Abstract
During a normal cell cycle, entry into S phase is dependent on completion of mitosis and subsequent activation of cyclin-dependent kinases (Cdks) in G1. These events are monitored by checkpoint pathways. Recent studies and data presented herein show that after treatment with microtubule inhibitors (MTIs), cells deficient in the Cdk inhibitor p21Waf1/Cip1 enter S phase with a ≥4N DNA content, a process known as endoreduplication, which results in polyploidy. To determine how p21 prevents MTI-induced endoreduplication, the G1/S and G2/M checkpoint pathways were examined in two isogenic cell systems: HCT116 p21+/+ and p21−/− cells and H1299 cells containing an inducible p21 expression vector (HIp21). Both HCT116 p21−/− cells and noninduced HIp21 cells endoreduplicated after MTI treatment. Analysis of G1-phase Cdk activities demonstrated that the induction of p21 inhibited endoreduplication through direct cyclin E/Cdk2 regulation. The kinetics of p21 inhibition of cyclin E/Cdk2 activity and binding to proliferating-cell nuclear antigen in HCT116 p21+/+ cells paralleled the onset of endoreduplication in HCT116 p21−/− cells. In contrast, loss of p21 did not lead to deregulated cyclin D1-dependent kinase activities, nor did p21 directly regulate cyclin B1/Cdc2 activity. Furthermore, we show that MTI-induced endoreduplication in p53-deficient HIp21 cells was due to levels of p21 protein below a threshold required for negative regulation of cyclin E/Cdk2, since ectopic expression of p21 restored cyclin E/Cdk2 regulation and prevented endoreduplication. Based on these findings, we propose that p21 plays an integral role in the checkpoint pathways that restrain normal cells from entering S phase after aberrant mitotic exit due to defects in microtubule dynamics.
Precise biochemical pathways have evolved in eukaryotic cells to coordinate the multiple events needed to ensure genomic stability. Fundamental to these biochemical pathways are checkpoints which serve to monitor the integrity of chromosomes and cell cycle progression (17). Defects in cell cycle checkpoints can result in gene mutations, chromosome damage, and aneuploidy, all of which can contribute to tumorigenesis (41). Aneuploidy is a common feature of human cancers, suggesting that the mechanisms that normally regulate the fidelity of mitotic exit and S-phase entry are frequently disrupted in tumor cells.
The eukaryotic cell cycle is regulated by the coordinated activity of protein kinase complexes, each consisting of a cyclin-dependent kinase (Cdk) and a cyclin (36, 46, 49). Cdks must bind a cyclin and undergo site-specific phosphorylation to be activated (1, 51), and they are negatively regulated by a family of functionally related proteins called Cdk inhibitors (CdkIs) (50, 59). These CdkIs fall into two categories: the INK4 inhibitors and the Cip/Kip inhibitors. There are four known INK4 family members, p16 (48), p15 (13, 24), p19 (21), and p18 (21), and three known Cip/Kip family members, p21Waf1/Cip1 (10, 60), p27Kip1 (44, 45, 53), and p57Kip2 (28, 31). The INK4 family can inhibit Cdk4 and Cdk6 activity, while the Cip/Kip family can inhibit Cdk2, Cdk4, Cdk6, and Cdc2. Both families of CdkIs have been shown to play regulatory roles during the G1/S cell cycle checkpoint (23, 50).
G1-phase progression is mediated by the combined activity of the cyclin D1/Cdk4,6 and cyclin E/Cdk2 complexes (49). Cyclin D1-associated kinase activity increases in mid-G1, while cyclin E/Cdk2 activity increases in late G1 and peaks in early S phase (8, 26). The G1/S transition is dependent on activation of the cyclin E/Cdk2 complex (40, 54). An important downstream target of the G1-phase cyclin/Cdk complexes is the retinoblastoma protein (pRb). pRb is a transcriptional repressor which, in its hypophosphorylated state, binds to members of the E2F transcription factor family (2, 19) and blocks E2F-dependent transcription of S-phase genes (19, 47). Upon sequential pRb phosphorylation by cyclin D1/Cdk4,6 and cyclin E/Cdk2 (58) during G1 progression, E2F and pRb dissociate and S-phase progression ensues (20, 57). Negative regulation of the cyclin E/Cdk2 complex plays a key role in G1/S checkpoint function (50). After exposure of normal cells to genotoxic agents (9, 56), the CdkI p21Waf1/Cip1 (p21) is induced and binds to cyclin E/Cdk2 complexes (12, 14, 60), resulting in pRb hypophosphorylation, which blocks S-phase entry and causes cell cycle arrest. p21 can also bind to proliferating-cell nuclear antigen (PCNA), a protein required for both DNA repair and replication. PCNA is an essential cofactor for DNA polymerases δ and ɛ during replication, enhancing polymerase processivity (55). Waga et al. have shown that p21 inhibits processive DNA synthesis in a PCNA-dependent manner in vitro (55). In the cell, cyclin-Cdk-PCNA-p21 complexes are found throughout the cell cycle (29, 61–63); p21 interacts with Cdks via its N terminus and with PCNA via its C terminus (3, 30). Cyclin A-Cdk2-PCNA-p21 complexes and cyclin B1-Cdc2-p21-PCNA complexes assemble in early S phase, whereas cyclin D1-Cdk4-p21-PCNA complexes persist in all phases of the cell cycle (29).
The mitotic spindle checkpoint monitors spindle microtubule structure, chromosome alignment on the spindle, and chromosome attachment to kinetochores during mitosis (5, 52). The spindle checkpoint delays the onset of chromosome segregation during anaphase until any defects in the mitotic spindle are corrected (11). Cells which have a defective spindle checkpoint can aberrantly exit from mitosis with a 4N DNA content (22). These cells may inappropriately continue to the next cell cycle division and enter S phase with a 4N DNA content; this process is known as endoreduplication.
Recent studies have shown that cells lacking p53, pRb, and the CdkIs p21 and p16 will undergo microtubule inhibitor (MTI)-induced endoreduplication. When p53−/− mouse embryo fibroblasts (MEFs) are treated with MTIs, they undergo endoreduplication, resulting in polyploid cells (6, 7, 42). However, in p53+/+ cells, p53 does not directly mediate the mitotic arrest induced by MTIs, since elevation in p53 protein levels occurs only after cells have exited the mitotic arrest and proceeded to G1 with a 4N DNA content (27, 33). Similarly, MEFs that are pRb−/− (7), p21−/− (25, 27), or p16−/− (25) endoreduplicate after MTI treatment. These studies suggest that the G1/S cell cycle checkpoint proteins prevent inappropriate S-phase entry following the mitotic slippage induced by prolonged MTI exposure.
To date, there is limited information available regarding the temporal deregulation of G1/S checkpoint pathways in relation to the onset of endoreduplication. The goal of the present study was to determine the biochemical pathway(s) regulated by p21 to prevent endoreduplication and the timing of such regulation. Our analysis of the kinases involved in G1/S and G2/M transitions in p21-deficient cells demonstrated that endoreduplication coincided with deregulated Cdk2 kinase activity. We found increased levels of p21 complexed with PCNA after MTI treatment in p21-containing cells. Furthermore, we were able to inhibit endoreduplication in p53-deficient cells by induction of ectopic p21 protein, which restored regulation of Cdk2 activity. The results suggest that p21 is able to maintain proper coupling of mitotic exit and S-phase entry through direct regulation of Cdk2 kinase activity and binding to PCNA.
MATERIALS AND METHODS
Growth conditions and MTI treatments for cell lines.
