Key Points
Question
Is early cochlear implantation associated with language development in children with prelingual single-sided deafness (SSD)?
Findings
In this longitudinal cohort study of 61 children, children with SSD with a cochlear implant achieved significantly better scores for grammar than children with SSD without an implant (difference in score, 0.76), similar to scores for children with bilateral normal hearing. Vocabulary and receptive language scores did not differ for the 3 groups.
Meaning
These findings suggest that early treatment with a cochlear implant is associated with improvements in the development of grammar skills among children with SSD.
This cohort study investigates whether early cochlear implantation is associated with language outcomes for children with prelingual single-sided deafness.
Abstract
Importance
Pediatric single-sided deafness (SSD) can seriously affect development, causing impaired spatial hearing skills, speech-language delays, and academic underachievement. Early cochlear implantation likely improves hearing-related outcomes, but its association with language development remains unclear.
Objective
To investigate whether early cochlear implantation is associated with language outcomes for children with prelingual SSD.
Design, Setting, and Participants
The Cochlear Implant for Children and One Deaf Ear study was initiated in 2015 and recruited participants at 4 academic hospitals in Flanders, Belgium, through 2019. This cohort study included 3 groups of children aged 2 to 5 years: children with SSD and a cochlear implant, children with SSD without a cochlear implant, and a control group with normal hearing. Language and hearing skills were assessed 1 to 2 times per year until the age of 10 years. Study completion rates were high (82%). Data analysis was performed from October to December 2020.
Exposure
Unilateral cochlear implant.
Main Outcomes and Measures
Longitudinal vocabulary, grammar, and receptive language scores. The implanted group was hypothesized to outperform the nonimplanted group on all language tests.
Results
During the recruitment period, 47 children with prelingual SSD without additional disabilities were identified at the participating hospitals. Fifteen of the 34 children with an intact auditory nerve received a cochlear implant (44%, convenience sample). Sixteen of the remaining children were enrolled in the SSD control group (50%). Data from 61 children (mean [SD] age at the time of enrollment, 2.08 [1.34] years; 26 girls [42%]) were included in the analysis: 15 children with SSD and a cochlear implant, 16 children with SSD without a cochlear implant, and 30 children with normal hearing. Children with SSD and a cochlear implant performed in line with their peers with normal hearing with regard to grammar. In contrast, children with SSD without a cochlear implant had worse grammar scores than the group with implants (−0.76; 95% CI, −0.31 to −1.21; P = .004) and the group with normal hearing (−0.53; 95% CI, −0.91 to −0.15; P = .02). The 3 groups had similar vocabulary and receptive language abilities.
Conclusions and Relevance
These findings suggest that early cochlear implantation is associated with normal grammar development in young children with prelingual SSD. Although further follow-up will reveal the long-term outcomes of the cochlear implant for other skills, the current results will help clinicians and policy makers identify the best treatment option for these children.
Introduction
Sensorineural hearing loss (HL) at birth affects approximately 1.86 newborns per 1000 in high-income countries, and an estimated 30% to 40% of these cases are unilateral impairments.1 Within this group with unilateral HL, 30% to 40% of newborns have single-sided deafness (SSD), in which case the child has normal hearing (NH) in 1 ear and profound HL (>90 dB HL) in the other ear.2 As a result of the absence of binaural hearing, children with SSD have difficulties with impaired sound localization and speech perception in adverse conditions,3 reduced balance skills,4 and significantly poorer language development than children with NH.5,6,7,8 For an overview, see van Wieringen et al.9
Cochlear implantation is the only treatment option that can partly restore binaural hearing and thereby facilitate language development. A cochlear implant directly stimulates the auditory nerve with electrical pulses. Over the past decades, it has become the standard of care for individuals with bilateral deafness and has proven to be a life-changing opportunity in terms of using spoken language and participating in a predominantly oral society.10 Although a cochlear implant is not standard of care for persons with SSD, an increasing body of research reports better sound localization and speech perception in noise with a cochlear implant for adults with acquired SSD11,12,13,14,15 and for children with SSD.16,17,18,19 Little is known about the association of the cochlear implant with language development in children with prelingual SSD.
Without treatment, SSD leads to cortical reorganization in favor of the NH ear.20,21,22 On the basis of the maturation of the auditory pathway, the estimated critical time frame for treatment is ages 3 to 4 years.23 Providing a cochlear implant within this early critical period may impede further preference for the NH ear and, possibly, reverse or restore cortical reorganization.23,24 Indeed, the best outcomes in children with congenital SSD are obtained if intervention is provided before the age of 3 years.25
The poorer language outcomes associated with untreated SSD may be caused by degraded auditory perception. In particular, the development of morphosyntax—that is, the set of rules that govern linguistic units—depends on the detection of subtle differences in speech, such as the addition of a suffix to conjugate verbs (eg, “you talk,” “he talks”). The aim of this study is to report on the language skills of young children with prelingual SSD with and without a cochlear implant. It was hypothesized that the cochlear implant would provide access to the subtle cues that are important for language. In turn, this would enhance the children’s implicit language learning, resulting in stronger language skills. Our research question was whether children with prelingual SSD benefit from early cochlear implantation in terms of language development. We hypothesized that children with SSD and a cochlear implant would outperform their peers without implants and perform in line with their peers with NH.
Methods
This cohort study was designed and conducted according to the Declaration of Helsinki.26 The study protocol was reviewed and approved by the medical-ethical committee of the University Hospitals Leuven and at all participating centers. Written informed consent was obtained from the parents of all participants.
Participants and Power Analysis
In a multicenter collaboration with 4 academic hospitals in Belgium, 31 children with prelingual SSD were recruited for this study between 2015 and 2019. At the time of inclusion, all children had confirmed NH thresholds in 1 ear (click-evoked auditory brainstem responses ≤35 dB normalized hearing level) and severe-to-profound HL in the other ear (auditory brainstem responses ≥80 dB normalized hearing level). Thresholds were later confirmed behaviorally using age-appropriate audiometric tests. Children were considered for cochlear implantation if they were younger than 3 years and if a magnetic resonance imaging scan confirmed the presence of an auditory nerve. In total, 47 children with prelingual SSD were identified at the participating hospitals, of whom 34 had an intact auditory nerve.
