Abstract
The surgical intervention to treat isolated severe tricuspid regurgitation (TR) is challenging due to the severe TR patients’ high-risk profile, hence associated with a high complication rate. Herein, we describe a first-in-human percutaneous deployment of a novel transcatheter prosthetic xenograft valve to treat severe TR. (Level of Difficulty: Advanced.)
Key Words: right coronary artery, right ventricular, transesophageal echocardiography, tricuspid regurgitation
Abbreviations and Acronyms: RCA, right coronary artery; RV, right ventricular; TEE, transesophageal echocardiography; TR, tricuspid regurgitation
Central Illustration
A 71-year-old female was referred from primary care to a tertiary medical center for tricuspid valve replacement surgery due to massive symptomatic tricuspid regurgitation (TR) (Video 1).
Learning Objectives
-
•
To describe the first-in-human experience with the percutaneous, transcatheter tricuspid valve replacement using the novel valve.
-
•
To show the feasibility and safety of the novel valve implantation through the internal jugular vein.
-
•
To show the immediate effect of the implanted novel valve on the right ventricular contraction following abrupt elimination of the severe regurgitation.
Medical History
The patient underwent surgical aortic valve replacement followed 4 years later by a transcatheter aortic “valve-in-valve” procedure. Recently she developed overt right heart failure despite attempted medical therapy, high-dose diuretics, low-sodium diet, and amiodarone therapy to maintain sinus rhythm. The physical examination on admission revealed the following findings: blood pressure: 110/70 mm Hg; irregular heart rate: 80 beats/min; typical signs of right ventricular (RV) failure; extensive jugular vein congestion; and severe lower limb pitting edema, however, without signs of ascites.
Investigation
Echocardiography revealed massive TR, RV dilation with borderline systolic dysfunction, moderate degenerative mitral valve stenosis, and normal function of the aortic prosthesis. The tricuspid valve’s diameters were 40 mm at the septum-lateral and 37 mm at the anterior-posterior dimensions. Invasive cardiac hemodynamic data are shown in Table 1.
Table 1.
Cardiac Hemodynamic Study Before Intervention
| Systemic blood pressure, mm Hg | 160/84 |
| Pulmonary artery pressure, mm Hg | 74/31 |
| Right ventricular pressure, mm Hg | 55/29 |
| Right atrial v-wave pressure, mm Hg | 40 |
| Right atrial mean pressure, mm Hg | 29 |
| Cardiac index, l/min/m2 | 2 |
| Stroke volume index, ml/m2 | 32 |
| Pulmonary artery pulsatility index | 1.48 |
| Hemoglobin at time of study, g/dL | 9.3 |
The local heart team estimated the patient’s surgical risk to be significantly high, mainly due to frailty, previous cardiac surgery, and the compromised RV contraction. Hence, tricuspid valve replacement using the novel valve (TriSol Medical, Yokneam, Israel) was selected.
Pre-procedural imaging included a transesophageal echocardiography (TEE) study explicitly focused on the right heart. The tricuspid valve was identified at the mid-esophageal level at 30° and the x-plane orthogonal view to allow simultaneous imaging of all 3 leaflets and obtain a 3-dimensional (3D) image of the valve through the 3D-zoom mode. The valve was also scanned on the 0° (short-axis) gastric view and 90° (long-axis) view. Acceptable TEE image quality was required with the patient in the supine position as during the procedure. Data for the right heart morphology were also obtained using cardiac computed tomography (CT) and further processed by using the 3mensio CT software (Esaote, Italy) to enable the novel valve’s projection onto the tricuspid valve’s annulus and ensure optimal adjustment between the 2. Finally, the operational staff trained on an in vivo porcine model and an in vitro simulator based on a 3D printed silicone heart model identical to the patient’s cardiac CT data. The local ethical committee and the Ministry of Health’s Ethical Committees approved the procedure.
