Abstract
Tyro3 is a member of the TAM subfamily of receptor tyrosine kinases alongside Axl and MerTK, which are activated by homologous ligands Gas6 and protein S. The TAMs activate signalling pathways that mediate diverse functions including cell survival, proliferation, phagocytosis and immune regulation, and defects in TAM-dependent processes are associated with the development of human autoimmune diseases and numerous cancers. In this study, we have focused on the signalling and functional roles of Tyro3, about which much remains unknown. For this purpose, we used cultured human cancer cell lines with different levels of TAM expression to reveal the relative significance of Tyro3 amongst the TAMs. Knockdown of Tyro3 expression by siRNA in MGH-U3 cells, which express Tyro3 as sole TAM, caused a reduction in cell viability, which could not be rescued by TAM ligand, demonstrating the dependence of these cells solely on Tyro3. In contrast, the reduced viability of SCC-25 cells upon Tyro3 knockdown could be rescued by Gas6 as these cells express both Tyro3 and Axl and hence Axl expression was preserved. An increase in the fraction of Tyro3 knockdown cells in the early apoptotic phase was observed in four different cell lines each with a different TAM expression profile, revealing a broad anti-apoptotic function of Tyro3. Furthermore, in the Tyro3-dependent cells, Tyro3 depletion caused a significant increase in cells in the G0/G1 phase of the cell cycle concomitant with decreases in the G2/M and S phases. In addition, a cancer pathway gene discovery array revealed distinct sets of genes that were altered in expression in cancer cells upon Tyro3 knockdown. Together, these results have elucidated further a role of Tyro3 in promoting multiple tumour-supporting pathways in human cancer cells, which differs in extent depending on the presence of other TAMs in the same cells.
Keywords: Tyro3, TAM receptor, Apoptosis, Cell cycle, Signal transduction, siRNA knockdown
Highlights
-
•
Knockdown of Tyro3 expression in human cancer cells reduced cell viability.
-
•
Reduced viability by Tyro3 knockdown can be rescued by Gas6 in cells also expressing Axl.
-
•
Tyro3 knockdown increased the fraction of cancer cells undergoing early apoptosis.
-
•
Tyro3 depletion shifted cells from G2/M and S phases of the cell cycle to G0/G1 phase.
-
•
Ligand-independent Tyro3 regulates cancer-related gene sets to promote tumorigenesis.
1. Introduction
The TAM receptor tyrosine kinases (RTKs), comprising Tyro3, Axl and MerTK, share considerable sequence identity, protein structural homology as well as common activating ligands Gas6 and protein S (ProS1) [1]. TAM RTKs are key negative regulators of innate immunity and inflammation, acting through limiting as well as resolving the immune response. They also mediate efferocytosis of apoptotic cells and debris, which helps prevent continuous inflammation as well as aiding tissue repair [2]. Both Axl and MerTK are normally expressed primarily by myeloid cells of the immune system such as macrophages, microglia and dendritic cells, whereas Tyro3 does not share this profile. Axl is also expressed in a wide variety of cell types and tissues, underlying its important role in mediating cell survival through Gas6/Axl signalling [1]. Furthermore, aberrant or ectopic expression of Axl occurs in a wide variety of human cancers and is associated with Axl’s well established role in promoting tumourigenesis [3]. However, relative to the other TAMs, the functional role of Tyro3 is not well defined. While it has a more restricted normal tissue expression profile, its oncogenic potential is evident through its aberrant expression in several cancers, including hepatocellular carcinoma [4], primary teratocarcinoma [5], chronic myelogenous leukaemia [6], ovarian cancer [7], colon cancer [8], melanoma [9], thyroid [10], and schwannoma [11]. Recently, we reported that ProS1 stimulates a Tyro3-ERK signalling pathway axis within cancer cells, whereas the other TAM ligand Gas6 was a weaker Tyro3 ligand but instead activated an Axl-Akt signalling axis within the same cells [12]. Furthermore, Tyro3 stimulation was able to additionally couple to Akt signalling in cancer cells which lacked the other two TAM receptors [12,13]. We also identified galectin-3 as an additional Tyro3 ligand, also activating ERK signalling via this receptor [13]. These studies showed that Tyro3 can regulate both cancer cell proliferation and survival in cells that express Tyro3 only, whilst its role appears to be more restricted to ERK signalling in cells where Axl is also present, which is the principal TAM receptor coupled to Akt-mediated survival and migratory signalling.
Further studies determined the impact of Tyro3 inhibition in different types of tumours, which can support the important role of Tyro3 in cancer cell survival and proliferation. Inhibition of Tyro3 by siRNA or shRNA decreased proliferation/growth of colorectal cancer [14], hepatocellular carcinoma [4], melanoma [15], and breast [16,17] and ovarian cancers [18]. Silencing of Tyro3 expression using siRNA in hepatocellular carcinoma cells was associated with decreased expression of cyclin D1, a cell cycle regulatory protein that promotes G1/S phase progression [4,19]. Studies using siRNA to suppress Tyro3 expression showed reduced migration and invasion in hepatocellular carcinoma cells [20]. Also, SNA1, a key regulator of epithelial-mesenchymal transition (EMT), was shown to be up- and downregulated by the overexpression and knockdown of Tyro3, respectively, in colon and pancreatic cancer cells [8,21], and it was shown that SNA1 mediated the Tyro3-induced EMT [8].
In contrast to ligand-stimulated signalling, RTKs may also be functional and signal through ligand-independent mechanisms, such as has been observed for Tyro3 autophosphorylation activity when overexpressed in cells [22,23]. Therefore, in the present study, we have extended our previous investigations on Tyro3 signalling activated by exogenous ligands to studying here basal Tyro3 signalling and functional role in human cancer cells. Here, we show that Tyro3 signalling is associated with cell cycle progression and anti-apoptosis via MAPK/ERK and PIK3/Akt signalling pathways. Also, a recent study has shown that Tyro3 is upregulated in a range of bladder carcinomas and that the majority of a panel of bladder cancer cell lines showed strong expression of Tyro3 relative to the other TAM RTKs [24]. Tyro3 siRNA knockdown in several of those cell lines inhibited experimental tumorigenesis in vitro and in vivo. As one of those cell lines, MGH-U3, was shown to exclusively express Tyro3 [12], we have therefore used this cell line to advantage for studying exclusively Tyro3 function in the absence of any hetero-interaction with the other TAMs. In addition, we also selected other cancer cell lines for the specific purpose of studying Tyro3 function when expressed together with other TAMs.