Fifteen children received a cochlear implant manufactured by Cochlear, Ltd (SSD plus cochlear implant group; 44%, convenience sample), at a mean (SD) age of 13.5 (4.6) months. All but 1 of these children had congenital SSD; only 1 child had acquired SSD after head trauma with temporal bone fracture at age 10 months. Eleven of the 15 children had congenital cytomegalovirus (CMV) infection, 2 of whom had clinical symptoms at birth. One child experienced progressive HL in the better ear, and 2 others received diagnoses of cerebral palsy after implantation. Data logging revealed that these children used their devices approximately 8 hours per day (median [interquartile range], 8.0 [5.9-9.2] hours), with individual use ranging between 2.1 and 12.2 hours. Sixteen other children did not receive a cochlear implant (SSD group; 50% of remaining children) because they had cochlear nerve deficiency (CND), defined as cochlear nerve hypoplasia or aplasia (9 children), because they were too old (1 child), or because their parents declined cochlear implantation (6 children). All children in the SSD without cochlear implant group had congenital SSD.
The study also included a control group of 30 children with bilateral NH. A power analysis confirmed that 15 children per group would suffice given the longitudinal study design (GPower statistical software version 3.31 [Franz Faul, University of Kiel]; F test analysis of variance [ANOVA] repeated measures, within-between interaction; effect size, f = 0.25 [corresponding to Cohen d = 0.50], α = .05, power = 0.90, 3 groups, and 3 measures). Study completion rates were high (82%).
Relevant child and family characteristics are summarized in Table 1. All children spoke Dutch at home. Although no additional therapy was provided as part of the longitudinal study, some children received individual support on preventive or curative grounds. Although these interventions may have influenced the language results, it was beyond the scope of the current study to control for this.
Table 1. Demographic Information of the Participants.
| Characteristic | Participants, No. (%) | |||
|---|---|---|---|---|
| SSD with cochlear implant (n = 15) | SSD without cochlear implant (n = 16) | Normal hearing (n = 30) | Total (n = 61) | |
| Sex | ||||
| Female | 7 (47) | 7 (44) | 12 (40) | 26 (42) |
| Male | 8 (53) | 9 (56) | 18 (60) | 35 (58) |
| Side of deafness | ||||
| Left | 8 (53) | 9 (56) | NA | 17 (55) |
| Right | 7 (47) | 7 (44) | NA | 14 (45) |
| Cause of hearing loss | ||||
| Cochlear nerve deficiency | 0 | 9 (56) | NA | 9 (29) |
| Cytomegalovirus infection | 11 (73) | 4 (25) | NA | 15 (48) |
| Cochlear malformation (incomplete partition type II) | 1 (7) | 0 | NA | 1 (3) |
| Petrous bone fracture (at age 10 mo) | 1 (7) | 0 | NA | 1 (3) |
| Ototoxicity | 0 | 1 (6) | NA | 1 (3) |
| Unknown | 2 (13) | 2 (13) | NA | 4 (13) |
| Comorbidities | ||||
| Premature birth | 2 (13) | 1 (6) | 0 | 3 (5) |
| Cerebral palsy | 2 (13) | 0 | 0 | 1 (2) |
| Oculo-auriculo-vertebral dysplasia | 0 | 1 (6) | 0 | 1 (2) |
| Maternal education level | ||||
| Secondary education | 2 (13) | 4 (25) | 5 (17) | 11 (18) |
| Higher education (academic or nonacademic) | 13 (87) | 12 (75) | 25 (83) | 50 (82) |
| Birth order | ||||
| First or only child | 3 (20) | 7 (44) | 15 (50) | 25 (41) |
| Second or later child | 12 (80) | 9 (56) | 15 (50) | 36 (59) |
Abbreviations: NA, not applicable; SSD, single-sided deafness.
Linguistic Skills
The full protocol of the study consisted of several language, cognitive, and hearing tests. Because of cochlear implantation at a very young age, combined with the gradual development of language and other skills, longitudinal follow-up was required to identify the true differences between the groups. In this article, we report on the linguistic skills of the children, based on the results of 1 receptive and 2 expressive language tests. The norm-referenced tests were administered every 6 months between the ages of 24 and 66 months.
Receptive language skills were examined using the Schlichting Receptive Language Test. The test contained 7 sections, which evaluated language comprehension skills at increasing levels of complexity. The tasks included pointing at certain objects (eg, “Where is the pencil”), manipulating toys (eg, “Put the monkey on the house”), selecting 1 of 4 pictures corresponding to a given statement (eg, “The bird is large but not white”), and manipulating colored shapes (eg, “Put the black flower next to the white apple”).
The children’s vocabulary was determined using the Word Development test from the Schlichting Expressive Language Test. The test consisted of black-and-white drawings to elicit specific words (eg, bread) or expressions (eg, in the middle).
Grammar was assessed using the Sentence Development test from the Schlichting Expressive Language Test. The test estimated the child’s level of morphosyntactic development based on a range of exercises of increasing complexity. The child was asked to repeat sentences (eg, “Now I want this one”), to complete sentences (eg, “A man with a beard and a man without a beard”), or to repeat sentences while changing 1 or more words (eg, “This is a picture of a man with a flower on his hat,” where the child changes “flower” to “feather”).
The test manuals mentioned good reliability and concept validity for all tests. Lambda-2 coefficients for internal consistency equaled 0.94 for receptive language, and 0.90 for vocabulary and grammar. The correlations between raw score and age were 0.85 for receptive language, 0.85 for vocabulary, and 0.78 for grammar.27,28
Statistical Analysis
We used R statistical software version 4.0.3 (R Project for Statistical Computing) to analyze the data and to create the graphs,29 along with packages tidyverse, lme4, car, MuMin, emmeans, deducorrect, eeptools, colorspace, and gridExtra.30,31,32,33,34,35,36,37,38 For all statistical tests (2 sided), the significance level was set to α ≤ .05. Raw test scores were transformed to age-adjusted scores and corresponding z scores, according to the standard values included in the test manual. The z scores represent the number of SDs by which the age-adjusted scores differ from the mean scores in the general population. Normal data distribution was confirmed using Shapiro-Wilk normality tests.