Description of the Novel Valve
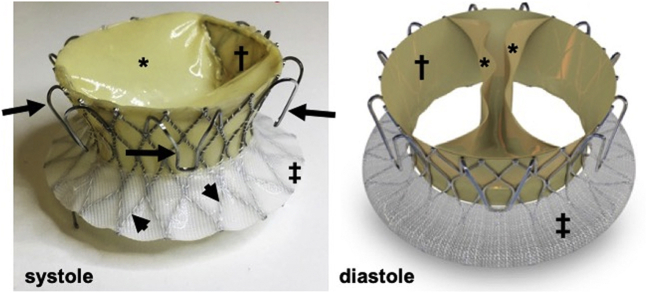
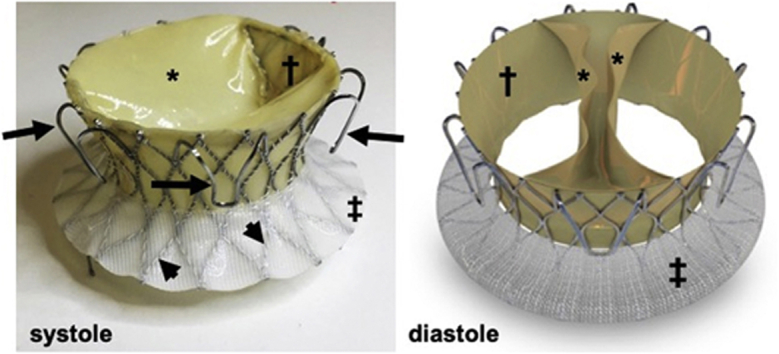
The valve apparatus (Figure 1) is a bioprosthesis consisting of a thin self-expanding nitinol alloy frame (height: 21 mm; inflow and outflow diameters: 62.5 and 50.3 mm, respectively; and inner waist diameter: 34.8 mm). A bovine pericardial monoleaflet valve is attached to the frame by 2 commissures. During diastole, the valve opens, with its 2 symmetrical segments coming into apposition on the ventricular aspect of the annular plane, forming 2 large orifices for the antegrade blood flow.
Figure 1.
The Novel Valve
Nitinol frame (arrowheads), fixation arms (arrows), skirts on the ventricular side (†) (pericardial) and atrial side (‡) (polyester), and the pericardial monoleaflet in systole and diastole (∗).
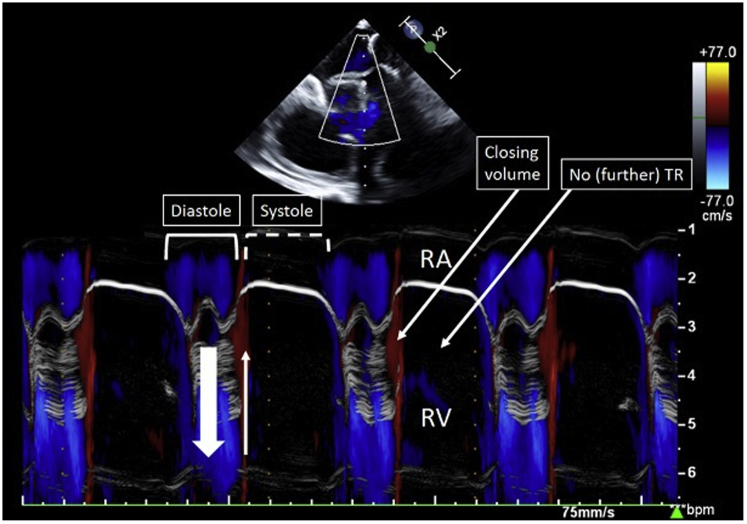
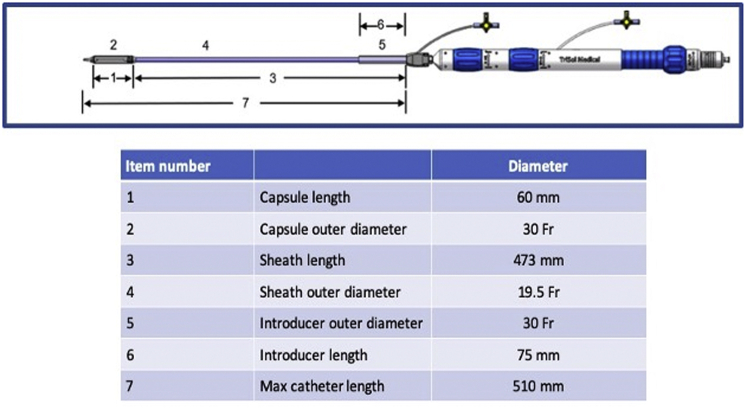
In systole, the ventricular contraction force pushes the leaflets back to form a circular line of coaptation, with the entire perimeter of the porcine pericardium-covered nitinol frame. The valve’s closing motion forms a closing volume approximating 5 ml (as measured in vitro), like a mechanical double-disk valve (Figure 2). Six arc-shaped fixation arms welded to the frame anchor the valve to the native annulus through engagement between the native leaflets and the adjacent walls, thus reducing the risk of compromising the right coronary artery (RCA) flow. The axial forces generated by blood flow through the valve and the frame’s conical shape aim to avoid impingement of the frame on the adjacent conduction system. The bioprosthesis is crimped and loaded into the distal capsule of the delivery system (Figure 3). The crimped valve is introduced from the internal jugular vein downstream into the RV cavity. The deployment begins with the exposure of the frame's ventricular edge. Once the 6 fixation arms capture the native leaflets, the frame's atrial edge is released. The device is collapsible and repositionable until the fixation arms are fully expanded. Currently, the prototype fits an annulus septum-lateral dimension in the range of 40 to 53 mm. An endovascular pacemaker does not prohibit the procedure but may add to its complexity.
