Graphical abstract
Keywords: Ultrasound, Degradation, Organic contaminants, Water bodies, Advanced oxidation processes
Highlights
-
•
Use of ultrasound (US) as an advanced oxidation process (AOPs).
-
•
Use of US coupled to other AOPs.
-
•
US application as an AOP in the last 20 years.
-
•
Degradation of drugs, dyes, pesticides, and aromatic compounds.
-
•
The use of US as an AOP must be further explored.
Abstract
The rising amount of persistent organic contaminants released into water reservoirs in the last years became a cause of concern for the industry, academy, and public administration, due to their bioaccumulation, mutagenicity, and photosynthesis reduction. Therefore, the search for processes that efficiently remove such contaminants became of primary importance. In this context, ultrasound (US) is one of the most promising and economically viable alternatives to degrade organic pollutants in varied environments. Whereas the use of other advanced oxidation processes (AOPs), such as Fenton and photocatalysis, has been widely reported for this purpose, only a few papers deal with ultrasound application as a possible AOP. In this review, a general overview of ultrasound is provided, covering the last twenty years. It includes fundamental aspects of ultrasound and applications, individually or combined with other AOPs, to deplete organic pollutants from various classes in an aqueous environment. Finally, the review concludes by indicating that additional research should be conducted worldwide to explore the full potential of ultrasound as a useful AOP.
1. Introduction
The amount of residues launched in the aquatic environment has significantly increased in the last decades [1]. The textile industry, domestic sewage, and agribusiness are the sectors that have most contributed to water pollution [2], [3]. The textile sector is responsible for discarding large amounts of wastes in the environment. The presence of dyes in wastewater, as well as fragments of these molecules, even at low concentrations, represents a severe unsolved issue [4], [5]. There is a growing consensus that domestic sewage and residues of organic contaminants are potential threats to aquatic organisms [6]. The main classes of organic contaminants in these effluents are pharmaceutical products and pesticides [6], a potential risk due to their high hazardousness [7], [8], [9], [10], [11], [12].
Because conventional water and sewage treatments do not satisfactorily remove organic contaminants [13], the development of processes for remediation is required. In this sense, advanced oxidation processes (AOPs) have stood out in recent years [13]. It consists of a set of processes, which have in common the ability to generate highly oxidizing radicals in an aqueous solution, mainly the hydroxyl radical (OH•) [14]. Among the main AOPs, the Fenton process, heterogeneous photocatalysis, ozonation, and ultraviolet (UV) radiation are highlighted [15], [16], [17], [18].
The use of ultrasound waves to generate hydroxyl radicals in an aqueous solution is a promising approach. This methodology can be easily applied on large scales, especially in water and sewage treatment plants. An exciting feature is that the ultrasound is a green process. Its use reduces the consumption of oxidizing chemicals, mineral acids, ferrous salts, and some semiconductors (TiO2 and Nb2O5) when coupled with other AOPs [4], [19], [20], [21], [22]. In 2010 Mahamuni and Adewuy demonstrated the economic viability of some systems that employ ultrasound to degrade phenol, trichloroethylene, and azo dyes [23]. The authors compared different technologies and concluded that less costly processes could result upon ultrasound application [23]. Over the past few years, several authors have verified the feasibility of ultrasound as an advanced oxidation method to degrade various organic contaminants in aqueous media [24], [25], [26], [27], [28], [29], [30], [31], [32]. For this purpose, several manuscripts have described the use of ultrasound alone or in conjunction with other AOPs [29], [33].
The present review aims to provide an overview of ultrasound applications to deplete organic contaminants in water media in the last two decades. The present review displays the following topics: (1) a brief introduction of the fundamentals of ultrasound as an AOP; (2) some manuscripts that report the use of the ultrasound (alone or coupled with other AOPs) to degrade organic contaminants; (3) the final considerations and future perspectives.
2. Mechanism of hydroxyl radical generation in ultrasound
Ultrasound differs from traditional sources of energy, such as heat, light, or ionizing radiation. Its use has become increasingly common in the last decades, mainly in environmental remediation applications [33].
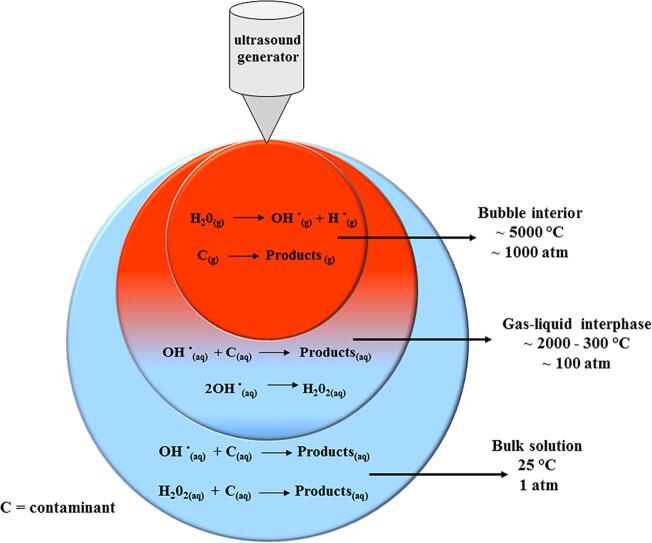
Technically, one can define ultrasound as any sound wave with a frequency above 16 kHz, imperceptible to the human ears [34]. Ultrasound waves are split into three frequency ranges: low (20–100 kHz), high (2–10 MHz), generally used in diagnostic medicine, and medium (300–1000 kHz). The sonochemical phenomenon occurs mainly in the medium frequency region and, to a lesser extent, in the low-frequency range [34], [35]. In liquids such as water, a phenomenon known as acoustic cavitation is produced, resulting from the application of intense sound waves from an ultrasound generator with frequencies in the range 20–1000 kHz [27]. The wave passing through the vibrating material medium (water) is transmitted to the adjacent molecules, which, before returning to the equilibrium position, pass on this movement to the surrounding molecules. This periodic movement creates compression and expansion cycles, characteristic of the cavitation phenomenon [27]. Microbubbles are then formed due to these successive cycles [27], [29], which grow and collapse in a fraction of seconds. The cavity's implosion generates very high temperatures and localized pressures, around 5000 K and 1000 atm, respectively. Therefore, acoustic cavitation concentrates diffuse ultrasonic energy in small and short-lived hot spots that behave as micro-reactors [29]. Fig. 1 illustrates the phenomenon of acoustic cavitation in water.
Fig. 1.
Acoustic cavitation in water.
As illustrated in Fig. 1, cavitation in an aqueous solution generates three regions, where different reactions occur:
Bubble cavity core (gaseous region). Volatile substances and hydrophobic molecules are degraded in this region via pyrolytic reactions due to the high temperatures inside the cavity. Hydroxyl radicals are also formed in this region due to the decomposition of vaporized water molecules inside the bubble.
Bubble-liquid interface. In this region, reactions with hydroxyl radicals are predominant to form H2O2 and oxidize organic substrates.
Liquid volume. In this region, the free radicals that migrate from the bubble–liquid interface create secondary sonochemical reactions, mainly between oxidizing species and organic substrates [27,29].
Ultrasound, therefore, provides a convenient method for treating wastewater or effluents containing persistent organic pollutants. Some of the main reactions that occur are summarized below [23], [27], [33], [34]:
| H2O + )))→H• + HO•(1) |
| O2 + )))•→2O• (2) |
| H2O + O•→ 2HO• (3) |
| O2 + H• → O•+ HO• (4) |
| HO•+ organic contaminant →Degradation products (5) |
| 2HO•→H2O2 (6) |
3. Application of the ultrasound as an advanced oxidation process (AOP)
As mentioned, several strategies have been used in recent years to apply ultrasound as a technology to degrade organic contaminants in aqueous media. Its use is exemplified in Table 1, alone or coupled to other AOPs.
Table 1.
Summary of publications in the last twenty years about using ultrasound to deplete typical organic pollutants in aqueous media, alone or coupled to other AOP's.