The HCT116 p21+/+ human colon carcinoma cell line and a derivative line, HCT116 p21−/−, in which both p21Waf1/Cip1 alleles have been deleted through homologous recombination (56) were kindly provided by Bert Vogelstein (John Hopkins Oncology Center). The HCT116 cell lines were maintained at 37°C under 5% CO2 in monolayer culture in McCoy’s 5A modified medium supplemented with 10% fetal bovine serum (FBS). H1299 human large-cell lung carcinoma cells (American Type Culture Collection) have a partial homozygous deletion of the p53 gene; p53 protein expression is not detectable (34). H1299 cells were maintained at 37°C under 5% CO2 in monolayer culture in F-12 medium supplemented with 10% FBS. WI-38 human lung fibroblasts (American Type Culture Collection) were maintained at 37°C under 5% CO2 in monolayer culture in Dulbecco modified Eagle medium supplemented with 10% FBS. When indicated, cells were treated with nocodazole (Sigma) diluted in dimethyl sulfoxide and added directly to cell media.
Flow cytometry.
Control and treated cells were trypsinized, the trypsin was inactivated, and 106 cells were aliquoted for flow cytometry. The remaining cells were processed for protein analysis (see below). Cells were incubated with 20 μg of propidium iodide (Sigma) per ml, and the DNA content was measured with a FACSCaliber instrument (Becton-Dickson). Data were plotted with Cell Quest software (Becton-Dickson); 15,000 events were analyzed for each sample.
Western analysis.
Cells were lysed in kinase lysis buffer (KLB) [50 mM Tris (pH 7.4), 150 mM NaCl, 0.1% Triton X-100, 0.1% Nonidet P-40, 4 mM EDTA, 50 mM NaF, 0.1 mM NaV, 1 mM dithiothreitol, and the protease inhibitors antipain (10 μg/ml), leupeptin (10 μg/ml), pepstatin A (10 μg/ml), chymostatin (10 μg/ml), phenylmethylsulfonyl fluoride (50 μg/ml) (Sigma), and 4-(2-aminoethyl)-benzenesulfonylfluoride (200 μg/ml) (Calbiochem-Novabiochem Corp.)]. Total-cell protein extracts were normalized for concentration by the Bradford assay (Bio-Rad) and 50 μg of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon-P membrane (Millipore). The membranes were incubated with mouse monoclonal antibodies against pRb (LM95.1), p53 (Pab1801), p21 (EA10), and PCNA (PC-10) (Calbiochem, Oncogene Research Products); mouse monoclonal antibodies against cyclin D1 (HD11), cyclin B1 (GNS1), cyclin E (HE12), and Cdc2 (clone 17) (Santa Cruz); and rabbit polyclonal antibodies against Cdk2 (M2), Cdk4 (C-22), and Cdk6 (C-21) (Santa Cruz). Primary antibodies were detected with goat anti-mouse or goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Pierce) and subjected to enhanced chemiluminescence detection.
Kinase assays.
For each sample, 300 μg of total-cell protein extract was precleared for 2 h at 4°C with 50 μg of rabbit immunoglobulin G (anti-Cdk2, anti-Cdk4, and anti-Cdk6) or 5 μg of mouse immunoglobulin G (anti-cyclin B1 and anti-cyclin E) prebound to protein A-Sepharose (PAS; Pharmacia Biotech). Precleared lysates were transferred to new microcentrifuge tubes and incubated with anti-Cdk2, anti-Cdk4, anti-Cdk6, anti-cyclin B1, or anti-cyclin E (E172) with mixing for 2 h at 4°C; the anti-cyclin E (E172) mouse monoclonal antibody was kindly provided by Joyce Slingerland (Sunnybrook Health Science Centre, Toronto, Canada). PAS was added, and the samples were mixed for 2 h at 4°C. The immunoprecipitates were washed twice with KLB and twice with kinase buffer (100 mM Tris [pH 7.4], 20 mM MgCl2, 2 mM dithiothreitol) before being incubated with 5 μg of glutathione S-transferase (GST)-pRb (amino acids 792 to 928) (32) (Cdk2, Cdk4, Cdk6, cyclin E) or 5 μg of histone H1 (Boehringer Mannheim) (cyclin B1) and 15 nM ATP for 10 min at 25°C. Samples were incubated with 2 μCi of [γ-32P]ATP at 30°C for 10 min (Cdk2 and cyclin B1) or 10 μCi of [γ-32P]ATP at 30°C for 30 min (Cdk4, Cdk6, and cyclin E), the reaction was stopped by addition of 2× Laemmli sample buffer, and the products were resolved by SDS-PAGE. The kinase assay mixtures were quantified with an Instant Imager (Packard Instruments) before autoradiography.
Immunoprecipitation of cyclin/Cdk complexes.
Cdk2 and cyclin D1 immunoprecipitations were performed as described for the kinase assays. Before immunoprecipitation, the PCNA and p21 antibodies were cross-linked to PAS beads. For cross-linking, the PAS beads and antibody were incubated overnight with mixing at 4°C. The antibody-bound PAS beads were washed twice with 500 mM sodium borate (pH 9.0) and resuspended in 500 mM sodium borate containing 100 mM dimethyl pimelimidate (Pierce), and the pH was adjusted to 8.2. The beads were mixed with 100 mM dimethyl pimelimidate for 2 h at 25°C, washed twice with 200 mM ethanolamine (pH 8.0), resuspended in ethanolamine, and mixed for 2 h at 25°C. The cross-linked PAS beads were washed twice with phosphate-buffered saline and resuspended in equal volume of phosphate-buffered saline prior to use in immunoprecipitations. All immunoprecipitates were washed three times in KLB and separated by SDS-PAGE, and Western analysis was performed as described above.
Generation of HIp21-inducible p21Waf1 stable transfectants.
The HIp21 (H1299-Inducible p21) cell line was generated by using the ecdysone (muristerone)-inducible expression system (Invitrogen). Full-length XhoI-NotI human p21Waf1/Cip1 cDNA (10) was ligated into the pIND vector (Invitrogen). H1299 cells were transfected with the pVgRXR vector (Invitrogen) by using Lipofectamine (Gibco BRL), and clones were selected in zeocin (250 μg/ml; Invitrogen). pVgRXR clones were transfected with the pIND-p21 vector, and stable cotransfectants were selected in zeocin and G418 sulfate (400 μg/ml; Mediatech, Inc.). Cotransfected clones were screened for muristerone (10 μM)-inducible p21 expression by Western analysis. The HIp21 clone was maintained in monolayer culture at 37°C under 5% CO2 in F-12 medium supplemented with 10% FBS, zeocin (250 μg/ml), and G418 sulfate (400 μg/ml).
RESULTS
MTIs induce endoreduplication in HCT116 p21−/− cells.