2. Results
2.1. Transient knockdown of Tyro3 in cancer cells
Four cancer cell lines were selected for this study: MGH-U3 which expresses Tyro3 as sole TAM, SCC-25 cells which express Axl and Tyro3 but not MerTK, 786-0 cells which express all three TAMs and WM9 cells which showed the highest expression of Tyro3 [12]. To determine whether depletion of Tyro3 expression in cancer cell influences their functions and signalling, Tyro3 knockdown was performed using three different small interfering (si) RNAs (Supplementary Table 1). Cells were transfected with Tyro3 siRNA (siTyro3) constructs whilst control transfections used a pool of non-targeting siRNAs at the same concentration (siControl). Using the cell line 786-0 as it expresses all three TAM receptors, western blotting was performed to verify the specificity of Tyro3 knockdown by the different siRNAs as compared to levels of the other TAMs as well as other RTKs. At the concentration tested, all three Tyro3 siRNA constructs caused a minimum 75% knockdown of Tyro3 protein with no effect on levels of Axl, MerTK and EGFR proteins (Supplementary Fig. 1), with the exception of siTyro3 #1, which also caused partial depletion of Axl protein; therefore, this particular construct was left out of subsequent experiments.
In order to establish the optimal Tyro3 siRNA knockdown conditions for subsequent experiments, a time-course of incubation experiment was performed combined with testing different concentrations of siRNA. A clear siRNA concentration dependence of Tyro3 knockdown was observed in SCC-25 (express Axl and Tyro3) and MGH-U3 (express Tyro3 only) cells, with roughly 50% protein knockdown at the lowest concentration (10 nM) and up to 90% knockdown at the highest concentration (50 nM) (Supplementary Fig. 2). Furthermore, equal levels of knockdown were already achieved after 48h with no further effects after 72h. According to these observations, siTyro3 #2 at 50 nM with 48h incubation was selected as optimal for use in subsequent experiments to investigate the influence of Tyro3 knockdown on cell functions, signalling pathways and gene programmes.
2.2. Tyro3 knockdown inhibits cell viability and induces apoptosis
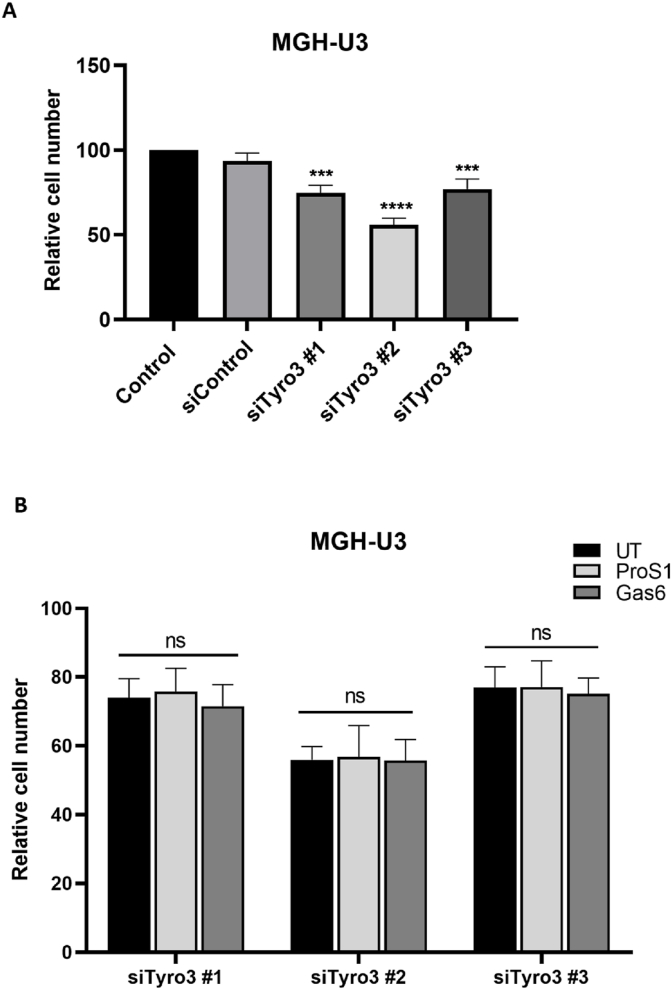
All three Tyro3 siRNAs caused a significant decrease in cell viability in MGH-U3 cells over 48h, with siTyro3 #2 having the greatest effect (Fig. 1A). Knockdown was also performed on MGH-U3 cells with ProS1 and Gas6 added for the last 24h of incubation. In these cells, we have shown that both TAM ligands stimulated cell survival and activated Tyro3 and Akt signalling [12,13]. In contrast to when Tyro3 is normally expressed in MGH-U3 cells, neither ligand significantly enhanced cell viability when Tyro3 was knocked down (Fig. 1B).
Fig. 1.
The effect of siRNA knockdown of Tyro3 on MGH-U3 cell viability. (A) MTS assay of cell viability after 48h incubation with control and three Tyro3 siRNAs. (B) MTS assay of cell viability after 48h incubation with control siRNA and three different Tyro3 siRNAs, in the absence (UT) or presence of ProS1 (7.5 nM) or Gas6 (5.7 nM). Data are mean ± SEM; one-way ANOVA with Tukey multiple comparisons post-hoc analysis; ***p<0.001 vs siControl; ns, not significant for comparisons indicated by lines. (n = 3 separate experiments).