Figure 2.
Color M-Mode Echocardiography of the Novel Valve
The blue jet represents the diastolic laminar inflow. The very-early systolic red jet represents the closing volume.
Figure 3.
The Delivery System
The Procedure
A multidisciplinary team composed of interventional and noninvasive cardiologists, a vascular surgeon, an anesthesiologist, and auxiliary staff performed the procedure.
The patient was anesthetized and intubated. External pacemaker patches were applied to the patient's chest. TEE and fluoroscopy monitored the procedure. An activated clotting time of 300 seconds was maintained during the procedure.
The Procedure Included 4 Main Stages
-
1.
Wiring of the RCA served to mark the tricuspid annulus during fluoroscopy.
-
2.
The device was inserted into the right internal jugular vein after surgical exposure and cut down in the vein.
-
3.
The roadmap obtained by the valve’s x-plane and 3D-zoom TEE images enabled precise steering of the device's fixation arms to achieve complete motion restriction of the native leaflets. Then, the deployment was completed by exposing the frame’s atrial side and detachment of the valve’s delivery system (Video 2).
-
4.
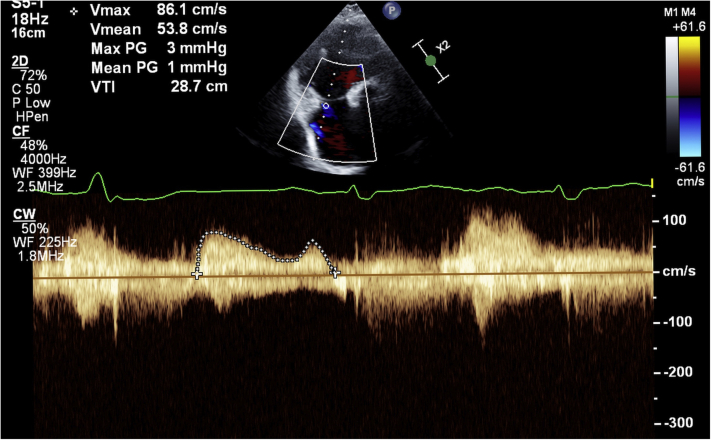
Upon completing deployment, the TEE confirmed the appropriate valve’s position, stability, and function. Low transvalvular pressure gradients (peak: 2 mm Hg; and mean: 1 mm Hg) and a trace residual perivalvular regurgitation were noted under stable hemodynamic conditions (Videos 3 and 4, Figure 4). The RV contraction did not deteriorate immediately after the abrupt elimination of the TR. The jugular vein was managed surgically following retrieval of the valve delivery system. The procedure lasted 210 minutes, requiring 41:29 minutes of fluoroscopy and 98 ml of contrast medium.
Figure 4.
Low Transvalvular Pressure Gradients 14 Days After the Implantation
Discussion
Patients with severe TR are at high risk for heart failure and death (1, 2, 3). However, their high-risk profile (4) attenuates the survival benefit of surgical intervention for TR (5). Hence, transcatheter alternatives represent an unmet need (6,7).
The abrupt elimination of severe TR may often unmask RV decompensation due to an acute rise in afterload. This complication significantly clouds immediate and long-term procedural success (8). In the present case, the RV did not severely decompensate despite altered baseline function. We assumed that the valve's unique closing volume might have moderated the impact of RV’s increased afterload. This hypothesis merits further study.
Follow-Up
At the end of the procedure, the patient was monitored in the cardiac intensive care unit. A constant course of clinical improvement and absence of new-onset conductance abnormality characterized the following days. On the sixth day, the patient was discharged from the hospital. The patient was instructed to continue with the pre-procedure diuretic regime and coumadin (target international normalized ratio of 2 to 3).
Serial echocardiograms starting from the procedure and up to 2 weeks post-procedurally revealed the following 3 insights (Table 2):
-
1.