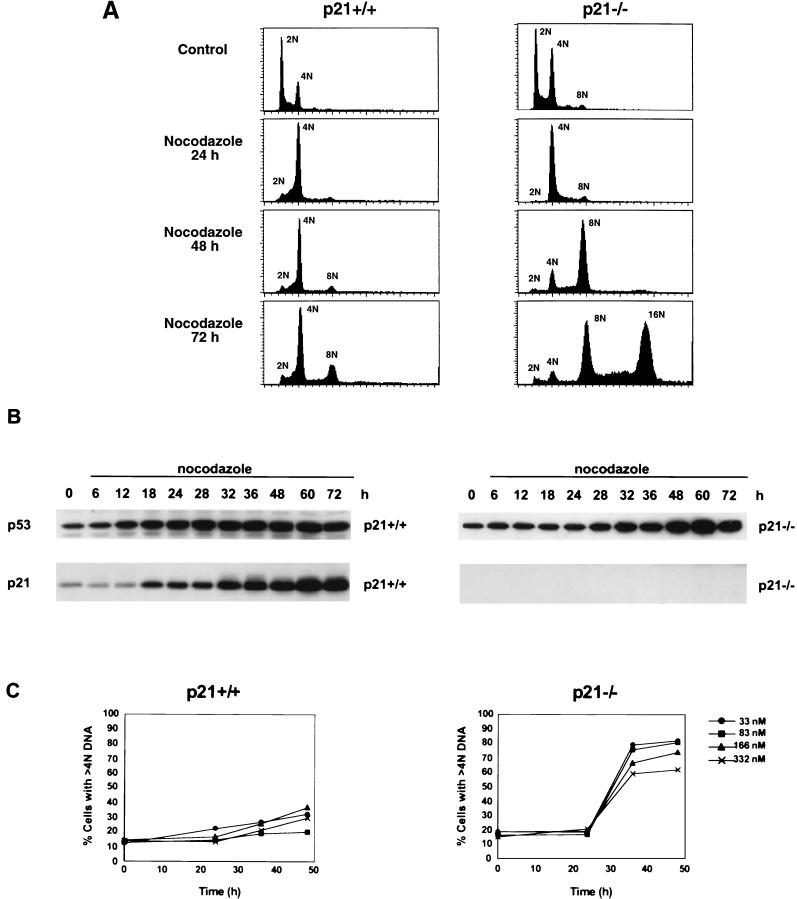
To determine if p21 is a downstream effector of the p53-dependent inhibition of endoreduplication, HCT116 p21+/+ and HCT116 p21−/− cells were treated with nocodazole, which prevents microtubule polymerization and activates the spindle checkpoint. After 48 h of nocodazole treatment, the HCT116 p21+/+ cells maintained a persistent 4N DNA content as assessed by flow cytometric analysis (Fig. 1A). This cell cycle arrest was accompanied by elevations in both p53 and p21 protein levels after 18 h of treatment (Fig. 1B). In contrast, nocodazole-treated HCT116 p21−/− cells endoreduplicated (Fig. 1A). HCT116 p21−/− cells had a 4N DNA content through 24 h of nocodazole treatment; however, by 48 h, a distinct 8N population had accumulated. By 72 h, endoreduplication in HCT116 p21−/− cells resulted in the generation of a 16N population; 32N populations were not observed, since cells died after achieving a 16N status (data not shown). The onset of endoreduplication in HCT116 p21−/− cells coincided with the timing of p21 protein induction in nocodazole-treated HCT116 p21+/+ cells (compare Fig. 1A and B). Similar flow-cytometric and Western blot analysis results were obtained when the cells were treated with the chemotherapeutic MTIs vincristine and taxol (data not shown).
FIG. 1.
HCT116 p21−/− cells endoreduplicate after MTI treatment. (A) Flow-cytometric analysis of asynchronous cultures of HCT116 p21+/+ and HCT116 p21−/− cells treated with nocodazole (83 nM). The cells were harvested at the indicated times; 15,000 events were analyzed for each sample. Data are representative of three independent experiments. DNA content is represented on the x axis; the number of cells counted is represented on the y axis. The subdiploid populations have been excluded; thus, the histograms for later time points represent fewer cells. (B) Western analysis of p53 and p21 proteins from HCT116 p21+/+ and HCT116 p21−/− cells. Asynchronous cells were treated with nocodazole (83 nM) for the indicated times, and the protein was harvested. (C) Analysis of relative polyploidy following nocodazole treatment. Asynchronous cultures of HCT116 p21+/+ and HCT116 p21−/− cells were treated with nocodazole at the concentrations shown and processed for flow-cytometric analysis as described for panel A. Representative data are shown. Data were plotted as the percentage of cells with >4N DNA content as a function of time. The DNA content was quantified by gating events with >4N DNA content on histogram plots.
Analysis of the percentage of HCT116 p21−/− cells with >4N DNA content after MTI treatment demonstrated an inverse correlation between the nocodazole concentration and the percentage of polyploid cells (Fig. 1C). This inverse correlation was due to an increased sensitivity of p21-null over p21-containing cells to nocodazole. By 36 h, there was a three- to fourfold increase in the number of HCT116 p21−/− cells with >4N DNA content. In comparison, there was minimal accumulation of HCT116 p21+/+ cells with >4N DNA content after exposure to all doses of nocodazole (Fig. 1C). The endoreduplication seen at later time points in the HCT116 p21+/+ cells was probably due to the absence of p16 protein expression in these cells, as recently reported by Myöhänen et al. (37).
Analysis of G1/S checkpoint proteins during endoreduplication.
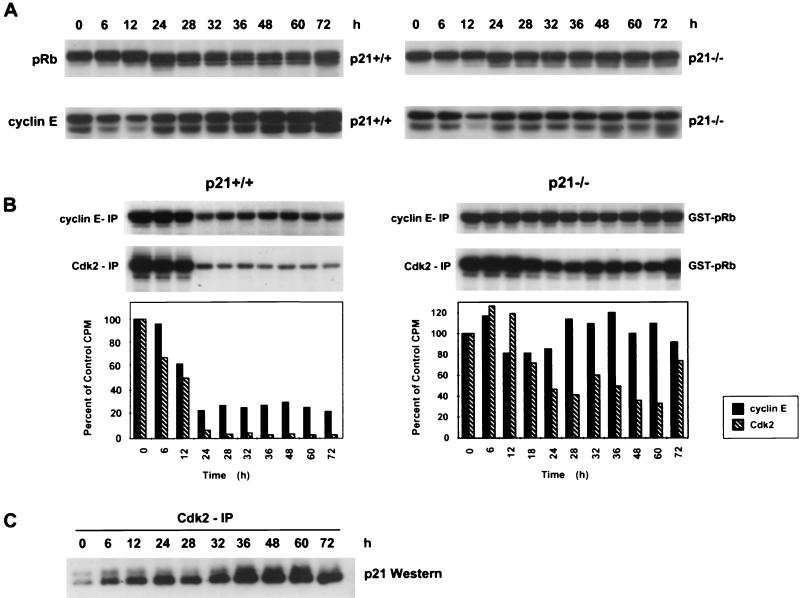
To dissect the biochemical events controlling mitotic and S-phase coupling in cells, we examined the levels and function of cell cycle proteins involved in the G1/S transition following treatment with nocodazole. To determine if the HCT116 p21−/− cells that underwent endoreduplication had reentered a G1-like biochemical state in contrast to the arrested HCT116 p21+/+ cells, protein levels of Cdk2, cyclin E, and pRb were examined (Fig. 2A). In both cell lines, Cdk2 protein levels remained constant at all time points (data not shown). Both cell lines also showed a decrease in cyclin E protein levels at 12 h, indicative of the loss of G1 cells and accumulation of cells in G2/M. However, the cyclin E protein levels increased again in both cell lines by 24 h, consistent with the presence of cells in a G1-like biochemical state (Fig. 2A).
FIG. 2.
Loss of p21 results in deregulated cyclin E/Cdk2 kinase activity during MTI-induced endoreduplication. (A) Western analysis of pRb and cyclin E. Asynchronous cells were treated with nocodazole (83 nM) for the indicated times, and protein was harvested. (B) Cyclin E and Cdk2 kinase activity in HCT116 p21+/+ and p21−/− cells. Anti-cyclin E or anti-Cdk2 antibody was used to immunoprecipitate kinase complexes; GST-pRb was used as a substrate. Quantification of the autoradiogram signals is presented in the histograms. Results are representative of three independent experiments. (C) Whole-cell lysates from HCT116 p21+/+ cells were immunoprecipitated with anti-Cdk2, separated by SDS-PAGE, and analyzed for coimmunoprecipitation of p21 by Western blot analysis.