| System | Ultrasound Power | Frequency (KHz) | Ultrasound Type | Contaminant | pH | Temperature(oC) | Initial Concentration | Removal Efficiency (%) | Time (min) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| US | 88.0 W.L-1 | 375 | uninformed | LosartanValsartan | 6.5 | 20.0 | 40 μM | 60–70 | 30 | [48]a, b |
| US | 100.0 W.L-1 | 20 | probe | DiclofenacAmoxicillinCarbamazepine | 3.0 | 24.0 | 2.5 mg.L-110.0 mg.L-15.0 mg.L-1 | ~ 50 | 60 | [49]a, b |
| US0.62 s pulse, 2.5 s pulse repetition | 7.9 W.cm−2 | 640 | probe | Diphenhydramine | natural | 10.0 | 2.8 μM | 50 | 3.9 | [30] |
| US | 45.0 W.L-1 | 205 | flat plate transducer | FluorouracilIbuprofenClonidineEstriolNifedipineLovastatin | 7.7 | 20.0 | 10 μM | Uninformed | Uninformed | [50] |
| US | 80 W | 300 | flat plate transducer | 4-Cumilphenol | 6.5 | 20.0 | 0.05 mg.L-1 | 100 | 35 | [51] |
| US | 32 W | 574 | ultrasound generator connected to a stainless steel transducer | ParacetamolLevodopa | Uninformed | 20.0 | 25.0 mg.L-1 | 100% | 480 | [52] |
| US | 43 W | 520 | bath | Carbamazepine | 7.0 | 20.0 | 8.05 μg.L-1 | 90.0 | 116 | [53] |
| US | 200 W | 200 | uninformed | Methylene BlueMethyl OrangePhenolic compounds | 7.0 | 10.0 | 160 μM | >87.5 (MB) | 30 with O2 bubbling | [31] |
| US | 14 W | 1700 | piezoelectricdisc | Naphthol Blue Black | Sea water | 65.0 | 15.0 mg.L-1 | 96.5 | 40 | [28] |
| US | 0.16 W mL−1 | 35 | bath | Rhodamine-B | 2.0 | 25.0 | 1.0 mg.L-1 | 67% chemical oxygen demand | 180 | [54] |
| US | 270 | 20 | probe | Dichlorvos | 2.0 | 25.0 | 20.0 mg.L-1 | 52.9 | 120 | [55] |
| US | 55.2 W | 600 | ultrasound generator connected to a stainless steel-made transducer | Parathion | 7.0 | 25.0 | Uninformed | Uninformed | Uninformed | [56] |
| US | 150 W | 80 | probe | NaphthaleneAcenaphthenePhenanthrene | Uninformed | 25.0 | 150 μ g.L-1 | 99.299.998.2 | 60 | [57] |
| US | 300 W cm−2 | 20 | probe | Dibenzothiophene | 9 | 25.0 | 6.5 μM | 72 | 120 | [58] |
| US/SWNT | 180 W | 28 | ultrasound generator connected to a stainless steel-made transducer | AcetaminophenNaproxen | 6 | 25.0 | 5.0 μM5.0 μM | 44.590.3 | 10 | [59] |
| US/Pd-TiO2, and US/Au-TiO2 | 1.14 Wcm2 | 861 | probe | Paracetamol | 6.5 | Uninformed | 35.0 μM | close to 100 depending on conditions | 30 | [60] |
| US/pre-Feo/(PS) | 60 W | 40 | probe | Sulfamethazine | 10.0 | 25.0 | 0.5 mg.L-1 | 99.6 | 30 | [12] |
| US/UV/TiO2 | 100 W | 20 | probe | Salicylic acid | Uninformed | 30.0 | 10.0 mM | close to 60.0 | 180 | [61] |
| US/Fe2+/UV/TiO2 | 80 W | 300 | piezoelectric disc | Ibuprofen | 3.0 | 25.0 | 0.019 mM | 92.0 DOC removal | 240 | [62] |
| US/O3/Co2+/SO4-• | 200 W | 20 | probe | Amoxicillin | 7.0 | 24.0 | 0.095 mM | ~ 98.0 of COD | 60 | [63] |
| US/UV/Fe2+/H2O2 | 200 W | 24 | probe | Reactive Blue 4 | 3.0 | 30.0 | 30 mg.L-1 | 94.0 of TOC | 60 | [64] |
| US/TiO2/UV | 600 W | 200 | bath | Orange Acid 52 | Uninformed | 25.0 | 25 mg.L-1 | 87.0 of TOC | 480 | [65] |
| US/UVC/sodium percarbonate | 100 W | 20 | probe | Orange Acid 7 | 6.0 | Uninformed | 25 mg.L-1 | 93 | 90 | [66] |
| US/ZnO/UV/Air | 170 W | 42 | bath | MethylOrange | Uninformed | 25.0 | 20 mg.L-1 | 99.45 | 40 | [67] |
| US/ZnO/UV | 1 W m−2 | 59 | bath | Rhodamine B | Uninformed | 20.0 | 2.5 mg.L-1 | 100 | 10 | [68] |
| US/LuFeO3 | 240 W | 60 | bath | Rhodamine B | 6.5 | 40.0 | 5.0 mg.L-1 | 98 | 90 | [69] |
| US/CuOUS/TiO2 | 170 W | 50 | bath | Rhodamine 6G | 12.5 | Uninformed | 10.0 mg.L-1 | 84 of COD89 of COD | 180 | [70] |
| US/S-TiO2/UV | 100 W | 35 | bath | Reactive Blue 19 | 3.0 | 25.0 | 20.0 mg.L-1 | 90 | 120 | [71] |
| Cr-MIL-101@NiO/13X/US/H2O2 | 100 W | 37 | bath | Methylene BlueRhodamine BMethyl Orange | 7.0 | 25.0 | 25.0 mg.L-125.0 mg.L-125.0 mg.L-1 | 97.294.385.1 | 8080100 | [72] |
| US/TiO2 (powder and nanotubes) | 100 W | 42 | bath | Congo RedReactive Blue 4Methyl OrangeRhodamine BMethylene Blue | 7.0 | 30.0 | 50.0 mg.L-1 | 57.7861.9012.5038.2473.26COD removal | 60 | [73] |
| US/CO32–/H2O2 | 400 W | 25 | probe | Orange Acid 8Orange IIRed Acid 9Yellow Acid 11 | 10.4 | 30.0 | 0.1 mM | >95>9582.07.0 | 120 | [74] |
| US/zirconia nanotubes | 0.5 Wcm−2 | 20 | bath | Methyl Orange | 2.0 | Uninformed | 20.0 mg.L-1 | 97.6 | 480 | [75] |
| US/Au-TiO2/UV | Uninformed | 42 | bath | Simazine | ~ 6.0 | Uninformed | 5.0 mg.L-1 | 43.0 of TOC | 420 | [76] |
| US/TiO2solar/TiO2US/solar/TiO2US/H2O2US/FentonUS/ozônio | 150 W | 36 | bath | Dichlorvos | 3.0 | 25.0 | 20.0 mg.L-1 | 7.4578.4283.1220.0081.19100.00 | 120 | [7] |
| US/Sch/H2O2 | 130 W | 20 | probe | Bisphenol A | 3.0 | 20.0 | 0.2 mM | 98.00 | 60 | [77] |
| US/peroxydisulfate and US/O2(aq) | 60 W | 28 | Uninformed | Bisphenol S | Uninformed | 20.0 | 40.0 μM | Uninformed | 60 | [78] |
a - In some works, the operational parameters were evaluated in more than one condition. Table 1 shows only the best conditions found in each work.
b - Some works used power density (W L-1) instead of power.
3.1. Applications of ultrasound alone in the degradation of organic pollutants in aqueous media
3.1.1. Drugs
Serna-Galvis et al. demonstrated the degradation of two antihypertensive drugs, Losartan and Valsartan, using ultrasound (Fig. 2a-b), even in complex simulated matrices, such as seawater and hospital effluents [36]. The authors used high-frequency ultrasound (at 375 kHz and 88.0 kHz) to promote these two drugs' degradation and found that it was not affected by the matrix constituents. The results showed that Losartan degraded at a higher rate than Valsartan. After 30 min, the percentage of Losartan and Valsartan degraded was 70 and 60%, respectively. The authors attributed this distinct behavior to the difference in both molecules' hydrophobicity, which determines its proximity to the bubble. The more hydrophobic molecule, Losartan, remains closer to the cavitation bubble, thus undergoing a higher degradation rate [36]. According to the authors, the degradation products formed are less lipophilic, indicating less toxicity and greater degradability.
Fig. 2.
Molecular structures of the drugs mentioned in the review.
Naddeo et al. investigated the degradation of three drugs, Diclofenac, Amoxicillin, and Carbamazepine, in pure water and urban wastewater effluents (Fig. 2c-e) [37]. The authors employed an ultrasound generator with a fixed frequency (20 kHz) and evaluated the influence of operational factors, such as power density (25–100 W.L-1), initial substrate concentrations (2.5–10 mg.L-1), initial solution pH (3–11), and air sparging. To measure the process efficiency, the authors evaluated the percentage of degradation via absorbance measurements and the degree of mineralization. Besides, the authors assessed the biodegradability and toxicity of solutions before and after sonication. They concluded that the low frequency seems to benefit the reaction mechanism via hydroxyl radicals. The ideal conditions consisted of a medium with low pHs and with air bubbling. Under ideal conditions, the authors obtained a degradation of approximately 50% of a mixture of the three drugs. The results also showed that sonication increased the samples' biodegradability, indicating that ultrasound can be used as an efficient pretreatment for subsequent biological oxidation or other AOPs [37].
Cui et al. investigated the sonochemical degradation of Diphenhydramine, a first-generation antihistamine (Fig. 2f) [30]. The authors used a piece of equipment that produced ultrasonic waves at 640 kHz and performed a detailed study of the reaction pathways. They found that the drug's degradation involves direct pyrolysis and mainly reactions mediated by the hydroxyl radical. The authors reported that the ultrasound effectively degraded the drug in aqueous solutions even at high concentrations. Under the best conditions, a degradation rate of 50% in just 3.9 min was achieved [30].
Xiao et al. studied the ultrasound degradation of six pharmaceutical compounds: Fluorouracil, Ibuprofen, Clonidine, Estriol, Nifedipine, and Lovastatin (Fig. 2g-m) in deionized water and wastewater effluent [38]. The authors used ultrasonic waves at 205 kHz in two different modes, continuous and pulsed. For targets with moderate hydrophobicity and with molecular sizes smaller than those of the sample components, matrix effects slightly inhibit degradation. The pharmaceutical compounds with high diffusivity and low hydrophobicity (Fluorouracil) or low diffusivity and high hydrophobicity (Lovastatin) are benefited by the additional time from the pulsed ultrasound to fill the water-bubble interface [38].
Chiha et al. investigated the ultrasound degradation of the endocrine disruptor 4-Cumyl Phenol (Fig. 2n) in an aqueous solution [39]. The authors studied the influence of operational parameters, such as initial concentration, frequency, power, pH, temperature, and reaction medium's saturation gas. The extent of degradation was inversely proportional to the initial concentration of the substrate. Besides, the degradation rate was dependent on the ultrasonic frequency. The degradation rate increased proportionally by increasing the ultrasonic power from 20 to 100 W and temperature from 20 and 50 °C. Moreover, the acidic medium and the presence of air instead of argon or nitrogen favored the reaction. With the optimized operational parameters, the authors managed to degrade 100% of the contaminant in 35 min of reaction. The authors also demonstrated that the ultrasound was more efficient in experiments carried out in natural than in pure water [39].