On the basis of the longitudinal study design with repeated measures, data were analyzed using linear mixed models.39 These models are well suited for longitudinal development follow-up because they can account for variability between and across participants while being robust to missing data.40 Accordingly, data from all participants were included in the analyses, regardless of study completion. Our linear mixed models included group and age as fixed variables, and we used random intercepts with 1 level per participant to account for participant-specific differences. Maternal education level (secondary or higher), birth order (firstborn or later-born), and sex (male or female) were added to the model as covariates.
We applied a stepwise procedure to select the best model for each outcome measure. Initially, we constructed a model using all aforementioned variables. Nonsignificant factors were identified using a Wald χ2 test. Next, we constructed a new model without these variables and compared it with the old model using ANOVA. If the ANOVA result was not significant, the new model estimated the data as accurately as the old model, and the Wald χ2 test was repeated for the new model. If the ANOVA result was significant (ie, the old model estimated the data better), the original model was kept. This procedure was repeated until the most restricted model was found that accurately estimated the data.
Normality and homogeneity of the residual variances were examined to ensure that the selected models were appropriate. The coefficient of determination was calculated using the R package MuMin to obtain both marginal (R2m, fixed factors) and conditional (R2c, fixed and random factors) R2 values.33,41,42 Subgroup analyses were performed through Bonferroni-corrected pairwise t tests of the estimated marginal means. Cohen d effect sizes were equal to the difference in z scores for all pairwise comparisons, as the z score expressed by how many SDs the child’s score differed from the population mean. Data analysis was performed from October to December 2020.
Results
Results for 1 or more language tests were available for 61 children (26 girls [42%]; mean [SD] age at the time of enrollment, 2.08 [1.34] years): 15 with SSD and a cochlear implant, 16 with SSD without a cochlear implant, and 30 with NH. Eight scores from 3 different children (1 from each group) were classified as outliers because of testing abnormalities and were excluded from the analyses. Data from a subset of this sample have been previously published.43
Receptive language z scores (241 scores for 61 children) were normally distributed. The scores improved with age (0.21 point per year; 95% CI, 0.14-0.28 point per year; χ21 = 32.6735; P < .001) and were higher for children with older siblings (0.43 point; 95% CI, 0.01-0.85 point; P = .04). Children from mothers with a higher academic degree had higher scores, although the difference was not significant after Bonferroni correction (0.54 point; 95% CI, 0.00-1.09 points; P = .05). There was no difference between the 3 groups. The model explained 74% of the variance (R2m = 0.14; R2c = 0.74). Figure 1 shows the children’s receptive language scores.
Figure 1. Individual Receptive Language Scores of Children With Single-Sided Deafness (SSD) Compared With Children With Normal Hearing.
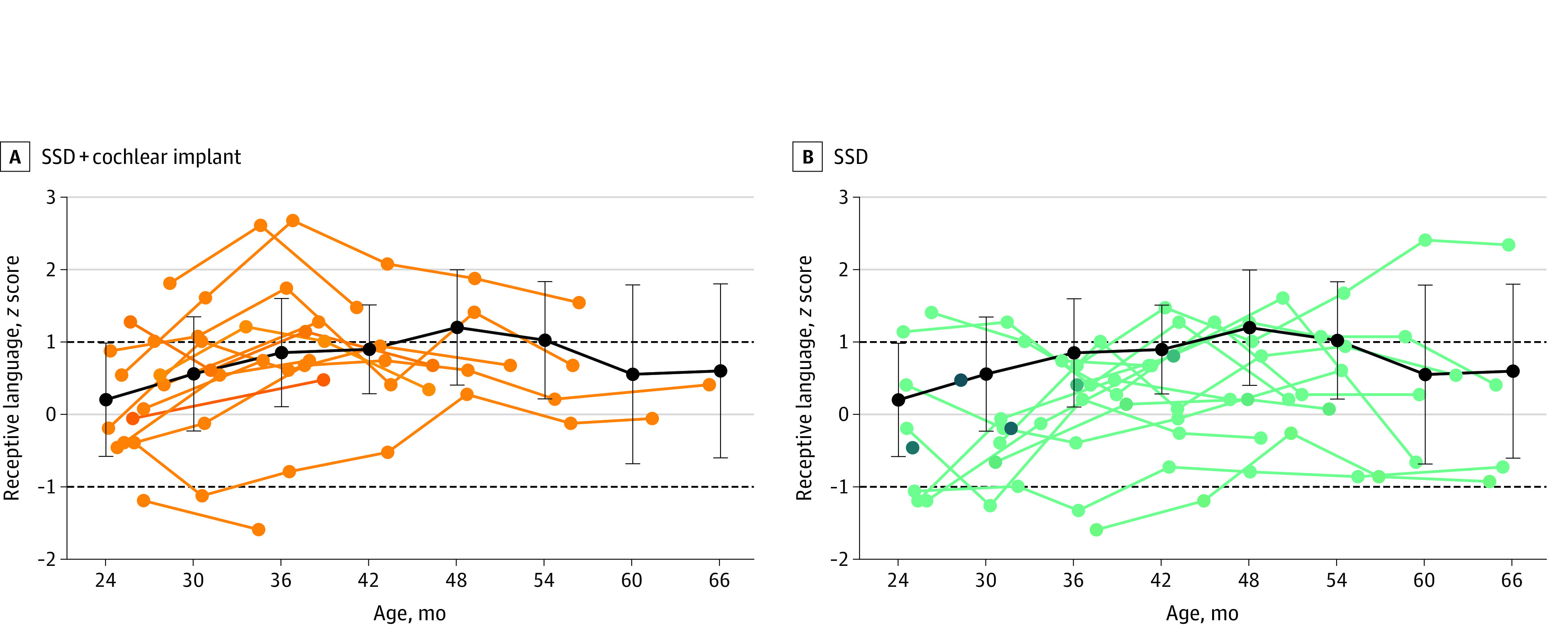
Individual receptive language scores (z scores) are shown for children with SSD and cochlear implants (A) and children with SSD without cochlear implants (B), as a function of age. The black lines and error bars indicate the mean (SD) score of the children with normal hearing, averaged per age interval of 6 months (24 months, 20 children; 30 months, 20 children; 36 months, 14 children; 42 months, 15 children; 48 months, 15 children; 54 months, 14 children; 60 months, 13 children; and 66 months, 14 children). The dashed lines at 1 and −1 indicate the range of average performance, according to the test’s standard values.