Another marker of cell cycle position is the pRb phosphorylation status. pRb phosphorylation is required for the G1-to-S-phase transition (18). After a 12-h nocodazole treatment of HCT116 p21+/+ and p21−/− cells, pRb remained in a slower-migrating or hyperphosphorylated form (Fig. 2A). However, between 24 and 72 h of drug treatment, there was a change in the pRb status in the HCT116 p21+/+ cells to faster-migrating, less phosphorylated forms of pRb, which have previously been shown to bind E2F and inhibit its activity (19, 47). The appearance of this hypophosphorylated pRb in HCT116 p21+/+ cells coincided with maintenance of 4N DNA content (compare Fig. 1A and 2A). In contrast, a relatively hyperphosphorylated or inactive form of pRb was observed in the HCT116 p21−/− cells 24 to 72 h after nocodazole treatment (Fig. 2A). This inactive form of pRb in HCT116 p21−/− cells coincided with the accumulation of polyploid cells (Fig. 1A).
Deregulated cyclin E/Cdk2 kinase activity during endoreduplication in HCT116 p21−/− cells.
Combined results of the flow-cytometric and Western blot analyses demonstrated that loss of p21 in the HCT116 cells led to endoreduplication following MTI treatment. We hypothesized that p21 prevented endoreduplication through binding and inhibition of the cyclin E/Cdk2 complex, thus preventing pRb phosphorylation and the subsequent G1-to-S-phase transition. Therefore, loss of p21 would lead to deregulated cyclin E/Cdk2 kinase activity and inappropriate S-phase entry. To test this hypothesis, cyclin E and Cdk2 kinase activities from control and nocodazole-treated HCT116 p21+/+ and p21−/− cells were measured. Cyclin E or Cdk2 was immunoprecipitated from the cells, and the kinase activity was measured by determining the ability of the immunoprecipitates to phosphorylate the GST-pRb substrate. In HCT116 p21+/+ cells, cyclin E kinase activity was inhibited to less than 30% of control levels after 24 to 72 h of nocodazole treatment whereas Cdk2 kinase activity was inhibited to less than 10% of control levels at the same time points (Fig. 2B). The inhibition of cyclin E and Cdk2 kinase activities paralleled the time-dependent decrease of pRb phosphorylation in the HCT116 p21+/+ cells (Fig. 2A).
After 12 h of nocodazole treatment in HCT116 p21−/− cells, cyclin E-dependent kinase activity was initially reduced to 80% of control levels, consistent with the mitotic arrest induced by nocodazole (Fig. 2B). After 24 h of nocodazole treatment, total Cdk2 kinase activity was transiently inhibited to less than 50% of control levels (Fig. 2B). However, at later times, both cyclin E-dependent and Cdk2 kinase activities increased, with the cyclin E-dependent activity staying at or above control levels after 24 h and the Cdk2 activity returning to approximately 80% of control activity at 72 h (Fig. 2B). This restimulation of cyclin E/Cdk2 activity paralleled the observed endoreduplication that began after 24 h of nocodazole treatment (Fig. 1A).
To determine if the decrease in Cdk2 kinase activity in nocodazole-treated HCT116 p21+/+ cells was due to p21 binding to the Cdk2 complex, Cdk2 immunoprecipitations (IP) were performed followed by Western analysis to evaluate coimmunoprecipitated p21. After nocodazole treatment, there was a time-dependent increase in the levels of p21 associated with the Cdk2 complex (Fig. 2C). The levels of p21 protein associated with Cdk2 inversely correlated with the observed changes in kinase activity (Fig. 2B and C). These data suggest that loss of p21 permitted deregulated Cdk2 activity in the HCT116 p21−/− cells.
Cdk4 and Cdk6 kinase activities are not deregulated during endoreduplication in HCT116 p21−/− cells.
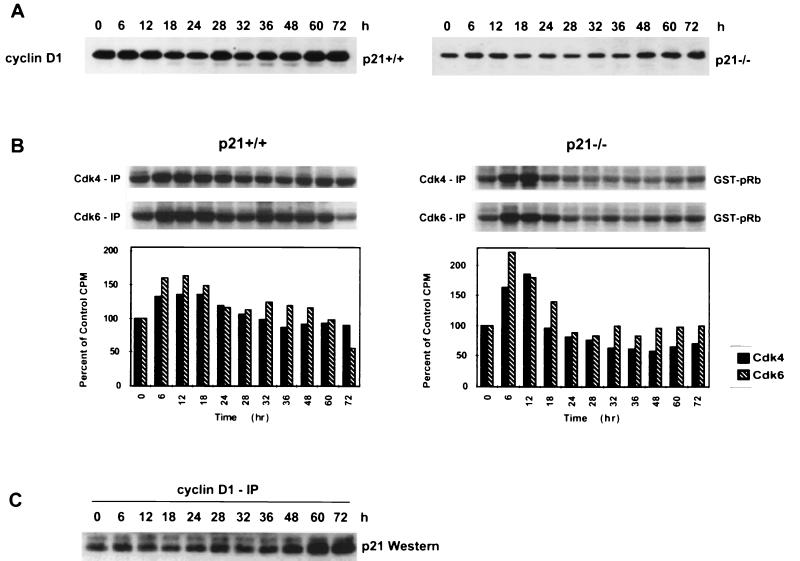
Because p21 can also bind to and inhibit cyclin D1/Cdk4 and cyclin D1/Cdk6 complexes during G1 progression, we evaluated the levels and activities of these proteins during nocodazole-induced endoreduplication. In both cell lines, Cdk4 and Cdk6 protein levels remained constant at all time points examined (data not shown). Both cell lines also had a small decrease in cyclin D1 protein levels at 18 h, consistent with the loss of G1 cells and accumulation of cells in G2/M. However, the cyclin D1 protein levels increased again in both cell lines by 32 h, consistent with the presence of cells in a G1-like biochemical state (Fig. 3A).
FIG. 3.
Cdk4 and Cdk6 kinases are not deregulated during endoreduplication in HCT116 p21−/− cells. (A) Western analysis of cyclin D1. Asynchronous cells were treated with nocodazole (83 nM) for the indicated times, and protein was harvested. (B) Cdk4 and Cdk6 kinase activity in HCT116 p21+/+ and p21−/− cells. Anti-Cdk4 or anti-Cdk6 antibody was used to immunoprecipitate kinase complexes; GST-pRb was used as a substrate. Quantification of the autoradiogram signals is presented in the histograms. Results are representative of three independent experiments. (C) Whole-cell lysates from HCT116 p21+/+ cells were immunoprecipitated with anti-cyclin D1, separated by SDS-PAGE, and analyzed for coimmunoprecipitation of p21 by Western blot analysis.
To determine if p21 also negatively regulated cyclin D1 kinase to prevent endoreduplication, Cdk4 and Cdk6 kinase activities from control and nocodazole-treated HCT116 p21+/+ and p21−/− cells were measured. Cdk4 or Cdk6 was immunoprecipitated from the cells, and kinase activity was measured by determining the ability of the immunoprecipitates to phosphorylate the GST-pRb substrate. In HCT116 p21+/+ cells, both Cdk4 and Cdk6 kinase activity initially increased between 6 and 18 h of nocodazole treatment and then remained near control levels at all time points after 24 h (Fig. 3B). Similarly, between 6 and 18 h of nocodazole treatment in HCT116 p21−/− cells, Cdk4 and Cdk6 kinase activity increased above control levels, but it remained at or below control levels after 18 h (Fig. 3B).
To determine if p21 exhibited increased binding to the cyclin D1 complexes during endoreduplication, cyclin D1 IP followed by Western analysis were performed to evaluate coimmunoprecipitated p21. The IP-Western analysis demonstrated that after nocodazole treatment the levels of p21 associated with the cyclin D1 complexes remained constant until 48 h, after which increased amounts of p21 were observed in the complex (Fig. 3C). Higher basal levels of p21 protein were associated with cyclin D1 complexes than those seen with cyclin E/Cdk2 complexes (compare Fig. 2C and 3C). In addition, the amount of p21 associated with cyclin D1 complexes did not correlate with the observed changes in kinase activity (Fig. 3B). Taken together, these data suggest that cyclin D1-associated kinase activities are not differentially regulated in the two cells lines after nocodazole treatment.