Paracetamol, a non-steroidal anti-inflammatory, and Levodopa, most often used to treat Parkinson's disease, had their ultrasound degradation in an aqueous solution investigated by Isariebel et al. (Fig. 2o-p) [40]. The authors evaluated the influence of ultrasonic frequency (574, 860, and 1134 kHz), power (9, 17, 22, and 32 W), and the initial concentration of the target molecules (25, 50, 100, and 150 mg.L-1). The temperature was kept constant at 20 °C. The process efficiency was estimated by varying the analytes' initial concentration and measuring chemical oxygen demand. The degradation of both molecules follows a kinetic of pseudo-first-order, and in some cases, it was possible to obtain complete degradation. However, there was still some amount of residual dissolved organic carbon, which evidences recalcitrant compounds in solution. The authors achieved the best results using the ultrasound with a frequency of 574 kHz. Experiments using 1-Butanol as a radical scavenger were also performed, suggesting that degradation occurs mainly via radical reactions and that the presence of H2O2 has a beneficial effect on the degradation process [40].
Tran et al. analyzed the sonochemical oxidation of Carbamazepine in an aqueous solution (Fig. 2d) [41]. They investigated the influence of power, treatment time, pH, and initial concentration of Carbamazepine on the process efficiency. The authors used a fixed bed ultrasound reactor, where the solution was recirculated. They used a 24 factorial matrix to determine the best conditions for degradation (rate of approximately 90%): power (43 W), treatment time (116 min), pH (7), and initial concentration (8.05 μg.L-1). The authors also verified that Carbamazepine was transformed mainly into Anthranilic Acid and Acridine. They further observed that the increase in power increased the degradation of the target molecule. According to them, the increased power of ultrasonic radiation results in a more vigorous collapse of cavitation bubbles.[41].
3.1.2. Dyes
The ultrasound degradation of Methylene Blue (MB), Methyl Orange (MO), and phenolic compounds (PC) in an aqueous solution was studied by Uddin and Okitsu (Fig. 3a-b) [31]. MB was degraded using a constant ultrasonic frequency at 200 kHz. The degradation rate depends on the gas dissolved in the solution, following the order at 10 °C Ar > O2 > Ne > He. In the presence of argon, the degradation rate decreases as the temperature increases. The authors also investigated the effect of CCl4 and C6F14 on the sonochemical degradation of MB. They verified that the presence of CCl4 in solution improved the degradation rates, probably due to chlorine radical formation. On the other hand, the initial rate of MB degradation was not enhanced by the addition of C6F14. Under suitable conditions, the authors achieved>80% degradation. [31].
Fig. 3.
Molecular structures of the dyes mentioned in the review.
The ultrasound degradation of Naphthol Blue-Black (NBB) (Fig. 3c) in an aqueous solution was investigated by Ferkous, Merouani, and Hamdaoui, using a frequency of 1700 kHz [28]. The authors evaluated the effect of the addition of NaCl, Na2SO4, and persulfate ions (PS) in different matrices (natural water and seawater) in the sonochemical degradation of the dye. They also investigated the influence of the initial dye concentration and the temperature on the extent of the NBB removal. They observed that the NBB degradation rate increased by increasing the initial dye concentration in the range of 3 to 15 mg.L-1. Near 65 °C, 96.5% of the starting material was depleted after 45 min of sonication, against 51% at 25 °C. The presence of salts (NaCl or Na2SO4) had a beneficial effect on the NBB degradation. For instance, the presence of NaCl (0.5 and 1 mol.L-1) improved 3 to 6 times the degradation rate. The authors also reported that the complete NBB degradation took just 40 min in seawater and 60 min in natural water, compared to 210 min in distilled water. The addition of persulfate significantly improved the process due to the formation of sulfate radicals (SO4•) in the solution. The PS/US combination resulted in 100% NBB degradation after 60 min [28].
Behnajady et al. investigated the US degradation of Rhodamine-B (Fig. 3d) in an aqueous solution and evaluated several parameters [42]. The results showed that the discoloration follows a kinetic reaction of pseudo-first-order. The apparent reaction rate constant (kap) increases with decreasing pH and decreases with increasing initial dye concentration. Besides, the power density was a critical parameter, as its increase caused a considerable increase in removal efficiency. Finally, the authors observed changes in the UV–vis spectra in the reaction course. They concluded that the primary process was the aromatic ring cleavage, followed by N-de-ethylation. Under the best conditions, they obtained a 67% decrease in the chemical oxygen demand in 180 min of reaction. [42].
3.1.3. Pesticides
Nisharg and Gogate used a sonochemical process to degrade Dichlorvos (Fig. 4a) in wastewater [43]. The authors conducted the experiments using a simple ultrasound probe operating at 20 kHz and a nominal power of 270 W. Among the operational parameters evaluated, pH, temperature, and power density stand out. Besides, the authors evaluated the influence of additives such as H2O2, Fenton reagent, and CCl4. The authors observed that low-frequencies are efficient in treating wastewaters contaminated with Dichlorvos. Moreover, acidic conditions, optimal temperature values, energy dissipation, and the introduction of additives favor degradation. The combination of ultrasound and Fenton's reagent led to complete degradation of the initial pesticide load. Studies with radical traps suggested that degradation occurred via a radical mechanism. Finally, the authors concluded that the system cost and stability are advantages of low-frequency sonochemical reactors [43].
Fig. 4.
Molecular structures of the pesticides mentioned in the review.
Yao et al. studied Parathion's ultrasonic degradation (Fig. 4b) in an aqueous solution [44]. The authors observed that the degradation rate decreased with the increase in the initial concentration and decreased power. Furthermore, they observed that the ideal frequency for degradation was 600 kHz. According to the authors, degradation occured predominantly through radical reactions in the interfacial region of the cavitation bubble and, to a lesser extent, inside the bubbles. It was described as a heterogeneous gas/liquid reaction that obeys a kinetic based on the Langmuir-Hinshelwood model. Also, the presence of nitrogen promoted the formation of nitrite (NO2•) radicals. This species participated in the degradation of Parathion to form Paraoxon and 4-Nitrophenol by two different pathways. This result is in agreement with the theoretical calculations based on molecular orbitals energies [44].
3.1.4. Aromatic compounds
Naphthalene, acenaphthene, and phenanthrene (Fig. 5 a-c) had their sonochemical degradation in an aqueous solution investigated by Psillakis et al. [45]. The authors studied the influence of operational parameters such as the initial concentration of substrates, the reaction system's temperature, applied power, ultrasound frequency, and other species' presence in the medium (1-Butanol, NaCl and FeSO4). In the investigated conditions (initial concentrations of 150, 300, and 450 μg.L-1, temperatures of 20 and 40 °C, applied power of 45, 75, and 150 W, and ultrasound frequencies of 24 and 80 kHz), the degradation of the three aromatic hydrocarbons occurred within 120 min. The authors verified that degradation decreased with increasing temperature and decreasing power and frequency. An excess of dissolved NaCl also reduced degradation. Conversely, ferrous ions at low concentrations increased the degradation due to Fenton-type reactions' contribution. Finally, experiments in the presence of 1-Butanol revealed that the reaction was suppressed, showing the vital role of radicals in this process [45].
Fig. 5.
Molecular structures of the aromatic compounds mentioned in the review.
Kim, Huang, and Chiu investigated Dibenzothiophene's ultrasound decomposition (Fig. 5d) in an aqueous solution [46]. The sonochemical decomposition followed a first-order kinetic model. The rate constant increased by the increase of the ultrasonic energy, temperature, and solution pH. However, the decomposition decreased with an increase in the initial concentration of Dibenzothiophene. The activation energy was 12.6 kJ mol−1 in the temperature range of 10–15 °C, suggesting that the reaction is under diffusion control. The authors identified Hydroxy-dibenzothiophenes and Dihydroxy-dibenzothiophenes as by-products, arising from Dibenzothiophene's oxidation by the hydroxyl radicals. Finally, the kinetic analysis suggested that approximately 72% of Dibenzothiophene decomposition was due to the hydroxyl radical's participation [46].
3.2. Use of ultrasound coupled to other AOPs
3.2.1. Drugs
Kwon Im et al. investigated the ultrasound degradation of the analgesics Acetaminophen (AAP) and Naproxen (NPX) (Fig. 2 q-r) in the presence of single-walled carbon nanotubes (SWNTs) in an aqueous solution [47]. The authors found that the drugs' degradation depends on the ultrasonic frequency, the solution pH, and the system temperature. The highest degradation rates for both molecules in the absence of SWNTs occurred at high frequencies (1000 kHz) and in acidic conditions (pH 3). Conversely, at 25 °C and in the absence of SWNTs, degradation of 5.2% for AAP and 10.6% for NPX at 28 kHz occurred. When the frequency was 1000 kHz, and the temperature was 35 °C, the degradation was 29.1% and 46.2% for AAP and NPX, respectively. The addition of SWNTs significantly improved degradation rates. The authors managed to degrade 44.5% and 90.3% of AAP and NPX, respectively, at 25 °C and 28 kHz. The higher degradation rate provided by the SWNT/US system is associated with nanotubes dispersed in solution, which function as nuclei during the reaction, increasing the production of H2O2 [47].
The research group of Yavas used commercial TiO2 (P-25) with nanoparticles of Pd and Au on its surface, generated by sonochemical reduction, for degradation of an aqueous solution of Paracetamol (Fig. 2o) [48]. The authors performed ultrasound degradation for comparison purposes. The degradation promoted by P-25 solely was minimal and similar to sonolysis and photolysis. However, the combination of ultrasound and photocatalysis (with the composites Pd-TiO2 and Au-TiO2) showed considerably superior results. The composites favor degradation due to their small particle sizes that assist the formation and collapse of cavitation bubbles. The addition of persulfate further improved the Paracetamol degradation, showing that this species can generate radicals that improve the overall efficiency [48].
