Abstract
We present the case of a symptomatic young woman with mitral stenosis and regurgitation due to a congenital mitral arcade. Multimodality imaging with echocardiography and computed tomography were used for diagnosis and surgical planning. The patient underwent successful bioprosthetic valve replacement. (Level of Difficulty: Intermediate.)
Key Words: congenital heart defect, imaging, echocardiography, computed tomography, mitral valve
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; CT, computed tomography; ECG, electrocardiogram; MVA, mitral valve area; PASP, pulmonary artery systolic pressure; PHT, pressure half-time; RBBB, right bundle branch block; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram
Central Illustration
A 34-year-old woman presented with 2 years of progressive dyspnea on exertion and decreasing exercise capacity, limiting her walking to 1 block. Six months before presentation, she experienced a productive cough with foamy, blood-speckled sputum as well as palpitations, orthopnea, and paroxysmal nocturnal dyspnea.
Learning Objectives
-
•
To recognize the clinical presentation and differential diagnosis of mitral stenosis to diagnose congenital mitral arcade.
-
•
To use multimodality imaging in characterizing the mitral valve and submitral apparatus to guide treatment of mitral valve disease.
Physical examination revealed sinus tachycardia, 2/6 apical systolic murmur, and bilateral wheezes, with no jugular venous distention or edema. Her blood pressure was normal, with a respiratory rate of 22 breaths/min and oxygen saturation 95% on room air. Her admission electrocardiogram (ECG) showed sinus tachycardia, left atrial enlargement, right axis deviation, and incomplete right bundle branch block (RBBB) (Figure 1).
Figure 1.
Admission Electrocardiogram
A point-of-care transthoracic echocardiogram (TTE) performed in the emergency room was concerning for mitral valve restriction with mitral stenosis, and she was referred for a complete TTE and transesophageal echocardiogram (TEE). A chest radiograph demonstrated cardiomegaly, bilateral interstitial opacities, and a right upper lobe calcified granuloma (Figure 2).
Figure 2.
Chest Radiograph and Computed Tomographic View
Medical History
The patient’s medical history was significant for childhood asthma. There was no history of tonsilitis. She had antibodies consistent with a recent infection of coronavirus disease-2019 (COVID-19) and recalled anosmia as her only symptom, which had since resolved. She had been born and brought up in New York City. She had a 15 pack-year history of smoking regular and electronic cigarettes.
Differential Diagnosis
Differential diagnosis included rheumatic mitral valve, anomalous mitral arcade, or other congenital mitral disease such as parachute mitral valve.
Investigations
The TTE showed severe mitral stenosis with bowing and restriction of the mitral leaflet in parasternal long views (Figure 3, Video 1) and a “fish-mouth” appearance of the mitral orifice, which can be seen in rheumatic valve disease. However, sequential interrogation of the left ventricle in the parasternal short axis revealed hypertrophied papillary muscles with diminutive chordal insertion into leaflet tips and a superiorly displaced anterolateral papillary muscle (Figure 4). There was no evidence of commissural fusion or calcification, which would be more commonly associated with rheumatic valve disease. Left ventricular function was preserved, and right ventricular function was mildly reduced. There was moderate pulmonary hypertension; pulmonary artery systolic pressure (PASP) was 49 mm Hg.
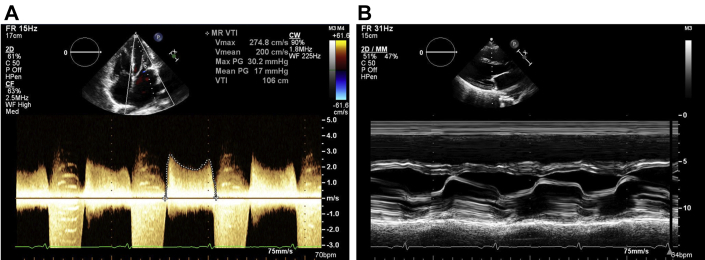
Figure 3.
Transmitral Gradient and M-Mode of Mitral Valve
(A) Mean transmitral gradient 17 mm Hg at a heart rate of 86 beats/min. (B) M-mode of leaflet tips.
Figure 4.
Sequential Transthoracic Echocardiogram Parasternal Short Axis and 3-Dimensionial Transesophageal Echocardiogram Reconstruction of Mitral Valve
“Fish-mouth” appearance of the mitral orifice leading to short thickened chordae tendinae and superiorly displaced anterolateral papillary muscle. Mitral valve area estimated by pressure half-time 1.1 cm2 and measured 1.0 cm2 by 3-dimensional multiplanar reconstruction. Estimated regurgitant orifice area 20 cm by proximal isovelocity surface area method, regurgitant volume 39 mL, regurgitant fraction 43%.