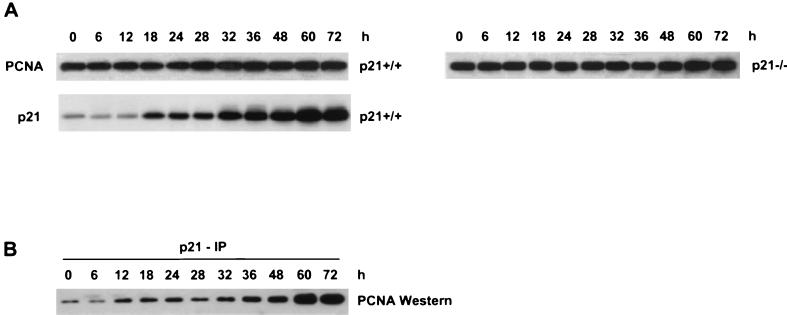
Increased p21 binding to PCNA coincides with inhibition of endoreduplication in HCT116 p21+/+ cells.
Previous studies have shown that p21 can bind PCNA and inhibit its ability to function in in vitro replication assays (55). To determine if the nocodazole-induced elevation in the p21 level led to increased p21-PCNA complex formation, both PCNA protein levels and the amount of PCNA associated with p21 were measured in HCT116 p21+/+ cell lysates. The levels of PCNA protein remained constant after nocodazole treatment in both HCT116 p21+/+ and p21−/− cells (Fig. 4A). p21 IP followed by Western analysis were performed to evaluate coimmunoprecipitated PCNA. IP-Western analysis demonstrated that after nocodazole treatment there was a time-dependent increase in the levels of p21 associated with PCNA (Fig. 4B). The increased levels of p21 associated with PCNA correlated with the inhibition of endoreduplication in the HCT116 p21+/+ cells, suggesting that p21 regulates both cyclin E/Cdk2 kinase activity and PCNA to prevent initiation of DNA synthesis in cells with a 4N DNA content.
FIG. 4.
Increased binding of p21 to PCNA after nocodazole treatment in HCT116 p21+/+ cells. (A) Western analysis of PCNA and p21. Asynchronous cells were treated with nocodazole (83 nM) for the indicated times, and protein was harvested. (B) Whole-cell lysates from HCT116 p21+/+ cells were immunoprecipitated with anti-p21, separated by SDS-PAGE, and analyzed for coimmunoprecipitation of PCNA by Western blot analysis.
p21 does not directly regulate cyclin B1/Cdc2 kinase activity during inhibition of endoreduplication in HCT116 p21+/+ cells.
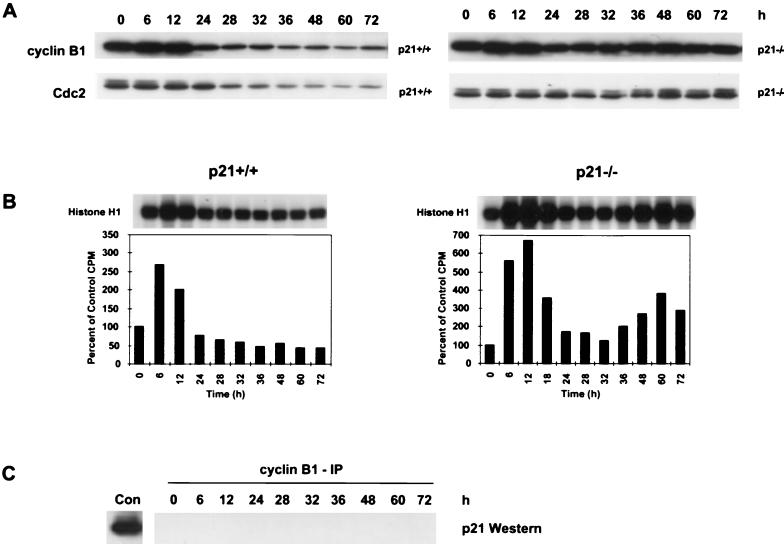
To determine if p21 was also regulating the G2/M transition, cyclin B1 and Cdc2 protein levels and function were evaluated following nocodazole treatment. The cyclin B1/Cdc2 kinase complex is a known regulator of the G2/M transition (35, 39). The exit from mitosis is marked by a decrease in the cyclin B1 protein levels and thus in Cdc2 kinase activity (43). After 12 h of nocodazole treatment, cyclin B1 protein levels in HCT116 p21+/+ cells decreased (Fig. 5A). The reduction in cyclin B1 protein levels in HCT116 p21+/+ cells was accompanied by a loss of Cdc2 phosphorylation and decrease in Cdc2 protein levels after 24 h (Fig. 5A). The cyclin B1 protein levels in nocodazole-treated HCT116 p21−/− cells showed only a transient decrease before they increased again as the cells endoreduplicated after 24 h (compare Fig. 5A with Fig. 1A). There was no significant change in the Cdc2 phosphorylation state or protein levels in nocodazole-treated HCT116 p21−/− cells, consistent with the continuous cyclin B1 expression (Fig. 5A).
FIG. 5.
Loss of p21 results in cyclical Cdc2 kinase activity during MTI-induced endoreduplication. (A) Western analysis of cyclin B1 and Cdc2 proteins. Asynchronous cells were treated with nocodazole (83 nM) for the indicated times, and protein was harvested. (B) Cyclin B1-associated kinase activity in HCT116 p21+/+ and p21−/− cells. Anti-cyclin B1 antibody was used to immunoprecipitate kinase complexes; histone H1 was used as a substrate. Quantification of the autoradiogram signals is presented in the histograms. Results are representative of three independent experiments. (C) Whole-cell lysates from HCT116 p21+/+ cells were immunoprecipitated with anti-cyclin B1, separated by SDS-PAGE, and analyzed for coimmunoprecipitation of p21 by Western blot analysis. Con, control representing p21 protein coimmunoprecipitated with anti-cyclin B1 from a whole-cell extract of WI-38 fibroblasts.
To evaluate cyclin B1-dependent Cdc2 kinase activity during nocodazole-induced endoreduplication, cyclin B1 was immunoprecipated from control and treated HCT116 p21+/+ and p21−/− cells. The associated kinase activity was measured by determining the ability of the cyclin B1 immunoprecipitates to phosphorylate histone H1 substrate. After 12 h of nocodazole treatment, the cyclin B1/Cdc2 kinase activity of HCT116 p21+/+ cells increased twofold over control levels as the cells arrested in mitosis (Fig. 5B). The kinase activity then decreased to 80% of control levels by 24 h, consistent with cells reentering a G1-like biochemical state. From 36 to 72 h, the cyclin B1/Cdc2 kinase activity was inhibited to approximately 50% of control levels (Fig. 5B). This reduction in cyclin B1/Cdc2 kinase activity was consistent with the observed decrease in the levels of cyclin B1 and Cdc2 proteins (Fig. 5A).
In HCT116 p21−/− cells, cyclin B1/Cdc2 kinase activity increased sixfold over control levels by 12 h, consistent with the mitotic arrest induced by nocodazole (Fig. 5B). However, following a transient decrease in activity between 24 and 32 h, the cyclin B1/Cdc2 kinase activity increased to twofold over control levels by 36 h and fourfold by 60 h (Fig. 5B). This restimulation of Cdc2 activity in HCT116 p21−/− cells was consistent with the endoreduplication that began after 24 h of nocodazole treatment (Fig. 1A).