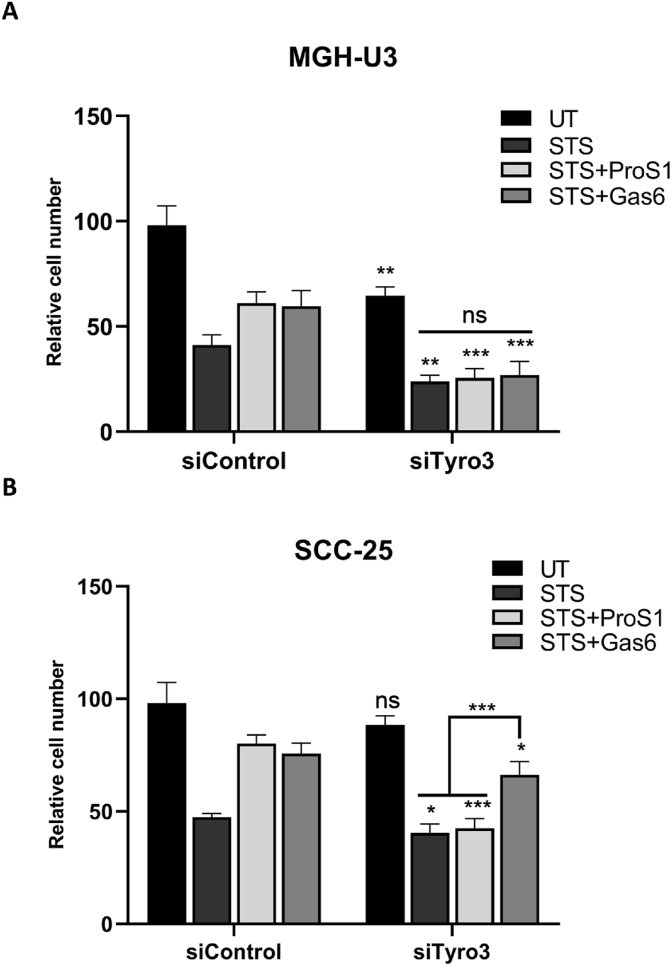
We have previously reported that both ProS1 and Gas6 significantly protected cells from apoptosis induced by staurosporine in both MGH-U3 and SCC-25 cancer cell lines [12]. Here, the baseline function of Tyro3 without added exogenous ligand was investigated in the same model through siRNA knockdown. Of the two cell lines, MGH-U3 cells showed a significantly decreased survival upon Tyro3 knockdown, which reflects the greater role of Tyro3 in these cells as compared to SCC-25 cells, which can also rely on Axl signalling. However, both cell lines showed significantly further reduced survival upon Tyro3 depletion when undergoing apoptosis by staurosporine (Fig. 2). Moreover, the presence of either TAM ligand had no effect on the degree of staurosporine-induced apoptosis in MGH-U3 cells with Tyro3 depleted. In contrast, Gas6 significantly protected SCC-25 cells undergoing staurosporine-induced apoptosis with Tyro3 depleted whereas ProS1 failed to do so (Fig. 2).
Fig. 2.
The effect of Tyro3 knockdown on cancer cell survival following staurosporine-induced apoptosis, and impact of added TAM ligand. MTS assay of cell viability of MGH-U3 (A) and SCC-25 (B). Cells were transfected with control siRNA (siControl) or Tyro3 siRNA #2 (siTyro3) for 48 h at 50 nM. Cells were then treated with staurosporine (STS; 0.1 μM) in the presence of ProS1 (7.5 nM) or Gas6 (5.7 nM) for the last 20 h. Data are mean ± SEM; Student's two-tailed t-test; ***p<0.001, **p<0.01, *p<0.05 and ns, not significant vs respective siControl for each treatment or as indicated by lines (n = 3 separate experiments).
The results showed that Tyro3 activates anti-apoptotic signalling and promotes cell survival both at basal levels of activity as well as, in addition, with its activity enhanced by added ligand stimulation. Furthermore, these experiments have distinguished between the respective roles of ProS1 and Gas6 via the Tyro3 receptor, revealing the additional ability of Gas6 to act via Axl activation. Analysis of apoptotic cell populations by flow cytometry also revealed that Tyro3-depleted cells were less viable as a general phenomenon. An increase in the percentage of Tyro3 knockdown cells in the early apoptotic phase was observed in four different cell lines (MGH-U3, SCC-25, 786-0, WM9), each of which has a different TAM expression profile. In all cell lines, knockdown of baseline Tyro3 increased the apoptotic cell fraction (MGH-U3: control siRNA 10%, Tyro3 knockdown 28%), as well as also further increasing the fraction after staurosporine-induced apoptosis (MGH-U3: control siRNA 56%, Tyro3 knockdown 82%), with the exception of SCC-25 cells (Fig. 3). As in previous experiments, SCC-25 presented as the cell line that was least dependent on Tyro3 for survival, although nevertheless, the more sensitive FACS-based apoptosis assay employed here did reveal a Tyro3 anti-apoptotic function in these cells too.
Fig. 3.
Flow cytometry assay of staurosporine-induced apoptosis following Tyro3 knockdown – comparison amongst four cancer cell lines. Representative dot plot diagrams obtained by flow cytometry with Annexin V-FITC/PI double-staining of MGH-U3, SCC-25, WM9 and 786-0 cells after incubation with Tyro3 (siTyro3) and control (siControl) siRNAs at 50 nM for 48h, and then treatment with STS 0.1 μM for 24h. Lower left quadrant A-/PI- corresponds to living cells, lower right quadrant A+/PI- corresponds to early apoptotic cells, upper right quadrant A+/PI + corresponds to late apoptotic cells and upper left quadrant A-/PI + corresponds to necrotic cells. Graphs below show proportions (%) of healthy, apoptotic and necrotic cells. Percentages are presented as the mean ± SEM (n = 3 experiments); p values were calculated using Student's two-tailed t-test; **p<0.01, *p<0.05, ns, not significant vs respective siControl under the same treatment as indicated by lines.
Apoptosis assays by flow cytometry were also performed following Tyro3 siRNA knockdown in cells in the presence or absence of ligands. In MGH-U3 cells, Tyro3 knockdown caused an increased proportion of cells to undergo apoptosis (28%) versus control siRNA-transfected cells (8%), as measured through Annexin V-FITC/PI staining (Supplementary Fig. 3). Furthermore, neither Gas6 nor ProS1 had any influence on the increased apoptotic cell fraction upon Tyro3 knockdown, whereas they did decrease this fraction in control knockdown cells, although weakly. However, in SCC-25 cells, Tyro3 knockdown did not significantly alter the relative cell populations nor did TAM ligands have any effects (Supplementary Fig. 3); this is likely due to a lesser impact of Tyro3 depletion in the presence of serum as measured by the FACS assay. Nevertheless, Fig. 2 and our previous report [12] have shown dependence of the SCC-25 cells on Gas6-Axl signalling.
2.3. Tyro3 knockdown induces cell cycle arrest
In three cancer cell lines tested, Tyro3 knockdown altered the relative proportions of cells in the respective phases of the cell cycle, G0/G1, S and G2/M, but with differences (Fig. 4). In MGH-U3 cells, Tyro3 knockdown caused a significant increase in cells in the G0/G1 phase concomitant with a significant decrease in cells in the G2/M phase. The same effect was observed in WM9 cells, with additionally a reduced proportion in the S phase. By contrast, in SCC-25 cells, Tyro3 knockdown caused a significant increase in cells in the S phase. The 786-0 cell line was not studied here as the data from Fig. 3 showed that this cell line was barely affected by Tyro3 depletion, suggesting redundancy for cell survival signalling amongst potentially others through its expression of all three TAMs.
Fig. 4.