The TEE revealed thickened mitral leaflets with short chordal attachments to the papillary muscles, which appeared hypertrophied and abnormally positioned (Video 2). Color flow Doppler demonstrated moderate mitral regurgitation and multiple jets of mitral inflow fractionated through reduced interchordal spaces (Video 3).
Electrocardiogram-gated computed tomography (CT) demonstrated normal coronary arteries. The mitral leaflets were mildly thickened, with diminutive chordae, and there was a fibrous band between the papillary muscles, creating an “arc-like” configuration (Video 4, Figure 5).
Figure 5.
Cardiac Computed Tomographic Short Axis
Cardiac computed tomographic view short axis showing fibrous band of tissue (arrow) between the papillary muscles.
Given the patient’s chronic cough, hemoptysis, and smoking history, chest CT angiography was performed, which showed extensive bilateral ground-glass opacities, smooth interlobular septal thickening, and lobular areas of consolidations. There was no evidence of pulmonary embolism (Figure 2).
Management
The patient was referred to cardiothoracic surgery and underwent mitral valve replacement. After administration of systemic heparinization and antegrade cardioplegia, the left atrium was opened through a longitudinal incision in the Sondergaard groove. The mitral valve was assessed. Both the anterior and the posterior leaflets appeared thickened and retracted. Scant calcifications were noted at the annular level, more pronounced on the posterior annulus. Two hypertrophied and fibrotic papillary muscles were noted to be displaced cranially nearly at the annular level. The papillary muscles merged into a common trunk (arch) that gave origin to short chordae tendinae anchored to both leaflets. These short chords tethered the leaflets down into the ventricle and were responsible for the relative immobility of the mitral apparatus, causing both stenosis and insufficiency. The gross appearance closely correlated with the echocardiographic and CT images (Figure 6). The anterior leaflet was completely removed (Figure 7), and the chords were resected, whereas the subvalvular apparatus of the posterior leaflet was preserved.
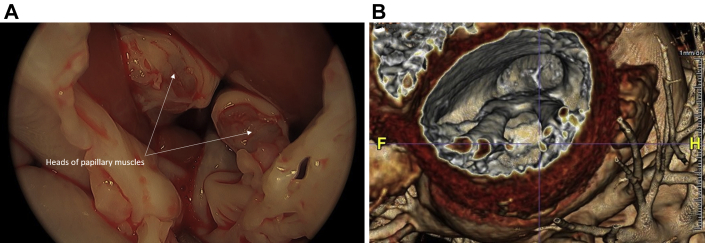
Figure 6.
Correlation of Surgical and Computed Tomographic Reconstruction
(A) Surgical view of the heads of the hypertrophied papillary muscles after resection of chords to the anterior leaflet, (B) 3-dimensional cardiac computed tomographic reconstruction.
Figure 7.
Gross Appearance of Mitral Arcade
Mitral valve after partial resection of the anterior leaflet showing thick, fibrotic, and cranially displaced papillary muscles with diminutive, thickened chordae.
A 27-mm bioprosthetic valve was selected and sutured in place in the usual fashion. Three lung wedges were obtained for biopsy. Intraoperative TEE showed a well-functioning valve with no residual gradient or regurgitation. The patient underwent extubation on postoperative day 1, and her postoperative course was uncomplicated.
Pathologic Findings
Histologic examination of the anterior mitral valve leaflet demonstrated fibrous thickening with focal myxoid changes (Figure 8). Microscopic examination results of the lung were notable for diffuse panlobular interstitial fibrosis with intra-alveolar hemosiderin-laden macrophages (Figure 9). There was no evidence of vaping-induced lung injury, organizing pneumonia, or bronchiolitis.
Figure 8.
Histology of Resected Mitral Valve
Mitral valve with focal myxoid change and fibrosis (hematoxylin and eosin, A: magnification ×100, B: magnification ×200).
Figure 9.
Histology of Biopsied Lung
Lung with intra-alveolar hemosiderin-laden macrophages (hematoxylin and eosin, magnification ×200).
Follow-up
The patient returned for outpatient follow-up 2 weeks postoperatively and reported improving symptoms.
Discussion
We have described a rare case of mitral arcade with anomalously displaced papillary muscles in an adult woman with accelerating symptoms of severe mitral stenosis.
Congenital mitral arcade is thought to result from the arrested embryonic development of the mitral valve before the lengthening and attenuation of the chordae tendinae (1). The shortened chords decrease the interchordal spaces, causing abnormal leaflet excursion and coaptation that can result in both valvular stenosis and insufficiency. A fibrous bridge may connect the 2 papillary muscles, creating an “arcade” when viewed from the left ventricle. In the case presented here, the cranially displaced papillary muscles were caused by improper delamination of the papillary muscle from the left ventricular wall.