To determine if the decrease in cyclin B1/Cdc2 activity in HCT116 p21+/+ cells was due to p21 binding to the cyclin B1/Cdc2 complex, IP-Western analysis was performed. Cyclin B1 was immunoprecipitated from nocodazole-treated cells, and the immunoprecipitates were analyzed by Western blotting for coimmunoprecipitated p21. p21 was not detected in the cyclin B1 immunoprecipitates at any of the time points examined (Fig. 5C), and it was not detected when the IP were performed with Cdc2 (data not shown). As a control to verify that the cyclin B1 antibody could coimmunoprecipitate p21, cyclin B1 IP were performed with protein lysates from WI-38 human lung fibroblasts and p21 was coimmunoprecipitated with cyclin B1/Cdc2 (Fig. 5C, Con). These data suggest that p21 does not regulate the mitotic arrest induced by MTIs through direct interaction with the cyclin B1/Cdc2 complex. Thus, the loss of cyclin B1/Cdc2 activity in HCT116 p21+/+ cells is an indirect effect of the G1/S arrest induced by p21 inhibition of the cyclin E/Cdk2 kinase complex.
p21 induction prevents endoreduplication in p53-deficient cells.
The data presented thus far suggest that p21 is sufficient to inhibit the endoreduplication induced by MTIs. However, H1299 cells that contain functional p21 but lack p53 protein still endoreduplicate in the presence of MTIs. We hypothesized that endoreduplication occurs in these p53-null cells because the levels of p21 are below a threshold required to negatively regulate cyclin E/Cdk2 activity. To test this hypothesis, we induced p21 expression above basal levels in p53-deficient cells. To elevate p21 protein levels, we generated an H1299 derivative cell line, containing an ecdysone-inducible p21 expression vector, called HIp21. After treatment of HIp21 cells with the ecdysone analog muristerone, p21 protein levels were induced in a dose-dependent manner, resulting in a G1 arrest after 24 h (data not shown).
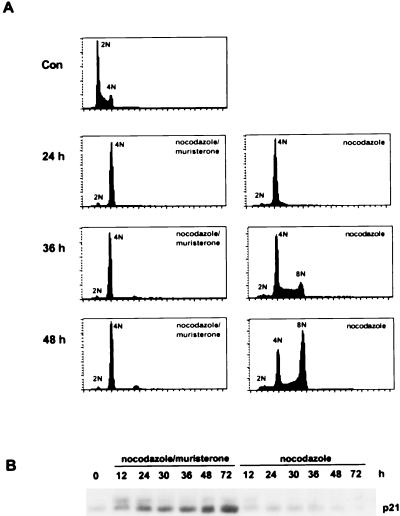
Noninduced HIp21 cells treated with nocodazole underwent endoreduplication, resulting in the accumulation of an 8N population by 36 h (Fig. 6A). Endogenous p21 protein was not induced by nocodazole treatment (Fig. 6B). In contrast, muristerone induction of p21 protein in HIp21 cells led to the maintenance of a 4N DNA content after nocodazole treatment (Fig. 6A). Western analysis showed time-dependent p21 induction by muristerone (Fig. 6B). These data indicate that expression of p21 over basal levels can rescue nocodazole-induced endoreduplication in p53-deficient cells.
FIG. 6.
p21 induction in p53-deficient cells prevents MTI-induced endoreduplication. (A) Asynchronous cultures of HIp21 cells were treated with nocodazole (83 nM) in the presence or absence of muristerone (7.5 μM). At the indicated times, cells were harvested and processed for flow-cytometric analysis; 15,000 events were analyzed for each sample. Data are representative of three independent experiments. DNA content is represented on the x axis; the number of cells counted is represented on the y axis. The subdiploid populations have been excluded; thus, the histograms for later time points represent fewer cells. (B) Western analysis of p21 protein from HIp21 cells treated with nocodazole (83 nM) in the presence or absence of muristerone (7.5 μM). Asynchronous cells were treated for the indicated times, and protein was harvested.
p21 induction in p53-deficient cells restores the regulation of cyclin E/Cdk2 kinase activity.
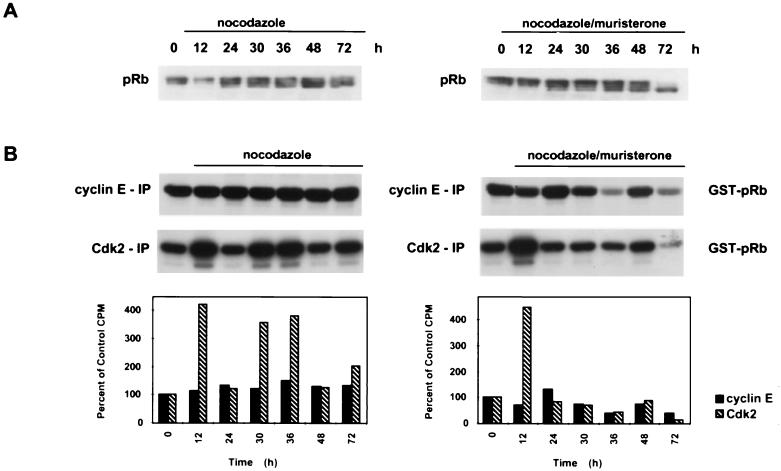
To determine if the HIp21 cells reentered a G1-like biochemical state prior to endoreduplication, levels of Cdk2, cyclin E, and pRb in control and nocodazole-treated cells were examined. In the presence and absence of muristerone, nocodazole-treated HIp21 cells had relatively constant Cdk2 and cyclin E protein levels (data not shown). However, in the muristerone-induced HIp21 cells, there was a change in the pRb phosphorylation state to a faster-migrating, hypophosphorylated form of the protein, most evident at 72 h (Fig. 7A). This hypophosphorylated form of pRb would probably be capable of binding E2F and blocking S-phase entry. In contrast, a relatively hyperphosphorylated form of pRb was observed in the noninduced HIp21 cells at all time points (Fig. 7A). This hyperphosphorylated pRb would probably be unable to bind E2F and repress S-phase progression, thus leading to the endoreduplication observed by 36 h (Fig. 6A).
FIG. 7.
p21 induction in p53-deficient cells during MTI-induced endoreduplication negatively regulates Cdk2 kinase activity. (A) Western analysis of pRb. Asynchronous cells were treated with nocodazole (83 nM) for the indicated times, and protein was harvested. (B) Cyclin E and Cdk2 kinase activity in the presence and absence of muristerone (7.5 μM) in nocodazole-treated HIp21 cells. Anti-cyclin E or anti-Cdk2 antibody was used to immunoprecipitate kinase complexes; GST-pRb was used as a substrate. Quantification of the autoradiogram signals is presented in the histograms. Results are representative of three independent experiments.
To determine if p21 induction restored the regulation of cyclin E/Cdk2 kinase activity, kinase assays were performed with cyclin E and Cdk2 immunoprecipitates from induced and noninduced HIp21 cells following nocodazole treatment. In the noninduced cells, cyclin E activity remained elevated at all time points, indicative of continuously cycling cells (Fig. 7B). In contrast, p21 induction resulted in reduced cyclin E kinase activity to below control levels after 24 h (Fig. 7B). In cells treated with nocodazole alone, there was an approximately fourfold increase in Cdk2 activity over control levels between 30 and 36 h, the time interval during which the cells were undergoing endoreduplication (Fig. 6A and 7B). However, induction of p21 resulted in continuous inhibition of Cdk2 activity and absence of endoreduplication after 24 h (Fig. 6A and 7B). These data parallel the cyclin E/Cdk2 kinase regulation observed in the nocodazole-treated HCT116 p21+/+ cells (Fig. 2B). Taken together, these data demonstrate that induction of p21 protein in a p53-deficient cell line was sufficient to restore the regulation of cyclin E/Cdk2 kinase activity to prevent endoreduplication.
p21 induction in p53-deficient cells results in decreased Cdc2 kinase activity.