Cell cycle distribution analysis by flow cytometry in cancer cells following Tyro3 knockdown. MGH-U3 (A), SCC-25 (B) and WM9 (C) cells were treated for 48h with Tyro3 and control siRNAs. Cells were harvested and fixed and stained with propidium iodide, and their DNA contents analysed by flow cytometry. The result of one representative assay from three similar independent experiments is shown. x- and y-axes denote DNA content and cell number, respectively. Each phase was calculated by using FlowJo software. The percentages of cells in G0/G1, S, and G2/M are also shown in graphs below, presented as the mean ± SEM (n = 3 experiments); Student's two-tailed t-test **p<0.01, *p<0.05 and ns, not significant for pairwise comparisons as indicated by lines.
2.4. Intracellular signalling pathway discovery analysis following Tyro3 knockdown in human cancer cells
A qPCR array was employed to identify signalling pathway genes in cancer cells affected by Tyro3 depletion. MGH-U3 cells were transfected with siTyro3 #2 and non-targeting siRNA (siControl) for 48h at 50 nM. From each of these two samples, 90 genes (Supplementary Table S4) were analysed in the RT [2] Profiler™ PCR Array Human Cancer Pathway Finder array to identify pathways activated via the Tyro3 receptor in the absence of any influence by both Axl and MerTK. The results showed that Tyro3 depletion caused downregulation of MAPK pathway (MAP2K1, MAP2K3, MAPK14) and CCND2 genes, which are involved in promoting proliferation and the cell cycle respectively. Conversely, Tyro3 knockdown resulted in upregulation of genes that are involved in promoting apoptosis including APAF1, CASP9 and BCL2L11, as well as downregulation of anti-apoptotic gene BIRC3 (Table 1 and Fig. 5A). Furthermore, other novel genes that were altered in expression upon Tyro3 depletion in MGH-U3 cells were identified by the array and grouped according to functions with which they are associated (Fig. 5B).
Table 1.
Genes which are upregulated (denoted by a + sign), or downregulated (denoted by a - sign) in MGH-U3 cells transfected with Tyro3 siRNA.
| Gene symbol | Fold change | The protein encoded by the gene |
|---|---|---|
| BCL2L11 | +9.89 | BCL2-like 11 |
| CASP9 | +4.16 | Caspase 9, apoptosis-related cysteine peptidase |
| APAF1 | +2.28 | Apoptotic peptidase activating factor 1 |
| ERCC5 | +2.42 | Excision repair cross-complementing rodent repair deficiency, complementation group 5 |
| LIG4 | +7.09 | Ligase IV, DNA, ATP-dependent |
| OCLN | +7.65 | Occludin |
| MAPK14 | −3.74 | Mitogen-activated protein kinase 14 |
| MAP2K1 | −2.66 | Mitogen-activated protein kinase kinase 1 |
| MAP2K3 | −3.21 | Mitogen-activated protein kinase kinase 3 |
| CCND2 | −2.72 | Cyclin D2 |
| BIRC3 | −5.68 | Baculoviral IAP repeat-containing 3 |
| SNAI2 | −2.08 | Snail homolog 2 |
| TBX2 | −3.49 | T-box 2 |
Fig. 5.
Effects Tyro3 knockdown on the expression of cancer signalling pathway genes in cancer cells. MGH-U3 cells transfected with siTyro3 #2 (siTyro3) compared with non-targeting siRNA (siConrtol) for 48h at 50 nM. Gene expression was measured using the Qiagen RT [2] Profiler PCR array. Data are shown as a fold-change expression of genes in cells following siTyro3 vs siControl treatment in A. Cancer pathway genes that are unique and regulated by Tyro3 receptor in B. are shown as a fold-change expression of genes in cells following siTyro3 vs siControl treatment (n = 1 experiment). Genes displayed are grouped together according to the functions they are most associated with. Fold change (2-ΔΔCt) is the normalised gene expression (2-ΔCt) in the test sample (siTyro3) divided by the normalised gene expression in the control sample (siControl). A fold-change value of greater than two was deemed to indicate an up-regulation, whilst a fold-change value of less than two indicates a down-regulation (negative inverse of the fold-change).
2.5. Effect of Tyro3 knockdown on genes involved in cell cycle progression and apoptosis
The PCR discovery array elucidated the genomic endpoints of signalling downstream of Tyro3, which confirmed our observations at the cell biology and protein signalling levels. Furthermore, we carried out the same array experiment on cancer cells undergoing apoptosis to analyse the effects of coincubation with TAM ligands (data not shown). We observed that ProS1 caused upregulation of anti-apoptotic and pro-proliferative genes as well as downregulation of pro-apoptotic genes. Therefore, those array analyses validate each other for revealing the role of Tyro3 in cancer pathway gene regulation. Moreover, as a separate validation of the PCR discovery array analysis following Tyro3 knockdown experiments, standard RT-qPCR of individually selected genes in MGH-U3 cells was also performed. This would moreover confirm the data for certain genes detected in the array. The genes analysed included those for cyclin D1 (CCND1), and cyclin-dependent kinase Inhibitor 2A (CDKN2A), as well as those regulating cell survival/apoptosis, including BAX, APAF-1, and BCLXL. In both SCC-25 and MGH-U3 cells, Tyro3 knockdown resulted in downregulation of the respective cell cycle progression and anti-apoptotic genes CCND1 and BCLXL, whereas there was upregulation of BAX, APAF-1 and CDKN2A (Fig. 6).
Fig. 6.
Quantitative RT-PCR analysis of CCND1, BAX, BCLXL, APAF-1 and CDKN2A gene expression in MGH-U3 (A) and SCC-25 (B) cells. Cells were transfected with siTyro3 #2 (siTyro3) and siRNA control (siControl) for 48h at 50 nM. Fold change (2-ΔΔCt) is the normalised gene expression (2-ΔCt) in the test samples (siTyro3 divided by the normalised gene expression (2-ΔCt) in the control sample (siControl). Data are mean ± SEM, normalised against reference gene (GAPDH) and underwent Student’s two-tailed t-test of the replicate; **p<0.01, *p<0.05 versus siControl-treated sample for each gene (n = 3 experiments).
