Abstract
Post-transplant lymphoproliferative disorder (PTLD) is an often fatal complication of cardiac transplantation that occurs in 2% to 6% of transplant recipients. We report a case in which PTLD led to pulmonary artery external compression and multimodality imaging showed key features in the diagnosis, management, and follow-up. (Level of Difficulty: Intermediate.)
Key Words: biopsy, computed tomography, echocardiography, external compression, post-transplant lymphoproliferative disease, pulmonary artery
Abbreviations and Acronyms: CT, computed tomography; EBV, Epstein-Barr virus; Ig, immunoglobulin; PTLD, post-transplant lymphoproliferative disorder; TAPSE, tricuspid annular plane systolic excursion; TTE, 2-dimensional transthoracic echocardiogram
Central Illustration
Case Description
A 22-year-old woman, with previous postpartum cardiomyopathy followed by successful heart transplantation 1 year earlier, presented to the hospital with a 2-month history of a painful, expanding right submandibular mass. She had enlarged cervical nodes and a new loud systolic murmur with gallop rhythm.
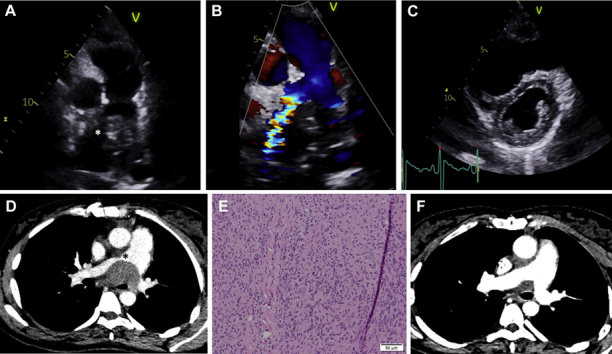
A 2-dimensional transthoracic echocardiogram (TTE) parasternal short-axis modified view showed an echogenic structure causing obstruction (asterisk) at the level of pulmonary bifurcation (Figure 1A, Video 1). Color Doppler imaging displayed flow acceleration (Figure 1B), with a continuous wave Doppler peak velocity of 4 m/s. A parasternal short-axis view (Figure 1C) presented a dilated right ventricle with a flattened D-shaped interventricular septum during end-systole suggestive of right ventricular pressure overload. The right ventricle was mildly dilated, measuring 45 mm at the base. Global right ventricular systolic function appeared impaired, and the tricuspid annular plane systolic excursion (TAPSE) was reduced, measuring 6 mm.
Figure 1.
Multimodality Imaging of PLTD Leading to Pulmonary Artery External Compression
(A) A 2-dimensional transthoracic echocardiogram, parasternal short-axis modified view, showing an echogenic structure causing obstruction (asterisk) at the level of the pulmonary bifurcation. (B) Color Doppler imaging displaying flow acceleration. (C) A 2-dimensional transthoracic echocardiogram, parasternal short-axis view, showing a dilated right ventricle with a flattened interventricular septum. (D) Computed tomography revealing multiple large, necrotic masses, the largest measuring 6.2 cm and causing partial effacement and narrowing (asterisk) of the right and left pulmonary arteries. (E) Histologic features. (F) Post-treatment computed tomography. PLTD = post-transplant lymphoproliferative disorder.
Computed tomography (CT) scan revealed multiple large, necrotic masses in the mediastinum and right side of the neck, the largest measuring 6.2 cm and causing partial effacement and narrowing (asterisk) of the right and left pulmonary arteries and narrowing of the distal trachea and left main bronchus (Figure 1D, Video 2).
Biopsy yielded Epstein-Barr virus (EBV)–positive monomorphic B-cell post-transplant lymphoproliferative disorder (PTLD) (1) (with features of diffuse large B-cell lymphoma, activated B-cell subtype). The specimen was necrotic and partly crushed, and it comprised large cells with irregular nuclei and nucleoli. The malignant cells expressed CD20, CD79a, PAX5, BCL-6 (weak subset), MUM-1, CD30, BCL-2, EBER, immunoglobulin (Ig) M and possibly IgG with a MIB-1 proliferation index of 60% (Figure 1E). Her presentation was rapid and clinically aggressive, consistent with the high-grade histologic features. Serologic evidence of previous EBV infection demonstrated before heart transplantation (EBV IgM antibodies not detected, viral capsid antigen [VCA] IgG antibodies detected, and EBV nuclear antigen [EBNA] IgG antibodies not detected) was consistent with the subsequent EBV-positive monomorphic PTLD manifesting 1 year following heart transplantation.
The PTLD was treated with immunochemotherapy. No structural interventions were performed because the patient was hemodynamically stable, with no clinical suggestion of right-sided heart failure, despite the imaging features. After 2 months of treatment, multimodality imaging showed an excellent response, with resolution of the right ventricular outflow obstruction. Further CT imaging demonstrated significant reduction in the mediastinal disease (Figure 1F), whereas TTE highlighted normal laminar flow across the pulmonary outflow tract (Video 3), a right ventricle of normal size (35 mm at base), and improvement in TAPSE from 6 to 10 mm.
Management of compression of cardiovascular structures caused by PTLD masses requires multidisciplinary management, and this case illustrates that structural intervention may not be required.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Dimosthenis Pandis, MD, MS, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
2D Transthoracic Echocardiography at Initial Diagnosis. Modified parasternal short-axis view, focused on the valve and pulmonary branches, showed an echogenic structure causing obstruction at the level of the pulmonary bifurcation
Computed Tomography Scan at Initial Diagnosis. Chest scan with contrast showed a 6.2-cm necrotic mediastinal mass causing compression of the pulmonary arteries and left main bronchus, with a further nodal mass on the right side of the neck causing jugular vein effacement, right paratracheal and supraclavicular lymphadenopathy, and multiple liver lesions
2D Transthoracic Echocardiography After 2 Months of Treatment. Modified parasternal short-axis view, focused on the valve and pulmonary branches, demonstrated normal laminar flow across the pulmonary outflow tract
Reference
- 1.Swerdlow S.H., Campo E., Harris N.L. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2D Transthoracic Echocardiography at Initial Diagnosis. Modified parasternal short-axis view, focused on the valve and pulmonary branches, showed an echogenic structure causing obstruction at the level of the pulmonary bifurcation
Computed Tomography Scan at Initial Diagnosis. Chest scan with contrast showed a 6.2-cm necrotic mediastinal mass causing compression of the pulmonary arteries and left main bronchus, with a further nodal mass on the right side of the neck causing jugular vein effacement, right paratracheal and supraclavicular lymphadenopathy, and multiple liver lesions
2D Transthoracic Echocardiography After 2 Months of Treatment. Modified parasternal short-axis view, focused on the valve and pulmonary branches, demonstrated normal laminar flow across the pulmonary outflow tract