Pan and his research group investigated the synergistic effect of combining ultrasound with Fe/persulfate (PS) to degrade the antibacterial drug Sulfamethazine (Fig. 2s) in an aqueous solution [12]. The authors used the Fe/PS system to generate sulfate radicals, efficiently removing persistent contaminants [12]. The authors showed that the systems US, Fe/US, pre-magnetized-Fe/US, and PS/US systems removed 1.6%, 4.1%, 6.2%, and 7.2% of Sulfamethazine after 1 h, respectively. The results indicated that the US, Fe/US, pre-magnetized-Fe/US, and PS/US could not deplete Sulfamethazine efficiently. The combination of pre-magnetized-Fe with PS increased the removal of Sulfamethazine to 49.3% within 1 h. The ultrasound's application enhanced the Fe/PS and pre-magnetized-Fe/PS systems' efficiency to 49.8% and 98.3% Sulfamethazine removal, respectively. [12]
Davydov et al. investigated the effect of the ultrasound on Salicylic Acid photodegradation using four different commercial TiO2 materials as a photocatalyst [49]. The authors verified that the ultrasound, combined with photocatalysis, had a pronounced effect on Salicylic Acid depletion (Fig. 2t) compared to photocatalysis solely. The authors proposed that the increase in activity may be related to the breakdown of aggregates (particles of photocatalysts) and oxidizing species' formation due to ultrasonic waves. Combining the ultrasound with photocatalysis produced synergistic effects with smaller particle sizes. However, photocatalysts with larger particle sizes (anatase from Sigma-Aldrich) produced no improvement. Degussa's P25 exhibited the highest activity to degrade Salicylic Acid, but the increase was moderate when combined with ultrasound. The authors observed reaction intermediates in solution during the photocatalytic degradation of phenol. The sonication of the system, however, allowed for the elimination of toxic intermediates [49].
Arriaga et al. investigated Ibuprofen's degradation (Fig. 2h) using various advanced oxidation hybrid systems [50]. The authors used the following systems: TiO2/US, photo-Fenton/US, and TiO2/photo-Fenton/US. The results indicated that the photo-Fenton/US system caused degradation of 95% and mineralization of 60% of Ibuprofen. The TiO2/US system led to the complete removal of Ibuprofen and mineralization of 50%. The TiO2/photo-Fenton/US system caused complete removal of Ibuprofen and mineralization of 92% within 240 min of reaction. The authors argued that hybrid advanced oxidation processes seem to be suitable systems for the degradation of recalcitrant micro-contaminants [50].
Su et al. investigated the degradation of aqueous solutions of the antibiotic Amoxicillin (Fig. 2e), induced by sulfate radicals under the action of ultrasonic waves [51]. The systems used were: ozone, cobalt-activated ozone (ozone/Co2+), ozone combined with US (ozone/US), and finally, the three combined systems (ozone/Co2+/US). The authors optimized the following parameters: temperature, ultrasound power, initial concentration of amoxicillin, ozone concentration, and the catalyst. The results showed that the removal efficiency of chemical oxygen demand (COD) followed the order: ozone < ozone/Co2+ < ozone/Co2+/US. Under the optimized conditions, the authors achieved 98% COD removal after 60 min. Comparative analysis revealed that the sulfate radicals had a high oxidation potential, and the use of the US reduced the energy barrier of the reaction, increasing COD removal efficiency [51].
3.2.2. Dyes
Monteagudo et al. investigated the degradation of the dye Reactive Blue 4 (Fig. 3e) by coupling the homogeneous photo-Fenton system with the ultrasound [52]. The authors accessed the degradation process's efficiency by determining the total organic carbon (TOC) content decrease. The influence of the initial concentrations of Fe2+, pH, and H2O2 on the dye's mineralization was appraised. In the optimized conditions, 94% of the TOC removal occurred in 60 min. The authors postulated that the mineralization of the dye by the photo-Fenton/US system occurred by a set of mechanisms: (i) mineralization by direct photolysis, (ii) oxidative species generated by the ultrasound, (iii) direct oxidation reactions with H2O2, (iv) radical reactions (mainly with the hydroxyl radical) and (v) direct pyrolysis inside the cavitation bubble. The authors also found that radical reactions (in solution and the bubbles' vicinity) were responsible for mineralization, contributing to 93.60%, followed by reactions with H2O2 (2.56%). Photolysis and reactions with oxidative species generated by the ultrasound contributed to 1.92% [52].
Maezawa et al. investigated the degradation of the dye Orange Acid 52 (Fig. 3b) in an aqueous solution [53]. The authors investigated how photocatalysis, ultrasound, or both processes combined affect dye degradation. The photocatalysis was not efficient in consuming the dye since approximately 35% of its initial concentration persisted after 480 min. On the other hand, the US allowed its complete decomposition after 300 min, but the concentration of TOC decreased by only 13% in 480 min. The coupled process (photocatalysis/US) allowed total degradation in 240 min, but TOC decreased only to about 13% in 480 min. The authors concluded that the coupling of TiO2 photocatalysis combined with the ultrasound presented a remarkable synergistic effect [53].
Eslami et al. used a relatively new method that has gained attention in recent years, consisting of sodium percarbonate (Na2CO3·1.5H2O2) to generate radicals in solution [54]. The US was used to activate the photocatalyst and promote Orange Acid 7 degradation (Fig. 3f) in an aqueous solution [54]. According to the authors, ultrasound and UV radiation emitted from a diode (UVC-LEDs) were efficient in generating radicals in the presence of a photocatalyst. Under the best conditions (pH 6.0, photocatalyst concentration of 1.5 mmol.L-1, ultrasound power of 100 W, and the reaction time of 90 min), a dye degradation rate of 93% was achieved. The results also indicated that nitrate and chloride ions contributed to the process's improvement, proportional to the ionic concentrations. The authors also observed that nitrate ions' photolysis and sonolysis could degrade the dye, indicating that the hydroxyl radical is generated even in photocatalyst absence. According to the results, hydroxyl and superoxide are the radicals with the highest concentration in the process. This work demonstrated that the photocatalyst/UVC/US system could be a new efficient method to degrade organic pollutants in water [54].
Kumar et al. investigated the photocatalytic/US activity of ZnO nano-aggregates used to degrade the Methyl Orange dye (Fig. 3b) in an aqueous solution under UV light [55]. The results revealed a strong synergistic effect between UV light and ZnO nano-aggregates in dye degradation. The generation of hydroxyl radicals increased with sonication, contributing to the synergistic effect [55].
Lops et al. used nano and microstructures of ZnO in different morphologies for the ultrasound degradation of Rhodamine-B (Fig. 3d) promoted by reactive oxygen species (ROS) [56]. These species are usually generated by ultrasonic stimulation of different ZnO structures and unveiled by electronic paramagnetic resonance spectroscopy (PRS) [56]. According to the authors, five ZnO structures had their activity investigated. The structure, named desert roses (DRs), showed the best activity. This system generated the highest proportion of hydroxyl radicals, degrading 100% rhodamine B (RhB) in 180 min under US exposure. Surprisingly, 100% degradation was obtained in just 10 min when carried in the presence of solar radiation, revealing a tremendous positive synergy between the photocatalytic and ultrasound mechanisms. The authors concluded that such a photocatalytic/ US system, under solar radiation, shows great potential as an efficient method for depleting dyes in wastewaters [56].
Zhou et al. investigated the activity of the LuFeO3 material (particles with an average size of 200 nm) exposed to ultrasound on the degradation of Rhodamine B (Fig. 3d) [57]. Several experimental parameters, such as ultrasonic frequency, solution temperature, catalyst concentration, initial dye concentration, and solution pH, were evaluated. According to the authors, four factors significantly influence such a process, pH being of little importance. The best conditions for the dye degradation were: frequency of 60 kHz, temperature 40 °C, catalyst concentration 4 g.L-1, and initial concentration of dye 5 mg.L-1. The authors used Terephthalic Acid and fluorimetry to detect hydroxyl radicals. The experiment revealed the production of hydroxyl radicals on the LuFeO3 particles under ultrasonic radiation. There was also a decrease in dye degradation with ethanol that probably consumes part of the hydroxyl radicals. This result revealed that hydroxyl radicals are the main species in dye degradation indeed. [57].
Bokhale et al. investigated the sonocatalytic and photosonocatalytic degradation of Rhodamine 6G (Fig. 3g) in an aqueous solution, using the CuO and TiO2 catalysts [58]. The authors performed experiments under a constant pH (12.5) and varied the catalyst concentration (ranging from 1.5 to 4.5 g.L-1). They achieved the maximum degradation rate with CuO and TiO2 concentrations of 1.5 and 4.0 g.L-1, respectively. Under these conditions, the dye's concentration decreased by 52.2 and 51.2%, respectively, after a reaction time of 180 min. The use of scavengers indicated that the degradation process occurs mainly by a radical mechanism. In coupled assays (US/UV) using TiO2 as a catalyst, the authors achieved 63.3% degradation. They concluded that, in general, coupled processes promote superior degradation compared to isolated ones [58].
The sonophotocatalytic degradation of the Reactive Blue 19 dye (Fig. 3h) was studied in the presence of sulfur-doped TiO2 nanoparticles (anatase) (S-TiO2) [59]. The efficiency of several methodologies carried out individually for the dye's degradation was investigated, such as sonolysis, photolysis, catalysis, sonocatalysis, and sonophotochemical coupling. The authors evaluated parameters such as pH, catalyst dosage, initial dye concentration, ultrasound power, and the effect of TiO2 doping. The sonophotocatalytic process stands out over the others, degrading 90% of the dye in 120 min of reaction. According to the authors, the process was considered feasible, efficient, and economical to degrade complex dyes such as reactive blue 19 [59].