3. Discussion
The aberrant expression of TAM receptors is frequently observed in several cancers and has been associated with overall poor patient survival, aggressive cancer phenotypes, and the development of drug resistance [reviewed in Ref. [25]]. This study used an siRNA knockdown approach to study the functions and signalling of Tyro3 in different human cancer cell lines with varying Tyro3 expression levels. Previously, we have shown through ligand stimulation experiments that Tyro3 is linked to the ERK and Akt signalling pathways in human cancer cells [12,13]. In the present study, Tyro3 knockdown inhibited cell survival and enhanced apoptosis in cancer cells. Tyro3 knockdown had the most pronounced effect on these outcomes in MGH-U3 cells, which express Tyro3 as the sole TAM receptor. Tyro3 knockdown in cells systematically resulted in a significant decrease in cell growth/survival in three different cancer cell lines tested, regardless of Axl and MerTK expression levels in these cells, as previously reported for melanoma cells [15]. Notably, the viability of MGH-U3 cells was drastically reduced by Tyro3 depletion, an effect that was more pronounced when cells were also undergoing apoptosis induced by staurosporine. Moreover, both ligands failed to protect cells from staurosporine-induced apoptosis upon Tyro3 knockdown. In contrast, this reduced survival effect was lesser in the SCC-25 cell line, in which Gas6 but not ProS1, was able to protect the cells from the effects of Tyro3 knockdown cells, owing to the additional utilisation of these cells of Axl signalling. Moreover, 786-0 cells were hardly affected as they express all three TAMs, thus suggesting that Tyro3 plays a minor role in these cells compared with cells that only express Tyro3 as the sole TAM receptor.
Tyro3 knockdown also further exacerbated apoptosis induced by staurosporine. Furthermore, the use of four different cancer cell lines, each with a different TAM expression profile, helped to reveal the relative role of each TAM for cell survival according to its level of expression in the cells, as well as its presence as sole TAM receptor or not. All four cell lines showed a significant increase in apoptosis upon Tyro3 knockdown; however, the degrees of responses varied, ranging from a dramatic increase in cells where Tyro3 is expressed as sole TAM (MGH-U3) or expressed considerably stronger than the other TAM receptors (WM9). There is also the possibility that Tyro3 may hetero-interact with another RTK. Indeed, as we have previously reported for Axl and EGFR [26], we have also observed hetero-interaction between Tyro3 and EGFR in human brain tumour cells, as well as additionally observed that Axl knockdown in cells leads to Tyro3 protein depletion without affecting Tyro3 mRNA levels (unpublished observations). This therefore suggests stability and protection from degradation may be conferred through the presence of multiple interacting RTKs. In support of this, downregulation of both Axl and Tyro3 was able to reverse taxol resistance in ovarian cancer [18,27]. In addition, Tyro3 overexpression in rat fibroblasts resulted in increased cell proliferation in the presence of Axl [28]. Also, Tyro3 co-immunoprecipitated with Axl and enhanced Axl activation by their common ligand Gas6, and Axl was able to phosphorylate Tyro3 [28]. Together, these data suggest the relative importance of Tyro3 may depend on its co-expression with other RTKs (e.g. Axl in SCC-25, Axl and MerTK in 786-0). This therefore has implications for the therapeutic targeting of tumours in individual cancer patients.
We also found that Tyro3 depletion negatively affected the cell cycle. Tyro3 knockdown caused an increase of cells in the G1 phase of the cell cycle, or in the S phase, with a concomitant decrease in the G2/M population. These results are in keeping with other cell cycle analysis studies on Tyro3. Tyro3 knockdown inhibited cell cycle progression in bladder cancer cells, including MGH-U3 cells [24], as well as the proliferation of melanoma cells [15]. In a breast cancer study, proliferation inhibition upon Tyro3 knockdown appeared to be due to G0-G1/S cell-cycle arrest with a decrease in cyclin D1 17. Cyclin D1 promotes cell proliferation by activating CDK4 and CDK6; consequently, the formed complex phosphorylates the tumour suppressor retinoblastoma protein (pRB) that regulates the transcription factors E2F, hence promoting gene expression required for entry of cells into the S phase of the cell cycle [reviewed in Refs. [29,30]]. All these data together showed that Tyro3 knockdown by siRNA inhibited factors involved in cell cycle progression. Here, upon Tyro3 knockdown, we observed cell cycle arrest in the G0/G1 phase in MGH-U3 and WM9 cells and an increased number of cells in the S phase in SCC-25 cells. Concomitantly, fewer cells progressed through the G2/M phase, indicating an additional impact on regulators of this phase of the cell cycle such as cyclin B/CDK1 that is important for cells to enter mitosis and therefore cell division.
To support our observations and to confirm this role of Tyro3 at the genome level, a further experiment was applied using a PCR discovery array. Moreover, this discovery array was a tool to identify genes involved in novel, alternative signalling pathways influenced by Tyro3. The qPCR array experiment revealed distinct sets of cancer signalling pathway genes to be regulated by the Tyro3 receptor. Some of these genes were involved in proliferation/growth and cell cycle progression and were downregulated upon Tyro3 knockdown; these included MAP2K1 and CCND2 which are linked to the MAPK pathway which promotes cell cycle progression [reviewed in Ref. [31]]. The gene CCND2 encodes a cyclin D2 protein, and like other cyclins, its activity is required for G1/S transition in the cell cycle [29,32]. Furthermore, genes for pro-apoptotic mediators, such as CASP9, APAF1 and BCL2L11 [33], were shown to be upregulated in Tyro3 knockdown cells, whilst the anti-apoptotic gene BIRC3 was downregulated. Moreover, BIRC3 was upregulated by ProS1 in SCC-25 cells which suggests that this gene might be regulated mainly via Tyro3 [12]. BIRC3 encodes cIAP2 protein which inhibits apoptosis through interfering with the activation of caspases [33].
The array experiments were furthermore validated through follow-on RT-qPCR analysis of individual genes involved in similar pathways, such as BCLXL that was down-regulated upon Tyro3 knockdown. This effect on BCLXL was observed previously (data not shown) where it was upregulated by both ligands in SCC-25 cells and by ProS1 in MGH-U3 cells. Increasing evidence supporting that overexpression of Tyro3 contributes to resistance to targeted therapies, Tyro3 was shown to be involved in chemoresistance in breast [17] and ovarian [18] cancers. Ovarian cancer cells overcome treatment resistance via upregulation of Tyro3, Akt phosphorylation, and BCLXL expression [27]. In addition, both BAX and APAF-1 genes were upregulated in Tyro3 knockdown cells, further confirming the discovery array results. Collectively, the results of this study and those from our previously reported observations [12] demonstrate that Tyro3 activates signalling pathways for cancer cell survival, proliferation and EMT.
