primary intra-aortic balloon support versus inotropes for decompensated heart failure and low output: a randomised trial. EuroIntervention 2019;15:586–593. [DOI] [PubMed] [Google Scholar]
- 47.Pilarczyk K, Boening A, Jakob H, Langebartels G, Markewitz A, Haake N, Heringlake M, Trummer G, et al. Preoperative intra-aortic counterpulsation in high-risk patients undergoing cardiac surgery: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2016;49:5–17. [DOI] [PubMed] [Google Scholar]
- 48.Gatti G, Morra L, Castaldi G, Maschietto L, Gripshi F, Fabris E, Perkan A, Benussi B, Sinagra G, Pappalardo A.. Preoperative intra-aortic counterpulsation in cardiac surgery: insights from a retrospective series of 588 consecutive highrisk patients. J Cardiothorac Vasc Anesth 2018;32:2077–2086. [DOI] [PubMed] [Google Scholar]
- 49.Goyal D, Nadar SK, Wrigley B, Koganti S, Banerjee P.. Successful use of intra-aortic counter pulsation therapy for intractable ventricular arrhythmia in patient with severe left ventricular dysfunction and normal coronary arteries. Cardiol J 2010;17:401–403. [PubMed] [Google Scholar]
- 50.Fotopoulos GD, Mason MJ, Walker S, Jepson NS, Patel DJ, Mitchell AG, Ilsley CD, Paul VE.. Stabilisation of medically refractory ventricular arrhythmia by intra-aortic balloon counterpulsation. Heart 1999;82:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meani P, Gelsomino S, Natour E, Johnson DM, Rocca H-PBL, Pappalardo F, Bidar E, Makhoul M, Raffa G, Heuts S, Lozekoot P, Kats S, Sluijpers N, Schreurs R, Delnoij T, Montalti A, Sels JW, van de Poll M, Roekaerts P, Poels T, Korver E, Babar Z, Maessen J, Lorusso R.. Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail 2017;19:84–91. [DOI] [PubMed] [Google Scholar]
- 52.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW; 2004 Writing Committee Members. Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing group to review new evidence and update the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction. Circulation 2008;117:296–329. [DOI] [PubMed] [Google Scholar]
- 53.Flaherty JT, Becker LC, Weiss JL, et al. Results of a randomized prospective trial of intraaortic balloon counterpulsation and intravenous nitroglycerin in patients with acute myocardial infarction. J Am Coll Cardiol 1985;6:434–46. [DOI] [PubMed] [Google Scholar]
- 54.O’Rourke MF, Norris RM, Campbell TJ, et al. Randomized controlled trial of in traaortic balloon counterpulsation in early myocardial infarction with acute heart fail ure. Am J Cardiol 1981;47:815–20. [DOI] [PubMed] [Google Scholar]
- 55.Ohman EM, George BS, White CJ, et al. Use of aortic counterpulsation to improve sustained coronary artery pa tency during acute myocardial infarction. Results of a randomized trial. The Randomized IABP Study Group. Circulation 1994;90:792–9 [DOI] [PubMed] [Google Scholar]
- 56.Prondzinsky R, Lemn H, Swyter M.. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med 2010;38:152–160. [DOI] [PubMed] [Google Scholar]
- 57.Sjauw KD, Engstrom AE, Vis MM, van der Schaaf RJ, Baan J, Koch KT, de Winter RJ, Piek JJ, Tijssen JGP, Henriques JPS.. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J 2008;30:459–468. [DOI] [PubMed] [Google Scholar]
- 58.Keeley EC, Boura JA, Grines CL.. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 59.Thiele H, Zeymer U, Thelemann N, Neumann F-J, Hausleiter J, Abdel-Wahab M, Meyer-Saraei R, Fuernau G, Eitel I, Hambrecht R, Böhm M, Werdan K, Felix SB, Hennersdorf M, Schneider S, Ouarrak T, Desch S, de Waha-Thiele S, Alkisoglu Z, Follath F, Frey S, Haerting J, Huber K, Maisch B, Messemer B, Ourrak T, Schuler G, Vonderschmitt K, Werdan K; On behalf of the IABP-SHOCK II Trial (Intraaortic Balloon Pump in Cardiogenic Shock II) Investigators. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation 2019;139:395–403. [DOI] [PubMed] [Google Scholar]
- 60.Abdel-Wahab M, Saad M, Kynast J, Geist V, Sherif MA, Richardt G, Toelg R.. Comparison of hospital mortality with intra-aortic balloon counterpulsation insertion before versus after primary percutaneous coronary intervention for cardiogenic shock complicating acute myocardial infarction. Am J Cardiol 2010;105:967–971. [DOI] [PubMed] [Google Scholar]