Sadeghi et al. used a novel nanocomposite (Cr-MIL-101 immobilized on NiO/13X zeolite) for the sonocatalytic degradation of dyes, Methylene Blue (Fig. 3a), Rhodamine B (Fig. 3d), and Methyl Orange (Fig. 3b), in an aqueous solution with H2O2 [60]. The authors evaluated the influence of several parameters, such as contact time and the concentrations of dye, catalyst, and H2O2. They achieved a rate constant (k) of 0.0323 min−1 and a half-life time (t1/2) of 21.4551 min for Methylene Blue degradation by the Cr-MIL-101@NiO/13X/US/H2O2 system. The optimized concentrations that provide the highest degradation rate (97.26%) were: catalyst (0.5 g.L-1), hydrogen peroxide (40 mmol.L-1), and Methylene Blue (25 mg.L-1). Besides, an additional investigation proved that hydroxyl radical is in charge of the degradation process. [60].
Pang and Abdullah investigated the sonochemical degradation of various dyes: Congo Red (CR, Fig. 3i) Reactive Blue 4 RB-4, Fig. 3e), Methyl Orange (MO, Fig. 3b), Rhodamine B (RhB, Fig. 3d), and Methylene Blue (MB, Fig. 3a), using TiO2 (powder and nanotubes) as a catalyst [61]. Sonocatalytic activity was elucidated based on the degradation of dyes. The powder was more efficient for degrading anionic dyes, while nanotubes were more efficient for degrading cationic dyes. They also observed that the sonocatalytic activity of the TiO2 nanotubes could be up to four times higher than the TiO2 powder under the ultrasound power of 100 W and a frequency of 42 kHz. It was explained considering a high electrostatic attraction between the dye molecules and TiO2 nanotubes. They concluded that TiO2 nanotubes have a high potential to be used as a sonocatalyst to degrade organic dyes. In conclusion, dyes with a xanthene structure are more likely to be destroyed by the ultrasound, followed by diazo, triazine, azo and similar structures to anthraquinone [61].
Zhaoet et al. investigated the discoloration of azo dyes, Acid Orange 8 (AO-8), Orange II (O-II), Acid Red 9 (AR-9), and Acid Yellow 11 (AY-11) (Fig. 3j-n) using hydrogen peroxide and sodium carbonate under the influence of low-frequency ultrasound radiation [62]. The percentage of discoloration of AO-8 was higher than 90% after 2 h of ultrasound irradiation. The discoloration rate was 0.023 min−1 under the influence of ultrasonic irradiation, which was approximately twice as high as without ultrasound. The authors also showed that the discoloration rate decreased following the order: AO-8 ≈ O-II > AR-9 ≫ AY-11, probably due to structural differences in dyes. According to the authors, unlike other works, hydroxyl radicals played a secondary role in degradation. The carbonate radical, generated by mixing sodium carbonate and hydrogen peroxide, was the predominant chemical species in the degradation process, contributing to 56.52%, followed by CO42− (32.61%) and 1O2 (singlet oxygen) (10.87%). Another difference is that carbonate radicals (CO3•-) arise from the cleavage of peroxy-carbonate or peroxy-dicarbonate (CO42- and C2O62-) under ultrasonic radiation instead of the reaction between the hydroxyl radical and carbonate [62].
Zhao et al. studied the zirconia nanotubes' effect in the sonochemical degradation of Methyl Orange (Fig. 3b) [63]. The authors used zirconia nanotubes with 25 µm in length, 80 nm in internal diameter, and walls with a thickness of 35 nm. The results showed synergism between the presence of nanotubes and ultrasonic waves for the degradation of methyl orange since, in the presence of ultrasound, the degradation is approximately 7 times higher. The authors also observed that the degradation was more accentuated in an acid medium. At pH 2 and an initial concentration of 20 mg.L-1, the percentage of Methyl Orange discoloration reached 94.6% and 97.6% after 6 h and 8 h of reaction, respectively. Under the same conditions, the percentage of degradation decreased with increasing concentration of the dye solution. According to the authors, the synergistic effect is related to the dye's adsorption by zirconia nanotubes, which would lead to a straightforward degradation under the influence of ultrasound [63].
3.2.3. Pesticides
Sathishkumar et al. worked with nanostructures of TiO2 and TiO2 with Au on the surface (Au-TiO2) for the sonocatalytic, photocatalytic and sonophotocatalytic degradation of the pesticide Simazine (Fig. 4c) [64]. The calculated rate constants allowed the authors to estimate the decrescent order for simazine degradation: sonophotocatalysis > sonocatalysis > photocatalysis. Mineralization in sonophotocatalysis was 1.65 and 1.38 times higher than photocatalysis and catalysis, respectively. Sonophotocatalytic degradation resulted in a 43% decrease in total organic carbon in 7 h of reaction [64].
Patil and Gogate used several processes to promote the degradation of Dichlorvos' commercial solutions (Fig. 4a) at pH 3 and the initial concentration of 20 mg.L-1 [7]. The authors used an ultrasound device with an operating power of 150 W and a 36 kHz frequency. They combined several processes such as TiO2/US, TiO2/solar radiation, TiO2/solar radiation/US, H2O2/US, Fenton/US, and ozone/US. A significant removal was observed for the TiO2/solar radiation (78.42%) and Fenton/US (81.19%) processes in 2 h of treatment, compared to only the ultrasound (6.4%) or TiO2 (3%). The authors demonstrated the beneficial aspects of combined over non-combined processes. Hence, they achieved 93% mineralization by submitting an aqueous Dichlorvos solution with the ozone/US system for 2 h [7].
3.2.4. Degradation of polycyclic aromatic compounds
Li et al. used bio-synthesized schwertmannite (Sch) to promote the degradation of Bisphenol-A (BPA) (Fig. 5e) using iron-oxy-hydroxy-sulfate as a Fenton-type catalyst, combined with the ultrasound [65]. The authors reported a synergistic effect between the ultrasound and Sch in the activation of H2O2. The catalytic system's efficiency was mainly affected by the pH, dosage of Sch, and temperature, whereas the H2O2 concentration was less relevant. The results indicated that hydroxyl radicals were the main species that caused the degradation of Bisphenol-A. The authors verified that hydroxyl radicals arise from both homogeneous and heterogeneous pathways. They argue that the proportional effect of the ultrasound on Fenton-type BPA degradation is due to the following reasons: (1) the ultrasound increases the generation of radicals through cavitation; (2) the ultrasound decreases the activation energy of the degradation reaction; (3) the ultrasound accelerates the dissolution of iron; (4) the US continuously removes impurities from the catalyst surface [65].
Lu et al. studied the degradation of Bisphenol S (Fig. 5f) under the influence of the ultrasound in the presence of dissolved oxygen (DO) or peroxy-disulfate (PDS) [66]. The authors noticed that for the PDS/US system, the PDS concentration increase resulted in faster degradation of Bisphenol S. They also investigated the formation of hydroxyl and sulfate radicals in the PDS/US system. The results showed that the sulfate radical was predominant, but the dissolved oxygen effectively improved the degradation of bisphenol S. The hydroxyl radicals, instead of O2•-, are the main reactive oxygen species. The generation of hydroxyl radicals may be related to the reaction of dissolved oxygen with H•, which decreases the recombination rate with the hydroxyl radical [66].
3.3. Factors affecting reactions in aqueous media promoted by US
3.3.1. Hydrophobicity and volatility
In general, the sonochemical degradation of chemical compounds can occur through two distinct pathways: oxidation by hydroxyl radicals and pyrolytic decomposition. Hydrophobic and volatile compounds tend to migrate towards the bubble and predominantly degrade via hydroxyl radical and pyrolytic reactions occurring at the bubble–liquid interfacial region and inside the bubble, while hydrophilic and non-volatile compounds tend to accumulate in the liquid phase, where they degrade via hydroxyl radical reactions [45].
3.3.2. Presence of other species in solution
Other species in solution can contribute to the removal of the target molecule in the US degradation process. If the species forms radicals that contribute to degradation, it will have a synergistic effect [12], [44], [54], [62]. For example, solutions that have nitrate, chloride, or release chlorine in solution, can contribute to degradation due to the formation of highly oxidizing species [28], [31], [54]. For example, carbon tetrachloride undergoes degradation in the presence of ultrasonic irradiations and results in the generation of additional oxidizing species in the reaction system. It is believed that CCl4 is entrapped in the cavitating bubble and is dissociated into Cl• and •CCl3. •CCl3 further dissociates into Cl• and •CCl2 radicals. Cl• and Cl• can also combine to form a potent oxidizing agent as Cl2. Chlorine, so produced during pyrolysis, can further combine with H2O to form HOCl and HCl. It is also possible that •CCl3 combines with H•, yielding CHCl3. Cl2 and HOCl are strong oxidizing agents which are independently capable of oxidizing organic compounds in the solution [43]. It has also been shown that bubbling nitrogen in the solution can increase the extent of degradation, promoting the formation of nitrite (NO2•) radicals, which contribute to radical degradation [44]. It has also been reported that the use of Feo/persulfate (PS) together with the US, significantly increases and the degradation of some organic molecules in aqueous media, as it generates radicals SO4• - [12]. An innovative technology that has been used successfully is oxone (2KHSO5.KHSO4.K2SO4) to generate sulfate radicals has gained significant importance and is a more efficient and powerful oxidant than hydroxyl radicals in water treatment. Oxone is the source of the potent oxidant peroxymonosulfate (HSO-5) and can generate sulfate radicals (SO4• -) with higher standard redox potentials than hydroxyl radicals (HSO-5/SO4• - +1.82 V, H2O2/H2O + 1.76 V) [51], [66]. More recent studies also demonstrated that CO32− in solution, under the US's action, generates carbonate radical (CO3•-) [62]. This species is an active radical with a low redox potential (E0) of 1.63 V at pH 8.4 and 1.59 at pH 12 [54].