Upregulation of pro-apoptotic genes and downregulation of anti-apoptotic genes upon Tyro3 knockdown in MGH-U3 (express Tyro3 as sole TAM) suggested that Tyro3 is involved in cell survival in cancer. Other genes encoding important regulators of the cell cycle such as CCND1, which is required for G1 to S transition in the cell cycle, and CDKN2A, which blocks traversal from G1 to S phase, were downregulated or upregulated by Tyro3 depletion. The expression ratio of CCND1 to CDKN2A mRNA regulates the activity of retinoblastoma product (RB1), which is a crucial regulator of cell cycle progression [34]. Our results showed that Tyro3 depletion caused significant upregulation of CDKN2A in SCC-25 cells and downregulation of CCND1 in both SCC-25 and MGH-U3 cells, which further supports the notion of a key role for Tyro3 in cell cycle progression.
In addition, the array analysis suggested Tyro3 regulates genes that are involved in EMT, a biological event in which epithelial cells lose their polarity and cell-cell adhesions and simultaneously acquire mesenchymal features, which is thought to play an important role in cancer progression. Upon Tyro3 knockdown, the genes SNAI2 and OCLN were downregulated and upregulated, respectively. SNAI2 (SLUG) is an EMT transcription factor that represses the cell adhesion molecule E-cadherin and promotes cell differentiation and migration whilst occludin, the OCLN gene product, is a protein important for tight junction function [35]. Other genes that were affected by Tyro3 depletion included those involved in DNA damage and repair such as LIG4 and ERCC5. LIG4 gene encodes an ATP-dependent DNA ligase, which is known for its role in the repair of double‐stranded DNA breaks, although its role in cancer has not been established in a particular direction [36]. ERCC5 is an essential DNA repair gene of the nucleotide excision repair (NER) pathway [37]. Therefore, the observed upregulation of these genes upon Tyro3 knockdown might indicate a reaction in the cells to avoid DNA damage-induced apoptosis. Also, a new functional role for Tyro3 may have been revealed through the detected change in expression of the TBX2 gene, which has been implicated in various cancers [38]. T-box (TBX)2 is a member of the T-box gene family that has been shown to function as either transcriptional activators or repressors [39]. A growing number of studies has reported dysregulation of T-box factors in different cancers [reviewed in Ref. [40]]. TBX2 overexpression promotes proliferation and invasion through EMT and ERK signalling, and also a correlation with poor patient survival [38]. The array also showed a decrease in the expression of the BIRC3 gene upon Tyro3 knockdown, whereas it was increased in expression upon ProS1 treatment (data not shown). Therefore, such single-gene examples of apparent discrepancies between signalling output downstream of Tyro3 in ligand-dependent vs –independent contexts are worthy of further investigation and delineation (Fig. 7; Supplementary Fig. 4).
Fig. 7.
Proposed mechanism of Tyro3-mediated cell proliferation and cell survival signalling. Tyro3 signalling regulates pathways for anti-apoptosis/cell survival and DNA repair (PI3K/Akt pathway), cell proliferation (Ras/Raf/MEK/Erk pathway) and EMT [41].
In conclusion, the results of present study demonstrate a role for Tyro3 in cancer cell cycle progression and survival, with the greatest role in cells that express Tyro3 as sole TAM receptor. These findings have elucidated further the nature of Tyro3 signalling in cancer cells, thus highlighting its importance as a therapeutic target in several cancers.
4. Materials and methods
4.1. Cell culture
A variety of human cancer cell lines from various sources were grown in culture (Supplementary Table S1). Cells were normally cultured in “complete” medium, comprising Dulbecco’s Modified Eagle Medium (Fisher Scientific, Loughborough, UK), supplemented with 10% foetal bovine serum (FBS) (Lonza, Slough, UK), and 1% penicillin/streptomycin (Fisher Scientific). Cells were routinely maintained at 37 °C in a humidified incubator with 5% CO [2] and were typically passaged once they reached 80% confluency, through dissociation with trypsin/EDTA (Fisher Scientific).
4.2. RNA extraction and quantitative real-time polymerase chain reaction
Cellular total RNA was isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer ’s protocol, and purity and concentration were determined using a spectrophotometer (ND-1000; NanoDrop Technologies, Wilmington, DE, USA). cDNA was synthesised from the total RNA (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. cDNA was used directly for PCR amplification or stored at −20 °C for later use. Quantitative PCR (qPCR) amplification from cDNA was performed using hydrolysis probes in a 96-well plate and run on a LightCycler®96 System (Roche, Burgess Hill, UK). Reactions were assembled together with a mastermix for probes (Roche). The genes were investigated using pre-designed primers/probes (Supplementary Table S2). For each reaction, the GAPDH gene was utilised as an endogenous control (reference) gene. The amplification conditions included 95 °C for15 s (1 cycle) followed by 60 °C for 1 min (45 cycles). qPCR amplification data were analysed using the2−ΔCt method, as previously described [12].
4.3. Cell transfection with short interfering RNA (siRNA)
Four cancer cell lines were selected for this study: MGH-U3 which expresses Tyro3 as sole TAM, SCC-25 cells which express Axl and Tyro3 but not MerTK, 786-0 cells which express all three TAMs and WM9 cells which showed the highest expression of Tyro3 [12]. For transient knockdown of protein expression, cells were transfected with individually designed 21-nucleotide siRNA constructs targeting Tyro3 (Tyro3 siRNA #1, Tyro3 siRNA #2, Tyro3 siRNA #3) (Life Technologies, Glasgow, UK) (Supplementary Table S3), as well as a pool of non-targeting 20–25 nucleotide siRNAs (control siRNA) (Santa Cruz; CA, USA) (Table 2.9). Indicated siRNA concentrations were delivered to cells mixed with jetPRIME transfection reagent and buffer (Polyplus, Reading, UK) with presence of serum, and with cells at 60–80% confluency as per manufacturer’s instructions. Cells were then incubated with the mixture for 48h or 72h before cell lysis for mRNA or protein extraction for subsequent RT-qPCR or Western blot analysis respectively.
4.4. Cell growth/viability assay
MTS assay was used to determine the metabolic activity of cells by measuring the reduction of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy methoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) compound (Fisher Scientific) in the presence of phenazine methosulfate (PMS) (Sigma), which relies on NAD(P)H-dependent oxidoreductase enzymes. This correlates with cell viability and proliferation [42]. The MTS assay was used in order to determine the effects of Tyro3 siRNA on cell survival during apoptosis induced chemically with staurosporine [43]. Briefly, 2000–10,000 cells, depending on cell type, were seeded in each well of a 96-well culture plate (and incubated overnight, prior to indicated treatments for various periods, after which MTS (0.4 μM) was added to cells together with PMS (0.3 nM) and incubated further for 2h, and absorbance was measured at 490 nm using a spectrophotometric microplate reader (Synergy; BioTek, Potton, UK). Viability changes upon treatments were determined as a percentage of untreated wells after subtraction of background values (no cells).