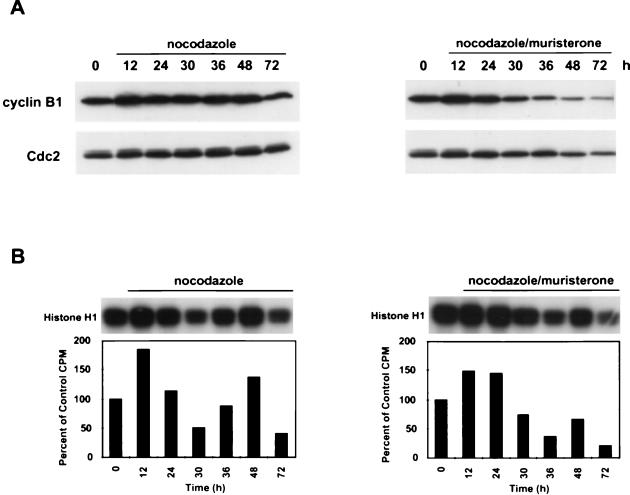
To determine if p21 induction in HIp21 cells altered cyclin B1 and Cdc2 protein levels and kinase activity, nocodazole-treated HIp21 cells were evaluated in the presence and absence of muristerone induction. Noninduced nocodazole-treated HIp21 cells had relatively constant levels of both cyclin B1 and Cdc2 proteins (Fig. 8A). After 12 h of nocodazole treatment, there was an initial twofold increase in cyclin B1/Cdc2 kinase activity, consistent with the transient mitotic arrest induced by nocodazole (Fig. 8B). After 24 h, the cyclin B1/Cdc2 kinase activity became cyclical, peaking again at 48 h with a 1.5-fold increase in activity over control levels. This cyclical pattern of cyclin B1/Cdc2 kinase activity paralleled the continued cell cycle movement (Fig. 6A).
FIG. 8.
p21 induction in p53-deficient cells during MTI-induced endoreduplication reduces cyclin B1/Cdc2 kinase activity. (A) Western analysis of cyclin B1 and Cdc2. Asynchronous cells were treated with nocodazole (83 nM) for the indicated times, and protein was harvested. (B) Cyclin B1-associated kinase activity in the presence and absence of muristerone (7.5 μM) in nocodazole-treated HIp21 cells. Anti-cyclin B1 antibody was used to immunoprecipitate kinase complexes; histone H1 was used as a substrate. Quantification of the autoradiogram signals is presented in the histograms. Results are representative of three independent experiments.
Analysis of muristerone- and nocodazole-cotreated HIp21 cells showed that induction of p21 protein led to a substantial decrease in cyclin B1 and Cdc2 protein levels and cyclin B1/Cdc2 kinase activity after 24 h (Fig. 8A and B). Initially, a 1.5-fold increase in cyclin B1/Cdc2 kinase activity was observed between 12 and 24 h after nocodazole treatment, again consistent with the transient mitotic arrest observed (Fig. 6A). However, by 36 h, the cyclin B1/Cdc2 kinase activity decreased to approximately 35% of control levels, and it failed to increase above control levels at subsequent time points.
DISCUSSION
Aneuploidy and loss of cell cycle checkpoint control are hallmarks of human tumor cells (15, 16). Previous studies have reported that the loss of cell cycle checkpoint proteins can result in MTI-induced endoreduplication. Cross et al. showed that p53 loss led to MTI-induced endoreduplication (6), and subsequent studies confirmed this initial observation (7, 25). Chen et al. reported that p21 overexpression in a p53-defective human astrocytoma cell line reduced polyploidy and rescued the malignant phenotype (4). Recent studies demonstrated that p21−/− MEFs (25, 27) and pRb-deficient cells (7, 25) endoreduplicated in the presence of MTIs. Our results significantly extend these findings by demonstrating that p21 inhibition of MTI-induced endoreduplication is dependent on p21-mediated temporal regulation of cyclin E/Cdk2 activity and increased PCNA-p21 complex formation. In addition, our data demonstrate that induction of p21 protein in a p53-deficient cell line is sufficient to prevent MTI-induced endoreduplication.
In HCT116 p21−/− cells, endoreduplication was accompanied by continuous cyclin E/Cdk2 activity and pRb hyperphosphorylation. In contrast, MTI-treated HCT116 p21+/+ cells were characterized by coassociation of p21 with Cdk2 and PCNA, decreased Cdk2 activity, hypophosphorylated pRb, and maintenance of 4N DNA content. The kinetics of p21 binding to Cdk2 and PCNA in HCT116 p21+/+ cells paralleled the onset of endoreduplication in HCT116 p21−/− cells. The observed increase of p21-PCNA complex formation in cells in which endoreduplication was inhibited was consistent with results of previous studies showing p21 inhibition of PCNA-dependent processive DNA synthesis in vitro (55).
We observed differential regulation of cyclin B1/Cdc2 kinase activity in the HCT116 cell lines; however, we were unable to detect p21 association with this kinase complex. These results suggest that the p21-mediated regulation of Cdc2 activity was indirect and potentially due to a cell cycle-dependent change in cyclin B1 availability. Previous studies have shown that p21 is not associated with cyclin B1/Cdc2 complexes in transformed cells and p53-deficient cells from patients with Li-Fraumeni syndrome (62).
Our results indicate that p21 is sufficient to inhibit MTI-induced endoreduplication. However, HIp21 cells that contain functional p21 but lack p53 still endoreduplicate in the presence of MTIs. Inhibition of endoreduplication in these cells, through induction of exogenous p21, indicates that p53-null cells endoreduplicate because the basal levels of p21 are insufficient to inhibit cyclin E/Cdk2 kinase activity and prevent pRb phosphorylation. Based upon these findings, we propose that p21 is necessary to properly regulate cyclin E/Cdk2 kinase activity and prevent uncoupling of mitosis and S-phase pathways. However, the inhibitory effects of p21 depend on the integrity of downstream targets. Clearly, if p21 is induced in pRb-deficient cells treated with MTIs, cells will still be able to enter S phase; this was confirmed by a recent study in which endoreduplication occurred despite overexpression of p21 in pRb-deficient cells (38).
Our data support the model that a temporal order of events must occur to maintain the normal diploid state in cells treated with MTIs. First, the inhibition of microtubule dynamics during mitosis engages the spindle checkpoint. After this initial mitotic arrest induced by MTIs, cells biochemically exit mitosis, as evidenced by the decrease of both cyclin B1 protein and cyclin B1/Cdc2 kinase activity. After exiting mitosis with a 4N DNA content, cells reenter a G1 biochemical state with the accumulation of G1 cyclins. The entry of cells with a 4N DNA content into G1 results in activation of p53 and its downstream target p21, through an as yet undetermined mechanism. If this set of events occurs in cells containing p53, p21 is induced and a G1/S arrest results from p21 binding and inhibition of cyclin E/Cdk2 and PCNA. This negative regulation of cyclin E/Cdk2 results in hypophosphorylation of Rb, repression of E2F-mediated transcription, and lack of S-phase progression. Furthermore, downstream Cdk activities will also be affected. This was evident from our cyclin B1/Cdc2 activity data, which demonstrated loss of this G2/M kinase activity only in p21-containing cells. Taken together, the results suggest an ordered biochemical pathway in which p21 plays a pivotal role in preventing endoreduplication after aberrant mitotic exit of cells with a ≥4N DNA content.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health institutional training grant GM07347 (to Z.A.S.), American Cancer Society Award 96-46 (to S.D.L.), National Institutes of Health grants CA70856 (to J.A.P.) and ES00267 and CA68485 (Core Services), and a Burroughs Wellcome Fund Grant (to J.A.P.).