However, other species can act as traps for radicals formed during sonication in aqueous media and decrease US as AOP potential [31], [56]. It is still possible that the combination of HO• with other species present in the aqueous medium forms radicals with less oxidation power for a given system than the radical HO• [57].
3.3.3. Medium pH
The solution pH and the molecules pKa are determinants of species' polarity in solution [47]. By changing the medium's pH, the molecular structure is modified so that chemical species can become positively or negatively charged, form a zwitterionic species, or even become neutral. Besides, pH strongly influences the generation or elimination of radicals in the solution [43].
For some molecules the rate of degradation increases with decreasing pH [42], [43], [47], [59], [63], [65]. This may be related to the enhanced generation of free radicals under acidic conditions or the higher oxidation potential of hydroxyl radical (HO•) in acidic conditions. Also, a high pH value may create more free radical scavengers (i.e., CO32–, HCO3–), resulting in a decrease in the concentration of HO• [43]. Hence, molecules that are preferentially degraded via hydroxyl radicals may have superior removal efficiency under acidic conditions. Moreover, the dependence of degradation with pH indicates that pKa has an important role in the process. The variation of the medium's pH can make the molecule more hydrophobic or more hydrophilic, and this alters the region where the molecule will predominantly react [47]. This also depends on factors such as volatility and diffusivity. The medium's pH can affect the target molecule's structure and solids' surface charge in sonophotocatalytic processes. For instance, the pH decrease results in greater adsorption of a given molecule on the catalyst surface, thus contributing to a greater degradation efficiency [59].
Other studies, however, show that degradation increases by increasing the solution pH [46]. These conflicting observations may be explained by the parent compounds' solubility and their intermediates at different pHs. Assuming that most reactions occur at the bubble–liquid interface, compounds that become increasingly ionized (and more soluble) at higher pHs (e.g., nitrophenols) would have a smaller partition in the interface exhibiting lower reaction rates at higher pHs. On the other hand, polar products can become ionizable and occupy the bubble–liquid interface at higher pHs [46].
3.3.4. Target molecule concentration
Some authors have observed that the degradation rate of target molecules in some US processes increases with increasing initial concentration [28], [45]. However, an antagonistic behavior is also observed [12], [42], [44], [46], [56], [57], [59], [60], [63]. Assuming that most reactions occur at the bubble–liquid interface, the decomposition may be limited by the available interfacial area in these cases. Therefore, higher initial concentrations of the target molecule and corresponding higher concentrations of intermediates at the interface can inhibit the target molecule's decomposition [46]. More precisely, the degradation efficiency can be influenced by several factors, such as: (i) at high concentrations, the formation of reaction intermediates increases, so the competition begins between the intermediates and the target molecules for the occupation of active sites; (ii) hydroxyl radicals react with both the target molecules and the reaction intermediates, so the degradation efficiency decreases because hydroxyl radicals concentration reaches a steady value; (iii) at high concentrations, the solution becomes intensively colored, and fewer photons reach the surface of the photoactive materials, reducing the photocatalytic contribution in sonophotocatalytic processes [56], [59]; (iv) in sonophotocatalytic processes, adsorption of the target molecule can occur on the photocatalyst surface decreasing the adsorption of H2O/OH−, thus decreasingly the holes to generate HO• radicals [57], [59].
3.3.5. US frequency
Although the frequency is a complex issue associated with bubble formation dynamics, higher frequencies may increase free radicals' production by increasing the cavitation events [57]. Moreover, as bubble lifetime is shorter at higher frequencies, free radicals have a greater chance to escape from the bubble and migrate towards the bulk solution before undergoing any recombination reactions [45].
However, other authors have shown that low-frequency is efficient to degrade some target molecules and have superior stability and lower operational cost than high-frequency systems. [43]. Therefore, considering the frequency parameter alone, more volatile and hydrophobic molecules, which tend to migrate into the cavitation bubble, may have their degradation improved at lower frequencies. Conversely, less-volatile and more hydrophilic molecules, which tend to remain in the liquid phase or migrate to the bubble–liquid interface, tend to undergo degradation at higher frequencies.
3.3.6. US power
The beneficial effect of power on degradation rates is probably due to an increased cavitational activity occurring at higher power levels. As power increases, the number of collapsing cavities also increases, leading to enhanced degradation rates [45]. Several reports show the benefit of increased power or the US's power density on the degradation of organic compounds in an aqueous medium [42], [46], [59]. Moreover, in sonophotocatalytic processes, the removal rate increase is also attributed to the photocatalyst solid's breakdown. At higher powers, solid particles tend to de-agglomerate, generating a superior number of active sites and improving degradation rates [59].
3.3.7. Temperature
Some studies show that temperature has little influence on the sonochemical degradation of organic compounds in water [43]. Moreover, although this influence is not very large, an increase in the temperature results in a decrease in the extent of degradation [43]. These results can be explained based on the dominant contribution of the cavitational activity reduction at higher operating temperatures. At higher operating temperatures, the vapor content increases, which exerts a cushioning effect on the collapsing bubble, i.e., increases the resistance for the inward motion of the bubble during the collapse phase. The energy released decreases and subsequently reduces the generation of free radicals [43]. In addition to this, increased temperatures are likely to favor the liquid phase's degassing, thus reducing the number of gas nuclei available for bubble formation. [45].
The relationship between the increase in temperature and the extent of degradation often occurs in the same direction [51], [46]. In these cases, the molecules have low apparent activation energy in the studied temperature range, and the diffusion step probably controls the decomposition. The apparent rate most likely reflects the rate at which molecules migrate from the bulk solution to the bubble–liquid interface where temperature and radical hydroxyl concentration are high. [46]. Therefore, more molecules migrating to the interfacial region results in a greater extent of removal.
However, the two trends are observed in some works, depending on the temperature range studied [57]. The increase in temperature is translated into an increase in the target molecule's degradation to some extent. However, after a specific value, degradation decreases with increasing temperature. Generally, chemical reactions have their rates increased with increasing temperature. However, in processes in which sonoluminescence plays a significant role in the excitation of electrons from the valence to the conduction band, the degradation decreases with increasing temperature, because lower temperatures will lead to an increased generation of sonoluminescence [57].
4. Quantitative of publications that have used the ultrasound as AOP in the last twenty years
Fig. 6 shows the number of articles published in the last twenty years using the US as an advanced oxidation process, either individually or in combination with other AOPs. Starting from 2000, one can note an increasing number of publications, with the maximum (13) reached in 2010. From 2010, there has been a noticeable oscillation in the number of publications each year, but with a downward trend. For instance, at the end of 2020, when writing this article, the number of publications was only 3. Moreover, especially in the last decade, there has been an increase in the number of works using US combined with other systems. In many cases, the US's coupling with other systems results in a synergistic effect that helps remove organic contaminants in aqueous media [7], [12], [58], [59], [60], [61], [62], [63], [64], [65], [66], [47], [48], [51], [52], [54], [55], [56], [57].
Fig. 6.
Articles published each year from 2000 to 2020.
Throughout the period evaluated (2000–2020), coupled processes represent approximately two-thirds of the articles published (Fig. 7). These publications clearly show the superior efficiency of coupled processes compared to isolated ultrasound. The higher production of hydroxyl radicals in coupled processes undoubtedly causes a synergistic effect that explains their remarkable capability in depleting organic contaminants in an aqueous medium. However, when ultrasound is combined with another AOP, pyrolytic degradation may occur, especially for hydrophobic molecules that tend to occupy the core of the cavitation bubbles [45].
Fig. 7.
The proportion of publications that used only ultrasound and ultrasound coupled with other AOPs.
The degradation of organic contaminants in aqueous media, in coupled processes with US, can occur by several mechanisms: (i) mineralization by direct photolysis, (ii) oxidative species generated by the ultrasound, (iii) direct oxidation reactions with H2O2, (iv) radical reactions (mainly with the hydroxyl radical, generated by different processes) and (v) direct pyrolysis inside the cavitation bubble [52]. Besides, some ions in solution can increase the rate of removal of organic contaminants in water [12], [44], [54], [62].
5. Conclusions and future directions
We herein prepared a review on the use of ultrasound as an advanced oxidation process covering the period 2000–2020. Ultrasound has shown to be technically feasible and economically viable, being less expensive than other AOPs. In the revised period, most of the publications (approximately two-thirds) used ultrasound combined with another AOP, which seems to be the most promising arrangement. Although the potential of ultrasound as AOP is evident, the number of publications is still relatively small compared to other advanced oxidation processes. The need for additional work is latent, as it is still necessary to understand several parameters that govern US as an AOP, alone or coupled to other systems. There is a need for cooperation between researchers from the most fundamental areas (physics and chemistry), with those from more applied areas (engineering) to implement US as AOP on a large scale. In addition to the mentioned challenges, reactors' design is also an aspect that will demand efforts soon. Finally, based on the present review's exposure, we believe that the application of ultrasound in the degradation of organic contaminants in aqueous media has enormous potential and should be increasingly encouraged.