Vocabulary z scores (237 scores for 59 children) were not normally distributed across groups. However, normal distribution was present within each group. Vocabulary scores were higher for older children (0.22 point per year; 95% CI, 0.15-0.30 point per year; P < .001) and children from mothers with a higher academic degree (0.65 point; 95% CI, 0.11-1.20 points; P = .02). The model showed that the groups were significantly different from each other with regard to their vocabulary scores (χ22 = 6.441; P = .04). However, the direction of this difference could not be determined using post-hoc comparisons, because none of these comparisons was significant after Bonferroni correction. The model explained 74% of the variance (R2m = 0.18; R2c = 0.74). Figure 2 presents the children’s vocabulary scores.
Figure 2. Individual Vocabulary Scores of Children With Single-Sided Deafness (SSD) Compared With Children With Normal Hearing.
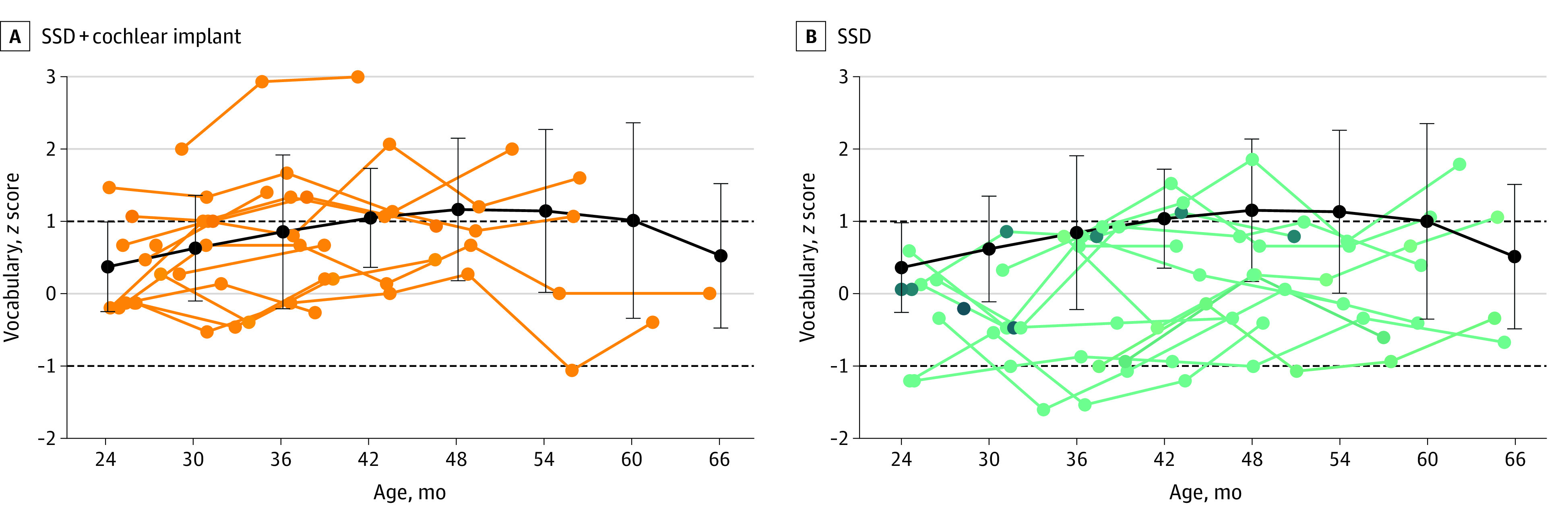
Individual vocabulary scores (z scores) for children with SSD and cochlear implants (A) and children with SSD without cochlear implants (B) are shown as a function of age. The black lines and error bars indicate the mean (SD) score of the children with normal hearing, averaged per age interval of 6 months (24 months, 19 children; 30 months, 20 children; 36 months, 15 children; 42 months, 13 children; 48 months, 16 children; 54 months, 15 children; 60 months, 13 children; and 66 months, 12 children). Two outliers were removed in correspondence with the linear mixed models used in the data analysis. The dashed lines at 1 and −1 indicate the range of average performance, according to the test’s standard values.
Grammar z scores (233 scores for 60 children) were normally distributed and were higher if children had a mother with a higher academic degree (0.50 point; 95% CI, 0.15 to 1.85 points; P = .02). Scores were significantly different across groups (χ22 = 12.699; P = .002). The SSD without cochlear implant group had worse scores compared with the SSD plus cochlear implant group (−0.76 point; 95% CI, −1.21 to −0.31 point; P = .004) and the NH group (−0.53 point; 95% CI, −0.91 to −0.15 point; P = .02). The difference between the scores of the SSD plus cochlear implant group and the NH group was not significant, and scores were not associated with age. The model explained 62% of the variance (R2m = 0.19; R2c = 0.62). The longitudinal grammar results are shown in Figure 3.
Figure 3. Individual Grammar Scores of Children With Single-Sided Deafness (SSD) Compared With Children With Normal Hearing.