The novel valve remained properly positioned, maintaining low-pressure transvalve gradients (peak and mean: 3 and 1 mm Hg, respectively) and a very mild residual perivalvular leak.
-
2.
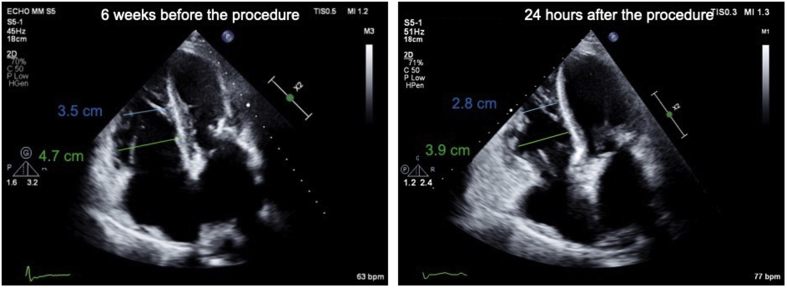
Stable RV systolic function was noticed despite the profound reduction in TR. Furthermore, a reduction in the RV’s size was already noticed after 24 hours (Figure 5), reflecting acute reverse remodeling.
-
3.
The procedure was associated with a 9% rise in the forward RV stroke volume after 14 days.
Table 2.
Echocardiographic Characteristics Before and 2 Weeks After the Valve Was Implanted
| Pre-Implantation | Post-Implantation | |
|---|---|---|
| Left ventricular ejection fraction, % | 65 | 70 |
| Forward RV stroke volume, ml | 64 | 70 |
| Mean transmitral pressure gradient, mm Hg | 3 | 7 |
| TAPSE, mm | 15 | – |
| Right ventricular fractional area change, % | 43 | 36 |
| Mean transtricuspid pressure gradient, mm Hg | – | 1 |
| Inferior vena cava width, cm | 2.6 | 2.6 |
RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion
Figure 5.
Reduction in the Right Ventricle’s Size 24 Hours After the Procedure
Conclusions
The successful first-in-human novel valve implantation constitutes an important milestone toward a shifting paradigm, enabling a worthy alternative to surgery for high-risk patients. The unique features of this valve may potentially better adapt to the challenges of long-standing severe TR.
Funding Support and Author Disclosures
Dr. Vaturi is a founder and medical director of TriSol Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Massive tricuspid regurgitation documented before the novel valve implantation
Fluoroscopy of the novel valve’s deployment
The right heart chambers 14 days after the implantation.
Very mild residual perivalvular regurgitation. The closing volume is present in laminar blue jets.
References
- 1.Wang N., Fulcher J., Abeysuriya N. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J. 2019;40:476–484. doi: 10.1093/eurheartj/ehy641. [DOI] [PubMed] [Google Scholar]
- 2.Topilsky Y., Inojosa J.M., Benfari G. Clinical presentation and outcome of tricuspid regurgitation in patients with systolic dysfunction. Eur Heart J. 2018;39:3584–3592. doi: 10.1093/eurheartj/ehy434. [DOI] [PubMed] [Google Scholar]
- 3.Topilsky Y., Nkomo V.T., Vatury O. Clinical outcome of isolated tricuspid regurgitation. J Am Coll Cardiol Img. 2014;7:1185–1194. [Google Scholar]
- 4.Zack C.J., Fender E.A., Chandrashekar P. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol. 2017;70:2953–2960. doi: 10.1016/j.jacc.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Axtell A.L., Bhambhani V., Moonsamy P. Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J Am Coll Cardiol. 2019;74:715–725. doi: 10.1016/j.jacc.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mylotte D. The forgotten valve no more. EuroIntervention. 2017;12:e1799–e1801. doi: 10.4244/EIJV12I15A292. [DOI] [PubMed] [Google Scholar]
- 7.Rodes-Cabau J., Hahn R.T., Latib A. Transcatheter therapies for treating tricuspid regurgitation. J Am Coll Cardiol. 2016;67:1829–1845. doi: 10.1016/j.jacc.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg Y.H., Ho E., Chau M., Latib A. Update on transcatheter tricuspid valve replacement therapies. Front Cardiovasc Med. 2021;8:619558. doi: 10.3389/fcvm.2021.619558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Massive tricuspid regurgitation documented before the novel valve implantation
Fluoroscopy of the novel valve’s deployment
The right heart chambers 14 days after the implantation.
Very mild residual perivalvular regurgitation. The closing volume is present in laminar blue jets.