- 61.Gul B, Bellumkonda L.. Usefulness of intra-aortic balloon pump in patients with cardiogenic shock. Am J Cardiol 2019;123:750–756. [DOI] [PubMed] [Google Scholar]
- 62.van Nunen LX, van ’t Veer M, Schampaert S, Rutten MCM, van de Vosse FN, Patel MR, Pijls NHJ.. Intra-aortic balloon counterpulsation reduces mortality in large anterior myocardial infarction complicated by persistent ischaemia: a CRISP-AMI substudy. EuroIntervention 2015;11:286–292. [DOI] [PubMed] [Google Scholar]
- 63.Romeo F, Acconcia MC, Sergi D, Romeo A, Muscoli S, Valente S, Gensini GF, Chiarotti F, Caretta Q.. The outcome of intra-aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock according to the type of revascularization: a comprehensive meta-analysis. Am Heart J 2013;165:679–692. [DOI] [PubMed] [Google Scholar]
- 64.Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A.. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584–1588. [DOI] [PubMed] [Google Scholar]
- 65.Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJS, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BAJM, Tijssen JGP, Henriques JPS.. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017;69:278–287. [DOI] [PubMed] [Google Scholar]
- 66.Manzo-Silberman S, Fichet J, Mathonnet A, Varenne O, Ricome S, Chaib A, Zuber B, Spaulding C, Cariou A.. Percutaneous left ventricular assistance in post cardiac arrest shock: comparison of intra aortic blood pump and IMPELLA Recover LP2.5. Resuscitation 2013;84:609–615. [DOI] [PubMed] [Google Scholar]
- 67.Thiele H, Jobs A, Ouweneel DM, Henriques JPS, Seyfarth M, Desch S, Eitel I, Pöss J, Fuernau G, de Waha S.. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 2017;38:3523–3531. [DOI] [PubMed] [Google Scholar]
- 68.Schrage B, Schneider S, Zeymer U, Thiele H, Westermann D.. Response by Scharge et al to letter regarding article, “Impella support for acute myocardial infarction complicated by cardiogenic shock: a matched-pair IABP-SHOCK II trial 30-day mortality analysis”. Circulation 2019;140:e559–60. [DOI] [PubMed] [Google Scholar]
- 69.Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JP, Berkowitz A, Masoudi FA, Messenger JC, Parzynski CS, Ngufor C, Girotra S, Amin AP, Shah ND, Desai NR.. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarctioncomplicated by cardiogenic shock. JAMA 2020;323:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amin AP, Spertus JA, Curtis JP, Desai N, Masoudi FA, Bach RG, McNeely C, Al-Badarin F, House JA, Kulkarni H, Rao SV.. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation 2020;141:273–284. [DOI] [PubMed] [Google Scholar]
- 71.Bochaton T, Huot L, Elbaz M, Delmas C, Aissaoui N, Farhat F, Mewton N, Bonnefoy E, IMPELLA-STIC Investigators. Mechanical circulatory support with the ImpellaR LP 5.0 pump and an intra-aortic balloon pump for cardiogenic shock in acute myocardial infarction: the IMPELLA-STIC randomized study. Arch Cardiovasc Dis 2020;113:237–243. [DOI] [PubMed] [Google Scholar]
- 72.Udesen NJ, Møller JE, Lindholm MG, Eiskjær H, Schäfer A, Werner N, Holmvang L, Terkelsen CJ, Jensen LO, Junker A, Schmidt H, Wachtell K, Thiele H, Engstrøm T, Hassager C, DanGer Shock Investigators. Rationale and design of DanGer Shock: Danish- German cardiogenic shock trial. Am Heart J 2019;214:60–68. [DOI] [PubMed] [Google Scholar]
- 73.Sheu JJ, Tsai TH, Lee FY, et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med 2010;38:1810–1817. [DOI] [PubMed] [Google Scholar]
- 74.Baek MS, Lee S-M, Chung CR, Cho WH, Cho Y-J, Park S, Koo S-M, Jung J-S, Park SY, Chang Y, Kang BJ, Kim J-H, Oh JY, Park SH, Yoo J-W, Sim YS, Hong S-B.. Improvement in the survival rates of extracorporeal membrane oxygenation-supported respiratory failure patients: a multicenter retrospective study in Korean patients. Crit Care 2019;23:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 2016;42:370–8. [DOI] [PubMed] [Google Scholar]