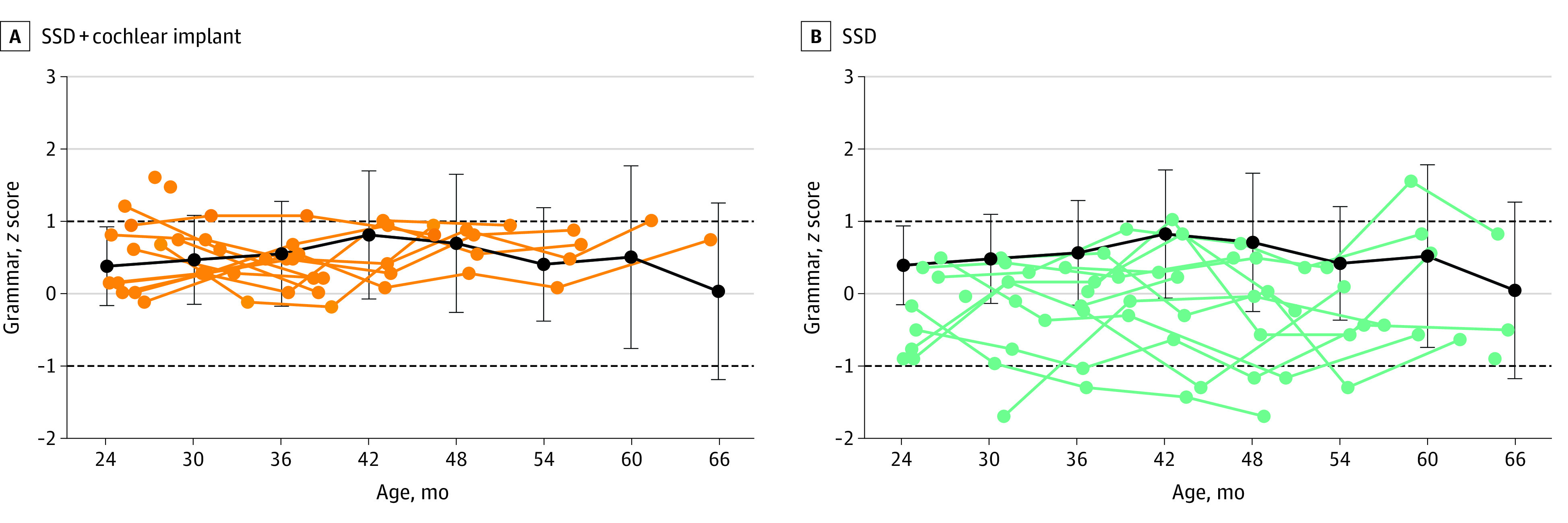
Individual grammar scores (z scores) for children with SSD and cochlear implants (A) and children with SSD without cochlear implants (B) are shown as a function of age. The black lines and error bars indicate the mean (SD) score of the children with normal hearing, averaged per age interval of 6 months (24 months, 19 children; 30 months, 21 children; 36 months, 14 children; 42 months, 14 children; 48 months, 16 children; 54 months, 15 children; 60 months, 13 children; and 66 months, 12 children). Six outliers were removed in correspondence with the linear mixed models used in the data analysis. The dashed lines at 1 and −1 indicate the range of average performance, according to the test’s standard values.
The associations of the factors with each of the outcome measures are summarized in Table 2. Group was significantly associated with both vocabulary and grammar, with children in the SSD plus cochlear implant group outperforming children with SSD but without cochlear implants in grammar in particular (0.41 point [95% CI, 0.05 to 0.77 point] vs −0.36 point [95% CI, −0.68 to −0.03 point]). Maternal education level was associated with the results on all language domains, with children from mothers with a higher education degree obtaining the higher scores than children from mothers with only a secondary education (receptive language, 0.49 point [95% CI, 0.26 to 0.72 point] vs −0.05 point [95% CI, −0.55 to 0.44 point]; vocabulary, 0.58 point [95% CI, 0.35 to 0.82 point] vs −0.07 point [95% CI, −0.57 to 0.43 point]; grammar, 0.33 point [95% CI, 0.14 to 0.51 point] vs −0.18 point [95% CI, −0.56 to 0.21 point]). Birth order was only associated with receptive language, with firstborn children performing worse than children with 1 or more older siblings (0.00 point [95% CI, −0.35 to 0.36 point]) vs 0.43 point [95% CI, 0.10 to 0.76 point]).
Table 2. Estimated Language Scores for Each Factor.
| Factor | Score, estimated mean (95% CI)a | ||
|---|---|---|---|
| Receptive language | Vocabulary | Grammar | |
| Group | |||
| SSD with cochlear implant (n = 15) | 0.36 (−0.11 to 0.83) | 0.57 (0.10 to 1.03) | 0.41 (0.05 to 0.77) |
| SSD without cochlear implant (n = 16) | 0.02 (−0.40 to 0.44) | −0.14 (−0.55 to 0.28) | −0.36 (−0.68 to −0.03) |
| Normal hearing (n = 30) | 0.28 (−0.07 to 0.63) | 0.34 (−0.01 to 0.69) | 0.17 (−0.10 to 0.45) |
| Maternal education level | |||
| Secondary (n = 11) | −0.05 (−0.55 to 0.44) | −0.07 (−0.57 to 0.43) | −0.18 (−0.56 to 0.21) |
| Higher (n = 50) | 0.49 (0.26 to 0.72) | 0.58 (0.35 to 0.82) | 0.33 (0.14 to 0.51) |
| Difference | 0.54 (0.00 to 1.09) | 0.65 (0.11 to 1.20) | 0.50 (0.07 to 0.93) |
| Birth order | |||
| First child (n = 25) | 0.00 (−0.35 to 0.36) | NA | NA |
| Later child (n = 36) | 0.43 (0.10 to 0.76) | NA | NA |
| Difference | 0.43 (0.01 to 0.85) | NA | NA |
Abbreviations: NA, not applicable; SSD, single-sided deafness.
Scores are estimated according to the corresponding linear mixed models, for each level of each estimated variable. For maternal education level and birth order, the significant pairwise differences are given as well.
Discussion
Untreated SSD in children is associated with hearing difficulties, speech-language delays, and cognitive and academic underachievement.3,9,44,45,46 Although the benefit of a cochlear implant in terms of hearing outcomes is well studied,16 the current study is the first, to our knowledge, to report language skills in young children with SSD with and without a cochlear implant. In the group of 31 children with prelingual SSD, early intervention with a cochlear implant preserved grammatical skills. Children with prelingual SSD and a cochlear implant performed significantly better on grammar tasks than those without a cochlear implant, and in line with the children with normal hearing. This is particularly striking given the young age of the children (24-66 months). Although this trend was already visible when the first results of our study were reported,43 we were now able to confirm these group differences using statistical methods that account for between-participant variability. Overall, our results are in line with previous research showing an expressive language deficit for children with untreated SSD.5,7,44
We hypothesized that difficulties with the perception of subtle auditory cues lie at the basis of this language deficit. Especially in noisy environments, humans rely on binaural cues to separate sounds and improve speech perception. Impaired speech perception may impede implicit language learning, delaying the formation of the children’s internal set of rules. Given that young children typically spend much time in noisy environments,47,48,49 even subtle auditory difficulties could have an extensive negative impact. In addition, such difficulties might trigger higher listening effort, which, in turn, could lead to higher rates of fatigue and behavioral problems, as seen in some children with SSD.50 Similarly, increased listening effort and language problems have also been found in children with mild bilateral HL.51,52 Improving the speech perception of children with SSD using a cochlear implant may reduce listening effort and aid language processing. This would explain why children with SSD seem to benefit from early cochlear implantation with regard to their language development.