Mitral arcade was first described in 3 infants by Layman and Edwards (2) in 1967 as a rare cause of congenital mitral insufficiency. Since then, this entity has been infrequently reported in adults. The uncommonness of congenital mitral arcade can lead to an under-recognition of this defect as the cause of mitral valve disease. In recently published case series, mitral arcades in adults have been reported to be unexpectedly encountered during cardiac surgery, necessitating a change in operative approach (3).
A clinical history that is inconsistent with prior rheumatic fever and findings of abnormal subvalvular apparatus such as anomalous papillary muscles or chordae should raise suspicion for mitral valve arcade. Imaging with multiple modalities can be critical in making the diagnosis. Although TTE may be sufficient to differentiate the morphology and hemodynamic effects of the aberrant valve, multiplanar and 3-dimensional TEE along with cardiac CT can provide further spatial resolution and reconstruction of the arcade to confirm the diagnosis and guide surgical management. Echocardiography and cardiac CT can also be useful in identifying associated abnormalities such as septal defects or supravalvular mitral rings, which have been described in up to two thirds of patients with congenital mitral stenosis (4).
Our patient’s presentation was confounded by a recent COVID-19 infection and her history of vaping; however, the radiographic and pathologic findings were consistent with pulmonary changes associated with chronic mitral valve stenosis. Longstanding mitral stenosis resulting in pulmonary parenchymal manifestations of diffuse alveolar hemorrhage, hemosiderosis, and ossification have been described radiographically and should prompt a work-up for unrecognized mitral valve disease (5).
Conclusions
Mitral arcade should be considered in cases where the cause of mitral stenosis or insufficiency is unclear, especially in the context of abnormal mitral valve subvalvular apparatus. Multimodality imaging, along with a high index of suspicion, is crucial in recognizing and differentiating uncommon causes of mitral stenosis from more common ones such as rheumatic mitral stenosis.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
TTE parasternal long axis. Bowing and restriction of the mitral leaflets.
TEE midesophageal multiplanar and 3D TTE apical 2-chamber views. Mildly thickened mitral leaflets with short chordal attachments to hypertrophied and anomalously positioned papillary muscles (arrows).
TEE midesophageal multiplanar and 3D TTE apical 2-chamber views. Mildly thickened mitral leaflets with short chordal attachments to hypertrophied and anomalously positioned papillary muscles (arrows).
TTE and TEE color Doppler images. Mitral inflow and regurgitation fractionated into multiple jets.
TTE and TEE color Doppler images. Mitral inflow and regurgitation fractionated into multiple jets.
Cardiac CT 2-chamber. Short thickened chordae and a fibrous band connecting the 2 papillary muscles, creating an “arcade” configuration.
References
- 1.Hakim F.A., Krishnaswamy C., Mookadam F. Mitral arcade in adults: a systematic overview. Echocardiography. 2013;30:354–359. doi: 10.1111/echo.12126. [DOI] [PubMed] [Google Scholar]
- 2.Layman T.E., Edwards J.E. Anomalous mitral arcade: a type of congenital mitral insufficiency. Circulation. 1967;35:389–395. doi: 10.1161/01.cir.35.2.389. [DOI] [PubMed] [Google Scholar]
- 3.Dokollari A., Cameli M., Bisleri G. Mitral arcades unexpectedly encountered during cardiac surgery. J Cardiothorac Vasc Anesth. 2021;35:914–916. doi: 10.1053/j.jvca.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Collins-Nakai R.L., Rosenthal A., Castaneda A.R., Bernhard W.F., Nadas A.S. Congenital mitral stenosis: a review of 20 years' experience. Circulation. 1977;56:1039–1047. doi: 10.1161/01.cir.56.6.1039. [DOI] [PubMed] [Google Scholar]
- 5.Woolley K., Stark P. Pulmonary parenchymal manifestations of mitral valve disease. Radiographics. 1999;19:965–972. doi: 10.1148/radiographics.19.4.g99jl10965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TTE parasternal long axis. Bowing and restriction of the mitral leaflets.
TEE midesophageal multiplanar and 3D TTE apical 2-chamber views. Mildly thickened mitral leaflets with short chordal attachments to hypertrophied and anomalously positioned papillary muscles (arrows).
TEE midesophageal multiplanar and 3D TTE apical 2-chamber views. Mildly thickened mitral leaflets with short chordal attachments to hypertrophied and anomalously positioned papillary muscles (arrows).
TTE and TEE color Doppler images. Mitral inflow and regurgitation fractionated into multiple jets.
TTE and TEE color Doppler images. Mitral inflow and regurgitation fractionated into multiple jets.
Cardiac CT 2-chamber. Short thickened chordae and a fibrous band connecting the 2 papillary muscles, creating an “arcade” configuration.