Funding
This work had the financial support of the Brazilian agencies, CNPq, FAPEMIG, and CAPES.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Nasir A.M., Jaafar J., Aziz F., Yusof N., Salleh W.N.W., Ismail A.F., Aziz M. A review on floating nanocomposite photocatalyst: Fabrication and applications for wastewater treatment. J. Water Process Eng. 2020;36:101300. doi: 10.1016/j.jwpe.2020.101300. [DOI] [Google Scholar]
- 2.Dashairya L., Sharma M., Basu S., Saha P. Enhanced dye degradation using hydrothermally synthesized nanostructured Sb2S3/rGO under visible light irradiation. J. Alloys Compd. 2018;735:234–245. doi: 10.1016/j.jallcom.2017.11.063. [DOI] [Google Scholar]
- 3.Domínguez I., Arrebola F.J., Martínez Vidal J.L., Garrido Frenich A. Assessment of wastewater pollution by gas chromatography and high resolution Orbitrap mass spectrometry. J. Chromatogr. A. 2020;1619:460964. doi: 10.1016/j.chroma.2020.460964. [DOI] [PubMed] [Google Scholar]
- 4.de Andrade F.V., de Lima G.M., Augusti R., Coelho M.G., Assis Y.P.Q., Machado I.R.M. A new material consisting of TiO2 supported on Nb2O5 as photocatalyst for the degradation of organic contaminants in aqueous medium. J. Environ. Chem. Eng. 2014;2(4):2352–2358. doi: 10.1016/j.jece:2014.02.004. [DOI] [Google Scholar]
- 5.De Andrade F.V., De Lima G.M., Augusti R., Coelho M.G., Ardisson J.D., Romero O.B. A versatile approach to treat aqueous residues of textile industry: The photocatalytic degradation of Indigo Carmine dye employing the autoclaved cellular concrete/Fe 2O 3 system. Chem. Eng. J. 2012;180:25–31. doi: 10.1016/j.cej.2011.10.089. [DOI] [Google Scholar]
- 6.Nsenga Kumwimba M., Meng F., Iseyemi O., Moore M.T., Zhu B., Tao W., Liang T.J., Ilunga L. Removal of non-point source pollutants from domestic sewage and agricultural runoff by vegetated drainage ditches (VDDs): Design, mechanism, management strategies, and future directions. Sci. Total Environ. 2018;639:742–759. doi: 10.1016/j.scitotenv.2018.05.184. [DOI] [PubMed] [Google Scholar]
- 7.Patil P.N., Gogate P.R. Degradation of Dichlorvos using hybrid advanced oxidation processes based on ultrasound. J. Water Process Eng. 2015;8:e58–e65. doi: 10.1016/j.jwpe.2014.10.012. [DOI] [Google Scholar]
- 8.Collings A.F., Gwan P.B. Ultrasonic destruction of pesticide contaminants in slurries. Ultrason. Sonochem. 2010;17(1):1–3. doi: 10.1016/j.ultsonch.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 9.A novel TiO2_autoclaved cellular concrete composite_ From a precast building material to a new floating photocatalyst for degradation of organic water contaminants _ Elsevier Enhanced Reader.pdf, (n.d.).
- 10.Serna-Galvis E.A., Botero-Coy A.M., Martínez-Pachón D., Moncayo-Lasso A., Ibáñez M., Hernández F., Torres-Palma R.A. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019;154:349–360. doi: 10.1016/j.watres.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 11.Monteagudo J.M., Durán A., San Martín I. Mineralization of wastewater from the pharmaceutical industry containing chloride ions by UV photolysis of H2O2/Fe(II) and ultrasonic irradiation. J. Environ. Manage. 2014;141:61–69. doi: 10.1016/j.jenvman.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Pan Y., Zhang Y., Zhou M., Cai J., Li X., Tian Y. Synergistic degradation of antibiotic sulfamethazine by novel pre-magnetized Fe0/PS process enhanced by ultrasound. Chem. Eng. J. 2018;354:777–789. doi: 10.1016/j.cej.2018.08.084. [DOI] [Google Scholar]
- 13.Dewil R., Mantzavinos D., Poulios I., Rodrigo M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manage. 2017;195:93–99. doi: 10.1016/j.jenvman.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Kanakaraju D., Glass B.D., Oelgemöller M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manage. 2018;219:189–207. doi: 10.1016/j.jenvman.2018.04.103. [DOI] [PubMed] [Google Scholar]
- 15.Sun Q., Hong Y., Liu Q., Dong L. Synergistic operation of photocatalytic degradation and Fenton process by magnetic Fe 3 O 4 loaded TiO 2. Appl. Surf. Sci. 2018;430:399–406. doi: 10.1016/j.apsusc.2017.08.085. [DOI] [Google Scholar]
- 16.Asghar A., Raman A.A.A., Daud W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015;87:826–838. doi: 10.1016/j.jclepro.2014.09.010. [DOI] [Google Scholar]
- 17.Rivas F.J., Solís R.R., Beltrán F.J., Gimeno O. Sunlight driven photolytic ozonation as an advanced oxidation process in the oxidation of bezafibrate, cotinine and iopamidol. Water Res. 2019;151:226–242. doi: 10.1016/j.watres.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Rosa J.M., Tambourgi E.B., Vanalle R.M., Carbajal Gamarra F.M., Curvelo Santana J.C., Araújo M.C. Application of continuous H2O2/UV advanced oxidative process as an option to reduce the consumption of inputs, costs and environmental impacts of textile effluents. J. Clean. Prod. 2020;246:119012. doi: 10.1016/j.jclepro.2019.119012. [DOI] [Google Scholar]
- 19.Zhang M.-H., Dong H., Zhao L., Wang D.-x., Meng D.i. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019;670:110–121. doi: 10.1016/j.scitotenv.2019.03.180. [DOI] [PubMed] [Google Scholar]
- 20.Nakagomi F., Cerruti S.E., de Freitas M.R., Freitas Neto E.S., de Andrade F.V., Siqueira G.O. Niobium pentoxide produced by a novel method microwave assisted combustion synthesis. Chem. Phys. Lett. 2019;729:37–41. doi: 10.1016/j.cplett.2019.05.003. [DOI] [Google Scholar]
- 21.Resende S.F., Augusti R., de Lima G.M., Gouveia R.L., Oliveira B.S., Krambrock K., Siqueira G.O., de Andrade F.V. Visible-light driven catalytic activity of two novel Cu(II) and Ni(II) titanium niobates. J. Environ. Chem. Eng. 2019;7(3):103065. doi: 10.1016/j.jece:2019.103065. [DOI] [Google Scholar]
- 22.Coelho M.G., de Andrade F.V., de Lima G.M., Augusti R., Ferreira M.P., Maria D.A., Ardisson J.D. Preparation of a new composite by reaction of SnBu3Cl with TiCl4 in the presence of NH4OH-photocatalytic degradation of indigo carmine. Appl. Organomet. Chem. 2011;25(3):220–225. doi: 10.1002/aoc.v25.310.1002/aoc.1745. [DOI] [Google Scholar]
- 23.Mahamuni N.N., Adewuyi Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010;17(6):990–1003. doi: 10.1016/j.ultsonch.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Naddeo V., Belgiorno V., Kassinos D., Mantzavinos D., Meric S. Ultrasonic degradation, mineralization and detoxification of diclofenac in water: Optimization of operating parameters. Ultrason. Sonochem. 2010;17(1):179–185. doi: 10.1016/j.ultsonch.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Villaroel E., Silva-Agredo J., Petrier C., Taborda G., Torres-Palma R.A. Ultrasonic degradation of acetaminophen in water: Effect of sonochemical parameters and water matrix. Ultrason. Sonochem. 2014;21(5):1763–1769. doi: 10.1016/j.ultsonch.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Kandasamy K., Tharmalingam K., Velusamy S. Treatment of tannery effluent using sono catalytic reactor. J. Water Process Eng. 2017;15:72–77. doi: 10.1016/j.jwpe.2016.09.001. [DOI] [Google Scholar]
- 27.Joseph C.G., Li Puma G., Bono A., Krishnaiah D. Sonophotocatalysis in advanced oxidation process: A short review. Ultrason. Sonochem. 2009;16(5):583–589. doi: 10.1016/j.ultsonch.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Ferkous H., Merouani S., Hamdaoui O. Sonolytic degradation of naphthol blue black at 1700kHz: Effects of salts, complex matrices and persulfate. J. Water Process Eng. 2016;9:67–77. doi: 10.1016/j.jwpe.2015.11.003. [DOI] [Google Scholar]
- 29.González-García J., Sáez V., Tudela I., Díez-Garcia M.I., Esclapez M.D., Louisnard O. Sonochemical treatment of water polluted by chlorinated organocompounds. A review, Water (Switzerland) 2010;2:28–74. doi: 10.3390/w2010028. [DOI] [Google Scholar]
- 30.Cui D., Mebel A.M., Arroyo-Mora L.E., Zhao C., De Caprio A., O'Shea K. Fundamental study of the ultrasonic induced degradation of the popular antihistamine, diphenhydramine (DPH) Water Res. 2018;144:265–273. doi: 10.1016/j.watres.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 31.Uddin M.H., Okitsu K. Effect of CCl4 or C6F14 on sonochemical degradation of dyes and phenolic compounds in an aqueous solution. J. Water Process Eng. 2016;12:66–71. doi: 10.1016/j.jwpe.2016.05.001. [DOI] [Google Scholar]
- 32.Torres R.A., Abdelmalek F., Combet E., Pétrier C., Pulgarin C. A comparative study of ultrasonic cavitation and Fenton's reagent for bisphenol A degradation in deionised and natural waters. J. Hazard. Mater. 2007;146(3):546–551. doi: 10.1016/j.jhazmat.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 33.Eren Z. Ultrasound as a basic and auxiliary process for dye remediation: A review. J. Environ. Manage. 2012;104:127–141. doi: 10.1016/j.jenvman.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Ince N.H., Tezcanli G., Belen R.K., Apikyan İ.G. Ultrasound as a catalyzer of aqueous reaction systems: The state of the art and environmental applications. Appl. Catal. B Environ. 2001;29(3):167–176. doi: 10.1016/S0926-3373(00)00224-1. [DOI] [Google Scholar]