Previous studies5,6 also reported a deficit in receptive language skills for children with SSD, but we could not identify such a deficit in our sample. Given that children with SSD have 1 NH ear on which to rely, they make far more subtle errors compared with children with bilateral deafness, and some tests may not be sensitive enough to detect those errors. Alternatively, receptive language skills may be more robust to the perceptual disadvantage associated with SSD than expressive skills. For example, failing to detect a suffix may not hinder comprehension (eg, “he drink water” is equally clear as “he drinks water”), while omitting that same suffix in a spoken utterance is grammatically incorrect. Finally, most children were still very young, and receptive language deficits may emerge only later in life.
Because CND is often treated as an exclusion criterion for cochlear implantation because of mixed outcomes,53 none of the children with CND in our sample received a cochlear implant. Although CMV infection has been associated with mixed outcomes after implantation too,54 the need for intervention may be greater in this group given the risk of developmental problems. In addition, the cochlear implant can serve as a backup in case the hearing in the better ear deteriorates, increasing its potential benefit for these children. Importantly, all children in our sample seemed to benefit from the cochlear implant, and none were nonusers. We, therefore, argue that cochlear implantation is a viable treatment option in children with SSD due to CMV.
This study focused specifically on children with SSD who received implants at an early age. The children are now at an age that allows us to address some very important research questions using detailed behavioral tests. However, further follow-up is critical to understand the long-term associations of the cochlear implant with various language skills. These should also include more complex language skills, such as story-telling or phonological awareness.
Limitations
This study has limitations that should be addressed. This type of study entails sources of variability that are difficult to eliminate, and certain child and family characteristics may have influenced the children’s language scores.55 On average, girls tend to develop language skills more rapidly compared with boys.56 However, our results did not reveal an association between sex and any of the outcomes. Second, high maternal education levels typically benefit children’s development in general and language skills in particular.57 Indeed, having a mother with a higher education degree was associated with higher language scores in our sample. Finally, firstborn children often have stronger expressive vocabulary skills.55 In contrast, birth order was not associated with expressive language in our sample, and firstborn children had lower receptive language scores than children who had at least 1 older sibling.
A second limitation is the considerable imbalance between the groups regarding causes of SSD. The most common causes of congenital SSD are CND and CMV infection. In contrast to CND, which typically reflects an isolated anatomical abnormality, congenital CMV infection is associated with the risk of additional developmental sequelae, especially if the child has clinical symptoms at birth.58 Additionally, children with SSD due to CMV have an increased risk of delayed-onset and progressive HL in the contralateral ear.59,60,61 In our sample, 11 of 15 children in the SSD plus cochlear implant group had congenital CMV, of whom 2 had apparent clinical symptoms at birth. In this group, 1 child experienced progressive HL in the better ear, and 2 others received diagnoses of cerebral palsy after implantation. Although most children in the SSD group (9 of 16 children) had CND, the SSD plus cochlear implant group still outperformed the SSD group.
Conclusions
In this cohort study of young children with prelingual SSD, early cochlear implantation was associated with normal grammar development. Longer follow-up is essential to understand the long-term outcomes associated with the cochlear implant and to identify any remaining language deficits in these children. Given the risk for lasting language problems associated with untreated SSD, the current results are especially encouraging. They highlight that a cochlear implant can preserve expressive language skills for children with SSD. Combined with the existing evidence for hearing-related advantages, these results support the notion of cochlear implantation becoming the standard of care for children with prelingual SSD.
References
- 1.Morton CC, Nance WE. Newborn hearing screening: a silent revolution. N Engl J Med. 2006;354(20):2151-2164. doi: 10.1056/NEJMra050700 [DOI] [PubMed] [Google Scholar]
- 2.Van Kerschaver E, Stappaerts L. Jaarrapport Gehoor 2009-2010-2011: universele gehoorscreening in Vlaanderen—doelgroepbereik, testresultaten en resultaten van de verwijzingen. Published 2011. Accessed July 21, 2021. https://www.kindengezin.be/img/rapportGehoor2009-2010-2011.pdf
- 3.Reeder RM, Cadieux J, Firszt JB. Quantification of speech-in-noise and sound localisation abilities in children with unilateral hearing loss and comparison to normal hearing peers. Audiol Neurootol. 2015;20(1)(suppl):31-37. doi: 10.1159/000380745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolter NE, Cushing SL, Vilchez-Madrigal LD, et al. Unilateral hearing loss is associated with impaired balance in children: a pilot study. Otol Neurotol. 2016;37(10):1589-1595. doi: 10.1097/MAO.0000000000001218 [DOI] [PubMed] [Google Scholar]