We thank Liying Yang for expert technical assistance in generation of the HIp21 cell line and Scott Hiebert, Hal Moses, and members of the Pietenpol laboratory for critical reading of the manuscript.
REFERENCES
- 1.Arellano M, Moreno S. Regulation of CDK/cyclin complexes during the cell cycle. Int J Biochem Cell Biol. 1997;29:559–573. doi: 10.1016/s1357-2725(96)00178-1. [DOI] [PubMed] [Google Scholar]
- 2.Chellappan S P, Hiebert S, Mudryj M, Horowitz J M, Nevins J R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Jackson P K, Kirschner M W, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Willingham T, Shuford M, Bruce D, Rushing E, Smith Y, Nisen P D. Effects of ectopic overexpression of p21WAF1/CIP1 on aneuploidy and the malignant phenotype of human brain tumor cells. Oncogene. 1996;13:1395–1403. [PubMed] [Google Scholar]
- 5.Cohen-Fix O, Koshland D. The metaphase-to-ananphase transition: avoiding a mid-life crisis. Curr Opin Cell Biol. 1997;9:800–806. doi: 10.1016/s0955-0674(97)80080-4. [DOI] [PubMed] [Google Scholar]
- 6.Cross S M, Sanchez C A, Morgan C A, Schimke M K, Ramel S, Idzerda R L, Raskind W H, Reid B J. A p53-dependent mouse spindle checkpoint. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- 7.Di Leonardo A, Khan S H, Linke S P, Greco V, Seidita G, Wahl G M. DNA rereplication in the presence of mitotic spindle inhibitors in human and mouse fibroblasts lacking either p53 or pRb function. Cancer Res. 1997;57:1013–1019. [PubMed] [Google Scholar]
- 8.Dulic V, Lees E, Reed S I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 9.El-Deiry W S, Harper J W, O’Connor P M, Velculescu V E, Canman C E, Jackman J, Pietenpol J A, Burrell M, Hill D E, Wang Y, Wiman K G, Mercer W E, Kastan M B, Kohn K W, Elledge S J, Kinzler K W, Vogelstein B. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 10.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 11.Gorbsky G J. Cell cycle checkpoints: arresting progress in mitosis. Bioessays. 1997;19:193–197. doi: 10.1002/bies.950190303. [DOI] [PubMed] [Google Scholar]
- 12.Gu Y, Turck C W, Morgan D O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- 13.Hannon G J, Beach D. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 14.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 15.Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992;71:543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- 16.Hartwell L H, Kastan M B. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 17.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 18.Helin K, Harlow E. The retinoblastoma protein as a transcriptional repressor. Trends Cell Biol. 1993;3:43–46. doi: 10.1016/0962-8924(93)90150-y. [DOI] [PubMed] [Google Scholar]
- 19.Hiebert S W, Chellappan S, Horowitz J M, Nevins J R. The interaction of RB with E2F coincides with inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- 20.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 21.Hirai H, Roussel M F, Kato J-Y, Ashmun R A, Sherr C J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyt M A, Totis L, Roberts B T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 23.Hunter T, Pines J. Cyclins and cancer II: cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 24.Kamb A, Gruis N A, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian S V, Stockert E, Day R S, III, Johnson B E, Skolnick M H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- 25.Khan S H, Wahl G M. p53 and pRb prevent rereplication in response to microtubule inhibitors by mediating a reversible G1 arrest. Cancer Res. 1998;58:396–401. [PubMed] [Google Scholar]
- 26.Koff A, Giordano A, Desai D, Yamashita K, Harper J W, Elledge S, Nishimoto T, Morgan D O, Franza B R, Roberts J M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- 27.Lanni J S, Jacks T S. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee M-H, Reynisdóttir I, Massagué J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Jenkins C W, Nichols M A, Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene. 1994;9:2261–2268. [PubMed] [Google Scholar]
- 30.Luo Y, Hurwitz J, Massagué J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995;375:159–161. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka S, Edwards M C, Bai C, Parker S, Zhang P, Baldini A, Harper J W, Elledge S J. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- 32.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minn A J, Boise L H, Thompson C B. Expression of Bcl-xL and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 1996;10:2621–2631. doi: 10.1101/gad.10.20.2621. [DOI] [PubMed] [Google Scholar]
- 34.Mitsudomi T, Steinberg S M, Nau M M, Carbone D, D’Amico D, Bodner S, Oie H K, Linnoila R I, Mulshine J L, Minna J D, Gazdar A F. p53 gene mutations in non-small-cell lung cancer cell lines and their correlation with the presence of ras mutations and clinical features. Oncogene. 1992;7:171–180. [PubMed] [Google Scholar]
- 35.Moreno S, Hayles J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- 36.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 37.Myöhänen S K, Baylin S B, Herman J G. Hypermethylation can selectively silence individual p16ink4A alleles in neoplasia. Cancer Res. 1998;58:591–593. [PubMed] [Google Scholar]
- 38.Niculescu A B, III, Chen X B, Smeets M, Hengst L, Prives C, Reed S I. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 40.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulovich A G, Toczyski D P, Hartwell L H. When checkpoints fail. Cell. 1997;88:315–321. doi: 10.1016/s0092-8674(00)81870-x. [DOI] [PubMed] [Google Scholar]
- 42.Pellegata N S, Antoniono R J, Redpath J L, Stanbridge E J. DNA damage and p53-mediated cell cycle arrest: a reevaluation. Proc Natl Acad Sci USA. 1996;93:15209–15214. doi: 10.1073/pnas.93.26.15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pines J, Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- 44.Polyak K, Kato J, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 45.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 46.Reed S I. The role of p34 kinases in the G1 to S-phase transition. Annu Rev Cell Biol. 1992;8:529–561. doi: 10.1146/annurev.cb.08.110192.002525. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz J K, Devoto S H, Smith E J, Chellappan S P, Jakoi L, Nevins J R. Interactions of the p107 and Rb proteins with E2F during the cell proliferation response. EMBO J. 1993;12:1013–1020. doi: 10.1002/j.1460-2075.1993.tb05742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serrano M, Hannon G J, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 49.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 50.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 51.Solomon M J, Harper J W, Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993;12:3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorger P K, Dobles M, Tournebize R, Hyman A A. Coupling cell division and cell death to microtubule dynamics. Curr Opin Cell Biol. 1997;9:807–814. doi: 10.1016/s0955-0674(97)80081-6. [DOI] [PubMed] [Google Scholar]
- 53.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 54.Tsai L, Lees E, Faha B, Harlow E, Riabowol K. The cdk2 kinase is required for the G1-to-S-phase transition in mammalian cells. Oncogene. 1993;8:1593–1602. [PubMed] [Google Scholar]
- 55.Waga S, Hannon G J, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 56.Waldmann T, Kinzler K W, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5195. [PubMed] [Google Scholar]
- 57.Wang J Y. Retinoblastoma protein in growth suppression and death protection. Curr Opin Genet Dev. 1997;7:39–45. doi: 10.1016/s0959-437x(97)80107-4. [DOI] [PubMed] [Google Scholar]
- 58.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 59.Xiong Y. Why are there so many CDK inhibitors? Biochim Biophys Acta Rev Cancer. 1996;1288:1–5. doi: 10.1016/0304-419x(96)00012-1. [DOI] [PubMed] [Google Scholar]
- 60.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 61.Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- 62.Xiong Y, Zhang H, Beach D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993;7:1572–1583. doi: 10.1101/gad.7.8.1572. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Xiong Y, Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993;4:897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]