4.5. Apoptosis assay
Following experimental treatments, cells were analysed for apoptotic cell populations by flow cytometry, in which suspended cells were double-stained with a fluorescent conjugate of Annexin V (Annexin V-FITC) as well as propidium iodide (PI) to stain DNA (Abcam, Cambridge, UK). In this system, live cells are both Annexin V-FITC and PI negative (A-/PI), whereas cells in the early stage of apoptosis have an intact cell membrane and hence do not take up PI but do stain with Annexin V-FITC (A+/PI-). In late apoptosis, the cell membrane additionally loses its integrity, hence cells will be stained with both Annexin V-FITC and PI (A+/PI+). Cells with only PI staining (A-/PI+) are considered necrotic/dead cells. Following treatments, cells were washed with PBS, trypsinised and collected by centrifugation. Then cells were centrifuged at 400×g for 5 min and re-suspended in 500 μl Annexin V binding buffer (Abcam). Finally, cells were double-stained by adding 5 μl Annexin V-FITC and 5 μl PI and incubated at RT for 15 min in the dark according to the manufacturer’s instructions. Then, flow cytometry was used to analyse Annexin V-FITC binding at the relevant wavelengths for PI (FL1) and FITC (FL2) and signal detection according to the manufacturer’s protocol (Abcam) (PI: excitation 493nm/emission 636 nm, Annexin V-FITC: excitation 488 nm/emission 530 nm). Flow cytometry was performed using a BD FACSCalibur™ instrument (BD Biosciences, New Jersey, USA), and the software FlowJo-V10 (BD Life Sciences, Ashland, OR, USA) was used for analysis.
4.6. Cell cycle analysis
Following experimental treatments, cell cycle analysis was performed on suspended cells by flow cytometry. Cells were first seeded 24h before being transfected with siRNA control or siRNA Tyro3 for 48h alone or in combination with staurosporine (0.1 μM) for a further 22h. Following treatments, cells were harvested and washed in PBS followed by fixation in 70% ethanol for at least 2 h at 4 °C. After this, cells were washed twice in PBS and re-suspended in 500 μl of FxCycle™ PI/RNase Staining Solution containing RNase A (Thermo Fisher Scientific). Cell populations were analysed on the flow cytometer for PI fluorescence according to the manufacturer’s protocol.
4.7. RT 2 Profiler™ PCR Array Human Cancer Pathway Finder™
The downstream signalling pathways activated via Tyro3 were investigated by a qPCR-based array (RT2 Profiler PCR array; Qiagen) to screen for a focused panel of relevant pathway genes. Each well of a pre-prepared 96-well plate contained primer pairs for qPCR amplification of different genes (Supplementary Table S4) together with SYBR Green mastermix for uniform simultaneous amplification across the plate. Cellular sample cDNA was synthesised from 500 ng total RNA using the same reverse transcription kit as described before. Equal volumes of cDNA were added to wells of the plate, followed by real-time PCR. The amplification procedure entailed pre incubations at 95 °C for 10 s (1 cycle), followed by denaturation 95 °C for 10 s and 60 °C for 20 s, 72 for 30 s (annealing and extension) (45 cycles) then 40 °C for 10 s (cooling). Fold change in gene expression was calculated using the using 2-ΔΔCt formula as previously described. The selection of the internal control/housekeeping genes for the analysis was by the average arithmetic mean of five housekeeping genes consistently across experiments, as provided by the manufacturer.
Declaration of competing interest
None.
Acknowledgements
This study was supported by a University of Portsmouth PhD bursary awarded to NK. We also wish to thank the Council for At-Risk Academics (Cara) for supporting NK’s postgraduate development. We are also grateful to Prof. Margaret Knowles, University of Leeds for the kind gift of MGH-U3 cells.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.101111.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Hafizi S., Dahlbäck B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Rothlin C.V., Carrera-Silva E.A., Bosurgi L., Ghosh S. TAM receptor signaling in immune homeostasis. Annu. Rev. Immunol. 2015;33:355–391. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu C., Wei Y., Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol. Canc. 2019;18:1–22. doi: 10.1186/s12943-019-1090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan Y. Overexpression of Tyro3 and its implications on hepatocellular carcinoma progression. Int. J. Oncol. 2016;48:358–366. doi: 10.3892/ijo.2015.3244. [DOI] [PubMed] [Google Scholar]
- 5.Dai W., Pan H., Hassanain H., Gupta S.L., Murphy M.J. Molecular cloning of a novel receptor tyrosine kinase, tif, highly expressed in human ovary and testis. Oncogene. 1994;9:975–979. [PubMed] [Google Scholar]
- 6.Polvi A. The human TYROS gene and pseudogene are located in chromosome 15q14-q25. Gene. 1993;134:289–293. doi: 10.1016/0378-1119(93)90109-g. [DOI] [PubMed] [Google Scholar]
- 7.Kim N.Y., Lee H.Y., Lee C. Metformin targets Axl and Tyro3 receptor tyrosine kinases to inhibit cell proliferation and overcome chemoresistance in ovarian cancer cells. Int. J. Oncol. 2015;47:353–360. doi: 10.3892/ijo.2015.3004. [DOI] [PubMed] [Google Scholar]
- 8.Chien C.W. Targeting TYRO3 inhibits epithelial-mesenchymal transition and increases drug sensitivity in colon cancer. Oncogene. 2016;35:5872–5881. doi: 10.1038/onc.2016.120. [DOI] [PubMed] [Google Scholar]
- 9.Zhu S. A genomic screen identifies TYRO3 as a MITF regulator in melanoma. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17025–17030. doi: 10.1073/pnas.0909292106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avilla E. Activation of TYRO3/AXL tyrosine kinase receptors in thyroid cancer. Canc. Res. 2011;71:1792–1804. doi: 10.1158/0008-5472.CAN-10-2186. [DOI] [PubMed] [Google Scholar]
- 11.Ammoun S. Axl/Gas6/NFκB signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene. 2014;33:336–346. doi: 10.1038/onc.2012.587. [DOI] [PubMed] [Google Scholar]