- 35.Gogate P.R. Cavitational reactors for process intensification of chemical processing applications: A critical review. Chem. Eng. Process. Process Intensif. 2008;47(4):515–527. doi: 10.1016/j.cep.2007.09.014. [DOI] [Google Scholar]
- 36.Serna-Galvis E.A., Isaza-Pineda L., Moncayo-Lasso A., Hernández F., Ibáñez M., Torres-Palma R.A. Comparative degradation of two highly consumed antihypertensives in water by sonochemical process. Determination of the reaction zone, primary degradation products and theoretical calculations on the oxidative process. Ultrason. Sonochem. 2019;58:104635. doi: 10.1016/j.ultsonch.2019.104635. [DOI] [PubMed] [Google Scholar]
- 37.Naddeo V., Meriç S., Kassinos D., Belgiorno V., Guida M. Fate of pharmaceuticals in contaminated urban wastewater effluent under ultrasonic irradiation. Water Res. 2009;43(16):4019–4027. doi: 10.1016/j.watres.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Xiao R., Wei Z., Chen D., Weavers L.K. Kinetics and mechanism of sonochemical degradation of pharmaceuticals in municipal wastewater. Environ. Sci. Technol. 2014;48(16):9675–9683. doi: 10.1021/es5016197. [DOI] [PubMed] [Google Scholar]
- 39.Chiha M., Hamdaoui O., Baup S., Gondrexon N. Sonolytic degradation of endocrine disrupting chemical 4-cumylphenol in water. Ultrason. Sonochem. 2011;18(5):943–950. doi: 10.1016/j.ultsonch.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Isariebel Q.-P., Carine J.-L., Ulises-Javier J.-H., Anne-Marie W., Henri D. Sonolysis of Levodopa and paracetamol in aqueous solutions. Ultrason. Sonochem. 2009;16(5):610–616. doi: 10.1016/j.ultsonch.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Tran N., Drogui P., Zaviska F., Brar S.K. Sonochemical degradation of the persistent pharmaceutical Carbamazepine. J. Environ. Manage. 2013;131:25–32. doi: 10.1016/j.jenvman.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 42.Behnajady M.A., Modirshahla N., Tabrizi S.B., Molanee S. Ultrasonic degradation of Rhodamine B in aqueous solution: Influence of operational parameters. J. Hazard. Mater. 2008;152(1):381–386. doi: 10.1016/j.jhazmat.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Golash N., Gogate P.R. Degradation of Dichlorvos containing wastewaters using sonochemical reactors. Ultrason. Sonochem. 2012;19(5):1051–1060. doi: 10.1016/j.ultsonch.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Yao J.-J., Gao N.-Y., Li C., Li L., Xu B. Mechanism and kinetics of parathion degradation under ultrasonic irradiation. J. Hazard. Mater. 2010;175(1-3):138–145. doi: 10.1016/j.jhazmat.2009.09.140. [DOI] [PubMed] [Google Scholar]
- 45.Psillakis E., Goula G., Kalogerakis N., Mantzavinos D. Degradation of polycyclic aromatic hydrocarbons in aqueous solutions by ultrasonic irradiation. J. Hazard. Mater. 2004;108:95–102. doi: 10.1016/j.jhazmat.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Kim I.-K., Huang C.-P., Chiu P.C. Sonochemical decomposition of Dibenzothiophene in aqueous solution. Water Res. 2001;35(18):4370–4378. doi: 10.1016/S0043-1354(01)00176-2. [DOI] [PubMed] [Google Scholar]
- 47.Im J.K., Heo J., Boateng L.K., Her N., Flora J.R.V., Yoon J., Zoh K.D., Yoon Y. Ultrasonic degradation of acetaminophen and naproxen in the presence of single-walled carbon nanotubes. J. Hazard. Mater. 2013;254–255:284–292. doi: 10.1016/j.jhazmat.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Ziylan-Yavas A., Mizukoshi Y., Maeda Y., Ince N.H. Supporting of pristine TiO2 with noble metals to enhance the oxidation and mineralization of paracetamol by sonolysis and sonophotolysis. Appl. Catal. B Environ. 2015;172–173:7–17. doi: 10.1016/j.apcatb.2015.02.012. [DOI] [Google Scholar]
- 49.Davydov L., Reddy E.P., France P., Smirniotis P.G. Sonophotocatalytic destruction of organic contaminants in aqueous systems on TiO2 powders. Appl. Catal. B Environ. 2001;32(1-2):95–105. doi: 10.1016/S0926-3373(01)00126-6. [DOI] [Google Scholar]
- 50.Méndez-Arriaga F., Torres-Palma R.A., Pétrier C., Esplugas S., Gimenez J., Pulgarin C. Mineralization enhancement of a recalcitrant pharmaceutical pollutant in water by advanced oxidation hybrid processes. Water Res. 2009;43(16):3984–3991. doi: 10.1016/j.watres.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 51.Su S., Guo W., Yi C., Leng Y., Ma Z. Degradation of amoxicillin in aqueous solution using sulphate radicals under ultrasound irradiation. Ultrason. Sonochem. 2012;19(3):469–474. doi: 10.1016/j.ultsonch.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Monteagudo J.M., Durán A., Martín I.S., García S. Ultrasound-assisted homogeneous photocatalytic degradation of reactive blue 4 in aqueous solution. Appl. Catal. B Environ. 2014;152–153:59–67. doi: 10.1016/j.apcatb.2014.01.014. [DOI] [Google Scholar]
- 53.Maezawa A., Nakadoi H., Suzuki K., Furusawa T., Suzuki Y., Uchida S. Treatment of dye wastewater by using photocatalytic oxidation with sonication. Ultrason. Sonochem. 2007;14(5):615–620. doi: 10.1016/j.ultsonch.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Eslami A., Mehdipour F., Lin K.-Y., Sharifi Maleksari H., Mirzaei F., Ghanbari F. Sono-photo activation of percarbonate for the degradation of organic dye: The effect of water matrix and identification of by-products. J. Water Process Eng. 2020;33:100998. doi: 10.1016/j.jwpe.2019.100998. [DOI] [Google Scholar]
- 55.Kumar R., Kumar G., Akhtar M.S., Umar A. Sonophotocatalytic degradation of methyl orange using ZnO nano-aggregates. J. Alloys Compd. 2015;629:167–172. doi: 10.1016/j.jallcom.2014.12.232. [DOI] [Google Scholar]
- 56.Lops C., Ancona A., Di Cesare K., Dumontel B., Garino N., Canavese G., Hérnandez S., Cauda V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nanoparticles of ZnO. Appl. Catal. B Environ. 2019;243:629–640. doi: 10.1016/j.apcatb.2018.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou M., Yang H., Xian T., Li R.S., Zhang H.M., Wang X.X. Sonocatalytic degradation of RhB over LuFeO3 particles under ultrasonic irradiation. J. Hazard. Mater. 2015;289:149–157. doi: 10.1016/j.jhazmat.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 58.Bokhale N.B., Bomble S.D., Dalbhanjan R.R., Mahale D.D., Hinge S.P., Banerjee B.S., Mohod A.V., Gogate P.R. Sonocatalytic and sonophotocatalytic degradation of rhodamine 6G containing wastewaters. Ultrason. Sonochem. 2014;21(5):1797–1804. doi: 10.1016/j.ultsonch.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Khan M.A.N., Siddique M., Wahid F., Khan R. Removal of reactive blue 19 dye by sono, photo and sonophotocatalytic oxidation using visible light. Ultrason. Sonochem. 2015;26:370–377. doi: 10.1016/j.ultsonch.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Sadeghi M., Zabardasti A., Farhadi S., Yekta S., Mirzaei D. Immobilization of Cr-MIL-101 over the NiO/13X zeolite nanocomposite towards ultrasound-assisted destruction of organic dyes in aqueous media. J. Water Process Eng. 2019;32:100946. doi: 10.1016/j.jwpe.2019.100946. [DOI] [Google Scholar]
- 61.Pang Y.L., Abdullah A.Z. Comparative study on the process behavior and reaction kinetics in sonocatalytic degradation of organic dyes by powder and nanotubes TiO 2. Ultrason. Sonochem. 2012;19(3):642–651. doi: 10.1016/j.ultsonch.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Zhao T., Li P., Tai C., She J., Yin Y., Qi Y., Zhang G. Efficient decolorization of typical azo dyes using low-frequency ultrasound in presence of carbonate and hydrogen peroxide. J. Hazard. Mater. 2018;346:42–51. doi: 10.1016/j.jhazmat.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Zhao J., Wang X., Zhang L., Hou X., Li Y., Tang C. Degradation of methyl orange through synergistic effect of zirconia nanotubes and ultrasonic wave. J. Hazard. Mater. 2011;188(1-3):231–234. doi: 10.1016/j.jhazmat.2011.01.100. [DOI] [PubMed] [Google Scholar]
- 64.Sathishkumar P., Mangalaraja R.V., Mansilla H.D., Gracia-Pinilla M.A., Anandan S. Sonophotocatalytic (42kHz) degradation of Simazine in the presence of Au-TiO2 nanocatalysts. Appl. Catal. B Environ. 2014;160–161:692–700. doi: 10.1016/j.apcatb.2014.06.027. [DOI] [Google Scholar]
- 65.Li X., Zhang Y., Xie Y., Zeng Y., Li P., Xie T., Wang Y. Ultrasonic-enhanced Fenton-like degradation of bisphenol A using a bio-synthesized schwertmannite catalyst. J. Hazard. Mater. 2018;344:689–697. doi: 10.1016/j.jhazmat.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 66.Lu X., Zhao J., Wang Q., Wang D.a., Xu H., Ma J., Qiu W., Hu T. Sonolytic degradation of bisphenol S: Effect of dissolved oxygen and peroxydisulfate, oxidation products and acute toxicity. Water Res. 2019;165:114969. doi: 10.1016/j.watres.2019.114969. [DOI] [PubMed] [Google Scholar]