- 5.Lieu JEC, Tye-Murray N, Karzon RK, Piccirillo JF. Unilateral hearing loss is associated with worse speech-language scores in children. Pediatrics. 2010;125(6):e1348-e1355. doi: 10.1542/peds.2009-2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick EM, Gaboury I, Durieux-Smith A, Coyle D, Whittingham J, Nassrallah F. Auditory and language outcomes in children with unilateral hearing loss. Hear Res. 2019;372:42-51. doi: 10.1016/j.heares.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 7.Sangen A, Royackers L, Desloovere C, Wouters J, van Wieringen A. Single-sided deafness affects language and auditory development: a case-control study. Clin Otolaryngol. 2017;42(5):979-987. doi: 10.1111/coa.12826 [DOI] [PubMed] [Google Scholar]
- 8.Fischer C, Lieu J. Unilateral hearing loss is associated with a negative effect on language scores in adolescents. Int J Pediatr Otorhinolaryngol. 2014;78(10):1611-1617. doi: 10.1016/j.ijporl.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Wieringen A, Boudewyns A, Sangen A, Wouters J, Desloovere C. Unilateral congenital hearing loss in children: challenges and potentials. Hear Res. 2019;372:29-41. doi: 10.1016/j.heares.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 10.O’Donoghue G. Cochlear implants: science, serendipity, and success. N Engl J Med. 2013;369(13):1190-1193. doi: 10.1056/NEJMp1310111 [DOI] [PubMed] [Google Scholar]
- 11.Grossmann W, Brill S, Moeltner A, Mlynski R, Hagen R, Radeloff A. Cochlear implantation improves spatial release from masking and restores localization abilities in single-sided deaf patients. Otol Neurotol. 2016;37(6):658-664. doi: 10.1097/MAO.0000000000001043 [DOI] [PubMed] [Google Scholar]
- 12.van Zon A, Peters JPM, Stegeman I, Smit AL, Grolman W. Cochlear implantation for patients with single-sided deafness or asymmetrical hearing loss: a systematic review of the evidence. Otol Neurotol. 2015;36(2):209-219. doi: 10.1097/MAO.0000000000000681 [DOI] [PubMed] [Google Scholar]
- 13.Bernstein JGW, Schuchman GI, Rivera AL. Head shadow and binaural squelch for unilaterally deaf cochlear implantees. Otol Neurotol. 2017;38(7):e195-e202. doi: 10.1097/MAO.0000000000001469 [DOI] [PubMed] [Google Scholar]
- 14.Prejban DA, Hamzavi J-S, Arnoldner C, et al. Single sided deaf cochlear implant users in the difficult listening situation: speech perception and subjective benefit. Otol Neurotol. 2018;39(9):e803-e809. doi: 10.1097/MAO.0000000000001963 [DOI] [PubMed] [Google Scholar]
- 15.Dirks C, Nelson PB, Sladen DP, Oxenham AJ. Mechanisms of localization and speech perception with colocated and spatially separated noise and speech maskers under single-sided deafness with a cochlear implant. Ear Hear. 2019;40(6):1293-1306. doi: 10.1097/AUD.0000000000000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benchetrit L, Ronner EA, Anne S, Cohen MS. Cochlear implantation in children with single-sided deafness: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147(1):58-69. doi: 10.1001/jamaoto.2020.3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arndt S, Prosse S, Laszig R, Wesarg T, Aschendorff A, Hassepass F. Cochlear implantation in children with single-sided deafness: does aetiology and duration of deafness matter? Audiol Neurootol. 2015;20(1)(suppl):21-30. doi: 10.1159/000380744 [DOI] [PubMed] [Google Scholar]
- 18.Thomas JP, Neumann K, Dazert S, Voelter C. Cochlear implantation in children with congenital single-sided deafness. Otol Neurotol. 2017;38(4):496-503. doi: 10.1097/MAO.0000000000001343 [DOI] [PubMed] [Google Scholar]
- 19.Beck RL, Aschendorff A, Hassepaß F, et al. Cochlear implantation in children with congenital unilateral deafness: a case series. Otol Neurotol. 2017;38(10):e570-e576. doi: 10.1097/MAO.0000000000001597 [DOI] [PubMed] [Google Scholar]
- 20.Gordon K, Henkin Y, Kral A. Asymmetric hearing during development: the aural preference syndrome and treatment options. Pediatrics. 2015;136(1):141-153. doi: 10.1542/peds.2014-3520 [DOI] [PubMed] [Google Scholar]
- 21.Kral A, Kronenberger WG, Pisoni DB, O’Donoghue GM. Neurocognitive factors in sensory restoration of early deafness: a connectome model. Lancet Neurol. 2016;15(6):610-621. doi: 10.1016/S1474-4422(16)00034-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kral A, Hubka P, Tillein J. Strengthening of hearing ear representation reduces binaural sensitivity in early single-sided deafness. Audiol Neurootol. 2015;20(1)(suppl):7-12. doi: 10.1159/000380742 [DOI] [PubMed] [Google Scholar]
- 23.Kral A, Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012;35(2):111-122. doi: 10.1016/j.tins.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polonenko MJ, Gordon KA, Cushing SL, Papsin BC. Cortical organization restored by cochlear implantation in young children with single sided deafness. Sci Rep. 2017;7(1):16900. doi: 10.1038/s41598-017-17129-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauch AK, Arndt S, Aschendorff A, et al. Long-term results of cochlear implantation in children with congenital single-sided deafness. Eur Arch Otorhinolaryngol. Published October 20, 2020. doi: 10.1007/s00405-020-06409-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.Schlichting L, Spelberg HL. Schlichting Test Voor Taalbegrip: Vlaamse Aanvulling. Bohn Stafleu van Loghum; 2011. [Google Scholar]
- 28.Schlichting L, Spelberg HL. Schlichting Test Voor Taalproductie-II: Vlaamse Aanvulling. Bohn Stafleu van Loghum; 2011. [Google Scholar]
- 29.R Core Team . R: a language and environment for statistical computing. Published 2020. Accessed July 21, 2021. https://www.r-project.org/
- 30.Wickham H, Averick M, Bryan J, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4(43):1686. doi: 10.21105/joss.01686 [DOI] [Google Scholar]
- 31.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 32.Fox J, Weisberg S. An R Companion to Applied Regression. 3rd ed. Sage Publications; 2019. [Google Scholar]
- 33.Barton K. MuMIn: multi-model inference. Published 2020. Accessed July 21, 2021. https://cran.r-project.org/package=MuMIn