- 12.Al Kafri N., Hafizi S. Tumour-secreted protein S (ProS1) activates a tyro3-erk signalling Axis and protects cancer cells from apoptosis. Cancers (Basel). 2019;11:1843. doi: 10.3390/cancers11121843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Kafri N., Hafizi S. Galectin-3 stimulates Tyro3 receptor tyrosine kinase and erk signalling, cell survival and migration in human cancer cells. Biomolecules. 2020;10:1035. doi: 10.3390/biom10071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin A., Qian W. MicroRNA-7 inhibits colorectal cancer cell proliferation, migration and invasion via TYRO3 and phosphoinositide 3-kinase/protein B kinase/mammalian target of rapamycin pathway suppression. Int. J. Mol. Med. 2018;42:2503–2514. doi: 10.3892/ijmm.2018.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demarest S.J. Evaluation of Tyro3 expression, Gas6-mediated akt phosphorylation, and the impact of anti-Tyro3 antibodies in melanoma cell lines. Biochemistry. 2013;52:3102–3118. doi: 10.1021/bi301588c. [DOI] [PubMed] [Google Scholar]
- 16.Smart S.K., Vasileidadi E., Wang X., DeRychere D., Graham D.K. The emerging role of TYRO3 as A therapeutic target in cancer. Cancers (Basel) 2018;1–27 doi: 10.1634/theoncologist.2007-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekyalongo R.C. TYRO3 as a potential therapeutic target in breast cancer. Antiancer Res. 2014;34:3337–3345. [PubMed] [Google Scholar]
- 18.Lee C. Overexpression of Tyro3 receptor tyrosine kinase leads to the acquisition of taxol resistance in ovarian cancer cells. Mol. Med. Rep. 2015;12:1485–1492. doi: 10.3892/mmr.2015.3542. [DOI] [PubMed] [Google Scholar]
- 19.Fu M., Wang C., Li Z., Sakamaki T., Pestell R.G. Minireview: cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 20.Kabir T.D. A microRNA-7/growth arrest specific 6/TYRO3 axis regulates the growth and invasiveness of sorafenib-resistant cells in human hepatocellular carcinoma. Hepatology. 2018;67:216–231. doi: 10.1002/hep.29478. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto M. Oncogenic role of TYRO3 receptor tyrosine kinase in the progression of pancreatic cancer. Canc. Lett. 2020;470:149–160. doi: 10.1016/j.canlet.2019.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Toshima J., Ohashi K., Iwashita S., Mizuno K. Autophosphorylation activity and association with src family kinase of sky receptor tyrosine kinase. Biochem. Biophys. Res. Commun. 1995;209:656–663. doi: 10.1006/bbrc.1995.1549. [DOI] [PubMed] [Google Scholar]
- 23.Taylor I.C.A., Roy S., Varmus H.E. Overexpression of the Sky receptor tyrosine kinase at the cell surface or in the cytoplasm results in ligand-independent activation. Oncogene. 1995;11:2619–2626. https://pubmed.ncbi.nlm.nih.gov/8545119/ [PubMed] [Google Scholar]
- 24.Dufour F. TYRO3 as a molecular target for growth inhibition and apoptosis induction in bladder cancer. Br. J. Canc. 2019;120:555–564. doi: 10.1038/s41416-019-0397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham D.K., Deryckere D., Davies K.D., Earp H.S. The TAM family: phosphatidylserine-sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Canc. 2014;14:769–785. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- 26.Vouri M. Axl-EGFR receptor tyrosine kinase hetero-interaction provides EGFR with access to pro-invasive signalling in cancer cells. Oncogenesis. 2016;5 doi: 10.1038/oncsis.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh Y.A., Jo S.Y., Lee H.Y., Lee C. Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells. Int. J. Oncol. 2015 doi: 10.3892/ijo.2014.2808. [DOI] [PubMed] [Google Scholar]
- 28.Brown J.E., Krodel M., Pazos M., Lai C., Prieto A.L. Cross-phosphorylation, signaling and proliferative functions of the Tyro3 and Axl receptors in Rat2 cells. PloS One. 2012;7:1–11. doi: 10.1371/journal.pone.0036800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozar K., Sicinski P. Cell cycle progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell Cycle. 2005:4 388–391. doi: 10.4161/cc.4.3.1551. [DOI] [PubMed] [Google Scholar]
- 30.Musgrove E.A., Caldon C.E., Barraclough J., Stone A., Sutherland R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Canc. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 31.Molina J.R., Adjei A.A. The Ras/Raf/MAPK pathway. J. Thorac. Oncol. 2006:1 7–9. [PubMed] [Google Scholar]
- 32.Schmidt E.E., Ichimura K., Reifenberger G., Collins V.P. CDKN2 (p16MTSI) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Canc. Res. 1994;54:6321–6324. [PubMed] [Google Scholar]
- 33.Jourdan M. Gene expression of anti- and pro-apoptotic proteins in malignant and normal plasma cells. Br. J. Haematol. 2009;145:45–58. doi: 10.1111/j.1365-2141.2008.07562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuarai S. Expression ratio of CCND1 to CDKN2A mRNA predicts RB1 status of cultured cancer cell lines and clinical tumor samples. Mol. Canc. 2011;10 doi: 10.1186/1476-4598-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno-Bueno G., Portillo F., Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008:27 6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 36.Assis J. Ovarian cancer and DNA repair: DNA ligase IV as a potential key. World J. Clin. Oncol. 2013 doi: 10.5306/wjco.v4.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh C.S. ERCC5 is a novel biomarker of ovarian cancer prognosis. J. Clin. Oncol. 2008 doi: 10.1200/JCO.2007.13.5806. [DOI] [PubMed] [Google Scholar]
- 38.Liu X. TBX2 overexpression promotes proliferation and invasion through epithelial-mesenchymal transition and ERK signaling pathway. Exp. Ther. Med. 2018 doi: 10.3892/etm.2018.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papaioannou V.E. The t-box gene family: emerging roles in development, Stem cells and cancer. Dev. 2014;141:3819–3833. doi: 10.1242/dev.104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abrahams A., Parker M.I., Prince S. The T-box transcription factor Tbx2: its role in development and possible implication in cancer. IUBMB Life. 2010:62 92–102. doi: 10.1002/iub.275. [DOI] [PubMed] [Google Scholar]
- 41.Zhou W., Gross K.M., Kuperwasser C. Molecular regulation of Snai2 in development and disease. J. Cell Sci. 2019;132 doi: 10.1242/jcs.235127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berridge M.V., Herst P.M., Tan A.S. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Annu. Rev. 2005:11 127–152. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- 43.Kabakov A.E., Kudryavtsev V.A., Gabai V.L. Methods in Molecular Biology. vol. 787. 2011. Determination of cell survival or death; pp. 231–244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.