- 34.Lenth RV. emmeans: Estimated marginal means, aka least-squares means. Published 2020. Accessed July 21, 2021. https://cran.r-project.org/package=emmeans
- 35.van der Loo M, de Jonge E, Scholtus S. deducorrect: Deductive correction, deductive imputation, and deterministic correction. Published 2015. Accessed July 21, 2021. https://cran.r-project.org/package=deducorrect
- 36.Knowles JE. eeptools: Convenience functions for education data. Published 2020. Accessed July 21, 2021. https://cran.r-project.org/package=eeptools
- 37.Zeileis A, Fisher JC, Hornik K, et al. colorspace: A toolbox for manipulating and assessing colors and palettes. J Stat Softw. 2020;96(1):1-49. doi: 10.18637/jss.v096.i01 [DOI] [Google Scholar]
- 38.Auguie B. gridExtra: Miscellaneous functions for “Grid” graphics. Published 2017. Accessed July 21, 2021. https://cran.r-project.org/package=gridExtra
- 39.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. 2nd ed. Springer; 2000. [Google Scholar]
- 40.Walker EA, Redfern A, Oleson JJ. Linear mixed-model analysis to examine longitudinal trajectories in vocabulary depth and breadth in children who are hard of hearing. J Speech Lang Hear Res. 2019;62(3):525-542. doi: 10.1044/2018_JSLHR-L-ASTM-18-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa S, Johnson PCD, Schielzeth H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface. 2017;14(134):20170213. doi: 10.1098/rsif.2017.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133-142. doi: 10.1111/j.2041-210x.2012.00261.x [DOI] [Google Scholar]
- 43.Sangen A, Dierckx A, Boudewyns A, et al. Longitudinal linguistic outcomes of toddlers with congenital single-sided deafness: six with and twelve without cochlear implant and nineteen normal hearing peers. Clin Otolaryngol. 2019;44(4):671-676. doi: 10.1111/coa.13347 [DOI] [PubMed] [Google Scholar]
- 44.Lieu JEC. Permanent unilateral hearing loss (UHL) and childhood development. Curr Otorhinolaryngol Rep. 2018;6(1):74-81. doi: 10.1007/s40136-018-0185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell PL, Shinn JR, Davis GE, Sie KCY. Children with unilateral hearing loss may have lower intelligence quotient scores: a meta-analysis. Laryngoscope. 2016;126(3):746-754. doi: 10.1002/lary.25524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anne S, Lieu JEC, Cohen MS. Speech and language consequences of unilateral hearing loss: a systematic review. Otolaryngol Head Neck Surg. 2017;157(4):572-579. doi: 10.1177/0194599817726326 [DOI] [PubMed] [Google Scholar]
- 47.Busch T, Vanpoucke F, van Wieringen A. Auditory environment across the life span of cochlear implant users: insights from data logging. J Speech Lang Hear Res. 2017;60(5):1362-1377. doi: 10.1044/2016_JSLHR-H-16-0162 [DOI] [PubMed] [Google Scholar]
- 48.Ganek HV, Cushing SL, Papsin BC, Gordon KA. Cochlear implant use remains consistent over time in children with single-sided deafness. Ear Hear. 2020;41(3):678-685. doi: 10.1097/AUD.0000000000000797 [DOI] [PubMed] [Google Scholar]
- 49.Picard M, Boudreau C. Characteristics of the noise found in day-care centers. J Acoust Soc Am. 1999;105(2):1127. doi: 10.1121/1.425260 [DOI] [Google Scholar]
- 50.Bess FH, Davis H, Camarata S, Hornsby BWY. Listening-related fatigue in children with unilateral hearing loss. Lang Speech Hear Serv Sch. 2020;51(1):84-97. doi: 10.1044/2019_LSHSS-OCHL-19-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hick CB, Tharpe AM. Listening effort and fatigue in school-age children with and without hearing loss. J Speech Lang Hear Res. 2002;45(3):573-584. doi: 10.1044/1092-4388(2002/046) [DOI] [PubMed] [Google Scholar]
- 52.Halliday LF, Tuomainen O, Rosen S. Language development and impairment in children with mild to moderate sensorineural hearing loss. J Speech Lang Hear Res. 2017;60(6):1551-1567. doi: 10.1044/2016_JSLHR-L-16-0297 [DOI] [PubMed] [Google Scholar]
- 53.Walton J, Gibson WPR, Sanli H, Prelog K. Predicting cochlear implant outcomes in children with auditory neuropathy. Otol Neurotol. 2008;29(3):302-309. doi: 10.1097/MAO.0b013e318164d0f6 [DOI] [PubMed] [Google Scholar]
- 54.Shin JJ, Keamy DG Jr, Steinberg EA. Medical and surgical interventions for hearing loss associated with congenital cytomegalovirus: a systematic review. Otolaryngol Head Neck Surg. 2011;144(5):662-675. doi: 10.1177/0194599811399241 [DOI] [PubMed] [Google Scholar]
- 55.Urm A, Tulviste T. Sources of individual variation in Estonian toddlers’ expressive vocabulary. First Lang. 2016;36(6):580-600. doi: 10.1177/0142723716673951 [DOI] [Google Scholar]
- 56.Bornstein MH, Hahn C-S, Haynes OM. Specific and general language performance across early childhood: stability and gender considerations. First Lang. 2004;24(3):267-304. doi: 10.1177/0142723704045681 [DOI] [Google Scholar]
- 57.Dollaghan CA, Campbell TF, Paradise JL, et al. Maternal education and measures of early speech and language. J Speech Lang Hear Res. 1999;42(6):1432-1443. doi: 10.1044/jslhr.4206.1432 [DOI] [PubMed] [Google Scholar]
- 58.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355-363. doi: 10.1002/rmv.544 [DOI] [PubMed] [Google Scholar]
- 59.Lanzieri TM, Chung W, Flores M, et al. ; Congenital Cytomegalovirus Longitudinal Study Group . Hearing loss in children with asymptomatic congenital cytomegalovirus infection. Pediatrics. 2017;139(3):e20162610. doi: 10.1542/peds.2016-2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goderis J, Keymeulen A, Smets K, et al. Hearing in children with congenital cytomegalovirus infection: results of a longitudinal study. J Pediatr. 2016;172:110-115.e2. doi: 10.1016/j.jpeds.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 61.Riga M, Korres G, Chouridis P, Naxakis S, Danielides V. Congenital cytomegalovirus infection inducing non-congenital sensorineural hearing loss during childhood: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;115(May):156-164. doi: 10.1016/j.ijporl.2018.10.005 [DOI] [PubMed] [Google Scholar]


